Abstract
Background:
The correlation between fibrinogen levels and adrenocortical carcinoma (ACC) remains unclear. This study aimed to explore the value of preoperative plasma fibrinogen as a biomarker for ACC.
Methods:
We identified 40 patients with ACC and 170 patients with adrenal adenoma (AA) who underwent surgery at our institution between 2015 and 2022. Plasma fibrinogen levels and postoperative tumor recurrence information of the patients were also recorded. For intergroup comparisons, data obtained from the AA and ACC groups were evaluated using a t-test. The cutoff value of fibrinogen level was determined using a receiver operating characteristic (ROC) curve.
Results:
Mean fibrinogen levels in the AA and ACC groups were 2.81 ± 0.59 g/L and 3.88 ± 1.75 g/L, respectively (P < .001). Fibrinogen level, which can help distinguish between AA and ACC, was evaluated using the ROC curve. The cutoff fibrinogen level was estimated as 3.87 g/L according to the Youden index. With this value, the sensitivity was 62.5%, specificity was 95.7%, and the area under the ROC curve (AUC) was 0.74 (P < .001). Fibrinogen level, which can help distinguish between recurrence and non-recurrence, was evaluated using the ROC curve. The cutoff fibrinogen level was estimated as 3.96 g/L according to the Youden index. The sensitivity, specificity, and AUC were 90%, 71.4%, and 0.85, respectively (P < .001).
Conclusion:
According to the data in this study, plasma fibrinogen could be used to distinguish ACC from AA. Most importantly, plasma fibrinogen may be used to identify recurrence of postoperative ACC.
Keywords: Fibrinogen, adrenocortical carcinoma, adrenal adenoma, recurrence, ROC curve
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy with a poor prognosis and an incidence rate of 0.5 to 2 per million per year. 1 Owing to the insidious onset of ACC, the disease usually does not cause any clinical symptoms until it advances to progressive stages. Therefore, there is an urgent need for the early detection and diagnosis of early diagnostic markers for clinical use. Most patients with ACC have poor prognosis; the expected 5-year survival rate of stage I patients is 80%, whereas that of stage IV patients is only 13%. 2 Even patients with stage I to III tumors still have a high risk of recurrence after surgical treatment, and approximately 40% of them can develop recurrence and metastasis within 2 years. 3 Surgery is the main treatment for most early-stage patients, and mitotane can be used as an initial treatment for patients with ACC who have no surgical opportunity or cannot completely remove the tumor. Mitotane alone may delay or prevent recurrence. 4 However, Glenn et al 5 conducted a retrospective review of 576 patients with ACC who underwent resection of stage I to III ACC and found that 70% of the patients developed disease recurrence.
Activation of the coagulation system is closely related to the occurrence, development, and metastasis of tumors. Fibrinogen is a key glycoprotein produced by the liver that functions as a nonspecific acute-phase reactant and is involved in inflammatory responses, clotting pathways, and tumor formation. Fibrinogen is involved in the proliferation and metastasis of malignant tumors by participating in the composition of the extracellular matrix in the tumor microenvironment, which is recognized by various integrins, activating the coagulation system, and promoting the metastasis of circulating tumor cells. 6 A large number of studies have suggested fibrinogen as a valuable marker for detecting cancers, informing prognoses, and monitoring treatment responses.7 -9 Patients with malignant tumors often exhibit varying degrees of blood hypercoagulability. Ma et al 10 conducted a retrospective analysis of penile cancer and found that preoperative fibrinogen level can be used as an independent prognostic marker to predict the survival outcome of patients. Masaaki Yamamoto et al 11 found that preoperative plasma fibrinogen level had the highest predictive value for recurrence among seven known prognostic markers (C-reactive protein, platelet count, platelet/lymphocyte ratio, and neutrophil/lymphocyte ratio), which would be useful for predicting prognosis after gastric cancer surgery. However, the correlation between fibrinogen levels and ACC remains unclear. In this study, we investigated the correlation between fibrinogen levels and ACC.
Methods
We retrospectively evaluated the data of 40 patients with ACC and 170 patients with adrenal adenoma (AA) who underwent resection at Tianjin Medical University General Hospital and Peking Union Medical College Hospital between January 1, 2015 and May 1, 2022. Tumors were graded according to the Union for International Cancer Control TNM 2004 staging system. All pertinent laboratory and pathological results and medical data were obtained from hospital databases. Data obtained from the patients’ routine preoperative test results included plasma fibrinogen levels, age, sex, body mass index (BMI), type of surgery, marginal condition, mitotane-based specific chemotherapy, and radiotherapy. The time points for blood collection to assess fibrinogen levels was 1 day before surgery. Fibrinogen levels were measured by enzyme-linked immunosorbent assay (ELISA) using the assay kits “KA0475” from Abnova. Patients with coexisting hematologic diseases and those in the inflammatory phase were excluded from this study. The cutoff value of fibrinogen level was determined using a receiver operating characteristic (ROC) curve to distinguish between AA and ACC. Patients with ACC were divided into recurrence and non-recurrence groups according to whether they experienced recurrence 1 year after surgery. Preoperative plasma fibrinogen levels were used to distinguish postoperative recurrence of ACC, and the cutoff value of the ROC curve was obtained.
Statistical analyses were performed using IBM SPSS Statistics for Windows (version 22.0; IBM Corp., Armonk, NY, USA). For data comparison within the inter-group comparison (AA and ACC groups), data obtained from patients were evaluated using Student’s t-test. Statistical significance was set at P < .05.
Results
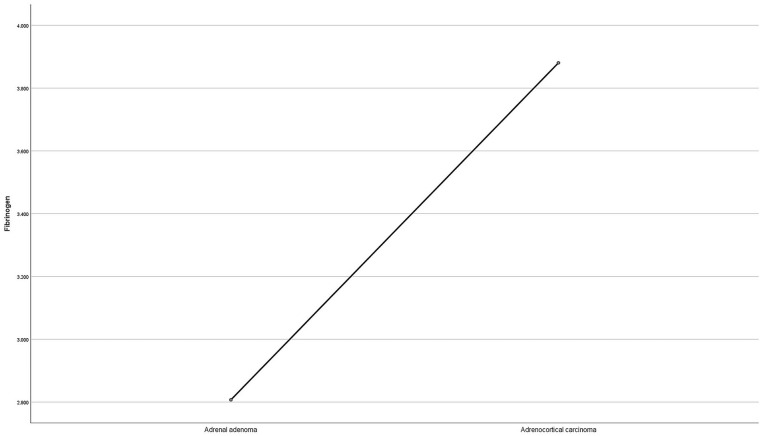
The data of the 210 cases are presented in Table 1 (including fibrinogen levels, sex, age, BMI, stage, and recurrence information). ACC consisted of 40 patients (there were 46 patients, 6 of whom were excluded), with the diameter from 3.5 to 19.0 cm and an average diameter of 6.67 ± 3.70 cm; whereas AA consisted of 170 patients (there were 204 patients, 34 of whom were excluded), with the diameter from 0.7 to 5.0 cm and an average diameter of 2.11 ± 0.60 cm. The mean fibrinogen of the AA and ACC groups were 2.81 ± 0.59 g/L and 3.88 ± 1.75 g/L, respectively (P < .001; see Figure 1).
Table 1.
Basic characteristic of patients.
| Groups | ACC | AA |
|---|---|---|
| Number of cases | 40 | 170 |
| Gender | ||
| M | 21 | 79 |
| F | 19 | 91 |
| Age (mean years) | 62.93 ± 5.37 | 55.97 ± 11.36 |
| BMI (mean) | 24.68 ± 2.26 | 25.74 ± 4.99 |
| Fibrinogen (mean, g/L) | 3.88 ± 1.75 | 2.81 ± 0.59 |
| Marginal condition (positive/negative) | 6/34 | — |
| Mitotane-based specific chemotherapy (Yes vs No) | 31/9 | — |
| Stage (cases) | ||
| I-II | 26 | — |
| III-IV | 14 | — |
| Postoperative recurrence (1 year) | ||
| Yes | 24 | — |
| No | 16 | — |
Abbreviations: AA, adrenal adenoma; ACC, adrenocortical carcinoma.
Figure 1.
The mean fibrinogen value of the AA and ACC groups. The fibrinogen levels are higher in patients with ACC than in patients with AA. AA indicates adrenal adenoma; ACC, adrenocortical carcinoma.
Fibrinogen level, which can help distinguish between AA and ACC, was evaluated using the ROC curve. The cutoff value of fibrinogen level was estimated as 3.87 g/L according to the Youden index (see Figure 2). With this value, the sensitivity was 62.5%, specificity was 95.7%, and the area under the ROC curve (AUC) was 0.74 (P < .001, confidence interval = 0.628-0.857).
Figure 2.
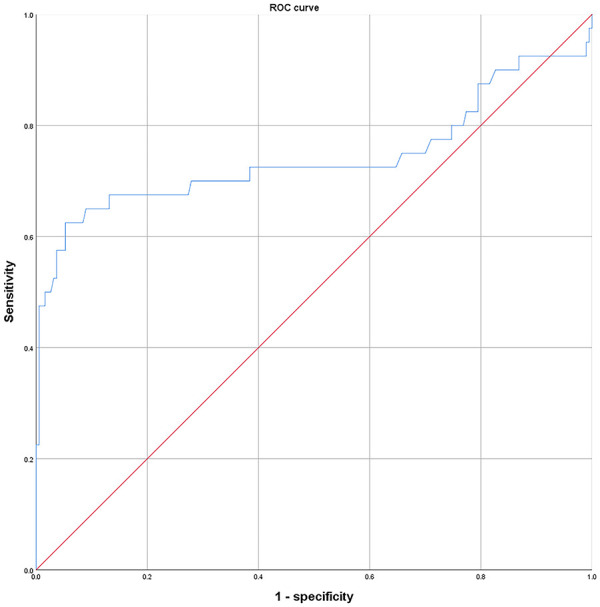
ROC curves of fibrinogen for distinguishing between AA and ACC. According to the cutoff value of fibrinogen, sensitivity was found to be 62.5%, specificity was 95.7%, and area under ROC curve (AUC) was 0.74 (P < .001, confidence interval = 0.628-0.857).
AA indicates adrenal adenoma; ACC, adrenocortical carcinoma; ROC, receiver operating characteristic.
In ACC tumors, the fibrinogen level can help distinguish between postoperative recurrence and no recurrence, which was evaluated using the ROC curve. The cutoff value of fibrinogen level was estimated as 3.96 g/L according to the Youden index (see Figure 3). The sensitivity, specificity, and AUC were 90%, 71.4%, and 0.85 (confidence interval = 0.690-1.0), respectively (P < .001)
Figure 3.
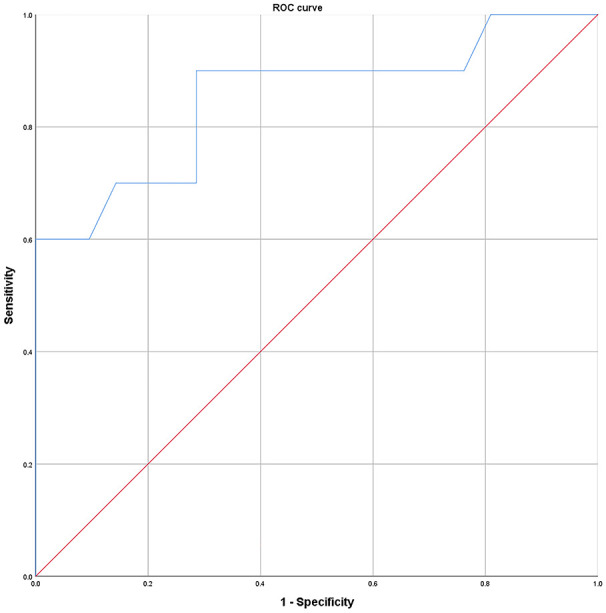
ROC curves of fibrinogen for distinguishing between postoperative tumor recurrence and no-recurrence. According to the cutoff value of fibrinogen, sensitivity was found as 90%, specificity was 71.4%, and AUC was 0.85 (P < .001, confidence interval = 0.690-1.0).
AUC indicates area under the ROC curve; ROC, receiver operating characteristic.
Discussion
Patients with tumors are often in a hypercoagulable state.12,13 Some studies have shown that fibrinogen levels are higher in patients with tumors than in patients without tumors.14 -16 To the best of our knowledge, this is the first study to examine the correlation between fibrinogen levels and ACC. This study confirmed these findings. First, the mean fibrinogen of ACC was significantly higher than AA (3.88 ± 1.75 g/L vs 2.81 ± 0.59 g/L, respectively; P < .001). Fibrinogen levels can help distinguish between AA and ACC using an ROC curve. The cutoff fibrinogen level was estimated as 3.87 g/L. Second, fibrinogen level can help distinguish between postoperative recurrence and non-recurrence. The cutoff fibrinogen level was estimated as 3.96 g/L.
One of the major clinical features of ACC is its low rate of early diagnosis, and it is difficult to distinguish ACC from benign adenomas using preoperative computed tomography (CT) or magnetic resonance imaging (MRI). Approximately half of the patients were initially treated for metastatic symptoms. Another characteristic of patients with ACC is poor prognosis. Postoperative in situ recurrence of ACC is mainly related to late tumor stage and surgical technique. Surgical resection of patients with locally advanced ACC can be performed according to the R0 resection principles. If necessary, the adjacent organs that may be involved should be excised, which may reduce the risk of local recurrence. Misperception of benign adrenal tumors before surgery and failure to observe the principle of no tumor during surgery increases the risk of local recurrence after surgery. These guidelines are not the preferred recommendations for minimally invasive surgery of ACC. Because ACC could not be accurately judged before surgery, minimally invasive surgical treatment was adopted, and intraoperative tumor rupture or R0 resection could not be achieved, which is an important cause of local recurrence. 17 The reasons about ACC patients the high risk of recurrence in patients with ACC include a ruptured capsule, large-size or high-grade histology. 18 Telomerase reverse transcriptase (TERT) has recently been shown to immortalize cells via telomerase activation and is associated with the recurrence of ACC. 19 Based on microarray analysis of lncRNA expression profiles, Glover et al 20 speculated that three genes, BUB1B, IGFL2, and IGFBP5, were significantly correlated with differentially expressed lncRNAs. In patients without recurrence who tolerate mitotane therapy, it is recommended that the drug be administered for at least 2 years 21 and up to 5 years. 22 It is difficult to determine whether therapeutic benefits result from mitotane alone. In a meta-analysis including six retrospective studies, mitotane therapy resulted in improved mortality but not in decreased recurrence. 22
Many studies have focused on using preoperative blood tests (red blood cell distribution width, lymphocyte-to-monocyte ratio, mean platelet volume, neutrophil/lymphocyte ratio, and platelet/lymphocyte ratio) to predict outcomes in patients with ACC.23 -25 However, no study has evaluated whether fibrinogen can differentiate AA from ACC and predict postoperative ACC recurrence. Fibrinogen is synthesized in the liver as a 350 kDa glycoprotein, which is converted to insoluble fibrin by activating thrombin. Fibrinogens play an important role in blood coagulation, thrombosis, wound healing, and platelet aggregation. In recent years, the relationship between hypercoagulability and malignant tumor progression has attracted considerable attention. Plasma fibrinogen is the main protein involved in coagulation in humans. The coagulation function of patients with malignant tumors is enhanced, and the body is in a state of hypercoagulation, which can easily lead to thrombosis. Fibrin plays an important role in inflammation and blood agglutination reactions. In addition, recent studies have shown the relationship between fibrinogen and ovarian cancer, 26 breast cancer, 27 renal cell carcinoma,28,29 cervical cancer, 30 esophageal cancer, 31 colon cancer, 32 prostate cancer, 33 non-small cell lung cancer, 34 and bladder cancer, 35 as potential factors to predict the therapeutic efficacy and prognosis of patients with many types of cancer. In this study, ROC analysis revealed that preoperative plasma fibrinogen level was of great value in the differential diagnosis of ACC and AA and in the identification of postoperative recurrence of ACC. To the best of our knowledge, this is the only study to evaluate the relationship between ACC and fibrinogen levels. In addition, it is unclear whether fibrinogen level is an independent prognostic factor for survival. Previous studies have described the mechanisms underlying hyperfibrinogenemia in patients with malignant tumors.36,37 Malignant cells usually have high levels of a fibrinogen receptor called intercellular adhesion molecule 1 (ICAM-1), whereas platelets have a fibrinogen receptor called aIIb3 integrin. Malignant cells bind to platelets via fibrinogen. These clumps form tumor clots that bind to the endothelium at the original site of the adrenal gland, adjacent tissues, and other organs, and hide in the target organ, preventing the immune system from attacking. Through this mechanism, fibrinogen plays a role in tumor progression, recurrence, and metastasis.
This study has some limitations. First, owing to the retrospective nature of this study, the attributable factors suffered from recall bias and could not be analyzed. Second, long-term exposure to elevated glucocorticoid levels leads to alterations in fibrinogen levels and function, possibly similar to tumor-associated inflammatory responses. To some extent, our findings may have been influenced by the functional status of the tumor and resulting glucocorticoid overproduction. Third, the values of systemic coagulation markers are highly influenced by many factors; therefore, small increases or decreases in these values may have affected the assessment. We believe that more comprehensive and extensive patient studies may more clearly reveal the correlation between fibrinogen levels and tumor volume in ACC.
Conclusions
ACC is a rare malignancy with a poor prognosis. Preoperative plasma fibrinogen level had the highest predictive value for recurrence among seven known prognostic markers, which would be useful for predicting prognosis after cancer surgery. However, the correlation between fibrinogen levels and ACC remains unclear. In this study, we investigated the correlation between fibrinogen levels and ACC. According to the data in this study, it reminds us that plasma fibrinogen could be used to distinguish ACC from AA. Most importantly, plasma fibrinogen may be used to identify recurrence of postoperative ACC.
Acknowledgments
Not applicable.
Footnotes
Author Contributions: Study conception and design was done by CM and QM. Data acquisition was done by CM and BY. BY contributed toward software. Data analysis and interpretation of results was done by all authors. Initial drafting of article was done by CM. Critical revision of article was done by CM and QM. All authors read and approved the final article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Scientific Research Project of Tianjin Municipal Education Commission (grant no. 2023KJ115) and sponsored by Tianjin Health Research Project (Grant No. TJWJ2024QN001).
Data Availability Statement: The datasets used and/or analyzed during this study are available from the corresponding author on reasonable request.
Ethical Statement: This study was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments. Local ethical approval was obtained from the Ethics Committee of the Peking Union Medical College Hospital (2021-022801) and the Ethics Committee of Tianjin Medical University General Hospital (Institutional Review Board 2023-KY-088).
Informed Consent: Written informed consent was obtained from all patients for being included in the study.
ORCID iD: Chengquan Ma  https://orcid.org/0000-0003-3612-610X
https://orcid.org/0000-0003-3612-610X
References
- 1. Fassnacht M, Assie G, Baudin E, et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1476-1490. doi: 10.1016/j.annonc.2020.08.2099 [DOI] [PubMed] [Google Scholar]
- 2. Lafemina J, Brennan MF. Adrenocortical carcinoma: past, present, and future. J Surg Oncol. 2012;106:586-594. doi: 10.1002/jso.23112 [DOI] [PubMed] [Google Scholar]
- 3. Boulle N, Logié A, Gicquel C, Perin L, Le Bouc Y. Increased levels of insulin-like growth factor II (IGF-II) and IGF-binding protein-2 are associated with malignancy in sporadic adrenocortical tumors. J Clin Endocrinol Metab. 1998;83:1713-1720. doi: 10.1210/jcem.83.5.4816 [DOI] [PubMed] [Google Scholar]
- 4. Puglisi S, Calabrese A, Basile V, et al. New perspectives for mitotane treatment of adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2020;34:101415. doi: 10.1016/j.beem.2020.101415 [DOI] [PubMed] [Google Scholar]
- 5. Glenn JA, Else T, Hughes DT, et al. Longitudinal patterns of recurrence in patients with adrenocortical carcinoma. Surgery. 2019;165:186-195. doi: 10.1016/j.surg.2018.04.068 [DOI] [PubMed] [Google Scholar]
- 6. He SS, Wang Y, Yang L, et al. Plasma fibrinogen correlates with metastasis and is associated with prognosis in human nasopharyngeal carcinoma. J Cancer. 2017;8:403-409. doi: 10.7150/jca.17028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhu LR, Li J, Chen P, Jiang Q, Tang XP. Clinical significance of plasma fibrinogen and D-dimer in predicting the chemotherapy efficacy and prognosis for small cell lung cancer patients. Clin Transl Oncol. 2016;18:178-188. doi: 10.1007/s12094-015-1350-7 [DOI] [PubMed] [Google Scholar]
- 8. Yuan C, Huang M, Wang H, Jiang W, Su C, Zhou S. Pretreatment fibrinogen-albumin ratio (FAR) associated with treatment response and survival in advanced non-small cell lung cancer patients treated with first-line anti-PD-1 therapy plus platinum-based combination chemotherapy. Cancer Manag Res. 2022;14:377-386. doi: 10.2147/CMAR.S347547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu W, Bao L, Qian Z, Wang D. Neutrophil-lymphocyte ratio combined with plasma fibrinogen is an useful predictor for the diagnosis of liver metastases from gastric cancer. Asian J Surg. 2022;45:2378-2379. doi: 10.1016/j.asjsur.2022.05.058 [DOI] [PubMed] [Google Scholar]
- 10. Ma C, Zhou Y, Zhou S, Zhao K, Lu B, Sun E. Preoperative peripheral plasma fibrinogen level is an independent prognostic marker in penile cancer. Oncotarget. 2017;8:12355-12363. doi: 10.18632/oncotarget.12563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yamamoto M, Kurokawa Y, Miyazaki Y, et al. Usefulness of preoperative plasma fibrinogen versus other prognostic markers for predicting gastric cancer recurrence. World J Surg. 2016;40:1904-1909. doi: 10.1007/s00268-016-3474-5 [DOI] [PubMed] [Google Scholar]
- 12. Falanga A, Marchetti M. Cancer-associated thrombosis: enhanced awareness and pathophysiologic complexity. J Thromb Haemost. 2023;21:1397-1408. doi: 10.1016/j.jtha.2023.02.029 [DOI] [PubMed] [Google Scholar]
- 13. Liz-Pimenta J, Tavares V, Neto BV, et al. Thrombosis and cachexia in cancer: two partners in crime? Crit Rev Oncol Hematol. 2023;186:103989. doi: 10.1016/j.critrevonc.2023.103989 [DOI] [PubMed] [Google Scholar]
- 14. Asanuma K, Matsumine A, Nakamura T, et al. Impact of plasma fibrinogen levels in benign and malignant soft tissue tumors. Cancer Biomark. 2016;16:453-458. doi: 10.3233/CBM-160584 [DOI] [PubMed] [Google Scholar]
- 15. Hefler-Frischmuth K, Lafleur J, Hefler L, et al. Plasma fibrinogen levels in patients with benign and malignant ovarian tumors. Gynecol Oncol. 2015;136:567-570. doi: 10.1016/j.ygyno.2014.12.041 [DOI] [PubMed] [Google Scholar]
- 16. Záhorec R, Marek V, Waczulikova I, et al. Predictive model using hemoglobin, albumin, fibrinogen, and neutrophil-to-lymphocyte ratio to distinguish patients with colorectal cancer from those with benign adenoma. Neoplasma. 2021;68:1292-1300. doi: 10.4149/neo_2021_210331N435 [DOI] [PubMed] [Google Scholar]
- 17. Schimmack S, Strobel O. Resektionsstrategie bei Nebennierenrindenkarzinomen [Resection strategies for adrenocortical carcinoma]. Chirurg. 2019;90:9-14. doi: 10.1007/s00104-018-0712-4 [DOI] [PubMed] [Google Scholar]
- 18. Kreissl MC, Bastholt L, Elisei R, et al. Efficacy and safety of vandetanib in progressive and symptomatic medullary thyroid cancer: post hoc analysis from the ZETA trial. J Clin Oncol. 2020;38:2773-2781. doi: 10.1200/JCO.19.02790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Svahn F, Paulsson JO, Stenman A, et al. TERT promoter hypermethylation is associated with poor prognosis in adrenocortical carcinoma. Int J Mol Med. 2018;42:1675-1683. doi: 10.3892/ijmm.2018.3735 [DOI] [PubMed] [Google Scholar]
- 20. Glover AR, Zhao JT, Ip JC, et al. Long noncoding RNA profiles of adrenocortical cancer can be used to predict recurrence. Endocr Relat Cancer. 2015;22:99-109. doi: 10.1530/ERC-14-0457 [DOI] [PubMed] [Google Scholar]
- 21. Basile V, Puglisi S, Altieri B, et al. What is the optimal duration of adjuvant mitotane therapy in adrenocortical carcinoma? An unanswered question. J Pers Med. 2021;11:269. doi: 10.3390/jpm11040269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fassnacht M, Dekkers OM, Else T, et al. European Society of Endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179:G1-G46. doi: 10.1530/EJE-18-0608 [DOI] [PubMed] [Google Scholar]
- 23. Gaitanidis A, Wiseman D, El Lakis M, Nilubol N, Kebebew E, Patel D. Preoperative systemic inflammatory markers are prognostic indicators in recurrent adrenocortical carcinoma. J Surg Oncol. 2019;120:1450-1455. doi: 10.1002/jso.25760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. de Jong MC, Mihai R, Khan S. Neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte ratio (PLR) as possible prognostic markers for patients undergoing resection of adrenocortical carcinoma. World J Surg. 2021;45:754-764. doi: 10.1007/s00268-020-05868-6 [DOI] [PubMed] [Google Scholar]
- 25. Sisman P, Bicer B, Gul OO, et al. May hemocytometer parameters be a biomarker in distinguishing between adrenal adenomas and carcinomas and in prognosis of adrenocortical carcinomas? Acta Clin Croat. 2020;59:439-444. doi: 10.20471/acc.2020.59.03.07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen W, Shan B, Zhou S, Yang H, Ye S. Fibrinogen/albumin ratio as a promising predictor of platinum response and survival in ovarian clear cell carcinoma. BMC Cancer. 2022;22:92. doi: 10.1186/s12885-022-09204-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dirix LY, Oeyen S, Buys A, et al. Coagulation/fibrinolysis and circulating tumor cells in patients with advanced breast cancer. Breast Cancer Res Treat. 2022;192:583-591. doi: 10.1007/s10549-021-06484-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsimafeyeu IV, Demidov LV, Madzhuga AV, Somonova OV, Yelizarova AL. Hypercoagulability as a prognostic factor for survival in patients with metastatic renal cell carcinoma. J Exp Clin Cancer Res. 2009;28:30. doi: 10.1186/1756-9966-28-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Z, Fan H, Wang W, et al. High preoperative plasma fibrinogen independently predicts a poor prognosis in patients with nonmetastatic RCC. J Cancer. 2020;11:2401-2407. doi: 10.7150/jca.40961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhao K, Deng H, Qin Y, Liao W, Liang W. Prognostic significance of pretreatment plasma fibrinogen and platelet levels in patients with early-stage cervical cancer. Gynecol Obstet Invest. 2015;79:25-33. doi: 10.1159/000365477 [DOI] [PubMed] [Google Scholar]
- 31. Hoshino S, Matsuda S, Kawakubo H, et al. Elevation of the prognostic factor plasma fibrinogen reflects the immunosuppressive tumor microenvironment in esophageal squamous cell carcinoma. Ann Surg Oncol. 2022;29:6894-6904. doi: 10.1245/s10434-022-11974-7 [DOI] [PubMed] [Google Scholar]
- 32. Zheng X, Xu K, Zhou B, et al. A circulating extracellular vesicles-based novel screening tool for colorectal cancer revealed by shotgun and data-independent acquisition mass spectrometry. J Extracell Vesicles. 2020;9:1750202. doi: 10.1080/20013078.2020.1750202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Y, Chen W, Hu C, et al. Albumin and fibrinogen combined prognostic grade predicts prognosis of patients with prostate cancer. J Cancer. 2017;8:3992-4001. doi: 10.7150/jca.21061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang W, Liu P, Zong M, et al. Combining lactate dehydrogenase and fibrinogen: potential factors to predict therapeutic efficacy and prognosis of patients with small-cell lung cancer. Cancer Manag Res. 2021;13:4299-4307. doi: 10.2147/CMAR.S300153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li X, Shu K, Zhou J, et al. Preoperative plasma fibrinogen and D-dimer as prognostic biomarkers for non-muscle-invasive bladder cancer. Clin Genitourin Cancer. 2020;18:11-19. doi: 10.1016/j.clgc.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 36. Liu X, Shi B. Progress in research on the role of fibrinogen in lung cancer. Open Life Sci. 2020;15:326-330. doi: 10.1515/biol-2020-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madani M, Goldstein D, Stefanescu R, Woodman SE, Rojas-Hernandez CM. Characterization of coagulopathy and outcomes in cancer patients with severe COVID-19 illness: longitudinal changes in hospitalized cancer patients. Cancer Med. 2022;11:3771-3785. doi: 10.1002/cam4.4753 [DOI] [PMC free article] [PubMed] [Google Scholar]