Introduction
Acetaminophen (N-acetyl-p-aminophenol (APAP)), also known as paracetamol, is the most common over-the-counter medication taken during pregnancy to relieve fever, pain, or discomfort.1 In the USA and Europe, it was estimated that 50–65% of women reported using APAP at least once during pregnancy, with about 20% taking APAP in all three trimesters.2 Despite the high prevalence of APAP intake in pregnancy, long-term safety data concerning offspring health are limited. The exact mechanism explaining the therapeutic effects of APAP is not fully understood.3 APAP may act upon the inhibition of cyclooxygenase (COX) enzymes and prostaglandin synthesis, the endocannabinoid system, or the serotonergic pathways in the brain, for exerting its pain-relieving and fever-reducing effects.1 Recently, adverse effects of APAP exposure on fetal development have been raised. Studies have reported that APAP and its metabolites can cross the human placental barrier exposing the fetus during critical period of development. The APAP-induced oxidative stress is amplified during fetal life because of the main pathways for APAP metabolism is not active in the fetus until mid-gestation, and that sex-specific endocrine-disruptive effects of APAP are reported in animal studies.1
A growing body of epidemiological research have investigated the associations between prenatal exposure to APAP and adverse reproductive or neurological outcomes in childhood.1 However, previous epidemiological studies have predominantly relied on using self-reported data or single-biomarker measurements to characterize APAP exposure, raising concerns over exposure misclassification.3 On one hand, self-reported maternal intakes are susceptible to under-reporting or recall bias. On the other hand, APAP metabolites have a short half-life (ie, <4 hours), thus a single biological measurement may only reflect recent or frequent intake. To address this methodological concern and advance our understanding of the assessment of prenatal APAP exposure, we conducted a study to compare three APAP metabolite levels in paired maternal urine and cord blood samples, and maternal self-reported frequent APAP intake data.
Methods
We analyzed questionnaire data and biological samples collected from the Nutrition in Pregnancy (NIP) Study, a cohort with a total of 2291 pregnant individuals recruited at 56 obstetric practices and 15 clinics in Connecticut and Western Massachusetts between September 1996 and January 2000. Detailed cohort information has been published elsewhere.4 The only exclusion criteria of the cohort were a gestational age over 24 weeks at enrollment, maternal insulin-dependent diabetes mellitus, inability of to speak either English or Spanish, and an intent to terminate the pregnancy. Briefly, all participants included were interviewed at home and had urine samples collected by a trained research assistant before 25 weeks gestation (interquartile range [IQR] of enrollment 12–17 weeks). In the baseline interview, the mothers were asked to report all prescription and non-prescription medication intake (including APAP) and the estimated total days of use in each of the first three months in the first trimester (T1; gestational weeks 1 to 12). If by the time of the baseline interview, the mother had not completed the T1, the mother was asked again around gestational week 20 to report the medication use in the months of T1. Additionally, a postpartum interview was conducted in the hospital or by telephone within one month of delivery. In the postpartum interviews, the same information on medication intake was collected for each of the last three months in the third trimester (T3; gestational weeks 29 to delivery). Umbilical cord blood samples were collected at delivery by the obstetrician. Venous and arterial cord blood was drained into a tube and refrigerated immediately. Serum was separated, spun off within 24 hours of collection, and immediately frozen and stored in a −80°C freezer biobank managed by the Yale Center for Perinatal, Pediatric and Environmental Epidemiology. Written inform consents were collected from all research participants at the time of enrollment. The initial data collection was approved by the Human Investigation Committee of Yale University (protocol ID #7991). The Yale Human Research Protection Program approved our secondary analysis project (protocol ID #2000031118). Our study complies with the Declaration of Helsinki.
From this cohort database, we first classified mothers into three exposure groups: (1) mothers reported never using APAP during each month in T1 and T3, (2) mothers reported using APAP frequently, defined as more than 14 days of intake, in T1, and (3) mothers reported using APAP frequently in T3. Due to the study cost constraints, we have a priori determined to randomly sample 10 pregnancies from each of the three exposure groups for analyses.
For the 30 selected pregnancies, we analyzed three specific APAP metabolites in the matched pairs of maternal urine and umbilical cord serum samples retrieved from the biobank. The three APAP metabolites we measured included the parent acetaminophen compound (APAP), acetaminophen glucuronide (APAP-gluc), and 3-(N-acetyl-l-cystein-S-yl)-acetaminophen (APAP-cys). These APAP metabolites were selected based on findings from the Boston Birth Cohort study that had recently reported cord biomarkers of APAP measured in the same laboratory as used in our study, with an untargeted metabolomic analyses workflow.5 The analyses were conducted by the Broad Institute Metabolite Profiling Laboratory at the Massachusetts Institute of Technology using liquid chromatography-mass spectrometry (LC-MS) techniques.6 The urine and serum samples were gently inverted 10–20 times to disrupt concentration gradients formed during thawing, divided into 100 μL urine or serum aliquots, and transferred into separate 2 mL cryovials. The aliquot cryovials were labeled with a random number sequence blinded to the APAP status and sorted in random order before being shipped overnight to the Broad Institute laboratory. LC-MS samples were prepared by protein precipitation of a 10μL sample portion with the addition of nine volumes of 74.9:24.9:0.2 v/v/v acetonitrile/methanol/formic acid containing stable isotope-labeled internal standards (valine-d8, Isotec; and phenylalanine-d8, Cambridge Isotope Laboratories). The samples were centrifuged (10 min, 9000g, 4°C), and the supernatants injected directly onto a 150 × 2-mm Atlantis HILIC column (Waters). The column was eluted isocratically at a flow rate of 250 μL/min with 5% mobile phase A (10 mm ammonium formate and 0.1% formic acid in water) for 1 min followed by a linear gradient to 40% mobile phase B (acetonitrile with 0.1% formic acid) over 10 min. MS analyses were carried out using electrospray ionization in the positive ion mode using full scan analysis over m/z 70–800 at 120,000 resolution and 3-Hz data acquisition rate. Additional MS settings are: ion spray voltage, 3.5 kV; capillary temperature, 350°C; probe heater temperature, 300°C; sheath gas, 40; auxiliary gas, 10; and S-lens RF level 40.
For statistical analysis, we first compared metabolite level differences in peak intensities between the self-reported frequent APAP use groups, and the non-user group, for each metabolite using the nonparametric Wilcoxon-Mann–Whitney test. Next, we estimated the Spearman correlation coefficients among the three APAP metabolite levels in maternal urine or cord serum samples. Finally, we estimated the Spearman correlation coefficients among the maternal urinary or cord serum APAP metabolite levels with the reported cumulative days of APAP intake in T1 or T3.
Results
The median age of pregnancy was 33 years, about 80% of the participants self-reported as non-Hispanic white, and 73% had some college or higher education (Supplementary Table 1).
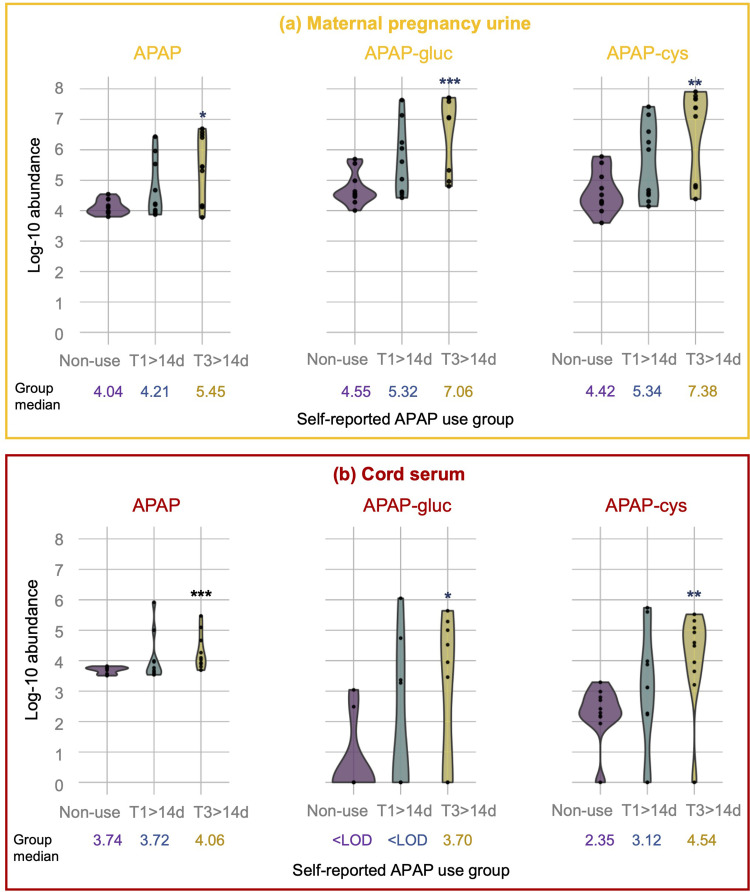
The distribution of the three APAP metabolites (indicated as log-10 abundance) according to the self-reported APAP use groups is presented in Figure 1. We found that the three APAP markers were detected in all (100%) of the 30 maternal urine samples analyzed. In contrast, only the parent APAP compound was detected in all (100%) of the 30 cord serum samples analyzed, while APAP-cys and APAP-gluc were detected in 25 (87%) and 12 (40%) of the 30 cord sera analyzed, respectively. Using the nonparametric Wilcoxon-Mann–Whitney test, we found that the three metabolite levels detected in both maternal urine and cord sera samples were higher in the T3 frequent users compared with the non-users (P<0.05) (Figure 1). In contrast, the urinary or serum APAP metabolite levels among T1 frequent users were more similar to the levels measured in the non-user group (P>0.05).
Figure 1.
Log-10 transformed abundance of APAP metabolites in maternal pregnancy urine (a) and cord serum (b) by self-reported APAP use groups. The median log-10 abundance of each APAP metabolite is noted in the figure for each APAP self-reported use group. The group differences between the frequent users and the non-users using the non-parametric Wilcoxon-Mann–Whitney test are marked with * for a P-value <0.05, ** for a P-value <0.01, and *** for a P-value <0.001.
Abbreviations: APAP, acetaminophen; APAP-gluc, acetaminophen glucuronide; APAP-cys, 3-(N-acetyl-l-cystein-S-yl)-acetaminophen.
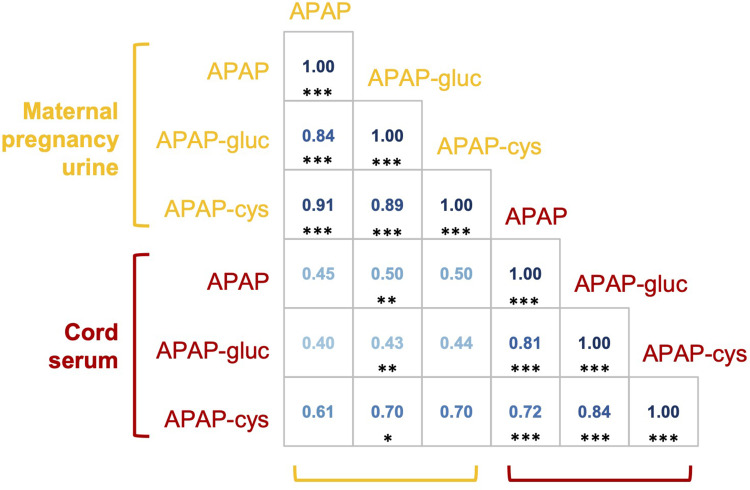
The Spearman correlation coefficients were high when comparing the levels of the three APAP markers in maternal urines (r=0.84–0.91, P<0.001), or the cord sera (r=0.72–0.84, P<0.001) (Figure 2). However, the correlations were moderate when comparing the level of the same type of APAP metabolites in the maternal urine with cord sera (APAP-cys r=0.70, P=0.065; APAP r=0.45, P=0.082; and APAP-gluc r=0.43, P=0.064).
Figure 2.
Spearman correlation coefficients for APAP metabolite levels detected in maternal mid-pregnancy urinary and cord serum. The correlation coefficients are marked with * for a P-value <0.05, ** for a P-value <0.01, and *** for a P-value <0.001.
Abbreviations: APAP, acetaminophen; APAP-gluc, acetaminophen glucuronide; APAP-cys, 3-(N-acetyl-l-cystein-S-yl)-acetaminophen.
The reported cumulative days of use of APAP in T3 were strongly correlated with cord serum APAP metabolites, especially APAP and APAP-cys (r>0.70), and moderately with maternal mid-pregnancy urinary APAP metabolites (r= 0.57–0.69) (Supplementary Table 2). The reported cumulative days of use in T1 were only weakly correlated with either maternal mid-pregnancy urinary or cord serum APAP metabolites (r=0.18–0.34). However, the P-values for these correlation measures were large (P>0.05) and chance errors are possible.
Discussion
In this study, we compared maternal self-reported frequent APAP intake and three specific APAP metabolites measured in paired maternal urine and umbilical cord serum samples. We confirmed that several APAP metabolites were widely detectable in stored human biological samples (cord blood and urine) in a biobank for more than two decades. Furthermore, we found that the same type of APAP metabolites in maternal urine were moderately to highly correlated with those found in the cord sera within mother-child pairs. All three urinary and cord serum APAP metabolite levels were more strongly correlated with self-reported frequent APAP use in late pregnancy than early pregnancy intake. Overall, our study suggests validity for collecting detailed self-reported APAP data in epidemiologic research, especially concerning frequent intake. On the other hand, biological APAP measures also reflect frequent APAP intake close to the time of sample collection.
This study is consistent with a pregnancy study conducted in Sweden that correlated maternally reported APAP use with urinary APAP concentrations (n=111).7 The Swedish study showed that parent APAP compound was detectable in all maternal urine samples analyzed, even among mothers who did not report taking APAP, suggesting environmental sources of exposure. Moreover, the study found that the reported number of pills of APAP intake was correlated with urinary parent APAP concentrations in gestational weeks 4–19 (r = 0.29, P <0.01). However, the Swedish study did not examine other APAP metabolites. While we could not rule out in our study that some mothers have used APAP during the second trimester which was not adequately captured in the cohort data collection, it is unlikely that all mothers have used acetaminophen during pregnancy. Pharmaceutical contamination including ubiquitous environmental occurrences of acetaminophen in the food and drinking water system has been reported, with major sources from human wastes, domestic and industrial wastewater. Furthermore, we also showed a slightly lower abundance of APAP-gluc than APAP and APAP-cys in cord serum samples.5 Studies of therapeutic doses of APAP in adults have shown that pharmacologically inactive APAP-gluc is primarily excreted in the urine, and a smaller fraction of APAP-gluc is transported by the intestine into the blood. In contrast, APAP-cys adducts are the end products formed when the toxic acetaminophen byproduct N-acetyl-p-benzoquinone imine (NAPQI) is conjugated by glutathione and then binds to cysteine. Therefore, high APAP-cys in serum have been used as a marker to indicate APAP overdose toxicity. As expected, we found that a spot cord-serum measure of APAP metabolites is a valid marker to indicate frequent APAP intake in late gestation, but APAP metabolites in cord serum are less predictive of early gestational APAP intake.
In this cohort, the spot measure of maternal mid-pregnancy urinary APAP metabolites also showed a higher agreement with self-reported frequent APAP intake in late gestation than early gestation. One plausibility is that the participants might not have recalled their medication intake as accurately for the first few weeks of gestation, especially, before the pregnancy was confirmed medically. Moreover, the lack of second-trimester APAP intake data may have added variability to our results concerning APAP metabolites in mid-pregnancy urines. Thus, the collection of prenatal APAP exposure data in epidemiological studies is recommended to start from early pregnancy whenever possible to reduce exposure misclassification bias concerning APAP exposure in the early gestational period.
Some limitations need to be considered for the present study. First, the sample size of this study was small. Larger studies are needed to explore whether the correlations between self-reported APAP intake and APAP metabolite levels would vary by maternal characteristics. Our study focused on the APAP parent compound and its two main metabolites, while several other APAP metabolites could also be studied. The study focused on frequent users and our results do not apply to sporadic/infrequent use. Studies of shorter-term or less frequent APAP intake would likely be further challenged by a higher degree of exposure misclassification bias. The cohort we analyzed did not collect self-reported medication data in the second trimester, thus, we were not able to assess the cumulative use of APAP use throughout the entire pregnancy.
Conclusions
Our findings suggest that the APAP parent compound and its two metabolites are widely detected and correlated in paired maternal urine and umbilical cord serum among samples of never and frequent APAP users in pregnancy. One-time measures of these APAP metabolites in either the maternal urine or the cord serum can be used to represent frequent APAP intake close to the time of sample collection. Collecting detailed maternal-reported APAP intake data derived from repeated maternal interviews is still an efficient and valid measure to indicate frequent APAP exposure in pregnancy, but data collection is recommended to start from early gestation. When study resources permit, collecting repeated self-reported intake data and validating it with biological APAP measures is recommended to improve the exposure classification of APAP during the entire pregnancy period.
Acknowledgment
We thank Drs. Michael B. Bracken and Brian Leaderer for their contribution to establishing the NIP cohort in Yale Center for Perinatal, Pediatric and Environmental Epidemiology (CPPEE).
Funding Statement
Dr. Liew’s lab is supported by the NIH/NICHD award (R01HD109213).
Abbreviations
APAP, acetaminophen, N-acetyl-p-aminophenol; APAP-cys, 3-(N-acetyl-l-cystein-S-yl)-acetaminophen; APAP-gluc, acetaminophen glucuronide; NAPQI, N-acetyl-p-benzoquinone imine.
Data Sharing Statement
Data supporting this manuscript are available to all NIP cohort investigators. Data are available from Dr Zeyan Liew upon request.
Ethics Approval and Informed Consent
All study participants were informed about the purpose of the study. Confirmation of consent forms were received from study participants. Our study complies with the Declaration of Helsinki.
Consent for Publication
We confirmed that details of words, tables, figures included in the manuscript can be published.
Author Contributions
All authors made a significant contribution to the work reported. All authors took part in drafting, revising or critically reviewing the article, whether that is in the conception of study design, execution, acquisition of data, analysis, and interpretation of the results. All authors gave final approval of the version to be published and have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this communication. A related abstract of this paper was presented at the 2022 international society for environmental epidemiology (ISEE). The abstract was published in ‘Conference Abstracts’ in Environmental Health Perspectives (EHP): https://ehp.niehs.nih.gov/doi/10.1289/isee.2022.O-OP-100.
References
- 1.Bauer AZ, Swan SH, Kriebel D, et al. Paracetamol use during pregnancy - A call for precautionary action. Nat Rev Endocrinol. 2021;17(12):757–766. doi: 10.1038/s41574-021-00553-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zafeiri A, Mitchell RT, Hay DC, Fowler PA. Over-the-counter analgesics during pregnancy: a comprehensive review of global prevalence and offspring safety. Hum Reprod Update. 2021;27(1):67–95. doi: 10.1093/humupd/dmaa042 [DOI] [PubMed] [Google Scholar]
- 3.Liew Z, Ernst A. Intrauterine exposure to acetaminophen and adverse developmental outcomes: epidemiological findings and methodological issues. Curr Environ Health Rep. 2021;8(1):23–33. doi: 10.1007/s40572-020-00301-5 [DOI] [PubMed] [Google Scholar]
- 4.Grosso LM, Triche E, Benowitz NL, Bracken MB. Prenatal caffeine assessment: fetal and maternal biomarkers or self-reported intake? Ann Epidemiol. 2008;18(3):172–178. doi: 10.1016/j.annepidem.2007.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ji Y, Azuine RE, Zhang Y, et al. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry. 2020;77(2):180–189. doi: 10.1001/jamapsychiatry.2019.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Stražar M, Mohamed AMT, et al. Gut microbiome and metabolome profiling in Framingham heart study reveals cholesterol-metabolizing bacteria. Cell. 2024;187(8):1834–1852.e1819. doi: 10.1016/j.cell.2024.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bornehag CG, Reichenberg A, Hallerback MU, et al. Prenatal exposure to Acetaminophen and children’s language development at 30 months. Eur Psychiatry. 2018;51:98–103. doi: 10.1016/j.eurpsy.2017.10.007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this manuscript are available to all NIP cohort investigators. Data are available from Dr Zeyan Liew upon request.