Abstract
Background:
Encorafenib plus cetuximab (EC) is the standard of care for pre-treated BRAF V600E mutated metastatic colorectal cancer (mCRC). Depth of response (DpR) and early tumour shrinkage (ETS) previously showed a strong correlation with survival outcomes of first-line chemotherapy ± biological agents.
Objectives:
We aimed to assess potential predictors of primary resistance to EC ± binimetinib (B) and relationships of DpR/ETS with survival outcomes and clinical characteristics.
Design:
This is a retrospective real-world cohort study of BRAF V600E mutated mCRC patients treated with second-line EC ± B at 20 Italian centres.
Methods:
Measurable disease according to Response Evaluation Criteria In Solid Tumour (RECIST) 1.1 at baseline and at least one subsequent computed tomography (CT) scan were mandatory for inclusion. Clinical features associated with primary resistance, DpR and ETS were investigated. Relationships of DpR and ETS, both as binary, according to conventional (30% for DpR and 20% for ETS) and median cut-off values, and continuous variables, with progression-free (PFS), overall survival (OS) and duration of response (DoR) were assessed in non-primary resistant patients.
Results:
A total of 105 patients were included. The primary resistance rate was 28% (29/105) and was associated with baseline peritoneal metastases (p = 0.04). Disease control and overall response rates were 72% (76/105) and 24% (25/105), respectively, with a median DpR of 15% and an ETS rate of 37% (28/76). Mucinous histology was associated with a significantly lower magnitude of DpR (p = 0.005) and a lower rate of ETS (p = 0.002). In the multivariable models, DpR significantly correlated with longer PFS as a dichotomous variable, according both to conventional (hazard ratio (HR)DpR ⩾30%: 0.52, 95% CI: 0.30–0.90, p = 0.02) and median cut-off values (HRDpR⩾15%: 0.55, 95% CI: 0.33–0.92, p = 0.03), and as a continuous variable (HR per 10% increment: 0.88, 95% CI: 0.78–0.98, p = 0.02), while correlations with OS were not confirmed. DpR was also significantly associated with longer DoR (pDpR⩾30% = 0.04; pDpR⩾15% = 0.04; pcont. = 0.02), whereas no relationships of ETS with PFS, OS or DoR were detected.
Conclusion:
A DpR of at least 15% independently predicts PFS benefit in BRAF V600E mutated mCRC patients treated with second-line EC ± B.
Keywords: BRAF V600E mutation, depth of response, early tumour shrinkage, metastatic colorectal cancer, real-world analysis
Introduction
The BRAF (v-Raf murine sarcoma viral oncogene homolog B1) V600E mutation–where valine (V) is substituted by glutamic acid (E) at amino acid position 600–commonly referred to as BRAFV600E, occurs in approximately 10% of metastatic colorectal cancers (mCRC) and defines a clinically and molecularly distinct subgroup of tumours with poor prognosis and refractoriness to cytotoxic agents.1,2 Some advances have been recently achieved with a combinatory targeted strategy consisting of the BRAF inhibitor encorafenib plus the anti-epidermal growth factor receptor (anti-EGFR) cetuximab (EC) ± the mitogen-activated protein kinase kinase (MEK) inhibitor binimetinib (B), that in the pivotal phase III BEACON CRC trial showed improved overall survival (OS), progression-free survival (PFS) and overall response rate (ORR) as compared to a standard irinotecan plus cetuximab-based treatment in pre-treated patients with BRAF V600E -mutated mCRC.3,4 Since the addition of binimetinib to EC did not result in better survival outcomes and, at the same time, was related to a less favourable safety profile, EC is now a guideline-recommended therapeutic option for mCRC patients with BRAF V600E mutated tumours who received at least a prior treatment regimen. 5 Nevertheless, a significant proportion of patients experience primary resistance or short-term clinical benefit when receiving EC.
To date, several efforts have been conducted towards the identification of potential predictive and prognostic biomarkers to stratify patient candidates for this therapeutic approach. A previously published real-world series 6 suggested worse Eastern Cooperative Oncological Group Performance Status (ECOG PS), peritoneal metastases, and more than one prior treatment as independent prognostic factors. Additionally, inactivating mutations in ring finger protein 43 (RNF43)–which encodes a negative regulator of the Wingless-related integration site (WNT) pathway–are suggested to be related to a better prognosis in patients with proficient mismatch repair/microsatellite stable (pMMR/MSS) BRAFV600E mutated mCRC treated with the anti-BRAF targeted approach.7,8 Most recently, a secondary analysis of the BEACON trial 9 reported potential associations between clinical outcomes of patients with BRAF V600E -mutated mCRC treated with EC ± B and immune gene signatures but not with RNF43 mutational status. Molecular correlates of acquired resistance were also characterized in depth suggesting a context of adaptive mutability as the potential reason for targeted treatment failure. 9 Furthermore, novel radiological parameters emerged as tools able to implement the conventional Response Evaluation Criteria In Solid Tumours (RECIST)-defined metrics in the assessment of the dynamics of tumour response. Among them, early tumour shrinkage (ETS) and depth of response (DpR) have been developed to describe temporal and quantitative longitudinal changes in disease burden during anti-tumour treatments. Post hoc exploratory analyses of several randomized clinical trials exploring first-line strategies with chemotherapy ± targeted agents in mCRC showed that both ETS and DpR are valuable predictors of survival outcomes in mCRC patients.10–14 More remarkably, increasing evidence suggests that these parameters may more accurately measure the quality and magnitude of response to targeted therapies in mCRC than the conventional RECIST metrics.15,16
This concept takes particular relevance in patients with BRAF V600E mutated tumours treated with EC ± B, characterized by relatively short-term clinical benefit and poor outcomes following resistance to targeted therapy.3,4 However, no data linking DpR and ETS to survival outcomes in patients with BRAF V600E mutated mCRC treated with EC ± B are currently available.
Based on these considerations, we aimed to investigate DpR and ETS as tumour dynamic response parameters and assess their relationships with baseline characteristics and clinical outcomes in a retrospective well-annotated cohort of patients with BRAF V600E mutated mCRC treated in a real-world setting with EC ± B after progression to one previous systemic therapy.
Methods
Study design and population
This was a retrospective cohort study in consecutive patients with BRAF V600E mutated mCRC treated with EC ± B at 20 Italian hospitals in a real-life setting, between May 2019 and October 2022. Patients were initially treated with EC ± B within a nominal use program, launched in Italy in May 2019, and since February 2020 they received only EC, as per clinical indication. For the present analysis, patients who had received only one systemic treatment for metastatic disease prior to EC ± B, with at least one measurable lesion according to the RECIST 1.1 criteria and availability of at least one radiological disease re-assessment using computed tomography (CT) scan as per clinical practice, were selected. Treatment was administered until disease progression, unacceptable toxicities or the patient’s refusal. Investigators retrospectively collected data on all consecutively enrolled patients from medical records after obtaining their informed consent. A waiver of consent was considered for deceased patients or those lost to follow-up. The study was approved by local Ethic Committees (Oncologic Institute of Veneto, code 2017/34) and its reporting conforms with the ESMO Guidance for Reporting Oncology real-World Evidence (ESMO-GROW) (Supplemental File). 17
Endpoints
The primary endpoint was to assess the impact of ORR, DpR and ETS on PFS and OS. Secondary endpoints were to explore the influence of ORR, DpR and ETS on the tailor-defined duration of response (DoR) and to characterize potential predictors of primary resistance to treatment, magnitude of DpR and rate of ETS. PFS was calculated as the time from the start of treatment with EC ± B to the evidence of progressive disease (PD) or death from any cause, whichever occurred first. Patients alive and not experiencing disease progression at the time of the analysis were censored at the date of the last tumour assessment.
OS was calculated as the time from the start of treatment with EC ± B to death from any cause. Censoring was performed at the date of the last follow-up for patients who were alive at the time of the analysis. DoR was defined as the time from the first documentation of tumour shrinkage (i.e., ⩾1%) to PD or death from any cause.
Assessment of radiological parameters
Tumour objective response dynamics were based on investigator-reported measurements and assessed according to the RECIST 1.1 criteria by the evaluating medical oncologist. ORR was calculated as the percentage of patients achieving complete response (CR) or partial response (PR). ETS rate was defined as the percentage of patients achieving a decrease of at least 20% in the sum of the longest diameters of the RECIST target lesions at the first radiological re-assessment as compared to baseline, and DpR was defined as the relative change in the sum of the longest diameters of the RECIST target lesions at the nadir, in the absence of new lesions or progression of non-target lesions, as compared with baseline.
The impact of ORR, ETS and DpR on survival outcomes (i.e., PFS, OS and DoR) was assessed only in the subset of patients achieving at least disease stabilization (SD) as the best response. Patients experiencing PD at the first radiological assessment (i.e., primary resistance) were analysed separately.
Statistical analysis
DpR and ETS were considered either binary (adopting the median and conventional values as cut-off) or continuous variables, with 10 levels based on decile distribution. When both parameters were analysed as continuous variables, hazard ratios (HRs) were referred to each increment of one decile point. According to RECIST 1.1 criteria, patients were dichotomized as responders (i.e., achieving CR or PR) or non-responders (i.e., achieving SD). Analyses involving response-related parameters were based on an 8-week landmark approach (i.e., coinciding with the earliest time of CT scan reassessment for tumour response).
PFS and OS curves were plotted with the Kaplan-Meier method and compared by the log-rank test. HRs and relative 95% confidence intervals (CIs) were estimated by the Cox proportional hazards model. Median values and interquartile ranges (IQR) were adopted to describe the distribution of continuous variables. Chi-squared test, Fisher’s exact test, Kruskal-Wallis and Mann-Whitney U tests were adopted as appropriate to examine baseline differences between groups. Covariates with p < 0.10 at univariable analyses were included in multivariable Cox proportional hazard models. Statistical significance was set at a p-value of 0.05. All analyses were carried out with MedCalc v22.002 (https://www.medcalc.org/), RStudio 2023.06.1+524 ‘Mountain Hydrangea’ Release (https://www.R-project.org/) and JMP PRO 17 (https://www.jmp.com/).
Results
In all, 105 patients were eligible for the present analysis. Their baseline demographic, clinical, molecular and treatment characteristics are summarized in Supplemental Table 1.
Overall, the median age at the beginning of targeted therapy was 66 years (IQR 57.4–74.1), 54% were female, and 91% had an ECOG PS of 0 or 1. Tumours were mostly right-sided (69%) and involved more than one metastatic site in 73% of cases, including the peritoneum in 49% of patients. Deficient mismatch repair (dMMR)/Microsatellite instability-high (MSI-H) status and mucinous histology were detected in 7% and 28% of patients, respectively. Among four patients with dMMR/MSI-H tumour, none of them received immune-checkpoint inhibitors (ICIs) before or after the study treatment, because in the timeframe of their clinical course, ICIs were not a standard of care for this molecular subgroup. Previous treatment consisted of a triplet, doublet or single-agent chemotherapy backbone in 44 (42%), 56 (53%) and 5 (5%) patients, respectively. Sixteen (15%) patients received binimetinib in combination with EC.
At the time of this analysis (data cut-off: February 6th, 2023), the median follow-up was 19.3 months (IQR 12.6–28.7). Ninety-two (88%) and 75 (71%) events of disease progression and death occurred, respectively. In the overall population, mPFS and mOS were 5.2 (95% CI: 4.6–5.8) and 10.3 (95% CI: 7.8–11.6) months, respectively (Supplemental Figure 1A and B).
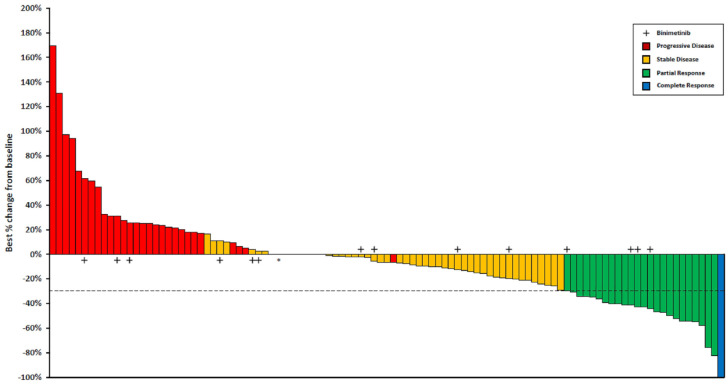
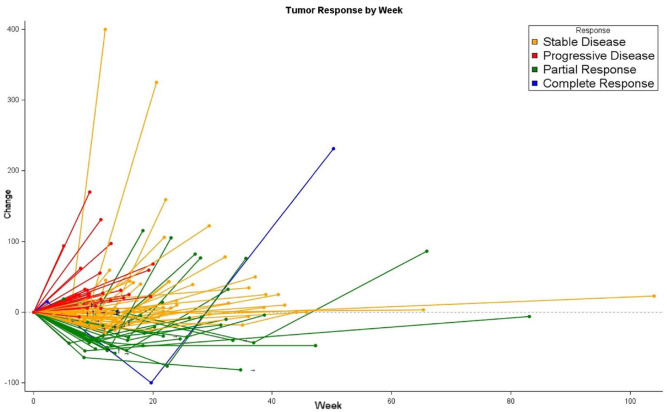
Tumour response and its dynamics at different timepoints are summarized in Figures 1 and 2. Seventy-six (72%) patients achieved disease control; among them, 25 (24%) and 51 (48%) had an objective response and disease stabilization, respectively. Primary resistance was observed in 29 (28%) subjects.
Figure 1.
Waterfall plot for response to encorafenib plus cetuximab ± binimetinib.
Tumour response in 105 evaluable patients. The bars show the best percentage change in target lesions from baseline. The dashed horizontal line at −30% indicates the threshold value to define PR.
*One patient with 0% as best percentage change in target lesions from baseline had progressive disease due to the appearance of new metastatic lesions.
Figure 2.
Spider plot for response to encorafenib plus cetuximab ± binimetinib.
Dynamics of tumour response according to best RECIST response in 105 evaluable patients. The individual lines represent the percentage variation of the sum of the longest diameters of target lesions at different time points, as compared to the baseline.
→Treatment ongoing; †Last known dimensional data.
Predictors of primary resistance to treatment
Baseline characteristics of patients experiencing primary resistance (n = 29) are reported in Supplemental Table 2; they did not significantly differ from those of patients achieving clinical benefit, except for a higher prevalence of baseline peritoneal metastases (66% versus 43%, p = 0.04).
Tumour response parameters in patients achieving disease control
Among 76 patients experiencing at least disease control, the median DpR was 15% (IQR 2–37), and a reduction in the sum of the highest diameters of the target lesions (i.e., ⩾1%) was observed in 61 (80%) out of 76 cases. ETS occurred in 28 (37%) patients and the median ETS was 12% (IQR 0–28).
Baseline patients’ characteristics according to ETS and DpR are detailed in Supplemental Table 3. No significant association was observed between baseline characteristics and radiological parameters, except for the lower magnitude of ETS (0% versus 14%, p = 0.002) and DpR (2% versus 19%, p = 0.005) in the subgroup of tumours with mucinous histology (Supplemental Table 3).
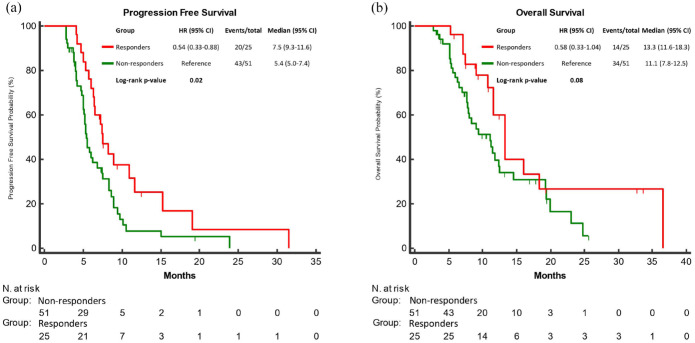
Expectedly, responders (n = 25) experienced longer PFS (mPFS: 7.5 versus 5.4 months; HR: 0.54, 95% CI: 0.33–0.88; p = 0.02) and OS (mOS: 13.3 versus 11.1 months; HR: 0.58, 95% CI: 0.33–1.04; p = 0.08) as compared to non-responders (n = 51) (Figure 3(a) and (b)). The association of RECIST response with PFS but not with OS was confirmed in the multivariable analyses (Table 1).
Figure 3.
PFS (a) and OS (b) according to RECIST response (responders (CR/PR) vs non-responders (SD)) to encorafenib plus cetuximab ± binimetinib.
CR, complete response; OS, overall survival; PFS, progression-free; PR, partial response.
Table 1.
Uni- and multivariable analyses for progression-free survival and overall survival according to RECIST response to encorafenib plus cetuximab ± binimetinib.
| Uni- and multivariate analyses for progression-free survival and overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression-free survival | Overall survival | ||||||||||
| Characteristics | N | Median (months) |
Univariable analysis | Multivariable analysis | Median (months) |
Univariable analysis | Multivariable analysis | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| Sex | |||||||||||
| Male | 37 | 5.8 | 1.29 | 0.34 | – | – | 7.8 | 1.70 | 0.09 | 1.72 | 0.13 |
| Female | 39 | 6.3 | (0.76–2.19) | – | – | 11.2 | (0.93–3.13) | (0.86–3.44) | |||
| Primary tumour location | |||||||||||
| Right | 51 | 6.2 | 0.99 | 0.97 | – | – | 8.8 | 1.10 | 0.76 | – | – |
| Left | 25 | 5.4 | (0.57–1.72) | – | – | 9.1 | (0.59–2.06) | – | – | ||
| Mucinous histology | |||||||||||
| Yes | 19 | 6.0 | 0.86 | 0.58 | – | – | 9.1 | 0.88 | 0.70 | – | – |
| No | 56 | 5.9 | (0.50 – 1.48) | – | – | 8.9 | (0.47–1.66) | – | – | ||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| MS status | |||||||||||
| High | 4 | 4.8 | 3.46 | 0.02 | 4.47 | 0.007 | 7.4 | 3.23 | 0.03 | 6.05 | 0.002 |
| Low | 71 | 6.2 | (1.21–9.90) | (1.54–13.52) | 9.1 | (1.13–9.27) | (1.90–19.22) | ||||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| ECOG PS before the beginning of targeted therapy | |||||||||||
| 0 | 34 | 7.3 | 0.83 | 0.47 | – | – | 11.4 | 0.78 | 0.41 | – | – |
| ⩾1 | 42 | 5.4 | (0.50–1.38) | – | – | 7.7 | (0.44–1.40) | – | – | ||
| Peritoneal mts | |||||||||||
| Yes | 33 | 5.9 | 1.66 | 0.07 | 1.38 | 0.29 | 8.2 | 1.54 | 0.16 | – | – |
| No | 43 | 6.2 | (0.96–2.88) | (0.75–2.53) | 10.8 | (0.85–2.81) | – | – | |||
| Sites of mts | |||||||||||
| >1 | 55 | 5.7 | 1.68 | 0.08 | 1.48 | 0.23 | 8.2 | 2.07 | 0.05 | 1.92 | 0.09 |
| 1 | 21 | 7.2 | (0.95–3.00) | (0.78–2.80) | 11.1 | (1.00–4.29) | (0.90–4.10) | ||||
| ⩾8 months between mts diagnosis and beginning of targeted therapy | |||||||||||
| Yes | 50 | 6.6 | 0.55 | 0.03 | 0.50 | 0.02 | 11.0 | 0.40 | 0.004 | 0.40 | 0.01 |
| No | 26 | 5.3 | (0.31–0.96) | (0.28–0.89) | 7.2 | (0.21–0.75) | (0.19–0.82) | ||||
| Ascites at the beginning of targeted therapy | |||||||||||
| Yes | 12 | 5.4 | 1.43 | 0.28 | – | – | 7.9 | 1.81 | 0.12 | – | – |
| No | 64 | 6.0 | (0.74–2.77) | – | – | 9.2 | (0.86–3.80) | – | – | ||
| Binimetinib in combination with encorafenib and cetuximab | |||||||||||
| Yes | 13 | 5.3 | 1.91 | 0.04 | 2.27 | 0.02 | 7.8 | 2.03 | 0.04 | 2.77 | 0.005 |
| No | 63 | 6.3 | (1.03 – 3.56) | (1.17–4.43) | 9.4 | (1.05–3.93) | (1.35–5.71) | ||||
| Schedule of targeted therapy administration | |||||||||||
| Weekly | 41 | 5.9 | 1.34 | 0.26 | – | – | 7.7 | 1.35 | 0.32 | – | – |
| Biweekly | 35 | 6.2 | (0.80–2.23) | – | – | 9.4 | (0.74–2.45) | – | – | ||
| CEA at the beginning of targeted therapy | |||||||||||
| ⩾5 | 57 | 5.8 | 1.10 | 0.77 | – | – | 8.9 | 1.14 | 0.71 | – | – |
| <5 | 13 | 6.4 | (0.56–2.19) | – | – | 9.0 | (0.54–2.40) | – | – | ||
| NA | 6 | – | – | – | – | – | – | – | – | – | – |
| Ca 19.9 at the beginning of targeted therapy | |||||||||||
| ⩾40 | 48 | 5.4 | 1.37 | 0.31 | – | – | 8.8 | 1.00 | 1.00 | – | – |
| <40 | 18 | 6.6 | (0.74–2.53) | – | – | 9.1 | (0.51–1.97) | – | – | ||
| NA | 10 | – | – | – | – | – | – | – | – | – | – |
| RECIST response | |||||||||||
| Yes | 25 | 7.4 | 0.54 | 0.02 | 0.52 | 0.02 | 10.8 | 0.58 | 0.08 | 0.70 | 0.31 |
| No | 51 | 5.3 | (0.33–0.88) | (0.30–0.90) | 8.0 | (0.33–1.04) | (0.36–1.38) | ||||
Ca 19.9, carbohydrate antigen sialyl Lewis a; CEA, carcinoembryonic antigen; DpR, depth of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; MS, microsatellite status; Mts, metastases; NA, not available.
Statistically significant p values (i.e., <0.10 and <0.05 for uni- and multivariable analyses, respectively) are highlighted in bold.
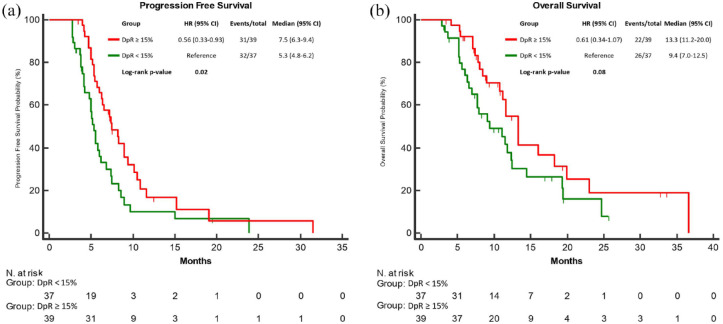
Furthermore, a highly significant association of DpR as a binary variable, according to the median value (15%), with both PFS and OS was found. Indeed, patients with a DpR ⩾15% reported longer PFS (mPFS: 7.5 versus 5.3 months; HR: 0.56, 95% CI: 0.33–0.93; p = 0.02) and OS (mOS: 13.3 versus 9.4 months; HR: 0.61, 95% CI: 0.34–1.07; p = 0.08) as compared to those with a DpR < 15% (Figure 4(a) and (b)). Comparable results were observed when DpR was considered as a continuous variable, with a significant association with both PFS (HR per 10% increment: 0.87, 95% CI: 0.77–0.96, p = 0.01) and OS (HR per 10% increment: 0.88, 95% CI: 0.76–0.99, p = 0.04) (Table 2). Both as a continuous and a binary variable, DpR was independently correlated with PFS in the multivariable analyses (Tables 2 and 3), while the same correlations with OS were not confirmed (Tables 2 and 3).
Figure 4.
PFS (a) and OS (b) according to tumour shrinkage using a 15% DpR threshold for response to encorafenib plus cetuximab ± binimetinib.
CI, confidence interval; DpR, depth of response; HR, hazard ratio; OS, overall survival; PFS, progression-free.
Table 2.
Uni- and multivariable analyses for progression-free survival and overall survival according to DpR as a continuous variable.
| Uni- and multivariable analyses for progression-free survival and overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression-free survival | Overall survival | ||||||||||
| Characteristics | N | Median (months) | Univariable analysis | Multivariable analysis | Median (months) | Univariable analysis | Multivariable analysis | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| Sex | |||||||||||
| Male | 37 | 5.8 | 1.29 | 0.34 | – | – | 7.8 | 1.70 | 0.09 | 1.72 | 0.13 |
| Female | 39 | 6.3 | (0.76–2.19) | – | – | 11.2 | (0.93–3.13) | (0.86–3.44) | |||
| Primary tumour location | |||||||||||
| Right | 51 | 6.2 | 0.99 | 0.97 | – | – | 8.8 | 1.10 | 0.76 | – | – |
| Left | 25 | 5.4 | (0.57–1.72) | – | – | 9.1 | (0.59–2.06) | – | – | ||
| Mucinous histology | |||||||||||
| Yes | 19 | 6.0 | 0.86 | 0.58 | – | – | 9.1 | 0.88 | 0.70 | – | – |
| No | 56 | 5.9 | (0.50–1.48) | – | – | 8.9 | (0.47–1.66) | – | – | ||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| MS status | |||||||||||
| High | 4 | 4.8 | 3.46 | 0.02 | 4.47 | 0.007 | 7.4 | 3.23 | 0.03 | 6.05 | 0.002 |
| Low | 71 | 6.2 | (1.21–9.90) | (1.54–13.52) | 9.1 | (1.13–9.27) | (1.90–19.22) | ||||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| ECOG PS before the beginning of targeted therapy | |||||||||||
| 0 | 34 | 7.3 | 0.83 | 0.46 | – | – | 11.4 | 1.28 | 0.41 | – | – |
| ⩾1 | 42 | 5.4 | (0.50–1.38) | – | – | 7.7 | (0.72–2.28) | – | – | ||
| Peritoneal mts | |||||||||||
| Yes | 33 | 5.9 | 1.66 | 0.07 | 1.38 | 0.29 | 8.2 | 1.54 | 0.16 | – | – |
| No | 43 | 6.2 | (0.96–2.88) | (0.75–2.53) | 10.8 | (0.85–2.81) | – | – | |||
| Sites of mts | |||||||||||
| >1 | 55 | 5.7 | 1.68 | 0.08 | 1.48 | 0.23 | 8.2 | 2.07 | 0.05 | 1.92 | 0.09 |
| 1 | 21 | 7.2 | (0.95–3.00) | (0.78–2.80) | 11.1 | (1.00–4.29) | (0.90–4.10) | ||||
| ⩾8 months between mts diagnosis and beginning of targeted therapy | |||||||||||
| Yes | 50 | 6.6 | 0.55 | 0.03 | 0.50 | 0.02 | 11.0 | 0.40 | 0.004 | 0.40 | 0.01 |
| No | 26 | 5.3 | (0.31–0.96) | (0.28–0.89) | 7.2 | (0.21–0.75) | (0.19–0.82) | ||||
| Ascites at the beginning of targeted therapy | |||||||||||
| Yes | 12 | 5.4 | 1.43 | 0.28 | – | – | 7.9 | 1.81 | 0.12 | – | – |
| No | 64 | 6.0 | (0.74–2.77) | – | – | 9.2 | (0.86–3.80) | – | – | ||
| Binimetinib in combination with encorafenib and cetuximab | |||||||||||
| Yes | 13 | 5.3 | 1.91 | 0.04 | 2.27 | 0.02 | 7.8 | 2.03 | 0.04 | 2.77 | 0.005 |
| No | 63 | 6.3 | (1.03–3.56) | (1.17–4.43) | 9.4 | (1.05–3.93) | (1.35–5.71) | ||||
| Schedule of targeted therapy administration | |||||||||||
| Weekly | 41 | 5.9 | 1.34 | 0.26 | – | – | 7.7 | 1.35 | 0.32 | – | – |
| Biweekly | 35 | 6.2 | (0.80–2.23) | – | – | 9.4 | (0.74–2.45) | – | – | ||
| CEA at the beginning of targeted therapy | |||||||||||
| >5 | 57 | 5.8 | 1.10 | 0.77 | – | – | 8.9 | 1.14 | 0.71 | – | – |
| ⩽5 | 13 | 6.4 | (0.56–2.19) | – | – | 9.0 | (0.54–2.40) | – | – | ||
| NA | 6 | – | – | – | – | – | – | – | – | – | – |
| Ca 19.9 at the beginning of targeted therapy | |||||||||||
| >40 | 48 | 5.4 | 1.37 | 0.31 | – | – | 8.8 | 1.00 | 1.00 | – | – |
| ⩽40 | 18 | 6.6 | (0.74–2.53) | – | – | 9.1 | (0.51 – 1.97) | – | – | ||
| NA | 10 | – | – | – | – | – | – | – | – | – | – |
| DpR (continuous variable)* | 76 | 6.0 | 0.87 (0.77–0.96) |
0.01 | 0.88 (0.78–0.98) |
0.02 | 9.1 | 0.88 (0.76–0.99) |
0.04 | 0.93 (0.80–1.08) |
0.29 |
For a 10% increment.
Ca 19.9, carbohydrate antigen sialyl Lewis a; CEA, carcinoembryonic antigen; DpR, depth of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; MS, microsatellite status; Mts, metastases; NA, not available.
Statistically significant p values (i.e., <0.10 and <0.05 for uni- and multivariable analyses, respectively) are highlighted in bold.
Table 3.
Uni- and multivariable analyses for progression-free survival and overall survival according to a 15% DpR threshold for response to encorafenib plus cetuximab ± binimetinib.
| Uni- and multivariable analyses for progression-free survival and overall survival | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Progression-free survival | Overall survival | ||||||||||
| Characteristics | N | Median (months) | Univariable analysis | Multivariable analysis | Median (months) | Univariable analysis | Multivariable analysis | ||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | ||||
| Sex | |||||||||||
| Male | 37 | 5.8 | 1.29 | 0.34 | – | – | 7.8 | 1.70 | 0.09 | 1.69 | 0.15 |
| Female | 39 | 6.3 | (0.76–2.19) | – | – | 11.2 | (0.93–3.13) | (0.83–3.41) | |||
| Primary tumour location | |||||||||||
| Right | 51 | 6.2 | 0.99 | 0.97 | – | – | 8.8 | 1.10 | 0.76 | – | – |
| Left | 25 | 5.4 | (0.57–1.72) | – | – | 9.1 | (0.59–2.06) | – | – | ||
| Mucinous histology | |||||||||||
| Yes | 19 | 6.0 | 0.86 | 0.58 | – | – | 9.1 | 0.88 | 0.70 | – | – |
| No | 56 | 5.9 | (0.50–1.48) | – | – | 8.9 | (0.47–1.66) | – | – | ||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| MS status | |||||||||||
| High | 4 | 4.8 | 3.46 | 0.02 | 5.10 | 0.003 | 7.4 | 3.23 | 0.03 | 5.88 | 0.003 |
| Low | 71 | 6.2 | (1.21–9.90) | (1.71–15.04) | 9.1 | (1.13–9.27) | (1.85–18.72) | ||||
| NA | 1 | – | – | – | – | – | – | – | – | – | – |
| ECOG PS before the beginning of targeted therapy | |||||||||||
| 0 | 34 | 7.3 | 0.83 | 0.46 | – | – | 11.4 | 0.78 | 0.41 | – | – |
| ⩾1 | 42 | 5.4 | (0.50–1.38) | – | – | 7.7 | (0.44–1.40) | – | – | ||
| Peritoneal mts | |||||||||||
| Yes | 33 | 5.9 | 1.66 | 0.07 | 1.42 | 0.26 | 8.2 | 1.54 | 0.16 | – | – |
| No | 43 | 6.2 | (0.96–2.88) | (0.77–2.61) | 10.8 | (0.85–2.81) | – | – | |||
| Sites of mts | |||||||||||
| >1 | 55 | 5.7 | 1.68 | 0.08 | 1.48 | 0.23 | 8.2 | 2.07 | 0.05 | 1.98 | 0.08 |
| 1 | 21 | 7.2 | (0.95–3.00) | (0.78–2.79) | 11.1 | (1.00–4.29) | (0.93–4.20) | ||||
| ⩾8 months between mts diagnosis and beginning of targeted therapy | |||||||||||
| Yes | 50 | 6.6 | 0.55 | 0.03 | 0.52 | 0.03 | 11.0 | 0.40 | 0.004 | 0.39 | 0.01 |
| No | 26 | 5.3 | (0.31–0.96) | (0.29–0.94) | 7.2 | (0.21–0.75) | (0.19–0.79) | ||||
| Ascites at the beginning of targeted therapy | |||||||||||
| Yes | 12 | 5.4 | 1.43 | 0.28 | – | – | 7.9 | 1.81 | 0.12 | – | – |
| No | 64 | 6.0 | (0.74–2.77) | – | – | 9.2 | (0.86–3.80) | – | – | ||
| Binimetinib in combination with encorafenib and cetuximab | |||||||||||
| Yes | 13 | 5.3 | 1.91 | 0.04 | 2.12 | 0.03 | 7.8 | 2.03 | 0.04 | 2.76 | 0.006 |
| No | 63 | 6.3 | (1.03–3.56) | (1.09–4.09) | 9.4 | (1.05–3.93) | (1.34–5.68) | ||||
| Schedule of targeted therapy administration | |||||||||||
| Weekly | 41 | 5.9 | 1.34 | 0.26 | – | – | 7.7 | 1.35 | 0.32 | – | – |
| Biweekly | 35 | 6.2 | (0.80–2.23) | – | – | 9.4 | (0.74–2.45) | – | – | ||
| CEA at the beginning of targeted therapy | |||||||||||
| >5 | 57 | 5.8 | 1.10 | 0.77 | – | – | 8.9 | 1.14 | 0.71 | – | – |
| ⩽5 | 13 | 6.4 | (0.56–2.19) | – | – | 9.0 | (0.54–2.40) | – | – | ||
| NA | 6 | – | – | – | – | – | – | – | – | – | – |
| Ca 19.9 at the beginning of targeted therapy | |||||||||||
| >40 | 48 | 5.4 | 1.37 | 0.31 | – | – | 8.8 | 1.00 | 1.00 | – | – |
| ⩽40 | 18 | 6.6 | (0.74–2.53) | – | – | 9.1 | (0.51–1.97) | – | – | ||
| NA | 10 | – | – | – | – | – | – | – | – | – | – |
| DpR | |||||||||||
| ⩾15% | 39 | 7.4 | 0.56 | 0.02 | 0.55 | 0.02 | 13.3 | 0.61 | 0.08 | 0.78 | 0.42 |
| <15% | 37 | 5.3 | (0.33–0.93) | (0.33–0.92) | 9.4 | (0.34–1.07) | (0.43–1.42) | ||||
Ca 19.9, carbohydrate antigen sialyl Lewis a; CEA, carcinoembryonic antigen; DpR, depth of response; ECOG PS, Eastern Cooperative Oncology Group Performance Status; HR, hazard ratio; MS, microsatellite; mts, metastases; NA, not available.
Statistically significant p values (i.e., <0.10 and <0.05 for uni- and multivariable analyses, respectively) are highlighted in bold.
Conversely, achieving ETS (⩾20%) was not associated with either PFS (HR: 0.81, 95% CI: 0.49–1.34, p = 0.41) or OS (HR: 0.82, 95% CI: 0.46–1.47, p = 0.51) (Supplemental Figure 2A and B). Consistently, when the median value was adopted as a cut-off, patients achieving an ETS ⩾12% had the same outcome as those achieving an ETS < 12%, in terms of both PFS (HR: 0.81, 95% CI: 0.49–1.32, p = 0.38) and OS (HR: 0.87, 95% CI: 0.49–1.53, p = 0.62) (Supplemental Figure 3A and B). Similarly, no correlation between ETS, as a continuous variable, and both PFS (HR per 10% increment: 0.94, 95% CI: 0.84–1.04, p = 0.24) and OS (HR per 10% increment: 0.95, 95% CI: 0.83–1.08, p = 0.41) was detected.
Finally, relationships between DpR and DoR were explored. While RECIST response was expectedly correlated with longer DoR (HR: 0.56, 95% CI: 0.33–0.97, p = 0.04), comparable outcomes were also reported in patients achieving a DpR ⩾ 15% (HR: 0.60, 95% CI: 0.36–0.99, p = 0.04). These observations were confirmed when DpR was employed as a continuous variable (HR per 10% increment: 0.88, 95% CI: 0.79–0.98, p = 0.02).
Discussion
Although achieving an objective response is often regarded as a minimally meaningful endpoint in a purely palliative setting, among pre-treated patients with BRAF V600E -mutated mCRC achieving an early and deep tumour shrinkage may be crucial since the high burden and intrinsic aggressive behaviour of the disease make the relief of tumour-related symptoms and the prevention of patient’s clinical deterioration the primary objective of treatment.
To this regard, size-based RECIST criteria – commonly adopted in the daily practice to estimate the ability of a treatment regimen to induce tumour shrinkage – have been widely debated for their inability to fully characterize tumour response during targeted therapies, both in terms of timing and magnitude of depth over time. To explore alternative metrics that could capture different patterns of tumour dynamics potentially related to long-term outcomes, ETS and DpR have first been investigated in mCRC patients receiving first-line treatment with chemotherapy ± biologic agents.10,14 Conversely, their correlation with the outcome of patients treated with targeted agents is yet to be determined.
The present analysis is the first attempt to challenge the concepts of DpR and ETS in a retrospective, well-annotated and clinically homogeneous cohort of patients with BRAF V600E mutated mCRC receiving EC ± B as second-line treatment, which is currently the optimal positioning of this targeted approach. The prognostic accuracy of both radiological dynamic parameters in patients achieving disease control at the first radiological tumour assessment was investigated.
To the best of our knowledge, we provided the first evidence of the independent impact of both DpR and RECIST response on the clinical outcomes of this subgroup of patients. In particular, we observed that achieving a RECIST response or a DpR of at least 15% is associated with better outcomes in terms of PFS, even when adjusting for other established prognostic variables. From a clinical perspective, patients achieving a tumour shrinkage of at least 15% have a chance of disease control similar to that of patients achieving a RECIST response. This suggests that a less stringent shrinkage cut-off for response may still provide clinicians with a reassuring treatment marker of benefit from EC ± B. Further confirmation of the biological reliability of these observations is offered by the significant impact of DpR on PFS also as a continuous variable (i.e., per 10% increment in tumour shrinkage), which perfectly aligns with the underlying definition of DpR itself.
Nevertheless, in contrast with previous reports,10–12,15 we failed to demonstrate a significant association of DpR and RECIST response with OS. We hypothesize that the inherent disease aggressiveness after progression to second-line EC ± B and the overall dismal prognosis may have clouded the apparent impact of targeted therapy on the disease burden, with patients rapidly deteriorating regardless of previous response dynamics. Similarly, the limited sample size and potential confounding effect of subsequent lines of therapy might have hampered the results of these analyses.
Moreover, ETS was not associated with survival outcomes. This finding mirrors a historical pitfall of targeted therapies in the therapeutic management of mCRC probably due to the early selection of resistant clones leading to disease progression that becomes evident at the following radiological staging, irrespective of the initial dimensional reduction of lesions. Conversely, the reason why DpR analyses could intercept at least a confirmed PFS benefit in our cohort may be likely because this parameter can be measured at any timepoint and is, therefore, able to identify a subgroup of patients achieving confirmed and more durable responses, as shown in our dataset.
Focusing on the subgroup of patients who derived no benefit from EC ± B, we observed an enrichment of peritoneal involvement, corroborating the well-known aggressiveness of tumours spreading to this site. 1 Nevertheless, no definitive conclusions could be drawn regarding a potential negative predictive role since all patients in our population received the same EGFR- and BRAF- inhibitor-based treatment strategy. Moreover, among patients achieving clinical benefit, the presence of mucinous histology yielded a negative impact on tumour shrinkage, reflecting once again the well-established refractoriness to systemic treatments of mucinous mCRC.18,19
We acknowledge some limitations of our work, including the retrospective nature of the study, the lack of a blinded independent central review of CT scans, the unclear suitability of version 1.1 as compared to 1.0 of the RECIST criteria when evaluating tumour response dynamics, the not fully standardized timing of CT scans that followed standard clinical practice. Despite a relatively large sample size (considering the prevalence of BRAF V600E mutation in mCRC), hypothesis-generating conclusions can be drawn from these analyses given the overall limited number of included patients. However, it should be pointed out that the multicenter academic effort, and the consistency of activity and survival outcomes with those reported in the pivotal BEACON trial, mitigate the above-mentioned weak points and reassure on the reliability of our data. 20
Conclusion
While these results report for the first time the role of DpR as a predictor of clinical outcome in a cohort of patients with BRAF V600E mutated mCRC treated with targeted therapy, their further validation in post hoc analyses of randomized trials would be warranted.3,21
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-7-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-3-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-4-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-6-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors acknowledge all participating centres for their contributions and the GONO and ARCO Foundations for their support in the conduction of this study.
Footnotes
ORCID iDs: Guglielmo Vetere  https://orcid.org/0000-0001-6943-2897
https://orcid.org/0000-0001-6943-2897
Carlotta Antoniotti  https://orcid.org/0000-0001-6881-3188
https://orcid.org/0000-0001-6881-3188
Lisa Salvatore  https://orcid.org/0000-0003-3864-0719
https://orcid.org/0000-0003-3864-0719
Filippo Pietrantonio  https://orcid.org/0000-0002-8530-8420
https://orcid.org/0000-0002-8530-8420
Sara Lonardi  https://orcid.org/0000-0002-7593-8138
https://orcid.org/0000-0002-7593-8138
Filippo Ghelardi  https://orcid.org/0009-0001-1820-4402
https://orcid.org/0009-0001-1820-4402
Francesco Giulio Sullo  https://orcid.org/0000-0002-6478-0638
https://orcid.org/0000-0002-6478-0638
Chiara Boccaccio  https://orcid.org/0000-0003-1019-5983
https://orcid.org/0000-0003-1019-5983
Ada Taravella  https://orcid.org/0009-0000-2153-3867
https://orcid.org/0009-0000-2153-3867
Mario Scartozzi  https://orcid.org/0000-0001-5977-5546
https://orcid.org/0000-0001-5977-5546
Chiara Cremolini  https://orcid.org/0000-0002-0520-4841
https://orcid.org/0000-0002-0520-4841
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Guglielmo Vetere, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Marco Maria Germani, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Carlotta Antoniotti, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Lisa Salvatore, Medical Oncology, Università Cattolica del Sacro Cuore, Rome, Italy; Comprehensive Cancer Center, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Filippo Pietrantonio, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Sara Lonardi, Department of Oncology, Veneto Institute of Oncology IOV – IRCCS, Padua, Italy.
Maria Bensi, Comprehensive Cancer Center, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Filippo Ghelardi, Medical Oncology Department, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy.
Maria Alessandra Calegari, Comprehensive Cancer Center, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome, Italy.
Rossana Intini, Department of Oncology, Veneto Institute of Oncology IOV – IRCCS, Padua, Italy.
Alessandro Minelli, Clinical Oncology Unit, Hospital San Paolo, Civitavecchia, Rome, Italy.
Francesco Giulio Sullo, Department of Medical Oncology, IRCCS Istituto Romagnolo per lo Studio dei Tumori (IRST) “Dino Amadori,” Meldola, Italy.
Chiara Boccaccio, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Ada Taravella, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Pisa, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy.
Alberto Puccini, Medical Oncology Unit 1, IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Daniele Lavacchi, Clinical Oncology, University Hospital Careggi, Florence, Italy.
Laura Noto, UOC Oncologia Medica, Policlinico “G. Rodolico” – AOU Policlinico S. Marco, Catania, Italy.
Massimiliano Salati, Division of Medical Oncology, University Hospital of Modena, Modena, Italy.
Mario Scartozzi, Medical Oncology Unit, University Hospital and University of Cagliari, Cagliari, Italy.
Chiara Cremolini, Department of Translational Research and New Technologies in Medicine and Surgery, University of Pisa, Via Roma 67, Pisa 56126, Italy; Unit of Medical Oncology 2, Azienda Ospedaliero-Universitaria Pisana, Via Roma, 67, Pisa 56126, Italy.
Declarations
Ethics approval and consent to participate: The study was approved by local ethic committees (Oncologic Institute of Veneto, code 2017/34). Investigators retrospectively collected data on all consecutively enrolled patients from medical records after obtaining their informed consent. A waiver of consent was considered for deceased patients or those lost to follow-up.
Consent for publication: Not applicable.
Author contributions: Guglielmo Vetere: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Marco Maria Germani: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Carlotta Antoniotti: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Lisa Salvatore: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Filippo Pietrantonio: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Sara Lonardi: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Maria Bensi: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Filippo Ghelardi: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Maria Alessandra Calegari: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Rossana Intini: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Alessandro Minelli: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Francesco Giulio Sullo: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Chiara Boccaccio: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Ada Taravella: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Alberto Puccini: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Daniele Lavacchi: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Laura Noto: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Massimiliano Salati: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Mario Scartozzi: Data curation; Investigation; Resources; Validation; Writing – review & editing.
Chiara Cremolini: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the European Union – Next-GenerationEU through the Italian Ministry of University and Research under PNRR – M4C2-I1.3 Project PE_00000019 ‘HEAL ITALIA’ [grant number CUP: I53C22001440006 to CC]. The views and opinions expressed are those of the authors only and do not necessarily reflect those of the European Union or the European Commission. Neither the European Union nor the European Commission can be held responsible for them. The study was supported by GONO and ARCO Foundations (no grant number).
L. S. reported current support by the Associazione Italiana per la Ricerca sul Cancro (AIRC) under My First Grant (MFAG) No. MFAG27367 and consulting or advisory role for Pierre-Fabre, AstraZeneca, Bayer, SERVIER, Merck, Amgen, GSK, Incyte, Leopharma, MSD, Takeda. F. P. reported institutional research grants from BMS, Incyte, Agenus, Amgen, Lilly and AstraZeneca, and personal fees from BMS, MSD, Amgen, Merck-Serono, Pierre-Fabre, Servier, Bayer, Takeda, Astellas, Johnson&Johnson, Rottapharm, Ipsen, AstraZeneca, GSK, Daiichi-Sankyo, Seagen/Pfizer, Beigene. S. L. reported consulting or advisory roles for Amgen, Merck Serono, Eli Lilly, AstraZeneca, Incyte, Daiichi-Sankyo, Bristol Myers Squibb, Servier, Merck Sharp & Dohme, Astellas, and Takeda; is on the speakers’ bureau for Roche, Eli Lilly, Bristol Myers Squibb, Servier, Merck Serono, Pierre Fabre, GlaxoSmithKline, and Amgen; research funding from Amgen, Merck Serono, Bayer, Roche, Lilly, AstraZeneca, and Bristol-Myers Squibb. L. N. reported advisory board/honoraria from Astra-Zeneca, Pierre-Fabre, Bayer and travel grants from Astra-Zeneca, MSD. C. C. reported honoraria from Amgen, Bayer, Merck, Merck, MSD, Pierre Fabre, Roche, and Servier; consulting or advisory roles for Amgen, Bayer, MSD, Nordic Bioscience, Pierre Fabre, and Roche; is on the speakers’ bureau for Merck, Pierre Fabre, Servier; research funding from Bayer, Merck, Roche, and Servier. All other authors have declared no conflicts of interest.
Availability of data and materials: Datasets supporting the results of this work are available to editors, referees and readers promptly upon reasonable request.
References
- 1. Clarke CN, Kopetz ES. BRAF mutant colorectal cancer as a distinct subset of colorectal cancer: clinical characteristics, clinical behavior, and response to targeted therapies. J Gastrointest Oncol 2015; 6: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cohen R, Liu H, Fiskum J, et al. BRAF V600E mutation in first-line metastatic colorectal cancer: an analysis of individual patient data from the ARCAD aatabase. J Natl Cancer Inst 2021; 113: 1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kopetz S, Grothey A, Yaeger A, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E–mutated colorectal cancer. N Engl J Med 2019; 381: 1632–1643. [DOI] [PubMed] [Google Scholar]
- 4. Tabernero J, Grothey A, Van Cutsem E, et al. Encorafenib plus cetuximab as a new standard of care for previously treated BRAF V600E-mutant metastatic colorectal cancer: updated survival results and subgroup analyses from the BEACON study. J Clin Oncol 2021; 39: 273–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol 2023; 34: 10–32. [DOI] [PubMed] [Google Scholar]
- 6. Boccaccino A, Borelli B, Intini R, et al. Encorafenib plus cetuximab with or without binimetinib in patients with BRAF V600E-mutated metastatic colorectal cancer: real-life data from an Italian multicenter experience. ESMO Open 2022; 7: 100506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elez E, Ros J, Fernández J, et al. RNF43 mutations predict response to anti-BRAF/EGFR combinatory therapies in BRAFV600E metastatic colorectal cancer. Nat Med 2022; 28: 2162–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moretto R, Germani MM, Ros J, et al. Predictive impact of RNF43 mutations in patients with proficient mismatch repair/microsatellite stable BRAFV600E-mutated metastatic colorectal cancer treated with target therapy or chemotherapy. JCO Precis Oncol 2023; 7: e2300255. [DOI] [PubMed] [Google Scholar]
- 9. Kopetz S, Murphy DA, Pu J, et al. Molecular profiling of BRAF-V600E-mutant metastatic colorectal cancer in the phase 3 BEACON CRC trial. Nat Med 2024; 2024: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stintzing S, Modest DP, Rossius L, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol 2016; 17: 1426–1434. [DOI] [PubMed] [Google Scholar]
- 11. Cremolini C, Loupakis F, Antoniotti C, et al. Early tumor shrinkage and depth of response predict long-term outcome in metastatic colorectal cancer patients treated with first-line chemotherapy plus bevacizumab: results from phase III TRIBE trial by the gruppo oncologico del nord ovest. Ann Oncol 2015; 26: 1188–1194. [DOI] [PubMed] [Google Scholar]
- 12. Piessevaux H, Buyse M, Schlichting M, et al. Use of early tumor shrinkage to predict long-term outcome in metastatic colorectal cancer treated with cetuximab. J Clin Oncol 2013; 31: 3764–3775. [DOI] [PubMed] [Google Scholar]
- 13. Douillard J-Y, Siena S, Tabernero J, et al. Overall survival (OS) and tumour shrinkage outcomes in patients with symptomatic/asymptomatic metastatic colorectal cancer (MCRC): data from the prime study. Ann Oncol 2013; 24: iv32. [Google Scholar]
- 14. Mansmann UR, Sartorius U, Laubender RP, et al. Deepness of response: a quantitative analysis of its impact on post-progression survival time after first-line treatment in patients with mCRC. J Clin Oncol 2013; 31: 427–427. [Google Scholar]
- 15. Heinemann V, Stintzing S, Modest DP, et al. Early tumour shrinkage (ETS) and depth of response (DpR) in the treatment of patients with metastatic colorectal cancer (mCRC). Eur J Cancer 2015; 51: 1927–1936. [DOI] [PubMed] [Google Scholar]
- 16. Sagawa T, Sato Y, Hirakawa M, et al. Clinical impact of primary tumour location, early tumour shrinkage, and depth of response in the treatment of metastatic colorectal cancer with first-line chemotherapy plus cetuximab or bevacizumab. Sci Rep 2020; 10: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Castelo-Branco L, Pellat A, Martins-Branco D, et al. ESMO guidance for reporting oncology real-world evidence (GROW). Ann Oncol 2023; 34: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 18. Moretto R, Morano F, Ongaro E, et al. Lack of benefit from anti-EGFR treatment in RAS and BRAF wild-type metastatic colorectal cancer with mucinous histology or mucinous component. Clin Colorectal Cancer 2019; 18: 116–124. [DOI] [PubMed] [Google Scholar]
- 19. Wang C, Sandhu J, Fakih M. Mucinous histology is associated with resistance to anti-EGFR therapy in patients with left-sided RAS/BRAF wild-type metastatic colorectal cancer. Oncologist 2022; 27: 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tabernero J, Cutsem V, Yoshino T, et al. SO-29 Clinical characteristics, efficacy, and safety in patients receiving second- or third-line encorafenib plus cetuximab (E+C) vs control for metastatic colorectal cancer (mCRC): BEACON CRC post hoc analysis. Ann Oncol 2023; 34: S174. [Google Scholar]
- 21. Kopetz S, Grothey A, Yaeger R, et al. BREAKWATER: randomized phase 3 study of encorafenib (enco) + cetuximab (cetux) ± chemotherapy for first-line (1L) treatment (tx) of BRAF V600E-mutant (BRAFV600E) metastatic colorectal cancer (mCRC). J Clin Oncol 2021; 39: TPS3619–TPS3619. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-5-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-pdf-7-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-3-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-4-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology
Supplemental material, sj-tiff-6-tam-10.1177_17588359241299975 for Prognostic impact of depth of response and early tumour shrinkage in patients with BRAFV600E-mutated metastatic colorectal cancer treated with targeted therapy by Guglielmo Vetere, Marco Maria Germani, Carlotta Antoniotti, Lisa Salvatore, Filippo Pietrantonio, Sara Lonardi, Maria Bensi, Filippo Ghelardi, Maria Alessandra Calegari, Rossana Intini, Alessandro Minelli, Francesco Giulio Sullo, Chiara Boccaccio, Ada Taravella, Alberto Puccini, Daniele Lavacchi, Laura Noto, Massimiliano Salati, Mario Scartozzi and Chiara Cremolini in Therapeutic Advances in Medical Oncology