Abstract
Background
To evaluate the biosafety, reduction in anterior capsule opacification, and fluctuation in intraocular pressure (IOP) of a new phakic refractive lens (PRL) with a sinusoidal drainage groove design.
Methods
This self-controlled experiment was performed on eight eyes of four rabbits. Each rabbit was implanted with a sinusoidal PRL (PRL-S5) in the right eye and a conventional posterior chamber PRL (PC-PRL) in the left eye. Slit-lamp examinations, optical coherence tomography, and IOP evaluation were performed before surgery and at 1 day, 1 week, 3 months, 6 months, and 1 year postoperatively in each eye. Gross examination, histopathology, and electron microscopy of the capsule and PRL were performed 1 year postoperatively.
Results
On slit-lamp examination, the inflammatory reactions recovered one week after surgery. The PC-PRL group developed anterior subcapsular cataracts at 3 months postoperatively and diffuse and dense opacification of the cortex at 1 year. PRL-S5 showed mild local opacification in the optical zone 6 months postoperatively, which did not progress significantly. At 1 year, PC-PRLs had greater opacification (27.37–72.17%) than PRL S5 (6.63–66.96%). Three months after surgery, one eye in the PC-PRL group had scleral staphyloma, one eye had corneal edema, and one eye experienced nasal hepatic prolapse into the anterior chamber. One eye in the PRL-S5 group had papillary membranes but recovered 6 months postoperatively. Histopathological examination revealed liquefaction and necrosis of the opacified area in the center of the subcapsular membrane in both groups. Numerous granular bodies and fibrous precipitates were observed in epithelial cells in the opaque area. Electron microscopy showed that epithelial cells proliferated on the surface of all anterior capsule membranes, with no significant differences between the two groups. The capsular PC-PRL group showed anterior cortical proliferation and fibrosis. An IOP elevation was noted on the first postoperative day (18.8 to 37.9 mmHg). However, both the PC-PRL and PRL-S5 groups exhibited relatively stable IOP levels 1 week, 3 months, 6 months, and 1 year postoperatively.
Conclusions
The new PRL exhibited robust long-term biocompatibility. The sinusoidal groove design facilitated the maintenance of IOP stability without necessitating iridectomy and effectively mitigated the onset and progression of cataracts.
Keywords: Refractive surgery, Phakic Intraocular Lens, Phakic Refractive Lens, Complications, Intraocular pressure, Cataract
Background
With the advancement of technology and the continuous development of society, people’s lifestyles have undergone tremendous changes, and the increasing use of electronic devices has led to a rising prevalence of myopia [1]. In 2000, the global prevalence of myopia was 22.9% with a prevalence of high myopia at 2.7% [2]. China has the highest prevalence of myopia globally, reaching 57.1% in 2014 [3], with the prevalence among young people as high as 80–90%, and the prevalence of high myopia as high as 10–20% [4]. Thick glasses cause many inconveniences to patients with myopia, and long-term wear of corneal contact lenses carries potential risks. Therefore, the surgical correction of refractive errors has become an increasingly popular choice for adult patients with myopia. Currently, refractive surgeries include corneal refractive surgery and intraocular lens implantation.
Posterior chamber phakic intraocular lens implantation technology can meet the requirements of low-to-high myopia treatment with minimal impact on the intraocular tissue structure and reversibility of the surgery [5]. Implantable collamer lenses with a central hole (ICL V4c; STAAR Surgical Co.) are commonly used in the domestic market. The ICL V4c allows natural aqueous humor circulation through the central hole in the optical zone, eliminating the need for a peripheral iris incision [6, 7]. However, this type of intraocular lens has drawbacks, such as glare and compromised visual quality [8]. The third-generation posterior chamber phakic refractive lens (PC-PRL) is an intraocular lens with a density close to that of the aqueous humor that can be suspended between the iris and the lens, avoiding contact with the anterior surface of the crystalline lens. However, it often requires 1–2 peripheral incisions around the iris before surgery. This suspended design has a reduced rate of secondary cataracts [9] but many postoperative complications, including pigmentary glaucoma [10, 11], zonular dehiscence [12], and spontaneous dislocation [13].
Phakic Refractive Lens With Sinusoidal Design (PRL-S5) is the new generation of PC-PRL, with a groove added at the junction of the optical zone and the haptic [14]. A sine wave-shaped drainage groove was designed on the groove at fixed angles, which served as a channel for natural aqueous humor circulation without the need for peripheral iris incisions during surgery. Compared to ICL V4c, which sacrifices imaging quality to meet the aqueous humor circulation function, PRL-S5 has unique advantages. Its drainage groove position does not affect the optical zone of the intraocular lens and thus does not affect visual effects or imaging. In addition, the ICL corrects − 0.50∼-18.00D and has only four sizes (12.1, 12.6, 13.2, and 13.7 mm), which may not fully fit the patient’s ciliary sulcus. The PRL used in this study corrects − 3.00∼ -28.00 D of myopia and has five size models (10.4, 10.7, 11.0, 11.3, and 11.6 mm) that can be more adaptable. By controlling the width, depth, and number of grooves, the flow of aqueous humor can be better regulated, ensuring better IOP stability and reducing the risk of anterior capsule opacification.
In our previous studies, novel PRLs were proven safe and feasible for short-term implantation after surgery [14]. However, we did not observe its long-term postoperative safety and stability. In this study, we evaluated the postoperative one-year in vivo biocompatibility, reduction in anterior capsule opacification, and fluctuation of IOP in rabbit eyes.
Methods
Characteristics of PRL
The PRL is a foldable, intraocularly implantable optical medical device with a refractive range of -3.00 D to -28.00 D. It was made of silicone rubber (refractive index 1.46, specific gravity of approximately 1.01), and the finished product was sterilized by G 60 irradiation, dried, and preserved. The PRL-S5 consisted of a loop and an optical zone, and the refractive error was designed to be on the anterior surface and was implanted in the posterior chamber of the eye. The radius of curvature of the posterior chamber of the eye was similar to that of the anterior surface of a natural lens. PC-PRL was a prototype of PRL-S5, and its properties have been described previously [14].
We designed a specialized PC-PRL and PRL-S5 based on the biometric parameters of the rabbit eye, and the specific parameters are as follows. Conventional PRL: control group on sale, diameter of 11.7 mm, loop width 6.0 mm, refraction − 15.25 D. PRL-S5: experimental group, diameter of 11.7 mm, loop width 6.0 mm, refraction − 15.00D.
Implantation of the PRLs
This study was performed using eight eyes of four male New Zealand white rabbits (2.5 kg). The experiment animals were purchased from Shanghai Jiagan Biotechnology Co. They were kept individually in an animal room at 20 ± 1℃. A light-dark cycle alternating at 12-h intervals was maintained, food was provided twice daily, and water was provided ad libitum. After 1 month of acclimation, the experiments were initiated. All animal experiments were approved by the Animal Ethics Committee of the Eye and ENT Hospital of Fudan University, Shanghai, China (No. 2022114), and all experimental protocols, including care, transportation, and experiments of the animals, complied with the guidelines of the Animal Care and Use Committee of Fudan University.
Their eyes were grossly examined and found to be unremarkable before the surgical procedure. PRL S5 was implanted in the right eye of each rabbit, and control PC-PRL was implanted in the left eye. All surgeries were performed by two surgeons (WXY and ZXT) and recorded on a video system.
Before surgery and each examination, each rabbit was given 1–2 mL of an intramuscular injection of a 1:1 combination of tilestamide and zolazepam (20 mg/mL) for general anesthesia. Topicamide and oxybucaine hydrochloride eye drops were administered topically. Iridotomy was done on the left eye (implanted conventional PRL). After placing an ophthalmic viscosurgical device (OVD; Opegan, Santen, Osaka, Japan) into the anterior chamber, a PRL or PRL-S5 was implanted in the posterior chamber through a 3-mm clear corneal incision using an injector cartridge. The OVD was washed with a balanced salt solution, and pilocarpine eye drops were topically administered. Postoperatively, 0.1% dexamethasone and 0.5% levofloxacin eyedrops were topically administered four times daily for 1 week. Dexamethasone eye ointment was discontinued 23 days after surgery for a total duration of 3 weeks to prevent steroid-induced elevated IOP.
Follow-up and examinations
Slit-lamp examinations were performed preoperatively and at 1 day, 1 week, 3 months, 6 months, and 1 year postoperatively in each eye. Intraocular pressure (IOP) was measured using a handheld digital tonometer (Tono-Pen XL; Reichert Inc., Depew, NY, USA) under general and local anesthesia, as described above. Ocular inflammation and the relative location of the PRLs were observed by normal and slit-lamp examinations at 1 day, 1 week, 3 months, 6 months, and 1 year postoperatively. The relative positions of the PRLs in the posterior chamber were confirmed by using optical coherence tomography (OCT; CASIA 2; Tomey Corp, Nagoya, Japan).
Anterior subcapsular opacification was assessed and scored from 0 to 4 as previously described [15]. The other complications were assessed using a standard clinical scoring method, including corneal edema and the presence of cells and flares in the anterior chamber. Retro-illumination images of normal pupils were obtained for the photographic documentation of anterior subcapsular clouding (ASC) and other complications.
Histopathology and electron microscopy of capsular and PRL
After a follow-up of 12 months, the animals were anesthetized using a 1–2 mL intramuscular injection of a 1:1 combination of tilestamide and zolazepam (20 mg/mL) and then humanely euthanized with an intravenous injection of air. Their globes were enucleated, and the capsule was removed using an anterior continuous curvilinear capsulorhexis.
Representative gross color photographs of the eyes were obtained using a digital camera. The capsules of crystalline lenses of four eyes of two rabbits were immersed in a 10% formalin solution and fixed for a week. Capsules were mounted on slides and stained with hematoxylin and eosin for light microscopy.
The capsules of the crystalline lens of the other four eyes of two rabbits and explanted PRL were enucleated and fixed with 4% glutaraldehyde 0.1 M phosphate buffer. The specimens were dried using a Hitachi HCP-2 (Tokyo, Japan) critical-point drying apparatus and coated with platinum using an ion-sputtering machine (LEICA EM AG600, Germany). The specimens were examined using a scanning electron microscope (SEM; Hitachi SU8010, Japan).
Calculation of relative area of opacification of optic area
The file containing the image of the anterior surface of the rabbit crystalline lens observed with a stereomicroscope was opened using ImageJ (Wayne Rasband and contributors, National Institutes of Health, USA), and cut to the size of the PRL optic for binary processing. The opaque area (black part) was enclosed and pixelized, and its area relative to the optic area was calculated. To compare the proportion of opacifiation of optic area, repeated measures ANCOVA was applied with change from baseline as the dependent variable, and treatment, time and the treatment multiplied by time interaction as independent variables.
Results
Slit-lamp examinations
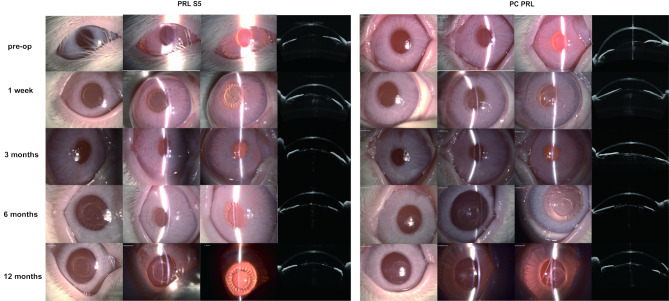
Figure 1 shows representative photographs of the ocular surface (columns 1 and 5), anterior chambers (columns 2 and 6), and posterior chambers (columns 3 and 7) observed using a slit-lamp and the crystalline lens observed using AS-OCT (columns 4 and 8) during the experimental period. The inflammatory reactions resolved 1 week after surgery. No abnormalities were observed in either of the groups (Fig. 1, line 1). From 3 months postoperatively, AS-OCT examination of the eye with PC-PRL showed cloudiness in the center of the anterior surface of the crystalline lens, but the slit-lamp microscopic observation showed no significant clinical signs. ASCs appeared in both groups 6 months after surgery (Fig. 1, line 2). The PC-PRL group developed diffuse ASC, whereas the PRL S5 group showed mild local opacification in the optical zone (Fig. 1, line 3). At 1 year postoperatively, there was no significant progression in the eyes of PRL S5, whereas ASC in the PC-PRL group gradually progressed toward the cortex, becoming denser (Fig. 1, line 4).
Fig. 1.
Slit-lamp and AS-OCT examination at 1 week, 3 months, 6 months, and 12 months postoperatively. Representative photographs of the ocular surface (columns 1 and 5), anterior chambers (columns 2 and 6), and posterior chambers (columns 3 and 7) observed using a slit lamp, and the crystalline lens observed using AS-OCT (columns 4 and 8) during the experimental period are shown. Photographs were captured from both eyes of the same rabbit
Table 1 shows complications in each eye during the study period. Three months after surgery, one eye in the PC-PRL group had scleral staphyloma and one eye in the PC-PRL group had corneal edema, which persisted throughout the observation period. Another rabbit experienced PC-PRL nasal hepatic prolapse into the anterior chamber, whereas the other PRL-S5 haptics were positioned normally in the space between the crystalline lens and the iris. One eye in the PRL-S5 group had papillary membranes but recovered 6 months postoperatively (Table 1).
Table 1.
Complications after PRL implantation during 1 year
| 1 Day | 1 week | 3 months | 6 months | 12 months | ||
|---|---|---|---|---|---|---|
| R1 | PRL S5 | / | / | ASC++ | ASC++ | |
| PC-PRL | / | ASC + | ASC++, PRL dislocation | ASC+++ | ASC+++ | |
| R2 | PRL S5 | / | / | papillary membranes | ASC+ | ASC+ |
| PC-PRL | / | / | ASC+, scleral staphyloma | ASC+++, scleral staphyloma | ASC++++, scleral staphyloma, cornea edema+ | |
| R3 | PRL S5 | / | / | ASC+ | ASC+ | ASC+ |
| PC-PRL | / | / | ASC+ | ASC+ | ASC++ | |
| R4 | PRL S5 | / | Contusive hyphema | ASC+ | ASC+++ | ASC+++ |
| PC-PRL | / | corneal edema++ | ASC+, cornea edema++ | ASC++, corneal edema++ | ASC++++, corneal edema++ |
PRL = phakic refractive lens; PRL S5 = phakic refractive lens with sinusoidal design; PC-PRL = posterior chamber phakic refractive lens; ASC = anterior subcapsular cataract
Comparison of opacity between PC-PRL and PRL S5
Table 2 shows the proportion of the opacity area accounting for the optic area in the two types of PRLs. At 1 week, two eyes with PC-PRLs had central opacification (3.40–5.63%), compared with none of PRL S5 had opacity (1.02–0.58%). At 3 months, all the PC-PRL led to central opacity (9.65–29.61%), whereas only three eyes with PRL S5 had minor opacity (3.43–12.49%). At 1 year, PC-PRLs had a higher proportion of opacification (27.37–72.17%) than PRL S5 (6.63–66.96%)(Table 2). After repeated measures ANCOVA, no statistically significant differences were observed between the PRL-S5 and PC-PRL groups at each follow up time point(P = 0.135). The opacification of optic area increased in both groups over time (P < 0.001). During the follow-up, the opacification of optic area in PRL-S5 showed no significant differences compared with PC-PRL (P = 0.580).
Table 2.
The proportion of the opacity area accounting for the optic area of the two PRLs
| PRL-S5 | PC-PRL | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| 1 day | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 week | 1.02 | 0.57 | 0.00 | 0.00 | 3.4 | 5.63 | 0.00 | 0.00 |
| 3 months | 3.43 | 5.76 | 9.60 | 12.49 | 9.65 | 15.16 | 14.83 | 29.61 |
| 6 months | 36.27 | 10.78 | 7.23 | 17.50 | 29.27 | 52.89 | 14.67 | 32.99 |
| 1 year | 40.22 | 16.49 | 6.63 | 66.96 | 56.05 | 38.95 | 27.37 | 72.17 |
PRL = phakic refractive lens; PRL S5 = phakic refractive lens with sinusoidal design; PC-PRL = posterior chamber phakic refractive lens; ASC = anterior subcapsular cataract
Gross examination
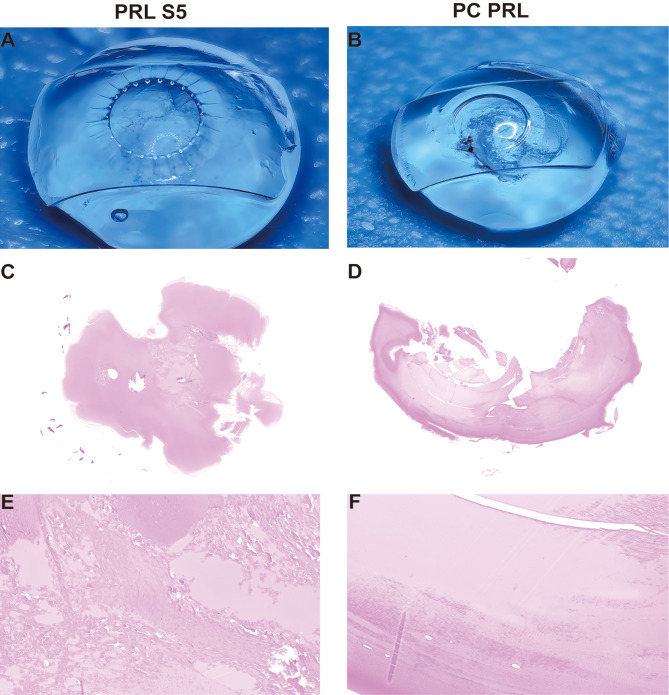
The results of the final observations indicated that both PC-PRL and PRL S5 exhibited varying degrees of opacity. Diffuse ASC was observed in both PRL S5 and PC-PRL; however, PC-PRL exhibited severe posterior adhesions between the proliferative mass in front of the crystalline lens, which was not observed in PRL S5 (Fig. 2A and B).
Fig. 2.
Gross examination and histopathology findings at 1 year postoperatively. A and B show diffuse anterior subcapsular cataracts in the PC-PRL and PRL-S5 groups; however, the PC-PRL group exhibited more severe posterior adhesions between the proliferative mass in front of the crystalline lens. C-F show hematoxylin and eosin-stained histopathological results of the specimens in A and B. Liquefaction and necrosis of the opacified area in the center of the subcapsular membrane in both groups
Histopathological findings
Figure 2C and F displays the histopathological results of the anterior capsule membrane for both the PRL S5 and PC-PRL groups. The capsules of both PC-PRL and PRL S5 groups had similar histopathological findings. Light microscopy revealed liquefaction and necrosis of the opacified area at the center of the subcapsular membrane (Fig. 2C and F). We also observed numerous granular bodies and fibrous precipitates in the epithelial cells in the opacity area in both groups (Fig. 3).
Fig. 3.
Inflammation and necrosis in the opacity area in PRL S5 and PC PRL. A was from PC-PRL. B-C were from PRL S5. Numerous granular bodies and fibrous precipitates were observed in the epithelial cells in both groups
Electronic microscopy examination
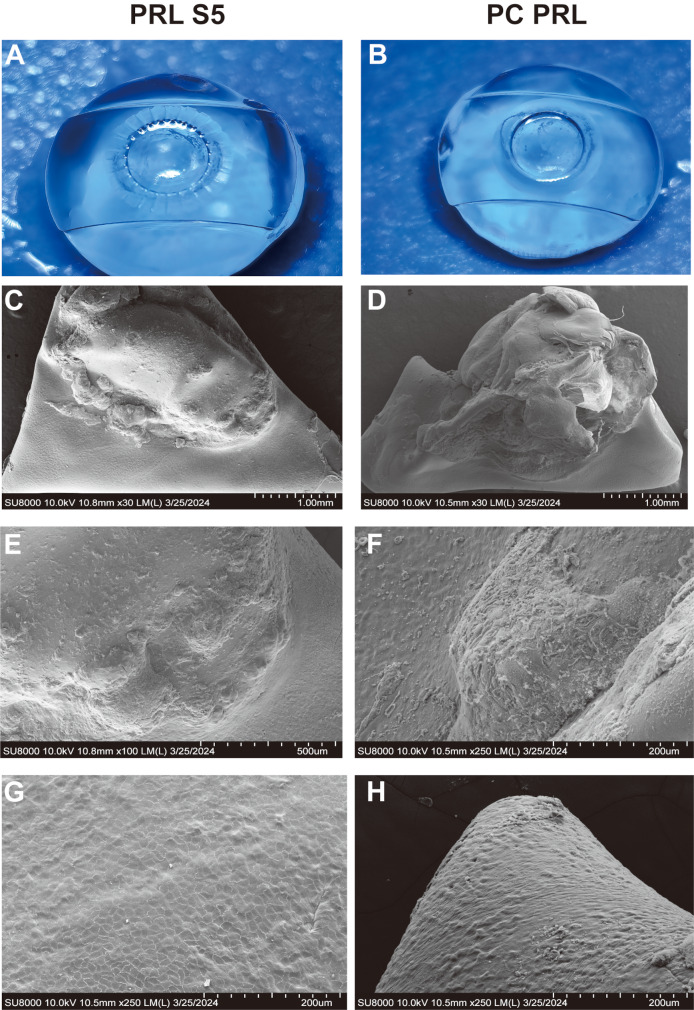
Electron microscopy showed that epithelial cells proliferated on the surface of all anterior capsule membranes, with no significant differences between the two groups (Fig. 4). More typical anterior cortical proliferation and fibrosis were noted on the surface of the PC-PRLs (Fig. 4F and H).
Fig. 4.
Scanning electron microscopy (SEM) observation of PRL-S5 and PC-PRL group at 1 year postoperatively. Panels A and B show gross photographs of the PRL-S5 and PC-PRL groups, respectively. Panels C, E, and G show SEM results for the PRL S5 group (from the same specimen as in A). Panels D, F, and H show the SEM images of the PC-PRL group (from the same specimen as in B), with panels F and H highlighting typical anterior cortical proliferation and fibrosis, respectively
Intraocular pressure
IOP was regularly monitored throughout the experimental period, and the results are shown in Fig. 5. IOP elevation was noted on the first postoperative day (18.8 to 37.9 mmHg). Both the PC-PRL and PRL-S5 groups exhibited relatively stable IOP levels 1 week, 3 months, 6 months, and 1 year postoperatively. (Fig. 5)
Fig. 5.
Intraocular pressure fluctuation of PRL-S5 and PC-PRL at different follow-up times. Blue line represents the PRL S5 group. The red line indicates the PC-PRL group. The symbols and error bars represent the mean and standard deviation of IOP at each time point
Discussion
Currently, the ICL is the most commonly used PIOL; however, there are visual quality problems owing to its central hole design and limited range of correction. Most previous studies on PRLs have demonstrated their short- and long-term effectiveness and predictability. Their suspension design avoids contact between the PRL and the anterior surface of the lens, thereby reducing the risk of cataracts and ensuring IOP stabilization. However, current PRLs still have many complications that require further improvement. In this study, a self-controlled experiment in rabbit eyes with PRL-S5 and conventional PC-PRLs was performed to observe in vivo safety 1 year postoperatively.
Secondary cataract is a common complication of posterior chamber IOLs that occurs mainly from inflammation resulting from contact between the PRL and its lens, leading to lens epithelial cell (LEC) metaplasia and migration. Another reason is metabolic damage from changes in aqueous humor dynamics. The PRL suspension design avoids the need for continuous contact between the pIOL and any specific area on the anterior surface of the lens. Therefore, the incidence of cataracts was lower than that of other posterior chamber types of IOLs [14]. The results of a meta-analysis showed that the incidence of postoperative cataracts in Chiron-Adatomed PIOL, ICL, and PRL was 25.66%, 8.48%, and 3.59%, respectively. Seven eyes in the PRL group had cataracts, of which six developed cataracts as a result of intraoperative trauma and the other eye developed cataracts 24 months postoperatively [16].
In our study, mild anterior subcapsular opacification of the crystalline lens appeared in the PC-PRL, beginning in the third postoperative month (12 weeks) and progressing gradually thereafter. PRL S5 developed opacification later and progressed slower than PC-PRL; however, there was no significant difference between the two in terms of the microscopic manifestations of histology at 1 year. Six weeks postoperatively in the rabbit eye is equivalent to 2 years postoperatively in the human eye. The overall observation time in our study amounted to 1 year, approximately 52 weeks, which is equivalent to 10 years for the human eye [17]. According to the morphological classification of cataracts, punctate ASC, which is usually non-progressive, may be associated with surgical trauma. Diffuse, annular, and centrally dense ASC, in contrast, progresses slowly and may be related to the impaired metabolism of the lens epithelium and mechanical damage caused by a vault that is too low [9]. The fact that the ASC in the present study occurred in the long-term period after surgery (12 weeks) and that both groups had a gradual progression with predominantly annular and diffuse shapes, suggests that the chordal design improved the flow of atrial fluid to a certain degree and maintained the metabolism of the crystalline lens over a longer period. However, an inflammatory response may still occur in contact with crystalline lens, ultimately leading to cataract development.
IOP is an important safety indicator. In our study, IOP was regularly measured in each group, and the results indicated that IOP in both groups stabilized within the normal range in rabbit eyes after 1 week. The IOP in anesthetized rabbits is 16 ± 2.1 mmHg (8.4–23.3) [18]. The increase in IOP after PRL surgery was associated with common postoperative events such as viscoelastic retention or reactions to steroid medications. In a previously published short-term PRL experiment, IOP returned to normal on the third day post-surgery but increased again on the 10th and 15th days post-surgery. However, in the short-term postoperative period, there was no significant difference in IOP among the different PRL designs [19]. The increase in IOP observed in that study may have resulted from reactions to steroids. In our study, steroids were used for three weeks post-surgery, and all rabbit eyes showed an IOP increase only on the day of surgery owing to viscoelastic retention. The IOP remained stable at 1 week, 3 months, 6 months, and 1 year post-surgery. This indicates that the sinusoidal drainage groove design of the new-generation PRL maintained a stable IOP without the need for iridotomy, resulting in a low risk of postoperative glaucoma and long-term safety.
The relationship between IOP and postoperative refraction was another worth noting point, which was mentioned by previous studies. The predicted refractive difference per 10 mm Hg IOP decrease was + 0.15 to 0.50 Diopter [20, 21]. Therefore, the potential risk of postoperative hyperopic shift should be taken into account when performing biometric measurements for IOL power calculation in patients with abnormally elevated IOP. Since this study was an animal study, the lens power calculation was not considered. Over the years, numerous IOL power calculation formulas have been developed to achieve the most accurate postoperative refractive outcomes [22]. PRL was a posterior chamber phakic intraocular lens. The Van Der Heijdei and Holladay formula has historically been used to calculate phakic lens power [23, 24]. Nowadays, artificial intelligence intraocular power calculation formular has been explored [25]. The PRL lens power calculation will be explored in the future research.
In this study, one rabbit eye experienced PRL dislocation in the anterior chamber, likely resulting from suboptimal placement during surgery. Previous studies reported an in-situ rate of 16.7% for small-sized PC-PRL (12.3 mm in diameter) and 100% for large-sized PC-PRL (13.3 mm in diameter). The in-situ rate for PRL S5 (13.3 mm in diameter) was 87.5%, with one case of dislocation on the 10th day post-surgery; however, the authors were unclear about the cause of this dislocation [14]. It is important to note that the rabbit eyes used in this study were not a high-myopia model, and the condition of the zonules was not reported. In addition, the OCT used in this study did not reveal the zonules. The contact between the PRL haptics and zonules may weaken zonular tension. The significant increase in tension on zonular fibers owing to axial elongation makes these fibers particularly fragile in patients with high myopia [12]. This can lead to zonular rupture and spontaneous dislocation of the PRL into the vitreous cavity [13, 26]. Therefore, further studies are required to enhance the implantation stability of PRL S5. Moreover, studies on optical quality and visual performance should be conducted to determine the long-term safety and effectiveness of this intraocular lens implant.
In our study, one eye exhibited corneal edema 1 week postoperatively, which may have resulted from intraoperative damage to the corneal endothelium, leading to endothelial decompensation. A transient episodes of elevated IOP often occur immediately after surgery due to residual viscoelastic materials. However, as the inflammatory response diminished, the IOP normalized, and no subsequent elevation in IOP was observed during follow-up. Additionally, one eye developed scleral staphyloma 3 months postoperatively, although the precise etiology remains uncertain. Both rabbit eyes experienced severe ASC, yet no increase in IOP was detected during the follow-up period.
Our study has several limitations. First, the sample size was relatively small, consisting of only four rabbits. The difference of opacification area between two groups of PRL was not significantly. conclusions need to be justified by larger sample sizes in the future. Second, no blank control group was included. However, the primary objective was to assess the safety of the novel PRL design, and the material of the PRL had already been validated, making a comparison with non-implanted rabbits unnecessary. Third, we did not report on the long-term impact of the PRL on corneal endothelial cell density (ECD). The ECD is a regular postoperative follow-up examination performed in clinical practice. The PRL is a posterior chamber PIOL that effectively avoids contact with corneal endothelial cells, and previous clinical applications have demonstrated its minimal effect on the corneal endothelium. The measurement of ECD in the rabbit eye was difficult; thus, it was replaced with a slit-lamp examination of the cornea. A detailed assessment of the endothelial cell status and provide a more comprehensive evaluation of the long-term effects on the cornea. Additionally, the rabbit eye model does differ from humans, which may restrict the application on human eyes. Rabbit corneal endothelial cells can regenerate, unlike those in humans [27]. Considering that the rabbit eye is closer in size to the human eye and is easier to perform ophthalmic surgical operations and observation, we used a rabbit eye model. We are willing to further observe the effect of PRL on corneal endothelial cells in future studieAs. Finally, previous studies have documented other potential complications associated with PRL, including secondary uveitis, retinal detachment [28], and choroidal neovascularization [29]. While IOP was stable postoperatively, this does not fully rule out the risk of glaucoma. Structural changes or damage to the optic nerve could still occur without elevated IOP, The retina and vitreous were not observed in our study. In future study, we will assess the optic nerve fiber to better evaluate the potential risk of glaucoma, and evaluate the retinal and uveal safety profile PRL thoroughly.
Conclusions
PRL exhibited robust long-term biocompatibility in rabbit eyes. The sinusoidal groove design facilitated the maintenance of IOP stability without requiring iridectomy and effectively mitigated the onset and progression of cataracts.
Acknowledgements
We would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
WXY and ZXT completed all the PRL implantation surgery. MHM and WXY made substantial contributions to the conception and design of the work. JYJ drafted the work. JYJ and CX substantively revised it. JYJ, CX, CMR, LYJ, LBL, ZXJ, XGH and MHM performed the slit-lamp observation, OCT examination and IOP measurement. JYJ, CX and MHM analyzed and interpreted the histopathological and electronic microscopy examination results. All authors read and approved the final manuscript.
Funding
1) Xiaoying Wang: National Natural Science Foundation of China (Grant No.82171095).
2) Xiaoying Wang: Project of Shanghai Science and Technology (Grant No. 23XD1400500).
3) Xiaoying Wang: Project of Shanghai Shenkang Hospital Development Center (Grant No. SHDC12024148).
4) Xingtao Zhou; National Natural Science Foundation of China (Grant No. 81770955).
5) Xingtao. Zhou; Project of Shanghai Science and Technology (Grant No.20410710100).
6) Xingtao Zhou; Research Plan of Shanghai Shenkang Hospital Development Center(SHDC) (Grant No. SHDC2020CR1043B).
7) Xingtao. Zhou: Project of Shanghai Xuhui District Science and Technology (Grant No. 2020-015, XHLHGG202104).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The animals were purchased from Shanghai Jiagan Biotechnology Co. and we have obtained their informed consent. All animal experiments were approved by the Animal Ethics Committee of the Eye and ENT Hospital of Fudan University, Shanghai, China (No. 2022114), and all experimental protocols, including care, transportation, and experiments of the animals, complied with the guidelines of the Animal Care and Use Committee of Fudan University.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huamao Miao, Email: miaohuamao@126.com.
Xiaoying Wang, Email: doctxiaoyingwang@163.com.
References
- 1.Zhou Y, Chen X, Huang X, Li L, Zhu Y, Cai Q, et al. Prevalence and association of uncorrected refractive error among Chinese adolescents: a cross-sectional study. BMC Public Health. 2024;24(1):2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. 2016;123(5):1036–42. [DOI] [PubMed] [Google Scholar]
- 3.Shi X, Gao Z, Leng L, Guo Z. Temporal and spatial characterization of myopia in China. Front Public Health [Internet]. 2022 Aug 16 [cited 2024 Mar 16];10. https://www.frontiersin.org/journals/public-health/articles/10.3389/fpubh.2022.896926/full [DOI] [PMC free article] [PubMed]
- 4.Morgan IG, French AN, Ashby RS, Guo X, Ding X, He M, et al. The epidemics of myopia: Aetiology and prevention. Prog Retin Eye Res. 2018;62:134–49. [DOI] [PubMed] [Google Scholar]
- 5.Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. OPTH. 2016;1059. [DOI] [PMC free article] [PubMed]
- 6.Igarashi A, Shimizu K, Kamiya K. Eight-year follow-up of posterior Chamber Phakic Intraocular Lens Implantation for Moderate to high myopia. Am J Ophthalmol. 2014;157(3):532–e5391. [DOI] [PubMed] [Google Scholar]
- 7.Guber I, Mouvet V, Bergin C, Perritaz S, Othenin-Girard P, Majo F. Clinical outcomes and cataract formation rates in eyes 10 years after posterior phakic Lens Implantation for Myopia. JAMA Ophthalmol. 2016;134(5):487. [DOI] [PubMed] [Google Scholar]
- 8.Zhao W, Zhao J, Han T, Wang J, Zhang Z, Zhou X. A Comprehensive investigation of contrast sensitivity and disk Halo in high myopia treated with SMILE and EVO Implantable Collamer Lens Implantation. Translational Vis Sci Technol. 2022;01(4):23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen LJ, Chang YJ, Kuo JC, Rajagopal R, Azar DT. Metaanalysis of cataract development after phakic intraocular lens surgery. J Cataract Refractive Surg. 2008;34(7):1181–200. [DOI] [PubMed] [Google Scholar]
- 10.Brandt JD, Mockovak ME, Chayet A. Pigmentary dispersion syndrome induced by a posterior chamber phakic refractive lens. Am J Ophthalmol. 2001;131(2):260–3. [DOI] [PubMed] [Google Scholar]
- 11.Jongsareejit A. Clinical results with the Medennium Phakic Refractive Lens for the correction of high myopia. J Refract Surg. 2006;22(9):890–7. [DOI] [PubMed] [Google Scholar]
- 12.Hoyos JE, Cigales M. Hoyos -Chacón Jairo. Zonular Dehiscence two years after phakic refractive Lens (PRL) implantation. J Refract Surg. 2005;21(1):13–7. [DOI] [PubMed] [Google Scholar]
- 13.Mart ínez CV, Elies D, Boixadera A, Garc ía AJ, Mauricio J, Cavero L, et al. Silicone posterior Chamber Phakic Intraocular Lens Dislocated into the vitreous cavity. J Refract Surg. 2004;20(6):773–7. [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Huang C, Miao H, Wu J, Xing C, Dai Z et al. Assessment of biosafety and implantation feasibility of novel phakic refractive lens. INTERNATIONAL OPHTHALMOLOGY. [DOI] [PMC free article] [PubMed]
- 15.Vargas LG, Peng Q, Apple DJ, Escobar-Gomez M, Pandey SK, Arthur SN, et al. Evaluation of 3 modern single-piece foldable intraocular lenses: clinicopathological study of posterior capsule opacification in a rabbit model. J Cataract Refract Surg. 2002;28(7):1241–50. [DOI] [PubMed] [Google Scholar]
- 16.Pallikaris IG, Kalyvianaki MI, Kymionis GD, Panagopoulou SI. Phakic refractive lens implantation in high myopic patients: one-year results. J Cataract Refractive Surg. 2004;30(6):1190. [DOI] [PubMed] [Google Scholar]
- 17.Gwon AE, Jones RL, Gruber LJ, Mantras C. Lens regeneration in juvenile and adult rabbits measured by image analysis. Investig Ophthalmol Vis Sci. 1992;33(7):2279–83. [PubMed] [Google Scholar]
- 18.Rogala MM, Danielewska ME, Antończyk A, Kiełbowicz Z, Rogowska ME, Kozuń M, et al. In-vivo corneal pulsation in relation to in-vivo intraocular pressure and corneal biomechanics assessed in-vitro. An animal pilot study. Exp Eye Res. 2017;162:27–36. [DOI] [PubMed] [Google Scholar]
- 19.Darcy K, Gunn D, Tavassoli S, Sparrow J, Kane JX. Assessment of the accuracy of new and updated intraocular lens power calculation formulas in 10 930 eyes from the UK National Health Service. J Cataract Refract Surg. 2020;46(1):2–7. [DOI] [PubMed] [Google Scholar]
- 20.Kim Csik, Kim KN, Kang TS, Jo YJ, Kim JY. Changes in axial length and refractive error after noninvasive normalization of intraocular pressure from elevated levels. Am J Ophthalmol. 2016;163:132–e1392. [DOI] [PubMed] [Google Scholar]
- 21.Chirapapaisan C, Eiamsamarng A, Chirapapaisan N, Raksong W, Sakiyalak D, Koodkaew S, et al. Effects of intraocular pressure change on intraocular lens power calculation in primary open-angle glaucoma and ocular hypertension. PLoS ONE. 2024;19(6):e0304169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stopyra W, Langenbucher A, Grzybowski A. Intraocular Lens Power calculation Formulas—A systematic review. Ophthalmol Ther. 2023;12(6):2881–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holladay JT. Refractive Power Calculations for Intraocular Lenses in the Phakic Eye. Am J Ophthalmol. 1993;116(1):63–6. [DOI] [PubMed] [Google Scholar]
- 24.Van Der Heijde GL. Some Optical aspects of Implantation of an IOL in a Myopic Eye. Eur J Implant Refractive Surg. 1989;1(4):245–8. [Google Scholar]
- 25.Stopyra W, Cooke DL, Grzybowski A. A review of intraocular Lens Power calculation formulas based on Artificial Intelligence. JCM. 2024;13(2):498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eleftheriadis H, Amoros S, Bilbao R, Teijeiro MA. Spontaneous dislocation of a phakic refractive lens into the vitreous cavity. J Cataract Refractive Surg. 2004;30(9):2013. [DOI] [PubMed] [Google Scholar]
- 27.Hadvina R, Estes A, Liu Y. Animal models for the study of Keratoconus. Cells. 2023;12(23):2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donoso R, Castillo P. Correction of high myopia with the PRL phakic intraocular lens. J Cataract Refractive Surg. 2006;32(8):1296. [DOI] [PubMed] [Google Scholar]
- 29.Ruiz-moreno JM, Tavolato M, Montero JA, Alio JL. Choroidal Neovascularization in myopic eyes after Phakic Refractive Lens and Iris-Claw Lens Implantation. Eur J Ophthalmol. 2004;14(2):159–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.