Abstract
Background:
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a rare, life-threatening congenital malformation primarily treated with abdominogenital repair. The optimal indication and timing of neurosurgical interventions for the associated spinal cord lesions remains insufficiently studied. We reviewed spinal dysraphism in OEIS to evaluate the best timing for neurosurgical intervention.
Methods:
We retrospectively reviewed 10 patients with OEIS, analyzing their clinical and imaging data, as well as surgical and pathological findings.
Results:
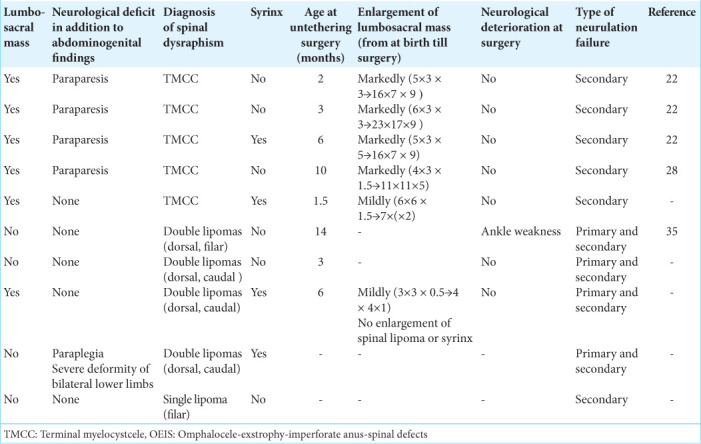
Terminal myelocystocele (TMCC) and spinal lipomas were observed in 5 patients each. Of the spinal lipomas, one had a single filar lipoma, while four had double lipomas (3 caudal and dorsal; 1 filar and dorsal). TMCC manifested with severe lower limb motor dysfunction in addition to abdominogenital disorder at birth, with the cyst-induced lumbosacral mass increasing over time. Spinal lipomas were less symptomatic except for abdominogenital issues and demonstrated minimal growth of the intraspinal lipoma over time. Untethering surgery was performed in 8 patients (5 TMCC; 3 spinal lipomas) at a median age of 3 (range, 2–10) months for TMCC and 6 (range, 2–14) months for spinal lipomas. One TMCC patient (surgery at 10 months) experienced postoperative cerebrospinal fluid leakage, necessitating three reoperations.
Conclusion:
Magnetic resonance imaging is essential to diagnose spinal cord malformations accurately. The necessity and timing of surgical intervention differ between TMCC and spinal lipomas. Since TMCC lesions tend to enlarge, surgery should be performed as soon as the patient’s abdominogenital condition stabilizes. For spinal lipomas, surgery should be considered carefully based on the patient’s neurological condition.
Keywords: Cloacal exstrophy, Double lipomas, Neurulation failure, Retained medullary cord, Spinal lipoma, Terminal myelocystocele

INTRODUCTION
Omphalocele-exstrophy-imperforate anus-spinal defects (OEIS) complex is a combination of defects comprising an omphalocele, exstrophy of the cloaca (cloacal exstrophy; CE), imperforate anus, and spinal defects.[5] OEIS complex considered the most severe form, has been utilized synonymously with CE, representing the same congenital anomaly.[6]
Omphalocele, CE, and imperforate anus are life-threatening conditions that necessitate urgent intervention by pediatric surgeons.[7,30] The most prevalent pathological condition of spinal dysraphism associated with the OEIS complex is occult spinal dysraphism, a deformity that is covered with normal skin. As such, neurosurgical intervention is typically deferred until recuperation from abdominogenital repair. Regarding occult spinal dysraphism, although terminal myelocystocele (TMCC) was previously reported as the most common,[7] recent studies indicate that spinal lipomas, such as the caudal or filar type, are also present.[15] However, the optimal indications and timing of neurosurgical intervention for such occult spinal dysraphism have been infrequently reported due to its rarity.[12,14]
We have previously reported spinal dysraphism in five patients with OEIS complex.[22,28,35] With the addition of five patients, we now summarize data from ten patients with OEIS complex and retrospectively evaluate the indications and timing of neurosurgical intervention by reviewing the clinical, neuroradiological, and histopathological findings.
MATERIALS AND METHODS
In this retrospective study, 10 patients treated at our institutions from 1995 to 2023 were examined, including five previously reported patients with OEIS complex[22,28,35] and five newly treated patients. Sex was comprehensively evaluated based on external genitalia, magnetic resonance imaging (MRI) of internal organs, and sex chromosome testing.
The diagnosis and classification of spinal dysraphism were based on an MRI performed after birth and the histopathological findings of the resected tethered cord region. The morphological classification of spinal lipomas was based on Arai’s classification.[2]
Routine MRI sequences included three-dimensional heavily T2-weighted imaging (3D-hT2WI) and 3D-T1-weighted imaging (3-mm slice thickness) of the whole spine in the sagittal plane, with axial sections through the region of interest. Additional sequences, including thin-slice reconstructed sagittal views of 3D-hT2WI, were performed as previously described.[10,11,23]
Intraoperative neurophysiological monitoring was performed as previously described.[22,23,28] Surgical specimens were stained using hematoxylin and eosin and immunostained for glial fibrillary acidic protein (GFAP) and S-100 protein in five patients (Patients 4–8) who recently underwent surgery.
RESULTS
Patient details
All 10 patients underwent surgical reconstruction to repair an ileostomy or colostomy, as well as enclosure and reduction of the omphalocele, urinary bladder, and urethra, performed by pediatric surgeons 0–5 days after birth. Postnatal MRI findings indicated that all patients exhibited occult spinal dysraphism with a tethered cord. No Chiari malformation or hydrocephalus was observed. Five patients presented with TMCC, while the remaining five possessed spinal lipomas. The clinical, neuroimaging, and histopathological findings are described below and summarized in Table 1 and categorized into two groups: TMCC and spinal lipoma.
Table 1:
Clinical profile of the patients with OEIS complex.
TMCC
The details of four of the five patients with TMCC (Patients 1–4) were previously reported[22,28], and the findings are concisely described herein. Patients 1–3 correspond to those in reference 22. The spinal deformity of Patient 1 in reference 22 was initially described as closed myelomeningocele (MMC); however, subsequent investigations led to a revised diagnosis of TMCC. All patients with TMCC presented with lumbosacral cystic lesions containing cerebrospinal fluid (CSF). Neurological findings revealed that all patients with TMCC, with one exception, exhibited lower limb paraparesis in addition to abdominogenital issues. All patients demonstrated typical MRI findings of TMCC, characterized by cystic dilatation of the terminal spinal cord in the shape of a trumpet (myelocystocele), with a neural placode at its base that communicated with the rostral dilated central canal [Figures 1a and b]. Untethering surgery was performed in all five patients with TMCC, with a median age at surgery of 3 (range: 2–10) months. During the surgical procedure, the neural placode with a dilated central canal was untethered from the nonfunctional portion of the cyst wall. Histopathological examination of Patients 4 and 5 revealed that the inner wall of the myelocystocele sac was lined with an ependymal layer, surrounded by GFAP-immunopositive neuroglial tissues, and the lesion was histologically confirmed as TMCC.
Figure 1:
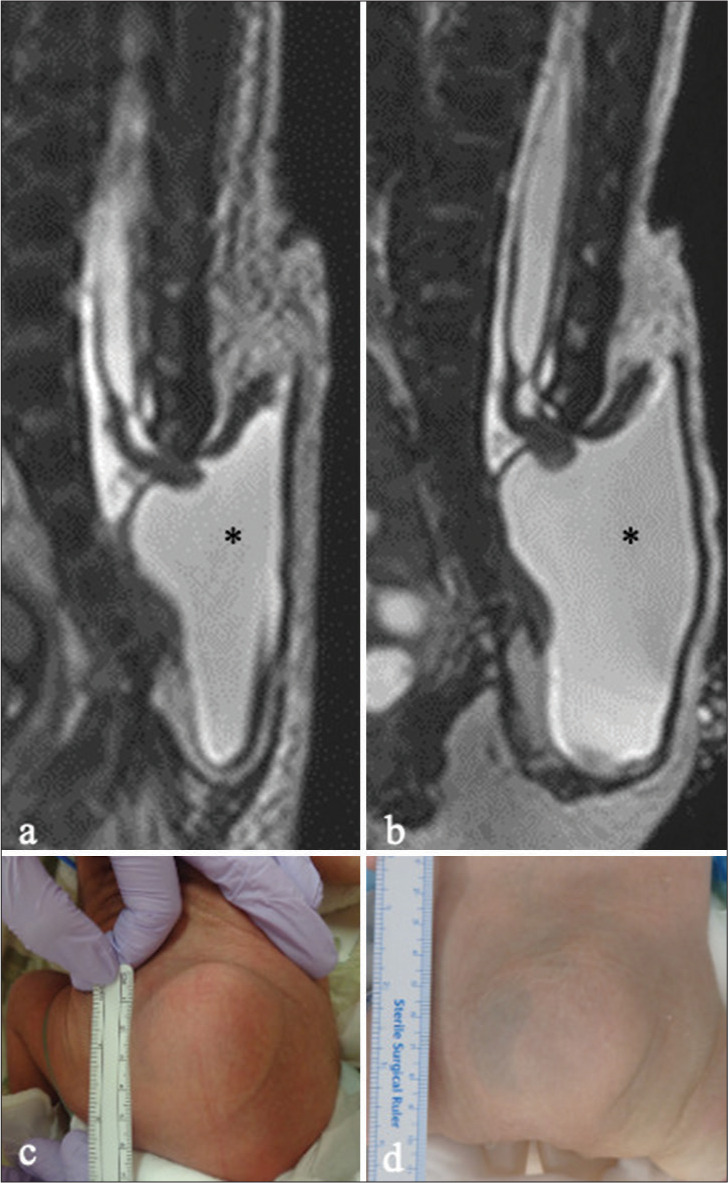
Patient 5. (a and b) Sagittal images of 3D-hT2WI obtained at 7 days (a) and 1.5 months (b) after birth reveal a low-lying hydromyelic cord extruding into the extraspinal space with a trumpet-shaped cystic cavity (myelocystocele; asterisk). Note the enlargement of the cystic cavity containing CSF during the 1.5-month interval (asterisk). (c, d) Photographs of the lumbosacral mass at 7 days (c) and 1.5 months (d) show a slight enlargement of the mass. (6 × 6 × 1.5 cm, and 7 × 6 × 2 cm, respectively). 3D-hT2WI: Three-dimensional heavily T2-weighted images, CSF: Cerebrospinal fluid.
In four patients (Patients 1–4), the CSF-filled cystic lesions significantly enlarged (more than doubled in size) during the waiting period for untethering surgery. In Patient 5, a case of relatively mild cyst enlargement during a brief period of 1.5 months is depicted in Figures 1c and d. This patient exhibited no neurological deterioration either before or after surgery. Similarly, all four patients with substantial cyst enlargement exhibited severe congenital dysfunction of the lower limbs but showed no neurological deterioration before or after surgery. In Patient 4, untethering surgery was delayed until 10 months due to abdominal conditions. During this period, the cyst continued to enlarge, necessitating three repair surgeries for postoperative CSF leakage.[28]
Spinal lipoma
Four out of five patients had double lipomas: three patients (Patients 7–9) possessed caudal and dorsal lipomas, while one patient (Patient 6) had filar and dorsal lipomas. Only Patient 10 had a single filar lipoma. The details of Patient 6 were previously reported.[35] No patients had a subcutaneous mass in the lumbosacral region at birth, except for Patient 8.
Three patients with double lipomas (Patients 6–8) underwent untethering surgery. They all exhibited no neurological symptoms at birth. MRI findings for each patient revealed a low-lying conus medullaris tethered by a terminal lesion, in addition to a dorsal-type lipoma, indicating the presence of two tethering lesions [Figures 2 and 3]. Therefore, we considered the risk of lower limb function deterioration high, and prophylactic surgery was deemed beneficial. The median age at surgery was 6 months (range: 3–14 months). Except for Patient 6, none of the patients experienced neurological deterioration before or after surgery. Patient 6, who underwent surgery at 14 months old, presented with mild ankle weakness at the time of surgery. There was no enlargement of the intraspinal lipoma or syrinx before untethering surgery in any of the patients, although the subcutaneous mass in Patient 8 slightly increased in size. Because the initial MRI of Patient 7 was performed 2 months after birth due to the absence of a lumbosacral mass coupled with no apparent neurological symptoms, untethering surgery was performed shortly after diagnosis, and there was no information regarding changes in the intraspinal lesions.
Figure 2:
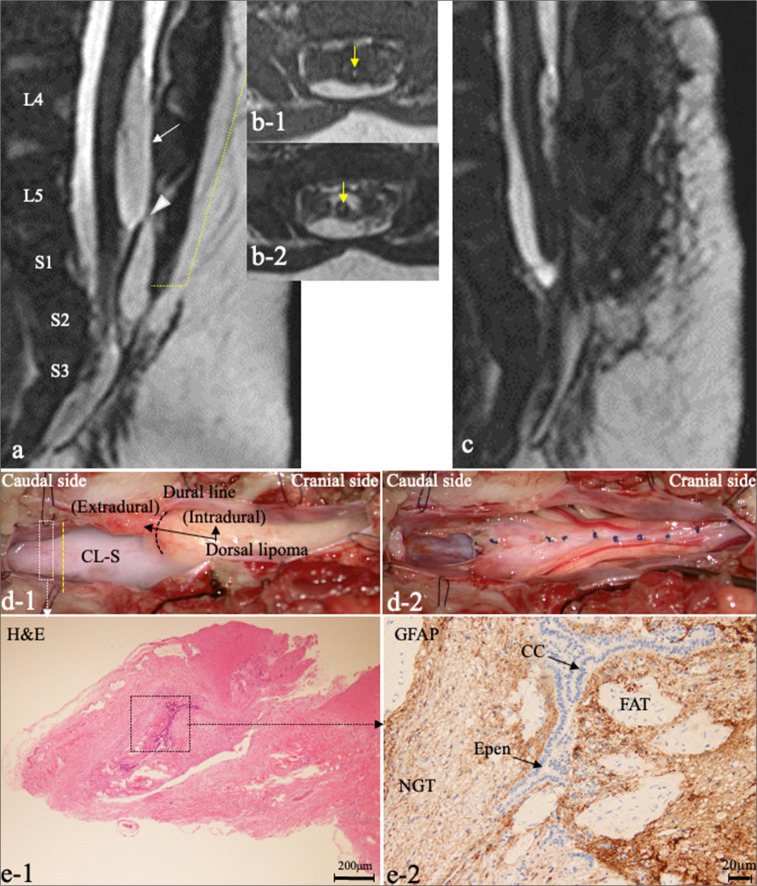
Patient 7. (a) Sagittal 3D-hT2WI reveals a low-lying conus medullaris and the C-LS terminating at the dural cul-de-sac at the S2 level, in addition to a dorsal-type lipoma (white arrows) at the L4–5 levels with a narrow connection (white arrowhead) between the intradural (white arrows) and extradural lipoma. (b) Axial images of T1WI (b-1) and 3D-hT2WI (b-2) of the C-LS show central lesions with high intensity (yellow arrows). (c) Postoperative sagittal 3D-hT2WI shows the untethered spinal cord and debulked dorsal lipoma. (d) Microscopic views of the operative findings. (d-1) Dura opening through laminoplastic laminotomy of L4–S3 (spina bifida below L5) reveals the dorsal lipoma and the C-LS extending to the dural cul-de-sac. The C-LS was cut at the electrophysiological border (yellow dotted line). (d-2) View after untethering. The dorsal lipoma was debulked and sutured from pia to pia. The cut-off surface of the C-LS was elevated. (e) Photomicrograph of the section of C-LS indicated by the white dotted square in (d-1) with H&E at 4 x magnification staining (e-1) and immunostaining for GFAP at 4 x magnification (e-2). (e-2) Higher magnification views of (e-1), which are the areas indicated by the black dotted square in (d-1) showing a central canal (CC)-like, the epen-lined canal (black arrows) with surrounding NGT with a small amount of FAT. 3D-hT2WI: Three-dimensional heavily T2-weighted images, C-LS: Cord-like structure, H&E: Hematoxylin and eosin, GFAP: Glial fibrillary acidic protein, Epen: Ependyma, NGT: Neuroglial tissue, FAT: Fibroadipose tissue, T1WI: T1-weighted imaging.
Figure 3:
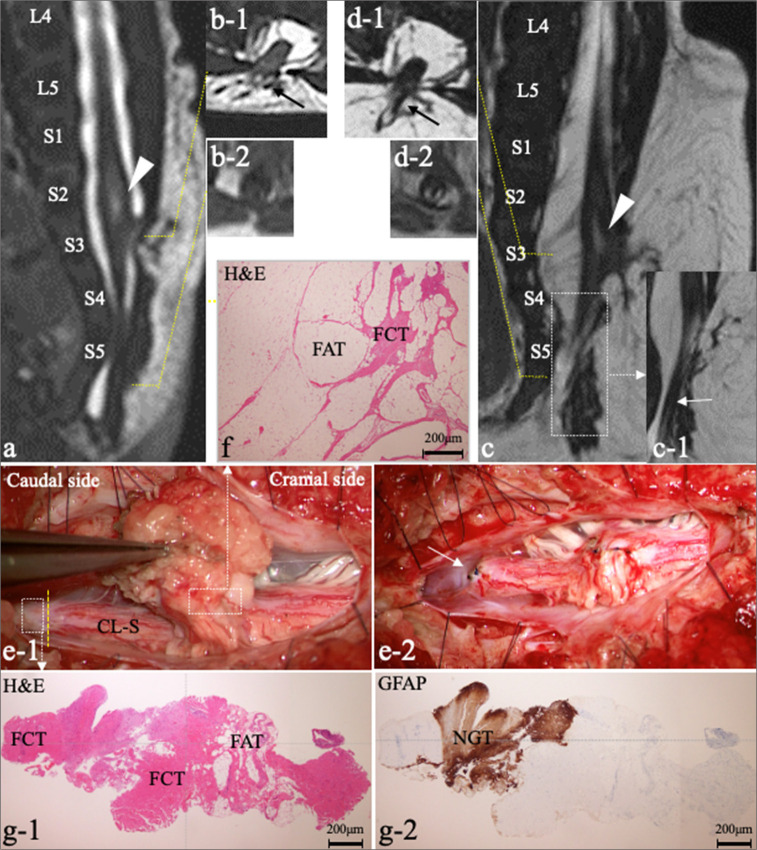
Patient 8. (a-d) Repeated MRI performed at 8 days (a and b) and 6 months (c and d) after birth. Sagittal views of 3D-hT2WI (a and c) and axial T2WI (b-1 and d-1) reveal a thick structure (black arrow) extending from the dorsal surface of the spinal cord through a laminar defect at S3 to the subcutaneous fat. Caudal to conus, a C-LS (white arrow) extends to the cul-de-sac at S5 (c-1: another slice of the area indicated by the dotted square in [c]). Axial T1WI (b-2 and d-2) reveals a small quantity of fat associated with C-LS and the fat became more distinct at 6 months compared to at 8 days. A syrinx is also present (a, c: White arrowhead). While the subcutaneous mass had enlarged, there was no enlargement of the intraspinal lipoma or syrinx between the MRI scans taken at 8 days and 6 months (a and c). (e) Microscopic view of the operative findings. (e-1) Dura opening through L5-S5 reveals the thick dorsal structure with lipomatous tissue and the C-LS extending to the dural cul-de-sac. The C-LS was cut at the nonfunctional region after confirming the electrophysiological border (yellow dotted line) between the functional and nonfunctional portions using intraoperative neurophysiological monitoring. (e-2) Intraoperative photograph shows the dorsal lipoma after debulking and pia-to-pia suturing (white arrow). The cut-off surface of the C-LS was elevated. (f) Histopathology of the dorsal lipoma indicated by the white dotted square in (e-1) with H&E at 4 x magnification staining shows FCT with FAT. (g) Histopathology of the section of C-LS indicated by the white dotted square in (e-1) with H&E staining (g-1) and immunostaining for GFAP at 4 x magnification (g-2) shows FAT with NGT. MRI: Magnetic resonance images, 3D-hT2WI: Three-dimensional heavily T2-weighted images, CL-S: Cord-like structure, H&E: Hematoxylin and eosin, FCT: Fibrocollagenous tissue, FAT: Fibroadipose tissue, GFAP: Glial fibrillary acidic protein, NGT: Neuroglial tissues, T1WI: T1-weighted imaging.
Detailed histopathological examination of the cord-like structure (C-LS) connected caudally to the conus medullaris in Patients 7 and 8 revealed features of a retained medullary cord (RMC), as well as adipose tissue. This included a central canal-like ependyma-lined lumen surrounded by neuroglial tissue [Figures 2 and 3]. In conjunction with the neuroimaging findings and the presence of an intraoperative electrophysiological border at the C-LS, we ultimately diagnosed caudal lipomas with RMC components, in addition to dorsal-type lipomas, in Patients 7 and 8.
Two patients (Patients 9 and 10) received conservative treatment. Patient 9, who presented with caudal and dorsal lipomas, exhibited severe congenital deformities and paraplegia of the bilateral lower limbs, necessitating bilateral femoral amputation by pediatric orthopedic surgeons. Due to the patient’s extremely severe bladder and rectal dysfunction at birth, it was decided that untethering surgery was not indicated, given the low risk of further worsening of tethering cord symptoms. The subcutaneous mass in the lumbosacral region appeared slowly and demonstrated a gradual increase in size over 5 years; however, there was no worsening of symptoms or enlargement of the intraspinal lipoma or syrinx [Figure 4]. Patient 10, who presented with a single filar lipoma with the conus medullaris located around the L2–L3 level and exhibited no neurological symptoms at birth, has demonstrated no worsening of symptoms over 20 years.
Figure 4:
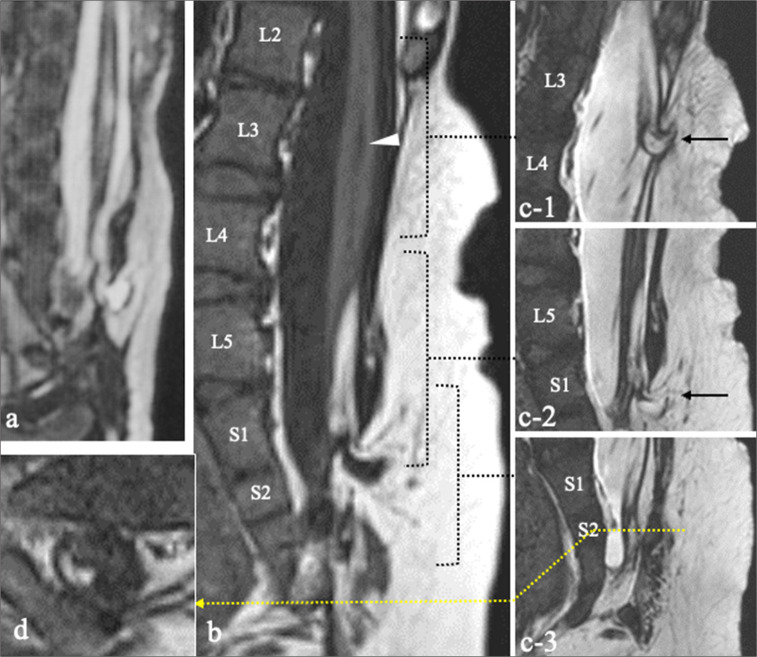
Patient 9. (a-c) Sagittal view of 3D-hT2WI taken at 20 days after birth (a), sagittal views of T1WI (b) and 3D-hT2WI (c), and axial view of T1WI (d), at the age of 5 years. (c: Another slice of the area indicated by the dotted square in [b]) Two isolated dorsal lipomas at the L3/4 level (c-1) and S1 level (c-2) (black arrows). (c-3) The C-LS extends down to the cul-de-sac at the S2 level. (d) A small amount of FAT is present at the termination of the C-LS, indicating a caudal lipoma. A syrinx is observed rostral to the spinal lipomas at the L3 level (b, white arrowhead). There was no change in the intraspinal lipoma or syrinx between the MRI at birth and at 5 years of age. 3D-hT2WI: Three-dimensional heavily T2-weighted images, CL-S: Cord-like structure, FAT: Fibroadipose tissue, MRI: Magnetic resonance imaging, T1WI: T1-weighted imaging.
DISCUSSION
Anorectal/sacral or urogenital anomalies are sometimes associated with spinal lipomas due to the overlapping timelines of the embryonic development periods of the cloaca and secondary neural tube formation (postovulatory 4–6 weeks and 4–7 weeks, respectively).[14,19,20] Although the precise embryopathogenesis of CE has not been fully elucidated, one hypothesis suggests that CE may develop from an early rupture of the cloacal membrane before 8 weeks of gestation;[1,7,20] while another hypothesis proposes that the failure of mesodermal migration leads to unfused inferior abdominal muscles, resulting in the development of CE after 7 weeks of gestation.[20,27] The embryopathogenesis of TMCC is considered to be a retained “terminal balloon” attached to the skin, which normally regresses but can persist due to late arrest of the secondary neurulation before the degenerative phase.[20,31] Given the overlap in developmental periods, it is understandable that TMCC is commonly reported as a type of spinal dysraphism associated with CE. Cohen et al. proposed an explanation for the association of CE and TMCC by hypothesizing that an injury to the notochord, due to being pulled by CE during the 2nd month of development, could interrupt the retrogressive differentiation of the secondary neural tube.[7]
Considering that secondary neurulation failure is also related to the development of filar and caudal lipomas, as well as RMC,[24,26,32] the observation that half of the patients with spinal dysraphism in the present study are patients with OEIS complex comprising caudal and filar lipomas is substantiated. Notably, most patients with spinal lipomas in this study presented with double lipomas. In cases of spinal lipoma, double lipomas are not common.[36] The combinations of double lipomas were caudal and dorsal or filar and dorsal, indicating that they were derived from both primary and secondary neurulation failure. This finding is consistent with a recent report by Kumar et al., which revealed that among 34 patients with CE, 20 possessed spinal lipomas, half of which were double lipomas, derived from both primary and secondary neurulation failure in eight patients.[15] Several studies suggest that the developmental period of CE does not overlap with that of the secondary neurulation as previously believed but is rather a manifestation of disturbances in morphogenesis occurring as early as blastogenesis.[17,34] If the spinal lipomas associated with CE are characterized as double lipomas caused by both primary and secondary neurulation failure,[9,15,25,26] it may support this earlier disorder.
On the other hand, despite being the same primary neurulation failure, there have been no reports accurately describing spinal lesions associated with CE as open spinal dysraphism, including MMC that required emergency surgery[3,38], nor was this observed in our series. Although both are caused by primary neurulation failure, the reason why MMC does not occur and is associated with CE, unlike dorsal lipoma, remains unclear. One possible explanation could be that the timing of CE development may be related to a specific narrow period, such as near the end of primary neurulation, between the formation periods of MMC and dorsal lipoma.[29]
Optimal indication and timing for neurosurgical intervention in TMCC and spinal lipomas
We posit that the optimal indication and timing for neurosurgical intervention are dependent on whether the diagnosis is TMCC or spinal lipomas. This is because TMCC is likely to cause more severe motor dysfunction in the lower limbs in addition to abdominogenital disorders at birth, presenting as a subcutaneous cystic mass from birth, which often increases dramatically over time. Consequently, there is a risk of postoperative complications, including CSF leakage, as observed in Patient 5. Furthermore, there is concern that cyst enlargement may lead to the pulling of the tip of the cord attached to the dome of the cyst, potentially resulting in neurological deterioration, as previously reported.[13,21,31] It is of utmost importance to consider the potential for rapid cyst enlargement in infants[18,22] and carefully monitor the progression of both the size of the cystic lesion and any neurological deterioration. Following recovery from life-threatening conditions, untethering surgery should be considered as soon as feasible.
In contrast, patients with spinal lipomas do not necessarily require untethering surgery during early infancy, and in some cases, conservative therapy may be a viable option. The main reason for this is that unlike TMCC, spinal lipomas associated with the OEIS complex are less likely to increase in size over time rapidly. In addition, the natural history of tethering by spinal lipomas is not completely understood, and whether prophylactic surgical untethering actually improves outcomes remains controversial.[33] Nevertheless, considering the high risk of spontaneous deterioration and the rarity of postoperative improvement once symptoms worsen, we believe that prophylactic surgery may be beneficial.
Regarding the timing of surgery, it may be prudent to monitor the condition conservatively while closely observing for the appearance of lower limb symptoms and utilizing this as an indicator for surgical intervention, particularly given that patients with OEIS complex already exhibit severe bladder and rectal dysfunction at birth. Delaying neurosurgery could potentially result in clinical deterioration before surgery, as observed in Patient 6.
Given that healthy infants attain peak fat accumulation at approximately 9 months of age,[16] and there are documented cases of enlarging spinal lipomas within a few months,[23,37] we typically performed surgery at approximately 6 months of age. Temporary discharge is sometimes employed to support mother-infant attachment.[4] For patients with more complex lipomas, such as transitional lipomas or those with a syrinx or CSF space, we conducted more frequent follow-ups with repeated MRI and earlier surgical intervention if warranted.[37]
In cases with severe deformities and paraplegia, such as Patient 9, aggressive surgical intervention may not be indicated. Although there is no absolute indication for surgery in the filar type, it is a curative and relatively simple procedure, and the child can be followed once adequate informed consent is granted by the parents to enable close attention to the emergence of symptoms, as in Patient 10.[8]
This study has several limitations, including its small sample size and retrospective nature. We can only speculate that the combination of events coincidentally occurs during different embryonic periods because when the patient cohort is small. However, as the number of patients increases, further considerations may become possible.
CONCLUSION
From the clinical course, neuroradiological, and histopathological findings of our patients, repeated MRI should be routinely performed for accurate diagnosis of spinal cord malformations associated with OEIS complex, even in the absence of lumbosacral masses, because the necessity and optimal timing of intervention may vary. As TMCC lesions tend to enlarge over time, surgery is recommended as soon as the patient’s abdominogenital condition stabilizes. In patients with spinal lipomas, surgical timing should be considered by weighing the benefits against the risks based on each patient’s neurological state. A notable embryopathological finding was that half of the spinal cord malformations associated with the OEIS complex consisted of spinal lipomas, most of which were double lipomas derived from both primary and secondary neurulation failure.
Footnotes
How to cite this article: Kurogi A, Murakami N, Morioka T, Shimogawa T, Mukae N, Suzuki SO, et al. Neurosurgical strategy based on the type of occult spinal dysraphism in omphalocele-exstrophy-imperforate anus-spinal defects complex: A review of 10 cases. Surg Neurol Int. 2024;15:472. doi: 10.25259/SNI_820_2024
Contributor Information
Ai Kurogi, Email: kurogi.a@fcho.jp.
Nobuya Murakami, Email: murakami.n@fcho.jp.
Takato Morioka, Email: takatons1227@gmail.com.
Takafumi Shimogawa, Email: shimogawa28@gmail.com.
Nobutaka Mukae, Email: mukaen0203@gmail.com.
Satoshi O. Suzuki, Email: sosuzuki@shouraikai.jp.
Koji Yoshimoto, Email: yoshimoto.koji.315@m.kyushu-u.ac.jp.
Statements and declarations
This manuscript is original and has not been published or presented elsewhere in part or whole.
Ethical approval
The research/study was approved by the Institutional Review Board at the Institutional Review Board at Fukuoka Children’s Hospital, number 2021-713, dated July 5, 2021.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript, and no images were manipulated using AI.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Journal or its management. The information contained in this article should not be considered to be medical advice; patients should consult their own physicians for advice as to their specific medical needs.
REFERENCES
- 1.Ambrose SS, O’Brien DP. Surgical embryology of the exstrophyepispadias complex. Surg Clin North Am. 1974;54:1379–90. doi: 10.1016/s0039-6109(16)40493-7. [DOI] [PubMed] [Google Scholar]
- 2.Arai H, Sato K, Okuda O, Miyajima M, Hishii M, Nakanishi H, et al. Surgical experience of 120 patients with lumbosacral lipomas. Acta Neurochir (Wien) 2001;143:857–64. doi: 10.1007/s007010170015. [DOI] [PubMed] [Google Scholar]
- 3.Bursac D, Bojanic K, Zmijanac Partl J, Lucic D, Duic Z, Horvat M. OEIS complex-using MRI in diagnostic: Two case reports. Radiol Case Rep. 2023;18:364–7. doi: 10.1016/j.radcr.2022.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bystrova K, Ivanova V, Edhborg M, Matthiesen A, Ransjö-Arvidson A, Mukhamedrakhimov R, et al. Early contact versus separation: Effects on mother-infant interaction one year later. Birth. 2009;36:97–109. doi: 10.1111/j.1523-536X.2009.00307.x. [DOI] [PubMed] [Google Scholar]
- 5.Carey JC, Greenbaum B, Hall BD. The OEIS complex (omphalocele, exstrophy, imperforate anus, spinal defects) Birth Defects Orig Artic Ser. 1978;14:253–63. [PubMed] [Google Scholar]
- 6.Carey JC. Exstrophy of the cloaca and the OEIS complex: One and the same. Am J Med Genet. 2001;99:270. doi: 10.1002/ajmg.1211. [DOI] [PubMed] [Google Scholar]
- 7.Cohen AR. The Mermaid malformation: Cloacal exstrophy and occult spinal dysraphism. Neurosurgery. 1991;28:834–43. [PubMed] [Google Scholar]
- 8.Cools MJ, Al-Holou WN, Stetler WR, Wilson TJ, Muraszko KM, Ibrahim M, et al. Filum terminale lipomas: Imaging prevalence, natural history, and conus position: Clinical article. J Neurosurg Pediatr. 2014;13:559–67. doi: 10.3171/2014.2.PEDS13528. [DOI] [PubMed] [Google Scholar]
- 9.Gupta A, Rajshekhar V. Fatty filum terminale (FFT) as a secondary tethering element in children with closed spinal dysraphism. Childs Nerv Syst. 2018;34:925–32. doi: 10.1007/s00381-017-3700-y. [DOI] [PubMed] [Google Scholar]
- 10.Hashiguchi K, Morioka T, Fukui K, Miyagi Y, Mihara F, Yoshiura T, et al. Usefulness of constructive interference in steady-state magnetic resonance imaging in the presurgical examination for lumbosacral lipoma. J Neurosurg. 2005;103:537–43. doi: 10.3171/ped.2005.103.6.0537. [DOI] [PubMed] [Google Scholar]
- 11.Hashiguchi K, Morioka T, Yoshida F, Miyagi Y, Mihara F, Yoshiura T, et al. Feasibility and limitation of constructive interference in steady-state (CISS) MR imaging in neonates with lumbosacral myeloschisis. Neuroradiology. 2007;49:579–85. doi: 10.1007/s00234-007-0225-1. [DOI] [PubMed] [Google Scholar]
- 12.Keppler-Noreuil KM, Conway KM, Shen D, Rhoads AJ, Carey JC, Romitti PA, et al. Clinical and risk factor analysis of cloacal defects in the National Birth Defects Prevention Study. Am J Med Genet A. 2017;173:2873–85. doi: 10.1002/ajmg.a.38469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim KH, Wang KC, Lee JY. Enlargement of extraspinal cysts in spinal dysraphism : A reason for early untethering. J Korean Neurosurg Soc. 2020;63:342–5. doi: 10.3340/jkns.2020.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kubota M. The current profile of persistent cloaca and cloacal exstrophy in Japan: The results of a nationwide survey in 2014 and a review of the literature. Pediatr Surg Int. 2017;33:505–12. doi: 10.1007/s00383-016-4053-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar N, Chatur C, Balani A, Bisharat M, Tahir Z, Johal N, et al. Patterns of spinal cord malformation in cloacal exstrophy. J Neurosurg Pediatr. 2021;28:236–43. doi: 10.3171/2021.1.PEDS20648. [DOI] [PubMed] [Google Scholar]
- 16.Kuzawa CW. Adipose tissue in human infancy and childhood: An evolutionary perspective. Am J Phys Anthropol. 1998;(Suppl 27):177–209. doi: 10.1002/(sici)1096-8644(1998)107:27+<177::aid-ajpa7>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Cottrell JR, Sanders RC, Meyers CM, Wulfsberg EA, Sun CC. OEIS complex (omphalocele-exstrophy-imperforate anus-spinal defects) in monozygotic twins. Am J Med Genet. 1999;84:29–33. [PubMed] [Google Scholar]
- 18.Lee JY, Phi JH, Kim SK, Cho BK, Wang KC. Urgent surgery is needed when cyst enlarges in terminal myelocystoceles. Childs Nerv Syst. 2011;27:2149–53. doi: 10.1007/s00381-011-1532-8. [DOI] [PubMed] [Google Scholar]
- 19.Marshall VF, Muecke EC. Variations in exstrophy of the bladder. J Urol. 1962;88:766–96. [Google Scholar]
- 20.Martinez-Frias ML, Bermejo E, Rodriguez-Pinilla E, Frias JL. Exstrophy of the cloaca and exstrophy of the bladder: Two different expressions of a primary developmental field defect. Am J Med Genet. 2001;99:261–9. doi: 10.1002/ajmg.1210. [DOI] [PubMed] [Google Scholar]
- 21.McLone DG, Naidich TP. Terminal myelocystocele. Neurosurgery. 1985;16:36–43. [PubMed] [Google Scholar]
- 22.Morioka T, Hashiguchi K, Yoshida F, Matsumoto K, Miyagi Y, Nagata S, et al. Neurosurgical management of occult spinal dysraphism associated with OEIS complex. Childs Nerv Syst. 2008;24:723–9. doi: 10.1007/s00381-007-0519-y. [DOI] [PubMed] [Google Scholar]
- 23.Morioka T, Hashiguchi K, Yoshida F, Nagata S, Miyagi Y, Mihara F, et al. Dynamic morphological changes in lumbosacral lipoma during the first months of life revealed by constructive interference in steady-state (CISS) MR imaging. Childs Nerv Syst. 2007;23:415–20. doi: 10.1007/s00381-006-0272-7. [DOI] [PubMed] [Google Scholar]
- 24.Morioka T, Murakami N, Kurogi A, Mukae N, Shimogawa T, Shono T, et al. Embryopathological relationship between retained medullary cord and caudal spinal lipoma. Interdiscip Neurosurg. 2022;29:101534. [Google Scholar]
- 25.Morioka T, Murakami N, Suzuki SO, Mukae N, Shimogawa T, Kurogi A, et al. Surgical histopathology of a filar anomaly as an additional tethering element associated with closed spinal dysraphism of primary neurulation failure. Surg Neurol Int. 2021;12:373. doi: 10.25259/SNI_340_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morota N, Ihara S, Ogiwara H. New classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2017;19:428–39. doi: 10.3171/2016.10.PEDS16247. [DOI] [PubMed] [Google Scholar]
- 27.Muecke EC. The role of the cloacae membrane in exstrophy: The first successful experimental study. J Urol. 1964;92:659–68. doi: 10.1016/S0022-5347(17)64028-X. [DOI] [PubMed] [Google Scholar]
- 28.Murakami N, Kurogi A, Kawakami Y, Noguchi Y, Hayashida M, Suzuki SO, et al. Refractory CSF leakage following untethering surgery performed 10 months after birth for enlarging terminal myelocystocele associated with OEIS complex. Surg Neurol Int. 2021;12:628. doi: 10.25259/SNI_995_2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Rahilly R, Müller F. Neurulation in the normal human embryo. Ciba Found Symp. 1994;181:70–82. doi: 10.1002/9780470514559.ch5. discussion 82-9. [DOI] [PubMed] [Google Scholar]
- 30.Pang D. Sacral agenesis and caudal spinal cord malformations. Neurosurgery. 1993;32:755–79. doi: 10.1227/00006123-199305000-00009. discussion 778-9. [DOI] [PubMed] [Google Scholar]
- 31.Pang D, Zovickian J, Lee JY, Moes GS, Wang KC. Terminal myelocystocele: Surgical observations and theory of embryogenesis. Neurosurgery. 2012;70:1383–405. doi: 10.1227/NEU.0b013e31824c02c0. [DOI] [PubMed] [Google Scholar]
- 32.Pang D, Zovickian J, Moes GS. Retained medullary cord in humans: Late arrest of secondary neurulation. Neurosurgery. 2011;68:1500–19. doi: 10.1227/NEU.0b013e31820ee282. [DOI] [PubMed] [Google Scholar]
- 33.Perera D, Craven CL, Thompson D. Lumbosacral lipoma in childhood, how strong is the evidence base? A systematic review. Childs Nerv Syst. 2024;40:715–28. doi: 10.1007/s00381-023-06203-9. [DOI] [PubMed] [Google Scholar]
- 34.Siebert JR, Rutledge JC, Kapur RP. Association of cloacal anomalies, caudal duplication, and twinning. Pediatr Dev Pathol. 2005;8:339–54. doi: 10.1007/s10024-005-1157-6. [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga S, Morioka T, Hashiguchi K, Samura K, Yoshida F, Miyagi Y, et al. Double lumbosacral lipomas of the dorsal and filar types associated with OEIS complex. Neurol Med Chir. 2009;49:487–90. doi: 10.2176/nmc.49.487. [DOI] [PubMed] [Google Scholar]
- 36.Tominey S, Kaliaperumal C, Gallo P. External validation of a new classification of spinal lipomas based on embryonic stage. J Neurosurg Pediatr. 2020;25:394–401. doi: 10.3171/2019.11.PEDS19575. [DOI] [PubMed] [Google Scholar]
- 37.Tu A, Hengel AR, Cochrane DD. Radiographic predictors of deterioration in patients with lumbosacral lipomas. J Neurosurg Pediatr. 2016;18:171–6. doi: 10.3171/2016.1.PEDS15614. [DOI] [PubMed] [Google Scholar]
- 38.Vliet R, Roelofs L, Rassouli-Kirchmeier R, De Gier R, Claahsen-Van Der Grinten H, Verhaak C, et al. Clinical outcome of cloacal exstrophy, current status, and a change in surgical management. Eur J Pediatr Surg. 2014;25:87–93. doi: 10.1055/s-0034-1387943. [DOI] [PubMed] [Google Scholar]