Abstract
Background: Rechallenge with immune checkpoint inhibitors (ICI) shows promise in various cancers, but data in esophageal squamous cell carcinoma (ESCC) is limited. This study aimed to evaluate the efficiency and safety of ICI rechallenge in ESCC.
Materials and Methods: This multicenter study analyzed ESCC patients rechallenged with ICI from January 2020 to March 2023 across two medical institutions. Patients were divided into rechallenge (R) and non-rechallenge (NR) groups. Key outcomes studied were progression-free survival (PFS), overall survival (OS), and safety.
Results: Among 329 included ESCC patients, 211 were in the R group and 118 in the NR group, with a median follow-up of 17.1 months. The R group exhibited significantly prolonged median PFS (4.7 vs. 3.2 months; p <.001) and OS (9.3 vs. 6.2 months; p <.001) compared to the NR group. Notably, for patients who initially received radiotherapy, the R group showed significantly longer mPFS (5.1 vs. 3.2 months; p <.001) and mOS (10.4 vs. 5.9 months; p <.001). Incidences of all-grade (64.5% vs. 66.1%; p = .764) and grade ≥3 adverse events (17.5% vs. 18.6%; p = .802) did not significantly differ between groups.
Conclusion: ICI rechallenge demonstrates efficacy and manageable safety in ESCC, particularly post-radiotherapy.
Keywords: esophageal squamous cell carcinoma, immune checkpoint inhibitor, Immunotherapy, rechallenge, retrospective analysis
Introduction
Global cancer statistics from 2020 indicate that esophageal cancer accounts for the sixth highest number of cancer-induced deaths 1. East Asia, especially China, has the highest incidence of esophageal cancer 2. Esophageal squamous cell carcinoma (ESCC) constitutes roughly 90% of all esophageal cancers 3. Most patients with esophageal cancer are diagnosed with advanced disease, and the 5-year survival rate is 26% 4.
Recently, immune checkpoint inhibitors (ICIs) remarkably prolonged OS in patients with malignancies, including those with esophageal cancer 5-8. Based on the findings from to the ESCORT, KEYNOTE-181, and ATTRACTION-3 studies 7,9,10, programmed death receptor 1 (PD-1) inhibitor monotherapy has shown better anti-tumor efficacy to chemotherapy in the second-line treatment of advanced esophageal cancer, with a favorable safety profile. Trials like ESCORT-1st, KEYNOTE-590, and CheckMate648 have propelled the search for first-line immunotherapy in advanced cases and shown significant efficacy. According to the findings, the combination of ICIs with chemotherapy has been advised as the preferred first-line treatment for advanced esophageal cancer, rather than chemotherapy alone 5-8.
However, only a portion of patients experience a durable response to first-line immunotherapy, and resistance to therapy and disease progression can still occur as time passes. When these occur, there are no established strategies to overcome drug resistance 11. Clinical researches show that repeated use of immunotherapy may be a more favorable option than traditional chemotherapy and radiotherapy 12. Some patients might benefit from an immunotherapy rechallenge. Successful ICI rechallenge has been reported in patients with several solid tumors, such as melanoma 13-16, lung cancer 17, hepatocellular 18 and renal cell carcinoma 19,20. However, as far as we know, there is currently a lack of data to approach this strategy in patients with ESCC. Our study aimed to evaluate the efficacy and safety of ICI rechallenge in patients with ESCC who discontinued first-line ICI treatment.
Materials and methods
Data collection
This study adhered to the principles outlined in the Declaration of Helsinki (revised in 2013). Due to the retrospective nature of the study, informed consent was deemed unnecessary. Data from patients with ESCC receiving PD-1/PD-L1 inhibitors as first-line treatment at Shandong Cancer Hospital and Institute and Shandong Provincial Hospital from January 2020 to April 2023 were retrospectively collected. Inclusion criteria were: (i) histologically confirmed ESCC; (ii) diagnosis of recurrent or metastatic ESCC; (iii) first-line treatment with anti-PD-1/PD-L1 inhibitors; (iv) discontinuation of anti-PD-1/PD-L1 inhibitors for any reason (disease progression, development of adverse events [AEs], protocol completion); (v) first-line treatment including at least two cycles of PD-1/PD-L1 inhibitors. The exclusion criteria were: (i) no tumor evaluation performed and (ii) presence of other primary tumor types. The enrolled patients were categorized into two groups: (i) the rechallenge group (R group, n=211), including patients who discontinued anti-PD-1/PD-L1 inhibitors and subsequently received anti-PD-1/PD-L1 inhibitors again and (ii) the non-rechallenge group (NR group, n=118), including patients who were not retreated with anti-PD-1/PD-L1 inhibitors. Patient data included age, sex, smoking and alcohol consumption history, Eastern Cooperative Oncology Group performance status (ECOG PS) score, lung/liver/bone metastases, best response to initial immunotherapy, reason for discontinuation of ICI-1, time to relapse after ICI-1, history of radiotherapy, first- and second-line therapeutic schedule, clinical T-stage and N-stage according to the AJCC TNM staging system (eighth edition).
Efficacy evaluation
The primary endpoints were PFS, OS and safety. PFS was defined as the duration time from the start of the second-line ICI treatment or another therapy to tumor progression or death from any causes. OS referred to the period from the start of the second-line treatment to death. The secondary endpoints were objective response rate (ORR) and disease control rate (DCR). Assessments of tumor response, including complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD), were conducted in accordance with the RECIST 1.1 criteria 21. ORR was determined by the proportion of individuals who obtained a CR and PR. DCR was determined by the proportion of individuals who obtained a CR, PR, or SD. The last follow-up date was November 30, 2023.
Statistical analysis
Baseline characteristics were described using the median and interquartile range (IQR) for continuous variables and frequency and percentage for categorical variables. Qualitative variables were analyzed using the chi-squared test or Fisher exact test. PFS and OS were assessed using the Kaplan-Meier method, and differences were compared using the log-rank test. Univariate and multivariate Cox regression analyses were conducted to identify predictors of PFS and OS, with hazard ratios (HR) and 95% confidence intervals (95% CIs) reported. Variables that reached a significance threshold of P<.05 in the univariate assessment were then included in the multivariate Cox regression model. Statistical significance was set at a two-tailed P-value below 0.05. The analyses were conducted utilizing the R statistical software, version 4.3.1.
Results
Patient clinical characteristics
Totally 329 patients with ESCC treated with ICIs met the criteria for inclusion. Among these, 211 and 118 were included in the R and NR groups, respectively. The baseline clinical features of both groups were well-matched (Table 1). There were 298 male patients (90.6%) and the median age of the overall study patients was 62 years. 172 (52.3%) patients with a smoking history. Most patients (96.4%) had an ECOG PS score of 0-1. Most patients received ICI combination therapy before first progression (95.7%), and 4.3% received immune monotherapy. Fewer patients in the NR group (71.6%) had received prior radiotherapy than those in the R group (77.1%); however, the difference did not reach statistical significance.
Table 1.
Baseline characteristics of the patients.
| All patients (n = 329) | Rechallenge(n=211) | Non-rechallenge(n=118) | p-value | |
|---|---|---|---|---|
| Age | ||||
| Median age (range), years | 62(56-68) | 62(57-69) | 62(55-67) | 0.116 |
| ≤60 | 133(40.4%) | 92(43.6%) | 41(34.7%) | |
| >60 | 196(59.6%) | 119(56.4%) | 77(65.3%) | |
| Sex | ||||
| Male | 298(90.6%) | 191(90.5%) | 107(90.7%) | 0.963 |
| Female | 31(9.4%) | 20(9.5%) | 11(9.3%) | |
| Smoking history | ||||
| Ever | 172(52.3%) | 106(50.2%) | 66(55.9%) | 0.321 |
| Never | 157(47.7%) | 105(49.8%) | 52(44.1%) | |
| Drinking history | ||||
| Ever | 172(52.3%) | 106(50.2%) | 66(55.9%) | 0.321 |
| Never | 157(47.7%) | 105(49.8%) | 52(44.1%) | |
| Best response to first line | ||||
| PR | 148(45.0%) | 100(47.4%) | 48(40.7%) | 0.362 |
| SD | 123(37.4%) | 73(34.6%) | 50(42.4%) | |
| PD | 58(17.6%) | 38(18.0%) | 20(16.9%) | |
| Liver metastasis | ||||
| Yes | 52(15.8%) | 34(16.1%) | 18(15.3%) | 0.838 |
| No | 277(84.2%) | 177(83.9%) | 100(84.7%) | |
| Lung metastasis | ||||
| Yes | 36(10.9%) | 22(10.4%) | 14(11.9%) | 0.689 |
| No | 293(89.1%) | 189(89.6%) | 104(88.1%) | |
| Bone metastasis | ||||
| Yes | 19(5.8%) | 13(6.2%) | 6(5.1%) | 0.688 |
| No | 310(94.2%) | 198(93.8%) | 112(94.9%) | |
| ECOG PS | ||||
| 0-1 | 317(96.4%) | 202(95.7%) | 115(97.5%) | 0.424 |
| 2 | 12(3.6%) | 9(4.3%) | 3(2.5%) | |
| Discontinuation reason | ||||
| Disease progression | 296(90.0%) | 191(90.5%) | 105(89.0%) | 0.893 |
| Toxicity | 6(1.8%) | 4(1.9%) | 2(1.7%) | |
| Others | 27(8.2%) | 16(7.6%) | 11(9.3%) | |
| Time to relapse after ICI-1 (months) | ||||
| <3 | 202(61.40%) | 130(61.61%) | 72(61.02%) | 0.915 |
| ≥3 | 127(38.60%) | 81(38.39%) | 46(38.98%) | |
| Radiotherapy | ||||
| Yes | 242(73.6%) | 151(71.6%) | 91(77.1%) | 0.273 |
| No | 87(26.4%) | 60(28.4%) | 27(22.9%) | |
| Treatment regimens of first-line | ||||
| ICIs monotherapy | 14(4.3%) | 7(3.3%) | 7(5.9%) | 0.442 |
| ICIs with Chemotherapy | 297(90.3%) | 194(91.9%) | 103(87.3%) | |
| ICIs with anti-VEGF | 4(1.2%) | 3(1.4%) | 1(0.8%) | |
| ICIs combined with chemotherapy plus anti-angiogenesis therapy | 14(4.3%) | 7(3.3%) | 7(5.9%) | |
| Treatment regimens of second-line | ||||
| ICIs monotherapy | 17(5.17%) | 17(8.06%) | 0(0%) | <0.001 |
| Chemotherapy with/without ICIs | 232(70.52%) | 137(64.93%) | 95(80.51%) | |
| Anti-VEGF with/without ICIs | 46(13.98%) | 37(17.54%) | 9(7.63%) | |
| Chemotherapy plus anti-angiogenesis therapy with/without ICIs | 34(10.33%) | 20(9.48%) | 14(11.86%) | |
| Clinical T stage | ||||
| 0-2 | 47(14.29%) | 31(14.69%) | 16(13.56%) | 0.778 |
| 3-4 | 282(85.71%) | 180(85.31%) | 102(86.44%) | |
| Clinical N stage | ||||
| 0-2 | 239(72.64%) | 157(74.41%) | 82(69.49%) | 0.337 |
| 3 | 90(27.36%) | 54(25.59%) | 36(30.51%) |
Abbreviations: ICI: immune checkpoint inhibitor; PR: partial response; SD: steady disease; PD: progressive disease; ECOG PS: Eastern Cooperative Oncology Group Performance Status
Efficacy evaluation
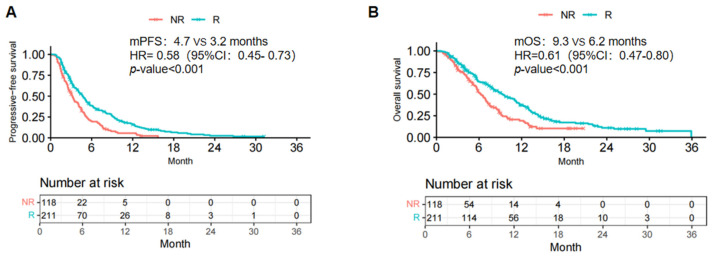
The median follow-up duration was 17.1 months. As of the follow-up date, 290 patients (88.1%) had experienced disease progression, including 180 (85.31%) and 110 (93.22%) in the R and NR groups, respectively. Additionally, 239 patients (72.64%) died (148 [70.14%], R group; 91 (77.12%), NR group). The mPFS and mOS were statistically prolonged in patients in the R group compared to those in the NR group within the overall study cohort (mPFS: 4.7 vs. 3.2 months; HR =0.58; 95% confidence interval [CI]: 0.45-0.73; P <.001; Fig. 1A; mOS: 9.3 vs. 6.2 months; HR =0.61; 95% CI: 0.47-0.80; P <.001; Fig. 1B).
Figure 1.
Kaplan-Meier curves of PFS (A) and OS (B) of ICI rechallenge in the total study population. R, rechallenge group; NR, non rechallenge group; mPFS, median progression-free survival; mOS, median overall survival; HR, hazard ratio; CI, confidence interval.
In the R group, no patients experienced CR (0%); 57, PR (23.7%); 107, SD (50.7%); and 54, PD (25.6%) (Table 2). Compared to the NR group, the R group exhibited significantly elevated ORR (23.7% vs. 9.3%; P=.001) and DCR (74.4% vs. 55.1%; P <.001).
Table 2.
Cox proportional hazards and logistic regression models for progression-free survival (PFS).
| Factors | PFS (Univariate analysis) | PFS (Multivariate analysis) | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |||
| rechallenge | 0.58(0.45- 0.73) | <0.001 | 0.55(0.43-0.70) | <0.001 | ||
| Age (≥65) | 0.94(0.73-1.20) | 0.602 | ||||
| Male gender | 1.45(0.95- 2.2) | 0.082 | ||||
| ECOG PS≥2 | 2.23(1.2-4.10) | 0.010 | 2.56(1.39-4.72) | 0.003 | ||
| Smoking history | 1.14(0.91-1.45) | 0.261 | ||||
| Drinking history | 1.14(0.90-1.44) | 0.278 | ||||
| Lung metastasis | 0.91(0.63-1.31) | 0.624 | ||||
| Bone metastasis | 1.26(0.77- 2.06) | 0.357 | ||||
| Liver metastasis | 1.30(0.96-1.77) | 0.095 | ||||
| Best response to first line | ||||||
| PR | 1 | |||||
| SD | 1.010(0.78-1.31) | 0.938 | ||||
| PD | 1.05(0.76-1.44) | 0.773 | ||||
| Primary tumor location | ||||||
| Cervical | 1 | |||||
| Upper | 1.05(0.54-2.04) | 0.876 | ||||
| Middle | 1.08(0.58-2.02) | 0.798 | ||||
| Lower | 0.95(0.51-1.77) | 0.878 | ||||
| Radiotherapy | 0.85(0.66-1.10) | 0.214 | ||||
| Treatment regimens of first-line | ||||||
| ICIs monotherapy | 1 | |||||
| ICIs with Chemotherapy | 1.28(0.69-2.35) | 0.435 | ||||
| ICIs with anti-VEGF | 1.81(0.50-6.56) | 0.364 | ||||
| ICIs with both Chemotherapy and anti-VEGF | 1.40(0.60-3.24) | 0.440 | ||||
| Discontinuation due to disease progression | 1.75(1.18-2.61) | 0.006 | 1.72(1.15-2.57) | 0.008 | ||
| Distant organ metastasis | 1.15(0.90-1.47) | 0.260 | ||||
| Time to relapse after ICI-1 (months) | ||||||
| <3 | 1 | |||||
| ≥3 | 0.90(0.72-1.12) | 0.327 | ||||
| Clinical T stage | ||||||
| 0-2 | 1 | |||||
| 3-4 | 1.03(0.75-1.40) | 0.862 | ||||
| Clinical N stage | ||||||
| 0-2 | 1 | |||||
| 3 | 1.76(1.35-2.29) | <0.001 | 1.68(1.29-2.19) | <0.001 | ||
PR: partial response; SD: steady disease; PD: progressive disease; ECOG PS: Eastern Cooperative Oncology Group Performance Status; HR: hazard ratio; CI: confidence interval.
The multivariate analysis indicated that anti-PD-1/PD-L1 inhibitor rechallenge (HR =0.55; 95% CI: 0.43-0.70, p<.001); ECOG PS score≥2 (HR =2.56; 95% CI: 1.39-4.72, P=.003), discontinuation due to disease progression (HR =1.72; 95% CI: 1.15-2.57; P=.008), and N3 stage (HR =1.68; 95% CI: 1.29-2.19; P<.001) were associated with PFS (Table 3). In addition, anti-PD-1/PD-L1 inhibitor rechallenge (HR =0.58; 95% CI: [0.44-0.76]; P <.001), ECOG PS score≥2 (HR =1.95; 95% CI: 1.06-3.62; P=.032), discontinuation due to disease progression (HR =1.84; 95% CI: 1.16-2.93; P=.010), and N3 stage (HR =1.73; 95% CI: 1.31-2.30; P<.001) were associated with OS (Table 4).
Table 3.
Cox proportional hazards and logistic regression models for overall survival (OS)
| Factors | OS (Univariate analysis) | OS (Multivariate analysis) | ||||
|---|---|---|---|---|---|---|
| HR (95%CI) | p-value | HR (95%CI) | p-value | |||
| rechallenge | 0.61(0.47-0.80) | <0.001 | 0.58(0.44-0.76) | <0.001 | ||
| Age (≥65) | 0.97(0.73-1.28) | 0.822 | ||||
| Male gender | 1.46(0.93-2.29) | 0.100 | ||||
| ECOG PS≥2 | 2.01(1.09-3. 70) | 0.025 | 1.95(1.06-3.62) | 0.032 | ||
| Smoking history | 1.15 (0.89-1.49) | 0.274 | ||||
| Drinking history | 1.12(0.87-1.44) | 0.395 | ||||
| Lung metastasis | 0.91(0.61-1.36) | 0.653 | ||||
| Bone metastasis | 1.62(0.94-2.80) | 0.081 | ||||
| Liver metastasis | 1.12(0.80-1.58) | 0.519 | ||||
| Best response to first line | ||||||
| PR | 1 | |||||
| SD | 0.94(0.71-1.25) | 0.689 | ||||
| PD | 0.98(0.69-1.39) | 0.897 | ||||
| Primary tumor location | ||||||
| Cervical | 1 | |||||
| Upper | 1.17(0.56-2.42) | 0.677 | ||||
| Middle | 1.10(0.55-2.18) | 0.793 | ||||
| Lower | 0.87(0.44-1.73) | 0.701 | ||||
| Radiotherapy | 0.87(0.66-1.15) | 0.333 | ||||
| Treatment regimens of first-line | ||||||
| ICIs monotherapy | 1 | |||||
| ICIs with Chemotherapy | 1.14(0.56-2.31) | 0.722 | ||||
| ICIs with anti-VEGF | 1.28(0.34-4.85) | 0.717 | ||||
| ICIs with both Chemotherapy and anti-VEGF | 1.14(0.46-2.84) | 0.782 | ||||
| Discontinuation due to disease progression | 1.89(1.19-2.98) | 0.007 | 1.84(1.16-2.93) | 0.010 | ||
| Distant organ metastasis | 1.15(0.88-1.50) | 0.307 | ||||
| Time to relapse after ICI-1 (months) | ||||||
| <3 | 1 | |||||
| ≥3 | 0.88(0.68-1.14) | 0.329 | ||||
| Clinical T stage | ||||||
| 0-2 | 1 | |||||
| 3-4 | 1.16(0.80-1.70) | 0.437 | ||||
| Clinical N stage | ||||||
| 0-2 | 1 | |||||
| 3 | 1.86(1.40-2.45) | <0.001 | 1.73(1.31-2.30) | <0.001 | ||
PR: partial response; SD: steady disease; PD: progressive disease; ECOG PS: Eastern Cooperative Oncology Group Performance Status; HR: hazard ratio; CI: confidence interval.
Table 4.
Short-term effect in total population.
| Best response, n (%) | Rechallenge (n=211) | Non-rechallenge (n=118) | p-value |
|---|---|---|---|
| CR | 0 | 0 | |
| PR | 50(23.7%) | 11(9.3%) | |
| SD | 107(50.7%) | 54(45.8%) | |
| PD | 54(25.6%) | 53(44.9%) | |
| ORR | 23.7% | 9.3% | 0.001 |
| DCR | 74.4% | 55.1% | <0.001 |
CR: complete response; PR: partial response; SD: steady disease; PD: progressive disease; ORR: objective response rate; DCR: disease control rate.
Subgroup analysis
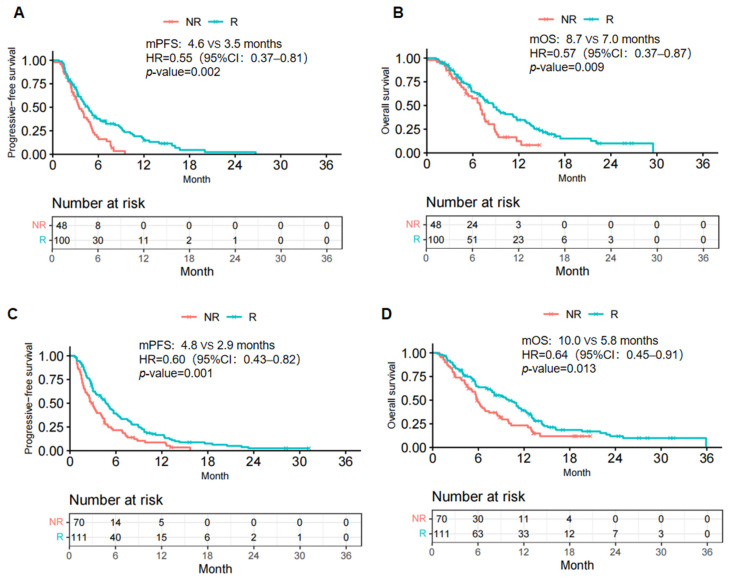
In the subgroup analysis, in those achieving CR/PR response to initial immunotherapy, ICI rechallenge exhibited significant advantages in both PFS and OS (mPFS: 4.6 vs. 3.5 months, HR=0.55, 95% CI: 0.37-0.81, p = .002; mOS: 8.7 vs. 7.0 months, HR=0.57, 95% CI: 0.37-0.87, p = .009) (Fig. 2A, B). Interestingly, in the subgroup achieving SD/PD response, the survival analysis demonstrated similar results (mPFS: 4.8 vs. 2.9 months, HR: 0.60, 95% CI: 0.43-0.82, p = .001; mOS: 10.0 vs. 5.8 months, HR: 0.64, 95% CI: 0.45-0.91, p = .013) (Fig. 2A, B). Therefore. ICI rechallenge resulted in clinical benefits regardless of the initial treatment response in this study cohort.
Figure 2.
Kaplan-Meier curves of PFS (A, C) and OS (B, D) from data in the CR/PR response (A, B) and SD/PD response (C, D) to initial immunotherapy subgroups. R, rechallenge group; NR, non-rechallenge group; mPFS, median progression-free survival; mOS, median overall survival; HR, hazard ratio; CI, confidence interval.
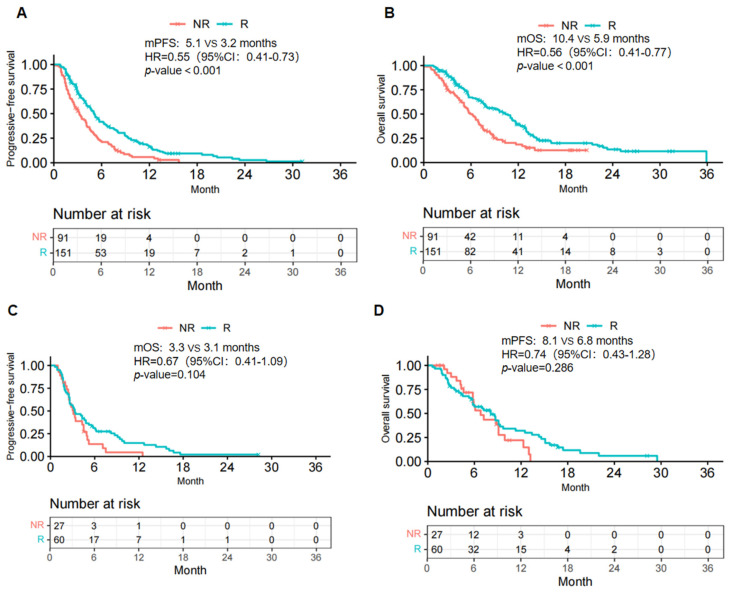
Subgroup analyses were conducted in the R and NR groups to investigate the effect of previous radiotherapy on ICI rechallenge. For patients who accepted radiotherapy in the initial treatment, the R group demonstrated a notable increase in mPFS (5.1 vs. 3.2 months; HR=0.55; 95% CI: 0.41-0.73, p <.001) and mOS (10.4 vs. 5.9 months; HR=0.56; 95%CI: 0.41-0.77, p <.001) (Fig. 3A, B). For patients who did not receive radiotherapy in the initial treatment, although the mPFS and mOS in the R group were longer than those in the NR group (mPFS: 3.3 vs. 3.1 months, HR: 0.67, 95% CI: 0.41-1.09, p = .104; mOS: 8.1 vs. 6.8 months, HR: 0.74, 95%CI: 0.43-1.28, p = .286), these differences did not show statistical significance (Fig. 3C, D).
Figure 3.
Kaplan-Meier curves of PFS (A, C) and OS (B, D) from data in the previously radiotherapy (A, B) and non-radiotherapy (C, D) subgroups. R, rechallenge group; NR, non-rechallenge group; mPFS, median progression-free survival; mOS, median overall survival; HR, hazard ratio; CI, confidence interval.
Safety of rechallenge with ICIs
Treatment-related adverse events (TRAEs) were observed in 136 out of 211 patients (64.5%) in the R group and 78 out of 118 patients (66.1%) in the NR group (p=.764). The most common AEs were diarrhea (n = 41[19.4%]), vomiting (n = 39[18.5%]), fatigue (n = 38[18.0%]), and neutropenia (n = 36[17.1%]) in the R group and fatigue (n = 21[17.8%]) and neutropenia (n = 20[16.9%]) in the NR group. Grade 3 or higher TRAEs occurred in 37 patients (17.5%) within the R group and 22 patients (18.6%) within the NR group (p=.802) (Table 5). Fifteen (7.1%) and eight patients (6.8%) discontinued at least one treatment component because of TRAEs (Supplementary Table 1). No treatment-related deaths occurred. Among the six patients who discontinued ICI therapy due to AEs, four accepted ICI rechallenge, one of whom experienced grade 3 diarrhea, which was also observed during first-line immunotherapy.
Table 5.
Adverse events
| No. (%) of patients | ||||
|---|---|---|---|---|
| R(n=211) | NR(n=118) | |||
| Any grade | ≥Grade 3 | Any grade | ≥Grade 3 | |
| Treatment-related adverse eventsb | 136(64.5%) | 37(17.5%) | 78(66.1%) | 22(18.6%) |
| Diarrhea | 41(19.4%) | 6(2.8%) | 8(6.8%) | 1(0.8%) |
| Vomiting | 39(18.5%) | 7(3.3%) | 17(14.4%) | 2(1.7%) |
| Fatigue | 38(18.0%) | 2(0.9%) | 21(17.8%) | 1(0.8%) |
| Neutropenia | 36(17.1%) | 4(1.9%) | 20(16.9%) | 4(3.4%) |
| Anorexia | 27(12.8%) | 3(1.4%) | 16(13.6%) | 2(1.7%) |
| Gastritis | 26(12.3%) | 3(1.4%) | 8(6.8%) | 1(0.8%) |
| Alopecia | 24(11.4%) | 0 | 15(12.7%) | 0 |
| Decreased white blood cells | 23(10.9%) | 3(1.4%) | 13(11.0%) | 6(5.1%) |
| Anemia | 18(8.5%) | 4(1.9%) | 12(10.2%) | 2(1.7%) |
| Decreased platelet count | 18(8.5%) | 2(0.9%) | 12(10.2%) | 3(2.5%) |
| Elevated liver enzymes | 11(5.2%) | 2(0.9%) | 2(1.7%) | 0 |
| Mucositis/stomatitis | 9(4.3%) | 1(0.5%) | 6(5.1%) | 0 |
| Fever | 8(3.8%) | 0 | 5(4.2%) | 0 |
| Arthritis/arthralgia/myalgia | 8(3.8%) | 0 | 3(2.5%) | 0 |
| Peripheral sensory neuropathy | 6(2.8%) | 0 | 0 | 0 |
| Increased blood creatinine | 5(2.4%) | 0 | 2(1.7%) | 0 |
| Hyponatremia | 5(2.4%) | 0 | 0 | 0 |
a. Adverse events were classified according to Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Grading ranges from 1 through 5 (1, mild; 2, moderate; 3, severe; 4. life-threatening; and 5, death). b. Treatment-related adverse events occurring in 1% or more of patients in either group are listed. Events are shown in descending order of frequency in R group. c. The numbers represent the number of patients with an adverse event.
Discussion
We performed this multicenter retrospective cohort study in patients with recurrent and metastatic ESCC and demonstrated the efficacy of ICI rechallenge after first-line immunotherapy. Among patients who discontinued the first-line treatment regimen including anti-PD-1/L1 antibody, rechallenge of ICI treatment achieved a higher response rate (ORR: 23.7% vs. 9.3%; P=.001; DCR: 74.4% vs. 55.1%; P<.001) and longer survival (mPFS: 4.7 vs. 3.2 months, HR =0.58, 95% CI: 0.45- 0.73, P<.001; mOS: 9.3 vs. 6.2 months, HR =0.61, 95% CI: 0.47-0.80, P<.001).
Based on the results of the subgroup analysis conducted according to the best response to the first-line immunotherapy, regardless of the initial response to first-line treatment, significant statistical differences still existed in the PFS and OS between the rechallenge and non-rechallenge groups. In terms of chemotherapy and targeted therapy, resistance is defined as disease progression after a duration of relevant medicine use that may not be applicable to immune treatment. Several studies on other solid tumors, such as lung cancer and renal cell cancer, have shown that the efficacy of ICI rechallenge is independent of the response to the first course of ICI 22-25. This may be due to a delay in the immune response caused by the time required for the immune system to initiate an anti-tumor response 26,27. Therefore, patients with ESCC who fail to achieve satisfactory tumor regression with initial immunotherapy may still benefit from the rechallenge treatment.
The subgroup analysis revealed that radiotherapy as the initial treatment is a critical factor in enhancing the efficacy of ICI rechallenge. In patients who initially underwent radiotherapy, the reintroduction of immunotherapy resulted in a significant survival benefit. However, this benefit was unclear in patients who did not receive radiotherapy. This finding is consistent with the commonly recognized synergistic effects of immunotherapy and radiotherapy. Besides its direct tumoricidal effects, ionizing radiation is deeply involved in the anti-tumor immune response. Relevant mechanisms include the release of tumor-associated antigens 28, activation of dendritic cells 29, upregulation of cytokines and chemokines 30, normalization of the tumor vasculature 31. Preclinical studies have shown that radiotherapy-induced remodeling of the tumor immune microenvironment helps build immune memory and overcome immune evasion, enhancing the anti-tumor efficiency of subsequent immunotherapy 32,33. The enhancement of immunotherapy provided by prior irradiation was also corroborated by reliable clinical evidence, namely, the Pacific trial, which established the value of ICI after chemoradiotherapy in local advanced Non-small cell lung cancer (NSCLC) 34. Similar conclusions have been drawn by Garon et al. 35. Accordingly, we inferred that initial radiotherapy is a favorable factor for immunotherapy rechallenge.
The findings of the multivariate analysis revealed that patients with better ECOG PS scores and earlier N stages benefited significantly from ICI rechallenge. Physical function and immune status are still crucial factors for the rechallenge of immunotherapy, which is consistent with previous findings. Imbalance of circulating T-lymphocyte subpopulations correlates with ECOG PS scores in patients with gastric cancer 36. A low ECOG-PS score serves as a negative prognostic indicator concerning the clinical outcomes of initial ICI therapy in NSCLC patients 37-40. Along with the disease extent, the N stage also reflects the extent of impairment in the efficiency of the anti-tumor immune response. A solid and convincing study found that dynamic CD8+ T-cell responses in normal lymph nodes, which are critical to immunotherapy, are disrupted in metastatic lymph nodes 41. A retrospective clinical analysis also found that retaining more normal lymph nodes after surgery was associated with elevated immunotherapy efficacy 42. The expression status of PD-L1 is considered one of the most reliable biomarkers for ICI treatments. Unfortunately, owing to the unavailability of PD-L1 status, we did not conduct a relevant analysis.
Our results showed similar rates of all-grade and high-grade TRAEs in both the groups. However, the types of AEs varied between the two groups, with gastrointestinal reactions and hematologic toxicity predominating in the R group and hematologic toxicity predominating in the NR group. Of the four patients who underwent ICI rechallenge after discontinuation due to AEs, one patient experienced a grade 3 gastrointestinal AE, similar to his initial AE. For patients who received radiotherapy during initial treatment, the incidence of AEs was similar to that of patients who did not receive radiotherapy, and no new AEs were identified. The Keynote001 study also demonstrated that there were no statistical differences in the frequency of pulmonary toxicity between patients who did and did not previously receive thoracic radiotherapy 35. Our study shows that ICI rechallenge has acceptable safety profiles regardless of whether patients received radiotherapy during their initial treatment. Close monitoring and adherence to standard treatment protocols are essential for identifying and managing the toxic effects associated with ICI rechallenge.
Our findings further contribute additional clinical evidence on the efficacy and safety of ICI rechallenge in patients with recurrent and metastatic ESCC. However, our study still has limitations. First, its retrospective design may have introduced recall bias and data gaps. Second, the lack of standardized rechallenge combination regimens may have affected the results and safety profiles. ICIs have only been approved as first-line immunotherapy options for esophageal cancer in China for a limited period. We have collected all currently eligible cases and will continue to expand the number of cases. In addition, prospective studies (NCT03736863) are ongoing, and further prospective clinical trials is warranted to explore the efficacy and safety of rechallenging with anti-PD-1/PD-L1 inhibitors.
In conclusion, rechallenge with ICIs is a viable option for patients with ESCC considering its encouraging efficacy and manageable safety, particularly in patients previously treated with radiotherapy. Further prospective trails are required to confirm these results.
Supplementary Material
Supplementary table.
Acknowledgments
Funding
This research was supported by National Natural Science Foundation of China (Grant number 82172865), Start-up fund of Shandong Cancer Hospital (Grant number 2020-B14), Clinical Research Special Fund of Wu Jieping Medical Foundation (Grant number 320.6750.2021-02-51 and 320.6750.2021-17-13), The Key Research and Development Program of Shandong (Major Science & Technology Innovation Project) (Grant number 2021SFGC05012021).
Author contributions
JM.Y., LL.W. and XJ.Z. made the study conception and design. Material preparation and data collection were performed XJ.Z. and JZ.Z. Data analysis was performed by XJ.Z. and JZ.Z. Statistical analysis was done by JY.H. The first draft of the manuscript was written by XJ.Z. and revised by JZ.Z. and LL.W. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Data availability statement
The data that support the findings of this study are available from the corresponding author, [Linlin Wang], upon reasonable request.
Ethical approval
This study was conducted in accordance with the Declaration of Helsinki and was approved by the independent ethics committees of Shandong Cancer Hospital and Institute, Shandong Provincial Hospital Affiliated with Shandong First Medical University. Owing to the retrospective design, the requirement for informed consent was waived.
Abbreviations
- AE
adverse events
- CR
complete response
- CI
confidence interval
- DCR
disease control rate
- ESCC
esophageal squamous cell cancer
- ECOG PS
Eastern Cooperative Oncology Group Performance Status
- HR
hazard ratio
- ICI
immune checkpoint inhibitor
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PFS
progressive-free survival
- PR
partial response
- SD
steady disease
- TRAE
treatment-related adverse event
References
- 1.Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen H-D, Yu Y-W. et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics. 2020. Chin Med J (Engl) 2021;134:783–91. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Obermannová R, Alsina M, Cervantes A. et al. Oesophageal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;33:992–1004. doi: 10.1016/j.annonc.2022.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Lin M-Q, Li Y-P, Wu S-G. et al. Differences in esophageal cancer characteristics and survival between Chinese and Caucasian patients in the SEER database. Onco Targets Ther. 2016;9:6435–44. doi: 10.2147/OTT.S112038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun J-M, Shen L, Shah MA. et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): a randomised, placebo-controlled, phase 3 study. The Lancet. 2021;398:759–71. doi: 10.1016/S0140-6736(21)01234-4. [DOI] [PubMed] [Google Scholar]
- 6.Luo H, Lu J, Bai Y. et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients With Advanced or Metastatic Esophageal Squamous Cell Carcinoma: The ESCORT-1st Randomized Clinical Trial. JAMA. 2021;326:916. doi: 10.1001/jama.2021.12836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kojima T, Shah MA, Muro K. et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. JCO. 2020;38:4138–48. doi: 10.1200/JCO.20.01888. [DOI] [PubMed] [Google Scholar]
- 8.Wang Z-X, Cui C, Yao J. et al. Toripalimab plus chemotherapy in treatment-naïve, advanced esophageal squamous cell carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell. 2022;40:277–288.e3. doi: 10.1016/j.ccell.2022.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Kato K, Cho BC, Takahashi M. et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. The Lancet Oncology. 2019;20:1506–17. doi: 10.1016/S1470-2045(19)30626-6. [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Xu J, Chen Y. et al. Camrelizumab versus investigator's choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): a multicentre, randomised, open-label, phase 3 study. The Lancet Oncology. 2020;21:832–42. doi: 10.1016/S1470-2045(20)30110-8. [DOI] [PubMed] [Google Scholar]
- 11.Schoenfeld AJ, Hellmann MD. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell. 2020;37:443–55. doi: 10.1016/j.ccell.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu H, Wang K, Jia R. et al. Current Status in Rechallenge of Immunotherapy. Int J Biol Sci. 2023;19:2428–42. doi: 10.7150/ijbs.82776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Michielin O, van Akkooi ACJ, Ascierto PA. et al. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30:1884–901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 14.Swetter SM, Thompson JA, Albertini MR. et al. NCCN Guidelines® Insights: Melanoma: Cutaneous, Version 2.2021. J Natl Compr Canc Netw. 2021;19:364–76. doi: 10.6004/jnccn.2021.0018. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan RJ, Atkins MB, Kirkwood JM. et al. An update on the Society for Immunotherapy of Cancer consensus statement on tumor immunotherapy for the treatment of cutaneous melanoma: version 2.0. J Immunother Cancer. 2018;6:44. doi: 10.1186/s40425-018-0362-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bimbatti D, Maruzzo M, Pierantoni F. et al. Immune checkpoint inhibitors rechallenge in urological tumors: An extensive review of the literature. Critical Reviews in Oncology/Hematology. 2022;170:103579. doi: 10.1016/j.critrevonc.2022.103579. [DOI] [PubMed] [Google Scholar]
- 17.Cai Z, Zhan P, Song Y. et al. Safety and efficacy of retreatment with immune checkpoint inhibitors in non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2022;11:1555–66. doi: 10.21037/tlcr-22-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheiner B, Roessler D, Phen S. et al. Efficacy and safety of immune checkpoint inhibitor rechallenge in individuals with hepatocellular carcinoma. JHEP Rep. 2023;5:100620. doi: 10.1016/j.jhepr.2022.100620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravi P, Mantia C, Su C. et al. Evaluation of the Safety and Efficacy of Immunotherapy Rechallenge in Patients With Renal Cell Carcinoma. JAMA Oncol. 2020;6:1606. doi: 10.1001/jamaoncol.2020.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gul A, Stewart TF, Mantia CM. et al. Salvage Ipilimumab and Nivolumab in Patients With Metastatic Renal Cell Carcinoma After Prior Immune Checkpoint Inhibitors. JCO. 2020;38:3088–94. doi: 10.1200/JCO.19.03315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eisenhauer EA, Therasse P, Bogaerts J. et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Abou Alaiwi S, Xie W, Nassar AH. et al. Safety and efficacy of restarting immune checkpoint inhibitors after clinically significant immune-related adverse events in metastatic renal cell carcinoma. J Immunother Cancer. 2020;8:e000144. doi: 10.1136/jitc-2019-000144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Liu T, Liu Q. et al. Rechallenge of immunotherapy beyond progression in patients with extensive-stage small-cell lung cancer. Front Pharmacol. 2022;13:967559. doi: 10.3389/fphar.2022.967559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akamatsu H, Teraoka S, Takamori S. et al. Nivolumab Retreatment in Non-Small Cell Lung Cancer Patients Who Responded to Prior Immune Checkpoint Inhibitors and Had ICI-Free Intervals (WJOG9616L) Clin Cancer Res. 2022;28:OF1–7. doi: 10.1158/1078-0432.CCR-22-0602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gobbini E, Toffart AC, Pérol M. et al. Immune Checkpoint Inhibitors Rechallenge Efficacy in Non-Small-Cell Lung Cancer Patients. Clin Lung Cancer. 2020;21:e497–510. doi: 10.1016/j.cllc.2020.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Kuczynski EA, Sargent DJ, Grothey A. et al. Drug rechallenge and treatment beyond progression—implications for drug resistance. Nat Rev Clin Oncol. 2013;10:571–87. doi: 10.1038/nrclinonc.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robert C, Schadendorf D, Messina M. et al. Efficacy and Safety of Retreatment with Ipilimumab in Patients with Pretreated Advanced Melanoma Who Progressed after Initially Achieving Disease Control. Clinical Cancer Research. 2013;19:2232–9. doi: 10.1158/1078-0432.CCR-12-3080. [DOI] [PubMed] [Google Scholar]
- 28.Formenti SC, Rudqvist N-P, Golden E. et al. Radiotherapy induces responses of lung cancer to CTLA-4 blockade. Nat Med. 2018;24:1845–51. doi: 10.1038/s41591-018-0232-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deng L, Liang H, Xu M. et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura S, Wang B, Kawashima N. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lugade AA, Moran JP, Gerber SA. et al. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 32.He K, Barsoumian HB, Sezen D. et al. Pulsed Radiation Therapy to Improve Systemic Control of Metastatic Cancer. Front Oncol. 2021;11:737425. doi: 10.3389/fonc.2021.737425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sezen D, Barsoumian HB, He K. et al. Pulsed radiotherapy to mitigate high tumor burden and generate immune memory. Front Immunol. 2022;13:984318. doi: 10.3389/fimmu.2022.984318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antonia SJ, Villegas A, Daniel D. et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:1919–29. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 35.Shaverdian N, Lisberg AE, Bornazyan K. et al. Previous radiotherapy and the clinical activity and toxicity of pembrolizumab in the treatment of non-small-cell lung cancer: a secondary analysis of the KEYNOTE-001 phase 1 trial. Lancet Oncol. 2017;18:895–903. doi: 10.1016/S1470-2045(17)30380-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Shen Y. Imbalance of circulating T-lymphocyte subpopulation in gastric cancer patients correlated with performance status. Clin Lab. 2013;59:429–33. [PubMed] [Google Scholar]
- 37.Inomata M, Hirai T, Seto Z. et al. Clinical Parameters for Predicting the Survival in Patients with Squamous and Non-squamous-cell NSCLC Receiving PD-1 Inhibitor Therapy. Pathol Oncol Res. 2020;26:327–33. doi: 10.1007/s12253-018-0473-x. [DOI] [PubMed] [Google Scholar]
- 38.Diem S, Schmid S, Krapf M. et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 39.Mezquita L, Auclin E, Ferrara R. et al. Association of the Lung Immune Prognostic Index With Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non-Small Cell Lung Cancer. JAMA Oncol. 2018;4:351–7. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Katayama Y, Yamada T, Tanimura K. et al. Impact of bowel movement condition on immune checkpoint inhibitor efficacy in patients with advanced non-small cell lung cancer. Thorac Cancer. 2019;10:526–32. doi: 10.1111/1759-7714.12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahim MK, Okholm TLH, Jones KB. et al. Dynamic CD8+ T cell responses to cancer immunotherapy in human regional lymph nodes are disrupted in metastatic lymph nodes. Cell. 2023;186:1127–1143.e18. doi: 10.1016/j.cell.2023.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Deng H, Zhou J, Chen H. et al. Impact of lymphadenectomy extent on immunotherapy efficacy in postresectional recurred non-small cell lung cancer: a multi-institutional retrospective cohort study. Int J Surg. 2024;110:238–52. doi: 10.1097/JS9.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary table.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [Linlin Wang], upon reasonable request.