ABSTRACT
Background and Aims
In the past decade, unmanned aerial systems (UASs), commonly known as drones, have found applications not only in military and agriculture but also in the transportation of medical supplies.
Purpose
The present study was conducted to assess the practicality of utilizing drones as a mode for the delivery of vaccines to combat the challenges.
Study Design
An exploratory study.
Methodology
Due to the COVID‐19 pandemic restrictions and paucity of availability of rules and regulations related to drones in India in 2021, this study was conducted as a exploratory study for which number of regulatory approvals are obtained and it involves five drone missions within the premises of the Indian Institute of Technology (IIT), Kanpur, India on a confined airstrip of 3 km2 to transport simulated vaccine vials using a multi‐rotor top‐load UAS in the normal weather conditions in daylight where dummy vaccine vials (COVID‐19) were packed with cool packs to maintain the temperature. Study was conducted to explore feasibility to carry vaccines through drones and any environmental impact on the vaccine vials while its transportation.
Results
The drones demonstrated a maximum flight endurance of 31 min while carrying a payload of up to 4.5 Kg, covering an aerial distance of 17 km at an average speed ranging at 12 m per second. Notably, the vaccine carrier box was able to maintain a recommended temperature of 3°C–4°C throughout the transportation process, and there is no impact of vibration on the physical integrity and leakage of the vaccine vials during flight.
Conclusions
These findings signify the potential for the drone‐based medical supply deliveries across confined and controlled environment conditions. This study provides the insights that there is no environmental impact such as humidity, temperature, wind etc on the drone and no impact on vibrations on the physical integrity and leakage of the dummy vaccine vials. There were few regulatory barriers that required special approvals from concerned authorities. The study was not designed to assess for cost‐effectiveness, also it was conducted in defined geography so all sorties were VLOS. Study has various limitations such as using simulated vaccine vials, regulatory barriers, operational barriers etc. Conducting the study in a controlled environment at IIT Kanpur limits generalizability. In spite of these limitations this study provides valuable insights and may explore a diverse environment that can help in strengthening health services especially in difficult terrains.
Keywords: COVID‐19, drones, immunization, unmanned aerial vehicle, vaccine, vaccine delivery
1. Introduction
Implementation of immunization programs often faces numerous challenges in delivering vaccines, especially in hard‐to‐reach terrains such as hills, islands, dense forests and remote villages [1, 2]. In India, road transportation serves as the most commonly deployed mode for vaccine mobility at the district level. Although such transportations works effectively in regions with established metallic road connectivity, challenges remain in hard‐to‐reach terrains [3, 4]. Natural disasters, social disruptions causing blockades on roads, extreme weather conditions etc. may interrupt timely delivery of vaccines in these terrains [5]. On the other hand, pandemic‐like situations such as COVID‐19 demands fast and efficient vaccine delivery with wider geographical and population level coverage as vaccination prevents progression to disease severity and reduces chance of hospitalization [6, 7].
Unmanned aerial systems (UAS) or drones, primarily developed for defense purposes, since drones are known for their rapid, cost‐effective and safe delivery of goods even in hard‐to‐reach terrains compared to other air transportation systems [8]. In geographically diverse countries like India, it could be one of the ways to overcome the transportation challenges. However, this study was conducted in late 2020 to early 2021 in India when using drones was challenging as it was not guided by rules differentiated to define the limits, restrictions or permission. Whereas, as per the Drone Rules 2021, the Aircraft Act, 1934 (Aircraft Act) and the Aircraft Rules, 1937 (Aircraft Rules) drone is that provides guidance and a legal framework where drones can be used as delivery of medical supplies…with few exceptions. The integration of drones into airspace raises regulatory and safety challenges. The literature discusses existing regulations, potential risks, and the need for frameworks to ensure safe operations, especially in populated areas [9].
As per the current Drone rules 2021, drones can be flown upto 400 feet above ground level (AGL) in green zone, where no special permission is required for government operations and research activities [10]. In case disaster sites are located far from the supply and when access to the disaster site is difficult, drones can be used for emergency medical supplies [11]. Drones can significantly reduce delivery times for medical supplies, particularly in rural or hard‐to‐reach areas. Studies highlight the ability of drones to bypass traffic and terrain obstacles, ensuring timely delivery of critical medical supplies such as blood, vaccines, and medications [12].
The advantages far outweigh the difficulties, once it is put to use, it might be helping in achieving the global goal of “Health for All”.
Some studies explored drone‐mediated vaccine delivery that underscored its potential to revolutionize healthcare accessibility, particularly in remote and hard‐to‐reach areas. Feasibility of using drones to transport vaccines in rural Mozambique, highlighting the ability of drones to overcome infrastructural challenges and significantly reduce delivery times compared to traditional methods like mobile real‐time surveillance done for Zika virus in Brazil [13]. The efficacy of drone delivery in maintaining vaccine cold chain integrity and reaching isolated communities was checked in rural Rwanda with limited road access [14]. Several case studies demonstrate the successful implementation of drone delivery systems. For example, Zipline operates in countries like Rwanda and Ghana, delivering blood and vaccines. These initiatives have shown promising results in improving healthcare access and reducing wastage of medical supplies [15]. Research indicates that drone delivery can improve health outcomes by ensuring timely access to essential supplies. Studies also explore the cost‐effectiveness of drone logistics compared to traditional methods [16]. While these studies provide valuable insights into the feasibility of drone‐based vaccine delivery, they predominantly focus on specific geographical contexts and operational parameters, leaving a research gap regarding the broader accessibility and feasibility of drones in healthcare delivery across diverse settings.
Some comparable studies that have been done in other nations regarding the viability of using drones to deliver vaccines show the difficulties encountered during the study. One feasibility study conducted by Thapa offered one potential strategy for deploying unmanned aerial vehicles (UAVs) to deliver vaccines to remote regions of Nepal [17]. In Sri Lanka, Kurunegala [18], was declared for a UAV deployment center that was advised as the starting point for implementing the proposed UAV‐inclusive vaccine delivery system [19]. These studies are being conducted to deal with the issues of traffic, truck load, to lessen human resource, and logistical issues. UAVs program is proposed as a solution to overcome system inefficiencies in Sri Lanka's current vaccine cold chain because of their faster speed and lower response time [18].
Various other institutions like All India Institute of Medical Sciences (AIIMS), Rishikesh, Tehri government hospital have also tried to understand the challenges associated with drone based medical supplies in the lower himalayan ranges, where it was difficult to reach by road due to difficult terrain [20]. Because of the future scope of drones as a transportation vehicle and growing interest, there is need for more ground level evidence on various feasibility aspects such as practicality, technical adaptability, effect of vibration, temperature effects, and any physical impact due to drones based transport. The literature on drone‐mediated medical deliveries illustrates a growing interest in leveraging drone technology to enhance healthcare delivery, particularly in challenging environments. While operational efficiencies and positive health outcomes are evident, regulatory frameworks and public acceptance remain critical areas for ongoing research [21]. Therefore, the present study aimed to conduct field study to understand how effective are indigenous drones in transporting dummy vaccine vials, and what impact does such transportation have on the cold chain maintenance within the Vaccine Carrier Box (VCB) and the physical integrity of the vaccine vials.
This research is necessary due to the existing gap in understanding the phenomenon by which the vaccines can be transported via drones under different time scenarios with few climatic conditions such as temperature, humidity, wind speed, and UV light and drone related factors such as vibration, and vaccine vials’ physical integrity and leakage. By investigating this vialsarea, the study aims to enhance our understanding of the usage of drones as a safe transportation mode which is crucial for practical implications in medical research and service delivery, policy‐making, intervention strategies, etc.
The primary objectives of this study are: to explore the capability of indigenous drones to carry dummy vaccine vials and evaluate the influence of this transportation on both the temperature conditions within the VCB and the physical state of the vaccine vials; and to contribute empirical data that can inform if drones can be an effective method to transport the vaccines without impact of climatic conditions, thus bridging the gap between research and practice.
This study is an initial kind of study which is conducted in regards to exploring the impact of environmental factors on the drone and of vibrations on the physical integrity and leakage of vaccine vials and was a success and demonstrated the effectiveness of drones in transporting simulated vaccine vials, demonstrating their operational capacity and temperature stability. The drones also demonstrated no physical damage, leakage, or vibration during transportation, indicating their ability to deliver sensitive medical supplies without compromising quality. The findings suggest potential for drone‐based deliveries in remote or underserved areas. However, challenges include obtaining regulatory approvals, a limited trial scope, VLOS constraints, and a lack of cost‐effectiveness analysis, which could hinder the widespread implementation of drone delivery in real‐world scenarios.
2. Materials and Methods
The present study was conducted in a controlled environment at Indian Institute of Technology Kanpur Uttar Pradesh India, to test the feasibility of drones to carry VCB in five different sorties and to explore the possibility of integrating drone based vaccine delivery in the existing vaccine delivery mechanism. The focus of the study was based on the practicality of the intervention in the use of drones in vaccine delivery. This study was conceptualized by the Indian Council of Medical Research (ICMR), New Delhi in December 2020 and implemented in May 2021. The study sites along with the sorties were pre‐decided for the planned flights.
2.1. Study Setting
The visible line of sight (VLOS) drones were used in the study where VLOS describes a flight in which the pilot maintains constant visual contact with the drone that is intended to prevent collisions with objects like buildings or trees. The VLOS drone sorties were conducted within the premises of the Indian Institute of Technology (IIT), Kanpur, India on a confined airstrip of 3 km2. The airstrip is a part of the aerospace laboratory, providing ambient air and land space for conducting drone trials (Figure 3).
-
A.
Stakeholders and Their Roles: To ensure the success of the research, three organizations participated as study partners. The following is a description of the various roles within organizations. The following is a description of the various roles within organizations:
-
1.
The Indian Council of Medical Research: ICMR, New Delhi, the apex body in India for the formulation, coordination and promotion of biomedical research, is one of the oldest medical research bodies in the world. The ICMR funded this study trying to find a practical solution to the vaccine delivery chain system to the areas that are hard to reach by road. ICMR obtained dummy Vaccine vials, Vaccine Storage Box and were provided by Bharat Biotech.
-
2.
IIT Kanpur & CD Space: Since the stakeholder partner is an aerospace engineer and the area includes an airstrip route accessible for drone‐related operations, the site was identified. In addition to providing knowledge in UAVs, CD Space and IIT Kanpur established a drone command center on the site, trained drone pilots, and handled other technical aspects. The multirotor drone was indigenously designed by IIT Kanpur for the study as there were restrictions at that time before Drone rules, 2021.
-
3.
Research Team: A multi‐disciplinary team comprising members from medical background such as doctor, health worker to ensure adherence to all SOPs related to packaging, transportation of VCB, loading and unloading of VCB; engineers consisted of a trained drone pilot and technical experts to conduct and manage drone flights, drone‐related activities, also to capture the data related to flying operation; drone partner to transport dummy vaccine vials. Multirotor drone (Figure 1a) was used that falls under the category of small unmanned aircraft vehicle as per the classification of Drone Rules (2021) of which characteristics, and specifications mentioned in Table 1 and drone configurations are mentioned in Table 2. The reason for selecting the multirotor drone are:
Multirotor drones were chosen for their stability, being the first trial by the ICMR for this study.
Their superior weight carrying capacity aligns perfectly with our specifications.
As it was indigenously designed by the IIT‐Kanpur team, the management of drones, including the drone team, benefits from in‐house expertize.
Figure 3.
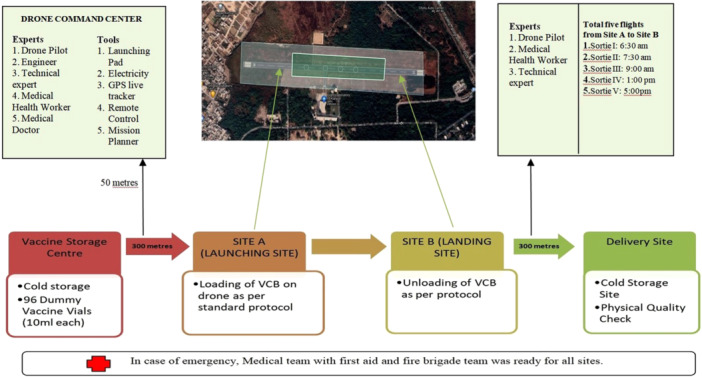
Flowchart showing the various sites for conducting VLOS drone flight sorties with different time slots.
Figure 1.
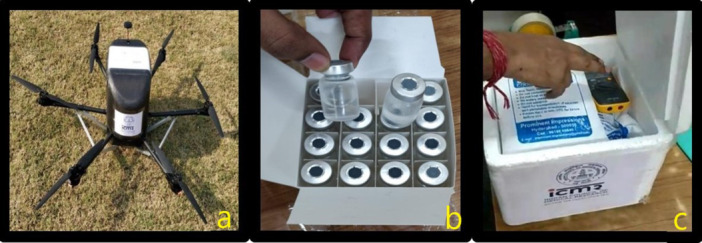
(a) Multirotor drone used in study, (b) Dummy vaccine vials (provided by Bharat Biotech) (c) Placement of electronic data logging monitor (EDLM) as per instructions.
Table 1.
Characteristics and technical specifications of drone.
| Particulars | Measures |
|---|---|
| Drone category | Small |
| Drone type | Multirotor |
| Drone's make | CD Space Robotics Pvt. Ltd. |
| Drone's model name | CDSHEXM001 |
| Drone's maximum weight | 24 kg |
| Overall dimension | 1400 × 1250 × 440 mm |
| Flight range | 12–15 km |
| Battery capacity | 32,000 mAh |
| Total number of batteries | Four (4) |
| GPS enabled | Yes |
Table 2.
Configurations of the drones.
| Manufacturer's name | CD Space Robotics Pvt. Ltd. |
|---|---|
| RPAS Model No. | CDSHEXM001 |
| Serial No(s). | D1DA00UB6 |
| Description | Multirotor Small Class RPAS |
| RPA details | |
| RPA category | Small |
| Maximum all‐up weigh (including payload) in kg | 24 Kg |
| Overall dimensions (l × b × h) in mm | 1400 × 1250 × 440 |
| Power plant details | |
| Engine details (Manufacturer details, model No.) | Hobbywing X6 |
| Engine/Motor | Motor |
| Power Rating | 2500 W |
| No. of Engines/Motors | 6 |
| Total fuel capacity (kg)/Battery capacity (mAh) | 32,000 mAh |
| Propeller details (Diameter, Max RPM) | 23 Inch, 6000 rpm |
2.2. Identification of Study Site
The selection of study sites and dummy vaccines was motivated by an attempt to look into the usage of drones as an aerial delivery mechanism. Since this study was carried out in 2021—before the Drone Regulations of that year—a location at IIT Kanpur was chosen, working with the aerospace engineering department, where a 1000‐m airstrip was accessible for use in drone‐related operations. The site was selected due to the number of restrictions of the Ministry of Aviation and pandemic impacts which limited our selection of site. To ensure that there were no inconsistencies in the drone flights, the Aerospace Engineering team and the drone crew, which included an operator and pilot, were present. Drones could only fly in designated areas before receiving government authority to fly anywhere. This was the rationale behind the site selection.
One of the objectives of the study was to monitor the effects of the environment on the drone and dummy vaccinations during flight, also to record the difficulties encountered. These difficulties will then be taken into consideration for field based study to strengthen the vaccine delivery system in the areas that are hard to reach. To develop a study in a real scenario, space was identified and designated as Vaccine Storage Center. In this vaccine storage center, a VCB was prepared that is used in the study.
-
1.
Vaccine Storage Center: Site was identified near the launching site of the airstrip (appx. 300 m away) where the cold storage refrigerator was maintained for the vaccine vials. The study was conducted with dummy vaccine vials (as supplied by the Bharat Biotech Pvt. Ltd) at the use of 96 dummy vaccine vials (960 mL; 10 mL in each vial) in 16 compact boxes (Figures 1b and 2) were taken out from the refrigerator which were kept in a cold chain maintained at 2°C–8°C and packed into the VCB along with 6 cool packs. VCB preparation was carried out as per the Universal Immunization Program protocol [22]. As per the COVID immunization program, the vaccines are available only in a 5 mL vial. A single dose of 0.5 mL of COVID‐19 vaccine is administered through the IM route. So each vial containing 5 mL have 10 doses per vial for administration. So the dummy vaccine vials provided by Bharat Biotech were used in the trial. Key Specifications of Dummy Vaccine vials and VCB used in the study for vaccine transportation are presented in Table 3. In addition, an electronic data logging monitor (EDLM) was placed for monitoring the temperature (Figure 1c).
-
2.
Drone Command Center: The drone command center was established near the take off site (50 m away) shown in Figure 3. It had sitting arrangements, electricity connection, laptops equipped Mission Planner, fire extinguishers, emergency vehicles ready and sheds for sittings. At this place a drone pilot, engineer and Medical officer were present to monitor drone behavior and vaccine transport respectively.
-
3.
Take Off Site: After packaging, VCB was transported by medical health workers to the take‐off site which was 300 m away from the cold chain room and handed over to the drone pilot for loading in the drone. The ground was cleared before take‐off with a command from the control room, and then a drone sortie took place.
-
4.
Landing Site: After completing the stipulated route, the drone landed on the designated landing site (see Figure 3). VCB was unloaded by the pilot who handed over the package to the medical health worker after checking the drone and VCB parameters.
-
5.
Delivery Site: VCB after landing was taken by medical health workers to the nearest cold storage site to examine the physical quality, leakage of vaccine or any vial damage which may happen during transport.
Figure 2.
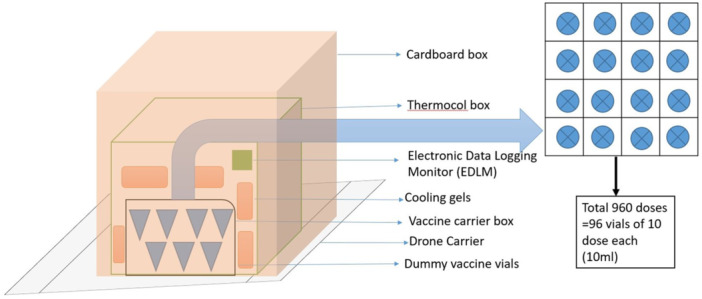
Shipping configuration and packaging of Vaccine vials in VCB (adopted from the literature provided by Bharat Biotech along with dummy vaccine vials and VCB).
Table 3.
The detailed description of the vaccine carrier box.
| Vaccine carrier box (Cold Chain) Specifications | |
|---|---|
| Weight of the carrier box (Vaccines, ice packs and temperature logger) | 4.5 kg (with shipper) |
| Number of doses of single vial | 10 doses |
| Number of vials per multi packs | 16 vials |
| Number of multi‐packs | 6 multi packs |
| Quantity of vials in one Thermocol box | 96 vials |
2.3. Sortie Plan
The flight trials consisted of five sorties where, each at different time points i.e. 06.30 a.m., 07.30 a.m., 08.30 a.m., 01.00 p.m. and 05.00 p.m., respectively, for Ist, IInd, IIIrd, IVth, and Vth sorties (Table 5). The timing of sorties was planned to expand over the entire daylight period to observe the effect of various climatic conditions like temperature, humidity, wind speed and ultra violet (UV) index on vaccine transportation and drone performance. As the study was conducted in the month of May, the temperature varies from morning to evening and the temperature range was 21°C–35°C due to which humidity also varies. The wind was more stable in the early morning time as compared to noon and evening. Therefore, different sorties were selected for the study to observe the impact of changing climate on the drone throughout the day (shown in Figure 4). The route for the flight started over the small circle of the selected site, which expanded with each sortie. Biggest round that covered the area was 4 km in diameter and the smallest was 400 m. There were no poles, no trees on the selected site and human habitation was also avoided for the selection of flight routes.
Table 5.
Details of flight parameters observed in different sorties during the study.
| S. No | Sortie | Time slot | Payload (kg) | Distance covered (KM) | Drone speed (meter/second) | Total time of flight (min) | Max. Altitude (M) |
|---|---|---|---|---|---|---|---|
| 1 | Sortie I | 06:30 a.m. | Nil | VTOL | NA | 5 | 40 |
| 2 | Sortie II | 07:30 a.m. | 4.8 | 0.400 | 5 | 1.40 | 10 |
| 3 | Sortie III | 08:30 a.m. | 4.8 | 6.2 | 5 | 24.47 | 60 |
| 4 | Sortie IV | 01:00 p.m. | 4.8 | 13.2 | 10 | 28.26 | 60 |
| 5 | Sortie V | 05:00 p.m. | 3.7 | 17 | 12 | 31.46 | 68 |
Figure 4.
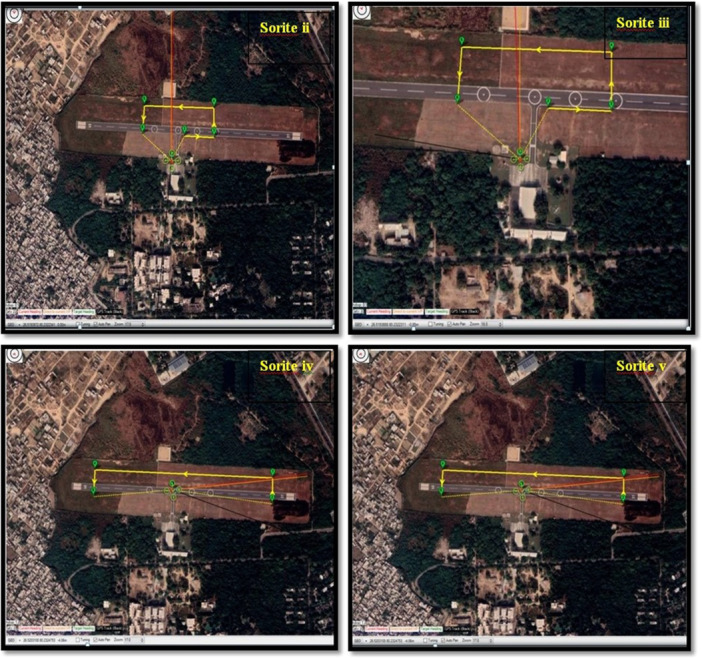
Mission planner images highlighting the waypoints of flight details in Sortie II, Sortie III, Sortie IV, and Sortie V.
The Ist sortie was conducted without any payload to check the technical feasibility of the drone flight. Followed by this, the remaining four sorties carried dummy vaccine vials with varied distance ranges, drone speed, flight time and altitude (Table 5). The drone traveled through a predefined flight path that was controlled by the drone command center. Data is mainly observed and recorded sortie wise and then it is interpreted by making comparison between the recorded values in these sorties.
2.4. Data Collection and Analysis
Descriptive methods are used for data analysis using mean, median and mode for measuring the values. The study parameters recorded in this study were the medical supplies, climate conditions, distance of the drone covered on the ground, and drone parameters including navigation, vibration, and flight dynamics of the drone. The data analysis process is not conducted using statistical tools, as the parameters observed and recorded were less in number and sorties so we performed descriptive analysis. The details of these parameters and the platform used for recording are given in Table 4.
Table 4.
Details of the parameters and platforms used to record the data.
| S. No. | Data sets related to | Parameters | Tools used for data recording |
|---|---|---|---|
| 1 | Medical supply | Temperature (outer surface of drone and VCB inside) | Paper checklist, Electronic Digital Temperature Logger, Infrared thermometer |
| 2 | Climate | Temperature, clouds, humidity, wind velocity, UV Index | Indian Meteorological Dept website |
| 3 | Drone | Navigation, Vibration, flight dynamics, GNSS, HDoP | Mission Planner |
| 4 | Distance and time | Horizontally distance covered on ground, time and endurance | Mission Planner |
2.5. Study Parameters
An overview of various data sets related to different scenarios or applications, along with the parameters being recorded and the tools used for data recording (see Table 4) are given as below.
2.5.1. Parameters for Medical Supply
Temperature (outer surface of drone and VCB inside) was recorded using the tools such as paper checklist, electronic digital temperature logger, and infrared thermometer. The rationale for this is that in the transportation of medical supplies via drones, maintaining the appropriate temperature is crucial to preserve the integrity of the supplies, especially sensitive items like vaccines or medications. Monitoring the outer surface temperature of the drone and the temperature inside the VCB ensures that the supplies are not exposed to extreme conditions that could affect their efficacy.
2.5.2. Parameters for Climate
Temperature of the area, clouds, humidity, wind velocity, and UV Index were recorded using Indian Meteorological Department website. Monitoring various climate parameters is essential for understanding weather conditions, especially when operating drones or conducting outdoor activities. Temperature, humidity, wind velocity, and cloud cover affect flight performance and safety. Additionally, UV Index monitoring is crucial for assessing potential sun exposure risks to both equipment and personnel.
2.5.3. Parameters for Drone
-
a.
Navigation, Vibration, flight dynamics, GNSS (Global Navigation Satellite System), HDoP (Horizontal Dilution of Precision) were recorded using the Mission Planner. For efficient and safe drone operations, it's essential to monitor various parameters related to the drone's performance and navigation. This includes tracking navigation data, assessing vibration levels to ensure mechanical integrity, analyzing flight dynamics for stability and control, and monitoring GNSS signals along with HDoP to gauge positioning accuracy. Mission Planner is a tool commonly used for mission planning and real‐time monitoring of drone flights, providing insights into these parameters.
-
b.
Horizontally distance covered on ground, time, and endurance were recorded using Mission Planner. Tracking distance covered on the ground, along with time and endurance metrics, provides essential data for assessing the efficiency and performance of drone missions. This data helps in planning routes, estimating mission durations, and optimizing drone operations for various applications, such as surveillance, delivery, or mapping.
These data sets and parameters, along with the specified tools for data recording, are critical for ensuring the safety, efficiency, and effectiveness of operations in diverse fields such as medical supply delivery, climate monitoring, drone navigation, and mission planning. Inside temperature of VCB was measured by Electronic Digital Temperature Logger (EDTL), the outside temperature of the surface was measured by an infrared thermometer, climate conditions were recorded using Indian Meteorological website, whereas drone parameters were recorded using Mission Planner. Multiple data were recorded that was related to sorties conducted at different time points that include environmental factors, UAS and VCB related data. The external temperature of the drone's outer cover, of VCB and the temperature of the vaccine vials were monitored during every take‐off and landing. In‐flight data such as distance traveled, drone speed, duration, horizontal alignment angle and altitude of the flight were also recorded. To analyse the technical feasibility and impact of transporting vaccines through drones, GNSS, flight dynamics, and vibration data were collected and analyzed after the completion of each sortie. Furthermore, data related to external temperature, humidity, wind velocity and UV index were collected for monitoring the environmental conditions.
At first, GNSS was used for aerial drone navigation and to verify accurate positioning to ensure the autonomous delivery of VCB following a predefined path. This was analyzed by Dilution of precision (DoP), which measures the error propagation as a mathematical effect of navigation satellites on positional measurement precision [23, 24]. In the present study, the HDoP providing horizontal position values of the drone were measured as the experiment conducted in horizontal circular motion within the controlled environment. The acceptable limit of HDoP is > 20. Then, the flight dynamics of drone flights were measured by analyzing the angle of rotations such as pitch and roll angle to understand the stability of the flight duration [25]. Pitch angle is the rotation of the vehicle fixed between the side‐to‐side axis (on a drone wingtip to wingtip) also called the lateral or transverse axis; this means the up and down movement of the vehicle's nose and tail. Roll angle is the rotation of the vehicle on the front to the back axis (nose to tail) also known as the longitudinal axis—a rolling movement up and down of the wings of the aircraft. The acceptable pitch and roll angle for the drone flight is < ±15°. At last, vibration measurements that are the most crucial metric for researching the viability of using drones to deliver vaccines were recorded. The triaxle (X, Y, and Z) vibration measurements were carried out during each sortie as a vibration could affect the quality of medical goods [26]. The acceptable vibration frequency should be < 15 Hzs in the X and Y direction and < 30 Hzs in the Z direction.
2.6. Institutional Ethic Approvals
The conditional exemption from UAS rules, 2017 was issued by the Ministry of Civil Aviation (MoCA) for conducting a dry run related to COVID‐19 vaccine delivery. Approvals and drone's Standard Operating Procedures (SOPs) were obtained from the Director‐General of Civil Aviation (DGCA) and the trials were restricted to up to 65 m altitude AGL and within VLOS. This pilot experiment further received approval from the ICMR, Central Ethic Committee on Human Research (CECHR) Bengaluru, India on 8 January 2021. Apart from these teams, the institutional fire brigade and the emergency vehicles were arranged where the emergency medical team was also informed and kept ready to manage unforeseen events (if any).
2.7. Regulatory Approval
Drone regulations were nonexistent at the time, thus ICMR had to obtain special permission from the ministry of civil aviation, local government, emergency services, and the medical community. Since drones cannot be transported by air, special authorization was obtained to carry the drone by road. The authorities—the police and hospitals—were consulted for authorization to perform the study during the pandemic. Unmanned pathway was selected in the Airstrip of IIT Kanpur.
3. Results
Total five sorties were conducted to see the various parameters related to feasibility, vaccine transportation, drone's capacity, vaccine vial condition and temperature maintenance. Table 5 depicts various parameters observed during flight. The average time required for the VCB preparation was 5–7 min and it took 3–4 min to transport the VCB to the take‐off site and to load into the drone, which was 300 m away from the cold storage site. Range of the external temperature of the VCB was 18°C–35°C and the range of temperature of drone outer surface was in the range of 20°C–38°C. The total weight of the drone was 24 kg including battery weight (5 kg). During the Ist sortie, vertical take‐off and landing were performed at 40 m altitude for 5 min without any payload, to check the flying condition of the drone. In the rest of the four sorties, the traveling distance, drone speed, total flight time and maximum flying altitude were progressively increased to observe the capacity of the drone to a safe landing.
The maximum traveled distance was 17 km (see Figure 5) with a drone speed of 12 m/s during the Vth sortie. The total time of the flight was 31.46 min at a maximum altitude of 68 m AGL (see Table 5). The ambient temperature ranged from 21°C to 36°C during the entire study period. The humidity varied from 25% to 27% throughout the study period. The wind velocity ranged from 1.38 to 3.05 m/s, the lowest velocity being recorded during the Ist and IInd sortie and the highest during Vth sortie. The UV index was low throughout the study period.
Figure 5.
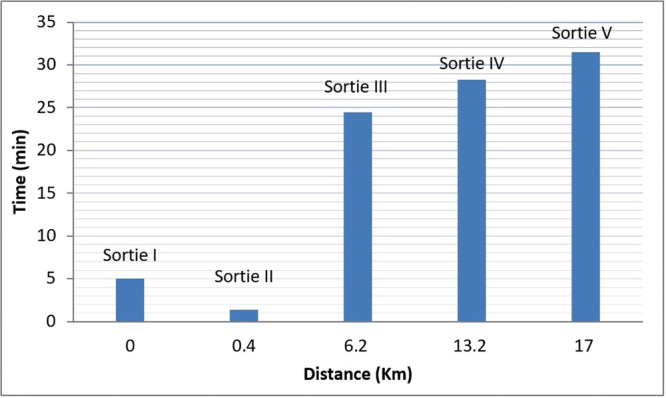
Graphical representation of time taken and distance covered by drone carrying VCB in each sortie.
The GNSS data for all stories were within the range of 14–20 HDoP. The flight dynamic data showed that all sorties were less than ±15°. The tri‐axle vibration data showed that vibration measures were within the acceptable range during all sorties.
While drone flight, no unexpected event happened. The Drone was able to follow the instruction given, and followed a predetermined path. After a successful and smooth landing, medical health workers unloaded the box from the drone and then evaluated it. Afterwards it was taken to the vaccination site room where physical inspection of vaccine vials was done and then these were transferred to a cold storage unit. Some of the important observational values before and after flight are given in Table 6.
Table 6.
Details of observational values to parameters of drone flights.
| S. No | Various parameters assessed | Observations |
|---|---|---|
| 1. | Total numbers of Sorties conducted | Five sorties |
| 2. | Maximum speed achieved (during fly) | 12 m/s |
| 3. | Minimum speed (during fly) | 0 m/s (Standstill) |
| 4. | Average temperature outside the VCB (before flight) | 31.4°C |
| 5. | Average temperature outside the VCB (after landing) | 35°C |
| 6. | Range of temperature maintained inside VCB | 3.3°C–3.8°C |
| 7. | Maximum altitude achieved | 68 m |
| 8. | The maximum duration of the drone flight | 31 min |
| 9. | The maximum distance covered by the flight | 17.1 km |
| 10. | Effect of vibration/Damage to vials | None |
| 11. | Any unexpected event happened | No |
| 12. | Deviation from the defined track | No |
| 13. | Average Payload | 4.5 Kg |
| 14. | Vaccine box details | Weight: 4.5 kg, 270 × 27 × 230 mm |
| 15. | No. of Vaccine vials | 96 vials (960 doses) |
Similar to loading, the average time required for unloading the VCB and transporting it to the vaccine storage center was 5 min. No damage, leakage or turbidity was observed in dummy vaccine vials after the drone transportation. Similarly, no internal or external physical damage was observed in the VCB. The seal of the VCB was intact, and the digital temperature logging monitor was functioning properly and was recording the temperature. The EDLM was analyzed for temperature charting. The data recorded from the EDLM showed the average temperature ranged from 3.5°C to 3.8°C during sorties carried out (Table 7). Average outside temperature of the VCB was 31.4°C and 34.5°C before and after flight respectively. The VCB was able to maintain the inside temperature between 3.3°C and 3.8°C.
Table 7.
Various parameters collected during the trial sorties along with a dummy vaccine.
| S. No. | Parameters | Sortie I | Sortie II | Sortie III | Sortie IV | Sortie V | |
|---|---|---|---|---|---|---|---|
| 1 | Weather conditions | Ambient temperature (°C) | 21 | 25 | 25 | 36 | 35 |
| Humidity | 25% | 25% | 25% | 27% | 25% | ||
| Wind velocity (direction) (km/hr) | 5 (NNE) | 5 (NNE) | 9 (NNE) | 8 (NNE) | 11 (NNE) | ||
| UV index (range) | Low | Low | Low | Low | Low | ||
| 2 | Take‐off | VCB loading and transportation time (min) | 4 | 5 | 4 | 4 | 5 |
| The temperature of the Drone's outer cover (°C) | 20 | 25 | 25 | 38 | 35 | ||
| The temperature of the VCB outer cover (°C) | 18 | 20 | 22 | 35 | 35 | ||
| 3 | Landing | VCB unloading time (min) | 8 | 8 | 8 | 8 | 8 |
| The temperature of the Drone's outer cover (°C) | 23 | 26 | 27 | 39 | 38 | ||
| The temperature of the VCB outer cover (°C) | 20 | 21 | 23 | 36 | 36 | ||
| 4 | Vaccine carrier box | Average temperature (°C) | 3.4 | 3.5 | 3.7 | 3.6 | 3.8 |
3.1. Challenges Faced
Number of studies describes the challenges they have faced while conducting drone studies for medical supplies. One of these explains these challenges, including vaccine costs, temperature requirements, packaging design, accessibility to appropriate local cooling equipment and maintenance, demand and delivery frequency, and availability [18] while the vaccine supply chain. Still, drones are cutting‐edge delivery systems that have the potential to improve the efficiency [27] of the present vaccination supply chain systems. Some of the challenges faced in our study are as follows:
-
1.
Operational Challenges Identified
-
–
Battery Related: Two sets of batteries were used for the drone flight (details given in Table 8) for which one person was required at the landing site to replace or charge the batteries whenever required. So, battery number should not be a limitation while usage of drones. Also, as per DGCA rules, transportation of lithium‐ion batteries is allowed only by road or train, not by air so, in case the concerned area for usage of drones is difficult to reach due to hard terrain or challenging areas. Therefore, there is a need to ensure drone batteries provide extended flight time, explore alternative transportation methods for lithium‐ion batteries to overcome logistical hurdles. Battery limitations and logistical hurdles in transportation not only affect operational efficiency but also impact the socio‐economic landscape. Addressing these challenges could enhance accessibility to healthcare resources, especially in remote or underserved areas.
-
–
Transportation of VCB to Landing Site: To minimize the transportation time and timely usage of vaccines, both the take‐off and landing sites should be proximal as much as possible to the cold storage points without compromising the safety and quality. There is a need to optimize the location of take‐off and landing sites near cold storage points for efficient transportation of vaccines.
-
2.
Implementation Challenges
-
–
Trained Manpower: As trained experts are required at both take‐off and landing sites to operate the drone, the automatic unloading of the payload and return to the home would have been the ideal option, as it would reduce the need for experts at the site of delivery. In future, there is a need to develop autonomous drone systems for payload unloading and return to base to reduce reliance on expert manpower at delivery sites.
-
–
Training and Orientation: Orientation around drone technology as well as healthcare delivery for both drone operators and healthcare workers are required for smooth functioning of the program and to delineate their respective roles. Therefore, need to provide comprehensive training on drone technology and healthcare delivery to both operators and healthcare workers to streamline operations. The transition to autonomous drone systems may raise concerns about job displacement among manual laborers. Efforts should be made to provide retraining opportunities and ensure that technological advancements benefit all stakeholders.
-
3.
Environmental Challenges
The wind was more stable in the morning and grew faster afterwards. High winds and clouds can be a challenging situation for drone flights. There is need to address fluctuating wind patterns and adverse weather conditions by scheduling drone flights during periods of stable weather conditions, such as in the morning. Understanding the ecological footprint of drone operations, including their energy consumption and potential disturbances to wildlife, is essential for sustainable implementation. Balancing environmental conservation with the benefits of drone technology is key. As climate change alters weather patterns, designing drones and flight schedules resilient to changing environmental conditions becomes imperative for reliable service delivery.
-
4.
Regulatory barriers
Table 8.
Details for performance details of drone with battery life.
| Maximum endurance (h/m) | 40 min |
| Maximum range (in km) | 20 km |
| Maximum speed (in m/s) | 15 m/s |
| Maximum height attainable/maximum ceiling height (in ft) | 1000 |
| Operating altitude (in ft) operational | 400 |
| Envelope | 400 ft |
| Engine limits (maximum RPM)/Max. battery temperature (in deg C) | Max RPM 6000/Max Battery Temp 70 deg Celsius |
| Propeller limits | Max RPM 10000 |
Drone‐based vaccination delivery systems face regulatory barriers, including approvals as per airspace rules, transport and safety requirements. Deployment was delayed due to bureaucratic processes, drone misuse concerns, airspace congestion, and liability issues due to which a number of approvals were taken. Also, in the absence of regulations at that time, there was matter of security and privacy issues which is why this study is conducted in confined Airstrip in IIT Kanpur. In future, to avoid any delay there is a need to streamline bureaucratic processes, address safety concerns, and ensure compliance with drone rules 2021.
Involving local communities in the planning and execution of drone operations can foster acceptance and support, mitigating potential resistance towards the technology.
4. Discussion
The study concludes that using drones for transporting medical supplies in remote areas can significantly reduce turnaround times for viral load testing. However, the cost of implementing a UAV‐based system is notably higher than traditional transport methods, indicating a need for careful consideration of financial implications alongside potential health benefits [28]. Many countries started integrating drones for medical delivery such as Rwanda is the first country to start a drone delivery program known as “Uber for blood” [29]. One study was conducted in restricted airspace in a military aircraft test range that investigates the stability of biological samples during prolonged drone flights where samples were kept in a cooling box for 3 h, but as a result glucose and potassium showed significant biases due to temperature differences [30]. One study conducted in challenging terrains such as mountainous regions and flood‐prone areas in Nepal, emphasized the adaptability of drones to various environmental conditions and highlighted the potential for scalable deployment in resource‐constrained settings [31]. The study primarily assessed technical aspects such as flight endurance and payload capacity. In this study, along with the flight endurance, and payload capacity the impact of climate on the vaccine vials was recorded while transportation where the average time required for the preparation of the VCB and the transportation from vaccine storage box to the take‐off site took around 11 min. Similarly, the unloading and transporting of VCB to the Vaccine Storage Center took about 6 min. One such strategy involves building and deploying a customized drone prototype in Nepal. The vaccine was transported by drone equipped with a vaccine carrier from Simikot Airport to the Local 18 Mission hospital, a 400‐m distance that takes 11–15 min [18]. Our study showed that the internal temperature of VCB was consistently maintained between 3°C and 4°C, during the VCB preparation and transportation, by adhering to the manufacturer's guidelines and UIP (Universal Immunization Program) standard operating procedures. Although the environmental and drone body temperature varied between 18°C and 39°C, the internal temperature of VCB was not affected by such temperature extremes. Also, the distance traveled and total flight time was 3.4–17 km and 5–32 min, respectively, which did not have an impact on VCB's internal temperature environment.
The environmental parameters monitored in our investigation such as ambient temperature, wind speed, and humidity, were within the permissible range [32]. The wind speed observed during the study period was in the range of 1.38–3.05 m/s, which was less than 10 m/s the maximum wind speed resistance allowed for common drones. In addition, the average air temperature 2 m above the ground level was recorded in the range of 21°C–36°C, which was within the operational temperature range of common drones i.e. 0°C–40°C [5]. However, we had sorties during different times of day in summer. The relative humidity and UV index were low, which did not hinder the drone flights under study situations. Noticeably, Global Flyability of drones has been reported to be higher in warm and dry conditions than in the oceans and at high latitudes [5].
The GNSS data collected during the drone flights revealed that the HDoP were within the acceptable limit of < 20, which confirmed the navigation of the drone following the predefined path by the drone operator. In addition, both roll and pitch angles of the drone during each sortie were < ±15°, which ensured that the dynamics of the drone did not have any plausible detrimental effect on the payload. The tri‐axle vibration data revealed that the maximum vibration frequency observed during the study period did not cross 15 Hz in the X and Y direction and 30 Hz in the Z direction. Conceivably, the safe wind speed observed throughout the study period might have played an important role in maintaining such parameters within acceptable limits. The operations during the current investigation were carried out by adhering to the given parameters in terms of payload, drone speed, endurance and altitude as defined by the drone manufacturer and SOPs issued by DGCA.
Furthermore, there was no external and internal damage either to the drone or the VCB. Importantly, there was no leakage in the cool packs or dummy vaccine vials. This indicates that the drone used in the study transported dummy vaccine vials and VCB safely within the range of capacity as defined by the manufacturer.
This study has a number of limitations because it was only done at one location and at different times; it could have been done at different locations, on different days, or during a different season. Also, usage of the simulated vaccine vials in this study results might differ for using the real vaccines so it should be tested in real conditions. After this study the implementation process of drone‐based delivery of medical supplies, operational challenges and innovations adopted by scientists in Manipur and Nagaland were elucidated in the study conducted by ICMR [33]. The team's experiences obtaining permission on a case‐by‐case basis and coordinating with state and federal aviation authorities, district administrations, and health authorities were noted. The deployment of appropriate drones, payload capacity, operational time management, and drone transportation were identified as the technical and logistical challenges associated with drones in difficult terrain. Medical supply deliveries via drones are proving to be timely, nevertheless, resolving operational issues that might offer a useful long‐term deployment plan [34].
4.1. Limitations
-
1.
Conducting the study in a controlled environment at IIT Kanpur limits generalizability. Future studies should include diverse geographical locations and real‐world conditions. While the study employed a Visual Line of Sight (VLOS) drone, acknowledging the need for BVLOS drones is crucial for extrapolating findings to real‐world scenarios. BVLOS capabilities are essential for covering larger areas efficiently, especially in regions with diverse geographical landscapes.
-
2.
This study used dummy vaccines that are needed to confirm in real case scenarios where real vaccines are to be delivered in real locations. The use of dummy vaccines in the study is understandable for initial experimentation and validation. However, transitioning to real‐world scenarios with genuine vaccines and delivery sites is imperative for confirming the practical applicability and effectiveness of the drone delivery system. This shift ensures that the findings accurately reflect the challenges and complexities encountered in real vaccine distribution efforts.
-
3.
Current study was conducted in the plains using a multirotor drone which was designed specifically for that terrain highlighting an important consideration. Different geographical landscapes across the country necessitate the adaptation of drone technologies to suit varying environmental conditions and topographies. In future geographical considerations by examining specific geographic contexts, such as urban areas, remote rural regions, or mountainous terrains is needed to present unique challenges and opportunities for drone‐based vaccine delivery. Discussing these variations in detail can provide insights into the feasibility and challenges of implementing drone delivery systems nationwide.
Key findings of drone usage in vaccine delivery system
|
Key features for future use of drone in vaccine delivery system
|
5. Conclusion and Way Forward
The study's conclusion underscores the successful adherence to vaccine transportation guidelines and drone rules [35] for transporting vaccine vials and VCB using drones in India. Despite various limitations, the study was successfully completed and no impact of environment found as such on drone and there was no physical damage of the vaccine vials or the VCB due to vibrations of the drone. The crucial aspect was the maintenance of the temperature of vaccine vials within the recommended range during transportation, despite external temperature variations, which highlights the safety and reliability of drones for vaccine delivery. Moreover, the observed absence of physical damage to VCB components, including dummy vaccine vials, emphasizes the safety and integrity of the delivery process. This finding may have significant implications for public health, as it opens avenues for more efficient and potentially widespread vaccine distribution that is possible if it is adhered to protocols and guidelines. According to this study, it may be feasible to control the vaccination distribution system in challenging terrain locations that are hard to reach by road. Additionally, using drones to maintain the immunization chain system may make it easier to reach remote locations. The study focused on VLOS operations, which limited coverage and efficiency in delivering vaccines to remote or inaccessible areas. Future research should explore BVLOS capabilities to assess their feasibility and effectiveness in real‐world scenarios. The study's specificity may limit its applicability to other locations with different logistical, environmental, and infrastructural challenges. Future assessments should consider the diversity of geographical landscapes and evaluate the suitability of various drone types for different case scenarios. The choice of a multirotor drone may introduce limitations in terms of range, payload capacity, and adaptability to different terrains. This study offers insights into the performance of multirotor drones, enabling recommendations in drone design for related uses. Evaluating multirotor drone performance against other varieties can help choose future drones tailored to specific delivery requirements and environmental factors. The study acknowledges that dummy vaccine vials may not accurately represent the intricacies of vaccine administration, such as fragility or temperature management. It encourages further research into the consequences of using actual vaccinations, including evaluating the system's capacity to preserve vaccine integrity during delivery and transportation.
The use of drones for vaccine delivery in healthcare has significant policy implications, including establishing a regulatory framework, integrating drone systems into existing health frameworks, promoting public awareness and trust, and advocating for public and private investment in drone technology and infrastructure. Government bodies, NGOs, and international organizations can collaborate to design pilot programs, establish guidelines, and engage local health departments in community‐specific needs assessments. The use of drones can also improve health access, healthcare delivery, and equity in healthcare. The potential economic and social impacts of drone‐mediated vaccine delivery include cost reduction, job creation, and improved health access. Regulatory changes, such as amending airspace regulations, operational guidelines, and data privacy and security, are necessary to ensure the sustainability and scalability of these programs.
Author Contributions
Sumit Aggarwal: conceptualization, methodology, writing–original draft, investigation, supervision, writing–review and editing, visualization, project administration, data curation. Prakamya Gupta: supervision, visualization, writing–review and editing, formal analysis, project administration, data curation, conceptualization, methodology. Sivaraman Balaji: project administration, formal analysis, data curation, writing–original draft, writing–review and editing. Saurabh Sharma: methodology, conceptualization, project administration. Ajoy Kanti Ghosh: resources, investigation. Simmy: writing–review and editing. Balram Bhargava: resources, funding acquisition, validation. Samiran Panda: Writing–review and editing.
Conflicts of Interest
The authors declare no conflicts of interest.
Transparency Statement
The lead author Sumit Aggarwal affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Supporting information
Supporting information.
Acknowledgments
We sincerely acknowledge the support from Bharat Biotech International. Ltd (BBIL), Telangana especially Dr V Krishna Mohan, Mr Sai Prasad, Mr T Srinivas, Dr VK Srinivas and Mr Pankaj Kumar Patel for providing the dummy vaccine vials (Including the technical details for vaccine vial packaging, temperature stability, Vaccine Carrier Box, cool packs etc.), Indian Institute of Technology (IIT), Kanpur and its start‐up CD Space Robotics Pvt. Ltd. for providing Air Strip and multi‐rotor drone for conducting the study. We are also thankful to Shri Amber Dubey, Mr. Thulasiraman, Mr. Subhasish Nath, Ministry of Civil Aviation, Mr. RP Kashyap, Mr. Nikhil Upadhye and the Directorate General of Civil Aviation for their constant support towards this endeavor. This research was funded and supported by the Indian Council of Medical Research, Department of Health Research, Ministry of Health and Family Welfare, Govt. of India. but had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; and the decision to submit the report for publication.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Data sharing is not applicable to this article as no new data were created or analyzed in this study. Data available on request from the authors.
References
- 1. Haidari L. A., Brown S. T., Ferguson M., et al., “The Economic and Operational Value of Using Drones to Transport Vaccines,” Vaccine 34, no. 34 (July 2016): 4062–4067, 10.1016/j.vaccine.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Michael O. T., Olusegun A., Paul O. A., et al., “The Use of UAV/Drones in the Optimization of Nigeria Vaccine Supply Chain,” International Journal of Scientific & Engineering Research 10, no. 10 (2019): 1273–1279, https://www.researchgate.net/profile/Timilehin-Omole/publication/337486853_The_Use_of_UAVDrones_in_the_Optimization_of_Nigeria_Vaccine_Supply_Chain/links/6014c80aa6fdcc071ba13339/The-Use-of-UAV-Drones-in-the-Optimization-of-Nigeria-Vaccine-Supply-Chain.pdf. [Google Scholar]
- 3. Amukele T. K., Sokoll L. J., Pepper D., Howard D. P., and Street J., “Can Unmanned Aerial Systems (Drones) be Used for the Routine Transport of Chemistry, Hematology, and Coagulation Laboratory Specimens,” PLoS One 10, no. 7 (July 2015): e0134020, 10.1371/journal.pone.0134020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hii M., Courtney P., and Royall P., “An Evaluation of the Delivery of Medicines Using Drones,” Drones 3, no. 3 (September 2019): 52, 10.3390/drones3030052. [DOI] [Google Scholar]
- 5. Gao M., Hugenholtz C. H., Fox T. A., Kucharczyk M., Barchyn T. E., and Nesbit P. R., “Author Correction: Weather Constraints on Global Drone Flyability,” Scientific Reports 11 (2021): 12092, 10.1038/s41598-021-91325-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ball P., “The Lightning‐Fast Quest for Covid Vaccines — and What It Means for Other Diseases,” Nature 589 (2021): 16–18, 10.1038/d41586-020-03626-1. [DOI] [PubMed] [Google Scholar]
- 7. Matter D. and Potgieter L., “Allocating Epidemic Response Teams and Vaccine Deliveries by Drone in Generic Network Structures, According to Expected Prevented Exposures,” PLoS One 16, no. 3 (2021): e0248053, 10.1371/journal.pone.0248053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lippi G. and Mattiuzzi C., “Biological Samples Transportation By Drones: Ready for Prime Time?,” Annals of Translational Medicine 4, no. 5 (2016): 92, 10.21037/atm.2016.02.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cacace J., Finzi A., and Lippiello V., “A Robust Multimodal Fusion Framework for Command Interpretation in Human‐Robot Cooperation,” In 2017 26th IEEE International Symposium on Robot and Human Interactive Communication (RO‐MAN) (August 2017): 372–377, 10.1109/ROMAN.2017.8172329. [DOI] [Google Scholar]
- 10. Government of India, Office of the Director General of Civil Aviation. Requirements for Operation of Civil Remotely Piloted Aircraft System (RPAS) (August 2018), https://public-prd-dgca.s3.ap-south-1.amazonaws.com/InventoryList/headerblock/drones/D3X-X1.pdf.
- 11. Das N. K., Patil R., Prasanna S., Das P., and Mukhida S., “Drones for Medical Supply During Disaster: A Game Changer in ‘Health for All’ Policy,” Health Services Insights 16 (2023): 1, 10.1177/11786329231160013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rajamohan K., “Review of Medical Drones in Healthcare Applications.” In Internet of Drones (CRC Press, 2023), 59–74, https://www.taylorfrancis.com/chapters/edit/10.1201/9781003252085-4/review-medical-drones-healthcare-applications-kavitha-rajamohan. [Google Scholar]
- 13. Faria N. R., Sabino E. C., Nunes M. R. T., Alcantara L. C. J., Loman N. J., and Pybus O. G., “Mobile Real‐Time Surveillance of Zika Virus in Brazil,” Genome Medicine 8 (2016): 97, 10.1186/s13073-016-0356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Griffith E. F., Schurer J. M., Mawindo B., Kwibuka R., Turibyarive T., and Amuguni J. H., “The Use of Drones to Deliver Rift Valley Fever Vaccines in Rwanda: Perceptions and Recommendations,” Vaccines 11, no. 3 (2023): 605, 10.3390/vaccines11030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gangwal A., Jain A., and Mohanta S., “Blood Delivery by Drones: A Case Study on Zipline,” International Journal of Innovative Research in Science, Engineering and Technology 8, no. 8 (2019): 2347–6710, 10.15680/IJIRSET.2019.0808063. [DOI] [Google Scholar]
- 16. Sanz‐Martos S., López‐Franco M. D., Álvarez‐García C., et al., “Drone Applications for Emergency and Urgent Care: A Systematic Review,” Prehospital and Disaster Medicine 37, no. 4 (2022): 502–508, 10.1017/S1049023X22000887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thapa P., “Feasibility Study of Using Uncrewed Aerial Vehicles to Deliver COVID‐19 Vaccines in Geographically Inaccessible Areas of Nepal,” Research Square 2021 (2021): 1, 10.21203/rs.3.rs-957476/v1. [DOI] [Google Scholar]
- 18. Gunaratne K., Hewage H. C., Nielsen I. I., Bocewicz G., Thibbotuwawa A., and Banaszak Z., “Location Suitability for the Implementation of Unmanned Aerial Vehicles in the Vaccine Supply Chain of Sri Lanka.” In International Conference on Intelligent Systems in Production Engineering and Maintenance (Cham: Springer Nature Switzerland, September 2023), 43–59, 10.1007/978-3-031-45021-1_4. [DOI] [Google Scholar]
- 19. Gunaratne K., Thibbotuwawa A., Vasegaard A. E., Nielsen P., and Perera H. N., “Unmanned Aerial Vehicle Adaptation to Facilitate Healthcare Supply Chains in Low‐Income Countries,” Drones 6, no. 11 (2022): 321, 10.3390/drones6110321. [DOI] [Google Scholar]
- 20.Accessed 24 November 2023, https://timesofindia.indiatimes.com/city/dehradun/drone-delivers-meds-from-aiims-rishikesh-to-uttarakhand-hospital/articleshow/97992202.cms.
- 21. Eichleay M., Evens E., Stankevitz K., and Parker C., “Using the Unmanned Aerial Vehicle Delivery Decision Tool to Consider Transporting Medical Supplies Via Drone,” Global Health, Science and Practice 7, no. 4 (2019): 500–506, 10.9745/GHSP-D-19-00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patra N., “Universal Immunization Programme in India: The Determinants of Childhood Immunization,” (2006). Available at SSRN 881224, https://ssrn.com/abstract=881224 or; 10.2139/ssrn.881224. [DOI]
- 23. Patrik A., Utama G., Gunawan A. A. S., et al., “Gnss‐Based Navigation Systems of Autonomous Drone for Delivering Items,” Journal of Big Data 6 (2019): 53, 10.1186/s40537-019-0214-3. [DOI] [Google Scholar]
- 24. Sheridan I., “Drones and Global Navigation Satellite Systems: Current Evidence From Polar Scientists,” Royal Society Open Science 7, no. 3 (2020): 191494, 10.1098/rsos.191494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang J., Ren L., Deng H., Ma M., Zhong X., and Wen P., “Measurement of Unmanned Aerial Vehicle Attitude Angles Based on a Single Captured Image,” Sensors 18, no. 8 (2018): 2655, 10.3390/s18082655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Oakey A., Waters T., Zhu W., et al., “Quantifying the Effects of Vibration on Medicines in Transit Caused by Fixed‐Wing and Multi‐Copter Drones,” Drones 5, no. 1 (2021): 22, 10.3390/drones5010022. [DOI] [Google Scholar]
- 27. Wurbel H., “Framework for the Evaluation of Cost‐Effectiveness of Drone Use for the Last‐Mile Delivery of Vaccines,” Master of Global Health University of Barcelona, ISGlobal (2017): 21–29, https://cdn.logcluster.org/public/gm_files/master_final_project_heike_wurbel_13_jun2017_003.pdf. [Google Scholar]
- 28. Parker C., Evens E., Stankevitz K., et al., “Adding Unmanned Aerial Vehicles to Hiv Supply Chains in Remote Settings: Modeling Feasibility and Cost in Turkana, Kenya,” Journal of Global Health Reports 5 (2021): e2021087, 10.29392/001c.28349. [DOI] [Google Scholar]
- 29. Poljak M. and Šterbenc A., “Use of Drones in Clinical Microbiology and Infectious Diseases: Current Status, Challenges and Barriers,” Clinical Microbiology and Infection 26, no. 4 (2020): 425–430, 10.1016/j.cmi.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 30. Amukele T. K., Hernandez J., Snozek C. L., et al., “Drone Transport of Chemistry and Hematology Samples Over Long Distances,” American Journal of Clinical Pathology 148, no. 5 (2017): 427–435, 10.1093/ajcp/aqx090. [DOI] [PubMed] [Google Scholar]
- 31. Pulsiri N. and Vatananan‐Thesenvitz R., “Drones in Emergency Medical Services: A Systematic Literature Review With Bibliometric Analysis,” International Journal of Innovation and Technology Management 18, no. 4 (2021): 2097001, 10.1142/S0219877020970019. [DOI] [Google Scholar]
- 32. Wang B. H., Wang D. B., Ali Z. A., Ting Ting B., and Wang H., “An Overview of Various Kinds of Wind Effects on Unmanned Aerial Vehicle,” Measurement and Control 52, no. 7–8 (2019): 731–739, 10.1177/0020294019847688. [DOI] [Google Scholar]
- 33.Accessed 29 November 2023, https://www.straitstimes.com/asia/south-asia/india-looks-to-drones-to-deliver-essential-medical-supplies-to-far-flung-areas.
- 34. Aggarwal S., Gupta P., Mahajan N., et al., “Implementation of Drone Based Delivery of Medical Supplies in North‐East India: Experiences, Challenges and Adopted Strategies,” Frontiers in Public Health 11 (2023): 1128886, 10.3389/fpubh.2023.1128886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Drone Rules 2021, The Gazette of India, The Directorate General of Civil Aviation, Ministry of Civil Aviation, India. 25 August, 2021, accessed 22 November 2023, https://www.dgca.gov.in/digigov-portal/jsp/dgca/homePage/viewPDF.jsp?page=InventoryList/headerblock/drones/Drone%20Rules%202021.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials. Data sharing is not applicable to this article as no new data were created or analyzed in this study. Data available on request from the authors.


