Abstract
Background:
Both the antibody–drug conjugate (ADC) enfortumab vedotin (EV) and programmed death-1 inhibitor pembrolizumab have been shown to provide survival benefits in patients previously treated with locally advanced or metastatic urothelial carcinoma (la/mUC). Cost-effectiveness is necessary to consider whether the increased efficacy of the two therapies will lead to higher prices for first-line treatment of previously untreated la/mUC.
Objectives:
To guide the choice of EV plus pembrolizumab or chemotherapy for patients with previously untreated la/mUC.
Design:
The cost-effective analysis.
Methods:
A Markov model was developed to simulate the lifetime of patients with previously untreated la/mUC to assess the overall cost and efficacy of EV plus pembrolizumab and chemotherapy based on the EV-302/KEYNOTE-A39 trial. Primary outcomes included total cost, life-years (LYs), quality-adjusted LYs (QALYs), the incremental cost-effectiveness ratio (ICER), and incremental net health benefits at the USA and Chinese willingness-to-pay threshold of $150,000/QALY and $35,173/QALY, respectively. Model stability was examined through sensitivity and subgroup analyses.
Results:
EV plus pembrolizumab and chemotherapy treatment regimens were associated with 2.07–2.16 and 1.04–1.06 QALYs with corresponding costs of $288,347–$532,362 and $24,773–$267,568, respectively. ICERs in the United States and China are $267,491/QALY and $254,339/QALY, respectively. The factors that most strongly influenced model outcomes in unidirectional sensitivity analyses were patient weight and the cost of EV. To achieve greater cost-effectiveness, EV costs would need to be reduced by over 75% and 10% in the United States and China, respectively.
Conclusion:
While first-line EV plus pembrolizumab has significant health benefits compared to chemotherapy for patients with previously untreated la/mUC, this regimen is not cost-effective at the current price in the United States or China.
Keywords: chemotherapy, cost-effectiveness, enfortumab vedotin, locally advanced or metastatic urothelial carcinoma, pembrolizumab
Introduction
Bladder cancer is the most prevalent urinary system malignancy in the world and among the 10 most common forms of cancer, with approximately 573,278 new diagnoses in 2020 alone, 1 including approximately 66,242 and 81,400 new diagnoses in China and the United States, respectively.2,3 Roughly 90% of bladder cancer cases are of the urothelial carcinoma (UC) subtype, 4 and ~5% of patients are initially diagnosed with metastatic disease, with a 5-year survival rate for this population of just 5%. 5 Platinum-based chemotherapy is currently the standard first-line treatment for locally advanced or metastatic UC (la/mUC). The survival of patients who undergo such treatment, however, tends to be poor, with median overall and progression-free survival (mOS and mPFS) intervals that generally do not exceed 9 and 6 months, respectively. 6 There thus remains a significant unmet need for improved therapeutic regimens that can yield better la/mUC patient outcomes.
The advent of immune checkpoint inhibitors (ICIs) and antibody–drug conjugates (ADCs) has profoundly reshaped the oncology treatment landscape.7,8 Pembrolizumab is an anti-PD-1 antibody, 7 while enfortumab vedotin (EV) is an ADC consisting of a nectin-4-specific antibody conjugated by a drug linker to a monomethyl auristatin E payload. 8 Both of these drugs have been shown to significantly improve the survival of la/mUC when used as per the recommendations included in many international guidelines.7 –10 This promising single-agent efficacy has led to interest in the potential benefits of their combined use. To address this topic, the open-label randomized phase III EV-302/KEYNOTE-A39 (NCT04223856) trial explored the safety and efficacy of EV plus pembrolizumab relative to chemotherapy when used as a first-line treatment for individuals with previously untreated la/mUC. In this trial, EV plus pembrolizumab significantly prolonged patient mOS (31.5 vs 16.3 months; hazard ratio (HR), 0.47; 95% confidence interval (CI), 0.38–0.58; p < 0.00001) and mPFS (12.5 vs 6.3 months; HR, 0.45; 95% CI, 0.38–0.54; p < 0.00001) without any increase in the incidence of unexpected treatment-related adverse events (AEs). 11 These promising results have the potential to significantly influence international guidelines and to influence clinical decision-making when managing la/mUC patients.
While promising clinical outcomes are significant from a healthcare perspective, these new therapies also entail high medical costs. It is thus essential that pharmacoeconomic evaluations be conducted to better guide the marketing and widespread application of innovative treatment options. These analyses can help identify appropriate pricing levels while improving patient acceptance of these emergent regimens. Accordingly, the present study was designed to evaluate the cost-effectiveness of EV plus pembrolizumab versus chemotherapy as a first-line treatment for la/mUC patients from the perspectives of the healthcare systems in the United States and China.
Materials and methods
The phase III EV-302/KEYNOTE-A39 trial was the source of the data used to conduct this study, as per the CHEERS 2022 guidelines11,12 (Table S1).
Study overview and design
This study included 886 patients with previously untreated la/mUC, an Eastern Cooperative Oncology Group performance status (ECOG PS) ⩽2, and a glomerular filtration rate ⩾30 ml/min was established. Of these patients, 442 underwent EV (1.25 mg/kg) plus pembrolizumab (200 mg) treatment, while the remaining 444 received chemotherapy (gemcitabine (1000 mg/m2) plus cisplatin (70 mg/m2) or carboplatin (area under the curve 5)). 11 Then, 8.7% and 13.6% of the patients in these respective groups developed progressive disease (PD) and were administered paclitaxel and pembrolizumab as a standard second-line treatment approach by international guidelines, while the remaining patients received the best supportive care (BSC)9,11 (Table S2). Drug doses were calculated based on the assumption that all patients were 69-year-old males with a creatinine clearance rate of 1 mg/dL, a body weight of 70 and 60 kg, and a body surface area of 1.84 and 1.72 m 2 in the United States and China, respectively13,14 (Table 1). Terminal care was administered to patients who died beginning 1 month before death.9,11
Table 1.
Clinical and health parameters.
| Variable | Baseline value (range) | Distribution |
|---|---|---|
| Clinical parameters | ||
| Weibull survival model for OS | ||
| Chemotherapy | Scale = 0.031710, Shape = 1.086298 11 | NA |
| EV + P | Scale = 0.013950, Shape = 1.126849 11 | NA |
| Weibull survival model for PFS | ||
| Chemotherapy | Scale = 0.085880, Shape = 1.110700 11 | NA |
| EV + P | Scale = 0.073244, Shape = 0.848184 11 | NA |
| Rate of post-discontinuation therapy | ||
| EV + P | 0.087 (0.070–0.104) 11 | Beta |
| Chemotherapy | 0.136 (0.101–0.163) 11 | Beta |
| Risk for main AEs in chemotherapy | ||
| Thrombocytopenia | 0.194 (0.155–0.233) 11 | Beta |
| Neutropenia | 0.300 (0.240–0.360) 11 | Beta |
| Anemia | 0.314 (0.251–0.377) 11 | Beta |
| Risk for main AEs in EV + P | ||
| Maculopapular rash | 0.077 (0.062–0.092) 11 | Beta |
| Health parameters | ||
| Utility and disutility | ||
| Utility of PFS | 0.842 (0.674–1.010)15,16 | Beta |
| Utility of PD | 0.840 (0.640–0.960)15,16 | Beta |
| Disutility of AEs (⩾3 grades) | 0.273 (0.218–0.328)13,17 | Beta |
AEs, adverse events; EV + P, enfortumab vedotin plus pembrolizumab; NA, not applicable; OS, overall survival; PD, progressive disease; PFS, progression-free survival.
Model construction and transition
A Markov model incorporating three health states (PFS, PD, and death) was developed to assess the cost-effectiveness of EV plus pembrolizumab. All patients were assumed to be treated with EV plus pembrolizumab or chemotherapy alone in the PFS state until disease progression. When the disease progressed or unacceptable AEs occurred, patients in both groups could receive subsequent comprehensive treatment until death. This model included a 3-week cycle period and was used to simulate the patient lifetime, and model construction was performed with TreeAge Pro (version 2021, Williamstown, MA, https://www.treeage.com; Figure S1). Study outcomes included life-years (LY), quality-adjusted life-years (QALYs), incremental cost-effectiveness ratios (ICERs), and incremental net health benefit (INHB) at a willingness-to-pay (WTP) threshold of $150,000/QALY (USA) and $35,173/QALY (three times the per capita gross domestic product of China).13,14 ICER refers to the incremental cost required to achieve an incremental effect, and it makes the difference between different treatments by comparing the ratio of the cost difference to the effective output. ICER = ∆C/∆E (∆C is the incremental cost, ∆E is the incremental effect). The calculated ICER results should also be correlated with the patient’s WTP to determine whether the intervention is cost-effective. Annual 3% and 5% discounts were applied to costs and utility values in the United States and China, respectively. 14 As novel therapies are very expensive, cost-effectiveness was also evaluated at prices corresponding to 100%, 70%, 60%, 50%, and 10% of current EV prices.
OS and PFS survival data from the EV-302/KEYNOTE-A39 trial were obtained using GetData Graph Digitizer (v 2.26, Graph Digitizer Pty Ltd, available at: http://www.getdata-graph-digitizer.com/index.php); MATLAB (v R2020a, The MathWorks, Inc. available at: https://ww2.mathworks.cn/products/matlab.html); R (v 4.2.2, R Foundation, available at: http://www.rproject.org), and the extracted data were used to calculate transition probabilities. These survival data were assessed through parametric curve fitting with the Weibull, Log-logical, Lognormal, Exponential, and Gompertz distributions, ultimately leading to the selection of the Weibull distribution as being most appropriate based on visual inspection and a combination of the Akaike information criterion and Bayesian information criterion (Figure S2 and Table S3). The Weibull distribution was then used for extrapolation with MATLAB (v R2020a). The shape (λ) and scale (γ) parameters were then computed with R (v 4.2.2) as follows:
where and represent the Markov period and the current model period, respectively13,14 (Table 1).
Estimates of cost and utility
The utility was used to reflect patients’ quality-of-life (QoL) weights in the natural history of the disease, on a scale of 0 (death) to 1 (total health). Utilities were used to obtain QALYs by discount LYs. Utility values for PFS and PD in this study were set at 0.842 and 0.800 for la/mUC patients, respectively, based on two prior studies15,18 (Table 1). AE-related disutility values in the first cycle were extracted from other studies and used for the present analyses.13,16 The utility of PFS was reduced by the duration-adjusted disutility (Table 1). Only direct medical costs were taken into consideration, including the costs of drugs, AE management, administration, PD-L1 testing, tumor imaging, BSC, and terminal care. Drug costs were obtained from the Centers for Medicare & Medicaid Services, RedBook, and DrugDataexpy,17,19,20 while other costs were based on literature sources.13,14,16,21,22 The Consumer Price Index was used to adjust costs to 2023 prices. 23
Sensitivity and subgroup analyses
Model robustness was assessed by performing sensitivity analyses. In one-way sensitivity analyses, individual parameters were varied within ±20% of the baseline. 14 For probabilistic sensitivity analyses, 10,000 Monte Carlo simulations were run. 13 Cost-effectiveness at various WTP thresholds was assessed with cost-effectiveness acceptability curves and scatter plots.
The EV-302/KEYNOTE-A39 trial included subgroup-specific HRs for OS and PFS to enable the calculation of ICER and INHB values for individual subgroups. 11 Factors considered in subgroup analyses included patient age, sex, ECOG PS, primary disease site of origin, liver metastases, PD-L1 expression, and cisplatin eligibility. Due to the lack of subgroup-specific data, baseline characteristics of subgroup patients and overall patients are considered to be the same, except for HRs of OS and PFS, according to Ding et al. 13 ’s approach.
Results
Cost-effectiveness results
Using the established model, the life expectancy of previously untreated la/mUC patients treated with EV plus pembrolizumab was 1.34 LYs (16 months) and 1.25 LYs (15 months) longer than that of patients treated with chemotherapy in the United States and China, respectively (Table 2). After adjusting for QoL and discounting, EV plus pembrolizumab was associated with respective increases of 1.1 and 1.04 QALYs relative to chemotherapy, with an incremental cost of $294,794 and $263,575 for corresponding ICERs of $267,491/QALY ($220,735/LY) and $254,339/QALY ($210,206/LY) and INHBs of −0.87 QALYs or −6.45 QALYs at traditional respective US and Chinese WTP thresholds of $150,000/QALY and $35,173/QALY (Table 2). In all cases, these calculated ICERs were above the selected WTP thresholds, indicating that EV plus pembrolizumab is not a cost-effective option for this patient population at current prices. However, this regimen was predicted to be cost-effective when the cost of EV was reduced by 40% and 90% in the United States and China, respectively (Table 2).
Table 2.
Cost and population assessment.
| Parameters | US value (range) | China value (range) | Distribution |
|---|---|---|---|
| Drug cost, $/cycle | |||
| Enfortumab vedotin | 24,534 (19,627–29,441) 17 | 18,827 (15,062–22,592) 19 | Gamma |
| Pembrolizumab | 11,282 (9026–13,538) 17 | 560 (448–672) 20 | Gamma |
| Chemotherapy | 99 (79–119) 17 | 41 (33–49) 20 | Gamma |
| Paclitaxel | 38 (30–46) 17 | 93 (74–112) 20 | Gamma |
| Cost of AEs | |||
| EV + P | 1375 (1100–1650) 16 | 5 (4–6) 16 | Gamma |
| Chemotherapy | 8713 (6970–10,456)13,14,16 | 693 (554–832)13,14,16 | Gamma |
| Administration per cycle | 209 (167–251) 16 | 14 (11–17) 16 | Gamma |
| PD-L1 tests per patient | 476 (381–571) 21 | 137 (110–164) 21 | Gamma |
| Tumor imaging per cycle | 942 (754–1130) 21 | 143 (114–172) 21 | Gamma |
| Best supportive care per cycle | 1374 (1099–1649) 16 | 711 (569–853) 16 | Gamma |
| Terminal care per patient | 6246 (4997–7495) 22 | 1761 (1409–2113) 21 | Gamma |
| Body weight, kg | 70 (35–140) 13 | 65 (33–130) 14 | Uniform |
| Body surface area, meters 2 | 1.840 (1.472–2.208) 13 | 1.720 (1.376–2.064) 14 | Uniform |
| Discount rate | 0.030 (0–0.050) 14 | 0.05 (0–0.080) 14 | Uniform |
AEs, adverse events; EV + P, enfortumab vedotin plus pembrolizumab; PD-L1, programmed cell death ligand-1.
Sensitivity analysis
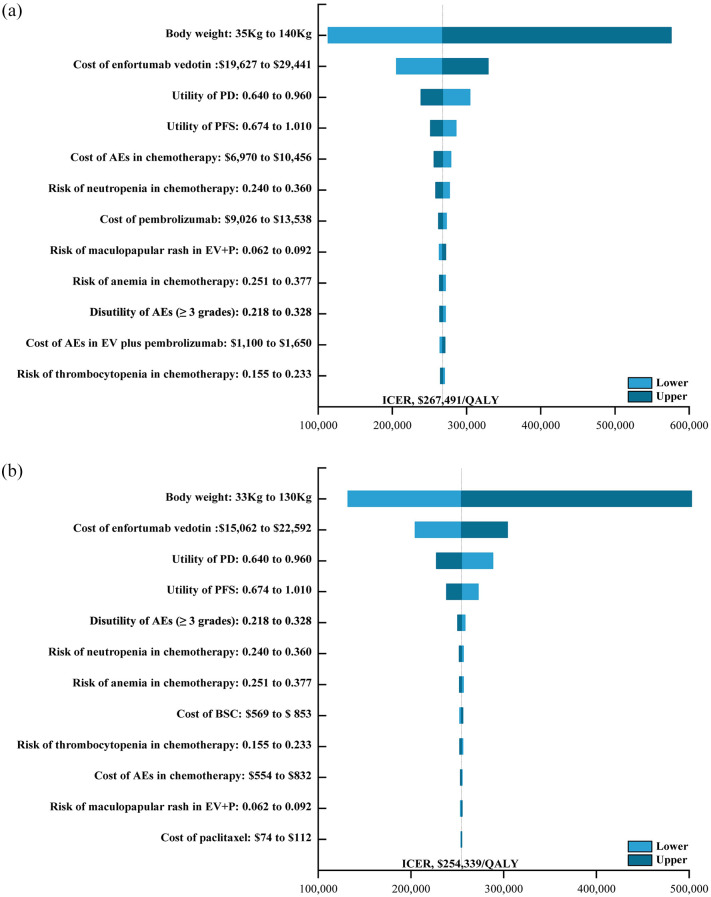
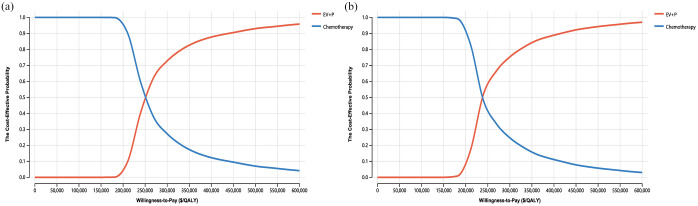
One-way sensitivity analyses indicated that ICERs were most strongly impacted by body weight ($113,254/QALY–$575,964/QALY), followed by the cost of EV and mean utility values (Figure 1). By contrast, outcomes were not particularly affected by the costs of chemotherapy, administration, or terminal care. Overall, these results support the relatively robust nature of the base-case analyses. Cost-effectiveness acceptability curves and scatter plots revealed that there was a 0% chance of EV plus pembrolizumab being a cost-effective alternative to chemotherapy at a WTP of $150,000/QALY (USA) or $35,173/QALY (China) (Figure 2 and Figure S3).
Figure 1.
The one-way sensitivity analyses for the enfortumab vedotin plus pembrolizumab compared to the chemotherapy in the United States (a) and China (b), respectively.
AEs, adverse events; BSC, best supportive care; EV, enfortumab vedotin; EV + P, enfortumab vedotin plus pembrolizumab; ICER, incremental cost-effectiveness ratio; PD, progressive disease; PFS, progression-free survival; QALY, quality-adjusted life-year.
Figure 2.
The cost-effectiveness acceptability curves of the enfortumab vedotin plus pembrolizumab compared to the chemotherapy in the United States (a) and China (b), respectively.
EV + P, enfortumab vedotin plus pembrolizumab; QALY, quality-adjusted life-year.
Subgroup analysis
Subgroup analyses were conducted using the HRs for patient OS and PFS in appropriate subgroups at the selected WTP thresholds of $150,000/QALY (USA) or $35,173/QALY (China). In these analyses, negative INHBs were observed in all cases, and the odds of EV plus pembrolizumab being cost-effective remained at 0%, with ICERs ranging from $221,233/QALY to $300,102/QALY (Table 3). EV plus pembrolizumab may be more economically effective when the risk of progression or death is higher for subgroups.
Table 3.
Cost-effectiveness results.
| Cost of EV + P | Incremental cost, $ a | Incremental benefits a | ICER a | INHB, QALYs a | Comments a | ||
|---|---|---|---|---|---|---|---|
| LYs | QALYs | $/LY | $/QALY | ||||
| The United States | |||||||
| Full cost (baseline results) | 294,794 | 1.34 | 1.10 | 220,735 | 267,491 | −0.87 | Not cost-effective |
| 70% cost | 192,806 | 1.34 | 1.10 | 144,369 | 174,949 | −0.19 | Not cost-effective |
| 60% cost | 158,810 | 1.34 | 1.10 | 118,914 | 144,102 | 0.04 | Cost-effective |
| 50% cost | 124,814 | 1.34 | 1.10 | 93,458 | 113,254 | 0.27 | Cost-effective |
| 10% cost | −11,170 | 1.34 | 1.10 | Dominant b | Dominant b | 1.18 | Cost-effective |
| China | |||||||
| Full cost (baseline results) | 263,575 | 1.25 | 1.04 | 210,206 | 254,339 | −6.45 | Not cost-effective |
| 70% cost | 186,418 | 1.25 | 1.04 | 148,672 | 179,887 | −4.26 | Not cost-effective |
| 60% cost | 160,699 | 1.25 | 1.04 | 128,161 | 155,069 | −3.53 | Not cost-effective |
| 50% cost | 134,981 | 1.25 | 1.04 | 107,650 | 130,251 | −2.80 | Not cost-effective |
| 10% cost | 32,106 | 1.25 | 1.04 | 25,605 | 30,980 | 0.13 | Cost-effective |
EV + P versus the chemotherapy.
Dominant, EV + P showed higher effectiveness and lower cost, as compared with chemotherapy.
EV + P, enfortumab vedotin plus pembrolizumab; ICER, incremental cost-effectiveness ratio; INHB, incremental net health benefits; LYs, life-years; QALYs, quality-adjusted life-years.
Discussion
The promising results of the phase III EV-302/KEYNOTE-A39 trial highlighted the promising risk-benefit profile of this novel ADC plus PD-1 inhibitor combination such that patients treated with EV plus pembrolizumab achieved improved OS and PFS relative to patients treated with chemotherapy. 11 These results suggest that this drug may significantly influence the global market for la/mUC patient treatment. The high cost of novel innovative cancer drugs, however, can place an imposing economic burden on patients and healthcare systems, resulting in the consumption of dramatically higher levels of medical resources. To enable the most effective utilization of limited resources, it is essential to evaluate the pharmacoeconomic characteristics of new therapies and expensive drugs. Accordingly, this study was conducted as the first analysis of the cost-effectiveness of EV plus pembrolizumab as a first-line approach to la/mUC patient management, providing valuable information that may inform the use of this combination regimen in a clinical setting.
Based on the model established in this study at current prices, the total cost of EV plus pembrolizumab is $562,362 and $288,347 in the United States and China, respectively, yielding 2.16 and 2.07 QALYs for corresponding ICERs of $267,491/QALY and $254,339/QALY. This suggests that this combination treatment regimen is not a cost-effective alternative to chemotherapy at the established WTP thresholds in either of these countries, with an overall cost-effectiveness probability of 0%. The higher costs associated with EV treatment were primarily a result of drug and BSC costs. In one-way sensitivity analyses, patient body weight and EV prices were identified as the factors that most strongly influence model results. EV plus pembrolizumab was more cost-effective for US and Chinese patients weighing less than 43 and 10 kg, highlighting ethical concerns regarding the need to charge less for life-sustaining treatments for thin patients. The costs of EV are also based on the number of vials used, rather than the actual dose. This thus suggests that it may make more sense for the cost of EV treatment to be paid on a per-patient or per-cycle basis, rather than a per-bottle basis.
To achieve similar odds of being cost-effective, the unit price for EV would need to be below $84.14/mg (60% of the current price) and $11.59/mg (10% of the current price) in the United States and China, respectively. While EV has been marketed in the United States, the same is not true in China. As such, Chinese EV prices in this model were based on its cost in Hong Kong. In these analyses, the cost of EV plus pembrolizumab was 10-fold higher than that of chemotherapy, contributing to very high total costs and poor cost-effectiveness. Even though EV plus pembrolizumab is not an economically efficient option in China, this does not indicate that patients should be administered less effective treatment regimens. The costs of many cancer drugs have dropped markedly following price negotiations and their entry into the Chinese market. 24 These changes in drug prices thus suggest that there is a possibility of EV-based regimens being more economically viable after they are available in China. Newer drugs also cost much more than extant drugs, and a lack of federal control together with limited transparency result in the United States having the highest drug prices.25,26 The appropriate balancing of the costs of innovative anticancer drugs in high- and middle-income countries is thus an important approach to overcoming this issue.
Several prior reports have explored the cost-effectiveness of pembrolizumab or EV when used as a second-line treatment option for mUC patients. Wu et al., 16 for example, found that EV treatment was not cost-effective relative to chemotherapy in the United States (ICER, $2,168,746.71/QALY), the UK (ICER, $2,164,494.38/QALY), or China (ICER, $1,775,576.56/QALY). Moreover, Ren et al. 27 (ICER, £67,068/QALY), Srivastava et al. 28 (ICER, €71,924/QALY), and Hale et al. 22 (ICER, $78,925/QALY) found the pembrolizumab was a cost-effective alternative to chemotherapy from the perspective of respective healthcare systems in the UK, Switzerland, and the United States. This high level of consistency across countries for these new drugs is surprising but also emphasizes the importance of approving new drugs not solely based on their clinical benefits, but also on their relative economic performance.
The remarkable EV-302/KEYNOTE-A39 study results have highlighted a promising new approach to the standard first-line treatment of la/mUC, employing a novel combination treatment regimen to benefit the entire study population irrespective of their PD-L1 expression status of cisplatin tolerance. The excellent performance of this EV plus pembrolizumab program offers new hope to most la/mUC patients while also increasing the confidence of clinicians in their ability to overcome clinical challenges. Overall, however, given the finding that EV plus pembrolizumab has a more favorable health advantage for patients with liver metastases, PD-L1 CPS scores <10, and upper urinary tract cancer, it is important to explore specific biomarkers and disease types to maximize the efficacy of EV treatment, thereby inevitably enhancing the cost-effectiveness of this drug.
The study has several important strengths worthy of highlighting. For one, this is the first and most up-to-date analysis of the cost-effectiveness of EV plus pembrolizumab relative to chemotherapy in patients with previously untreated la/mUC. ADCs and ICIs have emerged as promising tools for the treatment of a wide array of solid tumors. While EV and pembrolizumab received FDA approval for la/mUC patient treatment and are included in international guidelines, the economic viability of combining these treatments has not been established in any prior studies. Second, the cost-effectiveness of this combination treatment regimen was assessed from the perspectives of the medical systems in the United States and China owing to the marked differences in healthcare and other conditions between these nations. These results can be drawn upon by policymakers, individuals in the healthcare-related financial sector, and clinicians to better guide pricing and clinical decision-making efforts. Third, the pharmacoeconomic outcomes associated with EV plus pembrolizumab treatment for seven subgroups of patients defined in the EV-302/KEYNOTE-A39 trial were assessed. The resultant economic information may thus aid efforts to formulate appropriate individualized treatment regimens for these patient subsets. Lastly, changes in patient body weight and EV pricing were found to markedly alter the economic viability of this combination treatment regimen, suggesting that price reductions and appropriate drug dosing are important, informing future multi-party drug price negotiation and efforts to design treatment plans.
There are some limitations to this study. For one, short-term survival benefits were extrapolated to model the lifetime disease progression and survival of la/mUC patients, potentially reducing study robustness. To minimize this risk, appropriate modeling strategies were used to predict these outcomes, and many different variables were taken into consideration during sensitivity analyses. Second, the EV-302/KEYNOTE-A39 trial did not provide detailed patient QoL information, precluding efforts to directly extract health-related utility data such that these were derived from prior studies. Importantly, however, utility values did not significantly alter model outcomes in sensitivity analyses. Third, the established Markov model only considered grade 3+ AEs, potentially leading to the underestimation of AE management-related costs. Sensitivity analyses, however, suggested that these costs had a fairly small impact on the conclusions of this study. Lastly, the cost-effectiveness of EV plus pembrolizumab was assessed by calculating a discount in the model, and this price is expected to reach a minimum when approved. With the launch of EV in China, updates to this model will be required.
Conclusion
In conclusion, based on the available data, EV plus pembrolizumab is not expected to be a cost-effective option for the treatment of previously untreated la/mUC patients from the perspectives of payers in the United States or China at the appropriate WTP threshold. These results have the potential to guide clinical decision-making regarding the management of la/mUC patients or the establishment of appropriate pricing and medical reimbursement policies for these drugs. However, these results demonstrate that EV plus pembrolizumab treatment does afford patients significant clinical benefits while simultaneously imposing a heavy economic burden. Efforts to reduce EV costs may be highly effective as a means of improving the accessibility of this innovative therapeutic regimen in the clinic.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241295544 for Enfortumab vedotin plus pembrolizumab for previously untreated locally advanced or metastatic urothelial carcinoma: a cost-effectiveness analysis by Youwen Zhu, Kun Liu, Hong Zhu, Shan Li and Dan Yuan in Therapeutic Advances in Medical Oncology
Acknowledgments
None.
Footnotes
ORCID iDs: Youwen Zhu  https://orcid.org/0000-0001-8692-3591
https://orcid.org/0000-0001-8692-3591
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Youwen Zhu, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Kun Liu, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Hong Zhu, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan, China.
Shan Li, Department of Oncology, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China; National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, Hunan 410008, China.
Dan Yuan, Department of Oncology, Zhuzhou Second Hospital, Zhuzhou, Hunan 412000, China.
Declarations
Ethics approval and consent to participate: This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors, it does not require the approval of the independent ethics committee.
Consent for publication: Not applicable.
Author contributions: Youwen Zhu: Conceptualization; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Kun Liu: Conceptualization; Formal analysis; Investigation; Validation; Visualization; Writing – original draft; Writing – review & editing.
Hong Zhu: Conceptualization; Data curation; Funding acquisition; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Shan Li: Funding acquisition; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Dan Yuan: Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was partly supported by the Clinical Research Project of Xiangya Hospital (Grant/Award Number: 2016L06 to H.Z.) and the Changsha Natural Science Foundation of Hunan Provincial of China (Grant/Award Number: kq2208376 to H.Z.).
The authors declare that there is no conflict of interest.
Availability of data and materials: All authors had full access to all of the data in this study and took complete responsibility for the integrity of the data and the accuracy of the data analysis. The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. World Health Organization. Breast cancer, https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf (2020, accessed November 2022).
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020; 70(1): 7–30. [DOI] [PubMed] [Google Scholar]
- 3. Cao W, Chen HD, Yu YW, et al. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021; 134(7): 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep 2018; 8(1): 1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. NCI SEER Database. https://seer.cancer.gov/ (2023, accessed November 2023).
- 6. De Santis M, Bellmunt J, Mead G, et al. Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 2012; 30(2): 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017; 376(11): 1015–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021; 384(12): 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Bladder cancer, Version 3.2023, https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf (accessed 25 May 2023). [DOI] [PubMed]
- 10. US Food and Drug Administration. FDA approves pembrolizumab or enfortumab vedotin as second-line for patients with locally advanced or metastatic urothelial carcinoma. https://www.fda.gov/ (accessed November 2023).
- 11. Powles T, Valderrama BP, Gupta S, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med 2024; 390(10): 875–888. [DOI] [PubMed] [Google Scholar]
- 12. Husereau D, Drummond M, Augustovski F, et al. Consolidated Health Economic Evaluation Reporting Standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. Value Health 2022; 25(1): 3–9. [DOI] [PubMed] [Google Scholar]
- 13. Ding D, Hu H, Li S, et al. Cost-effectiveness analysis of durvalumab plus chemotherapy in the first-line treatment of extensive-stage small cell lung cancer. J Natl Compr Canc Netw 2021; 19(10): 1141–1147. [DOI] [PubMed] [Google Scholar]
- 14. Zhu Y, Liu K, Zhu H. Immune checkpoint inhibitor for patients with advanced biliary tract cancer: a cost-effectiveness analysis. Liver Int 2023; 43(10): 2292–2301. [DOI] [PubMed] [Google Scholar]
- 15. Qin S, Yi L, Li S, et al. Cost-effectiveness of atezolizumab plus chemotherapy as first-line therapy for metastatic urothelial cancer. Adv Ther 2021; 38(6): 3399–3408. [DOI] [PubMed] [Google Scholar]
- 16. Wu Q, Qin Y, Liao W, et al. Cost-effectiveness of enfortumab vedotin in previously treated advanced urothelial carcinoma. Ther Adv Med Oncol 2022; 14: 17588359211068733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/payment/fee-for-service-providers/part-b-drugs/average-drug-sales-price/asp-pricing-files (2023, accessed October 2023). [PubMed]
- 18. Patterson K, Prabhu V, Xu R, et al. Cost-effectiveness of pembrolizumab for patients with advanced, unresectable, or metastatic urothelial cancer ineligible for cisplatin-based therapy. Eur Urol Oncol 2019; 2(5): 565–571. [DOI] [PubMed] [Google Scholar]
- 19. RedBook. https://www.micromedexsolutions.com/micromedex2/librarian/ssl/true (2023, accessed October 2023).
- 20. DrugDataexpy. https://data.yaozh.com (accessed October 2023).
- 21. Wu B, Zhang Q, Sun J. Cost-effectiveness of nivolumab plus ipilimumab as first-line therapy in advanced renal-cell carcinoma. J Immunother Cancer 2018; 6(1): 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hale O, Patterson K, Lai Y, et al. Cost-effectiveness of pembrolizumab versus carboplatin-based chemotherapy as first-line treatment of PD-L1-positive locally advanced or metastatic urothelial carcinoma ineligible for cisplatin-based therapy in the United States. Clin Genitourin Cancer 2021; 19(1): e17–e30. [DOI] [PubMed] [Google Scholar]
- 23. US Bureau of Labor Statistics. CPI inflation calculator, https://www.bls.gov/data/inflation_calculator.htm (2023, accessed June 2023).
- 24. State Council of China. China’s centralized procurement leads to 50% drop in prices of over 100 drugs, http://english.www.gov.cn/statecouncil/ministries/202011/21/content_WS5fb86defc6d0f7257694042b.html (2020, accessed November 2023).
- 25. Prasad V, De Jesús K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol 2017; 14(6): 381–390. [DOI] [PubMed] [Google Scholar]
- 26. Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursed by commercial health plans, 2000–2014. JAMA Oncol 2016; 2(7): 960–961. [DOI] [PubMed] [Google Scholar]
- 27. Ren S, Squires H, Hock E, et al. Pembrolizumab for locally advanced or metastatic urothelial cancer where cisplatin is unsuitable: an evidence review group perspective of a NICE single technology appraisal. Pharmacoeconomics 2019; 37(9): 1073–1080. [DOI] [PubMed] [Google Scholar]
- 28. Srivastava T, Prabhu VS, Li H, et al. Cost-effectiveness of pembrolizumab as second-line therapy for the treatment of locally advanced or metastatic urothelial carcinoma in Sweden. Eur Urol Oncol 2020; 3(5): 663–670. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241295544 for Enfortumab vedotin plus pembrolizumab for previously untreated locally advanced or metastatic urothelial carcinoma: a cost-effectiveness analysis by Youwen Zhu, Kun Liu, Hong Zhu, Shan Li and Dan Yuan in Therapeutic Advances in Medical Oncology