Abstract
Biomarker‐based screening enables early detection of AL amyloidosis in intermediate/high‐risk MGUS patients. Our study shows that identifying pre‐symptomatic AL through biomarker longitudinal monitoring allows early treatment, leading to significant organ function recovery.
Keywords: AL amyloidosis, biomarkers, MGUS, screening
To the Editor,
Monoclonal gammopathy of undetermined significance (MGUS) is a widespread condition, often incidentally identified, with a risk of evolution in multiple myeloma (MM) or, although more rarely, in plasma cell‐related disorders including immunoglobulin light chain (AL) amyloidosis [1].
The outcome of AL amyloidosis is highly impacted by the extent of organ damage, in particular by cardiac involvement. Since the presenting symptoms of organ damage are highly heterogeneous and generally late, establishing an early diagnosis of AL amyloidosis can often be challenging. Nonetheless, late diagnosis, when organ involvement is advanced and the possibility of delivering effective therapy is limited, has a relevant impact on outcome [2]. Alteration of free light chain ratio (FLCr), a known risk factor for MGUS progression to MM, precedes the onset of AL amyloidosis by 4 years or more, thus offering a potential window for early diagnosis and treatment [3].
Alterations of biomarkers of organ dysfunction and damage, in particular N‐terminal‐pro‐natriuretic peptide type‐B (NT‐proBNP) for the heart, 24‐h proteinuria, predominantly albumin, for the kidney, and alkaline phosphatase (ALP) for the liver, can raise suspicion of initial pre‐symptomatic amyloid organ involvement in patients with MGUS. Implementing their use during the follow‐up in MGUS cases with abnormal FLCr could allow early identification of patients who deserve further diagnostic workup for AL amyloidosis [2, 4].
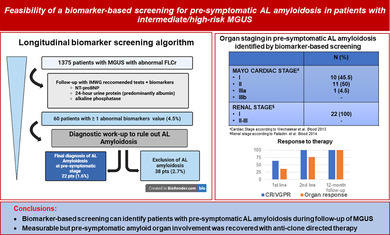
Given these premises, biomarker‐based screening for AL amyloidosis has been systematically integrated since 2012 in the follow‐up of MGUS with alteration of FLCr to explore their role for early capturing pre‐symptomatic AL amyloidosis cases [2]. We present data from our single‐center longitudinal cohort of 4742 patients with a diagnosis of MGUS, based on International Myeloma Working Group (IMWG) criteria, consecutively observed at our Division of Hematology between January 2012 and December 2021 (details regarding study methods are provided in the Supporting Information). The median follow‐up for the MGUS cohort was 4 years. The primary goal of our study was to determine the incidence rate of pre‐symptomatic AL amyloidosis identified through biomarker‐based longitudinal screening. Within this cohort, 1375 patients (28.9%) had altered FLCr and underwent sequential biomarker monitoring (refer to Figure 1 and to the Table S1 and Table S2 for details regarding baseline data for the entire cohort and for cohort of MGUS with altered FLCr, respectively).
FIGURE 1.
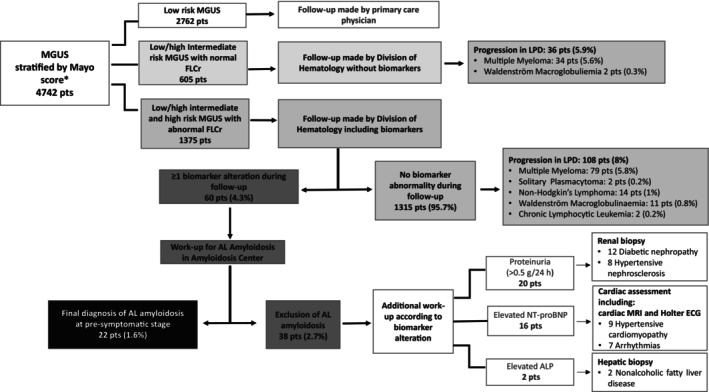
Flow chart of patient disposition during the study period. Baseline evaluation at the time of first detection of monoclonal component (MC) included complete blood count, serum calcium, serum creatinine, serum protein electrophoresis, serum and urine immunofixation, free light chain quantification and ratio (FLCr) and 24‐h proteinuria. Diagnosis of MGUS was established according to International Myeloma Working Group (IMWG) criteria. In cases of low‐risk Monoclonal Gammopathy of Undetermined Significance (MGUS) according to Mayo risk stratification (i.e., MC ≤ 1,5 g/dL, Immunoglobulin G [IgG] type, normal FLCr), no further tests were performed and patients were monitored after 6 months at the Division of Hematology and then, if stable, they were referred back to primary care physician for follow‐up. In patients with non‐low‐risk MGUS according to the Mayo risk score, baseline evaluation included skeletal imaging (low‐dose whole‐body computed tomography) along with bone marrow evaluation, to rule out multiple myeloma. Follow‐up of these patients was carried out at the Division of Hematology, firstly after 6 months and then every year in case of no significant change. Follow‐up tests included: complete blood count, serum calcium, serum creatinine, serum protein electrophoresis, 24‐h proteinuria, and FLC analysis. In intermediate (low/high) as well as high‐risk MGUS with altered FLCr, follow‐up tests were integrated by assessment of biomarkers of amyloid organ involvement (N‐terminal pro‐type‐B natriuretic peptide [NT‐proBNP], 24‐h proteinuria, and alkaline phosphatase). All MGUS patients with at least one abnormal biomarkers according to predefined cut‐off values (suspect kidney involvement: 24‐h proteinuria > 0.5 g/24 h, predominantly albumin; suspect liver involvement: Alkaline phosphatase concentration > 1.5 times the institutional upper limit of normal; suspect cardiac involvement: NT‐proBNP concentration > 332 ng/L) underwent a new follow‐up within three months, in case of confirmed biomarker alteration a multistep diagnostic workup, in particular abdominal fat biopsy, was performed at the Amyloidosis Research and Treatment Center. The diagnosis of AL amyloidosis was biopsy‐based and included amyloid typing by immuno‐electron microscopy or mass spectrometry. Patients with negative abdominal fat biopsy underwent minor salivary gland or organ biopsy as appropriate. Amyloid organ involvement was defined and graded according to the International Society of Amyloidosis guidelines. In cases in whom amyloidosis was excluded, diagnostic algorithm included additional multidisciplinary specific workup performed in accordance with type of biomarker alteration identified, to rule out alternative non‐hematological morbidities. MGUS, Monoclonal gammopathy of undetermined significance; FLCr, Free Light Chain ratio; Ps, patients; LPD, Lymphoproliferative Disorders; AL, Light Chain Amyloidosis; NT‐proBNP, N‐terminal pro‐type‐B Natriuretic Peptide; ALP, Alkaline phosphatase; MRI, Magnetic Resonance Imaging; ECG, electrocardiogram; h, hour.* According to the Mayo risk model, low‐risk MGUS meets all the following characteristics: Monoclonal Component < 1.5 g/dL, Immunoglobulin G subtype, normal FLCr (0.26–1.65), low‐intermediate risk MGUS had any one factor abnormal, high‐intermediate risk MGUS had any 2 factors abnormal, high‐risk MGUS had all three factors abnormal.
Notably, throughout the follow‐up period, none of the MGUS cases without biomarker alterations progressed to AL amyloidosis. The overall rate of progression to lymphoproliferative disorders was 8%, with multiple myeloma accounting for 5.8% of these cases. The median interval between MGUS diagnosis and the first suspicion of AL amyloidosis, based on emerging biomarker alterations, was 3.6 years, with a wide range of variability (0.8 to 10.2 years). Alteration of at least one biomarker was detected in 60 cases out of 1375 screened patients (4.3%) all of whom were subsequently referred to specific workups for amyloid detection. AL amyloidosis was confirmed, before any clinical symptoms appeared, in 22 patients (1.6%) while it was excluded in 38 pts. (2.8%) (refer to Figure 1 and, for more details, Table S3). The incidence rate of pre‐symptomatic AL amyloidosis was 2.5/1000 person‐years. Remarkably, the ratio of early AL diagnosis in our population of one case for approximately 4 cases of MM, underscores the screening's diagnostic accuracy. Focusing on the 22 patients with AL amyloidosis identified at the pre‐symptomatic stage, light chain isotype distribution was mostly lambda (95.5%), with only 1 case with kappa light chain isotype (4.5%). The biomarkers found abnormal during follow‐up were 24‐h proteinuria (predominantly albumin) in 12 patients, elevated NT‐proBNP in 9 patients, and increased ALP in 1 patient (for more details refer to Table S3 and Figure S1). Abdominal fat aspirate allowed identification of amyloid deposits of AL amyloidosis in most cases (18 patients, 82%). Patients with negative abdominal fat aspirate underwent a minor salivary gland biopsy that resulted positive in 2 cases (9%); kidney biopsy was required only in 2 patients (9%). None of the patients with cardiac involvement required endomyocardial biopsy for the final diagnosis. The liver was the only affected organ in the patient with abnormal ALP and the diagnosis was confirmed with abdominal fat aspirate. Importantly, all AL cases had limited organ involvement. In detail, all patients were renal stage I. 10 patients (45.5%) were cardiac Mayo/European stage I, 11 patients were stage II (50%), only one patient (4.5%) was cardiac stage IIIa, and none had advanced stage IIIb.
In three patients with normal (< 12 mm) median left ventricular wall thickness at echocardiogram, cardiac magnetic resonance confirmed the presence of typical amyloid involvement showing delayed gadolinium enhancement. Among patients with cardiac involvement, only one patient had a mild elevation of NT‐proBNP (556 ng/L) at the first assessment. In the remaining patients, an NT‐proBNP level of at least 650 ng/L was identified and subsequently confirmed during a second evaluation. Once addressed to anti‐clone‐directed therapy (for more details refer to Table S4), most patients (73%) achieved a hematologic response with 46% reaching very good partial response (VGPR) and 18% complete response (CR). Among patients with unsatisfactory responses, all achieved VGPR following second‐line therapy. Regarding organ response evaluation at the 12‐month time point, 27% of patients achieved cardiac complete response, 10% achieved a very good partial cardiac response, and 63% exhibited cardiac partial response. After a median follow‐up of 36 months, there were no amyloid‐related deaths, while three patients (13.6%) died from unrelated causes.
Focusing on 38 MGUS cases, with significant alterations of biomarkers, in whom the diagnosis of AL amyloidosis was ruled out, the light chain isotype was more frequently kappa (60.5%). In these subjects, the biomarkers abnormalities emerging during follow‐up were the following (for more details refer to Figure 1, Table S3, and Figure S1): 24‐h proteinuria (predominantly albumin) in 20 patients (52.6%), elevated NT‐proBNP in 16 patients (42.1%), and elevated ALP value in 2 patients (5.3%). Additional specific work‐up was tailored according to the emerging alterations (Figure 1). An organ biopsy was required for 22 patients with proteinuria and elevated ALP. The diagnoses in these cases were diabetic nephropathy in 12 patients, hypertensive nephrosclerosis in 8 patients, and non‐alcoholic fatty liver disease in 2 patients. No patients were found to have other monoclonal gammopathy of renal significance in the current series. Sixteen patients with high NT‐proBNP were not diagnosed with AL amyloidosis. In these subjects, hypertensive cardiomyopathy was diagnosed in 9 patients, valvular heart disease in 3 patients, and arrhythmias in 4 patients.
Overall, our data show that the implementation of easily available biomarker‐based screening for the follow‐up of MGUS with altered FLCr allows the identification of pre‐symptomatic AL amyloidosis. This result, higher than expected from Kyle et al. [1], suggests that switching from an exclusively symptom‐based approach to a red flag biomarker‐based screening will increase the chance of timely detection of AL amyloidosis, regardless of the expertise of the physicians, overcoming the limit of early phase subclinical underestimated disease manifestations.
Early diagnosis, with limited organ involvement, was associated with significant damage recovery, particularly cardiac, a clinically relevant endpoint associated with a substantial gain in life expectancy [2].
The variability in the timing of biomarker alteration development suggests that patients with MGUS remain at risk of developing AL amyloidosis throughout the course of follow‐up.
Remarkably, we did not observe any cases of non‐AL amyloidosis, specifically transthyretin wild‐type amyloidosis (ATTRwt), despite its higher prevalence and frequent association with monoclonal protein (20%–35%). This may be due to the exclusion of low‐risk MGUS, more common in ATTRwt, and the younger median age of our cohort (70 years) compared to ATTRwt patients (median 75 years) [5]. Additionally, some patients with ATTRwt amyloidosis may present with NT‐proBNP levels below the diagnostic cut‐off for cardiac amyloidosis and without heart failure symptoms. Thus cardiac biomarkers may be suboptimal to detect early ATTRwt heart involvement due to the distinct mechanism of organ damage in the two diseases. This emphasizes the need for in‐depth studies of monoclonal patterns in ATTR amyloidosis to identify patients who may require organ biopsy to rule out AL amyloidosis.
In addition, the high prevalence of lambda isotype in our series warrants further investigations in larger populations to assess the relevance of screening for AL amyloidosis in patients with kappa light chain isotype. For instance, cases with kappa light chain isotypes could be screened based on more specific light chain features, such as N‐glycosylation patterns [6].
Finally, biomarker screening has proven valuable in identifying relevant non‐hematological diseases, thereby minimizing the risk of overlooking asymptomatic conditions warranting intervention.
We acknowledge the limitations of our longitudinal case series conducted at a single‐center institution and the absence of a control group. However, our data suggest that pre‐symptomatic diagnosis of systemic AL amyloidosis based exclusively on biomarkers of organ dysfunction is possible. Larger international studies, such as randomized controlled studies or prospective registries are warranted to validate the efficacy and cost‐effectiveness of this approach for the early detection of AL amyloidosis. To this end, a prospective study involving four different hematology divisions is ongoing in Italy (NCT06383143) with the aim of promoting an early diagnosis of this disease.
Author Contributions
S.M. and G.P. were responsible for the study conception and design. Data preparation and collection were performed by S.M., P.M., C.S.C., P.B., M.P., V.M., M.B., G.P., M.V., M.V., and A.F., S.M., P.M., C.S.C., L.A., and G.P. participated in content planning, interpreted, and reviewed the data, and wrote the paper. S.M., P.M., C.S.C., G.M., L.A., and G.P. reviewed and commented on drafts. All the authors approved the final version of this paper for publication.
Ethics Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by our institutional independent ethics committee.
Consent
All patients provided written informed consent.
Conflicts of Interest
SM has received honoraria from Bristol‐Myers Squibb, Sanofi, AMGEN, GSK, Takeda, Pfizer, and Janssen, has served on the advisory boards for Sanofi, Takeda, Bristol‐Myers Squibb, Pfizer, and Janssen; GP received honoraria from Pfizer, Serbia, and Siemens and served on advisory boards Alexion, Argobio, GSK, Janssen, and Prothena. PM has received honoraria from Janssen, Pfizer (also research support), Prothena, and Siemens and served on the advisory board for Siemens. LA received honoraria from EUSA Pharma, and Novartis, and served on advisory boards and consultations from Roche, incite, EUSA Pharma, Kite/Gilead, Novartis, and Morphosys.
Supporting information
Data S1.
Funding: This work was supported by Fondazione Cariplo, 2018‐0257, GJC23044, Cancer Research UK 4013, C355/A26819, Italian Ministry of Research and Education, PRIN 20207XLJB2, Italian Ministry of Health, Ricerca Finalizzata, GR‐2018‐12368387 Associazione Italiana per la Ricerca sul Cancro, Accelerator Award Program Biomarker, European Union—Next Generation EU—PNRR M6C2—Investment 2.1 ‘Valorizzazione e potenziamento della ricerca biomedica del SSN, PNRR‐MR1‐2022‐12376853, the Telethon Foundation, GJC23044.
Silvia Mangiacavalli, Paolo Milani, Luca Arcaini and Giovanni Palladini contributed equally to this study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.
References
- 1. Kyle R. A., Larson D. R., Therneau T. M., et al., “Long‐Term Follow‐Up of Monoclonal Gammopathy of Undetermined Significance,” New England Journal of Medicine 378, no. 3 (2018): 241–249, 10.1056/NEJMoa1709974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Palladini G. and Merlini G., “How I Treat AL Amyloidosis,” Blood 139, no. 19 (2022): 2918–2930, 10.1182/blood.2020008737. [DOI] [PubMed] [Google Scholar]
- 3. Weiss B. M., Hebreo J., Cordaro D. V., et al., “Increased Serum Free Light Chains Precede the Presentation of Immunoglobulin Light Chain Amyloidosis,” Journal of Clinical Oncology 32, no. 25 (2014): 2699–2704, 10.1200/JCO.2013.50.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sharpley F. A., Fontana M., Martinez‐Naharro A., et al., “Cardiac Biomarkers Are Prognostic in Systemic Light Chain Amyloidosis With no Cardiac Involvement by Standard Criteria,” Haematologica 105 (2019): 1405–1413, 10.3324/haematol.2019.217695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gonzalez‐Lopez E., Escobar‐Lopez L., Obici L., et al., “Prognosis of Transthyretin Cardiac Amyloidosis Without Heart Failure Symptoms,” JACC CardioOncol 4, no. 4 (2022): 442–454, 10.1016/j.jaccao.2022.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nevone A., Girelli M., Mangiacavalli S., et al., “An N‐Glycosylation Hotspot in Immunoglobulin Kappa Light Chains Is Associated With AL Amyloidosis,” Leukemia 36, no. 8 (2022): 2076–2085, 10.1038/s41375-022-01599-w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


