Abstract
Purpose
Breast cancer radiation therapy (RT) techniques have historically delivered mean heart doses (MHDs) in the range of 5 Gy, which have been found to predispose patients to cardiopulmonary toxicities. The purpose of this study was to apply artificial intelligence (AI) cardiac substructure auto-segmentation to evaluate the corresponding substructure doses, whether there are laterality- and technique-specific differences in these doses, and if the doses are significantly associated with cardiorespiratory fitness after state-of-the-art RT planning and delivery for breast cancer.
Methods and Materials
Cardiopulmonary substructures were AI auto-segmented. Cardiorespiratory fitness was evaluated at a median of 2.3 (range, 1.1-9.8) years following RT from 2007 to 2021 among 65 breast cancer survivors. The associations between the mean dose to each of the 9 AI auto-segmented cardiopulmonary substructures, the contralateral, and the ipsilateral lung with cardiorespiratory fitness were evaluated using linear regression.
Results
The median MHD was 0.64 Gy (range, 0.12-7.1). Among the auto-segmented substructures, the highest mean doses were observed for the left ventricle (median, 0.88 Gy). The mean dose to each of the 11 structures was significantly higher for women treated with volumetric modulated arc therapy (MHD median, 3.8 Gy vs 0.57 Gy; P < .0001). Women with left-sided breast cancer had significantly higher MHDs (0.97 vs 0.38 Gy; P < .0001) due to higher doses in 3 of 4 cardiac chambers and also due to significantly higher pulmonary artery doses (median, 0.93 vs 0.32 Gy; P = .0003); women with right-sided breast cancer had significantly higher vena cava and right atrium doses (eg, right atrium median, 0.74 vs 0.29 Gy; P = .0002). No cardiopulmonary structure dose was significantly associated with reduced cardiorespiratory fitness after adjusting for age, chemotherapy agent, volumetric modulated arc therapy, RT position, and RT extent.
Conclusions
State-of-the-art breast cancer RT reduces cardiopulmonary dose, and there is a technique and cancer laterality RT dose dependence throughout the cardiopulmonary system.
Introduction
Breast is the most common cancer site among women, and every other woman diagnosed with breast cancer (BC) will receive radiation therapy (RT).1 Further, women with BC are at an elevated risk of dying from cardiovascular disease.2 In a large cohort study from 2013, among 2168 women treated with RT for BC between 1958 and 2001, the mean heart dose (MHD)3 was found to predispose patients to the risk of coronary events within a linear nonthreshold relationship and a 7% increased risk/Gy. State-of-the-art RT delivery techniques, in addition to more conservative dose/volume guidelines to the cardiopulmonary system, can better conform the therapeutic dose to the tumor and away from the heart and have together systematically reduced the MHD from ∼ 4.9 Gy, as observed in3, to ∼ 1.5 Gy for women with left-sided BC.4, 5, 6
Cardiac substructure auto-segmentation algorithms have recently emerged7 and have facilitated the determination of associations between cardiopulmonary substructure dose and cardiac toxicity among patients with lung cancer.8 While similar segmentation algorithms have been applied to assess the magnitude of cardiopulmonary irradiation in contemporary BC series,4, 5, 6 they have not been used to study the relationship between the associated RT dose and cardiac toxicity.
The goal of this study was to apply artificial intelligence (AI) cardiac substructure auto-segmentation to assess the corresponding substructure doses, whether there are laterality- and technique-specific differences in these doses, and if they, in turn, are significantly associated with cardiorespiratory fitness after contemporary RT for BC.
Methods and Materials
Patient cohort
The cohort data were leveraged from 3 clinical trials. This ancillary analysis was approved by the institutional review board at Memorial Sloan Kettering Cancer Center. The cohort consisted of 65 patients treated with definitive RT for stage I to III BC from December 2007 to February 2021. The added inclusion criterion for the current analysis was a receipt of RT. RT had typically been delivered as three-dimensional conformal RT (3DCRT; n = 58); the remaining 7 patients were treated with intensity modulated RT using either static fields or volumetric modulated arc therapy (VMAT). Forty of the 58 patients treated with 3DCRT were prescribed 2.65 Gy in each of 16 fractions, followed by a boost of 2 to 2.5 Gy over 4 to 5 fractions. The remaining patients treated with 3DCRT received 1.8 to 2 Gy in each of 25 to 28 fractions, with a portion receiving a 2 Gy boost in each of 5 to 7 fractions; 1 patient was prescribed 40 Gy over 10 fractions as partial breast irradiation. All 7 patients receiving VMAT treatments were treated in 2 Gy in each of 25 fractions without a boost (exception: 1 patient received a boost of 2 Gy in each of 5 fractions).
Cardiopulmonary substructure AI auto-segmentation and associated RT dose metrics
For each patient, 9 cardiopulmonary substructures (aorta [ascending and descending combined], atria [left {LA} and right {RA}], heart, pulmonary artery [PA], vena cava [inferior {IVC} and superior {SVC}], and ventricles [left {LV} and right {RV}]) were segmented using a previously developed AI open-source auto-segmentation algorithm.7 Because the segmentation algorithm had been developed from planning computed tomography (CT) scans of lung cancer patients scanned in a supine position, the planning CT scans for the 23 BC patients that had been scanned in a prone position were flipped in the anterior-posterior direction for segmentation inference. All segmentations were postprocessed to adhere to the anatomy. The mean dose was extracted for each of the 9 substructures and the tumor-defined contralateral and ipsilateral lung volumes and corrected for fractionation effects (the physical dose was converted to the equivalent dose in 2 Gy fractions assuming α/β = 3 Gy9, 10, 11).
Assessment of cardiorespiratory fitness
All patients had cardiorespiratory fitness assessed by a symptom-limited cardiopulmonary exercise test (CPET) on a treadmill with 12-lead electrocardiogram monitoring (Mac 5000, GE Healthcare) following standard testing procedures.12,13 The exercise capacity (henceforth referred to ‘VO2peak’ [ml O2/kgmin]) was defined as the highest recorded 30 seconds average output during the last 90 seconds of the CPET. All CPETs were conducted in a dedicated research laboratory by exercise physiologists. The median time between RT completion and VO2peak measurement was 2.3 (range, 1.1-9.8) years (Table 1).
Table 1.
Disease, patient, and treatment characteristics for the 65 included breast cancer patients
| Characteristics | Median (range) or n (%) |
|---|---|
| Age (y) | 55 (35-72) |
| Stage | |
| IA | 33 (51) |
| IIA | 13 (20) |
| IIB | 5 (8) |
| IIIA | 4 (6) |
| IIIB | 1 (2) |
| IIIC | 3 (5) |
| Localized (unspecified) | 4 (6) |
| Unknown | 2 (3) |
| Chemotherapy agent | |
| Anthracycline | 30 (46) |
| Other | 15 (23) |
| None | 20 (31) |
| RT treatment technique | |
| 3DCRT | 58 (89) |
| VMAT | 7 (11) |
| RT patient position | |
| Prone | 22 (34) |
| Supine | 43 (66) |
| RT extent | |
| Breast | 47 (72) |
| Breast + nodes* | 18 (28) |
| VO2peak (mL O2/kg.min) | 23 (12-31) |
| End of RT to VO2peak measurement (y) | 2.3 (1.1-9.8) |
Characteristics are represented as the median (range) or n (%) where applicable.
Abbreviations: 3DCRT = three-dimensional conformal radiation therapy; RT = radiation therapy; VMAT = volumetric modulated arc therapy; VO2peak = cardiorespiratory fitness.
Regional and internal mammary nodes.
Statistical analysis
The cardiac substructure and lung mean RT doses were each evaluated for association with VO2peak using linear regression analysis. Multivariable linear regression models were fit adjusting for age (continuous), chemotherapy agent (none n = 20; anthracycline n = 30; other n = 15), use of VMAT (yes n = 7; no n = 58), RT patient position (prone n = 22; supine n = 43), and RT extent (breast n = 47; breast and nodes n = 18). In addition, cardiopulmonary mean doses were compared by BC laterality (left n = 34; right n = 31) and VMAT using Wilcoxon rank sum tests. All tests were 2-sided .05-level tests. Statistical analyses were performed in SAS v9.4 (SAS Institute) and R v4.3.2 (R Foundation for Statistical Computing).
Results
The LV receives the highest doses among auto-segmented cardiopulmonary substructures
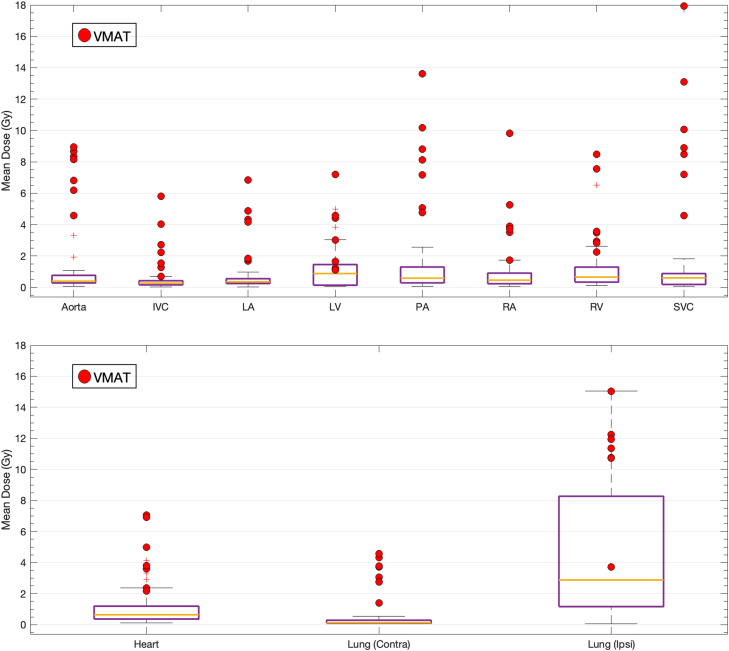
As demonstrated in Fig. 1, the median MHD was 0.64 (range, 0.12-7.1) Gy, while the contralateral and ipsilateral mean lung dose medians were 0.13 (range, 0.06-4.6) Gy and 2.9 (range, 0.06-15) Gy, respectively. Among all auto-segmented cardiopulmonary substructures, the IVC had the overall lowest doses (0.27; range, 0.03-5.8 Gy), while the highest doses were observed for the LV (0.88; range, 0.06-7.2 Gy).
Figure 1.
The mean doses to all studied cardiopulmonary substructures (upper) and the heart and lungs (lower). The 7 patients treated with VMAT are indicated with red dots.
Abbreviations: Contra = contralateral; Ipsi = ipsilateral; IVC = inferior vena cava; LA = left atrium; LV = left ventricle; PA = pulmonary artery; RA = right atrium; RV = right ventricle; SVC = superior vena cava; VMAT = volumetric modulated arc therapy.
VMAT increases the dose to all cardiopulmonary substructures
All cardiopulmonary substructure doses and lung doses were significantly higher for the 7 patients treated using VMAT compared with the remaining 58 patients treated with 3DCRT (all P < .01; eg, MHD median, 3.8 Gy vs 0.57 Gy; Fig. 1).
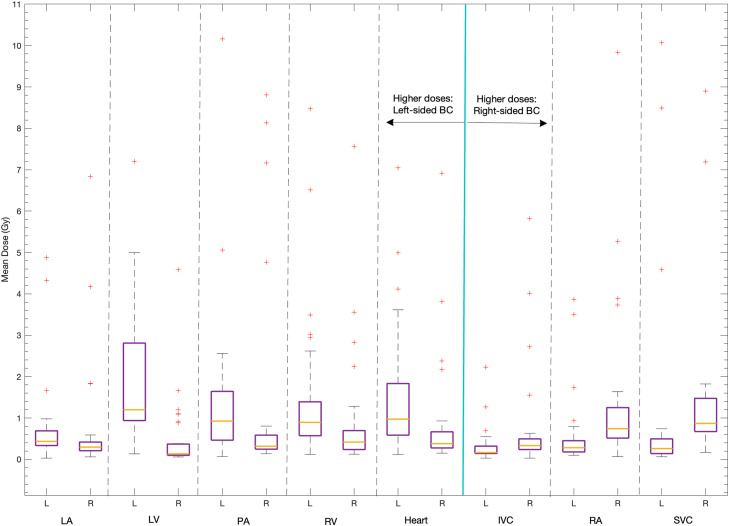
Left-sided BC is associated with higher LA, LV, PA, and RV doses
The 34 women with left-sided BC received significantly higher MHDs (median, 0.97 vs 0.38; P < .0001), which was due to higher LV doses followed by higher RV and, lastly, higher LA doses (median, 1.2 vs 0.13 Gy; 0.89 vs 0.42 Gy; 0.44 vs 0.30 Gy; P < .0001; P < .0007; P < .02, respectively; Fig. 2). A similar higher dose pattern for left-sided laterality was observed for the PA (median, 0.93 vs 0.32 Gy; P = .0003), while the IVC, SVC, and RA doses were significantly higher for women with right-sided BC (median, 0.33 vs 0.17 Gy; 0.87 vs 0.26 Gy; 0.74 vs 0.29 Gy; P = .001; P < .0001; P = .0002, respectively). No cancer laterality dose dependence was observed for the contralateral lung, ipsilateral lung, or the aorta.
Figure 2.
Cardiopulmonary substructure is doses that were significantly higher (left of the cyan line) or lower (right of the cyan line) for patients treated for left-sided breast cancer (BC). The y-axis has been truncated at 11 Gy, excluding 2 SVC mean doses (at 13 Gy and 18 Gy) and 1 PA mean dose (at 14 Gy).
Abbreviations: IVC = inferior vena cava; LA = left atrium; LV = left ventricle; PA = pulmonary artery; RA = right atrium; RV = right ventricle; SVC = superior vena cava.
Cardiac substructure doses are not associated with reduced cardiorespiratory fitness
When adjusting for age, chemotherapy agent, VMAT, RT patient position, and RT extent in the multivariable analysis, no substructure mean dose was significantly associated with VO2peak (Table 2).
Table 2.
Univariate linear regression results between cardiopulmonary substructure mean radiation therapy doses and VO2peak, and multivariate linear regression results adjusted for age, chemotherapy agent, volumetric modulated arc therapy, radiation therapy position, and radiation therapy extent
| Substructure mean dose (Gy) | Univariable β (95% CI) | P value | Multivariable β (95% CI) | P value |
|---|---|---|---|---|
| Aorta | 0.72 (0.32-1.1) | .0006 | 0.59 (−0.67 to 1.8) | .35 |
| IVC | 1.2 (0.17-2.2) | .02 | −0.18 (−1.5 to 1.1) | .79 |
| LA | 1.1 (0.28-1.8) | .008 | −0.03 (−1.3 to 1.1) | .96 |
| LV | 0.69 (0.01-1.4) | .05 | 0.11 (−0.64 to 0.86) | .77 |
| PA | 0.58 (0.23-0.92) | .002 | 0.12 (−0.61 to 0.84) | .75 |
| RA | 0.67 (0.06-1.3) | .03 | −0.49 (−1.4 to 0.38) | .27 |
| RV | 0.61 (0.04-1.2) | .04 | −0.14 (−0.83 to 0.56) | .69 |
| SVC | 0.47 (0.20-0.75) | .001 | −0.12 (−0.68 to 0.44) | .67 |
| Heart | 0.77 (0.14-1.4) | .02 | −0.10 (−1.0 to 0.83) | .83 |
| Contralateral lung | 1.27 (0.40-2.1) | .005 | 0.19 (−2.0 to 2.4) | .86 |
| Ipsilateral lung | 0.43 (0.24-0.63) | <.0001 | 0.40 (−0.08 to 0.88) | .10 |
Abbreviations: IVC = inferior vena cava; LA = left atrium; LV = left ventricle; PA = pulmonary artery; RA = right atrium; RV = right ventricle; SVC = superior vena cava.
Discussion
Based on AI auto-segmented cardiopulmonary substructures and associated RT doses from 65 women who had previously been treated with RT for BC, this study suggests that (1) the LV receives the highest doses of all cardiac substructures, (2) the use of VMAT increases RT dose to all cardiopulmonary structures, (3) left-sided BC is associated with higher LA, LV, PA, and RV doses, but lower IVC, SVC, and RA doses compared with right-sided BC, and (4) cardiopulmonary substructure doses are not associated with significantly reduced cardiorespiratory fitness post-RT.
Among all 9 auto-segmented cardiopulmonary substructures, the LV received the overall highest doses (median, 0.88 Gy), and a left-sided BC diagnosis translated into a significantly higher LV dose (median, 1.2 vs 0.13 Gy; P < .0001). Restricted to the 8 overlapping cardiopulmonary substructures, Finnegan et al6 observed these same 2 patterns but with higher median population LV doses (∼1.8 Gy) than those observed among our 34 left-sided BC patients. In another study based on data from 47 BC patients, systolic or diastolic indices of LV function from echocardiography were not found to be associated with the MHD.5 Despite using no LV substructure dose in that study, the lack of an association with cardiac function aligns with the results from this study in that no relationship was observed between dose to the LV (or to any cardiopulmonary substructure) and reduced cardiorespiratory fitness as quantified by VO2peak.
The VO2peak levels observed here are in the same range as those observed in a previous BC series (median, 23 vs 25 mL O2/kg.min).14 In that study, VO2peak was associated with age and body mass index but not with RT-related fatigue. Here, no association was observed between VO2peak and cardiopulmonary substructure dose. Further, the VO2peak was assessed at a median of 2.3 years after post-RT, and there was no difference in VO2peak before and after that time point (n = 32 vs 32; median VO2peak, 23.3 vs 21.5; P = .19).
For the 7 patients where VMAT was used, the dose to all 11 cardiopulmonary structures was significantly higher than observed among the remaining 58 patients treated with 3DCRT. For 10 replanned patients, Corradini et al15 also observed higher heart and lung doses for VMAT than 3DCRT, particularly when no deep-inspiration breath hold (DIBH) was used. In the current study, the 3 patients treated with VMAT without DIBH received the highest doses, followed by the 4 patients treated with VMAT plus DIBH (median across all 11 structures, 3.7 Gy vs 4.2 Gy).
Left-sided BC over right-sided BC has historically been associated with higher MHD.4,6 We observed a related dependence for MHD but also found a laterality dose dependence across the cardiopulmonary system, similar to Finnegan et al,6 with the LA, LV, PA, and RV doses being higher for left-sided BC, while the RA and SVC doses being higher for right-sided BC. In addition, this study also identified significantly higher IVC doses among right-sided BC patients (of note: Finnegan et al,6 did not assess IVC doses).
Limitations of the current study include a cardiorespiratory fitness test-tailored cohort of 65 patients. Further, our observed low-end cardiopulmonary doses may have precluded an association between cardiopulmonary substructure dose and cardiorespiratory fitness, and a false negative type II β error can, therefore, not be ruled out.
Conclusions
Our study suggests that state-of-the-art RT has successfully reduced the dose to the cardiopulmonary system with a median MHD of 0.64 Gy compared with historically delivered doses of 4.9 Gy.3 Our results suggest that cancer laterality should be considered when designing new cardiopulmonary substructure-specific dose/volume guidelines for BC, given that a left-sided cancer leads to significantly higher LA, LV, PA, and RV doses, and a right-sided cancer leads to significantly higher IVC, RA, and SVC doses.
Disclosures
Anthony F Yu reports a relationship with Genentech Inc that includes board membership and a relationship with American Heart Association Inc that includes funding grants. Laura Cervino reports a relationship with American Association of Physicists in Medicine that includes board membership. Chaya Moskowitz reports a relationship with National Cancer Institute that includes funding grants and a relationship with Radiological Society of North America that includes speaking and lecture fees. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Sources of support: This work was in part funded by the NIH/NCI Cancer Center Support/Core Grant (P30 CA009748).
References
- 1.Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 2.Stoltzfus KC, Zhang Y, Sturgeon K, et al. Fatal heart disease among cancer patients. Nat Commun. 2020;11:2011. doi: 10.1038/s41467-020-15639-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987–998. doi: 10.1056/NEJMoa1209825. [DOI] [PubMed] [Google Scholar]
- 4.Pierce LJ, Feng M, Griffith KA, et al. Recent time trends and predictors of heart dose from breast radiation therapy in a large quality consortium of radiation oncology practices. Int J Radiat Oncol Biol Phys. 2017;99:1154–1161. doi: 10.1016/j.ijrobp.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Yu AF, Ho AY, Braunstein LZ, et al. Assessment of early radiation-induced changes in left ventricular function by myocardial strain imaging after breast radiation therapy. J Am Soc Echocardiogr. 2019;32:521–528. doi: 10.1016/j.echo.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finnegan R, Lorenzen EL, Dowling J, et al. Analysis of cardiac substructure dose in a large, multi-centre Danish breast cancer cohort (the DBCG HYPO trial): Trends and predictive modelling. Radiother Oncol. 2020;153:130–138. doi: 10.1016/j.radonc.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Haq R, Hotca A, Apte A, Rimner A, Deasy JO, Thor M. Cardio-pulmonary substructure segmentation of radiotherapy computed tomography images using convolutional neural networks for clinical outcomes analysis. Phys Imaging Radiat Oncol. 2020;14:61–66. doi: 10.1016/j.phro.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hotca A, Thor M, Deasy JO, Rimner A. Dose to the cardio-pulmonary system and treatment-induced electrocardiogram abnormalities in locally advanced non-small cell lung cancer. Clin Transl Radiat Oncol. 2019;19:96–102. doi: 10.1016/j.ctro.2019.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thor M, Apte A, Haq R, Iyer A, LoCastro E, Deasy JO. Using auto-segmentation to reduce contouring and dose inconsistency in clinical trials: The simulated impact on RTOG 0617. Int J Radiat Oncol Biol Phys. 2021;109:1619–1626. doi: 10.1016/j.ijrobp.2020.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thor M, Deasy JO, Hu C, et al. Modeling the impact of cardiopulmonary irradiation on overall survival in NRG oncology trial RTOG 0617. Clin Cancer Res. 2020;26:4643–4650. doi: 10.1158/1078-0432.CCR-19-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose–volume effects in the lung. Int J Radiat Oncol Biol Phys. 2010;76:S70–S76. doi: 10.1016/j.ijrobp.2009.06.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott JM, Thomas SM, Peppercorn JM, et al. Effects of exercise therapy dosing schedule on impaired cardiorespiratory fitness in patients with primary breast cancer: A randomized controlled trial. Circulation. 2020;141:560–570. doi: 10.1161/CIRCULATIONAHA.119.043483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu AF, Flynn JR, Moskowitz CS, et al. Long-term cardiopulmonary consequences of treatment-induced cardiotoxicity in survivors of ERBB2-positive breast cancer. JAMA Cardiol. 2020;5:309–317. doi: 10.1001/jamacardio.2019.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leeman JE, Lapen K, Fuchs HE, et al. Cardiorespiratory fitness in patients with early-stage breast cancer and radiation therapy-related fatigue: A prospective pilot study. Int J Radiat Oncol Biol Phys. 2024;118:1060–1065. doi: 10.1016/j.ijrobp.2023.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corradini S, Ballhausen H, Weingandt H, et al. Left-sided breast cancer and risks of secondary lung cancer and ischemic heart disease: Effects of modern radiotherapy techniques. Strahlenther Onkol. 2018;194:196–205. doi: 10.1007/s00066-017-1213-y. [DOI] [PubMed] [Google Scholar]