Abstract
Background
Carotid artery stenosis (CAS) may cause many cerebrovascular diseases, and a biomarker for screening and monitoring is needed. This study focused on the clinical significance of long-chain non-coding RNA (lncRNA) non-coding RNA activated by DNA damage (NORAD) in patients with CAS and aimed to search for potential biomarkers of CAS.
Methods
Eighty-six asymptomatic patients with CAS and 60 healthy individuals were enrolled, with corresponding clinical data and serum samples collected. The expression of NORAD was detected by reverse transcription-quantitive PCR (RT-qPCR). All patients were followed up for 2 years to collected the occurrence data of cerebrovascular events, and Kaplan-Meier and Cox regression were used for data analysis. Receiver operator characteristic curve was used to analyze the diagnostic value of NORAD in distinguishing CAS patients from healthy people, and to evaluate the prediction accuracy of NORAD.
Results
NORAD is overexpressed in the serum of CAS patients, and associated with patients’ hypertension, TC, LDL-C levels and stenosis degree. NORAD has high sensitivity (88.37%) and specificity (80.00%) in the identification of CAS patients (AUC = 0.917). NORAD was independently related to the occurrence of cerebrovascular events (HR = 2.435, P = .003). a logistic regression risk model for predicting cerebrovascular events was constructed with the parameters including NORAD, TC and LDL.
Conclusion
NORAD can be used as a diagnostic and prognostic biomarker for CAS, and NORAD, total cholesterol (TC), and low density lipoprotein cholesterol (LDL-C) can be independently correlated to predict cerebrovascular events.
Keywords: lncRNA NORAD, carotid artery stenosis, cerebrovascular events, prognosis, diagnosis
Introduction
Carotid artery stenosis (CAS) is one of the leading causes of acute ischemic stroke, accounting for about 20% of cases. 1 CAS is mainly caused by atherosclerosis. 2 In atherosclerosis, lipids and plaque adhere to the lining of blood vessels and accumulate to block blood vessels, causing CAS. 3 When the plaque that has accumulated in the stenosis of the carotid artery breaks off, it may float down the blood stream to the cerebral artery and accumulate in the cerebral artery, resulting in a cerebral infarction. 4 Studies have shown that vascular smooth muscle cells (VSMCs) play a critical role in the integrity of the fiber cap, significantly affecting plaque stability. Therefore, the regulation of VSMCs function is also closely related to the etiology and progression of CAS.5,6 CAS will reduce the blood supply to the brain, dizziness, amaurosis, consciousness disorders, memory loss and other symptoms. It can also lead to cerebral infarction, and severe cerebral infarction can cause disability and even death. 7 However, the early diagnosis of CAS is still very difficult, leading to the development of a more severe encephalopathy with a poor prognosis. Therefore, finding an efficient, accurate and convenient method to predict cerebrovascular events is an important means to improve the prognosis of patients with CAS.
Long noncoding RNA (lncRNAs) is a class of functional noncoding RNA transcripts with a length greater than 200nt. 8 LncRNAs can regulate gene expression by regulating epigenetic modifications, transcription, and posttranscriptional events. 9 Studies have shown that lncRNAs play an important role in CAS, AS and other related diseases. Non-coding RNA activated by DNA damage (NORAD) was found to be a lncRNAs closely associated with vascular events.10,11 Zhang et al showed that knockdown of lncRNA-NORAD increased lipid disorders and atherosclerotic lesions in ApoE-/- mice. The results suggest that lncRNA-NORAD inhibits atherosclerosis through NF-κB and p53-p21 signaling pathways. 10 And, NORAD is upregulated in acute cerebral ischemic stroke, and strongly associated with severe development and poor prognosis. 12 In addition, other studies have shown that NORAD enhances H3K9 deacetylation by recruiting histone deacetylase 6 (HDAC6), thereby enhancing vascular endothelial cell injury and atherosclerosis. 13 NORAD may regulate the occurrence of Alzheimer's Disease by affecting glycometabolic reprogramming, and NORAD can be considered a good target for VSMC phenotypic intervention and Alzheimer's Disease therapy. 14 Li et al ‘s study showed that in patients with coronary heart disease, elevated NORAD was associated with increased stenosis, inflammatory status, and blood lipid. 15 These findings suggest that NORAD plays an important role in CAS progression and related diseases.
Therefore, the purpose of this study was to explore the relationship between NORAD and the disease progression of CAS, as well as the occurrence of cerebrovascular events. In addition, a novel predicting model for the onset of cerebrovascular events was also explored according to NORAD combined with other related parameters.
Materials and Methods
Patients and Sample Collection
The study enrolled 86 asymptomatic CAS patients and 60 healthy individuals from 2020 to 2021 in Liaocheng People's Hospital.
Patients’ inclusion criteria: (i) age > 18-year-old; (ii) ultrasound examination found carotid stenosis. Patients Exclusion criteria: (i) cases with temporary amaurosis; (ii) with severe chronic medical history of heart, brain, lung and other important organs; (iii) with malignant tumors; (iv) pregnancy or lactation; (v) had history of TIA or stroke. Patients with CAS who were diagnosed by Doppler ultrasound or CT angiography in Liaocheng People's Hospital and met one of the following indications :(1) stenosis ≥70%(color ultrasound) or 60% (CT angiography); (2) the degree of stenosis <70% by ultrasound but CT angiography indicated the unstable lesion.
Healthy individuals were enrolled from the group receiving a routine physical examination at our hospital without any indications of carotid artery abnormality. Venous blood samples of included asymptomatic CAS patients and health control patients were collected, serum samples were separated by centrifugation at 4°C for subsequent analysis. All protocols for blood samples collection and analysis were all in accordance with the guideline of the Ethics Committee and has approved by the Ethics Committee of Liaocheng People's Hospital. A signed informed consent has been obtained from the participants before sampling in this study.
Clinical Data Collection
Clinical data of patients and Healthy individuals were collected, including age, gender, smoking, hypertension, hyperlipidemia, diabetes, hypersensitive C-reactive protein (hs-CRP), total cholesterol (TC), Triglyceride (TG), low density lipoprotein cholesterol (LDL-C) and high density lipoprotein cholesterol (HDL-C).
Follow-up of Cerebrovascular Events
All patients with CAS were followed up by telephone or outpatient for 2 years, the development and status of the patients were tracked, and the occurrence of cerebrovascular events in all patients with CAS was counted. Cerebrovascular events include TIA, stroke, and death related to arterial stenosis. TIA was defined as transient episode of neurologic dysfunction due to the focal brain, spinal cord, or retinal ischemia without acute infarction or tissue injury, and the symptom can be disappeared within 24 h. Stroke was detected and diagnosed with the combination of clinical manifestations, imaging examination and laboratory examinations. The death recorded in this study defined all the deaths due to the CAS related diseases, but no other accident events. The occurrence of the above cerebrovascular events was the follow-up endpoint of this study.
RNA Extraction and Reverse Transcription Quantitative PCR (RT-qPCR)
In this study, total RNA was obtained from blood samples by RNeasy Mini Kit (Qiagen, Valencia, CA, USA). Following the manufacturer's guidelines, the RNA was reversely transcribed into cDNA using a reverse transcription kit (Takara, Tokyo, Japan)., and the reaction conditions were: room temperature for 10 min, 42 °C for 60 min, 95 °C for 5 min, and on ice for 5 min. qPCR was conducted using SYBR Premix Ex TaqTM II Kit (Takara) and a real-time fluorescent qPCR 7500 system (ABI, Foster City, CA, USA). GAPDH was used as an endogenous control for NORAD. The final expression value of NORAD was calculated using the 2−ΔΔCt method.
Statistical Analysis
All data were expressed as mean ± standard deviation (SD) and analyzed at SPSS 26.0 (SPSS, Inc., Chicago, Illinois) and GraphPad 9.0 (GraphPad Software, Inc., USA). Student's t test and Chi-square test were used to compare the differences between two groups in the clinical characteristics. A Pearson correlation coefficient was adopted to analyze the relationship between serum NORAD with CAS. The significance of serum NORAD in the identification of CAS patients and healthy individuals was evaluated by Receiver operating characteristic (ROC). Logistic analysis was used to construct a risk model for cerebrovascular events. Kaplan-Meier and Cox regression analysis were used to identify potential prognostic factors. A P value of less than .05 was considered as statistical significance.
Results
Baseline Clinical Characteristics of the Participants
The clinical characteristics of the study subjects are shown in Table 1. The results showed that the CAS group had significant differences in hyperlipidemia, hs-CRP, TC, and LDL compared with the healthy control group (P < .05). There was no significant difference in other indexes (age, gender, smoking status, hypertension, diabetes, TG, HDL) (P > .05).
Table 1.
Baseline Characteristics of the Participants.
| Characteristics | Health (n = 60) | CAS (n = 86) | P value |
|---|---|---|---|
| Age (years) | 61.62 ± 5.97 | 60.76 ± 7.24 | .450 |
| Gender | .441 | ||
| Female | 22 | 37 | |
| Male | 38 | 49 | |
| Smoking | 28 | 31 | .198 |
| Hypertension | 31 | 46 | .828 |
| Hyperlipidemia | 27 | 45 | .384 |
| Diabetes | 22 | 39 | .295 |
| hs-CRP (mg/L) | 9.59 ± 5.87 | 16.29 ± 9.80 | <.001 |
| TC (mM) | 4.19 ± 0.81 | 5.29 ± 1.34 | <.001 |
| TG (mM) | 1.47 ± 0.53 | 1.80 ± 0.68 | .002 |
| LDL-C (mM) | 2.45 ± 0.59 | 3.36 ± 0.61 | <.001 |
| HDL-C (mM) | 1.23 ± 0.20 | 1.19 ± 0.18 | .219 |
Elevated Expression Levels of NORAD in Patients with CAS
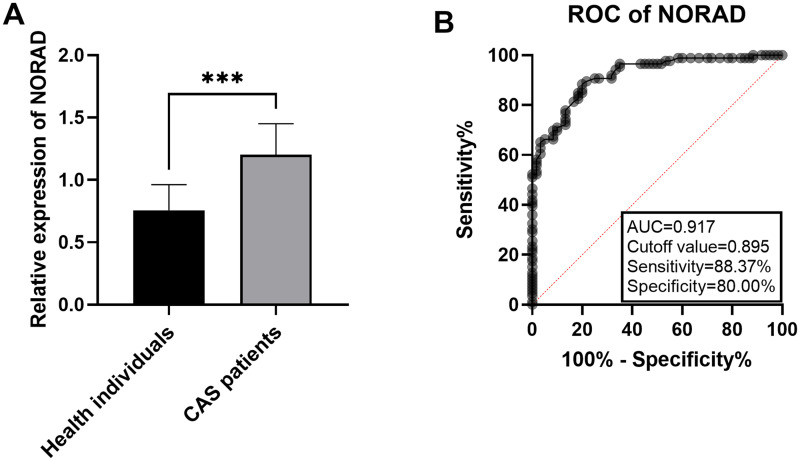
Histogram was drawn based on RT-qPCR data to analyze the significance of differences. RT-qPCR results displayed that the expression level of serum NORAD was markedly increased in asymptomatic CAS patients compared with healthy controls (Figure 1A, P < .001). ROC curves were drawn according to serum NORAD expression levels in the blood sample (Figure 1B). The results showed that when the AUC curve was 0.917 and the cutoff value was 0.895, the sensitivity was 88.37% and the specificity was 80.00%. The data suggest that serum NORAD had significant diagnostic value in asymptomatic CAS patients from healthy controls.
Figure 1.
Elevated expression levels of NORAD in patients with CAS. A. NORAD was significantly up-regulated in serum of CAS patients (***P < .001). B. NORAD showed sensitively and specifically diagnostic in patients with CAS.
Association of NORAD with Clinical Data of CAS Patients
CAS patients were divided into low- NORAD (n=40) and high- NORAD (n=46) groups according to the average serum NORAD levels in CAS patients. According to the data analysis, the expression levels of NORAD were significantly correlated with hyperlipidemia (P = .021), TC (P = .002), LDL (P = .020). But there was no obvious relationship of serum NORAD expression level with Age, Gender, Smoking, Hypertension, Diabetes, hs-CRP (mg/L), TG (mM), HDL-C (mM). The results are shown in Table 2.
Table 2.
Association of NORAD with Clinical Data of CAS Patients.
| Variables | NORAD expression | P value | |
|---|---|---|---|
| Low (n = 40) | High (n = 46) | ||
| Age (years) | 61.23 ± 8.59 | 60.35 ± 5.88 | .578 |
| Gender (male) | 22 | 27 | .730 |
| Smoking | 14 | 17 | .850 |
| Hypertension | 18 | 32 | .021 |
| Hyperlipidemia | 17 | 28 | .089 |
| Diabetes | 18 | 21 | .952 |
| hs-CRP (mg/L) | 15.05 ± 10.53 | 17.37 ± 9.10 | .275 |
| TC (mM) | 4.81 ± 0.90 | 5.70 ± 1.52 | .002 |
| TG (mM) | 1.65 ± 0.69 | 1.93 ± 0.64 | .059 |
| LDL-C (mM) | 3.20 ± 0.65 | 3.50 ± 0.53 | .020 |
| HDL-C (mM) | 1.22 ± 0.18 | 1.17 ± 0.17 | .180 |
Correlation of NORAD with Stenosis Degree in CAS Patients
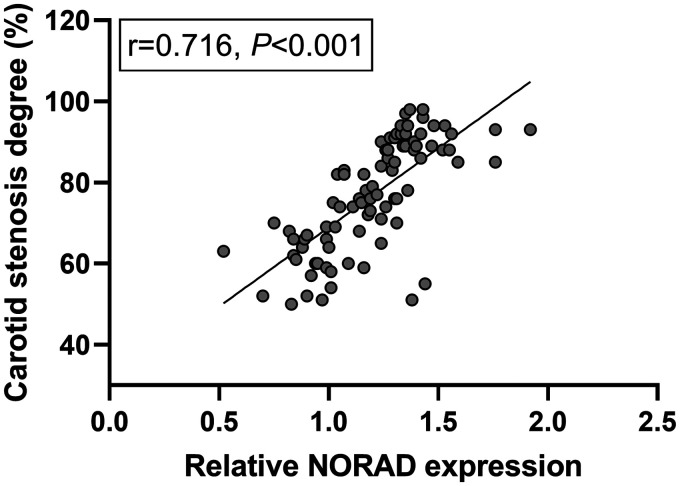
Pearson correlation coefficient results were shown in Figure 2. The analysis results showed that the expression level of NORAD was positively correlated with the degree of vascular stenosis in CAS patients (P < .001, r = 0.716).
Figure 2.
Correlation of NORAD with stenosis degree in CAS patients. NORAD was positively associated with stenosis in patients with CAS.
Predictive Value of NORAD for Cerebrovascular Events in CAS Patients
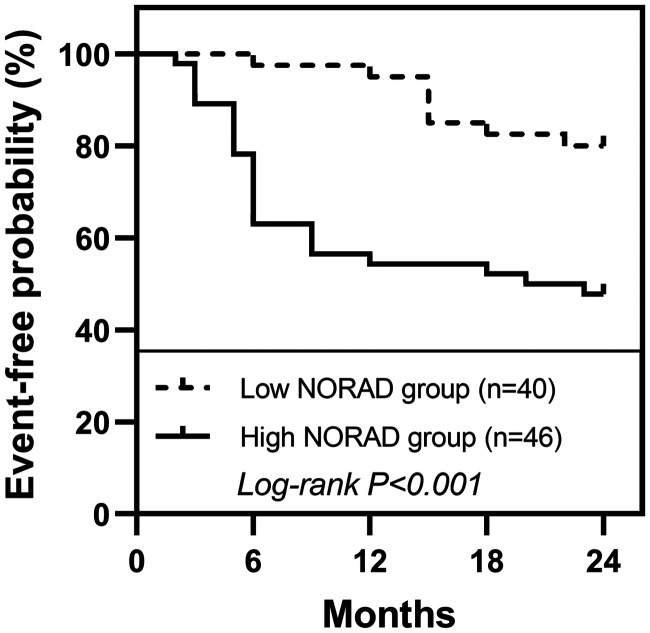
Additionally, 2-year follow-up data showed that 32 patients with CAS developed cerebrovascular events. As shown in Kaplan-Meier curve, CAS patients with high NORAD expression levels of had a significantly increased probability of cerebrovascular events in the group with lower NORAD expression levels (Figure 3) Among the results of Cox regression analysis, univariate regression analysis showed that hyperlipidemia, hs-CRP, TC, LDL and NORAD were correlated with cerebrovascular events. The results of multi-factor analysis showed that TC, LDL and NORAD were independent risk factors for cerebrovascular events (all P < .05, Table 3).
Figure 3.
Predictive value of NORAD for cerebrovascular events in CAS patients. Patients with CAS with higher NORAD expression levels had a higher probability of cerebrovascular events.
Table 3.
Cox Regression Analysis Results.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Age | 1.563 | 0.623-3.921 | .231 | – | – | – |
| Gender | 0.735 | 0.223-2.423 | .443 | – | – | – |
| Smoking | 1.859 | 0.741-4.664 | .301 | – | – | – |
| Hypertension | 1.236 | 0.863-1.770 | .096 | – | – | – |
| Hyperlipidemia | 1.694 | 1.085-2.645 | .049 | – | – | – |
| Diabetes | 1.879 | 0.639-5.525 | .238 | – | – | – |
| hs-CRP | 2.110 | 1.358-3.278 | .039 | 2.010 | 1.220-3.312 | .049 |
| TC | 1.996 | 1.299-3.067 | .028 | 1.874 | 1.105-3.178 | .042 |
| TG | 1.743 | 0.945-3.215 | .085 | – | – | – |
| LDL-C | 2.379 | 1.452-3.898 | .022 | 2.013 | 1.234-3.284 | .031 |
| HDL-C | 0.854 | 0.541-1.348 | .068 | – | – | – |
| NORAD | 2.849 | 1.557-5.213 | .001 | 2.435 | 1.407-4.214 | .003 |
A Logistic Risk Model Constructed Using NORAD, TC and LDL for Cerebrovascular Events
To facilitate the prediction of cerebrovascular events, the indicators that found to be independently associated with cerebrovascular events (NORAD, TC and LDL) were used to construct a prediction model according a multivariate logistic regression analysis (Table 4). The predicted probability (p) of presenting cerebrovascular events in CAS patients was estimated by the multivariate logistic regression model: p = 1/(1 − e−x), where X = −3.92 + 0.714×NORAD + 0.396×TC + 0.598×LDL-C. Thus, the final predictive equation was as follow: p = 1/[1 + e−(−3.92+0.714×NORAD+0.396×TC+0.598×LDL−C)].
Table 4.
Multivariate Logistic Regression Analysis Results.
| Variables | Estimated regression coefficient | OR | 95% CI | P value |
|---|---|---|---|---|
| NORAD | 0.714 | 3.012 | 2.109-4.302 | .003 |
| TC | 0.396 | 1.873 | 1.114-3.152 | .046 |
| LDL-C | 0.598 | 2.237 | 1.406-3.669 | .019 |
Discussions
Carotid atherosclerosis and carotid plaque formation are the main predisposing factors of stroke. 16 In addition, there is evidence that CAS is a major predisposing factor of cerebral circulatory insufficiency. 17 The degree of carotid atherosclerosis is the main risk factor for the malignant development of ischemic stroke. 18 The traditional view is that the degree of CAS stenosis is the main risk factor leading to the development of malignant cerebrovascular diseases, but some patients with mild stenosis often have cerebrovascular events. Thus, searching for diagnostic markers with high accuracy can help to screen patients with CAS in advance and further prevent the occurrence of encephalopathy. The results of this study show that NORAD is abnormally expressed in patients with CAS and can be used as a diagnostic and prognostic marker for CAS. Additionally, CAS patients with high levels of NORAD were found to have increased risk to suffer from cerebrovascular diseases.
A large number of lncRNAs have been found to be related to the pathogenesis and degree of stenosis of CAS. For example, THRIL is a diagnostic and prognostic biomarker of CAS and plays an important role in regulating oxidized low-density lipoprotein (OX-LDL)-induced proliferation, apoptosis, and inflammation of human aortic endothelial cells. 19 Dong et al found that the small nucleolar RNA host gene 1 (SNHG1)/miR-145 axis regulates the proliferation and migration of human carotid smooth muscle cells, suggesting its potential for the prevention and treatment of restenosis. 20 Long non-coding RNA HOX transcript antisense RNA (HOTAIR) may be a diagnostic biomarker in patients with CAS and is involved in the activity of vascular smooth muscle cells. 21 Li et al ‘s study showed that serum rhabdomyosarcoma 2 associated transcript (RMST) levels were elevated in patients with CAS. The detection of RMST expression level has a high value in predicting the occurrence and prognosis of CAS. LncRNA NORAD has been found to be closely related to vascular diseases and encephalopathy. Zhang et al ‘s results suggest that lncRNA-NORAD inhibits endothelial senescence, endothelial apoptosis, and atherosclerosis through NF-κB and pp53 p21 signaling pathways and IL-8. 10 Elevated NORAD acts as a sponge for miR-211-5p in hepatocellular carcinoma, thereby releasing the latter's inhibitory effect on its downstream targets forkhead box D1 (FOXD1) and vascular endothelial growth factor A (VEGF-A), ultimately promoting angiogenesis. 11 Therefore, we speculated that NORAD also plays an important role in CAS. The results of this study showed that NORAD was overexpressed in CAS patients, indicating that NORAD was closely related to CAS. In addition, the ROC data results showed that the AUC was 0.917, indicating that NORAD had a high diagnostic accuracy in distinguishing CAS patients from healthy people. At the same time, Kaplan-Meier curve showed that the higher the NORAD expression level in CAS patients, the greater the incidence of cerebrovascular events, indicating that NORAD can be used as a prognostic biomarker in CAS patients.
Cerebrovascular disease (CVD) has become a major social and public health problem worldwide, 22 including stroke, cerebrovascular abnormalities, malformations, and other disorders of cerebral blood circulation. 23 Patients with CAS have a higher risk of cerebrovascular events, and there are currently some indicators in the clinic to predict cerebrovascular events in patients with CAS. There is ample evidence that elevated levels of TC, and LDL are major risk factors for diabetes and can lead to arterial wall thickening. 24 Moreover, clinical data show that carotid stenosis is related to TC and LDL indexes, and the increase of the indexes indicates that the macromolecular substances in the blood will increase, and these substances are attached to the inner wall of the blood vessel, which is easy to thicken the inner wall of the blood vessel, but the width and diameter of the blood vessel remain unchanged, so artery stenosis occurs. 25 Therefore, TC and LDL are also important indicators to predict the degree of CAS. In this study, there were significant differences between CAS cases and healthy controls in terms of hyperlipidemia, hs-CRP, TC, and LDL, which were consistent with previous reports. With the development of molecular mechanisms, the identification of molecules related to CAS development has been extensively studied. In our study NORAD expression level was closely related to cerebrovascular events. Compared to the reported indicators, such as TC and LDL, which related with the development of CAS and related cerebrovascular events, NORAD might show more significant clinical value with its increased specificity. In addition, NORAD as a kind of ncRNAs, can be detected easily from serum samples, which was also a characteristic to serve as a suitable biomarker. Furthermore, this study established a logistic regression model based on NORAD, TC and LDL, which was hope to provide novel insight to predict the onset cerebrovascular events in patients with CAS.
The growth and movement of VSMCs are considered to be the main pathological basis of CAS and other arterial diseases.26,27 Previous studies have shown that NORAD is associated with the biological function of vascular endothelial cells and VSMCs. Li et al demonstrated that NORAD inhibits miR-136-5p, thereby up-regulating lysine demethylase 1A (KDM1A) by inducing phenotypic regulation of VSMCs to participate in the formation and rupture of intracranial aneurysms. 28 lncRNA NORAD can slow down endothelial cell senescence and apoptosis. 10 lncRNA NORAD enhances H3K9 deacetylation by recruiting HDAC6, thereby inhibiting VEGF gene transcription, thereby enhancing vascular endothelial cell injury and atherosclerosis. 13 So it was speculated that NORAD was probably involved in the occurrence and development of CAS and cerebrovascular events by regulating two kinds of cellular functions. This study was not able to carry out cell experiments for verification, which is a major limitation of this study. In the future, vascular endothelial cells and smooth muscle cells will be used as research models to conduct in-depth studies to explore the mechanism of NORAD function.
In summary, the up-regulation of NORAD in CAS can be used as a potential diagnostic biomarker to screen for the occurrence of CAS. The expression level of NORAD can predict the probability of encephalopathy in patients. Serum NORAD level was positively correlated with the degree of CAS stenosis, and could independently predict the occurrence of subsequent cerebrovascular events.
Acknowledgements
Not applicable.
Footnotes
Availability of Data and Materials: The data used and analyzed can be obtained from the corresponding author under a reasonable request.
Consent for Publication: Not applicable.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics Approval and Consent to Participate: The experimental procedures were all in accordance with the guideline of the Ethics Committee of Liaocheng People's Hospital and has approved by the Ethics Committee of Liaocheng People's Hospital. This study complies with the Declaration of Helsinki. A signed written informed consent was obtained from each patient.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lili Wang https://orcid.org/0009-0004-5088-9159
References
- 1.Arasu R, Arasu A, Muller J. Carotid artery stenosis: An approach to its diagnosis and management. Aust J Gen Pract. 2021;50(11):821-825. [DOI] [PubMed] [Google Scholar]
- 2.Aday AW, Beckman JA. Medical management of asymptomatic carotid artery stenosis. Prog Cardiovasc Dis. 2017;59(6):585-590. [DOI] [PubMed] [Google Scholar]
- 3.Fan J, Watanabe T. Atherosclerosis: Known and unknown. Pathol Int. 2022;72(3):151-160. [DOI] [PubMed] [Google Scholar]
- 4.Falk E. Pathogenesis of atherosclerosis. J Am Coll Cardiol. 2006;47(8 Suppl):C7-12. [DOI] [PubMed] [Google Scholar]
- 5.Han Z, Hu H, Yin M, et al. miR-145 is critical for modulation of vascular smooth muscle cell proliferation in human carotid artery stenosis. J Biol Regul Homeost Agents. 2018;32(3):506-516. [PubMed] [Google Scholar]
- 6.Liu Q, Yan S, Yuan Y, Ji S, Guo L. miR-28-5p improved carotid artery stenosis by regulating vascular smooth muscle cell proliferation and migration. Vascular. 2022;30(4):764-770. [DOI] [PubMed] [Google Scholar]
- 7.Halliday A. Treatment of asymptomatic carotid artery stenosis. Lancet Neurol. 2022;21(10):858-859. [DOI] [PubMed] [Google Scholar]
- 8.Bhan A, Soleimani M, Mandal SS. Long noncoding RNA and cancer: A new paradigm. Cancer Res. 2017;77(15):3965-3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Statello L, Guo CJ, Chen LL, Huarte M. Gene regulation by long non-coding RNAs and its biological functions. Nat Rev Mol Cell Biol. 2021;22(2):96-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bian W, Jing X, Yang Z, et al. Downregulation of LncRNA NORAD promotes ox-LDL-induced vascular endothelial cell injury and atherosclerosis. Aging. 2020;12(7):6385-6400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun DS, Guan CH, Wang WN, Hu ZT, Zhao YQ, Jiang XM. LncRNA NORAD promotes proliferation, migration and angiogenesis of hepatocellular carcinoma cells through targeting miR-211-5p/FOXD1/VEGF-A axis. Microvasc Res. 2021;134(1):104120. [DOI] [PubMed] [Google Scholar]
- 12.Liu D, Li L, Xu J, et al. Upregulated lncRNA NORAD can diagnose acute cerebral ischemic stroke patients and predict poor prognosis. Folia Neuropathol. 2023;61(1):105-110. [DOI] [PubMed] [Google Scholar]
- 13.Kai H, Wu Q, Yin R, et al. LncRNA NORAD promotes vascular endothelial cell injury and atherosclerosis through suppressing VEGF gene transcription via enhancing H3K9 deacetylation by recruiting HDAC6. Front Cell Dev Biol. 2021;9(1):701628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia SB, Tian ZB, Zhang W, Zhang H. NORAD Promotes the viability, migration, and phenotypic switch of human vascular smooth muscle cells during aortic dissection via LIN28B-mediated TGF-beta promotion and subsequent enhanced glycolysis. BioMed Res Int. 2022;6(1):5333928. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zhang X, Kan X, Shen J, Li J. Increased long non-coding RNA NORAD reflects serious cardiovascular stenosis, aggravated inflammation status, and higher lipid level in coronary heart disease. J Clin Lab Anal. 2022;36(11):e24717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saba L, Cau R, Murgia A, et al. Carotid plaque-RADS: A novel stroke risk classification system. JACC Cardiovasc Imaging. 2024;17(1):62-75. [DOI] [PubMed] [Google Scholar]
- 17.Hu W, Wei R, Wang L, Lu J, Liu H, Zhang W. Correlations of MMP-1, MMP-3, and MMP-12 with the degree of atherosclerosis, plaque stability and cardiovascular and cerebrovascular events. Exp Ther Med. 2018;15(2):1994-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H, Zhou J, Jiang W, Wang F. Analysis of the diagnostic and prognostic value of miR-9-5p in carotid artery stenosis. Bosn J Basic Med Sci. 2021;21(6):724-729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang F, Wang Y, Guo X. LncRNA THRIL functions as a marker for carotid artery stenosis and affects the biological function of human aortic endothelial cell. J Inflamm Res. 2023;16(1):2437-2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma H, Dong A. Dysregulation of lncRNA SNHG1/miR-145 axis affects the biological function of human carotid artery smooth muscle cells as a mechanism of carotid artery restenosis. Exp Ther Med. 2021;21(5):423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen D, Chen Q, Li J, Wang S, Song H, Wang F. Clinical value of long non-coding RNA HOTAIR in carotid artery stenosis and its role in vascular smooth muscle cell proliferation. Crit Rev Eukaryot Gene Expr. 2022;33(1):15-23. [DOI] [PubMed] [Google Scholar]
- 22.Thomas H, Diamond J, Vieco A, et al. Global atlas of cardiovascular disease 2000-2016: The path to prevention and control. Glob Heart. 2018;13(3):143-163. [DOI] [PubMed] [Google Scholar]
- 23.Onaolapo AY, Onaolapo OJ, Nathaniel TI. Cerebrovascular disease in the young adult: Examining melatonin’s possible multiple roles. J Exp Neurosci. 2019;13(1):1179069519827300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Chen Q. lncRNA PCA3 suppressed carotid artery stenosis and vascular smooth muscle cell function via negatively modulating the miR-124-3p/ITGB1 axis. Clin Appl Thromb/Hemost: Off J Int Acad Clin Appl Thromb/Hemost. 2023;29(1):10760296231190383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou A, Zhu W, Xu P, Zhao C, Jiang L, Yuan W. Carotid contrast-enhanced ultrasonography combined with sirtuin-3 in the diagnosis of plaques in carotid atherosclerosis. Adv Clin Exp Med: Off Organ Wroclaw Med Univ. 2022;31(12):1319-1326. [DOI] [PubMed] [Google Scholar]
- 26.Shigeno T, Mima T, Yanagisawa M, et al. Prevention of cerebral vasospasm by actinomycin D. J Neurosurg. 1991;74(6):940-943. [DOI] [PubMed] [Google Scholar]
- 27.Shi J, Yang Y, Cheng A, Xu G, He F. Metabolism of vascular smooth muscle cells in vascular diseases. Am J Physiol Heart Circ Physiol. 2020;319(3):H613-HH31. [DOI] [PubMed] [Google Scholar]
- 28.Lv C, Wang J, Dai S, Chen Y, Jiang X, Li X. Long non-coding RNA NORAD induces phenotypic regulation of vascular smooth muscle cells through regulating microRNA-136-5p-targeted KDM1A. Cell Cycle. 2021;20(20):2137-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]