Abstract
Background
Increasing syphilis infection rates are a concerning issue worldwide. Blood donation screening is an opportunity to monitor the burden of asymptomatic infections, providing information on contemporary factors associated with infection and public health insights into transmission.
Methods
Blood donations collected at five Brazilian blood centers between January 2020 and February 2022 were screened with treponemal or non-treponemal assays according to local protocols, followed by alternate Enzyme-Linked Immunosorbent Assay (ELISA); samples with reactive or indeterminate results in the alternate ELISA were further tested with the rapid plasma reagin (RPR), and categorized as RPR-positive or RPR-negative. RPR-positive donations were also grouped according to RPR titers (< 1:8 or ≥ 1:8). We report the prevalence of syphilis in first-time donors (FTD) and repeat donors (RD), as well as incidence in RD. Multivariable models were used to assess factors associated with RPR-positive syphilis. Additionally, we explored the relationship between syphilis positivity in FTD and syphilis cases registered by the Brazilian public health surveillance system from 2012 to 2022.
Findings
Of 862,146 donations, 10,771 (1.3%) were reactive or indeterminate on screening; 7,541 available samples underwent additional testing. Of those, 5,876 (77.9%) tested positive or indeterminate on the alternate ELISA; 907 (12.0%) were RPR-negative, 2,980 (39.5%) were RPR-positive < 1:8, and 1,989 (26.4%) were RPR-positive with titers ≥ 1:8. The prevalence of syphilis including RPR-positive and RPR-negative cases was 2.5% among FTD and 0.6% among RD. The incidence of syphilis in RD was 90/105 person-years (95% CI 86–95), with younger age, male gender, Black and Mixed race (relative to White) and lower education associated with incident syphilis in RD. Blood donors had lower rates of syphilis compared to the general population, with correspondence between numbers in blood donors and congenital syphilis rates registered by the Brazilian surveillance system between 2012 and 2022.
Conclusion
The prevalence of syphilis was < 3% among FTD and < 1% among RD. We found wide variability according to donor characteristics, with gender, age, race, and schooling significantly associated with prevalent and incident RPR-positive syphilis in multivariable models. Syphilis occurrence among blood donors can be used to assess disease patterns in low-risk populations.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12889-024-21114-3.
Keywords: Syphilis, Screening tests, Blood donation, Risk factors, Public health surveillance
Introduction
Despite the availability of effective treatments for more than 70 years, syphilis continues to be a major public health issue, with a worrying worldwide increase in cases observed in recent years [1, 2]. According to the World Health Organization (WHO), approximately 8 million adults acquired syphilis in 2022 alone [3]. The United States (US) Centers for Disease Control and Prevention reported an alarming 79% increase in syphilis cases in the country between 2018 and 2022 [4]. In Brazil, monitoring data have shown increasing syphilis detection rates, with a peak number of 213,129 reported cases in 2022; a notable exception was seen in 2020, with a decline in registered cases likely due to the COVID-19 pandemic [5, 6]. Among Brazilian blood donors, the prevalence of syphilis based on donation screening tests was 1.08% in 2020, the highest since 2013 [7].
Since most cases are asymptomatic, understanding the full burden of syphilis in the general population is challenging. Besides antenatal care, routine testing of asymptomatic individuals is only implemented in blood donors; hence, investigating the prevalence, incidence, and factors associated with syphilis positivity in blood donors may provide relevant information regarding the patterns of disease occurrence in low-risk groups, supporting the development of weighted estimates of syphilis in larger populations. However, the screening tests used in blood banks are usually unable to discriminate between active and resolved infections, and studies on the syphilis prevalence and incidence in blood donors using confirmatory tests are limited [8–10].
Here, we aimed to investigate the prevalence, incidence, and the factors associated with syphilis infection among Brazilian blood donors in five blood centers. We also explored the relationship between syphilis detection among blood donors and syphilis cases registered by the Brazilian surveillance system between 2012 and 2022.
Methods
Study design and setting
The Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) Brazil component includes blood donations collected at five Brazilian blood centers: Fundacao Pro-Sangue (São Paulo); Hemominas (Belo Horizonte); Hemope (Recife); Hemorio (Rio de Janeiro) and Hemoam (Manaus). Donor and donation data from participating blood centers are registered in a centralized electronic system, comprising information captured across all stages of the donation procedure, ranging from donor demographics reported at registration to the notification of serological findings. In this study we included data from all allogeneic donations collected between January 2020 and February 2022 within the REDS-IV-P Brazil program.
Routine blood donation and study procedures
Blood donation candidates are routinely screened using an initial clinical assessment which includes a detailed donor history questionnaire, a hematocrit/hemoglobin examination and a concise physical examination including vital signs. A complete description of blood donor screening criteria in Brazil is provided in Additional file 1.
We identified all blood donations with repeat reactive or indeterminate results in the routine serological screening for syphilis. These samples were selected for a confirmatory algorithm, as described in Fig. 1 and detailed below. We used the donation databases to extract information on the donors´ demographic characteristics (gender, age, self-reported race/skin color, and educational level at the date of donation), donor status (first time donor [FTD] or repeat donor [RD]), type of donation (community or replacement), and co-infections with other transfusion transmissible infections (TTIs).
Fig. 1.
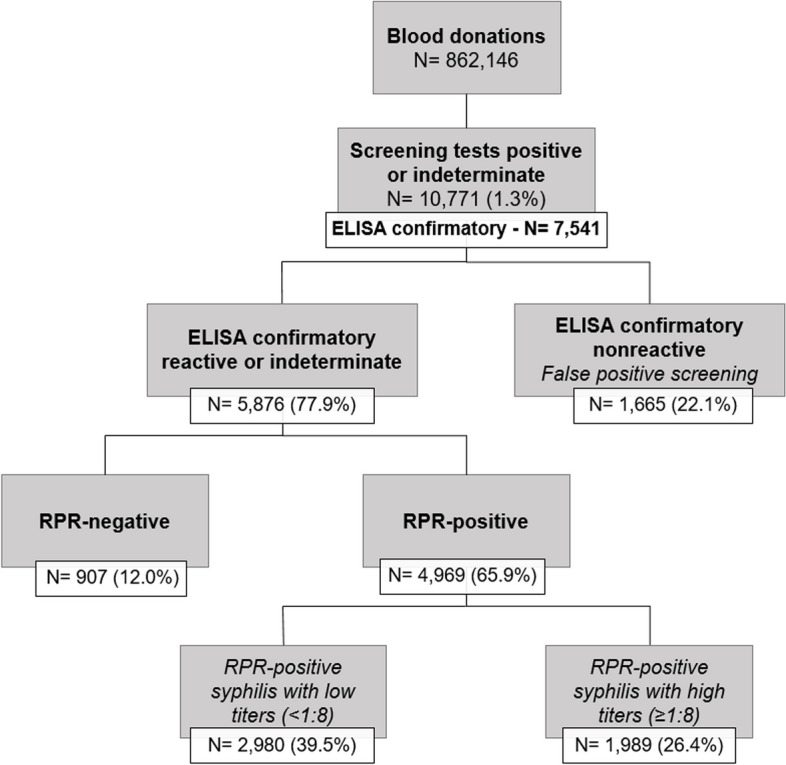
Algorithm for serological testing and prevalence of RPR-negative and RPR-positive syphilis infections
Laboratory methods
Routine laboratory screening of blood donations at the participating blood centers is performed using kits approved by the Brazilian Ministry of Health, with high sensitivity and specificity as described in the manufacturers’ package leaflets. A full description of laboratory screening tests used at the participating blood centers is presented in Additional file 2. Tests for TTIs include serologic tests for syphilis, HIV 1/2 (antigen/antibody combo assay), HTLV, HCV, HBV (hepatitis B surface antigen and anti-hepatitis B core antigen), and Chagas disease; and nucleic acid tests for hepatitis B, hepatitis C, and HIV 1/2.
The serological screening for syphilis is routinely conducted at the participating blood centers using treponemal tests (ELISA; Electrochemiluminescence Immunoassay [ECLIA]; Chemiluminescent Microparticle Immunoassay [CMIA]; chemiluminescence immunoassays [ChLIA]; or a nontreponemal test (Venereal Disease Research Laboratory [VDRL]). In the case of an initial reactive result, the test is repeated in duplicate. If the results are concordant, the sample is classified as positive; if the results are discordant or meet the criteria for indeterminate classification, the sample is labeled as indeterminate. Samples from blood donations with positive or indeterminate results in routine syphilis screening were identified and shipped to a centralized study laboratory for confirmatory testing. All samples were retested using an ELISA (Treponema pallidum Screen, Lübeck, Germany), an orthogonal test not used by any of the five blood centers, with comparable or higher sensitivity and specificity. Samples with reactive or indeterminate results were further tested with the Rapid Plasm Reagin (RPR) test (RPR Corado, Pinhais, Brazil) following the manufacturers´ instructions.
For the RPR, each sample was serially diluted at least six times (1:1, 1:2, 1:4, 1:8, 1:16, 1:32) for a systematic assessment of prozone effect, with further dilution applied for RPR titer ascertainment in samples that were still reactive at 1/32 dilution. Based on RPR test results, samples were categorized as RPR-negative or RPR-positive. RPR-positive donations were further grouped based on RPR titers (< 1:8 or ≥ 1:8). Samples with nonreactive results in the confirmatory ELISA were categorized as biological false positives [11]. For the assessment of incident syphilis in RD, the same testing algorithm was used, with incident cases characterized by positive or indeterminate results in routine syphilis screening and reactive results in both confirmatory tests in RD who had previously tested nonreactive in the screening test from their last donation.
Temporal trends of syphilis in the Brazilian general population and in blood donors
We used graphical analysis to evaluate the relationships between the rates of syphilis deferral in blood donors in Brazil; the rates of syphilis deferral in FTD in the REDS-IV-P blood centers; the incidence rate of congenital syphilis (cases in infants younger than 1 year old per 1,000 live births); the notified rates of sexually acquired syphilis (cases per 100,000 population); and the notified rates of syphilis in pregnant persons in Brazil between 2012 and 2020 (cases in pregnant persons per 1,000 live births).
The yearly rates of syphilis deferral among Brazilian blood donors were extracted from the Hemotherapy Bulletin issued by the Brazilian Ministry of Health [12]. The rates of syphilis deferral in FTD in the REDS-IV-P blood centers were extracted from the study database. Both deferral rates were based exclusively on screening test results. The incidence rates of congenital syphilis, detection rates of sexually acquired syphilis, and rates in pregnant persons in Brazil were obtained from the Syphilis Epidemiological Bulletin, published by the Brazilian Ministry of Health in 2023 [5].
Statistical analysis
Statistical analyses were performed by Westat, the data coordinating center for REDS-IV-P. Summary statistics were calculated from available data to describe demographic characteristics of participants stratified by syphilis test reactivity. Prevalence estimates were calculated as the number of cases per 100,000 donations.
Unadjusted and multivariable-adjusted modified Poisson regression models were used to determine the association between demographic and donation characteristics and syphilis reactivity, reported as prevalence ratios (PRs). The incidence rate of syphilis among RD was calculated using the number of seroconversions during the total at-risk person-time in the study period. The date of incident syphilis infection was assigned at the midpoint between the last negative and the first positive donation. We used multivariable Poisson models to calculate adjusted incidence rate ratios. Results were considered statistically significant based on α < 0.05. All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC).
Ethical aspects
The ethics committees at each blood center and the Brazilian National Ethics Research Committee reviewed and approved the study, including exemption of informed consent and authorization for further testing of repository samples for all donors included in the analysis (CAAE: 14561118.6.1001.0068). The institutional review board (IRB) at University of California San Francisco, which serves as Vitalant Research Institute’s IRB of record, approved this study (approval number 20–32768) as non-human subjects research with exemption of additional informed consent. Samples were coded and pseudonymized before shipment to testing facilities. The study investigators had no access to the keys that linked pseudonymized observations to identifying blood center data; therefore, all identifiable information of the blood donors included in the study were kept confidential.
Results
Donations included in the study and prevalence of RPR-positive and RPR-negative syphilis infections
A total of 862,146 blood donations were collected at the participating blood centers from January 2020 to February 2022. We identified 10,771 donations (1.3%) with reactive or indeterminate results in routine syphilis screening; frequencies and percentages, overall and by demographics and donation characteristics, are shown in Table 1. Of those, 7,541 had residual volume samples available for additional testing in a centralized laboratory. Overall, 5,876 (77.9%) samples tested positive or indeterminate on the alternate ELISA; 907 (12.0%) were RPR-negative syphilis infections, 2,980 (39.5%) were RPR-positive with titers < 1:8 and 1,989 (26.4%) were RPR-positive with titers ≥ 1:8 (Fig. 1). The prevalence of syphilis infection, including RPR-positive and RPR-negative cases, was 2.5% among FTD (95% CI 2.5–2.6) and 0.6% among RD (95% CI 0.6–0.6). The prevalence of syphilis RPR-positive infections was 1.2% in FTD (95% CI 1.2–1.3) and 0.2% in RD (95% CI 0.2–0.3). Among FTD, the prevalence rates of RPR-positive syphilis with high and low RPR titers were 466 and 745/100,000 donations; these rates were 4.4 and 5.6 times higher than those observed in RD (Additional file 3A). A total of 220 samples were categorized as indeterminate on screening; of those, 24 were positive on the confirmatory ELISA test and 20 were positive on RPR. The findings of the confirmatory testing algorithm among samples with indeterminate results are presented in detail in Additional file 3B.
Table 1.
Demographic characteristics of syphilis screening positive/indeterminate and negative donations by donor status
| Positive or indeterminate FTD n (%) | Negative FTD n (%) | Positive or indeterminate RD n (%) | Negative RD n (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Total Donations | 7522 | 2.5 | 288153 | 97.5 | 3249 | 0.6 | 553823 | 99.4 |
| Age | ||||||||
| < = 24 | 1506 | 20.0 | 92745 | 32.2 | 320 | 9.8 | 73524 | 13.3 |
| 25–34 | 2547 | 33.9 | 93435 | 32.4 | 837 | 25.8 | 151461 | 27.3 |
| 35–44 | 1690 | 22.5 | 60993 | 21.2 | 934 | 28.7 | 166265 | 30.0 |
| 45–54 | 1111 | 14.8 | 29573 | 10.3 | 690 | 21.2 | 109528 | 19.8 |
| > = 55 | 668 | 8.9 | 11407 | 4.0 | 468 | 14.4 | 53045 | 9.6 |
| Gender | ||||||||
| Female | 3333 | 44.3 | 146540 | 50.9 | 1000 | 30.8 | 203614 | 36.8 |
| Male | 4189 | 55.7 | 141613 | 49.1 | 2249 | 69.2 | 350209 | 63.2 |
| Race | ||||||||
| White | 1932 | 25.7 | 110921 | 38.5 | 924 | 28.4 | 201774 | 36.4 |
| Black | 932 | 12.4 | 23119 | 8.0 | 378 | 11.6 | 48941 | 8.8 |
| Mixed | 4398 | 58.5 | 143709 | 49.9 | 1684 | 51.8 | 277721 | 50.1 |
| Asian | 24 | 0.3 | 2524 | 0.9 | 13 | 0.4 | 5438 | 1.0 |
| Indigenous | 2 | 0.0 | 127 | 0.0 | 11 | 0.3 | 508 | 0.1 |
| Unknown/Refused | 234 | 3.1 | 7753 | 2.7 | 239 | 7.4 | 19441 | 3.5 |
| Educational Status | ||||||||
| Elementary School or Less | 884 | 11.8 | 19955 | 6.9 | 260 | 8.0 | 45189 | 8.2 |
| High. Tech. or Professional School | 2215 | 29.4 | 105169 | 36.5 | 910 | 28.0 | 190571 | 34.4 |
| University/Post-graduate Degree | 721 | 9.6 | 58875 | 20.4 | 337 | 10.4 | 125259 | 22.6 |
| Unknown/Refused | 3702 | 49.2 | 104154 | 36.1 | 1742 | 53.6 | 192804 | 34.8 |
| Donation type | ||||||||
| Replacement | 3902 | 51.9 | 118173 | 41.0 | 1276 | 39.3 | 173209 | 31.3 |
| Community | 3569 | 47.4 | 168356 | 58.4 | 1913 | 58.9 | 365159 | 65.9 |
| Other | 51 | 0.7 | 1624 | 0.6 | 60 | 1.8 | 15455 | 2.8 |
| Donation Year | ||||||||
| 2020 | 3204 | 42.6 | 133122 | 46.2 | 1504 | 46.3 | 250437 | 45.2 |
| 2021 | 3709 | 49.3 | 134856 | 46.8 | 1496 | 46.0 | 262869 | 47.5 |
| 2022 | 609 | 8.1 | 20175 | 7.0 | 249 | 7.7 | 40517 | 7.3 |
| Co-infections | 88 | 1.2 | 477 | 0.2 | 43 | 1.3 | 246 | 0.0 |
| HIV | 66 | 0.9 | 201 | 0.1 | 39 | 1.2 | 173 | 0.0 |
| HBV | 15 | 0.2 | 172 | 0.1 | 1 | 0.0 | 32 | 0.0 |
| HCV | 7 | 0.1 | 106 | 0.0 | 3 | 0.1 | 41 | 0.0 |
| Blood Center | ||||||||
| Hemope, Recife | 1930 | 25.7 | 62930 | 21.8 | 666 | 20.5 | 137212 | 24.8 |
| Hemominas, Minas Gerais | 641 | 8.5 | 35852 | 12.4 | 301 | 9.3 | 81371 | 14.7 |
| FPS, Sao Paulo | 1316 | 17.5 | 88860 | 30.8 | 492 | 15.1 | 148253 | 26.8 |
| Hemorio, Rio de Janeiro | 2658 | 35.3 | 69763 | 24.2 | 1142 | 35.1 | 97969 | 17.7 |
| Hemoam, Manaus | 977 | 13.0 | 30748 | 10.7 | 648 | 19.9 | 89018 | 16.1 |
Hemominas, Fundação Hemominas (Belo Horizonte); Hemoam, Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas (Manaus); Hemope, Fundação Hemope (Recife); Hemorio, Fundação Hemorio (Rio de Janeiro). Excludes 9,399 donations for which information on donor status or samples for confirmatory testing were unavailable
FTD First-time donos, RD Repeat donos, HIV Human Immunodeficiency Virus, HBV Hepatitis B Virus, HBC Hepatitis C Virus, FPS Fundação Pró-Sangue (São Paulo)
Supplemental Table 2 shows the frequencies and percentages of RPR-positive and RPR-negative syphilis infections according to RPR titers in FTD and RD, and distributions by demographic and donation characteristics. For all categories, a consistent predominance of donors aged 25 to 34 years old and mixed race was observed. The FTD had the highest prevalences in all syphilis definitions and HIV was the most common co-infection.
Factors associated with RPR-positive infections among FTD and RD in unadjusted and multivariable models
Table 2 shows the prevalence of RPR-positive syphilis infections per 100,000 donations according to demographics and donation characteristics, as well as the results of unadjusted and multivariable analyses investigating associated factors. Subgroups with the highest prevalence of RPR-positive syphilis were black donors (933/100,000 donations), donors with ≤ primary school education (782/100,000 donations) and replacement donors (815/100,000 donations).
Table 2.
Prevalence and factors associated with prevalent RPR-positive syphilis infections among FTD and RD models
|
Prevalence per 105 donations (95% CI) |
Unadjusted PR (95% CI) |
Adjusted PR (95% CI) |
Adjusted PR p-value |
|
|---|---|---|---|---|
| Gender | ||||
| Female | 578 (554–603) | Reference | Reference | - |
| Male | 575 (554–596) | 0.99 (0.94–1.05) | 1.15 (1.08–1.21) | < .0001 |
| Age category (years) | ||||
| ≤ 24 | 545 (510–580) | Reference | Reference | - |
| 25–34 | 699 (666–731) | 1.28 (1.18–1.39) | 1.70 (1.57–1.85) | < .0001 |
| 35–44 | 494 (465–522) | 0.91 (0.83–0.99) | 1.42 (1.30–1.55) | < .0001 |
| 45–54 | 510 (473–547) | 0.94 (0.85–1.03) | 1.59 (1.44–1.76) | < .0001 |
| ≥ 55 | 627 (567–687) | 1.15 (1.02–1.29) | 2.07 (1.83–2.34) | < .0001 |
| Race | ||||
| White | 418 (395–440) | Reference | Reference | - |
| Black | 933 (864–1002) | 2.23 (2.04–2.45) | 2.00 (1.82–2.20) | < .0001 |
| Mixed | 638 (615–662) | 1.53 (1.43–1.63) | 1.40 (1.30–1.50) | < .0001 |
| Asian | 160 (73–247) | 0.38 (0.22–0.66) | 0.56 (0.33–0.97) | 0.0394 |
| Indigenous | 608 (14–1202) | 1.46 (0.54–3.89) | 2.05 (0.77–5.44) | 0.1485 |
| Unknown/Refused | 603 (512–694) | 1.44 (1.23–1.7) | 2.15 (1.77–2.62) | < .0001 |
| Education level | ||||
| Elementary school or less | 782 (715–849) | 3.02 (2.67–3.42) | 2.36 (2.08–2.69) | < .0001 |
| High, technical, or professional | 517 (491–543) | 2 (1.81–2.21) | 1.73 (1.55–1.92) | < .0001 |
| University/post-graduate degree | 259 (236–282) | Reference | Reference | - |
| Unknown/refused | 785 (754–817) | 3.04 (2.75–3.35) | 1.37 (1.24–1.51) | 0.003 |
| Donation type | ||||
| Replacement | 815 (782–847) | 1.78 (1.68–1.88) | 1.42 (1.33–1.51) | < .0001 |
| Community | 458 (440–476) | Reference | Reference | - |
| Other | 189 (125–254) | 0.23 (0.16–0.33) | 0.52 (0.37–0.74) | 0.0002 |
| Donor Type | ||||
| First time | 1211 (1171–1250) | 5.09 (4.78–5.42) | 5.06 (4.72–5.42) | < .0001 |
| Repeat donor | 238 (225–251) | Reference | Reference | - |
| Blood Center | ||||
| Hemominas, Minas Gerais | 408 (372–445) | Reference | Reference | - |
| FPS, Sao Paulo | 381 (356–405) | 0.93 (0.84–1.05) | 1.09 (0.98–1.23) | 0.1096 |
| Hemoam, Manaus | 406 (371–442) | 0.99 (0.88–1.13) | 1.19 (0.98–1.43) | 0.0763 |
| Hemope, Recife | 577 (545–610) | 1.41 (1.27–1.57) | 1.12 (1.01–1.25) | 0.0331 |
| Hemorio, Rio de Janeiro | 1088 (1039–1137) | 2.66 (2.41–2.94) | 2.77 (2.31–3.31) | < .0001 |
Hemominas, Fundação Hemominas (Belo Horizonte); Hemoam, Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas (Manaus); Hemope, Fundação Hemope (Recife); Hemorio, Fundação Hemorio (Rio de Janeiro)
FTD First-time donos, RD Repeat donos, RPR Rapid Plasma Reagin, CI confidence interval, PR prevalence ratio, FPS Fundação Pró-Sangue (São Paulo)
In the unadjusted models, age, race, education, donor type, and donation type were significantly associated with RPR-positive syphilis infections. We found no statistically significant association between gender and RPR-positive syphilis in the unadjusted analysis (PR 0.99, 95% CI 0.94–1.05).
In the multivariable model, age, race, education, donor type, and type of donation were significantly associated with RPR-positive syphilis. Male donors had 1.15 times the prevalence of RPR-positive syphilis compared to female donors (95% CI 1.08–1.21; Table 2).
Supplementary Tables 3 and 4 show the prevalence of RPR-positive syphilis per 100,000 donations according to demographics and donation characteristics as well as the results of unadjusted and multivariable analyses by strata of donor status (FTD and RD). Apart from higher prevalence rates in FTD, stratum-specific findings regarding associated factors were similar.
Syphilis incidence in RD and associated factors
Table 3 shows the incidence rate of RPR-positive syphilis among RD according to demographics and donation characteristics, as well as the results of multivariable analysis investigating associated factors. The overall incidence of RPR-positive syphilis in RD was 90/100,000 person-years (95% CI 86–95). Incidence rates were higher in younger groups (< 24 and 25–34 years old), black and indigenous donors, and in donors with ≤ high school education. Results from the adjusted model suggest higher syphilis incidence in males compared to females; younger donors compared to older age groups; donors declaring Black or Mixed race compared to White/Caucasians; and donors with ≤ high school education compared to those with university-level or higher education. Additionally, we found differences by blood center, with higher incidence in Hemorio compared to Hemominas.
Table 3.
Incidence rates and factors associated with incident syphilis among RD
|
Incident RPR-positive syphilis N |
Person-years | Incidence Rate per 100,000 person-years (95% CI) |
Adjusted incidence rate ratios (95% CI) |
p-value | |
|---|---|---|---|---|---|
| Gender (%) | |||||
| Female | 425 | 548305.1 | 78 (71–85) | Reference | - |
| Male | 912 | 940948.8 | 97 (91–103) | 1.23 (1.09–1.38) | 0.0007 |
| Age category (%) | |||||
| ≤ 24 | 145 | 81046.6 | 179 (152–211) | Reference | - |
| 25–34 | 393 | 318332.7 | 124 (112–136) | 0.71 (0.59–0.86) | 0.0005 |
| 35–44 | 390 | 526515.6 | 74 (67–82) | 0.42 (0.34–0.51) | < .0001 |
| 45–54 | 254 | 387816.2 | 66 (58–74) | 0.35 (0.28–0.44) | < .0001 |
| ≥ 55 | 155 | 175561.3 | 88 (75–103) | 0.45 (0.35–0.57) | < .0001 |
| Race (%) | |||||
| White | 371 | 591541.0 | 63 (57–69) | Reference | - |
| Black | 186 | 134525.1 | 138 (120–160) | 1.92 (1.61–2.31) | < .0001 |
| Mixed | 699 | 672616.1 | 104 (97–112) | 1.48 (1.29–1.69) | < .0001 |
| Asian | 3 | 14216.8 | 21 (7–65) | 0.43 (0.14–1.35) | 0.1498 |
| Indigenous | 2 | 1199.2 | 167 (42–667) | 2.68 (0.67–10.81) | 0.1654 |
| Unknown/Refused | 76 | 75174.1 | 101 (81–127) | 1.69 (1.28–2.23) | 0.0002 |
| Education level (%) | |||||
| Elementary school or less | 114 | 133353.6 | 86 (71–103) | 1.93 (1.48–2.50) | < .0001 |
| High, technical, or professional | 432 | 495722.1 | 87 (79–96) | 1.79 (1.47–2.19) | < .0001 |
| University/post-graduate | 134 | 350969.1 | 38 (32–45) | Reference | - |
| Unknown/refused | 657 | 509227.6 | 129 (120–139) | 1.62 (1.24–2.10) | 0.0003 |
| Donation type (%) | |||||
| Replacement | 536 | 589027.4 | 91 (84–99) | 0.95 (0.84–1.07) | 0.4151 |
| Community | 784 | 878712.1 | 89 (83–96) | Reference | - |
| Other | 17 | 21532.7 | 79 (49–127) | 0.64 (0.40–1.04) | 0.0729 |
| Blood Center (%) | |||||
| Hemope, Recife | 307 | 353541.1 | 87 (78–97) | 1.19 (0.96–1.46) | 0.1127 |
| Hemominas, Minas Gerais | 134 | 210663.1 | 64 (54–75) | Reference | - |
| Fundacao Pro-Sangue, Sao Paulo | 222 | 430741.6 | 52 (45–59) | 1.01 (0.81–1.26) | 0.9925 |
| Hemorio, Rio de Janeiro | 499 | 318414.8 | 157 (144–171) | 2.56 (1.98–3.32) | < .0001 |
| Hemoam, Manaus | 175 | 175911.8 | 100 (86–115) | 1.30 (0.96–1.75) | 0.0904 |
Hemominas, Fundação Hemominas (Belo Horizonte); Hemoam, Fundação Hospitalar de Hematologia e Hemoterapia do Amazonas (Manaus); Hemope, Fundação Hemope (Recife); Hemorio, Fundação Hemorio (Rio de Janeiro)
RD Repeat donos, RPR Rapid Plasma Reagin, CI confidence interval, PR prevalence ratio, FPS Fundação Pró-Sangue (São Paulo)
Temporal trends of syphilis in blood donors and reported cases in Brazil between 2012 and 2022
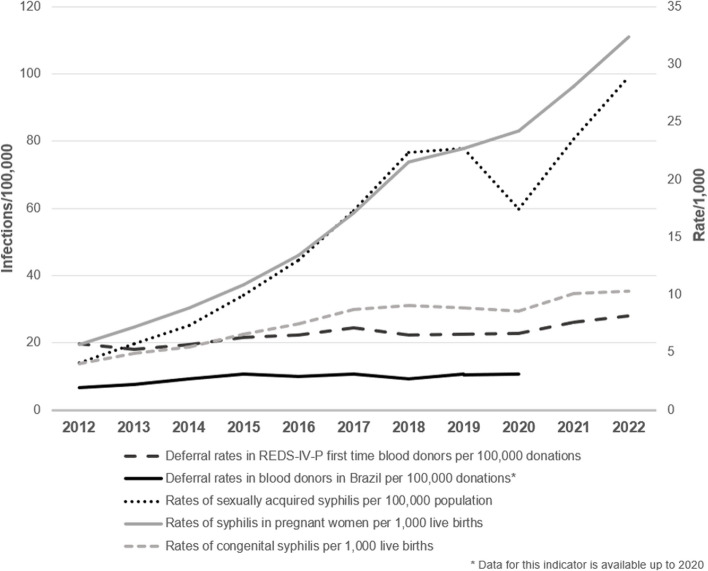
Figure 2 shows the temporal trends of reported syphilis cases in Brazil, deferral rates due to reactive syphilis among Brazilian blood donors, and deferral rates due to reactive screening tests in REDS-participating blood donors between 2012 and 2022. Throughout the period, an increase in syphilis detection was evident for all definitions, with a more pronounced increment for syphilis in pregnant persons (5.7-fold) and sexually acquired syphilis (7.0-fold), except for 2020, likely due to underreporting resulting from disruptions in health services and monitoring programs during the early stages of the COVID-19 pandemic.
Fig. 2.
Syphilis trends in Brazil and deferral rates among overall and REDS Brazilian blood donors
The rates of syphilis deferral in blood donors also had an upward trend in Brazil (1.6-fold) and in the REDS-IV-P Brazil program (1.4-fold), albeit in different proportions when compared to the rates of sexually acquired syphilis and congenital syphilis. Even though REDS-IV-P data represents only five Brazilian cities, our data reflected the country’s deferral patterns and were closely related to the congenital syphilis detection rates, which increased 2.5-fold between 2012 and 2022.
Discussion
In this study, we used screening and confirmatory tests to evaluate the prevalence, incidence, and factors associated with syphilis infection in blood donors within the REDS-IV-P Brazil program who donated between January 2020 and February 2022. The prevalence of syphilis infections including RPR-positive and RPR-negative cases was 2.5% among FTD and 0.6% among RD, and the incidence of syphilis in RD was 90/100,000 person-years. We observed that both the prevalence and incidence of RPR-positive syphilis were associated with male sex, black and mixed-race and lower schooling; regarding age, compared to donors ≤ 24 years old, those aged ≥ 55 had higher prevalence of RPR-positive syphilis, whereas younger donors had higher syphilis incidence compared to older donors. Our findings are also in line with the Brazilian Syphilis Epidemiological Bulletin, which showed higher rates of acquired syphilis in males and in persons of black or mixed race [5]. In the evaluation of relationships between syphilis detection in blood donors and cases reported in the general Brazilian population between 2012 and 2022, we found that rates have been increasing in all groups, with a close correlation between the trends in blood donors and congenital cases.
Other studies have investigated the prevalence of syphilis in blood donor populations. In a study conducted in the US including blood donations collected between 2020 and 2022, the prevalence of donations categorized as consensus positive and active syphilis was 28.4/100,000 and 11.7/100,000, respectively [8]. The prevalence of donations with positive treponemal tests was 1.86% in a Colombian study [13], and 0.33% in a Chinese study [14]. Our prevalence estimates were higher than those reported in prior Brazilian studies; compared to a study reporting data from blood donors in Sao Paulo city in 2021, the prevalence syphilis in FTD appears to have increased [10]. Other Brazilian studies evaluated deferral rates due to syphilis reactivity in screening serologies in Brazilian blood centers; rates reported in Goiania and in São Paulo were 1.09% [15] and 0.68% [10] respectively. In the most recently published national data, the deferral rate due to syphilis was 1.08%, the highest ever recorded [7]. Following the testing algorithm adopted in these reports, in our study the overall deferral rate was 1.22%. Internationally, one study conducted in Angola showed a 20% prevalence of syphilis positivity in donations collected between 2011 and 2016 [16]. The incidence of syphilis in RD in our study was approximately 8 times higher than in the US during the same period [8]. These heterogeneities reflect striking disparities in syphilis prevalence globally, changes in syphilis epidemiology with increasing rates in recent years [17], and differences in blood donation screening practices [18, 19].
As seen in our results, other studies have shown that the prevalence of syphilis in FTDs tends to be higher than in RDs [8, 10, 14, 20]. It is likely that this difference results from multiple factors, including differences in demographic characteristics, behavioral aspects (with repeat donors on average having fewer behavioral exposures), and the fact that repeat donors are tested for syphilis and other transmissible infections each time they donate; those who continue donating are a selected subgroup with lower risk of any transfusion transmissible infection.
Factors associated with syphilis seropositivity in blood donors have also been investigated by other authors. As seen in our study, RPR-positive syphilis in US blood donors was significantly associated with male sex, black race, first-time donations, and younger age (20–39 years old) [8]. Data from blood donors in Angola suggest higher prevalence of syphilis seropositivity with increasing age [16]. These associations should be interpreted carefully due to differences in testing algorithms; studies based on treponemal tests only will reflect the prevalence of lifetime exposure to syphilis rather than active infections, showing increasing rates in older age groups in any population. Lower levels of education were associated with higher prevalence of syphilis infection in blood donors in China [14], as seen in our results, likely resulting from limited access to health information and prevention in donors with lower schooling. Of note, age is a determinant of educational attainment, as younger donors may still be completing their education. In our study, the age group ≤ 24 years old had lower prevalence of RPR-positive syphilis in the adjusted analysis compared to older age groups, but a higher incidence rate (with lower adjusted incidence rate ratios for older age groups). This suggests that, while older groups are more likely to have prevalent active syphilis, incident syphilis is more likely to happen in the younger age group even after adjustment for educational levels. Younger populations are known to be a higher-risk group for syphilis and other STIs [21]. We also found a statistically significant association between RPR-positive syphilis and replacement donation. While community donors present for donation voluntarily, replacement donors are responding to a request; this seemingly subtle difference may explain differences in behaviors and awareness between community and replacement donors that align with our findings [22].
The blood donor population in our study had a lower prevalence of syphilis compared to the general population, with temporal trends closely matching the rates of congenital syphilis recorded by the Brazilian surveillance system between 2012 and 2022. It is plausible that the lower rates observed in blood donors compared to the general population is attributable to differences in behavior patterns and risk exposures resulting from higher schooling and socioeconomic status, and better access to healthcare and information [23]. We used graphical analysis to investigate temporal dynamics of syphilis cases in Brazil in relation to deferral rates due to syphilis, under the assumption that, despite a presumably lower risk of infection, blood donors would still reflect shifts in syphilis occurrence in the general population. Our findings support this hypothesis. A rise in congenital infection cases suggests that syphilis occurrence has increased among women of reproductive age, also indicating potential problems in the detection and management of cases among pregnant women during antenatal care. Although cases in FTD and congenital cases are measured on different scales (per 100,000 in blood donors and per 1,000 live births in congenital cases), the observation that trends in these populations have risen nearly proportionally is interesting and may indicate that blood donors can be monitored as sentinels of the underlying occurrence of syphilis in the general population, including congenital cases, particularly given the infrequent testing of syphilis in low-risk asymptomatic populations and imperfect case ascertainment of congenital infections.
Our study has limitations. The blood center donation screening tests were not the same across all participating blood centers, and 82 of the 10,771 positive or indeterminate donations identified for confirmatory testing in the centralized study laboratory had been initially tested with a non-treponemal assay. Not all donations with reactive or indeterminate results had samples available for confirmatory testing. However, all confirmation testing for this study was conducted in a single laboratory including both treponemal and non-treponemal tests. The criteria adopted to categorize syphilis infections in this study, based on RPR reactivity, was intended to discriminate cases more likely to reflect active (RPR-positive) and resolved (RPR-negative) infections. This categorization may have misclassified very recent infections (with reactive ELISA and nonreactive RPR) and recently treated infections (with RPR results still reactive). Our study was conducted during the COVID-19 pandemic, which impacted the behaviors of donors and nondonors and may have modified the prevalence, incidence, and factors associated with syphilis. Despite these limitations, our study reports results for a large number of individuals from five blood centers, representing three different regions of Brazil.
The blood donor population can be used as a sentinel to monitor syphilis in lower risk populations in different locations. Together with other studies, our findings highlight the increasing rates of syphilis, even in the blood donor population, emphasizing the importance of public health policies, including the identification of subgroups to be prioritized in education, prevention, and treatment interventions.
Supplementary Information
Additional file 1. Criteria for blood donation in Brazil according to Consolidated Ordinance No. 5. This file provides the detailed criteria for blood donation in Brazil, based on the regulations outlined in the Consolidated Ordinance No. 5. It includes eligibility requirements, restrictions, and procedural guidelines for blood donors.
Additional file 2. Laboratory kits used for syphilis screening at participating hemocenters. This file contains a list of laboratory kits employed for syphilis screening at the hemocenters participating in the study. It includes the methodology, trade names, specificity, sensitivity, manufacturer and date of Study Manage System.
Additional file 3. A. Prevalence rates of RPR-positive syphilis infection in FTD and RD according to RPR titers. This file presents the prevalence rates of syphilis infections, measured by RPR-positive results, among first-time donors (FTD) and repeat donors (RD). The RPR titers are classified into high titer (RPR ≥1:8) and low titer (RPR <1:8), and the prevalence is expressed per 100,000 donations, accompanied by 95% confidence intervals. B. Confirmatory testing results in 220 samples categorized as indeterminate on blood donation screening. Description of confirmatory test results among samples categorized as indeterminate on blood donation screening.
Additional file 4. Characteristics of blood donors by RPR reactivity and titers. This file provides detailed characteristics of blood donors based on RPR reactivity and titers. Donors are categorized by RPR-positive syphilis with titers ≥ 1:8, RPR-positive syphilis with titers < 1:8 and RPR-negative syphilis, along with demographic data.
Additional file 5. Prevalence and factors associated with RPR-positive syphilis infections in first-time donors. This file presents the prevalence of RPR-positive syphilis infections among first-time donors (FTD) expressed per 100,000 donations (95% CI). Additionally, it explores the factors associated with these infections.
Additional file 6. Prevalence and factors associated with RPR-positive syphilis infections in repeat donors. This file presents the prevalence of RPR-positive syphilis infections among repeat donors (RD), expressed per 100,000 donations (95% CI). It also explores the factors associated with these infections.
Acknowledgements
The authors acknowledge all participants and parents for their cooperation in the study. The authors also thank the staff at all participating Brazilian and American centers. Without their help, this study would not have been possible. We also thank the REDS-IV-P publications committee for reviewing the manuscript. This work was supported by the NHLBI Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P), with the participation of the following centers and investigators. Brazilian participants—Instituto de Medicina Tropical de São Paulo (USP)—Cecilia Alencar; Fundação Pró-Sangue (São Paulo) – Carla Dinardo, Ligia Capuani; Instituto de Tratamento do Câncer Infantil (São Paulo)—Miriam Park; Faculdade de Medicina da Universidade de São Paulo (São Paulo)—Paula Blatyta, Daiane Silva de Souza; Hemominas—Belo Horizonte (Minas Gerais)—Anna Bárbara de Freitas Carneiro-Proietti, Carolina Miranda Teixeira, Bárbara Victória Malta de Andrade, Poliane Gonçalves; Hemominas; Montes Claros (Minas Gerais) – Rosemere Afonso Mota, Amanda Barbosa; Hemominas—Juiz de Fora (Minas Gerais) – Daniela de Oliveira Werneck, Julia Carneiro; Fundação Hemope – Recife (Pernambuco) – Dahra Teles, Alessandra Ferraz de Sá, Regina Siqueira Nogueira Nunes Gomes; Hemorio – (Rio de Janeiro) – Fabiana Canedo; Fundação Hemoam – (Amazonas) – Theomário Theotonio Azevedo da Cruz; Instituto de Matemática e Estatística da Universidade de São Paulo—USP (São Paulo)—Mina Cintho Ozahata. US Investigators: Vitalant Research Institute and University of California San Francisco—Michael P. Busch, Shannon Kelly; Westat – Adelaide Amo-Mensah; National Heart, Lung, and Blood Institute, NIH – Simone A. Glynn.
Authors’ contributions
NAB, SOGM and BC conceived the study. DEW was the primary data analyst; RB, VIAS, EG, MS and BC revised data analysis. CAN, MR, LA, PL, NF, and ECS contributed with data acquisition; MKO contributed with data management. NAB, VIAS, and SOGM wrote the first draft of the manuscript. NAB produced the manuscript figures. All authors contributed with data interpretation. All authors revised and approved the final version of the manuscript.
Funding
This study was funded by NIH/NHLBI, under contract number 75N92019D00038.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The ethics committees at each blood center and the Brazilian National Ethics Research Committee reviewed and approved the study, including exemption of informed consent and authorization for further testing of repository samples for all donors included in the analysis (CAAE: 14561118.6.1001.0068). The institutional review board (IRB) at University of California San Francisco, which serves as Vitalant Research Institute’s IRB of record, approved this study (approval number 20–32768) as non-human subjects research with exemption of additional informed consent. Samples were coded and pseudonymized before shipment to testing facilities. The study investigators had no access to the keys that linked pseudonymized observations to identifying blood center data; therefore, all identifiable information of the blood donors included in the study were kept confidential.
Consent for publication
The authors confirm that all participants consented to blood safety screening at the time of donation, allowing publication of their data in the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tao YT, Gao TY, Li HY, Ma YT, Li HJ, Xian-Yu CY, et al. Global, regional, and national trends of syphilis from 1990 to 2019: the 2019 global burden of disease study. BMC Public Health. 2023;23(1):754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GOV.UK. Sexually transmitted infections (STIs): annual data tables. 2023. Available from: https://www.gov.uk/government/statistics/sexually-transmitted-infections-stis-annual-data-tables. [cited 2024 May 10].
- 3.World Health Organization. Implementing the global health sector strategies on HIV, viral hepatitis and sexually transmitted infections, 2022–2030. Geneva: World Health Organization; 2024. p. 69. Available from: https://www.who.int/publications/i/item/9789240094925.
- 4.Centers for Disease Control and Prevention (CDC). STI Statistics. 2024. Sexually Transmitted Infections Surveillance. 2022. Available from: https://www.cdc.gov/std/statistics/2022/default.htm. [cited 2024 May 10].
- 5.Ministério da Saúde do Brasil. Boletim Epidemiológico - Sífilis 2023. Brasília: Ministério da Saúde do Brasil; 2023. p. 56. Report No.: Número Especial. Available from: https://www.gov.br/saude/pt-br/centrais-de-conteudo/publicacoes/boletins/epidemiologicos/especiais/2023/boletim-epidemiologico-de-sifilis-numero-especial-out.2023/view. [cited 2024 May 10].
- 6.Seara-Morais GJ, Pousada BF, Escaleira FF, Doi AM, Welter EAR, Avelino-Silva VI. Mobility restrictions during the COVID-19 pandemic and reduced outpatient HIV and syphilis testing in Brazil. Braz J Infect Dis. 2023;27(3):102771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agência Nacional de Vigilância Sanitária (ANVISA). 9° Boletim de Produção Hemoterápica. Brasília: Ministério da Saúde do Brasil; 2022. p. 24. Report No.: 9. Available from: https://www.gov.br/anvisa/pt-br/assuntos/noticias-anvisa/2022/anvisa-divulga-9o-boletim-de-producao-hemoterapica. [cited 2024 May 10].
- 8.Conti G, Notari EP, Dodd RY, Kessler D, Custer B, Reik R, et al. Syphilis seroprevalence and incidence in US blood donors from 2020 to 2022. Transfusion. 2024;64(2):325–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu S, Luo L, Xi G, Wan L, Zhong L, Chen X, et al. Seroprevalence and risk factors on Syphilis among blood donors in Chengdu, China, from 2005 to 2017. BMC Infect Dis. 2019;19(1):509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Attie A, de Almeida-Neto C, S. Witkin S, Derriga J, Nishiya AS, Ferreira JE, et al. Detection and analysis of blood donors seropositive for syphilis. Transfus Med. 2021;31(2):121–8. [DOI] [PubMed] [Google Scholar]
- 11.Henao-Martínez AF, Johnson SC. Diagnostic tests for syphilis. Neurol Clin Pract. 2014;4(2):114–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agência Nacional de Vigilância Sanitária (ANVISA). Agência Nacional de Vigilância Sanitária - Anvisa. Relatórios de produção hemoterápica - Hemoprod. Available from: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/sangue-tecidos-celulas-e-orgaos/producao-e-avaliacao-de-servicos-de-hemoterapia. [cited 2024 May 24].
- 13.Martínez-Garcés JC, Macías-Vidal M, Maestre-Serrano R, la Hoz RÁD, Navarro-Jiménez E, Bula-Viecco J, et al. Serorreacción y prevalencia de sífilis en donantes de un banco de sangre de Barranquilla, Colombia. Bioméd. 2019;39(Sp. 1):163–71. [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Liu Q, Sun P, Yuan S, Liao H, Zhang X. Prevalence of syphilis infections among volunteer blood donors in Jinan blood center, China: a 15-year retrospective study. Infect Drug Resist. 2022;1(15):6431–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pessoni LL, de Aquino ÉC, de Alcântara KC. Prevalence and trends in transfusion-transmissible infections among blood donors in Brazil from 2010 to 2016. Hematol Transfus Cell Ther. 2019;41(4):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quintas E, Cogle ADC, Dias CC, Sebastiao A, Pereira ADC, Sarmento A, et al. Prevalence of syphilis in blood donors in Angola from 2011 to 2016. Clin Med Rep. 2018;1(4). Available from: http://www.oatext.com/prevalence-of-syphilis-in-blood-donors-in-angola-from-2011-to-2016.php. [cited 2024 Jun 5].
- 17.STI: Prevalence of active syphilis in 15-49 year olds (%). Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/prevalence-of-active-syphilis-in-individuals-(-). [cited 2024 Jun 21].
- 18.U.S. Food and Drug Administration (FDA), Center for Biologics Evaluation and Research. Recommendations for screening, testing, and management of blood donors and blood and blood components based on screening tests for syphilis. 2020. p. 15. Available from: https://www.fda.gov/media/85283/download. [cited 2024 Aug 7].
- 19.World Health Organization. Screening donated blood for transfusion-transmissible infections: recommendations. Geneva; 2009. p. 66. Available from: https://iris.who.int/handle/10665/44202. [cited 2024 Aug 7]. [PubMed]
- 20.Osei-Boakye F, Nkansah C, Appiah SK, Abbam G, Derigubah CA, Ukwah BN, et al. Self-reported high-risk behavior among first-time and repeat replacement blood donors; a four-year retrospective study of patterns. PLoS ONE. 2024;19(8):e0308453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention (CDC). STI Statistics. 2024. Sexually Transmitted Infections Surveillance, 2023. Available from: https://www.cdc.gov/sti-statistics/annual/index.html. [cited 2024 Dec 9].
- 22.Abdel Messih IY, Ismail MA, Saad AA, Azer MR. The degree of safety of family replacement donors versus voluntary non-remunerated donors in an Egyptian population: a comparative study. Blood Transfus. 2014;12(2):159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zucoloto ML, Gonçalez T, Custer B, McFarland W, Martinez EZ. Comparison of the demographic and social profile of blood donors and nondonors in Brazil. Health Soc Care Community. 2019;27(2):330–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Criteria for blood donation in Brazil according to Consolidated Ordinance No. 5. This file provides the detailed criteria for blood donation in Brazil, based on the regulations outlined in the Consolidated Ordinance No. 5. It includes eligibility requirements, restrictions, and procedural guidelines for blood donors.
Additional file 2. Laboratory kits used for syphilis screening at participating hemocenters. This file contains a list of laboratory kits employed for syphilis screening at the hemocenters participating in the study. It includes the methodology, trade names, specificity, sensitivity, manufacturer and date of Study Manage System.
Additional file 3. A. Prevalence rates of RPR-positive syphilis infection in FTD and RD according to RPR titers. This file presents the prevalence rates of syphilis infections, measured by RPR-positive results, among first-time donors (FTD) and repeat donors (RD). The RPR titers are classified into high titer (RPR ≥1:8) and low titer (RPR <1:8), and the prevalence is expressed per 100,000 donations, accompanied by 95% confidence intervals. B. Confirmatory testing results in 220 samples categorized as indeterminate on blood donation screening. Description of confirmatory test results among samples categorized as indeterminate on blood donation screening.
Additional file 4. Characteristics of blood donors by RPR reactivity and titers. This file provides detailed characteristics of blood donors based on RPR reactivity and titers. Donors are categorized by RPR-positive syphilis with titers ≥ 1:8, RPR-positive syphilis with titers < 1:8 and RPR-negative syphilis, along with demographic data.
Additional file 5. Prevalence and factors associated with RPR-positive syphilis infections in first-time donors. This file presents the prevalence of RPR-positive syphilis infections among first-time donors (FTD) expressed per 100,000 donations (95% CI). Additionally, it explores the factors associated with these infections.
Additional file 6. Prevalence and factors associated with RPR-positive syphilis infections in repeat donors. This file presents the prevalence of RPR-positive syphilis infections among repeat donors (RD), expressed per 100,000 donations (95% CI). It also explores the factors associated with these infections.
Data Availability Statement
No datasets were generated or analysed during the current study.