Abstract
Background/Aim
The recently published Node-Reporting and Data System (Node-RADS) can aid the characterization of lymph nodes in cross-sectional imaging. This study investigated the Node-RADS system in computed tomography (CT) to characterize lymph nodes in esophageal cancer.
Patients and Methods
Overall, 126 patients (15 female, 11.9%) with a mean age of 62.1±10.4 years comprised the patient sample. All patients underwent resection with curative intent and the lymph nodes were histopathologically analyzed during clinical routine. For every patient, the locoregional lymph nodes were scored in accordance with the Node-RADS classification. For statistical analysis, receiver-operating characteristics (ROC) with area under the curve (AUC) were used to test for diagnostic accuracy; inter-reader variability was assessed with Cohen’s kappa.
Results
Overall, 54 patients were nodal positive (42.9%), 72 patients were nodal negative (57.1%). Inter-reader agreement was substantial for the overall Node-RADS scoring (ĸ=0.65, p<0.001). ROC curve analysis for lymph node discrimination (N0 versus N1-3) showed an AUC of 0.69 (95% confidence interval=0.59-0.79). A threshold score of more than 2 resulted in a sensitivity of 0.77 and a specificity of 0.55 for correctly predicting nodal positivity. Node-RADS 1 category had a malignancy rate of 30%, Node-RADS 2 of 14%, Node-RADS 3 of 81%, Node-RADS 4 of 90.1% and Node-RADS 5 of 86.5%.
Conclusion
The Node-RADS score on staging CT is associated with the malignancy rate of lymph nodes in patients with EC with only moderate diagnostic accuracy. The inter-reader variability is moderate, which could pose difficulties for translation into clinical routine.
Keywords: Computed tomography, lymph node, diagnostic performance, esophageal cancer
Esophageal cancer (EC) is one of the most common malignancies and is the sixth most deadly cancer worldwide (1). Despite advances in diagnostic and therapeutic options and modalities, the overall 5-year survival rate for EC has improved only marginally and remains between 15 and 20% (2). Radical esophagectomy is the only curative treatment choice for patients with EC. However, surgery alone has a high perioperative mortality of approximately 7.7% (3). It is of great importance to characterise patients at risk of poor surgical and oncological outcomes and to correctly diagnose lymph node involvement.
Nodal positivity is an important prognostic factor in EC, with reported 5-year survival rates of 90% for patients having no lymph node metastasis, 52.2% for those with 1-4 lymph node metastases, and 28.9% for those with 5 or more lymph node metastases, respectively (p<0.05) (4). Of note, the accuracy of clinical modalities to correctly diagnose lymph node metastases in EC is only poor, with a reported sensitivity of 54.4% and specificity of 77.3% for contrast-enhanced computed tomography (CT) (5). There is a definite need to improve the diagnostic modalities in clinical routine.
The Node-Reporting and Data System (Node-RADS) is a recently proposed novel classification system for standardizing clinical reporting and possible categorization of lymph nodes in oncological imaging and staging investigations (6). It consists of two main features, size and configuration. A 5-point probability scale is used ranging from 1 (with a very low likelihood) up to 5 (with a very high likelihood) of malignant involvement (6). For other standardized radiological reporting systems, comprising breast imaging RADS (BI-RADS), prostate imaging RADS (PI-RADS), liver imaging RADS (LI-RADS) and thyroid imaging RADS (TI-RADS), various studies have analyzed the potential diagnostic benefits and potential shortcomings, as well as evaluating malignant probability in the different categories (7-10).
However, there is limited data regarding the diagnostic accuracy and potential clinical benefit of Node-RADS beyond the first description (11-16). There have been systematic publications with inconsistent results, promising results for lung cancer and gastric cancer, and rather weak results for colon cancer (11,13,16).
Notably, there is still demand for systematic evaluation in patients with EC. A better classification and characterization of lymph nodes may be crucial in patients with EC to improve treatment planning.
Therefore, the present study aimed to elucidate whether the Node-RADS classification on staging CT can characterize locoregional lymph nodes and improve the diagnostic performance in patients with EC.
Patients and Methods
Study design. This retrospective study was approved by the local Ethics Committee of the University Hospital of Leipzig (approval no.: 106-16-14032016). The study was conducted according to the ethical standards of the institutional and/or national research committee and with the Helsinki Declaration of 1964 and its subsequent amendments or equivalent ethical standards.
The radiological database of the University Hospital of Leipzig was screened for all consecutive patients with EC between August 2016 and January 2023.
The inclusion criteria for this study consisted of available pre-treatment CT images and histopathologically confirmed EC. All patients underwent surgery with curative intention, including locoregional lymphadenectomy and pathological evaluation during clinical routine. All analyzed CT scans were performed within 4 weeks before the surgical resection.
Surgery. All procedures were performed by consultant surgeons. Minimally invasive McKeown and Ivor Lewis resections included total minimally invasive procedures, hybrid procedures (where part of the operation is performed as an open procedure), and total minimally invasive robotic resections through an abdominal and right thoracic approach. Ivor Lewis resections were primarily performed using the hybrid approach (minimally invasive abdominal and open thoracic), with a change to totally minimally invasive surgery and robotically assisted esophagectomies starting in 2023. Open abdominal surgery was only used in cases of previous extensive abdominal surgeries or necessity for extensive lymphadenectomy. We routinely performed an end-side esophagogastrostomy with circular stapler anastomosis and nasogastric tube placement for early feeding (17).
CT imaging. Contrast-enhanced CT was performed on a 128-slice CT scanner (Ingenuity 128, Philips, Hamburg, Germany) or 256-slice CT scanner (iCT, Philips) during clinical routine. Intravenous iodinated contrast (90 mL Imeron 400 MCT; Bracco Imaging Germany GmbH, Konstanz, Germany) was administered at a rate of 4.0 ml/s via a peripheral venous line. Typical imaging parameters were 100 kVp; 125 mAs; slice thickness, 1 mm; slice spacing, 0.9. The staging CT included the thorax and abdomen and was acquired in the portal venous phase.
Node-RADS. The locoregional lymph nodes located in the mediastinum were scored according to the Node-RADS classification (6). The scoring was independently performed by two radiologists with 5 years and 3 years of experience in oncological CT imaging analysis, respectively. Both readers were blinded to the pathological results. In short, Node-RADS score ranges from 1 to 5: 1 − very low; 2 − low; 3 − equivocal; 4 − high; 5 − very high, as in the other RADS-classifications (7-10). The two imaging criteria size and configuration are the main imaging findings evaluated.
The size is measured as the short-axis diameter and is considered enlarged above the threshold value of 10 mm. Configuration is assessed as texture (homogenous, heterogeneous) and with border (smooth or irregular). All features taken together result in the final lymph node category. Figure 1 provides two cases to demonstrate the scoring of the patient samples.
Figure 1.
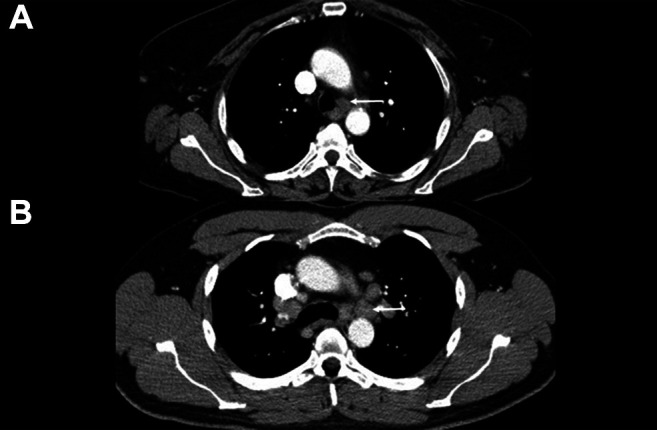
Representative cases from the patient cohort; white arrows indicate the lymph nodes that were assessed. (A) A 57-year-old female patient with T2, N0, M0 squamous cell carcinoma. The Node-RADS score was 3, and the short-axis diameter was 11 mm. (B) A 36-year-old male patient, with T3, N1, M0 squamous cell carcinoma. The Node-RADS score was 5 and the short-axis diameter was 16 mm.
Statistical analysis. Firstly, the collected data was analyzed with descriptive statistics. Discriminatory analysis was investigated with Mann-Whitney test and Fisher’s exact test where suitable. Inter-reader variability was analyzed with Cohen’s kappa. Diagnostic accuracy was further tested with receiver-operating characteristics (ROC) curve, with area under the curve (AUC) analysis as the outcome parameter. In every instance, two-sided values of p<0.05 were used to indicate statistical significance. All analyses and graphical creation were performed with SPSS (IBM, Version 25.0, Armonk, NY, USA).
Results
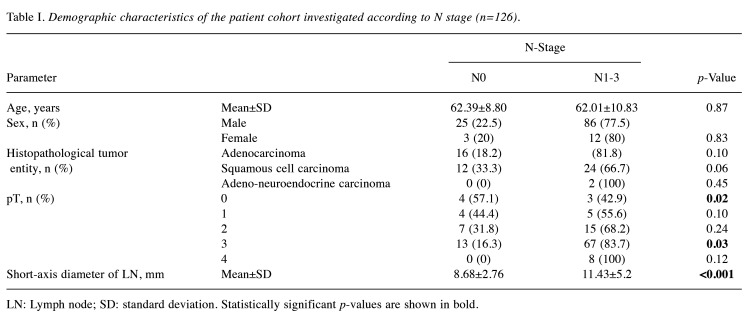
Overall, 126 consecutive patients (15 female, 11.9%) with a mean age of 62.1±10.4 years were included in the present analysis. Table I provides an overview of the demographics of the patient cohort. Among the cohort, 54 patients were nodal positive (42.9%), whereas 72 patients were nodal negative (57.1%). According to the American Joint Committee on Cancer (AJCC) TNM classification (18), N1 stage was present in 28 cases (22.2%), N2 in 20 (15.9%) and N3 stage in six (4.8%).
Table I. Demographic characteristics of the patient cohort investigated according to N stage (n=126).
LN: Lymph node; SD: standard deviation. Statistically significant p-values are shown in bold.
Regarding histopathological type, the tumors were squamous cell carcinoma in 36 (28.6%) cases, adenocarcinoma in 88 cases (69.8%) and a mixed adenoneuroendocrine carcinoma in two cases (1.6%).
Discriminatory analysis. In total, 182 lymph nodes (37 N0 stage, 101 N1, 35 N2, and 9 N3 stage) were scored with the Node-RADS system. For reader 1, the score was Node-RADS1 for 10 (5.5%), Node-RADS 2 for 43 (23.6%), Node-RADS 3 for 37 (20.3%), Node-RADS 4 for 55 (30.2%) and (20.3%) Node-RADS 5 for 37.
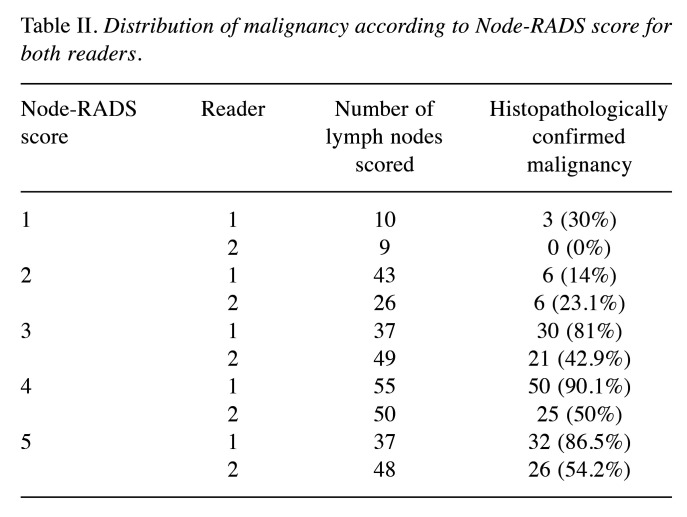
The Node-RADS results were as follows for reader 2: Node-RADS 1 for 9 (4.9%), Node-RADS 2 for 26 (14.3%), Node-RADS 3 for 49 (26.9%), Node-RADS 4 for 50 (27.5%) and Node-RADS 5 for 48 (26.4%). The distribution of the malignancy rate according to Node-RADS score is provided in Table II.
Table II. Distribution of malignancy according to Node-RADS score for both readers.
Inter-reader agreement. Overall, the inter-reader agreement was substantial for the Node-RADS scoring (ĸ=0.65, p<0.001). The inter-reader agreement for the main category size reached an excellent ĸ of 1.0 (p<0.001). Inter-reader agreement for the main category configuration reached moderate levels, with ĸ=0.52 (p<0.001). Inter-reader agreement for the subcategories of configuration were as follows: texture reached ĸ=0.62 (p<0.001), shape reached ĸ=0.74 (p=0.001) and scoring of the border resulted in ĸ=0.65 (p<0.001).
Malignancy rate according to Node-RADS. Node-RADS 1 had a malignancy rate of 0% for reader 1 and 30% for reader 2, whereas the corresponding malignancy rates were 14% and 23.1% for Node-RADS 2, 81% and 42.9% for Node-RADS 3, 90.1% and 50% for Node-RADS 4, and 86.5% and 54.2% for Node-RADS 5.
The resulting total Node-RADS score was statistically significant higher in node-positive compared to node-negative cases (mean score±standard deviation N0 versus N1-3: reader 1: 2.68±1.31 versus 3.54±1.11; reader 2: 3.05±1.31 versus 3.69±1.10; p<0.001). The short-axis diameter was statistically higher in node-negative compared to node-positive cases (N0 versus N1-3: mean 8.68±2.76 versus 11.43±5.2 mm, p<0.001).
Consequently, the Node-RADS subcategory size was also statistically significantly higher comparing N0 and N1-3 stages (N0 versus N1-3: mean of 1.27±0.45 versus 1.60±0.52, p<0.001).
Furthermore, the main subcategory configuration reached statistical significance in the discrimination of benign from malignant lymph nodes for reader 1 (scores of 1.41±0.99 for N0 and 1.95±0.80 for N1-3, p=0.001). For reader 2, there was no statistical significant difference (p=0.15). Differences in scores for the sub-subcategories texture (p=0.07), border (p=0.06) and shape (p=0.11) did not reach statistically significant values.
Diagnostic accuracy of Node-RADS. The ROC curve analysis revealed an AUC of 0.69 [95% (confidence interval) CI=0.59-0.79] for lymph node discrimination (N0 versus N1-3) for reader 1 (Figure 2). For reader 2, the AUC reached a value of 0.64 (95% CI=0.54-0.75). A threshold Node-RADS score of more than 2 resulted for reader 1 in a sensitivity of 0.77 and a specificity of 0.55. For reader 2, a threshold value of a Node-RADS score of more than 3 resulted in a sensitivity of 0.52 and a specificity of 0.83.
Figure 2.
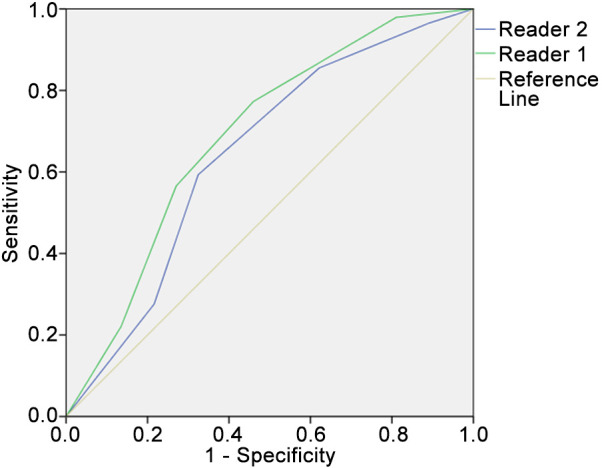
Results of the receiver operating characteristics curve analysis for discrimination of N0 versus N1-3 using the total Node-RADS score for both readers. The area under the curve for reader 1 was 0.69 (95% confidence interval=0.59-0.79) and for reader 2 was 0.64 (95% confidence interval=0.54-0.75). For reader 1, a threshold value of Node-RADS score 2 resulted in a sensitivity of 0.77 and a specificity of 0.55; for reader 2, a threshold value of a Node-RADS score of more than 3 resulted in a sensitivity of 0.52 and a specificity of 0.83.
ROC curve analysis using the short-axis diameter resulted in an AUC of 0.72 (95% CI=0.64-0.81; p<0.001). Employing the cut-off value of 8.5 mm, a sensitivity of 0.74 and specificity of 0.59 for lymph node discrimination was achieved (Figure 3). No statistically significant difference in discriminatory ability was demonstrated between the short-axis diameter and the Node-RADS score (DeLong test, p=0.29).
Figure 3.
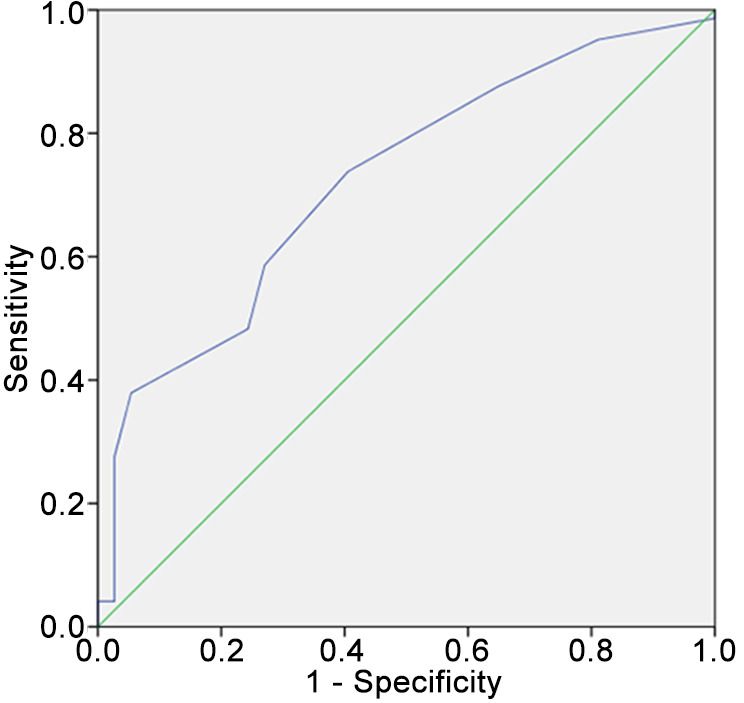
Results of the receiver operating characteristics curve analysis for discrimination of N0 versus N1-3 using the short-axis diameter as measured by reader 1. The resulting area under the curve was 0.72 (95% confidence interval=0.64-0.81, p<0.001). Using a threshold value of 8 mm, sensitivity reached 0.74 and specificity 0.59.
Discussion
The present analysis investigated the CT-Node-RADS score as a semiquantitative method for scoring lymph nodes in EC. The study demonstrated the CT-Node-RADS score had a moderate diagnostic accuracy with a good inter-reader agreement, which may warrant further evaluation in clinical routine.
It is well known that nodal status is independently of great prognostic relevance in patients with EC (4). Even very early T1-stage cancers can harbor lymph node metastasis in up to 29.4% of cases (19). In an unselected cohort of squamous cell cancer cases, there were 52.5% of patients with nodal positivity (20). Moreover, squamous cell cancers tend to have an even higher incidence of lymph node metastasis compared to adenocarcinomas of the esophagogastric junction (21). These findings highlight the critical need for imaging modalities to improve the non-invasive diagnosis of malignant lymph nodes. This would possibly alter preoperative decision-making and could deem some patients as having unresectable disease if lymph nodes outside the locoregional area were diagnosed as positive.
Conventional CT imaging has only poor accuracy for correctly diagnosing lymph node metastasis in EC, with a reported sensitivity of 54.4% and specificity of 77.3% for contrast-enhanced CT (5). [18F]-Fluorodeoxyglucose positron-emission tomography yielded in a higher accuracy, as provided by a meta-analysis of seven studies with pooled sensitivity of 0.62 (95% CI=0.40-0.79) and specificity of 0.96 (95% CI=0.93-0.98) (22,23).
Regarding size, it is of great importance that in 10% of male and 4% of female patients, thoracic lymph nodes can measure more than 10 mm in the short-axis diameter (24), which is the proposed cut-off for the Node-RADS score and which may be one reason for the low specificity of the present results.
Besides the size criterion, an important aspect of Node-RADS is the configuration criterion (6). Presumably, a higher heterogeneity of lymph nodes due to tumor deposits results in a higher CT heterogeneity, quantified by the texture aspect of the Node-RADS in particular.
One merit of the present study is that the size criterion had a very high inter-reader agreement, whereas the configuration criterion had only a moderate inter-reader agreement. These are important aspects of the Node-RADS classification to consider before further translation into clinical routine can be made.
Another important aspect of Node-RADS might be to allow a semiquantitative assessment of the malignancy rate. However, we demonstrated that malignant lymph nodes were present even in the lower groups of Node-RADS 1 and 2, which might limit the clinical benefit. We were unable to demonstrate a threshold for clearly differentiating benign lymph nodes from malignant ones.
The current study for the first time provides new representative results for the novel Node-RADS classification in patients with EC. Generally, the current results are in good comparison with published systematic results regarding the diagnostic accuracy of Node-RADS in other cancer entities.
However, the diagnostic accuracy of our study is lower compared to recent Node-RADS studies on gastric cancer or lung cancer (11,13). Beyond that, a recent study from Italy demonstrated a very good diagnostic accuracy of the Node-RADS score in colon cancer (25). Differences in regard to patient cohorts, different characteristics of the primary tumors, or center-specific criteria have to be discussed as potential reasons for the substantial differences of the diagnostic accuracy of the Node-RADS score. Nevertheless, distinctive differences between EC and colon cancer can also be assumed.
In gastric cancer, the study by Loch et al. showed Node-RADS 3 reached a sensitivity of 56.8% with a specificity of 90.7%, and Node-RADS 4 reached a 48.6% sensitivity and 98.1% specificity in discriminating between benign and malignant lymph nodes (13). For size and configuration, their study showed good to substantial inter-reader agreement (ĸ=0.73 and 0.67, p<0.01) (13), which is higher compared to the present results.
The present analysis cannot provide a clear threshold for determining malignancy in EC using Node-RADS, which is in agreement with previous results (11). In the best diagnostic scenario, Node-RADS 5 would result in almost 100% nodal positivity.
It remains uncertain why the diagnostic accuracy is low in EC. It may be due to the small size of positive mediastinal lymph nodes in EC. These small lymph nodes would also be expected to be homogenous in texture, which would consequently result in a low Node-RADS score. One could discuss whether Node-RADS might only reflect the clinical reading of the radiologists and might not translate into a superior diagnostic accuracy. In most cases with EC, the lymph nodes will be round and small, which poses the most diagnostic dilemma.
The findings of our study could potentially be enhanced by incorporating approaches from other research, such as that by Romeo et al., who employed a machine-learning algorithm based on texture-analysis features extracted from primary tumor lesions to predict nodal status, achieving high accuracy rates (over 90%) for the differentiation between benign and malignant lymph nodes (26).
The observation that texture analysis may be a potentially valuable tool is further supported by findings from a study by Shiozaki et al. on nonfunctional pancreatic neuroendocrine neoplasms. Their study demonstrated the utility of texture analysis based on preoperative dynamic computed tomography scans in predicting tumor histological grade with impressive sensitivity (1.00) and specificity (0.89) for grade 1 lesions, resulting in an area under the curve of 0.97 (27). These results further suggest that incorporating texture analysis into our methodology might enhance the predictive accuracy of lymph node characterization.
Clearly, Node-RADS needs to be further evaluated in a larger sample size in terms of malignancy frequency and should be revised to better diagnose malignancy.
There are some limitations of the current analysis to address. Firstly, it has a retrospective study design with possible known inherent bias. However, the Node-RADS score was performed blinded to the pathological results to reduce some bias. Secondly, the patient sample size is rather small. Thirdly, the Node-RADS classification was performed by resident radiologists with limited experience. However, it is not to be expected that senior radiologists will score the Node-RADS classification any differently from that which is well described in different categories by the original publication. However, there remains a slight uncertainty of the diagnostic accuracy of Node-RADS performed by senior radiologists.
In conclusion, the Node-RADS score derived from staging CT is associated with the malignancy of lymph nodes in patients with EC with, as yet, only moderate diagnostic accuracy. The inter-reader variability is moderate, which may pose difficulties for its translation into clinical routine.
Conflicts of Interest
The Authors have no conflict of interest.
Authors’ Contributions
Conception: HJM; study design: HJM and JL; data collection: MM, JL, BS, AKH and SN; data analysis: HJ and JL; data interpretation: DS, TD, HJM, PP, ST and JL, article writing: JL and HJM; article editing: All Authors. All Authors read and approved the final article.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Rogers JE, Sewastjanow-Silva M, Waters RE, Ajani JA. Esophageal cancer: emerging therapeutics. Expert Opin Ther Targets. 2022;26(2):107–117. doi: 10.1080/14728222.2022.2036718. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs HF, Harnsberger CR, Broderick RC, Chang DC, Sandler BJ, Jacobsen GR, Bouvet M, Horgan S. Simple preoperative risk scale accurately predicts perioperative mortality following esophagectomy for malignancy. Dis Esophagus. 2016;30(1):1–6. doi: 10.1111/dote.12451. [DOI] [Google Scholar]
- 4.Wang WP, He SL, Yang YS, Chen LQ. Strategies of nodal staging of the TNM system for esophageal cancer. Ann Transl Med. 2018;6(4):77. doi: 10.21037/atm.2017.12.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foley KG, Christian A, Fielding P, Lewis WG, Roberts SA. Accuracy of contemporary oesophageal cancer lymph node staging with radiological-pathological correlation. Clin Radiol. 2017;72(8):693.e1–693.e7. doi: 10.1016/j.crad.2017.02.022. [DOI] [Google Scholar]
- 6.Elsholtz FHJ, Asbach P, Haas M, Becker M, Beets-Tan RGH, Thoeny HC, Padhani AR, Hamm B. Introducing the Node Reporting and Data System 1.0 (Node-RADS): a concept for standardized assessment of lymph nodes in cancer. Eur Radiol. 2021;31(8):6116–6124. doi: 10.1007/s00330-020-07572-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Renzulli M, Clemente A, Brocchi S, Milandri M, Lucidi V, Vukotic R, Cappabianca S, Golfieri R. LI-RADS: a great opportunity not to be missed. Eur J Gastroenterol Hepatol. 2019;31(3):283–288. doi: 10.1097/MEG.0000000000001269. [DOI] [PubMed] [Google Scholar]
- 8.Jordan EJ, Fiske C, Zagoria RJ, Westphalen AC. Evaluating the performance of PI-RADS v2 in the non-academic setting. Abdom Radiol. 2017;42(11):2725–2731. doi: 10.1007/s00261-017-1169-5. [DOI] [Google Scholar]
- 9.Bent CK, Bassett LW, D’Orsi CJ, Sayre JW. The positive predictive value of BI-RADS microcalcification descriptors and final assessment categories. Am J Roentgenol. 2010;194(5):1378–1383. doi: 10.2214/AJR.09.3423. [DOI] [PubMed] [Google Scholar]
- 10.Barbosa TLM, Junior COM, Graf H, Cavalvanti T, Trippia MA, da Silveira Ugino RT, de Oliveira GL, Granella VH, de Carvalho GA. ACR TI-RADS and ATA US scores are helpful for the management of thyroid nodules with indeterminate cytology. BMC Endocr Disord. 2019;19(1):112. doi: 10.1186/s12902-019-0429-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer HJ, Schnarkowski B, Pappisch J, Kerkhoff T, Wirtz H, Höhn AK, Krämer S, Denecke T, Leonhardi J, Frille A. CT texture analysis and node-RADS CT score of mediastinal lymph nodes - diagnostic performance in lung cancer patients. Cancer Imaging. 2022;22(1):75. doi: 10.1186/s40644-022-00506-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leonardo C, Flammia RS, Lucciola S, Proietti F, Pecoraro M, Bucca B, Licari LC, Borrelli A, Bologna E, Landini N, Del Monte M, Chung BI, Catalano C, Magliocca FM, De Berardinis E, Del Giudice F, Panebianco V. Performance of node-RADS scoring system for a standardized assessment of regional lymph nodes in bladder cancer patients. Cancers (Basel) 2023;15(3):580. doi: 10.3390/cancers15030580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loch FN, Beyer K, Kreis ME, Kamphues C, Rayya W, Schineis C, Jahn J, Tronser M, Elsholtz FHJ, Hamm B, Reiter R. Diagnostic performance of Node Reporting and Data System (Node-RADS) for regional lymph node staging of gastric cancer by CT. Eur Radiol. 2024;34(5):3183–3193. doi: 10.1007/s00330-023-10352-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lucciola S, Pisciotti ML, Frisenda M, Magliocca F, Gentilucci A, Del Giudice F, Canale V, Scarrone E, Busetto GM, Carrieri G, Cormio L, Carbone A, Pastore A, De Nunzio C, Tubaro A, Leonardo C, Franco G, Di Pierro GB, Salciccia S, Sciarra A, Panebianco V. Predictive role of node-rads score in patients with prostate cancer candidates for radical prostatectomy with extended lymph node dissection: comparative analysis with validated nomograms. Prostate Cancer Prostatic Dis. 2023;26(2):379–387. doi: 10.1038/s41391-022-00564-z. [DOI] [PubMed] [Google Scholar]
- 15.Leonhardi J, Sabanov A, Schnarkowski B, Hoehn A, Sucher R, Seehofer D, Denecke T, Meyer H. CT texture analysis and node-RADS CT score of lymph nodes in patients with perihilar cholangiocarcinoma. Anticancer Res. 2023;43(11):5089–5097. doi: 10.21873/anticanres.16709. [DOI] [PubMed] [Google Scholar]
- 16.Leonhardi J, Mehdorn M, Stelzner S, Scheuermann U, Höhn AK, Seehofer D, Schnarkowski B, Denecke T, Meyer HJ. Diagnostic accuracy and reliability of CT-based Node-RADS for colon cancer. Abdom Radiol. 2024 doi: 10.1007/s00261-024-04485-4. [DOI] [Google Scholar]
- 17.van der Sluis PC, Schizas D, Liakakos T, van Hillegersberg R. Minimally invasive esophagectomy. Dig Surg. 2020;37(2):93–100. doi: 10.1159/000497456. [DOI] [PubMed] [Google Scholar]
- 18.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (eds.) AJCC Cancer Staging Manual, Eighth Edition. Springer. 2017 [Google Scholar]
- 19.Nguyen CL, Tovmassian D, Isaacs A, Falk GL. Risk of lymph node metastasis in T1 esophageal adenocarcinoma: a meta-analysis. Dis Esophagus. 2024;37(6):doae012. doi: 10.1093/dote/doae012. [DOI] [PubMed] [Google Scholar]
- 20.Li B, Chen H, Xiang J, Zhang Y, Li C, Hu H, Zhang Y. Pattern of lymphatic spread in thoracic esophageal squamous cell carcinoma: A single-institution experience. J Thorac Cardiovasc Surg. 2012;144(4):778–786. doi: 10.1016/j.jtcvs.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Ma Z, Chen C, Shang X, Yue J, Jiang H. Comparison of lymph node metastasis pattern from esophagogastric junction adenocarcinoma versus very low thoracic esophageal squamous cancer: a propensity-matched analysis. J Thorac Dis. 2023;15(2):442–451. doi: 10.21037/jtd-22-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi W, Wang W, Wang J, Cheng H, Huo X. Meta-analysis of 18FDG PET-CT for nodal staging in patients with esophageal cancer. Surg Oncol. 2013;22(2):112–116. doi: 10.1016/j.suronc.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Jiang C, Chen Y, Zhu Y, Xu Y. Systematic review and meta-analysis of the accuracy of 18F-FDG PET/CT for detection of regional lymph node metastasis in esophageal squamous cell carcinoma. J Thorac Dis. 2018;10(11):6066–6076. doi: 10.21037/jtd.2018.10.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen T, Nilsson M, Lindholm D, Sundström J, Hedberg J. Normal radiological lymph node appearance in the thorax. Dis Esophagus. 2019;32(10):1–6. doi: 10.1093/dote/doy120. [DOI] [Google Scholar]
- 25.Maggialetti N, Greco CN, Lucarelli NM, Morelli C, Cianci V, Sasso S, Rubini D, Scardapane A, Stabile Ianora AA. Applications of new radiological scores: The Node-RADS in colon cancer staging. Radiol Med. 2023;128(11):1287–1295. doi: 10.1007/s11547-023-01755-x. [DOI] [PubMed] [Google Scholar]
- 26.Romeo V, Cuocolo R, Ricciardi C, Ugga L, Cocozza S, Verde F, Stanzione A, Napolitano V, Russo D, Improta G, Elefante A, Staibano S, Brunetti A. Prediction of tumor grade and nodal status in oropharyngeal and oral cavity squamous-cell carcinoma using a radiomic approach. Anticancer Res. 2020;40(1):271–280. doi: 10.21873/anticanres.13949. [DOI] [PubMed] [Google Scholar]
- 27.Shiozaki H, Gocho T, Shirai Y, Takano Y, Ohki K, Suka M, Okamoto T, Fujioka S, Toya N, Ikegami T. A novel observational strategy for nonfunctional pancreatic neuroendocrine neoplasms with texture analysis: a multicenter retrospective study. Cancer Diagn Progn. 2023;3(5):543–550. doi: 10.21873/cdp.10253. [DOI] [PMC free article] [PubMed] [Google Scholar]