Abstract
The most common oncogenic driver in non-small cell lung cancer (NSCLC) is epidermal growth factor receptor (EGFR) gene mutations, which are more common in Asian (30–50%) than in Caucasian (10–15%) populations. Exon 19 deletion (ex19del) and exon 21 L858R (ex21 L858R) mutations account for ~45 and 40% of all EGFR mutations, respectively. Moreover, EGFR-tyrosine kinase inhibitors (TKIs) may be more effective and improve the quality of life of patients with NSCLC more than chemotherapy regimens. By contrast, patients with the ex21 L858R mutation may have a lower sensitivity and duration of response to EGFR-TKIs as well as a shorter survival compared with those with the ex19del mutation. However, current guidelines classify ex21 L858R and ex19del as the same condition and recommend the same treatment strategy for both. Aiming for precision medicine, the present review introduces and compares different EGFR-TKIs for the ex21 L858R mutation to assess more personalized treatment options for the population with this mutation.
Keywords: EGFR, ex19del, ex21 L858R, NSCLC, EGFR-TKIs
1. Introduction
According to estimates from the International Agency for Research on GLOBOCAN 2020, lung cancer was the second most commonly diagnosed cancer and the leading cause of cancer death in 2020, accounting for ~11.4% of diagnosed cancers and 18.0% of cancer deaths worldwide (1). China also faces a significant burden, with ~828,000 new lung cancer cases and 657,000 deaths reported in 2016, comprising ~20% of cancer incidence and 27% of mortality in the country (2). Non-small cell lung cancer (NSCLC) is the most prevalent type of lung cancer, accounting for ~85% of cases (3). Despite advancements in treatment options, the survival rates for patients with NSCLC remain low. The five-year survival rates for stage III and IV NSCLC are only ~15 and 10%, respectively (3).
The gene mutations of epidermal growth factor receptor (EGFR) are the most common cancer driver mutations in NSCLC (4). In normal cells, ligand binding to EGFR induces dimerization, subsequently leading to autophosphorylation of the tyrosine kinase domain (TKD), thereby transmitting pro-proliferative physiological signals. However, in the presence of an EGFR mutation, the TKD is homeostatically activated in a ligand-independent manner, resulting in the transmission of excessive pro-survival and pro-proliferation signals, which leads to cancer initiation and development (4). A subset of patients with NSCLC (10–15%) have EGFR mutations, which are more common among women and nonsmokers (3). Exon 19 deletions (ex19del) and exon 21 L858R (ex21 L858R) mutations are the most common, accounting for 85–90% of all NSCLC cases with an EGFR mutation.
EGFR-tyrosine kinase inhibitors (TKIs) are a class of small molecules that can inhibit the homeostatic activation of tyrosine kinase by reducing the relative affinity of TKD for adenosine triphosphate (ATP) (4). Targeted therapy with EGFR-TKIs has shown improved progression-free survival (PFS) outcomes compared with chemotherapy, making it the recommended first-line treatment for these patients (4). However, despite positive results in overall study populations from several randomized controlled trials (RCTs) and meta-analyses, patients with the ex21 L858R mutation have shown reduced sensitivity and duration of response to EGFR-TKIs, resulting in shorter survival compared with those with ex19del (5). As a result, strategies to address the challenges posed by the ex21 L858R substitution are still being sought. Nevertheless, a comprehensive review comparing survival benefits among different EGFR-TKIs and combination treatments for the ex21 L858R mutation has not been reported, to the best of our knowledge.
To introduce and discuss the current progress and prospects of using EGFR-TKIs for the treatment of patients with NSCLC with the ex21 L858R mutation, the present review performed a comprehensive literature search using PubMed/MEDLINE (https://pubmed.ncbi.nlm.nih.gov/), EMBASE (https://www.embase.com/), Cochrane Library (https://www.cochranelibrary.com/), ClinicalTrial.gov (https://clinicaltrials.gov/) and Google Scholar (https://scholar.google.com/) for all English-language studies published between December 31, 2010 and December 31, 2023. The search terms ‘NSCLC’, ‘EGFR’, ‘EGFR-TKI’, ‘EGFR mutation’, ‘anti-angiogenic’ and ‘chemotherapy’ were used, as well as their full names, synonyms and variations. Duplicated material, most preclinical studies, single-arm or phase I clinical trials, trials with no PFS data and review commentaries were excluded. After analyzing the abstracts and titles, 120 potentially eligible studies were carefully evaluated by full-text review. Finally, 85 articles were cited as references, including RCTs, retrospective clinical analyses, case reports, research articles and reviews.
2. Distinct molecular pathogenesis and clinical characteristics of ex21 L858R NSCLC
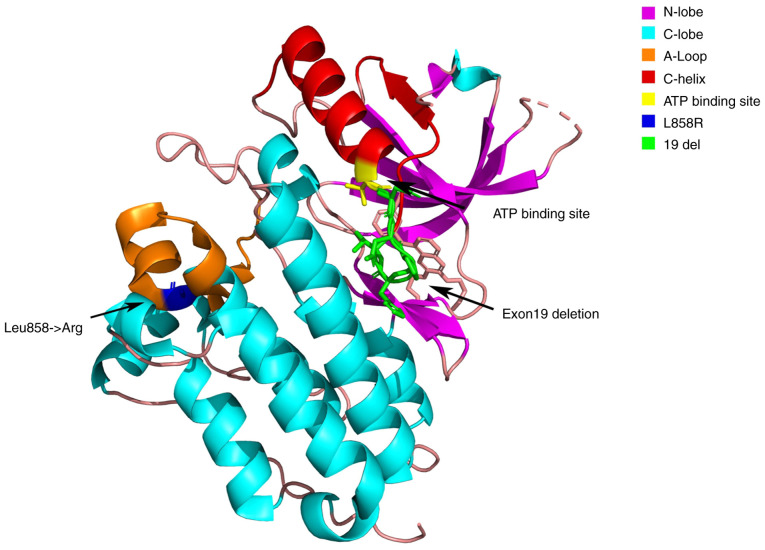
The L858R point mutation in exon 21 is a type II EGFR kinase domain mutation; it has a change of T to G in the 858th codon of the EGFR gene, resulting in the substitution of arginine for leucine at this position of the EGFR protein (6). As shown in Fig. 1, the ex21 L858R mutation is located in the activation loop (A-Loop) region of the hydroxy acid leaflet (C-Lobe) region of the EGFR kinase domain, away from the ATP-binding site, which may explain its resistance to TKIs (6). By contrast, ex19del is located in the C-helix region of the N-Lobe of EGFR, which is closer to the ATP-binding site and possesses high sensitivity to TKIs (6).
Figure 1.
Structure model of the N- and C-lobes in the epidermal growth factor receptor kinase domain, along with the mutation sites and functional areas. The N-lobe, located at the front, includes β-sheets and the C-helix adjacent to the ATP-binding site. The C-lobe, situated at the back, is mainly composed of α-helices and is crucial for substrate binding and catalysis. The ATP-binding site lies between the lobes. The L858R mutation is in the A-loop of the C-lobe, far from the site, whilst the exon 19 deletion is in the C-helix of the N-lobe, closer to the ATP-binding site.
Compared with tumor cells with the ex19del mutation, those with the ex21 L858R mutation exhibit distinct molecular pathogenesis characteristics that may be negatively associated with EGFR-TKI efficacy. For example, the ex21 L858R mutation is more frequently combined with co-mutations in tumor suppressor genes and is associated with a markedly shorter time to treatment failure on frontline TKI therapy (7). In addition, compared with ex19del, the degree of phosphorylation activation at EGFR phosphorylation site Y845 in an ex21 L858R cell line is greater, making TKI inhibition more challenging (8).
Compared with NSCLCs with the ex19del mutation, those with the ex21 L858R mutation exhibit less differentiation, greater malignancy and more aggressive clinical features. For example, ex21 L858R-mutated cells have increased expression of C-X-C motif chemokine receptor 4 (CXCR4) due to activation of the C-X-C motif chemokine ligand 12-CXCR4 pathway, thereby promoting the formation of malignant pleural effusion and enhancing the invasion capacity of cancer cells (9). Recently, by using single-cell RNA sequencing, Wang et al (10) demonstrated the nerve growth factor signaling pathway, which was exclusively detected in patients with the ex21 L858R mutation. By contrast, the tumor necrosis factor, Fas ligand, and semaphorin 3A signaling pathways were only detected in patients with ex19del. The uniquely identified pathways in the patients with ex19del were associated with anticancer activities, tumor necrosis and inhibition of angiogenesis. Such tumor microenvironment differences provide preliminary clues to further understand why patients with ex19del have improved clinical outcomes (10). Additionally, patients with ex21 L858R have been reported to have a shorter life expectancy and a worse prognosis than those with ex19del (11). As a result, more precise treatment for patients with NSCLC with the ex21 L858R mutation is required.
3. EGFR-TKI monotherapy for ex21 L858R NSCLC
A total of three generations of EGFR-TKIs
There are currently three generations of approved EGFR-TKIs: First-generation (1G) EGFR-TKIs, erlotinib and gefitinib; second-generation (2G) EGFR-TKIs, afatinib and dacomitinib; and third-generation (3G) EGFR-TKIs, osimertinib, aumolertinib, furmonertinib and befotertinib (12).
The quinazoline ring serves as the mother ring structure for both the 1G and 2G EGFR-TKIs (13). The 1G EGFR-TKIs bind to the ATP-binding site of EGFR reversibly and noncovalently, whereas the 2G EGFR-TKIs contain an acrylamide functional group that covalently binds to the ATP-binding domain Cys797 of EGFR for potent and long-lasting irreversible inhibition (13). The parent ring structure of the 3G EGFR-TKIs is 2-aminopyrimidine, and it retains the acrylamide functional group as an irreversible covalent-binding inhibitor (13). The several generations of EGFR-TKIs have different activity ranges due to their structural differences (14). The 1G EGFR-TKIs have the ability to inhibit EGFR-sensitive mutations. The 2G EGFR-TKIs are pan-human epidermal growth factor receptor (HER) inhibitors that cannot only irreversibly covalently inhibit multiple ErbB family receptors, including EGFR, ErbB2 and ErbB4, but also simultaneously inhibit the homodimerization and heterodimerization of HER family receptors, resulting in more potent antitumor activity by completely blocking the HER signaling pathway. The 3G EGFR-TKIs selectively inhibit the EGFR T790M mutation and the EGFR-sensitive mutation.
Monotherapy of 1G EGFR-TKIs in ex21 L858R NSCLC
The Iressa Pan-Asia Study (IPASS) is a phase III RCT that compared the 1G EGFR-TKI gefitinib to carboplatin/paclitaxel chemotherapy in previously untreated never-smokers and light ex-smokers with advanced lung adenocarcinoma (15). The IPASS results demonstrated a notable PFS benefit with gefitinib in patients with NSCLC with the ex21 L858R mutation [hazard ratio (HR), 0.55; 95% confidence interval (CI), 0.35–0.87], but no significant overall survival (OS) benefit was observed (15). Furthermore, the ENSURE study is a phase III RCT that compared first-line erlotinib to gemcitabine/cisplatin in patients with EGFR mutation-positive NSCLC from China, Malaysia and the Philippines. The study demonstrated that PFS, but not OS, was improved with erlotinib vs. gemcitabine + cisplatin chemotherapy. In patients with the 21 L858R mutation, the HR for PFS was 0.57 (95% CI, 0.31–1.05; median PFS, 8.3 vs. 7.1 months), whereas the HR for OS was 1.05 (95% CI, 0.60–1.84; median OS, 25.3 vs. 27.2 months) (16). However, neither the EURTAC (8.4 vs. 6.0 months; HR, 0.55; 95% CI, 0.29–1.02; P=0.054) nor the CONVINCE study (11.1 vs. 7.8 months; HR, 0.76; 95% CI, 0.43–1.33; P=0.331) demonstrated a significant PFS benefit with 1G EGFR-TKIs (17,18). Additionally, the INCREASE study evaluated the efficacy of high-dose icotinib (250 mg, three times/day), another 1G EGFR-TKI for patients with ex21 L858R-mutated. The results revealed a prolonged PFS compared with regular-dose icotinib (125 mg, three times/day; 12.9 vs. 9.2 months; HR, 0.75; 95% CI, 0.53–1.05; P<0.05) (19). The aforementioned results are presented in Table I.
Table I.
Characteristics of clinical trials evaluating epidermal growth factor receptor-tyrosine kinase inhibitor monotherapy in patients with non-small cell lung cancer with the ex21 L858R mutation.
| First author/s, year | Trial | Registration no. | Phase | Intervention arm | Control arm | Sample size of patients with ex21 L858R(Intervention/Control) | PFS HR (95% CI) | OS HR (95% CI) | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Fukuoka et al, 2011 | IPASS | NCT00322452 | III | Gefitinib | Carboplatin + paclitaxel | 64/67 | 0.55 (0.35–0.87) | NR | (15) |
| Rosell et al, 2012 | EURTAC | NCT00446225 | III | Erlotinib | Cisplatin/carboplatin + docetaxel/gemcitabine | 29/29 | 0.55 (0.29–1.02) | 0.99 (0.56–1.76) | (17) |
| Wu et al, 2015 | Ensure | NCT01342965 | III | Erlotinib | Gemcitabine + cisplatin | 52/46 | 0.57 (0.31–1.05) | 1.05 (0.60–1.84) | (16) |
| Shi et al, 2017 | CONVINCE | NCT01719536 | III | Icotinib | Cisplatin + pemetrexed | 68/63 | 0.76 (0.43–1.33) | NR | (18) |
| Li et al, 2020 | INCREASE | NCT02404675 | II | Icotinib-HD | Icotinib | 90/86 | 0.75 (0.53–1.05) | NR | (19) |
| Sequist et al, 2013 and Yang et al, 2015 | LUX-Lung3 | NCT00949650 | III | Afatinib | Cisplatin + pemetrexed | 91/47 | 0.73 (0.46–1.17) | 1.30 (0.80–2.11) | (20,22) |
| Wu et al, 2014 and Yang et al, 2015 | LUX-Lung6 | NCT01121393 | III | Afatinib | Gemcitabine + pemetrexed | 92/46 | 0.32 (0.19–0.52) | 1.22 (0.81–1.83) | (21,22) |
| Park et al, 2016 and Paz-Ares et al, 2017 | LUX-Lung7 | NCT01024413 | II | Afatinib | Gefitinib | 67/66 | 0.71 (0.48–1.06) | 0.91 (0.62–1.36) | (23,24) |
| Wu et al, 2017, Mok et al, 2018 and Mok et al, 2021 | ARCHER1050 | NCT01774721 | II | Dacomitinib | Gefitinib | 93/92 | 0.63 (0.44–0.88) | 0.67 (0.47–0.94) | (26–28) |
| Cheng et al, 2021 | ARCHER1050 (Asian) | NCT01774721 | II | Dacomitinib | Gefitinib | 71/73 | 0.50 (0.34–0.76) | 0.62 (0.42–0.93) | (29) |
| Pluzanski et al, 2021 | ARCHER1050 (SUB) | NCT01774721 | II | Dacomitinib | Gefitinib | 93/92 | 0.50 (0.33–0.75) | 0.47 (0.31–0.71) | (30) |
| Mok et al, 2017 | AURA3 | NCT02151981 | II | Osimertinib | Platinum-pemetrexed | 83/45 | 0.46 (0.30–0.71) | NR | (50) |
| Ramalingam et al, 2020 and Soria et al, 2018 | FLAURA | NCT02296125 | III | Osimertinib | Gefitinib/erlotinib | 97/90 | 0.51 (0.36–0.71) | 1.00 (0.71–1.40) | (37,36) |
| Lu et al, 2022 | AENEAS | NCT03849768 | III | Aumolertinib | Gefitinib | 74/74 | 0.60 (0.40–0.98) | NR | (38) |
CI, confidence interval; HD, high dose; HR, hazard ratio; NR, not reported; OS, overall survival; PFS, progression-free survival; IPASS, Iressa Pan-Asia Study.
Monotherapy of 2G EGFR-TKIs in ex21 L858R NSCLC
In patients with the ex21 L858R mutation, afatinib demonstrated a marked PFS benefit (HR, 0.32; 95% CI, 0.19–0.52) compared with chemotherapy in the Asian LUX-Lung 6 trial, but not in the global LUX-Lung 3 trial (HR, 0.73; 95% CI, 0.46–1.17), possibly due to differences in the study population or the efficacy of the chemotherapy comparators used in each study (20,21). However, neither of the two trials demonstrated a significant improvement in OS with first-line afatinib vs. chemotherapy for ex21 L858R (20–22). Afatinib was compared with gefitinib in the LUX-Lung 7 trial, and neither PFS (10.9 vs. 10.8 months; HR, 0.71; 95% CI, 0.48–1.06) nor OS benefit (25.0 vs. 21.2 months; HR, 0.91; 95% CI, 0.62–1.36) was observed in patients with the ex21 L858R mutation (23,24). In line with this finding, Lau et al (25) reported no significant improvement in OS for patients with the ex21 L858R mutation when they compared afatinib to gefitinib and erlotinib in a real-world study (median OS, 25.4 vs. 20.6 months).
In comparison, the encouraging results of dacomitinib, another 2G EGFR-TKI, warrant special attention. Notably, the ARCHER 1050 trial reported that dacomitinib outperformed gefitinib in terms of both PFS (12.3 vs. 9.8 months; HR, 0.63; 95% CI, 0.44–0.88) and OS (32.5 vs. 23.2 months; HR, 0.67; 95% CI, 0.47–0.94) in patients with the ex21 L858R mutation (26–28). According to a sub-analysis of ARCHER 1050, first-line dacomitinib was markedly associated with prolongation of PFS and improved OS compared with gefitinib in patients from Asian populations with the ex21 L858R mutation: The mean PFS for dacomitinib compared with gefitinib was 16.0 vs. 11.1 months, with an HR of 0.505 (95% CI, 0.337–0.758; P=0.0004), and the median OS was 36.5 months vs. 25.6 months, with an HR of 0.622 (95% CI, 0.415–0.931; P=0.01) (29). In another sub-analysis, improvements in PFS and OS with dacomitinib over gefitinib were observed in patients with dacomitinib dose reduction in the ex21 L858R mutation subgroup. Dacomitinib dose reduction improved PFS and OS in 67 and 68% of patients with ex21 L858R, respectively, making dacomitinib the first TKI to demonstrate improved PFS and OS over gefitinib in patients with dose reduction in the ex21 L858R subgroup (30).
Consistent real-world data report the benefits of dacomitinib in the ex21 L858R population, as demonstrated in ARCHER1050. In a multicenter retrospective analysis (TOPGAN2020-02), patients from nine Japanese institutions who received dacomitinib for advanced EGFR-mutant NSCLC that had progressed after EGFR-TKI treatment were included. Subset analysis indicated that patients with the ex21 L858R mutation had a longer PFS than those with ex19del (median PFS, 5.8 vs. 4.1 months; P=0.018) (31). In another real-world study in China, first-line dacomitinib treatment demonstrated a promising efficacy (median PFS, 16.3 months) and tolerable adverse events (AEs) among patients with NSCLC with the EGFR ex21 L858R mutation (32). The initial dose and baseline brain metastasis status did not have a significant impact on the PFS among these patients (32). ARIA is a noninterventional study of the real-world utilization of dacomitinib and the associated clinical outcomes in patients from Asian populations with advanced EGFR mutation-positive NSCLC (33). Its recent interim analysis reported that at data cutoff, the median real-world PFS was longer in patients with the ex21 L858R mutation than in those with ex19del (30.5 vs. 20.3 months) (33). The aforementioned results are presented in Table I.
3G EGFR-TKIs in ex21 L858R NSCLC
The acquired T790M mutation has been reported in 36.5% of patients with ex21 L858R after 1G or 2G TKI treatment (34). The 3G EGFR-TKIs are irreversible inhibitors that were developed to overcome the increased ATP and EGFR affinity caused by the T790M mutation (35). In the FLAURA study, patients with the ex21 L858R mutation who received osimertinib experienced a significant improvement in PFS (14.4 vs. 9.5 months; HR, 0.51; 95% CI, 0.36–0.71; P<0.001) but not OS (HR, 1.00; 95% CI, 0.71–1.40) compared with those treated with the 1G EGFR-TKI gefitinib or erlotinib (36,37). In the phase III trial AENEAS (NCT03849768), the median PFS for the aumolertinib and gefitinib groups was 13.4 and 8.3 months, respectively (HR, 0.60; 95% CI, 0.40–0.89; P=0.0102) (38). The 12-month OS rates for aumolertinib and gefitinib were similar, at 86.2% (95% CI, 80.8–90.2) and 85.3% (95% CI, 79.8–89.4), respectively. Additionally, the real-world research OSI-FACT retrospectively assessed osimertinib as a first-line treatment for EGFR-mutant NSCLC. In terms of mutation types, the median PFS for those with the ex21 L858R mutation was 16.9 months (95% CI, 13.6-not reported), demonstrating a comparable level of disease control to that observed in clinical trials (39). However, the superiority of osimertinib over the 2G EGFR-TKIs remains unknown in the real world. Ito et al (40) evaluated patients with NSCLC treated with osimertinib or afatinib as the first-line therapy at 15 Japanese institutions between May 2016 and October 2019. The survival curves for patients with ex21 L858R indicated that afatinib tended to provide an improved survival benefit than osimertinib. Additionally, there were marked differences in PFS and OS between the two treatments for patients with ex21 L858R without brain metastases, with HRs favoring afatinib (40). In another Dutch nationwide real-world cohort, Gijtenbeek et al (41) confirmed that the OS benefit for patients with ex21 L858R treated with osimertinib as the first-line therapy was not superior to that of 1G and 2G TKIs. The aforementioned results are presented in Table I.
Fourth generation (4G) EGFR-TKIs
The 4G EGFR-TKIs have been developed for targeting the osimertinib-resistant mutations T790M and C797S. In preclinical and/or phase I studies, a number of 4G EGFR-TKIs, including BLU-945, EAIO45, JIN-A02 and BBT176, have demonstrated inhibitory activities for classical EGFR mutations as well as T790M + C797S (42–44). Additional 4G TKI therapeutic data for ex21 L858R is expected in the future.
Safety of EGFR-TKI monotherapy
Rash, diarrhea, paronychia, oral mucositis, hepatotoxicity and interstitial lung disease are among the most common AEs associated with EGFR-TKIs, with different generations of EGFR-TKIs having different safety profiles (45,46). As 1G EGFR-TKIs are associated with an increased risk of hepatotoxicity and interstitial lung disease, they should be used with caution in patients with liver disease (45,46). Meanwhile, 2G TKIs are associated with common and tolerable AEs such as dermatitis, diarrhea and oral mucositis (47). Furthermore, 3G TKIs have a reduced prevalence of typical AEs such as rash and diarrhea, but they are coupled with a higher risk of cardiotoxicity (48). As a result, patients with pre-existing heart problems should use them with caution.
Comparison of the safety of different generations of EGFR-TKIs is undefined in the general study population that includes both patients with ex19del and ex21 L858R. In LUX-Lung 7, the frequency of grade ≥3 AEs was reported to be similar in patients receiving afatinib or gefitinib (57% vs. 52%) (23). However, grade ≥3 treatment-related AEs (TRAEs) were reported in 63% of patients given dacomitinib and in 41% of patients given gefitinib in ARCHER 1050 (26). In FLAURA, AEs of grade 3 or higher were less frequent in patients treated with osimertinib than with gefitinib or erlotinib (34% vs. 45%) (36), whilst patients in the aumolertinib and gefitinib groups experienced comparable grade ≥3 AEs in AENEAS (36.4% vs. 35.8%) (38). By contrast, patients who received EGFR-TKIs had apparently fewer grade ≥3 AEs than those who received chemotherapy, with 28.7% vs. 61.0% in IPASS (49), 9.5% vs. 24.8% in CONVINCE (18), 35.5% vs. 57.7% in ENSURE (16), 45% vs. 67% in EURTAC (17), 49% vs. 48% in LUX-Lung 3 (20), 36.0% vs. 60.2% in LUX-Lung 6 (21) and 23% vs. 47% in AURA3 (50). INCREASE is the only trial that reported safety evaluations in different mutation subpopulations: Patients in the L858R-high-dose group experienced notably more TRAEs (81%) than those in the L858R-reduced-dose group (55%) and ex19del-reduced-dose group (66%), whereas the incidences of grade 3/4 TRAEs were similar across all groups (19). Nevertheless, the potential for safety differences between EGFR mutation subgroups warrants further investigation.
Summary of EGFR-TKI monotherapy
Whilst the PFS benefits of 3G TKIs outperform those of 1G and 2G TKIs, only 2G dacomitinib has demonstrated OS benefits, and 3G TKI OS data remain unmatured. Certain preclinical evidence may explain the possible reason for the efficacy of dacomitinib in ex21 L858R NSCLC. An in-vitro study reported that dacomitinib can effectively inhibit EGFR phosphorylation, particularly hyperphosphorylation at the Y845 site, which is one of the reasons for the low sensitivity of the ex21 L858R mutation to TKIs (51). Another in-vitro study reported that dacomitinib had a sustained inhibitory effect on EGFR in cells expressing the ex21 L858R mutation for ≤30 min after drug withdrawal, whereas the inhibitory effect of the 1G and 3G TKIs vanished immediately (52). Additionally, the half-maximal inhibitory concentration value of 2G TKIs (2.6–4 nM) was much lower than that of 1G TKIs (16–26 nM) and the 3G TKI osimertinib (9 nM), especially for dacomitinib, indicating another explanation that dacomitinib has a stronger inhibitory effect on the ex21 L858R mutation than other TKIs (53). These preliminary findings support the use of dacomitinib in the treatment of ex21 L858R NSCLC.
Furthermore, studies have reported that sequential 3G TKIs following initial 2G TKI treatment may also result in long OS benefits. The ARCHER1050 study reported that the median OS of patients with the ex21 L858R mutation who were resistant to first-line dacomitinib therapy followed by osimertinib was 44.7 months, demonstrating the survival benefit of 2G sequential 3G TKI therapy (30). In addition, two previous noninterventional trials, GioTag (NCT03370770) and UpSwinG (NCT04179890), reported notable OS results in patients who received sequential afatinib/osimertinib. The GioTag observational study evaluated the role of sequential osimertinib after afatinib failure in patients with ex21 L858R mutations, reporting an OS of 33.0 (29.8–37.0) months for these patients (54). In the global study UpSwinG, first-line afatinib and second-line osimertinib resulted in a median OS of 33.1 (24.9–41.8) months in patients with the ex21 L858R mutation in regular clinical practice (55). Moreover, Miura et al (56) performed a combined analysis of patients from Asian populations from both studies, and the results indicated a median OS of 39.1 (29.3–48.5) months in patients with the ex21 L858R mutation. More research comparing first-line 3G EGFR-TKIs to sequential therapy for patients with NSCLC with the ex21 L858R mutation is expected (57,58).
4. Combination therapy of EGFR-TKIs in ex21 L858R NSCLC
Mechanism of EGFR-TKI combination therapy
Combination therapy is currently a common treatment option for NSCLC with EGFR mutations. Combining EGFR-TKIs with antiangiogenic therapy (T+A) or chemotherapy (T+C) can produce synergistic antitumor effects via several potential molecular mechanisms. By activating caspase-7, the combination of EGFR-TKIs and chemotherapy enhances apoptotic signaling, significantly inhibiting cell growth, promoting cell death and preventing resistance mediated by epithelial-mesenchymal transition (59,60). Additionally, antiangiogenic agents can normalize the vascular wall and network structure, enhancing the delivery and penetration of EGFR-TKIs into tumor tissues (61). EGFR is also expressed on tumor-associated endothelial cells, and its inhibition by EGFR-TKIs in combination with antiangiogenic agents can suppress tumor angiogenesis synergistically (62). Furthermore, antiangiogenic therapy can prevent compensatory increases in angiogenesis from both tumor and stromal sources caused by EGFR-TKIs (63).
Combination of EGFR-TKIs and chemotherapy
Several studies have assessed the clinical efficacy of combining EGFR-TKIs and chemotherapy for patients with EGFR mutations, and combination therapy shows clinical benefits compared with EGFR-TKI monotherapy. A significant PFS benefit was demonstrated for patients with L858R receiving combination therapy compared with patients receiving gefitinib in the JMIT trial (median PFS, 12.6 vs. 10.9 months; HR, 0.58; 95% CI, 0.33–1.01; P=0.054), NCT02148380 trial (HR, 0.31; 95% CI, 0.15–0.66) and NEJ009 trial (HR, 0.55; 95% CI, 0.38–0.80) (64–67). As presented at the 2023 World Conference on Lung Cancer, in FLAURA2, osimertinib in combination with chemotherapy demonstrated a marked PFS improvement compared with osimertinib alone for patients with the L858R mutation (median PFS, 24.7 vs. 13.9 months; HR, 0.63; 95% CI, 0.44–0.90), with a benefit degree similar to that of the ex19del subgroup (68). In addition, Yan et al (69) retrospectively analyzed 76 patients with NSCLC with EGFR mutations who received EGFR-TKI monotherapy (gefitinib, erlotinib or icotinib) or in combination with a platinum-based regimen (cisplatin or carboplatin + paclitaxel, docetaxel, pemetrexed or gemcitabine). The results demonstrated that the PFS and OS of patients with L858R in the combination group were significantly longer than in the monotherapy group (median PFS, 7.2 vs. 5.8 months; P=0.013; and median OS, 22.0 vs. 18.7 months; P=0.024). The aforementioned results are presented in Table II.
Table II.
Characteristics of clinical trials evaluating epidermal growth factor receptor-tyrosine kinase inhibitor combined therapy in patients with non-small cell lung cancer with the ex21 L858R mutation.
| First author/s, year | Trial | Registration no. | Phase | Intervention arm | Control arm | Sample size of patients with ex21 L858R (Intervention/Control) | PFS HR (95% CI) | OS HR (95% CI) | (Refs.) |
|---|---|---|---|---|---|---|---|---|---|
| Cheng et al, 2016 | JMIT | NCT01469000 | II | Gefitinib + pemetrexed | Gefitinib | 52/23 | 0.58 (0.33–1.01) | NR | (64) |
| Han et al, 2017 | NCT02148380 | NCT02148380 | II | Gefitinib + pemetrexed + carboplatin | Gefitinib | 19/20 | 0.31 (0.15–0.66) | NR | (65) |
| Hosomi et al, 2020 and Miyauchi et al, 2022 | NEJ009 | UMIN000006340 | III | Gefitinib + pemetrexed + carboplatin | Gefitinib | 69/67 | 0.55 (0.38–0.80) | 0.80 (0.53–1.20) | (66,67) |
| Jänne et al, 2023 | FLAURA2 | NCT04035486 | III | Osimertinib + pemetrexed + cisplatin or carboplatin | Osimertinib | NA | 0.63 (0.44–0.90) | NR | (68) |
| Zhao et al, 2021 | CTONG1706 | NCT02824458 | III | Gefitinib + apatinib | Gefitinib | 74/73 | 0.72 (0.48–1.09) | NR | (74) |
| Zhou et al, 2021 | CTONG1509 | NCT02759614 | III | Erlotinib + bevacizumab | Erlotinib | 75/75 | 0.50 (0.32–0.77) | 1.36 (0.53–3.47) | (70) |
| Saito et al, 2019 | NEJ026 | UMIN000017069 | III | Erlotinib + bevacizumab | Erlotinib | 56/57 | 0.57 (0.33–0.97) | NR | (71) |
| Seto et al, 2014 | JO25567 | JapicCTI-111390 | II | Erlotinib + bevacizumab | Erlotinib | 35/37 | 0.67 (0.38–1.18) | NR | (73) |
| Nakagawa et al, 2019 | RELAY | NCT02411448 | III | Erlotinib + ramucirumab | Erlotinib | 99/105 | 0.62 (0.44–0.87) | NR | (72) |
| Nishio et al, 2021 | RELAY Japanese | NCT02411449 | III | Erlotinib + ramucirumab | Erlotinib | 56/54 | 0.51 (0.32–0.84) | NR | (76) |
| Lee et al, 2023 | NCC2016-0107 | NCT03126799 | II | Erlotinib + ramucirumab | Erlotinib | 27/26 | 0.63 (0.35–1.13) | NR | (75) |
CI, confidence interval; HR, hazard ratio; NR, not reported; OS, overall survival; PFS, progression-free survival.
Combination of EGFR-TKIs and antiangiogenic therapy
The combination of EGFR-TKIs and antiangiogenic drugs has also shown efficacy in first-line treatment for patients with advanced EGFR-mutated NSCLC. A total of four clinical trials assessed the prognosis of the combination of erlotinib and bevacizumab compared with erlotinib, including the CTONG1509 trial, NEJ026 trial, JO25567 trial and a phase II trial in Japan. Moreover, NCT03126799 and the RELAY study evaluated the efficacy and safety of the combination of erlotinib and ramucirumab compared with erlotinib, and CTONG1706 was performed to compare apatinib + gefitinib with gefitinib monotherapy (70–75). For patients with the L858R mutation, a significant PFS benefit of combined therapy was demonstrated in the CTONG1509 trial (median PFS, 19.5 vs. 9.7 months; HR, 0.50; 95% CI. 0.32–0.77; P=0.001), NEJ026 trial (median PFS, 17.4 vs. 13.7 months; HR, 0.57; 95% CI. 0.33–0.97), RELAY trial (median PFS, 19.4 vs. 11.2 months; HR, 0.62; 95% CI, 0.44–0.87; P=0.006) and RELAY Japanese Subset (median PFS, 19.4 vs. 10.9 months; HR, 0.51; 95% CI, 0.32–0.84; P=0.006) (70–72,76). However, no significant OS benefit was demonstrated for the ex21 L858R mutation in these ongoing trials (70–72). Moreover, PFS benefits were not revealed to be significant in the exon 21 L858R subgroup in the JO25567 trial (median PFS, 13.9 vs. 7.1 months; HR, 0.67; 95% CI, 0.38–1.18; P=0.1653), CTONG1706 (median PFS, 11.9 vs. 10.1 months; HR, 0.72; 95% CI, 0.48–1.09; P=0.123) and NCT03126799 (HR, 0.63; 95% CI. 0.35–1.13; P=0.118) (73–75). The aforementioned results are presented in Table II.
Safety of EGFR-TKI combination therapy
In the general population, the safety profiles of EGFR-TKI combination therapy were consistent with the known profiles of both drugs, including liver dysfunction, neutropenia, fatigue, hematologic toxicities and skin rash. The safety findings in several studies are constant in that combined therapy has more AEs than monotherapy. For T+C trials, the proportion of grade ≥3 treatment-emergent AEs (TEAEs) was notably higher in the gefitinib + chemotherapy group than in the gefitinib group in both the JMIT study (42% vs. 19%) (64) and the NEJ009 study (65.3% vs. 31.0%) (66). Similarly, for the T+A regimen, patients receiving erlotinib + bevacizumab or ramucirumab experienced more grade ≥3 TEAEs than patients in the erlotinib-only group, with 54.8% vs. 26.1% in CTONG1509 (70), 88% vs. 46% in NEJ026 (71), 72% vs. 54% in RELAY (72), 91% vs. 53% in JO25567 (73) and 50.6% vs. 20.6% in NCC2016-0107 (75). Notably, in CTONG1706, grade ≥3 TEAEs were also reported more frequently in the apatinib + gefitinib group than in the gefitinib monotherapy group (84.1% vs. 37.7%) (74). However, no data on the safety differences between EGFR mutation subgroups have been published, to the best of our knowledge.
Summary of EGFR-TKI combination therapy
In general, studies have reported that both T+A and T+C combination therapies had improved PFS for patients with L858R compared with EGFR-TKI monotherapy (64–75). However, it is unknown whether monotherapy or combination therapy is a more effective first-line treatment for these patients in terms of OS benefits. Amivantamab is an EGFR- and mesenchymal-epithelial transition-bispecific antibody with immune cell-directing activity (33). The phase III trial MARIPOSA (NCT04487080) compared amivantamab + lazertinib, a central nervous system-penetrating 3G EGFR-TKI, to osimertinib in the first-line setting. Its early PFS data demonstrated that amivantamab + lazertinib outperformed osimertinib in patients with the ex21 L858R mutation (HR, 0.78; 95% CI, 0.59–1.02) or ex19del (HR, 0.65; 95% CI, 0.51–0.85) after a median follow-up of 22.0 months (33). Future studies combining EGFR-TKIs with treatments other than chemotherapy and antiangiogenic therapy deserve attention.
Additionally, despite their effectiveness in extending survival benefits, the combination of other treatments with EGFR-TKIs may result in additional AEs. A meta-analysis by Qi et al (77) revealed that compared with osimertinib, the combination of 1G T+A (HR, 2.40; 95% CI, 1.70–3.40) and 1G T+C (HR, 2.50; 95% CI, 1.60–4.60) significantly increased the risk of grade 3 or worse AEs, with the following risk rank: osimertinib < 1G EGFR-TKIs < 2G EGFR-TKIs < 1G T+A < 1G T+C. As a result, when prescribing combination treatments, clinicians should be more cautious about the increased toxicities as well as the toxicity profile of each treatment for clinical decision making and improved management.
Furthermore, regimens combining EGFR TKIs with additional EGFR-targeting therapies have been assessed to delay/prevent resistance and to achieve more intense suppression. For example, studies have begun to investigate combination therapy with EGFR TKIs and EGFR monoclonal antibodies (mAbs) due to their synergistic antitumor effects: EGFR mAbs inhibit ligand-induced activation of TKD by blocking ligand-EGFR binding extracellularly, whilst EGFR TKIs reduce the relative affinity of TKD for ATP intracellularly (78–80). This ‘sandwich’ strategy also enables a decreased dosage of each medication, facilitating the management of adverse effects without compromising the therapeutic efficacy. Preclinical data indicate that the afatinib-based ‘sandwich’ method notably delays drug resistance in transgenic mice carrying tumors with the EGFR L858R mutation (78). However, the phase II RCT SWOG S1403 reported that adding cetuximab to afatinib did not enhance outcomes in patients with NSCLC with classic mutations (exon 19del and exon 21 L858R), with 30% of patients discontinuing cetuximab due to intolerable AEs (79). Using comprehensive pharmacology profiling and molecular docking analyses, a recent study identified EGFR as a potential target of the key therapeutic component of Spirulina, suggesting that small molecules acting through several mechanisms to EGFR may be used as adjuvants to enhance the treatment effectiveness or sensitivities of EGFR TKIs (80).
5. Conclusions and perspectives
In summary, due to their distinct clinicopathological characteristics and response to EGFR-TKIs, patients with NSCLC with the ex21 L858R mutation should be treated as a separate subpopulation (81). However, current guidelines classify ex21 L858R and ex19del as the same condition and recommend the same treatment strategy for both (82). As a result, several issues must be addressed in future studies aimed at precision medicine. First, the mechanism of the poor response toward EGFR-TKIs in those with the ex21 L858R mutation is currently not fully understood, and further research is highly anticipated to help delay resistance and provide therapeutic benefits, particularly in terms of prolonging OS. As clinical evidence suggests that dacomitinib improves OS in patients with ex21 L858R, more research into using dacomitinib-based sequential or combination therapy to benefit this subgroup is warranted (26–30). For patients resistant to 1G or 2G EGFR-TKI therapy, T790M mutation detection should be performed to support the selection of 3G EGFR-TKI therapy (42,83).
Additionally, a number of ongoing phase III studies involving patients with ex21 L858R receiving combination therapy with 3G EGFR-TKIs merit attention. These include first-line combined chemotherapy (LAURA/NCT03521154 and NCT04923906), first-line combined antiangiogenic therapy (NCT04181060), first-line combined EGFR/mesenchymal-epithelial transition double antibody therapy (MARIPOSA/NCT04487080 and MARIPOSA-2/NCT04988295) and first-line combined EGFR/HER3 double antibody therapy (NCT05020769). A group of prospective trials focusing on the combination of osimertinib and antiangiogenic drugs (UMIN000028071 and NCT 0281579) is expected to improve efficacy and break the treatment bottleneck for patients with the L858R mutation in the first-line setting. As a result, the OS benefit of combining EGFR-TKIs with antiangiogenic therapy in patients with the ex21 L858R mutation warrants further investigation.
The release of the aforementioned research data will help to further clarify the efficacy and safety of several combination therapy modes of 3G EGFR-TKIs for patients with ex21 L858R. Notably, the first phase III clinical trial targeting only patients with the ex21 L858R mutation in NSCLC is ongoing (84). This study, REVOL858R (WJOG14420L), will enroll 230 patients with ex21 L858R to compare the clinical efficacy of erlotinib + ramucirumab to osimertinib monotherapy (84). The findings of the trial may aid in the discovery of more treatment options for these patients with ex21 L858R mutation.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AE
adverse event
- ATP
adenosine triphosphate
- CI
confidence interval
- CXCR4
C-X-C motif chemokine receptor 4
- EGFR
epidermal growth factor receptor
- TKIs
tyrosine kinase inhibitors
- ex21 L858R
exon 21 L858R
- ex19del
exon 19 deletion
- HER
human epidermal growth factor receptor
- HR
hazard ratio
- IPASS
Iressa Pan-Asia Study
- mAbs
monoclonal antibodies
- NSCLC
non-small cell lung cancer
- OS
overall survival
- PFS
progression-free survival
- RCTs
randomized controlled trials
- TEAE
treatment-emergent adverse event
- TKD
tyrosine kinase domain
- TRAE
treatment-related adverse event
- 1G
first-generation
Funding Statement
This report was supported by funding from Pfizer China.
Availability of data and materials
Not applicable.
Authors' contributions
JYL, SZW and HQY collected data and prepared the initial draft. JLL and PYX verified the authenticity of all original data and reviewed and edited the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
Dacomitinib is a Pfizer product. The authors have no additional competing interests to declare.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Zheng R, Zhang S, Zeng H, Wang S, Sun K, Chen R, Li L, Wei W, He J. Cancer incidence and mortality in China, 2016. J Natl Cancer Cent. 2022;2:1–9. doi: 10.1016/j.jncc.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duma N, Santana-Davila R, Molina JR. Non-Small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Nan X, Xie C, Yu X, Liu J. EGFR TKI as First-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer. Oncotarget. 2017;8:75712–75726. doi: 10.18632/oncotarget.20095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang CY, Yang JC, Yang PC. Precision management of advanced Non-small cell lung cancer. Annu Rev Med. 2020;71:117–136. doi: 10.1146/annurev-med-051718-013524. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell RA, Luwor RB, Burgess AW. Epidermal growth factor receptor: Structure-function informing the design of anticancer therapeutics. Exp Cell Res. 2018;371:1–19. doi: 10.1016/j.yexcr.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 7.Hellyer JA, White MN, Gardner RM, Cunanan K, Padda SK, Das M, Ramchandran K, Neal JW, Wakelee HA. Impact of tumor suppressor gene Co-mutations on differential response to EGFR TKI therapy in EGFR L858R and exon 19 deletion lung cancer. Clin Lung Cancer. 2022;23:264–272. doi: 10.1016/j.cllc.2021.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Hong W, Wu Q, Zhang J, Zhou Y. Prognostic value of EGFR 19-del and 21-L858R mutations in patients with non-small cell lung cancer. Oncol Lett. 2019;18:3887–3895. doi: 10.3892/ol.2019.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsai MF, Chang TH, Wu SG, Yang HY, Hsu YC, Yang PC, Shih JY. EGFR-L858R mutant enhances lung adenocarcinoma cell invasive ability and promotes malignant pleural effusion formation through activation of the CXCL12-CXCR4 pathway. Sci Rep. 2015;5:13574. doi: 10.1038/srep13574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang T, Zheng L, Wang Q, Xiao S. Single-cell analyses reveal tumor microenvironment differences between EGFR 19del and L858R mutations in lung adenocarcinoma. J Thorac Oncol. 2022;17((Suppl)):S606. doi: 10.1016/j.jtho.2022.07.1099. [DOI] [Google Scholar]
- 11.Rossi A, Mari E. EGFR-Mutant Non-small cell lung cancer: State-of-the-Art and future perspectives. EMJ. 2022;7:112–122. [Google Scholar]
- 12.Abourehab MAS, Alqahtani AM, Youssif BGM, Gouda AM. Globally approved EGFR inhibitors: Insights into their syntheses, target kinases, biological activities, receptor interactions, and metabolism. Molecules. 2021;26:6677. doi: 10.3390/molecules26216677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amelia T, Kartasasmita RE, Ohwada T, Tjahjono DH. Structural insight and development of EGFR tyrosine kinase inhibitors. Molecules. 2022;27:819. doi: 10.3390/molecules27030819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard N. Optimizing outcomes in EGFR mutation-positive NSCLC: Which tyrosine kinase inhibitor and when? Future Oncol. 2018;14:1117–1132. doi: 10.2217/fon-2017-0636. [DOI] [PubMed] [Google Scholar]
- 15.Fukuoka M, Wu YL, Thongprasert S, Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu DT, Saijo N, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS) J Clin Oncol. 2011;29:2866–2874. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 16.Wu YL, Zhou C, Liam CK, Wu G, Liu X, Zhong Z, Lu S, Cheng Y, Han B, Chen L, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: Analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26:1883–1889. doi: 10.1093/annonc/mdv050.44. [DOI] [PubMed] [Google Scholar]
- 17.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 18.Shi YK, Wang L, Han BH, Li W, Yu P, Liu YP, Ding CM, Song X, Ma ZY, Ren XL, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): A phase 3, open-label, randomized study. Ann Oncol. 2017;28:2443–2450. doi: 10.1093/annonc/mdx359. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Zhang L, Jiang D, Wang Y, Zang A, Ding C, Zhao M, Su W, Zhang Y, Zhong D, et al. Routine-dose and High-dose icotinib in patients with advanced Non-small cell lung cancer harboring EGFR Exon 21-L858R mutation: The randomized, phase II, INCREASE trial. Clin Cancer Res. 2020;26:3162–3171. doi: 10.1158/1078-0432.CCR-19-3064. [DOI] [PubMed] [Google Scholar]
- 20.Sequist LV, Yang JC, Yamamoto N, O'Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–3334. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 21.Wu YL, Zhou C, Hu CP, Feng J, Lu S, Huang Y, Li W, Hou M, Shi JH, Lee KY, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): An open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–222. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 22.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 23.Park K, Tan EH, O'Byrne K, Zhang L, Boyer M, Mok T, Hirsh V, Yang JC, Lee KH, Lu S, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): A phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–589. doi: 10.1016/S1470-2045(16)30033-X. [DOI] [PubMed] [Google Scholar]
- 24.Paz-Ares L, Tan EH, O'Byrne K, Zhang L, Hirsh V, Boyer M, Yang JC, Mok T, Lee KH, Lu S, et al. Afatinib versus gefitinib in patients with EGFR mutation-positive advanced non-small-cell lung cancer: Overall survival data from the phase IIb LUX-Lung 7 trial. Ann Oncol. 2017;28:270–277. doi: 10.1093/annonc/mdw611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lau SC, Chooback N, Ho C, Melosky B. Outcome differences between First- and Second-generation EGFR inhibitors in advanced EGFR mutated NSCLC in a large Population-based cohort. Clin Lung Cancer. 2019;20:e576–e583. doi: 10.1016/j.cllc.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Wu YL, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Tsuji F, Linke R, Rosell R, Corral J, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): A randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–1466. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 27.Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Lee M, Linke R, Rosell R, Corral J, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-Activating mutations. J Clin Oncol. 2018;36:2244–2250. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 28.Mok TS, Cheng Y, Zhou X, Lee KH, Nakagawa K, Niho S, Chawla A, Rosell R, Corral J, Migliorino MR, et al. Updated overall survival in a randomized study comparing dacomitinib with gefitinib as First-line treatment in patients with advanced Non-Small-cell lung cancer and EGFR-activating mutations. Drugs. 2021;81:257–266. doi: 10.1007/s40265-020-01441-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng Y, Mok TS, Zhou X, Lu S, Zhou Q, Zhou J, Du Y, Yu P, Liu X, Hu C, et al. Safety and efficacy of first-line dacomitinib in Asian patients with EGFR mutation-positive non-small cell lung cancer: Results from a randomized, open-label, phase 3 trial (ARCHER 1050) Lung Cancer. 2021;154:176–185. doi: 10.1016/j.lungcan.2021.02.025. [DOI] [PubMed] [Google Scholar]
- 30.Pluzanski A, Wu Y, Cheng Y, Zhou X, Migliorino MR, Niho S, Nakagawa K, Lee KH, Corral J, Rosell R, et al. Safety and efficacy of first-line dacomitinib in advanced non-small cell lung cancer by EGFR mutation subtype in ARCHER 1050. J Thorac Oncol. 2021;16((Suppl)):S617–S618. doi: 10.1016/j.jtho.2021.01.1124. [DOI] [Google Scholar]
- 31.Tanaka H, Sakamoto H, Akita T, Ohyanagi F, Kawashima Y, Tambo Y, Tanimoto A, Horiike A, Miyauchi E, Tsuchiya-Kawano Y, et al. Clinical efficacy of dacomitinib in rechallenge setting for patients with epidermal growth factor receptor mutant non-small cell lung cancer: A multicenter retrospective analysis (TOPGAN2020-02) Thorac Cancer. 2022;13:1471–1478. doi: 10.1111/1759-7714.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S, Wang Y, Wang L, Liu Y, Hu Y, Liu Z, Yao Y, Liang L, Liu J, Li J, et al. Efficacy and safety of dacomitinib as first-line treatment for advanced non-small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) 21L858R mutation: A multicenter, ambispective, consecutive case-series study. Ann Oncol. 2023;34((Suppl)):S1697. doi: 10.1016/j.annonc.2023.10.658. [DOI] [Google Scholar]
- 33.Cho BC, Felip E, Spira AI, Girard N, Lee JS, Lee SH, Ostapenko YV, Danchaivijitr P, Liu B, Alip A, et al. Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Ann Oncol. 2023;34((Suppl)):S1306. doi: 10.1016/j.annonc.2023.10.062. [DOI] [Google Scholar]
- 34.Ke EE, Zhou Q, Zhang QY, Su J, Chen ZH, Zhang XC, Xu CR, Yang JJ, Tu HY, Yan HH, et al. A higher proportion of the EGFR T790M mutation may contribute to the better survival of patients with exon 19 deletions compared with those with L858R. J Thorac Oncol. 2017;12:1368–1375. doi: 10.1016/j.jtho.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 35.Cross DA, Ashton SE, Ghiorghiu S, Eberlein C, Nebhan CA, Spitzler PJ, Orme JP, Finlay MR, Ward RA, Mellor MJ, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046–1061. doi: 10.1158/2159-8290.CD-14-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soria JC, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura F, Nogami N, Kurata T, et al. Osimertinib in untreated EGFR-Mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–125. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 37.Ramalingam SS, Vansteenkiste J, Planchard D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y, Chewaskulyong B, et al. Overall survival with osimertinib in untreated, EGFR-Mutated advanced NSCLC. N Engl J Med. 2020;382:41–50. doi: 10.1056/NEJMoa1913662. [DOI] [PubMed] [Google Scholar]
- 38.Lu S, Dong X, Jian H, Chen J, Chen G, Sun Y, Ji Y, Wang Z, Shi J, Lu J, et al. AENEAS: A randomized phase III trial of aumolertinib versus gefitinib as First-Line therapy for Locally Advanced or MetastaticNon-Small-Cell lung cancer with EGFR exon 19 deletion or L858R mutations. J Clin Oncol. 2022;40:3162–3171. doi: 10.1200/JCO.21.02641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sakata Y, Sakata S, Oya Y, Tamiya M, Suzuki H, Shibaki R, Okada A, Kobe H, Matsumoto H, Yokoi T, et al. Osimertinib as first-line treatment for advanced epidermal growth factor receptor mutation-positive non-small-cell lung cancer in a real-world setting (OSI-FACT) Eur J Cancer. 2021;159:144–153. doi: 10.1016/j.ejca.2021.09.041. [DOI] [PubMed] [Google Scholar]
- 40.Ito K, Morise M, Wakuda K, Hataji O, Shimokawaji T, Takahashi K, Furuya N, Takeyama Y, Goto Y, Abe T, et al. A multicenter cohort study of osimertinib compared with afatinib as first-line treatment for EGFR-mutated non-small-cell lung cancer from practical dataset: CJLSG1903. ESMO Open. 2021;6:100115. doi: 10.1016/j.esmoop.2021.100115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gijtenbeek RGP, Damhuis RAM, van der Wekken AJ, Hendriks LEL, Groen HJM, van Geffen WH. Overall survival in advanced epidermal growth factor receptor mutated non-small cell lung cancer using different tyrosine kinase inhibitors in The Netherlands: a retrospective, nationwide registry study. Lancet Reg Health Eur. 2023;27:100592. doi: 10.1016/j.lanepe.2023.100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Yu L, Zhang Z, Ren X, Smaill JB, Ding K. Targeting EGFR(L858R/T790M) and EGFR(L858R/T790M/C797S) resistance mutations in NSCLC: Current developments in medicinal chemistry. Med Res Rev. 2018;38:1550–1581. doi: 10.1002/med.21488. [DOI] [PubMed] [Google Scholar]
- 43.Shum E, Elamin YY, Piotrowska Z, Spigel DR, Reckamp KL, Rotow JK, Tan DSW, Lim SM, Kim TM, Lin CC, et al. A phase 1/2 study of BLU-945 in patients with common activating EGFR-mutant non-small cell lung cancer (NSCLC): SYMPHONY trial in progress. J Clin Oncol. 2022;40:TPS9156. doi: 10.1200/JCO.2022.40.16_suppl.TPS9156. [DOI] [Google Scholar]
- 44.Wang S, Song Y, Liu D. EAI045: The fourth-generation EGFR inhibitor overcoming T790M and C797S resistance. Cancer Lett. 2017;385:51–54. doi: 10.1016/j.canlet.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 45.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR-TKI treatment for EGFR mutation-positive non-small cell lung cancer. Lung Cancer. 2015;88:74–79. doi: 10.1016/j.lungcan.2015.01.026. [DOI] [PubMed] [Google Scholar]
- 46.Díaz-Serrano A, Gella P, Jiménez E, Zugazagoitia J, Paz-Ares Rodríguez L. Targeting EGFR in lung cancer: Current standards and developments. Drugs. 2018;78:893–911. doi: 10.1007/s40265-018-0916-4. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Li Y, Li L, Wu Z, Yang D, Ma H, Wang D. The effect of icotinib combined with chemotherapy in untreated non-small-cell lung cancer that harbored EGFR-sensitive mutations in a real-life setting: A retrospective analysis. Onco Targets Ther. 2018;11:2345–2353. doi: 10.2147/OTT.S157755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shinomiya S, Kaira K, Yamaguchi O, Ishikawa K, Kagamu H. Osimertinib induced cardiomyopathy: A case report. Medicine (Baltimore) 2020;99:e22301. doi: 10.1097/MD.0000000000022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–957. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 50.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. Osimertinib or Platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montermini L, Meehan B, Garnier D, Lee WJ, Lee TH, Guha A, Al-Nedawi K, Rak J. Inhibition of oncogenic epidermal growth factor receptor kinase triggers release of exosome-like extracellular vesicles and impacts their phosphoprotein and DNA content. J Biol Chem. 2015;290:24534–24546. doi: 10.1074/jbc.M115.679217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tjin Tham Sjin R, Lee K, Walter AO, Dubrovskiy A, Sheets M, Martin TS, Labenski MT, Zhu Z, Tester R, Karp R, et al. In vitro and in vivo characterization of irreversible mutant-selective EGFR inhibitors that are wild-type sparing. Mol Cancer Ther. 2014;13:1468–1479. doi: 10.1158/1535-7163.MCT-13-0966. [DOI] [PubMed] [Google Scholar]
- 53.Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: Perspectives for individualized treatment strategy. Cancer Sci. 2016;107:1179–1186. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hochmair MJ, Morabito A, Hao D, Yang CT, Soo RA, Yang JC, Gucalp R, Halmos B, Märten A, Cufer T. Sequential afatinib and osimertinib in patients with EGFR mutation-positive non-small-cell lung cancer: Final analysis of the GioTag study. Future Oncol. 2020;16:2799–2808. doi: 10.2217/fon-2020-0740. [DOI] [PubMed] [Google Scholar]
- 55.Popat S, Jung HA, Lee SY, Hochmair MJ, Lee SH, Escriu C, Lee MK, Migliorino MR, Lee YC, Girard N, et al. Sequential afatinib and osimertinib in patients with EGFR mutation-positive NSCLC and acquired T790M: A global non-interventional study (UpSwinG) Lung Cancer. 2021;162:9–15. doi: 10.1016/j.lungcan.2021.05.017. [DOI] [PubMed] [Google Scholar]
- 56.Miura S, Jung HA, Lee SY, Lee SH, Lee MK, Lee YC, Hochmair MJ, Yang CT, Märten A, Yang JC, et al. Sequential afatinib and osimertinib in asian patients with EGFR Mutation-Positive non-small cell lung cancer and acquired T790M: Combined analysis of two global non-interventional studies. Onco Targets Ther. 2022;15:873–882. doi: 10.2147/OTT.S362535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Haratake N, Misumi T, Yamanaka T, Seto T. Optimizing sequential treatment with EGFR tyrosine kinase inhibitor with a simulation of the T790M mutation rate in EGFR-mutated lung cancer. JTO Clin Res Rep. 2020;1:100085. doi: 10.1016/j.jtocrr.2020.100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hsu PC, Chang JW, Chang CF, Huang CY, Yang CT, Kuo CS, Fang YF, Wu CE. Sequential treatment in advanced non-small cell lung cancer harboring EGFR mutations. Ther Adv Respir Dis. 2022;16:17534666221132731. doi: 10.1177/17534666221132731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.La Monica S, Madeddu D, Tiseo M, Vivo V, Galetti M, Cretella D, Bonelli M, Fumarola C, Cavazzoni A, Falco A, et al. Combination of gefitinib and pemetrexed prevents the acquisition of TKI resistance in NSCLC cell lines carrying EGFR-activating mutation. J Thorac Oncol. 2016;11:1051–1063. doi: 10.1016/j.jtho.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 60.La Monica S, Minari R, Cretella D, Flammini L, Fumarola C, Bonelli M, Cavazzoni A, Digiacomo G, Galetti M, Madeddu D, et al. Third generation EGFR inhibitor osimertinib combined with pemetrexed or cisplatin exerts long-lasting anti-tumor effect in EGFR-mutated pre-clinical models of NSCLC. J Exp Clin Cancer Res. 2019;38:222. doi: 10.1186/s13046-019-1240-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stylianopoulos T, Munn LL, Jain RK. Reengineering the physical microenvironment of tumors to improve drug delivery and efficacy: From mathematical modeling to bench to bedside. Trends Cancer. 2018;4:292–319. doi: 10.1016/j.trecan.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Viloria-Petit A, Crombet T, Jothy S, Hicklin D, Bohlen P, Schlaeppi JM, Rak J, Kerbel RS. Acquired resistance to the antitumor effect of epidermal growth factor receptor-blocking antibodies in vivo: A role for altered tumor angiogenesis. Cancer Res. 2001;61:5090–5101. [PubMed] [Google Scholar]
- 63.Naumov GN, Nilsson MB, Cascone T, Briggs A, Straume O, Akslen LA, Lifshits E, Byers LA, Xu L, Wu HK, et al. Combined vascular endothelial growth factor receptor and epidermal growth factor receptor (EGFR) blockade inhibits tumor growth in xenograft models of EGFR inhibitor resistance. Clin Cancer Res. 2009;15:3484–3494. doi: 10.1158/1078-0432.CCR-08-2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Y, Murakami H, Yang PC, He J, Nakagawa K, Kang JH, Kim JH, Wang X, Enatsu S, Puri T, et al. Randomized Phase II trial of gefitinib with and without pemetrexed as first-line therapy in patients with advanced nonsquamous non-small-cell lung cancer with activating epidermal growth factor receptor mutations. J Clin Oncol. 2016;34:3258–3266. doi: 10.1200/JCO.2016.66.9218. [DOI] [PubMed] [Google Scholar]
- 65.Han B, Jin B, Chu T, Niu Y, Dong Y, Xu J, Gu A, Zhong H, Wang H, Zhang X, et al. Combination of chemotherapy and gefitinib as first-line treatment for patients with advanced lung adenocarcinoma and sensitive EGFR mutations: A randomized controlled trial. Int J Cancer. 2017;141:1249–1256. doi: 10.1002/ijc.30806. [DOI] [PubMed] [Google Scholar]
- 66.Hosomi Y, Morita S, Sugawara S, Kato T, Fukuhara T, Gemma A, Takahashi K, Fujita Y, Harada T, Minato K, et al. Gefitinib alone versus gefitinib plus chemotherapy for non-small-cell lung cancer with mutated epidermal growth factor receptor: NEJ009 study. J Clin Oncol. 2020;38:115–123. doi: 10.1200/JCO.19.01488. [DOI] [PubMed] [Google Scholar]
- 67.Miyauchi E, Morita S, Nakamura A, Hosomi Y, Watanabe K, Ikeda S, Seike M, Fujita Y, Minato K, Ko R, et al. Updated analysis of NEJ009: Gefitinib-alone versus gefitinib plus chemotherapy for Non-small-cell lung cancer with mutated EGFR. J Clin Oncol. 2022;40:3587–3592. doi: 10.1200/JCO.21.02911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Janne P, Planchard D, Cheng Y, Kulkarni D, Huang X, Kobayashi K. Osimertinib with/without platinum-based chemotherapy as first-line treatment in patients with EGFRm advanced NSCLC (FLAURA2) J Thorac Oncol. 2023;18:S36–S37. doi: 10.1016/j.jtho.2023.09.009. [DOI] [Google Scholar]
- 69.Yan X, Wang H, Li P, Zhang G, Zhang M, Yang J, Zhang X, Zheng X, Ma Z. Efficacy of first-line treatment with epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) alone or in combination with chemotherapy for advanced non-small cell lung cancer (NSCLC) with low-abundance mutation. Lung Cancer. 2019;128:6–12. doi: 10.1016/j.lungcan.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 70.Zhou Q, Xu CR, Cheng Y, Liu YP, Chen GY, Cui JW, Yang N, Song Y, Li XL, Lu S, et al. Bevacizumab plus erlotinib in Chinese patients with untreated, EGFR-mutated, advanced NSCLC (ARTEMIS-CTONG1509): A multicenter phase 3 study. Cancer Cell. 2021;39:1279–1291.e1273. doi: 10.1016/j.ccell.2021.07.005. [DOI] [PubMed] [Google Scholar]
- 71.Saito H, Fukuhara T, Furuya N, Watanabe K, Sugawara S, Iwasawa S, Tsunezuka Y, Yamaguchi O, Okada M, Yoshimori K, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): Interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi: 10.1016/S1470-2045(19)30035-X. [DOI] [PubMed] [Google Scholar]
- 72.Nakagawa K, Garon EB, Seto T, Nishio M, Ponce Aix S, Paz-Ares L, Chiu CH, Park K, Novello S, Nadal E, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi: 10.1016/S1470-2045(19)30634-5. [DOI] [PubMed] [Google Scholar]
- 73.Seto T, Kato T, Nishio M, Goto K, Atagi S, Hosomi Y, Yamamoto N, Hida T, Maemondo M, Nakagawa K, et al. Erlotinib alone or with bevacizumab as first-line therapy in patients with advanced non-squamous non-small-cell lung cancer harbouring EGFR mutations (JO25567): An open-label, randomised, multicentre, phase 2 study. Lancet Oncol. 2014;15:1236–1244. doi: 10.1016/S1470-2045(14)70381-X. [DOI] [PubMed] [Google Scholar]
- 74.Zhao H, Yao W, Min X, Gu K, Yu G, Zhang Z, Cui J, Miao L, Zhang L, Yuan X, et al. Apatinib plus gefitinib as first-line treatment in advanced EGFR-mutant NSCLC: The phase III ACTIVE study (CTONG1706) J Thorac Oncol. 2021;16:1533–1546. doi: 10.1016/j.jtho.2021.05.006. [DOI] [PubMed] [Google Scholar]
- 75.Lee Y, Kim HR, Hong MH, Lee KH, Park KU, Lee GK, Kim HY, Lee SH, Lim KY, Yoon SJ, et al. A randomized Phase 2 study to compare erlotinib with or without bevacizumab in previously untreated patients with advanced non-small cell lung cancer with EGFR mutation. Cancer. 2023;129:405–414. doi: 10.1002/cncr.34553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nishio K, Seto T, Nishio M, Reck M, Garon EB, Sakai K, Goto K, Kato T, Nakanishi Y, Takahashi T, et al. Ramucirumab plus erlotinib versus placebo plus erlotinib in patients with untreated metastatic EGFR-Mutated NSCLC: RELAY Japanese subset. JTO Clin Res Rep. 2021;2:100171. doi: 10.1016/j.jtocrr.2021.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Qi Y, Xia X, Shao L, Guo L, Dong Y, Tian J, Xu L, Niu R, Wei S. An updated network meta-analysis of EGFR-TKIs and combination therapy in the first-line treatment of advanced EGFR mutation positive non-small cell lung cancer. Front Oncol. 2022;12:616546. doi: 10.3389/fonc.2022.616546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang G, Yan B, Guo Y, Yang H, Li J. ‘Sandwich’ Strategy to intensify EGFR blockade by concurrent tyrosine kinase inhibitor and monoclonal antibody treatment in highly selected patients. Front Oncol. 2022;12:952939. doi: 10.3389/fonc.2022.952939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Goldberg SB, Redman MW, Lilenbaum R, Politi K, Stinchcombe TE, Horn L, Chen EH, Mashru SH, Gettinger SN, Melnick MA, et al. Randomized trial of afatinib plus cetuximab versus afatinib alone for First-Line treatment of EGFR-Mutant non-small-cell lung cancer: Final results from SWOG S1403. J Clin Oncol. 2020;38:4076–4085. doi: 10.1200/JCO.20.01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hassanin SO, Hegab AMM, Mekky RH, Said MA, Khalil MG, Hamza AA, Amin A. Combining in vitro, in vivo, and network pharmacology assays to identify targets and molecular mechanisms of spirulina-derived biomolecules against breast cancer. Mar Drugs. 2024;22:328. doi: 10.3390/md22070328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li WQ, Cui JW. Non-small cell lung cancer patients with ex19del or exon 21 L858R mutation: Distinct mechanisms, different efficacies to treatments. J Cancer Res Clin Oncol. 2020;146:2329–2338. doi: 10.1007/s00432-020-03296-6. [DOI] [PubMed] [Google Scholar]
- 82.Ganti AKP, Loo BW, Bassetti M, Blakely C, Chiang A, D'Amico TA, D'Avella C, Dowlati A, Downey RJ, Edelman M, et al. Small cell lung cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:1441–1464. doi: 10.6004/jnccn.2021.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haratake N, Hayashi H, Shimokawa M, Nakano Y, Azuma K, Oki M, Ota K, Yoshioka H, Sakamoto T, Yamamoto N, et al. Phase III Clinical trial for the combination of erlotinib plus ramucirumab compared with osimertinib in previously untreated advanced or recurrent non-small cell lung cancer positive for the L858R mutation of EGFR: REVOL858R (WJOG14420L) Clin Lung Cancer. 2022;23:e257–e263. doi: 10.1016/j.cllc.2021.10.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.