Abstract
Background:
Pain is a common symptom in people with dementia living in nursing homes, but cognitive impairment, including language and communication difficulties, challenges pain assessment and the ability to self-report pain.
Objectives:
This study aimed to identify and summarize patterns, advances, and gaps in research literature describing pain assessment in people with dementia living in nursing homes.
Design:
We conducted a scoping review following Arksey and O’Malley’s methodological framework.
Methods:
Systematic searches were conducted in CINAHL, Embase, MEDLINE, and PsycINFO. We included studies describing pain expressions in people with dementia and/or healthcare personnel assessment of pain in people with dementia, in a nursing home context. Charted data included demographics, methodological descriptions, ethical and quality assessment and relevant findings. Relevant findings were summarized using thematic analysis, and an overview of patterns, advances, and gaps in the research literature is presented.
Results:
Thirty-nine studies were included. The results describe three patterns: (1) pain awareness; (2) suspected pain and (3) pain mapping. Collectively, these patterns constitute a process of pain assessment, integrating pain expressions of people with dementia. Important perspectives on self-reporting are touched upon in several of the included studies, though direct descriptions of attempts to capture the residents’ own experience of pain are sparse.
Conclusion:
This scoping review provides a comprehensive description of pain assessment in people with dementia living in nursing homes as a process in three steps. We identified several knowledge gaps in the understanding of this process and provide concrete recommendations for further research. The results underpin the importance of pain assessment approaches that incorporate the flexibility to meet residents’ varying and potentially fluctuating ways of communicating pain.
Trial registration:
This scoping review is registered in the Open Science Framework (https://osf.io/8kaf5/).
Keywords: Dementia, nursing, nursing home, pain assessment, palliative care
Background
The nursing home population is characterized by a high degree of multimorbidity 1 and polypharmacy 2 and a large proportion of nursing home residents have a moderate-to-severe degree of dementia.3,4 Studies have documented a pain prevalence in people with dementia living in nursing homes of 35%–43%,2 –4 but a possible prevalence of 60%–80%. 5 Thus, pain assessment is an important part of care for this population. 6 The International Association for the Study of Pain defines pain as ‘an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage’. 7 Pain is a complex multidimensional phenomenon, influenced by physical, psychological, social, cultural, spiritual and existential factors. 8 Self-reported information is the most appropriate when assessing pain, as symptom experience is subjective and highly personal. 9 However, for people with dementia living in nursing homes, self-reporting represents a challenge due to cognitive impairment, including difficulties with language and communication.10 –12 People with dementia might express pain with different behavioural expressions or signs, such as agitation, apathy, restlessness or wandering.6,13
The use of assessment tools can supplement challenging pain assessment and support residents’ limitations in communicating verbally. Numerous observational assessment tools targeting pain in people with dementia have been developed and evaluated10,14 and systematic use of standardized observational tools has been recommended.6,15,16 However, assessment tools only capture fragments of the overall picture. 9 The ability of people with dementia to self-report is an individual resource that healthcare personnel (HCP) should engage, promote and support. 17 At some point in the dementia trajectory, extensive cognitive impairment will make self-reporting so difficult that HCP must depend on for instance observational assessment tools. 18 Nevertheless, HCP can work purposefully to use valid self-reporting for as long as possible.19,20
A scoping review by Pringle et al. exploring the complexity of pain recognition, assessment and treatment for people living in nursing homes, found a need for training and detailed guidelines for appropriate assessment of pain in the nursing home population in general. 21 However, they did not investigate people with dementia in particular, nor focused on knowledge and tools that emphasize accounting for individual variation and the ability to self-report. A systematic review by Tsai et al. investigated the effectiveness of interventions to improve pain assessment and management in people with dementia. 22 They found that comprehensive pain models improve nurses’ pain assessment and management. However, none of the included interventions emphasized a structured approach to safeguard individuals’ residual capacity to self-report, and the review was concerned about people with dementia in general and not particularly the nursing home population. 22 Hence, to the best of our knowledge, no study has reviewed the research literature with a comprehensive perspective on pain assessment in people with dementia living in nursing homes, and how the residents’ expressions of pain are integrated into the clinical practice of HCP. Thus, the aim of this scoping review was to identify and summarize patterns, advances and gaps in research literature describing pain assessment in people with dementia living in nursing homes.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) extension for Scoping Reviews checklist was used to prepare this manuscript. 23 The procedure presented in this section is derived and extended from a peer-reviewed protocol. 24 Two or more of the authors were involved in every step of the process, and methodological decisions were discussed extensively. We utilized the five first stages of Arksey and O’Malley’s methodological framework for scoping reviews, with Levac et al.’s recommendations for each stage: (1) Identifying the research questions; (2) Identifying relevant studies; (3) Study selection; (4) Charting the data and (5) Collating, summarizing and reporting the results.25,26 The method was additionally advanced by using the PAGER framework (Pattern, Advances, Gaps, Evidence for Practice and Research Recommendations). 27
Stage 1: Identifying the research questions
We searched an overview of pain assessment in people with dementia based on the clinical practice of HCP, and how it integrates pain expressions of people with dementia. To clarify the focus of the scoping review, we developed two research questions to target the broad aim of the review:
RQ1: How is the clinical practice regarding pain assessment in people with dementia living in nursing homes described in the research literature?
RQ2: How are pain expressions of people with dementia living in nursing homes described and included in the clinical practice regarding pain assessment?
Stage 2: Identifying relevant studies
A systematic search in the CINAHL, Embase, MEDLINE and PsycINFO databases was conducted. No time limit for publication was specified. We formed three main blocks in the search strategy: people with dementia (Population), pain expressions in people with dementia and/or HCP’s assessment of pain (Concept of interest) and nursing homes (Context). 28 The search strategy combines MeSH terms and synonyms within the respective blocks. When developing the search strategy, we observed that the utilization of the search terms in population and context sufficiently reduced the search results, enabling us to apply broad terms for the concept of interest, preventing the exclusion of relevant studies. The search strategy went through several rounds of revision and quality assurance in collaboration with experienced librarians and the full search strategy is available as Supplemental Material (Additional File 1). The main search was carried out in December 2022 and updated in May 2024. The reference lists of the included studies were manually searched.
Stage 3: Study selection
Inclusion and exclusion criteria are presented in Table 1.
Table 1.
Eligibility criteria guiding study selection.
| Eligibility criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Source | Peer-reviewed journals Published in English, Norwegian, Swedish or Danish |
Grey literature |
| Population | Healthcare personnel (such as registered nurses, assistive personnel and doctors) performing care for people with dementia in nursing homes AND/OR People with a diagnosis of dementia (e.g. patients, service users or residents), including people with a researcher diagnosis of dementia (e.g. use of the Mini-Mental State Examination 29 ) |
Mixed samples (e.g. mild cognitive impairment/cognitive impairment + dementia) Cognitive impairment not caused by dementia Mixed sample where results about people with dementia are not specifically defined in the results |
| Context | Nursing home | Mixed context where results about nursing homes are not specifically defined in the results |
| Concept | Research literature describing: Pain expressions in people with dementia living in nursing homes AND/OR Healthcare personnel’s assessment of pain in people with dementia living in nursing homes |
Studies that exclusively focus on development and psychometric testing of assessment tools |
| Study design | Primary research, all study designs | Editorials, commentaries or letters, discussion papers, opinion papers, literature reviews and nonempirical studies |
SE and CKO independently reviewed the first 300 abstracts prior to discussing and reaching consensus on the discrepancies. CKO solely reviewed the remaining abstracts. Rayyan 30 was used as a tool for team-based screening, and sources that subsequently matched the inclusion criteria were obtained for full-text assessment. If the relevance of a study was unclear from the title and abstract, the full article was reviewed. All full texts were independently assessed for eligibility by two researchers. Several calibration meetings were held during the selection process, and disagreements were discussed until consensus was reached.
Stage 4: Charting the data
Data from 13 studies, randomly selected among the included, were extracted and reviewed by two researchers (CKO and SE) to determine consistency in the understanding of the studies’ compatibility with the research questions and aim. Data from the remaining studies were charted by CKO alone. The final data-charting form was reviewed and approved by all authors, including demographics, aim and research questions, methodological descriptions and relevant findings. Levac et al. argue the importance of quality assessment in scoping reviews to achieve information on the quality of existing knowledge. 26 Therefore, all authors made an informal assessment of quality during the full-text review and noted any quality deficiencies. Study quality was then assessed using the Mixed Methods Appraisal Tool (MMAT). 31 SE and CKO independently assessed 10 studies, and CKO solely assessed the remaining. Reflecting the rationale for quality appraisal in scoping reviews, no studies were excluded based on the appraisals. 26 The importance of ethical awareness in reviews has been emphasized. 32 In response, we conducted an ethical mapping inspired by Westerdahl et al. 33 The ethical mapping considered the description of ethical approval, informed consent, data protection, financial support and conflict of interest.
Stage 5: Collating, summarizing and reporting the results
In this stage, we prepared an overview and summary of the extracted information, which is presented in the results section. The review includes both quantitative and qualitative data. The quantitative results have been transposed into descriptive phrases, and the descriptive summary is formulated in text. Our results are described and discussed in line with the PAGER framework. 27 Hence, a descriptive thematic analysis of the key findings, was conducted to identify patterns in the research literature; reading, rereading and coding the data, then generating initial themes, which were reviewed and refined several times. 34 As a scoping review intends to summarize, not synthesize, the results are presented descriptively on a semantic level, using the same terms as those used in the referenced studies where feasible. 25
Results
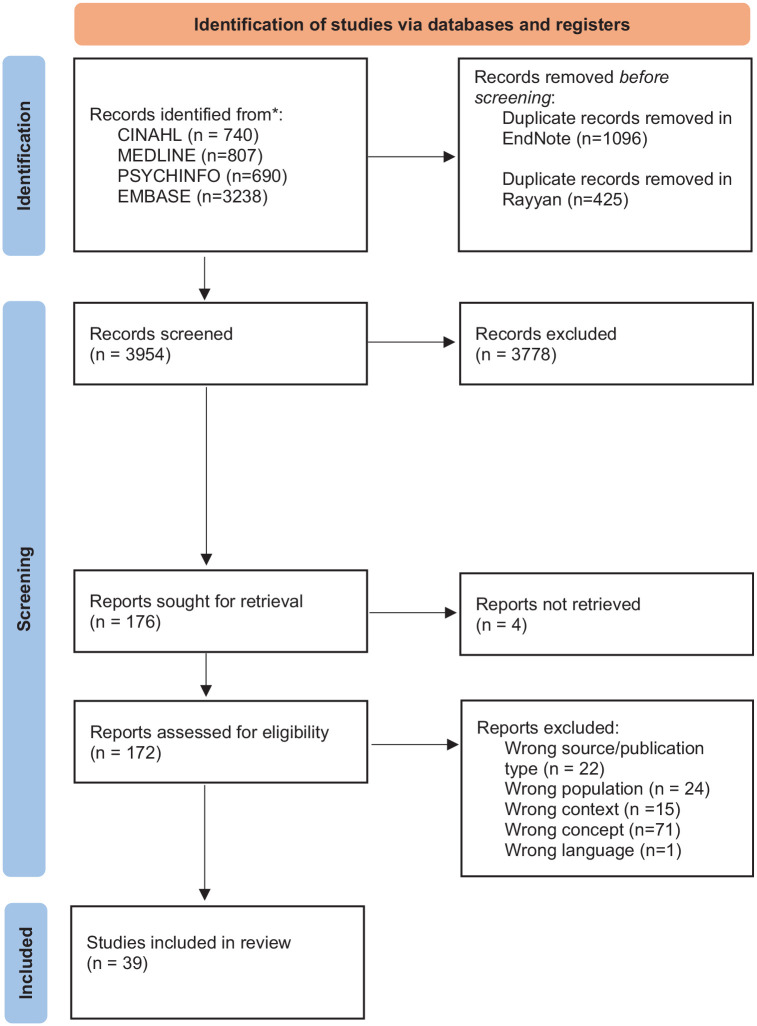
A total of 3954 unique records were assessed by title/abstract after duplicates were removed. The selection process is documented in a PRISMA flowchart (Figure 1). 35
Figure 1.
Overview of study selection process.
*The updated search (May 2024) identified a total of 446 records from the four databases.
Thirty-nine studies met all the inclusion criteria. Twenty-three had a quantitative approach, seven a qualitative approach and nine had a mixed or multiple-method approach. The studies were published between 2002 and 2024, in Asia (n = 8), Europe (n = 9), North America (n = 19) and Oceania (n = 3). The studies constitute a total sample of 1174 HCP and 37,174 people with dementia. One of the studies included 34,658 people with dementia. 36 Nursing staff in nursing homes include several different groups of HCP, with substantial international variations in title, level of education and tasks.37,38 In our study, we use the collective term HCP, including the diverse array of care providers employed in nursing homes. Where relevant in the presentation of results, we distinguish between registered nurses and assistive personnel, such as certified nursing assistants, nurse assistants and care aides. 37 Limitations identified with MMAT were mainly related to limited descriptions of methods. In relation to limitations in ethical assessment declaration of adequate data protection was the most common. For an overview of quality- and ethical appraisal, see Supplemental Material (Additional Files 2 and 3). An overview of the included studies is presented in Table 2. An extended version of Table 2, including relevant findings, is available as Supplemental Material (Additional File 4).
Table 2.
Presentation of studies included.
| First author, year, country | Aim/objectives | Participants | Design/method (including assessment tools) |
|---|---|---|---|
| Alexander, 2005, United States 54 | Develop, implement and evaluate a system for pain assessment and monitoring | 41 residents with dementia, 24 from secure unit 17 from open unit |
Quantitative Pilot study, nonexperimental design Coloured Visual Analogue Scale (CVAS) |
| Andrews, 2019, Australia 43 | Investigate the quality and completeness of pain documentation and assess the extent to healthcare personnel are engaged in documentation processes | 114 residents with moderate-to-severe dementia, across 4 facilities. 169 pain episodes | Quantitative Descriptive design Review of medical records |
| Apinis, 2014, United States 66 | Examine the agreement between the interdisciplinary evaluation and the validated observational pain tools PAINAD and PACSLAC | 67 residents with advanced dementia and moderate-to-severe communication disability, from 6 different nursing home wards | Quantitative Cross-sectional Pain Assessment in Advanced Dementia (PAINAD) Pain Assessment Checklist for Seniors with Limited Ability to Communicate (PACSLAC) |
| Burns, 2015, United Kingdom 56 | (1) Explore nurses’ knowledge about pain assessment for people with dementia, (2) determine the factors that may influence their knowledge and attitudes towards pain assessment, (3) identify nurses’ level of training and education in pain and dementia, (4) explore the perceived barriers of effective pain assessment | 32 registered nurses working in nursing home, regularly caring for people with dementia | Quantitative Cross-sectional survey design Questionnaire, including open-ended questions |
| Chang, 2011, South Korea 44 | To clarify and conceptualize pain identification in people with dementia by nurses | 13 nurses from 3 nursing homes | Quantitative Concept development Individual interviews |
| Chen, 2015, Taiwan 72 | Investigate the reliability and validity of self-reported pain across groups with different degrees of cognitive function, and to determine the important predictors of self-reported pain intensity in four cognition groups | 341 residents diagnosed with dementia from 12 dementia special care units, and 50 registered nurses Control: 73 cognitively intact residents, from 2 long-term care facilities |
Quantitative Cross-sectional Multifaceted measures to validate residents’ pain reports Verbal Descriptor Scale (VDS) Doloplus-2 |
| Chen, 2010, Taiwan 63 | Validate registered nurses’ and nurse assistants’ reports in assessing present pain and to investigate potential influencing factors | 304 residents with dementia from 6 dementia special care units 15 registered nurses, 21 nurse assistants |
Quantitative Prospective study Doloplus-2 |
| Closs, 2003, United Kingdom 65 | (1) Assess the usability of a range of approaches to pain assessments; (2) identify and develop appropriate verbal and/or nonverbal pain assessments in varying levels of cognitive impairment; (3) relate, where possible, the severity of cognitive impairment to the most appropriate methods of assessment | 113 nursing home residents | Quantitative Cross-sectional Verbal Rating Scale (VRS) Numerical Rating Scale (NRS) Colour Pain Analogue Scale (CS) Faces Pain Scale (FS) Mechanical Visual Analogue Scale (MVAS) |
| Cohen-Mansfield, 2008, United States 64 | Compare pain assessments using self-report, informant rating and observational assessments | 153 nursing home residents with dementia from 4 nursing homes 84 staff members |
Quantitative Cross-sectional Functional Pain Scale Present Pain Intensity Scale Verbal Descriptor Scale Global Pain Assessment Scale Pain Assessment for Dementing Elderly (PADE) Pain Assessment in Noncommunicative Elderly (PAINE) Pain Assessment in Advanced Dementia (PAINAD) The checklist of nonverbal pain indicators (CNPI) Observational Pain Behaviour Assessment Instrument (OPBAI) |
| Cohen-Mansfield, 2002, United States 45 | (1) To identify the behaviours and other observable indicators that are perceived by nurses to be manifestations of pain, (2) determine what cues are used to differentiate pain from other causes of unusual behaviour, (3) assess nurses’ perceptions of the prevalence and importance of specific indicators of pain, (4) validate the perceptions of nursing staff members concerning the applicability of the pain indicators provided in the previous studies, (5) to examine their perceptions of their own ability to identify pain in this population | 72 staff members from 3 nursing homes | Mixed or multiple methods Individual interviews, survey and focus groups |
| Cohen-Mansfield, 2002, United States 60 | Examine the reliability and validity of geriatricians’ assessments of pain | 79 nursing home residents. 31 with mild/moderate cognitive impairment and 48 with severe cognitive impairment 2 geriatricians |
Quantitative Cross-sectional |
| Corbett, 2016, United Kingdom 40 | Explore the current landscape of pain management in people with dementia living in nursing homes | 12 healthcare personnel, including junior care assistants, senior carers, nurses and care home managers | Mixed or multiple methods Triangulation of stakeholder consultation and quality review of pain management Focus groups with care home staff |
| Ersek, 2011, United States 69 | Explore whether a combination of pain indicators would be significantly better in predicting self-reported pain intensity than any single pain indicator | 326 residents, from 24 nursing homes | Quantitative Chart review, resident interviews, surrogate reports from certified nursing assistants Iowa pain thermometer Checklist for nonverbal pain indicators |
| Ford, 2015, United States 55 | Examine ethnic differences in the presentation and intensity of nonverbal pain behaviours among African Americans, Caucasians and Hispanics | 28 residents with moderate-to-severe dementia and pain-related diagnosis, from 4 nursing homes 6 certified nursing assistants |
Quantitative Cross-sectional Noncommunicative Patients Pain Assessment Instrument (NOPPAIN) |
| Gilmore-Bykovskyi, 2013, United States 46 | (1) Examine how nurses make decisions to pharmacologically treat pain, as well as identify the conditions that influence treatment decisions, (2) identify conditions that influence nurses’ actions related to pain management | 13 nurses from four facilities (3 licensed practice nurses and 10 registered nurses) | Qualitative In-depth interviews Grounded dimensional analysis |
| Kaasalainen, 2007, Canada 39 | Explore the decision-making process of pain management of physicians and nurses and how their attitudes and beliefs about pain affect their decisions about prescribing and administering pain medications | 24 registered nurses and 33 registered practice nurses from 4 nursing homes 9 physicians |
Qualitative Grounded theory Semi-structured, individual interviews |
| Karlsson, 2012, Sweden 41 | Interpret certified nursing assistants’ perception of pain | 12 certified nursing assistants working in dementia care | Qualitative Hermeneutic design Individual interviews |
| Lautenbacher, 2017, The Netherlands 47 | Identify which facial descriptors are used by caregivers to evaluate and influence their diagnostic decision-making process when assessing pain | 284 residents with dementia (mostly advanced stage) from 79 nursing homes | Quantitative Survey Questionnaire |
| Liu, 2012, China 76 | Report the development and implementation of an observational pain assessment protocol and its impacts on pain management. To report the opinions of the nursing home staff about the protocol | 11 healthcare personnel (8 nursing assistants, 2 registered nurses and 1 physiotherapist) 30 residents |
Mixed or multiple methods Intervention: Pre-/posttest Group interviews Chinese version of Pain Assessment in Advanced Dementia (C-PAINAD) |
| Lundin, 2021, Sweden 48 | Describe the experiences of nurses in caring for people with advanced dementia and pain at the end-of-life | 13 registered nurses from 12 nursing homes | Qualitative Descriptive explorative design Individual semi-structured interviews |
| Manfredi, 2003, United States 57 | (1) Identify a clinical condition consistently described as painful by residents who were able to verbally communicate the experience of pain (2) Assess the reliability and validity of facial expressions as pain indicators in residents with severe dementia undergoing a painful procedure |
39 residents with decubitus ulcers able to reliably answer questions about pain 9 residents with dementia and decubitus ulcers |
Quantitative |
| Mezinskis, 2004, United States 49 | Examine which formal and informal methods of pain assessment nurses and caregivers use | From 14 long-term care facilities: Sample A was 160 direct caregivers (35 registered nurses, 41 licensed practice nurses and 84 certified nursing assistants) Sample B was 307 residents in dementia units, with chronic painful illnesses |
Quantitative Survey/document analysis Sample A: Questionnaire Sample B: Chart review |
| Monroe, 2015, United States 50 | Assess nursing home personnel’s cues and practices to identify and alleviate pain | 29 healthcare personnel, including registered nurses and licensed practice nurses with direct care responsibilities, from two long-term care facilities | Qualitative Exploratory study Focus group interviews |
| Monroe, 2014, United States 74 | Determine if a diagnosis of dementia influenced pain self-reports and pain medication use | 52 nursing home residents able to self-consent, including 20 people with dementia | Quantitative Between groups, cross-sectional Discomfort Behaviour Scale |
| Monroe, 2012, United States 58 | Use medical records to assess advanced cancer pain at the end-of-life | 48 records from 9 nursing homes 43 people with Alzheimer’s dementia (90%), 4 people with vascular dementia (8%) and 1 person with Lewy body dementia (2%) |
Quantitative Retrospective between groups cross-sectional design Retrospective chart audit |
| Nakashima, 2019, United States 36 | Compare pain interventions (including assessment) between nursing home residents with and without dementia | 50,673 nursing home residents, 34,658 with dementia | Quantitative Cross-sectional |
| Neville, 2006, Australia 71 | A needs analysis of the pain management skills of regional nurses caring for older people with dementia | 197 staff members (120 unlicensed nurses, 19 enrolled nurses and 55 registered nurses) | Quantitative Survey Questionnaire |
| Parkman, 2020, United States 51 | (1) Explore the relationship between two observational pain scales, expressed need-driven behaviours and likelihood of medication administration, (2) examined nurses’ perceptions regarding ease of and barriers to use of the scales | 28 nursing home residents with dementia 4 registered nurses and 2 licensed practical nurses |
Mixed or multiple methods Abbey Pain Scale The Pain Assessment in Advanced Dementia (PAINAD) |
| Peisah, 2014, Australia 52 | Explore attitudes and processes relating to pain assessment and management | 20 staff members (10 registered nurses and 6 nurse assistants) | Quantitative Descriptive design A topical survey typology with semi-structured interviews |
| Rababa, 2019, Jordan 75 | Examine the relationship among comorbid burden, ability to self-report symptoms, severity of dementia and patient outcomes of pain and agitation | 78 nursing home residents with dementia | Quantitative Descriptive correlational design Discomfort-DAT |
| Rababa, 2018, Jordan 70 | Examine temporally based relationships between change in behaviour, the nurses’ level of certainty regarding pain, assessment scope and outcomes of pain | 76 nursing home residents with dementia and known pain or a known pain diagnosis | Quantitative Descriptive correlational design Discomfort-DAT |
| Rababa, 2018, Jordan 68 | Examine the associations of pain assessment scope, nurses’ certainty, patient outcomes, and cognitive and verbal characteristics | 76 nursing home residents with dementia and known pain/known pain diagnosis | Quantitative Descriptive correlational design Discomfort-DAT |
| Rostad, 2018, Norway 59 | Assess the effectiveness of regular pain assessment on analgesic use and pain score | 112 residents with dementia and unable to self-report, from 16 nursing homes that did not routinely use a pain assessment tool | Quantitative Single-blinded, parallel cluster randomized controlled trial Doloplus-2 |
| Scherder, 2004, The Netherlands 73 | Compare the assessment by nursing assistants of pain experienced by residents with the residents’ own evaluation | 20 residents with Alzheimer’s dementia and 17 residents without dementia, from 2 nursing homes. Both groups with chronic painful conditions | Quantitative Case–control study Checklist for Nonverbal Pain Indicators (CNPI) Coloured Analogue Scale (CAS) |
| Sloane, 2007, United States 53 | To describe the amount of staff time spent in care provision of morning care and the sources of discomfort and pain that were identified | 17 nursing home residents with dementia who were likely to have chronic pain | Mixed or multiple methods Study and analysis of 51 videotaped morning care and care plans |
| Vitou, 2022, France 61 | To analyse whether a diagnosis label of Alzheimer’s disease or the stage of the disease may bias pain assessment scores and empathic reactions of healthcare staff in nursing homes | 152 certified nursing assistants From 19 nursing homes |
Quantitative Experimental between subjects’ design Visual Analogue Scale (VAS) Algoplus |
| Vitou, 2021, France 62 | (1) Characterize pain assessment behaviours; (2) compare assessments with individuals with no professional experience in the field of care (controls) and (3) explore the impact of demographic, psychological and socio-professional determinants on pain assessment | 50 certified nursing assistants from 5 nursing homes Controls: 96 adults living in the community |
Quantitative Experimental between subjects’ design Visual Analogue Scale (VAS) Algoplus |
| Yang, 2024, China 42 | To elucidate the methodologies employed by nursing assistants in identification and management of pain | 17 nursing assistants | Qualitative Phenomenological design Semi-structured individual interviews |
| Zahid, 2020, Canada 67 | (1) Evaluate whether pain assessment frequency improved with the use of the tablet app compared with that for the paper-and-pencil method of administration of the PACSLAC-II, (2) evaluate the impact of each method of administration of the PACSLAC-II on frontline staff stress and burnout levels, (3) obtain the perspectives of healthcare personnel on each method of administration | 121 staff (33 registered nurses and 88 special care aides) | Mixed or multiple methods Case series design, quasi-experimental and exploratory design Pain Assessment Checklist for Seniors with Limited Ability to Communicate II (PACSLAC-II) |
We identified three patterns in the thematic analysis in which HCP are assessing pain in people with dementia living in nursing homes: (1) pain awareness; (2) suspected pain and; (3) pain mapping. Collectively, these patterns constitute a process of pain assessment, which integrate pain expressions of people with dementia. The following presentation of the results is conclusively summarized in an overview of patterns, advances and gaps (Table 4).
Table 4.
Patterns, advances and gaps in the included studies.
| Patterns | Advances | Gaps |
|---|---|---|
| Healthcare personnel’s clinical practice in pain assessment | ||
| Pain awareness Suspected pain Pain mapping |
How uncertainty around pain experience affects pain management processes Observational strategies to detect signs of pain, and the importance of knowledge regarding baseline behaviour HCP perspective on how people with dementia express/self-report pain The importance of continuity in information between shifts and healthcare personnel |
Knowledge on • the promotion of systematic individualized pain assessment and how to place the results of assessment tools into a larger context • The application of pain assessment tools in clinical practice (outside the context of participation in studies testing given tools) • how to support people with dementia in communicating their subjective experience of pain • how to assess the residual ability of people with dementia to self-report • how to integrate different pain assessment strategies at different degrees of residual capacity to self-report • how people with dementia experience pain assessment processes in nursing homes • prerequisites for relational continuity in relation to pain assessment • systematic approaches to ensure informational continuity throughout the pain assessment processes • strategies of systematic trial-error where this is unavoidable |
| (. . .in response to) Pain expressions in people with dementia | Signs of pain (observable, nonverbal) Descriptions of self-reporting focus on the presence and severity of pain Importance of individualized pain assessment |
Knowledge on • self-reports of aspects other than presence and severity of pain • cultural differences in pain expressions in people with dementia • the role of relatives in pain assessment |
Pattern 1: Pain awareness
HCP must actively search for pain in people with dementia. 39 ‘Pain awareness’ concerns how HCP are aware that pain might occur, as well as their alertness, knowledge and understanding of the situation. To discover pain, it must be prioritized, and it requires a combination of familiarity with the resident and professional expertise with pain and dementia.40,41 Pain awareness can also have a preventive and protective aspect, for example by checking positions to avoid painful pressure ulcers.41,42
Pattern 2: Suspected pain
‘Suspected pain’ refers to the moment when HCP recognize that a person with dementia might be in pain. The included studies describe several sources of suspected/recognized pain: (a) observation of behavioural changes39 –54; (b) verbal self-reports43,44,48,53,54; (c) observation of signs of pain 41,42,44,45,54 and (d) known indicators of pain.44,46,49 This categorization is based on the conceptual model of how HCP engage in identifying and deciding whether to treat the residents’ pain, developed by Gilmore-Bykovskyi and Bowers. 46 The model describes how the presence or absence of an obvious reason for pain, influences HCP’s levels of certainty regarding pain. Behavioural change in people with dementia might result in suspected pain but with a high degree of uncertainty – especially in the absence of an obvious reason. 46 Gilmore-Bykovskyi and Bowers present three groups of behavioural indicators: behaviours suggestive of pain (e.g. repetitive rubbing of a body part), behaviours highly suggestive of pain (e.g. intense guarding with care) and general behaviour changes (e.g. withdrawal or agitation). 46 Ford et al. compared behavioural pain expressions across different ethnic groups and found no significant differences, only the words used to describe pain. 55
Observable signs of pain are emphasized, and the most described are (a) behavioural changes that differ from baseline behaviour42,44 –46,49,52,56; and (b) facial expressions of pain.41,42,45,47,52 –54,57 ‘Knowing the person’ is highlighted as a crucial prerequisite for recognizing changes from baseline, to identify unique individual pain behaviours and detecting and interpreting pain-related changes in people with dementia.40 –42,44 –46,48,52 Family members are described as important resources,39,48,56 as they may be familiar with the residents’ earlier behaviours, and capable of interpreting their present behaviours. 48 However, though HCP can distinguish behavioural changes from baseline, the behavioural changes might have other causes.46,50,51 As Alzheimer’s dementia progresses, observable pain behaviours might diminish and the observation of pain behaviour will be even more difficult. 58
The different sources of pain identification reported by HCP in the included studies are presented in Table 3.
Table 3.
Sources of pain recognition reported by healthcare personnel.
| Sources of pain recognition46 | Examples as described in included studies |
|---|---|
| Observation of behavioural changes | |
| Unspecified39,40,43,50 –52 | |
| Behaviours suggestive of pain40 –42,44 –50,52 | Grimacing, repetitive rubbing or touching body parts, clenching jaw or fist, bracing body part, changing position, reluctance to move, unusual body movements, moaning, wincing when moved, grunting, whining, sudden limping, tossing and turning in chair or bed, moving head back and forth, body stiffens, sad eyes, dark eyes, empty look, mouth movements, hanging mouth, frowning, narrowed eyes, closed eyes, raising upper lip, opened mouth, tightened lips, empty gaze, seeming disinterested, teary eyed, looking tense, looking sad, looking frightened, curled up position |
| Behaviours highly suggestive of pain41,44 –46,48 –50,52 | Crying, intense guarding, suddenly inability to raise arms, painful look, screams, groaning |
| General behaviour changes40 –42,44 –46,48,49,51 | Withdrawal, restless behaviour, agitation, moodiness, irritability, pacing, sleep disturbance, refusal to eat, depression, unusual quietness, negative vocalizations, decreased participation in activities, changes in sociability, desire to be left alone, anxious behaviour, alterations in daily activities |
| Resident self-report | |
| Verbal self-report43,44,46,48 | Spontaneous self-report, resident response to staff asking about pain |
| Observation of signs of pain | |
| Visible signs of pain41,42,44,45 | Skin colour, oedema in joints, blood on diaper or clothing, changes in vital signs, trembling, falls, limited range of motion, perspiration, contractions |
| Known indicators of pain | |
| Visible/obvious reasons for pain44,45 | Surgery, fracture, terminal |
| Nonvisible/not obvious reason for pain44,46,49 | Knowledge of painfull diagnosis, increase in blood pressure |
Pattern 3: Pain mapping
Pain mapping is complex and refers to the specific and more comprehensive part of pain assessment. Pain mapping can be both regulatory-driven (i.e. ‘on admission’) or patient-driven (i.e. ‘the person appears to be in pain’), 52 where HCP builds upon their suspicion of pain, and/or attempts to determine the underlying cause of the residents stated pain or behaviour that suggests pain. One study found that pain assessment driven by regulation was prevalent. 52 The state of knowledge is unclear, but there is insufficient evidence to conclude that regular pain mapping using a pain assessment tool is not clinically relevant. 59
There are high validity, reliability and agreement between physicians in the pain assessment of people with dementia with mild/moderate levels of cognitive impairment, but these dropped in the assessment of residents with severe cognitive impairment. 60 Assistive personnel assigned less pain intensity and affective distress to the person in pain when the person was described as severely ill with Alzheimer’s dementia, compared to when the stage of dementia was not stated. 61
The perspective of pain mapping in dementia will further be described according to: (a) pain assessment tools; (b) a combination of pain mapping strategies; and (c) self-reporting.
Pain assessment tools
Several studies report the use of pain assessment tools as part of pain assessment in clinical practice.40,53,37,45,46,47,49 However, the included studies provide limited descriptions of the relationship between the clinical use of assessment tools, degree of dementia and residual capacity to self-report. There are significant differences in HCP use of standardized assessment tools, both interpersonal62,63 and between different types of assessment tools. 64 Registered nurses and assistive personnel using standardized assessment tools largely agreed on the presence of pain at the moment but agreed to a lesser extent on how often pain occurred in the past week. 63 One study reported poor agreement between tools based on observation compared to self-reports. 64 Registered nurses reported the use of assessment tools to a greater extent than assistive personnel. 49
A study by Closs et al found that two-thirds of the participants with moderate or severe dementia were able to use simple self-report assessment scales. 65 Many of those who when asked, claimed to have no pain indicated that they had pain when they used pain scales. 65 In contrast, another study found that participants with moderate-to-severe dementia unable to use verbal tools often could use nonverbal tools. 54
Combination of pain mapping strategies
Several of the included studies describe a combination of strategies, where HCP assess and integrate information from various sources including review of medical records40,50, physical examination44,45,66,67, medical history 44 and intuition. 48 The scope of registered nurses’ pain assessment increased with severe dementia and a high degree of uncertainty. 68 A study investigating the combination and weighting of different sources in pain assessment, found that mapping multiple indicators of pain was not necessarily more appropriate than one single proxy report. 69 Team meetings with interdisciplinary evaluations of pain for people with dementia report less pain than assessment with standardized observational tools. 66
Several of the included studies described trialling different combinations of pharmacological and nonpharmacological interventions targeting various potential underlying causes of changed behaviour, including pain.39,44,46,50,68 This is described as ‘trial and error’, and the goal is that the person with dementia will return to baseline functioning with the reduction or elimination of their behavioural symptoms.44,46
Self-reporting of pain
The use of self-reports was highlighted as the most meaningful, when possible. 40 At the same time, several of the included studies describe the difficulties HCP experience when communicating with people with dementia, and this is one of the major barriers to recognizing and assessing pain in the group.39,41,42,46,48,51 There are different points of view when it comes to self-reporting of people with dementia. Two studies stated that a large proportion of the included people with dementia were unable to verbally self-report,64,70 and 78% of HCP believed that people with dementia could not accurately provide a self-report of pain, 56 another study (44%) stated that people with dementia could verbalize at least ‘some pain’ if their pain management were ineffective. 71 Three of the included studies compared HCP reports of pain with the residents’ reports of pain, and the findings are contradictory.63,72,73 People with dementia reported higher prevalence, 63 intensity and frequency 72 compared to HCP. On the other hand, assistive personnel is found to score pain as significantly higher than the people with Alzheimer’s dementia themselves. 73 One study found no significant differences between the prevalence of self-reported pain symptoms when comparing people with and without dementia. People with dementia reported higher pain intensity, were less likely to tell HCP about their pain, and fewer reported that HCP asked about their pain, compared to people without dementia. 74
Two studies found that a large proportion of the included people with dementia were unable to verbally self-report.64,70 Cohen-Mansfield found significantly higher scores on the Mini-Mental State Examination 29 in the responders to self-report questions, than in non-responders. 64 Chen and Lin’s findings indicate that people with dementia with up to a moderate level of cognitive impairment may be able to self-report, despite limitations in communication and self-awareness. They highlight that HCP should accept the pain reports of people with dementia to promote adequate pain management, and in addition, use a multifaceted approach for those in the later stages of dementia. 72
Integrating the patterns into a coherent process of pain assessment
Collectively, the three identified patterns constitute a pain assessment process.
This process is largely characterized by uncertainty due to cognitive impairment affecting the person’s ability to verbally express pain, and difficulty establishing certainty regarding the underlying causes of pain.39,46,48,50,51,68,75 Significantly fewer pain assessments are carried out on people with dementia in nursing homes, compared to people without dementia. 36
The process of pain assessment involves different HCP disciplines and roles.39,40,42,52 To connect the various aspects, the process relies on continuity in relation to communication and information.39,40,52,60 Pain assessment is described as a complex network of communication channels in the nursing home, and communication between different disciplines is problematized in several studies.40 –42,52,67 Poor or inaccurate documentation and communication could be a barrier to effective pain assessment. 51 Andrews et al. found that 83% of the pain episodes investigated contained documentation only about the problem and the intervention. 43 The use of a pain management protocol may address these challenges, as it may provide a common language for staff to talk about pain across disciplines and help to strengthen the communication of pain observations.67,76 The use of an electronic systematic pain assessment protocol to help HCP identify visual patterns in pain scores over time has been promoted. This could also be a faster and easier way to store and access data. 67
Summary of results
We identified three patterns describing the current state and advances of research concerning the pain assessment process in people with dementia living in nursing homes: (1) pain awareness; (2) suspected pain and (3) pain mapping. Patterns, advances and gaps in the research literature concerning pain assessment in people with dementia living in nursing homes are summarized in Table 4.
Discussion
In this review, we aimed to identify and summarize patterns, advances and gaps in research literature describing pain assessment in people with dementia living in nursing homes. We included and examined 39 studies, finding that pain assessment is described as a process, facilitated by uninterrupted information transfer. We identified perspectives of importance on self-reporting, but direct descriptions of self-reporting and attempts to capture the patient’s own experience of pain were sparse.
Evidence for practice and research recommendations
Our findings highlight and illuminate aspects of pain assessment that are important to reflect on in clinical work with this patient group. Bradbury-Jones states that the evidence for practice using the PAGER framework also targets a broader understanding of the practice field, involving stakeholders beyond clinicians (e.g. researchers). 27 Evidence to inform practice and research recommendations seen in such a context can contribute by providing concrete recommendations for further research responding to identified knowledge gaps. 27 The gaps that need to be addressed are presented in Table 4, and the most prominent are elaborated and discussed in this section.
People with dementia’s limited ability to verbally communicate, constitute major challenges and this is highlighted in the literature as a problem that must be addressed. 19 Hence, the literature is focused on objective assessment alternatives when self-reporting cannot be carried out: these alternatives include the development, testing and implementation of assessment tools. 10 However, there are nuances between ‘fully capable of self-reporting’ and ‘not at all capable of self-reporting’. Our findings show limited descriptions of how to support people with dementia to communicate their subjective experiences of pain; how HCP can assess the ability/residual ability for self-reporting and how to integrate different pain assessment strategies at different degrees of the residual capacity of the target group to self-report. Self-reporting is mainly described as whether or not the person is able to confirm or deny the presence of pain and to describe the severity of the pain. Descriptions of self-reported pain in the included studies are largely quantified. Qualitative descriptions of the subjective experience of pain are not emphasized, either in those with mild or moderate dementia. Quantitative pain measures are vital in pain management but often overlook important attributes of the subjective experience, such as personal context and meaning, which can have a major impact on the experience of pain. 9 There is a knowledge gap regarding the promotion of systematic individualized pain assessment and how to place reported pain, the results of assessment tools or clinical examinations into a larger context. Wideman highlights the need for assessment models that specifically emphasize how to address subjectivity related to pain in general. 9 Our results show that this might be even more challenging in people with dementia. Nevertheless, we claim that models of pain management in this group and context can have the flexibility to meet individual residents’ varying and potentially fluctuating ways of communicating pain, as well as their individual need for assessment, intervention and evaluation.
The results describe ‘trial and error’ strategies: the use of interventions as part of an assessment to find the underlying cause of behavioural changes. Due to risk of delayed treatment, ‘trial and error’ should follow a thorough pain mapping. However, we found that pain mapping will not eliminate all uncertainty, and ‘trial and error’ can be appropriate for instances where uncertainty cannot be eliminated. There is a lack of knowledge concerning strategies for systematic implementation and evaluation of ‘trial and error’, where this is unavoidable. Sandvik et al. discuss how people with dementia receive painkillers as much as or more than people without dementia, in contrast to an earlier trend of undertreating pain due to assessment challenges. 77 People with dementia in nursing homes constitute a population with a high degree of multimorbidity that is vulnerable to pharmacological side effects.1,78 The evaluation of implemented measures is therefore particularly important. These factors highlight the importance of further developing and implementing models that facilitate the systematic evaluation and informational continuity of any pain intervention: both as a result of a specific pain assessment or ‘trial and error’.
We found that pain awareness in particular was described as having a preventive function. Systematic work to prevent pain in this population is described in the included studies to a limited extent. Pain prevention is outside the scope of this review, but in a patient group with such a high prevalence of pain, prevention should be a priority in both clinical practice and future research. 10 Liao et al. state that there is a lack of knowledge about dementia and pain among HCP, which can be solved with easy access to ongoing training. 79 Although competence-enhancing measures were outside the scope of this review, we acknowledge this as an important topic that should be highlighted in further studies.
Strengths and limitations
An important strength of this study was the guidance by a peer-reviewed protocol. 24 We used an established methodology25,26 and analysis method, 34 as well as standardized reporting guidelines. 23 To ensure transparency, the review process is described in detail.
This study has some limitations. First, searches, screening and selection of studies are open to error or bias. We acknowledge that this review may not have captured all relevant material, as we did not include grey literature, nor studies published in other languages than English and the Nordic languages. The search strategy resulted in a large volume and wide range of evidence. Another team of researchers might have included and chosen to emphasize other areas of the research field.
We conducted an assessment of quality and ethical standards. Levac et al. argue how quality appraisal is an important aspect of mapping and identifying gaps in the existing literature, giving comprehensive information on the nature and extent of those gaps. 26 The MMAT guidelines are standardized. 31 However, the appraisal is vulnerable to bias, as the result depends on the interpretation of the researcher. We sought rigour by involving all members of the research team in the quality appraisal. Studies with low methodological quality are not excluded in this scoping review, following methodological recommendations, 26 which contributes to a complementary description of the research field. Hence to this, a second limitation is that studies with less robust evidence and a high risk of bias are not excluded, and results must be used cautiously.
Conclusion
This scoping review provides a comprehensive picture of the existing research on pain assessment in people with dementia living in nursing homes as a process with three steps; it also contributes to the understanding of highly complex nursing processes in this group and context. It has identified several knowledge gaps in the understanding of this process and provides concrete recommendations for further research. The phenomenon of self-reporting in people with dementia is insufficiently explored, and there is limited knowledge on how HCP relates to varying degrees of residual capacity to self-report. The results underpin the importance of pain assessment approaches that have sufficient flexibility to meet individual residents’ varying and potentially fluctuating ways of communicating pain.
Supplemental Material
Supplemental material, sj-docx-1-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-2-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-3-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-4-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-pdf-5-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Acknowledgments
We would like to thank Annelie Ekberg-Andersson and Linda Borg (Information Specialists at Karlstad University) and Kari L. Mariussen (Head Librarian at Lovisenberg Diaconal University College) for their contribution to the search strategy presented in this scoping review. We would also like to thank the staff at the library at Lovisenberg Diaconal University College for helping us obtain articles to which we did not have access.
Appendix
List of abbreviations
HCP healthcare personnel
MMAT Mixed Methods Appraisal Tool
PAGER Patterns, Advances, Gaps, Evidence for Practice and Research Recommendations
PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses
Footnotes
ORCID iDs: Caroline Kreppen Overen  https://orcid.org/0000-0002-2270-4557
https://orcid.org/0000-0002-2270-4557
Maria Larsson  https://orcid.org/0000-0003-0417-6161
https://orcid.org/0000-0003-0417-6161
Adelheid Hummelvoll Hillestad  https://orcid.org/0000-0002-5780-1699
https://orcid.org/0000-0002-5780-1699
Siren Eriksen  https://orcid.org/0000-0002-5541-0934
https://orcid.org/0000-0002-5541-0934
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Caroline Kreppen Overen, Lovisenberg Diaconal University College, Lovisenberggata 15B, Oslo 0456, Norway Department of Health Sciences, Faculty of Health, Science and Technology, Karlstad University, Karlstad, Sweden.
Maria Larsson, Department of Health Sciences, Faculty of Health, Science and Technology, Karlstad University, Karlstad, Sweden.
Adelheid Hummelvoll Hillestad, Lovisenberg Diaconal University College, Oslo, Norway.
Ingela Karlsson, Department of Health Sciences, Faculty of Health, Science and Technology, Karlstad University, Karlstad, Sweden.
Siren Eriksen, Lovisenberg Diaconal University College, Oslo, Norway; The Norwegian National Centre for Ageing and Health, Tønsberg, Norway.
Declarations
Ethics approval and consent to participate: This study is a scoping review and therefore does not involve collection of primary data. Consequently, formal ethical approval was not required.
Consent for publication: Not applicable.
Author contributions: Caroline Kreppen Overen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Validation; Visualization; Writing – original draft; Writing – review & editing.
Maria Larsson: Conceptualization; Data curation; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing – review & editing.
Adelheid Hummelvoll Hillestad: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing – review & editing.
Ingela Karlsson: Data curation; Formal analysis; Investigation; Methodology; Supervision; Writing – review & editing.
Siren Eriksen: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: The data sets analysed as part of the current study are available from the corresponding author upon reasonable request.
References
- 1. Jørgensen LB, Thorleifsson BM, Selbæk G, et al. Physical diagnoses in nursing home residents-is dementia or severity of dementia of importance? BMC Geriatr 2018; 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helvik A-S, Šaltytė Benth J, Wu B, et al. Persistent use of psychotropic drugs in nursing home residents in Norway. BMC Geriatr 2017; 17: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergh S, Holmen J, Saltvedt I, et al. (2012). Dementia and neuropsychiatric symptoms in nursing-home patients in Nord-Trøndelag County. Tidsskr Nor Legeforen 2012; 132: 1956–1959. [DOI] [PubMed] [Google Scholar]
- 4. Helvik A-S, Engedal K, Benth JŠ, et al. Prevalence and severity of dementia in nursing home residents. Dement Geriatr Cogn Disord 2015; 40(3–4): 166–177. [DOI] [PubMed] [Google Scholar]
- 5. Helvik A-S, Bergh S, Tevik K. A systematic review of prevalence of pain in nursing home residents with dementia. BMC Geriatr 2023; 23(1): 641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Steen JT, Radbruch L, Hertogh CMPM, et al. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliat Med 2014; 28(3): 197–209. [DOI] [PubMed] [Google Scholar]
- 7. International Association for the Study of Pain. Pain terms and definitions, https://www.iasp-pain.org/resources/terminology/#pain (2011, accessed 27 October 2023)
- 8. Brant J. Holistic total pain management in palliative care: cultural and global considerations. Palliat Med Hosp Care Open J 2017; 1: S32–S38. [Google Scholar]
- 9. Wideman TH, Edwards RR, Walton DM, et al. The multimodal assessment model of pain: a novel framework for further integrating the subjective pain experience within research and practice. Clin J Pain 2019; 35(3): 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Felton N, Lewis JS, Cockburn S-J, et al. Pain assessment for individuals with advanced dementia in care homes: a systematic review. Geriatrics 2021; 6(4): 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sirsch E, Lukas A, Drebenstedt C, et al. Pain assessment for older persons in nursing home care: an evidence-based practice guideline. J Am Med Dir Assoc 2020; 21(2): 149–163. [DOI] [PubMed] [Google Scholar]
- 12. Woodward M. Aspects of communication in Alzheimer’s disease: clinical features and treatment options. Int Psychogeriatr 2013; 25(6): 877–885. [DOI] [PubMed] [Google Scholar]
- 13. Tible OP, Riese F, Savaskan E, et al. Best practice in the management of behavioural and psychological symptoms of dementia. Ther Adv Neurol Disord 2017; 10(8): 297–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Van der Steen JT, Sampson EL, Van den Block L, et al. Tools to assess pain or lack of comfort in dementia: a content analysis. J Pain Symptom Manage 2015; 50(5): 659-675.e653. [DOI] [PubMed] [Google Scholar]
- 15. Husebo B, Ostelo R, Strand LI. The MOBID-2 pain scale: reliability and responsiveness to pain in patients with dementia. Eur J Pain 2014; 18(10): 1419-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Torvik K, Kaasa S, Kirkevold Ø, et al. Validation of Doloplus-2 among nonverbal nursing home patients-an evaluation of Doloplus-2 in a clinical setting. BMC Geriatr 2010; 10(1): 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. McCormack B, McCane T, Martin S., What is. person-centredness? In McCormack B. (ed.), Fundamentals of person-centred healthcare practice. 1st ed. Oxford, UK: Wiley Blackwell, 2021, pp. 13–22. [Google Scholar]
- 18. Malara A, De Biase GA, Bettarini F, et al. Pain assessment in elderly with behavioral and psychological symptoms of dementia. J Alzheimer’s Dis 2016; 50(4): 1217–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Achterberg W, Lautenbacher S, Husebo B, et al. Pain in dementia. Pain Rep 2020; 5(1): e803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jansen BW, Brazil K, Passmore P, et al. Exploring healthcare assistants’ role and experience in pain assessment and management for people with advanced dementia towards the end of life: a qualitative study. BMC Palliat Care 2017; 16(1): 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pringle J, Mellado ASAV, Haraldsdottir E, et al. Pain assessment and management in care homes: understanding the context through a scoping review. BMC Geriatr 2021; 21: 431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsai YIP, Browne G, Inder KJ. The effectiveness of interventions to improve pain assessment and management in people living with dementia: a systematic review and meta-analyses. J Adv Nurs 2021; 77(3): 1127–1140. [DOI] [PubMed] [Google Scholar]
- 23. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for Scoping Reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169(7): 467–473. [DOI] [PubMed] [Google Scholar]
- 24. Overen CK, Larsson M, Hillestad AH, et al. Process of pain assessment in people with dementia living in nursing homes: a scoping review protocol. BMJ Open 2022; 12(9): e063230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arksey H, O’Malley L. Scoping studies: towards a methodological framework. Int J Social Res Methodol 2005; 8(1): 19–32. [Google Scholar]
- 26. Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci 2010; 5(1): 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bradbury-Jones C, Aveyard H, Herber OR, et al. Scoping reviews: the PAGER framework for improving the quality of reporting. Int J Soc Res Methodol 2021; 25: 1–14. [Google Scholar]
- 28. Peters MD, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020; 18(10): 2119–2126. [DOI] [PubMed] [Google Scholar]
- 29. Folstein M, Folstein S, McHugh Mini-Mental State Examination (MMSE). In: Larner AJ. (ed.), Manual of screeners for dementia. Cham, Switzerland: Springer Cham, 2020, pp. 51–69. [Google Scholar]
- 30. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan – a web and mobile app for systematic reviews. Syst Rev 2016; 5(1): 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hong QN, Fàbregues S, Bartlett G, et al. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Educ Inf 2018; 34(4): 285–291. [Google Scholar]
- 32. Weingarten MA, Paul M, Leibovici L. Assessing ethics of trials in systematic reviews. BMJ 2004; 328(7446): 1013–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Westerdahl F, Carlson E, Wennick A, et al. Teaching strategies and outcome assessments targeting critical thinking in bachelor nursing students: a scoping review protocol. BMJ Open 2020; 10(1): e033214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Braun V, Clarke V. Thematic analysis: a practical guide. Los Angeles, CA: Sage, 2022. [Google Scholar]
- 35. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 2021; 88: 105906. [DOI] [PubMed] [Google Scholar]
- 36. Nakashima T, Young Y, Hsu W-H. Do nursing home residents with dementia receive pain interventions? Am J Alzheimers Dis Other Demen 2019; 34(3): 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Laxer K, Jacobsen FF, Lloyd L, et al. Comparing nursing home assistive personnel in five countries. Ageing Int 2016; 41: 62–78. [Google Scholar]
- 38. World Health Organization. Classifying health workers: mapping occupations to the international standard classification. Geneva: WHO Press, 2019. [Google Scholar]
- 39. Kaasalainen S, Coker E, Dolovich L, et al. Pain management decision making among long-term care physicians and nurses. West J Nurs Res 2007; 29(5): 561–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Corbett A, Nunez K-M, Smeaton E, et al. The landscape of pain management in people with dementia living in care homes: a mixed methods study. Int J Geriatr Psychiatry 2016; 31(12): 1354–1370. [DOI] [PubMed] [Google Scholar]
- 41. Karlsson C, Sidenvall B, Bergh I, et al. Certified nursing assistants’ perception of pain in people with dementia: a hermeneutic enquiry in dementia care practice. J Clin Nurs 2013; 22(13): 1880–1889. [DOI] [PubMed] [Google Scholar]
- 42. Yang Q, Yi R, Wang N, et al. Perception, behavior and experience of nursing assistants towards pain of older adults with dementia: a qualitative study. Geriatr Nurs 2024; 56: 100–107. [DOI] [PubMed] [Google Scholar]
- 43. Andrews SM, Dipnall JF, Tichawangana R, et al. An exploration of pain documentation for people living with dementia in aged care services. Pain Manag Nurs 2019; 20(5): 475–481. [DOI] [PubMed] [Google Scholar]
- 44. Chang SO, Oh Y, Park EY, et al. Concept analysis of nurses’ identification of pain in demented patients in a nursing home: development of a hybrid model. Pain Manag Nurs 2011; 12(2): 61–69. [DOI] [PubMed] [Google Scholar]
- 45. Cohen-Mansfield J, Creedon M. Nursing staff members’ perceptions of pain indicators in persons with severe dementia. Clin J Pain 2002; 18(1): 64–73. [DOI] [PubMed] [Google Scholar]
- 46. Gilmore-Bykovskyi AL, Bowers BJ. Understanding nurses’ decisions to treat pain in nursing home residents with dementia. Res Gerontol Nurs 2013; 6(2): 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lautenbacher S, Sampson EL, Päahl S, et al. Which facial descriptors do care home nurses use to infer whether a person with dementia is in pain? Pain Med 2017; 18(11): 2105–2115. [DOI] [PubMed] [Google Scholar]
- 48. Lundin E, Godskesen TE. End-of-life care for people with advanced dementia and pain: a qualitative study in Swedish nursing homes. BMC Nurs 2021; 20(1): 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mezinskis PM, Keller AW, Luggen AS. Assessment of pain in the cognitively impaired older adult in long-term care. Geriatr Nurs 2004; 25(2): 107–112. [DOI] [PubMed] [Google Scholar]
- 50. Monroe TB, Parish A, Mion LC. Decision factors nurses use to assess pain in nursing home residents with dementia. Arch Psychiatr Nurs 2015; 29(5): 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Parkman S, Mastel-Smith B, McGuire A, et al. Insights to identifying and managing pain in persons with dementia in long-term care: a mixed methods study comparing the Abbey Pain Scale and Pain Assessment in Advanced Dementia Scale. J Gerontol Nurs 2021; 47(2): 21–30. [DOI] [PubMed] [Google Scholar]
- 52. Peisah C, Weaver J, Wong L, et al. Silent and suffering: a pilot study exploring gaps between theory and practice in pain management for people with severe dementia in residential aged care facilities. Clin Interv Aging 2014; 9: 1767–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sloane PD, Miller LL, Mitchell CM, et al. Provision of morning care to nursing home residents with dementia: opportunity for improvement? Am J Alzheimers Dis Other Demen 2007; 22(5): 369–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alexander BJ, Plank P, Carlson MB, et al. Methods of pain assessment in residents of long-term care facilities: a pilot study. J Am Med Dir Assoc 2005; 6(2): 137–143. [DOI] [PubMed] [Google Scholar]
- 55. Ford B, Snow AL, Herr K, et al. Ethnic differences in nonverbal pain behaviors observed in older adults with dementia. Pain Manag Nurs 2015; 16(5): 692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burns M, McIlfatrick S. Nurses’ knowledge and attitudes towards pain assessment for people with dementia in a nursing home setting. Int J Palliat Nurs 2015; 21(10): 479–487. [DOI] [PubMed] [Google Scholar]
- 57. Manfredi PL, Breuer B, Meier DE, et al. Pain assessment in elderly patients with severe dementia. J Pain Symptom Manage 2003; 25(1): 48–52. [DOI] [PubMed] [Google Scholar]
- 58. Monroe T, Carter M, Feldt K, et al. Assessing advanced cancer pain in older adults with dementia at the end-of-life. J Adv Nurs 2012; 68(9): 2070–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rostad HM, Utne I, Grov EK, et al. The impact of a pain assessment intervention on pain score and analgesic use in older nursing home residents with severe dementia: a cluster randomised controlled trial. Int J Nurs Stud 2018; 84: 52–60. [DOI] [PubMed] [Google Scholar]
- 60. Cohen-Mansfield J, Lipson S. Pain in cognitively impaired nursing home residents: how well are physicians diagnosing it? J Am Geriatr Soc 2002; 50(6): 1039–1044. [DOI] [PubMed] [Google Scholar]
- 61. Vitou V, Gély-Nargeot M-C, Jeandel C, et al. The influence of Alzheimer’s disease stigma on pain assessment in older persons. Dementia 2022; 21(8): 2418–2441. [DOI] [PubMed] [Google Scholar]
- 62. Vitou V, Gély-Nargeot M-C, Bayard S. Interrater variability in pain assessment of long-term care residents with dementia. Pain Manag Nurs 2021; 22(3): 377–385. [DOI] [PubMed] [Google Scholar]
- 63. Chen Y, Lin L, Watson R. Validating nurses’ and nursing assistants’ report of assessing pain in older people with dementia. J Clin Nurs 2010; 19(1): 42–52. [DOI] [PubMed] [Google Scholar]
- 64. Cohen-Mansfield J. The relationship between different pain assessments in dementia. Alzheimer Dis Assoc Disord 2008; 22(1): 86–93. [DOI] [PubMed] [Google Scholar]
- 65. Closs S, Barr B, Briggs M, et al. Evaluating pain in care home residents with dementia. Nurs Resid Care 2003; 5(1): 32–35. [Google Scholar]
- 66. Apinis C, Tousignant M, Arc M, et al. Can adding a standardized observational tool to interdisciplinary evaluation enhance the detection of pain in older adults with cognitive impairments? Pain Med 2014; 15(1): 32–41. [DOI] [PubMed] [Google Scholar]
- 67. Zahid M, Gallant NL, Hadjistavropoulos T, et al. Behavioral pain assessment implementation in long-term care using a tablet app: case series and quasi-experimental design. JMIR Mhealth Uhealth 2020; 8(4): e17108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rababa MJ. The association of nurses’ assessment and certainty to pain management and outcomes for nursing home residents in Jordan. Geriatr Nurs 2018; 39(1): 66–71. [DOI] [PubMed] [Google Scholar]
- 69. Ersek M, Polissar N, Neradilek MB. Development of a composite pain measure for persons with advanced dementia: exploratory analyses in self-reporting nursing home residents. J Pain Symptom Manage 2011; 41(3): 566–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rababa M. Association of comorbid burden and patient outcomes of residents with dementia in Jordanian nursing homes. J Gerontol Nurs 2018; 44(7): 50–58. [DOI] [PubMed] [Google Scholar]
- 71. Neville C, McCarthy A, Laurent K. Pain management skills of regional nurses caring for older people with dementia: a needs analysis. Collegian 2006; 13(2): 31–36. [DOI] [PubMed] [Google Scholar]
- 72. Chen Y-H, Lin L-C. The credibility of self-reported pain among institutional older people with different degrees of cognitive function in Taiwan. Pain Manag Nurs 2015; 16(3): 163–172. [DOI] [PubMed] [Google Scholar]
- 73. Scherder E, Van Manen F. Pain in Alzheimer’s disease: nursing assistants’ and patients’ evaluations. J Adv Nurs 2005; 52(2): 151–158. [DOI] [PubMed] [Google Scholar]
- 74. Monroe TB, Misra SK, Habermann RC, et al. Pain reports and pain medication treatment in nursing home residents with and without dementia. Geriatr Gerontol Int 2014; 14(3): 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rababa M, Al-Rawashdeh S. Nurses’ certainty and pain outcomes of nursing home residents with dementia: the mediating effect of pain assessment. Pain Manag 2019; 9(6): 559–567. [DOI] [PubMed] [Google Scholar]
- 76. Liu JYW, Pang PCP, Lo SKL. Development and implementation of an observational pain assessment protocol in a nursing home. J Clin Nurs 2012; 21(11): 1789–1793. [DOI] [PubMed] [Google Scholar]
- 77. Sandvik R, Selbaek G, Kirkevold O, et al. Analgesic prescribing patterns in Norwegian nursing homes from 2000 to 2011: trend analyses of four data samples. Age Ageing 2016; 45(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 78. Woolford SJ, Aggarwal P, Sheikh CJ, et al. Frailty, multimorbidity and polypharmacy. Medicine 2021; 49(3): 166–172. [Google Scholar]
- 79. Liao Y-J, Jao Y-L, Berish D, et al. A systematic review of barriers and facilitators of pain management in persons with dementia. J Pain 2023; 24(5): 730–741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-2-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-3-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-docx-4-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice
Supplemental material, sj-pdf-5-pcr-10.1177_26323524241308589 for The process of pain assessment in people with dementia living in nursing homes: a scoping review by Caroline Kreppen Overen, Maria Larsson, Adelheid Hummelvoll Hillestad, Ingela Karlsson and Siren Eriksen in Palliative Care and Social Practice