Abstract
Background
Breast cancer (BC) has exhibited varied epidemiological trends based on distinct age categories. This research aimed to explore the incidence and mortality rates of BC within pre-defined age groups in the Brazilian population.
Methods
BC incidence trends were assessed from 2010 to 2015 using Brazilian Population-Based Cancer Registries, employing age-standardized ratios and annual average percentage change (AAPC). Hospital-Based Cancer Registries provided clinical and sociodemographic data from 2000 to 2019. Mortality data were obtained from the National Mortality Information System from 2000 to 2020. Three groups were compared: < 40, 40–69, and ≥ 70 years.
Results
From 2010 to 2015, 205,966 new BC cases were recorded, with incidence rates of 7.1/100,000 for < 40, 156.5/100,000 for 40–69, and 247.5/100,000 for ≥ 70 years. The < 40 years group exhibited a significant increase in incidence rate (AAPC + 1.6; 95% CI: 1.0 to 2.2; p < 0.001). This age group also showed a higher proportion of black patients (53%, p < 0.001), alcohol consumption (20.5%, p < 0.001), proportion of patients treated at stages ≥ IIB (64.0%, p < 0.001), and a higher likelihood of receiving multiple treatment modalities (60.7%, p < 0.001). The ≥ 70 years group experienced a longer delay exceeding 60 days from diagnosis to treatment onset (54%, p < 0.001), while exhibiting a higher proportion of endocrine therapy utilization (45.3%, p < 0.01). Mortality rates increased across all subgroups, with the < 40 years group showing the most pronounced increase (AAPC + 1.8%; 95% CI: 1.6 to 2.1; p < 0.001).
Conclusion
These results highlight marked disparities in BC incidence, mortality rates, clinicopathological and sociodemographic characteristics between women under 40, and those in the 40–69 and ≥ 70 age groups in Brazil.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12885-024-13381-5.
Keywords: Breast cancer, Age groups, Incidence, Mortality
Background
Breast cancer (BC) emerges as the prevailing malignancy afflicting both genders. Projections extrapolated from the GLOBOCAN Cancer Tomorrow prediction tool anticipate a substantial surge of over 46% in global incident cases by the year 2040 [1]. Within the context of Brazil, the projected triennial period from 2023 to 2025 reveals 73,610 novel BC cases per year, entailing an approximate risk of 66.54 per 100,000 women, thereby establishing BC as the most incident malignancy in the country [2]. BC assumed a formidable position as the fourth most fatal malignancy worldwide, with an approximate global mortality count of 684,996 (constituting 6.9% of the total deaths) in 2020 [3]. Notably in Brazil, the year 2020 witnessed the registration of 18,032 BC-specific deaths, accounting for 1.2% of the overall mortality in the nation [4].
The rising global incidence rates of BC can be attributed, at least in part, to heightened exposure to various risk factors. However, the incidence of BC among young women remains relatively modest. According to 2019 data, women below the age of 40 constituted merely 2% of ductal carcinoma in situ (DCIS) cases and 4% of invasive BC cases [5]. Nonetheless, other sources suggest that BC is the most incident neoplasm in women aged 20–49 years and the primary cause of cancer-related mortality within the 30–49 age range [6]. Furthermore, recent investigations have revealed a rising incidence rate of BC in young women in Europe, which may be attributed to heightened exposure to oncogenetic and various reproductive risk factors that are potentially modifiable [7]. The increase in BC occurrence among older women is associated with a demographic transition marked by greater exposure to lifestyle-related risk factors and the enhanced implementation of BC screening programs, which were introduced later in numerous low/middle-income countries (LMICs) [8].
Data from the International Agency for Research on Cancer shows that breast cancer is the most frequent cause of cancer-related death in many nations [9]. LMICs encounter challenges such as restricted access to mammography screening, limited availability of healthcare resources, and delays in the onset of proper treatment, resulting in a more pronounced increase in BC-specific mortality rates as compared to high-income countries (HICs) [10].
This study aimed to explore the Brazilian patterns of BC incidence spanning a comprehensive 6-year period, while also examining the temporal trajectory of mortality rates across a 20-year timeframe, and describing a thorough clinical and sociodemographic profile of the patients. Epidemiological disparities were deeply investigated between young and elderly women, highlighting the objective of creating insights to support future research and guide formulation of efficient strategies for BC control.
Material and methods
Study design, eligibility criteria and data source
This epidemiological study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) [11] and was carried out utilizing data sourced from the national Population-Based Cancer Registries (PBCRs) [12], Hospital-Based Cancer Registries (HBCRs) [13], and the Mortality Information System (MIS) [14]. As only using de-identified data from public government sources, the need for ethical review board approval and individual consent was deemed unnecessary. This study included breast cancer cases and fatalities coded under ICD-O-3 (C50.0-C50.9).
Data curation involved histological diagnosis, diagnosis year, and gender as key factors. Exclusion criteria ensured data accuracy and relevance, including misclassification, noninvasive diseases, misdiagnosis, cases pre-2000, and data lacking diagnosis year. Data were gathered on female BC cases in March 2023 from the PBCRs specifically available for the Brazilian places where such registries exist (supplementary Table 1; supplementary figure). Then BC incidence was analyzed from 2010 to 2015, categorizing by age groups: < 40, 40–69, and ≥ 70 years. Incidence rates, per 100,000 women, were examined for trends over the study period. Data from 2016 onward were omitted due to limited population coverage (< 12%).
The study obtained comprehensive data encompassing the clinical and sociodemographic profiles of women with BC registered from 2000 to 2019. This data came from HBCRs, specialized centers that collect and analyze information on cancer patients in Brazilian hospitals. The retrieval of such data from the HBCRs integrator system occurred in June 2021. Mortality data for Brazilian women with BC from 2000 to 2020 were sourced from the MIS in March 2023. The data were categorized by age and year of diagnosis, offering an extensive overview of BC mortality trends nationwide. BC deaths with incomplete age information were excluded from the analysis to ensure data accuracy.
Statistical analysis
Joinpoint models were used to analyze incidence and mortality data, identifying trend changes and calculating average annual percent change (AAPC). The Joinpoint Regression Program helped with confidence intervals and p-values for AAPC. Crude breast cancer incidence rates per 100,000 women were calculated by dividing new cases by the population in RCBPs. Crude and age-standardized mortality rates per 100,000 women were calculated for different age groups and years of death. Multiple pairwise comparisons tested incidence rate variability, and chi-square tests assessed differences in clinical and sociodemographic variables between age groups. A p-value below 0.05 was considered significant. Microsoft Excel, Joinpoint Regression Program version 4.9.1.0, and SPSS Statistics version 24.0.0.0 were used for analysis.
Results
Incidence
During the analysis period, from 2010 to 2015, a total of 205,966 newly diagnosed cases of BC were documented across the 30 participating PBCRs. Within this timeframe, the overall occurrence rate of BC exhibited a wide range of variation, with values spanning from 53.49 per 100,000 (representing the lowest rate) to 65.66 per 100,000 (representing the highest rate) (supplementary Table 2). Out of the overall cases, the group of females under the age of 40 constituted 10.2% of the total, that aged 40–69 accounted for 69.6%, and the group of women aged ≥ 70 made up 20.2% of the cases. Crude incidence rates according to age group were 7.1 per 100,000 for patients under the age of 40, 156.5 per 100,000 for patients aged 40–69, and 247.5 per 100,000 for individuals aged ≥ 70 (Fig. 1).
Fig. 1.
Crude incidence rates of breast cancer stratified by age groups in Brazil, covering the years 2010 to 2015
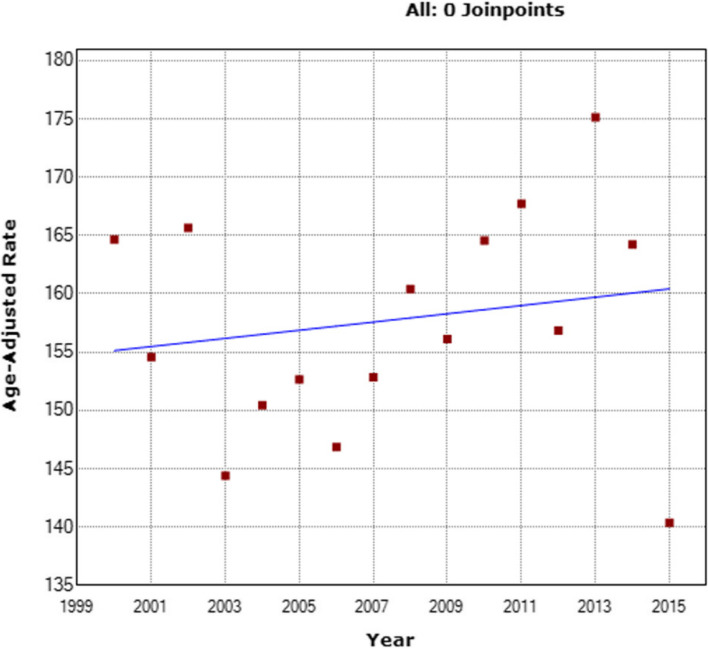
As indicated in Supplementary tables 3, the analysis period demonstrated a non-significant decline in overall incidence by 0.8% (95% confidence interval, CI: −2.6 to 1.0; p = 0.400) within the general population. Remarkably, the group of patients aged < 40 exhibited a significant increase in incidence rate by 1.6% annually (AAPC + 1.6; 95% CI: 1.0 to 2.2; p < 0.001), escalating from 6.68/100,000 to 7.63/100,000 (Fig. 2). Conversely, the incidence rates for the group aged 40–69 (AAPC −0.9; 95% CI: −2.8 to 1.1; p = 0.378) (Fig. 3) and the group aged ≥ 70 years (AAPC −0.5; 95% CI: −1.1 to 0.2; p = 0.138) (Fig. 4) did not display any significant variations. They evolved from 164.65/100,000 to 140.36/100,000 and from 275.32/100,000 to 225.17/100,000, respectively.
Fig. 2.
The evolving patterns of adjusted incidence rates for breast cancer in the female cohort < 40 years old from 2010 to 2015
Fig. 3.
The changing trends in adjusted breast cancer incidence rates for the female cohort aged 40–69 from 2000 to 2020
Fig. 4.
Changing patterns in adjusted breast cancer incidence rates for the female cohort aged > 69 from 2010 to 2015
Distribution of clinical and sociodemographic characteristics
Leveraging the expansive HBCR dataset, a carefully curated cohort of 611,589 patients, covering the period from 2000 to 2019, underwent an in-depth comparative analysis to investigate the clinical and sociodemographic characteristics across the predefined age groups. Notably, as evidenced by the data presented in Table 1, a majority of cases (74.6%) received treatment in regions classified as more developed, whereas patients aged under 40 exhibited a comparatively lower prevalence within these regions (70.0%, p < 0.001).
Table 1.
Features of breast cancer cases derived from Hospital-Based Cancer Registries categorized by age groups
| Age at diagnosis | |||||
|---|---|---|---|---|---|
| Variables |
< 40 years N (%) |
40–69 years N (%) |
≥ 70 years N (%) |
Total N (%) |
p value |
| Year of diagnosis | < 0.001 | ||||
| 2000–2004 | 9485 (14.5) | 58,917 (13.5) | 13,864 (13.7) | 82,266 (13.6) | |
| 2005–2009 | 15,080 (23.1) | 102,121 (23.3) | 23,275 (22.9) | 140,476 (23.2) | |
| 2010–2014 | 21,830 (33.4) | 151,018 (34.5) | 34,853 (34.3) | 207,701 (34.4) | |
| 2015–2019 | 18,947 (29.0) | 125,737 (28.7) | 29,514 (29.1) | 174,198 (28.8) | |
| Oncology units and centers geographic regiona | < 0.001 | ||||
| More developed region | 45,731 (70.0) | 328,243 (75.0) | 77,335 (76.2) | 451,309 (74.6) | |
| Less developed region | 19,611 (30.0) | 109,550 (25.0) | 24,171 (23.8) | 153,332 (25.4) | |
| Ethnicityb | < 0.001 | ||||
| White | 20,242 (47.0) | 151,875 (53.3) | 38,093 (58.2) | 210,210 (53.4) | |
| Black | 22,846 (53.0) | 133,070 (46.7) | 27,361 (41.8) | 183,277 (46.6) | |
| Schooling, years | < 0.001 | ||||
| ≤ 8 years | 20,989 (43.7) | 193,610 (61.7) | 58,420 (82.9) | 273,019 (63.1) | |
| > 8 years | 27,042 (56.3) | 120,205 (38.3) | 12,086 (17.1) | 159,333 (36.9) | |
| Alcohol consumption | < 0.001 | ||||
| Yes (current or past consumption) | 5637 (20.4) | 34,851 (19.5) | 4546 (11.5) | 45,034 (18.3) | |
| Never | 21,961 (79.6) | 143,919 (80.5) | 35,102 (88.5) | 200,982 (81.7) | |
| Tobacco consumption | < 0.001 | ||||
| Yes (current or past consumption) | 5705 (19.2) | 61,602 (31.5) | 10,167 (23.6) | 77,474 (28.9) | |
| Never | 23,956 (80.8) | 133,877 (68.5) | 32,837 (76.4) | 190,670 (71.1) | |
| Clinical stage | < 0.001 | ||||
| Initial (< IIB) | 17,881 (36.0) | 165,713 (49.2) | 37,536 (49.1) | 221,130 (47.8) | |
| Advanced (≥ IIB) | 31,817 (64.0) | 170,959 (50.8) | 38,957 (50.9) | 241,733 (52.2) | |
| Time from diagnosis to first treatment | < 0.001 | ||||
| ≤ 60 days | 28,431 (52.1) | 170,760 (47.2) | 37,279 (45.9) | 236,470 (47.6) | |
| > 60 days | 26,149 (47.9) | 190,702 (52.8) | 43,867 (54.1) | 260,718 (52.4) | |
| Treatment modalities used | < 0.001 | ||||
| 1 | 23,611 (39.2) | 164,713 (40.7) | 43,737 (47.9) | 232,061 (41.7) | |
| 2 + | 36,667 (60.8) | 240,441 (59.3) | 47,568 (52.1) | 324,676 (58.3) | |
| Surgery at some point | < 0.001 | ||||
| Yes | 33,634 (55.8) | 226,357 (55.9) | 48,445 (53.1) | 308,436 (55.4) | |
| No | 26,665 (44.2) | 178,884 (44.1) | 42,873 (46.9) | 248,422 (44.6) | |
| Radiotherapy at some point | < 0.001 | ||||
| Yes | 30,100 (49.9) | 201,895 (49.8) | 39,941 (43.7) | 271,936 (48.8) | |
| No | 30,199 (50.1) | 203,346 (50.2) | 51,377 (56.3) | 284,922 (51.2) | |
| Chemotherapy at some point | < 0.001 | ||||
| Yes | 41,220 (68.4) | 234,954 (58.0) | 31,597 (34.6) | 307,771 (55.3) | |
| No | 19,079 (31.6) | 170,287 (42.0) | 59,721 (65.4) | 249,087 (44.7) | |
| Hormonotherapy at some point | < 0.001 | ||||
| Yes | 19,196 (31.8) | 153,368 (37.8) | 41,484 (45.4) | 214,048 (38.4) | |
| No | 41,103 (68.2) | 251,873 (62.2) | 49,834 (54.6) | 342,810 (61.6) | |
| Death at the end of the first treatment cycle | < 0.001 | ||||
| Yes | 3683 (5.6) | 19,528 (4.5) | 7426 (7.3) | 30,637 (5.1) | |
| No | 61,659 (94.4) | 418,265 (95.5) | 94,080 (92.7) | 574,004 (94.9) | |
| Total | 65,862 (10.8) | 442,434 (72.3) | 103,293 (16.9) | 611,589 | |
aClassified according to the Human Development Index (HDI) in More developed regions: Southeast = 0.676, South = 0.660 and Midwest = 0.639 versus Less developed regions: North = 0.527 and North East 0.516)
bExcludes yellow race (n = 3313) and indigenous (n = 422)
Several distinct patterns emerged within the different age groups. Firstly, the group aged < 40 demonstrated a significantly higher proportion of black patients (53%, p < 0.001). Additionally, this age group exhibited a greater prevalence of females with more than 8 years of schooling (56.3%, p < 0.001), a heightened rate of alcohol consumption (20.4%, p < 0.001), and a higher proportion of patients diagnosed at advanced stages ≥ IIB (64.0%, p < 0.001). Furthermore, patients in this age group displayed a substantially increased likelihood of receiving two or more treatment modalities (60.8%, p < 0.001), as well as a greater proportional chance of receiving radiotherapy (49.9%, p < 0.001) and/or chemotherapy (68.4%, p < 0.001) at some point in their treatment journey.
Conversely, patients aged 40–69 exhibited a higher rate of smoking (31.5%, p < 0.001), distinguishing them from other age groups. Lastly, the elderly population of ≥ 70 years manifested a greater proportional prevalence within the group experiencing a time-lapse exceeding 60 days from diagnosis to the initiation of treatment (54.1%, p < 0.001). Moreover, this age group received a higher proportional prevalence of hormone therapy (45.4%, p < 0.001).
Mortality
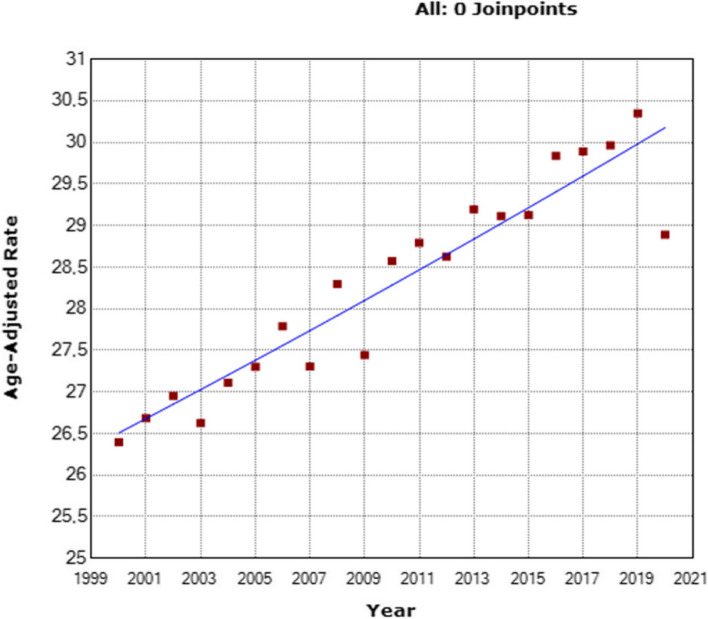
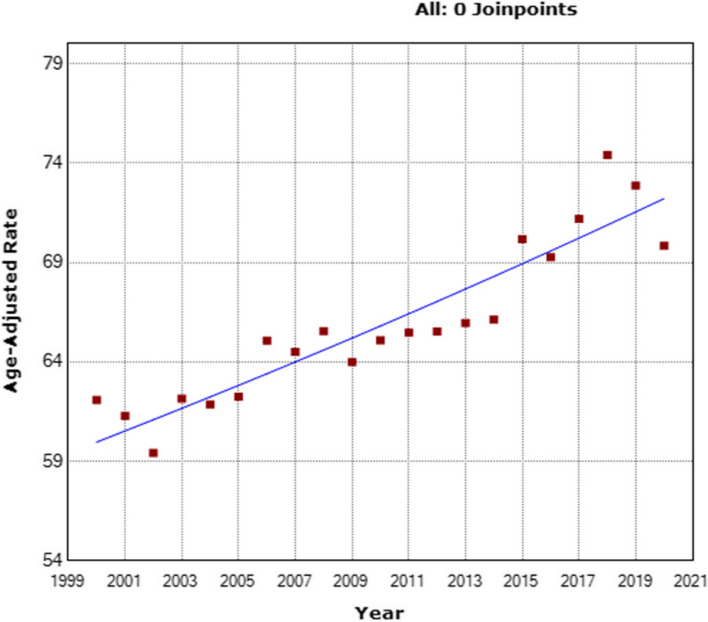
By using the comprehensive MIS dataset, an analysis of BC-related deaths between the years 2000 and 2020 revealed a total of 546,193 fatalities. The mortality rates varied across this period, ranging from 10.54/100,000 (the lowest rate) to 12.31/100,000 (the highest rate) (Supplementary Table 4). Of particular significance is the observed escalation in BC-specific mortality rates within the three examined age groups. Notably, a significantly higher increase was noted among young patients (< 40 years) with an AAPC of + 1.8% (95% CI: 1.6 to 2.1; p < 0.001) (Fig. 5) (Supplementary Table 5). Similarly, patients aged 40–69 exhibited a slightly elevated AAPC of + 0.7% (95% CI: 0.5 to 0.8; p < 0.001) (Fig. 6), and patients aged ≥ 70 also demonstrated a modest yet noteworthy AAPC of + 0.9% (95% CI: 0.7 to 1.1; p < 0.001) (Fig. 7).
Fig. 5.
Evolution of adjusted breast cancer mortality rates among women < 40, from 2010 to 2015
Fig. 6.
Changing trends in adjusted breast cancer mortality rates among women aged 40–69, from 2010 to 2015
Fig. 7.
Shifting patterns in adjusted breast cancer mortality rates among women aged > 69, from 2010 to 2015
Discussion
This study represents a pioneering examination of breast cancer patterns at a national level across specific age groups. It provides a comprehensive analysis of temporal trends in both incidence and mortality rates, while also investigating disparities in key sociodemographic and clinicopathological factors, as well as variations in treatment modalities. The findings reveal distinct trends in incidence and mortality rates that may have been influenced by age, also suggesting some variations in clinicopathological characteristics and sociodemographic profiles. Additionally, the results indicate different treatment strategies according to the age group analyzed.
The higher BC incidence among women ≥ 70 reaffirms advanced age as a significant risk factor, consistent with IARC research [7]. While BC incidence showed a declining trend overall, it remained stable for ages 40–69 and ≥ 70. However, patients < 40 experienced a sustained increase, possibly due to reproductive factors like delayed childbirth and lower parity or nulliparity [8], which are more prevalent in transitional economies [10]. The rising BC incidence among young women in HICs, like North America and Europe, is due to early mammography, driven by various screening practices [12, 13]. High-income Asian countries with early mammography recommendations are also shifting incidence to younger age groups [10].
Similar trends in Brazil compared to the United States and other Latin American countries have been observed [14–16]. Multiple factors, including genetics and modifiable risk factors like smoking and alcohol, contribute to these differences [17]. Notably, this study found higher alcohol use among women under 40. Chronic exposure to pesticides, prevalent in Brazil, is a concern [18, 19]. Research on genomics and environmental carcinogens is essential for understanding these factors and their impact on BC incidence. In contrast, declining BC incidence in postmenopausal women in some countries is linked to reduced hormone therapy replacement and the stabilizing effect of mammography screening [12, 20].
The higher frequency of BC in more high-income regions of Brazil is strongly linked to improved access to screening methods, as demonstrated by Nogueira et al. [21]. Disparities in mammographic screening coverage across micro-regions in Brazil suggest that areas with fewer wealth inequities and better healthcare access have higher screening rates. The higher prevalence of black women in the < 40 age group observed in this study corroborates previous findings [22]. DeSantis et al. conducted a population-based study utilizing data from the Surveillance, Epidemiology, and End Results (SEER) program, which revealed elevated BC incidence rates among black women under 45 compared to white women in the same age group [23]. Black women were diagnosed at a median age of 58 years, whereas white women were diagnosed at a median age of 62 years. This increased incidence of BC among young black women can be primarily attributed to a complex interplay of genetic, socioeconomic, and healthcare-related factors [24]. Genetic variations potentially contribute to disparities in BC susceptibility and tumor characteristics across different racial and ethnic groups. Furthermore, cultural and behavioral factors, including variations in reproductive patterns, obesity rates, and exposure to specific environmental factors, may also contribute to the observed discrepancy [22].
The higher incidence of advanced-stage BC among women < 40, in comparison to those aged 40–69 and ≥ 70 in the HBCR dataset, can be attributed to various factors [25, 26]. One contributing factor is the absence of regular screenings in this age group, as younger women are typically not included in routine mammography screening programs that primarily focus on women aged 50 and above. Consequently, breast cancer in younger women may remain undetected until symptoms become noticeable or the cancer has progressed. Another factor is the delayed diagnosis, whereby younger women are more prone to disregard or overlook early signs and symptoms of breast cancer due to its relative rarity in younger populations. Additionally, a higher proportion of aggressive subtypes, such as triple-negative breast cancer, is observed in younger women, exhibiting more rapid growth and metastasis compared to other subtypes. Moreover, younger women often have denser breast tissue, which poses challenges in tumor detection via mammograms. Furthermore, some younger women with breast cancer may possess genetic predispositions, such as BRCA1 or BRCA2 gene mutations, which increase the risk of developing breast cancer at a younger age and are often associated with more aggressive forms of the disease. Previous evidence suggests that older women typically present with less advanced-stage breast cancer at diagnosis, often exhibiting histology linked to a more favorable prognosis [27].
The findings of the current study indicate that older patients (≥ 70 years) faced delays in initiating treatment and had limited access to various treatment options such as surgery, radiotherapy, and chemotherapy, while hormonal therapy was more frequently administered. Bagegni and Peterson argue that the focus on avoiding overtreatment in older adults may contribute to age-related disparities in treatment outcomes, suggesting the need for individualized treatment approaches that consider patient preferences and functional status rather than relying exclusively on chronological age [27]. Malik et al.'s prospective cohort study revealed that elderly patients received fewer axillary samplings, mastectomies, and adjuvant radiation therapy, but had a higher prevalence of hormonal therapy [28]. Approximately 51% of the 382 elderly patients were deemed undertreated based on conventional criteria. Similarly, Van Leeuwen et al., in a cohort of 212 elderly women, estimated that 57% of patients received inadequate treatment according to institutional and national guidelines [29]. Bastiaannet et al., analyzing Dutch population data, identified poor patient selection and highlighted the possibility of undertreatment in physically fit elderly patients [30]. Some barriers to access to new effective therapies can also harm outcomes in the population [31].
The present study highlights a notable increase in the mortality rate among individuals below the age of 40, surpassing twice the rates observed in the age groups of 40–69 and ≥ 70 years. The multi-institutional AMAZONA III study (GBECAM 0115), employing a prospective registry, further reveals that Brazilian women under the age of 40 exhibit unfavorable clinicopathological characteristics at the time of BC diagnosis, characterized by more aggressive subtypes and advanced stages compared to older women [25]. Silva et al., in a Brazilian population study, demonstrate a real increase in cancer mortality among young women in Brazil, its regions, and states over the past two decades [32]. Mortality rates were higher in 2017 compared to 1996 for both age groups studied, with the Midwest region exhibiting the highest annual percentage change (APC) of 7.4% for women aged 20–29 and the North region with an APC of 3.7% for women aged 30–39. Balmant et al. observe higher BC mortality rates in Brazil and its regions for adolescents and young adults, particularly within the 25–29 age range, with rates exceeding 4.4 per million [33]. These findings underscore the increased mortality of young women from BC in Brazil, showing regional variations.
Conversely, while low and middle-income countries experience rising mortality rates, high-income countries demonstrate a contrasting trend. Notably, in several high-income countries, BC mortality rates have decreased due to significant advancements in treatment [33]. For instance, in France, although the incidence of BC among young women has increased by 1.1% annually from 1990 to 2018, mortality rates have declined by 1.3% during the same period, accounting for 5% of deaths in young women [34]. Similar trends have been observed in Shanghai, China, where mortality rates have shown a downward trend [35]. Additionally, a trend analysis of BC mortality among women aged 30 to 39 in Switzerland between 1996 and 2009 demonstrated a decline from 3 to 1.6 deaths per 100 thousand women [36]. Finally, Heer et al. conducted a population-based study revealing that countries with a very high Human Development Index (HDI) exhibited the highest incidence rates of BC among both premenopausal and postmenopausal women (30.6 and 253.6 cases per 100,000, respectively) [8]. In contrast, countries with low and medium HDI demonstrated the highest mortality rates for BC in the respective age groups (8.5 and 53.3 deaths per 100,000, respectively). These findings highlight the influence of socioeconomic factors on the implementation and adherence to BC prevention measures by women.
The present study demonstrates several notable strengths, including its population-based design encompassing extensive national coverage and the comprehensive analysis of a substantial time period capturing incidence and mortality rates recorded in the databases. Nonetheless, it is imperative to consider certain methodological constraints that may impact the interpretation of the data. Firstly, the accuracy and reliability of the data collected from registries and information systems may exhibit regional variability. Secondly, inherent limitations associated with observational studies, such as the inability to establish causality and account for unmeasured variables, should be considered when interpreting the findings. Furthermore, the absence of information on lifestyle factors, socioeconomic status, health insurance, screening practices, data on histological subtypes and molecular profiles, and family history could potentially influence the outcomes of the disparity analyses among the age group.
Conclusion
This study reveals a significant disparity in BC incidence and mortality among women < 40, 40–69, and ≥ 70 years old. Further research is needed to understand why BC sustains a high incidence among young women in Brazil. Identifying high-risk individuals can lead to targeted surveillance and screening policies. An enduring awareness policy is essential to educate vulnerable populations about BC risk factors. The study also shows undertreatment among elderly women, highlighting the need for interventions to reduce treatment delays and prioritize patient preferences and functional status over numerical age in treatment guidelines.
Supplementary Information
Acknowledgements
We extend our sincere appreciation to the public data source in Brazil for their crucial role in providing the extensive dataset employed in this study. It is essential to note that the authors assume complete responsibility for the content of this publication; any views or opinions articulated herein reflect solely those of the authors and should not be interpreted as official positions endorsed by any governmental entities.
Abbreviations
- BC
Breast cancer
- DCIS
Ductal carcinoma in situ
- PBCRs
Population-Based Cancer Registries
- HBCRs
Hospital-Based Cancer Registries
- MIS
Mortality Information System
- AAPC
Average annual percent change
- SEER
Surveillance, Epidemiology, and End Results
Authors’ contributions
Conceptualization/visualization: JLS, LCST and ACM. Methodology: JLS, LCST and ACM. Data curation/formal analysis/software: LCST and ACM. Investigation: JLS, LCST and ACM. LCST had access to raw data. LCST and JLS verified the data. Project administration/supervision: JLS, LCST and ACM. Writing the original draft: JLS, LCST and ACM. Reviewing and editing: JLS, LCST and ACM. All authors had final responsibility for the decision to submit for publication.
Funding
The authors declare that this study has received no financial support.
Data availability
The datasets used in this investigation are publicly available and can be accessed through the following sources. For information pertaining to incidence rates, please refer to the National Cancer Institute's database at https://www.inca.gov.br/BasePopIncidencias/Home.action. Data related to clinical and sociodemographic factors can be found at https://irhc.inca.gov.br/RHCNet/. Additionally, mortality data is accessible via the TABNET platform at http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obt10uf.def.
Declarations
Ethics approval and consent to participate
The study employed de-identified data obtained from publicly available government sources, thereby waiving the requirement for ethical review board approval and individual consent, in accordance with established ethical standards. The study adhered to good clinical practices.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jessé Lopes da Silva and Luiz Claudio Santos Thuler contributed equally to this work.
References
- 1.Cancer Tomorrow. [cited 2023 Jun 4]. Available from: https://gco.iarc.fr/tomorrow/en/dataviz/isotype?cancers=20&single_unit=100000
- 2.Santos M de O, Lima FC da S de, Martins LFL, Oliveira JFP, Almeida LM de, Cancela M de C. Estimativa de Incidência de Câncer no Brasil, 2023–2025. Revista Brasileira de Cancerologia. 2023;69(1):e-213700.
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 4.National Cancer Institute (Brazil). INCA Mortality Online Atlas. Rio de Janeiro: INCA; c2021. Available from: https://www.inca.gov.br/app/mortalidade. Accessed June 23, 2023.
- 5.American Cancer Society. Breast Cancer Facts&Figures 2019–2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2019-2020.pdf. (accessed on 4 June 2023).
- 6.Ward EM, Sherman RL, Henley SJ, Jemal A, Siegel DA, Feuer EJ, et al. Annual Report to the Nation on the Status of Cancer, Featuring Cancer in Men and Women Age 20–49 Years. JNCI: Journal of the National Cancer Institute. 2019;111(12):1279–97. [DOI] [PMC free article] [PubMed]
- 7.Merlo DF, Ceppi M, Filiberti R, Bocchini V, Znaor A, Gamulin M, et al. Breast cancer incidence trends in European women aged 20–39 years at diagnosis. Breast Cancer Res Treat. 2012;134(1):363–70. [DOI] [PubMed] [Google Scholar]
- 8.Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8(8):e1027–37. [DOI] [PubMed] [Google Scholar]
- 9.DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–51. [DOI] [PubMed] [Google Scholar]
- 10.Ciuba A, Wnuk K, Nitsch-Osuch A, Kulpa M. Health Care Accessibility and Breast Cancer Mortality in Europe. Int J Environ Res Public Health. 2022;19(20):13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 12.Instituto Nacional de Câncer - INCA. [cited 2023 Jun 4]. Registros de Câncer de Base Populacional. Available from: https://www.gov.br/inca/pt-br/assuntos/cancer/numeros/registros/base-populacional/registros-de-cancer-de-base-populacional
- 13.Integrador RHC ::. [cited 2023 Jun 4]. Available from: https://irhc.inca.gov.br/RHCNet/
- 14.DATASUS - SIM - Sistema de Informação sobre Mortalidade. [cited 2023 Jun 4]. Available from: http://sim.saude.gov.br/default.asp
- 15.Cancer today. [cited 2023 Jun 24]. Available from: http://gco.iarc.fr/today/home
- 16.Ma H, Bernstein L, Pike MC, Ursin G. Reproductive factors and breast cancer risk according to joint estrogen and progesterone receptor status: a meta-analysis of epidemiological studies. Breast Cancer Res. 2006;8(4):R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter P. “Westernizing” Women’s Risks? Breast Cancer in Lower-Income Countries. N Engl J Med. 2008;358(3):213–6. [DOI] [PubMed] [Google Scholar]
- 18.Oberaigner W, Buchberger W, Frede T, Knapp R, Marth C, Siebert U. Breast cancer incidence and mortality in Tyrol/Austria after fifteen years of opportunistic mammography screening. BMC Public Health. 2010;20(10):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qin J, White MC, Sabatino SA, Febo-Vázquez I. Mammography use among women aged 18–39 years in the United States. Breast Cancer Res Treat. 2018;168(3):687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joko-Fru WY, Jedy-Agba E, Korir A, Ogunbiyi O, Dzamalala CP, Chokunonga E, et al. The evolving epidemic of breast cancer in sub-Saharan Africa: Results from the African Cancer Registry Network. Int J Cancer. 2020;147(8):2131–41. [DOI] [PubMed] [Google Scholar]
- 21.Nogueira MC, Fayer VA, Corrêa CSL, Guerra MR, Stavola BD, Dos-Santos-Silva I, et al. Inequities in access to mammographic screening in Brazil. Cad Saude Publica. 2019;35(6):e00099817. [DOI] [PubMed] [Google Scholar]
- 22.Yedjou CG, Sims JN, Miele L, Noubissi F, Lowe L, Fonseca DD, et al. Health and Racial Disparity in Breast Cancer. Adv Exp Med Biol. 2019;1152:31–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeSantis CE, Fedewa SA, Goding Sauer A, Kramer JL, Smith RA, Jemal A. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66(1):31–42. [DOI] [PubMed] [Google Scholar]
- 24.Nnorom SO, Wilson LL. Breast Cancer in Black Women: Racial/Ethnic Disparities Affecting Survival. J Womens Health (Larchmt). 2022;31(9):1255–61. [DOI] [PubMed] [Google Scholar]
- 25.Franzoi MA, Rosa DD, Zaffaroni F, Werutsky G, Simon S, Bines J, et al. Advanced Stage at Diagnosis and Worse Clinicopathologic Features in Young Women with Breast Cancer in Brazil: A Subanalysis of the AMAZONA III Study (GBECAM 0115). J Glob Oncol. 2019;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orlandini LF, do Nascimento Antonio MV, Espreafico Jr CR, Bosquesi PL, Poli-Neto OB, de Andrade JM, et al. Epidemiological Analyses Reveal a High Incidence of Breast Cancer in Young Women in Brazil. JCO Glob Oncol. 2021;7:GO.20.00440.11111 [DOI] [PMC free article] [PubMed]
- 27.Bagegni NA, Peterson LL. Age-related disparities in older women with breast cancer. Adv Cancer Res. 2020;146:23–56. [DOI] [PubMed] [Google Scholar]
- 28.Malik MK, Tartter PI, Belfer R. Undertreated breast cancer in the elderly. J Cancer Epidemiol. 2013;2013:893104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Leeuwen BL, Rosenkranz KM, Feng LL, Bedrosian I, Hartmann K, Hunt KK, et al. The effect of under-treatment of breast cancer in women 80 years of age and older. Crit Rev Oncol Hematol. 2011;79(3):315–20. [DOI] [PubMed] [Google Scholar]
- 30.Bastiaannet E, Liefers GJ, de Craen AJM, Kuppen PJK, van de Water W, Portielje JEA, et al. Breast cancer in elderly compared to younger patients in the Netherlands: stage at diagnosis, treatment and survival in 127,805 unselected patients. Breast Cancer Res Treat. 2010;124(3):801–7. [DOI] [PubMed]
- 31.Debiasi M, Barrios CH. Estimated number of breast cancer deaths in the Brazilian Public Health System due to lack of access to adjuvant trastuzumab between 2005 and 2012. Journal of Clinical Oncology. 2015 May 20 [cited 2024 Nov 20]; Available from: https://ascopubs.org/doi/10.1200/jco.2015.33.15_suppl.e17601
- 32.Silva JDDE, de Oliveira RR, da Silva MT, Carvalho MD de B, Pedroso RB, Pelloso SM. Breast Cancer Mortality in Young Women in Brazil. Frontiers in Oncology. 2021 [cited 2023 Jun 25];10. Available from: https://www.frontiersin.org/articles/10.3389/fonc.2020.569933 [DOI] [PMC free article] [PubMed]
- 33.Balmant NV, Reis R de S, Santos M de O, Maschietto M, Camargo B de. Incidence and mortality of bone cancer among children, adolescents and young adults of Brazil. Clinics (Sao Paulo). 2019;74:e858. [DOI] [PMC free article] [PubMed]
- 34.Lanta Q, Arveux P, Asselain B. Epidemiology and socio-cultural specificities of young women with breast cancer. Bull Cancer. 2019;106(12S1):S4–9. [DOI] [PubMed]
- 35.Huang Z, Wen W, Zheng Y, Gao YT, Wu C, Bao P, et al. Breast cancer incidence and mortality: trends over 40 years among women in Shanghai. China Ann Oncol. 2016;27(6):1129–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bodmer A, Feller A, Bordoni A, Bouchardy C, Dehler S, Ess S, et al. Breast cancer in younger women in Switzerland 1996–2009: a longitudinal population-based study. Breast. 2015;24(2):112–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used in this investigation are publicly available and can be accessed through the following sources. For information pertaining to incidence rates, please refer to the National Cancer Institute's database at https://www.inca.gov.br/BasePopIncidencias/Home.action. Data related to clinical and sociodemographic factors can be found at https://irhc.inca.gov.br/RHCNet/. Additionally, mortality data is accessible via the TABNET platform at http://tabnet.datasus.gov.br/cgi/deftohtm.exe?sim/cnv/obt10uf.def.