Abstract
The aging population has led to a global issue of osteoarthritis (OA), which not only impacts the quality of life for patients but also poses a significant economic burden on society. While biotherapy offers hope for OA treatment, currently available treatments are unable to delay or prevent the onset or progression of OA. Recent studies have shown that as nanoscale bioactive substances that mediate cell communication, exosomes from stem cell sources have led to some breakthroughs in the treatment of OA and have important clinical significance. This paper summarizes the mechanism and function of stem cell exosomes in delaying OA and looks forward to the development prospects and challenges of exosomes.
Keywords: osteoarthritis, cartilage, exosomes, stem cells, regenerative medicine
Introduction
As the population ages, the prevalence of patients with osteoarthritis (OA) is on the rise, with >240 million people worldwide currently affected [1, 2]. OA is a multifaceted degenerative condition characterized by the deterioration of joint cartilage and inflammation, leading to impaired joint function and limited mobility [3, 4]. The management of OA presents significant clinical, societal, and economic challenges [5]. Being a complex disease influenced by various factors, OA affects both weight-bearing and non-weight-bearing joints and is closely associated with obesity [6]. Given the growing number of individuals affected by OA, the prevention and treatment of this condition have become important medical and social priorities.
The aim of OA treatment is to reduce symptoms, delay joint degeneration, and improve patients′ quality of life. Treatment primarily focuses on preventing cartilage wear and tear. It is divided into conservative treatment and surgical treatment [7]. Surgical treatment is recommended for patients who do not respond well to conservative treatment. In recent years, researchers have been searching for targets to effectively treat OA by inhibiting joint structural damage and achieving long-term improvement [8–10].
All cells release extracellular vesicles (EVs), including exosomes, which are involved in cell-to-cell communication and have various physiological functions [11, 12]. Exosomes play a crucial role in regulating environmental homeostasis, affecting diseases, and promoting processes such as tumor growth, migration, and tissue repair [13–16]. They contain active substances like mRNA, miRNA, DNA, and proteins, and are found in almost all body fluids. Exosomes serve as potential therapeutic targets and have shown potential in stem cell therapy [17, 18]. They participate in intercellular signal transduction, target specific cells, regulate the microenvironment, and promote tissue regeneration. Additionally, exosomes derived from different cell sources are implicated in the development and onset of OA disease [19–21]. The field of exosome research is rapidly advancing and holds significant clinical transformation potential. Therefore, this study aims to investigate the mechanisms and challenges of exosomes in OA, as well as the current state of research in this area.
Pathogenesis of OA
OA is a slowly progressing disease that affects the joint tissue, particularly as individuals age. The incidence of OA tends to increase with age. The development of OA is influenced by various factors such as biomechanics, biochemistry, mutations in inflammatory genes, and immunological factors. These factors interact with each other, leading to a cascade of degenerative reactions. As a result, patients with OA experience characteristic changes in the cartilage of their joints, affecting all joint structures [22]. Environmental factors also play a significant role in the pathogenesis of OA, including obesity, metabolic syndrome, dietary changes, and lack of exercise [23]. OA is a multifactorial total joint disease, characterized by the alteration of joint cartilage through complex pathological mechanisms. In addition to affecting the cartilage, OA also impacts the synovial, subchondral, ligament, and muscle tissues surrounding the joint. This complex disease involves inflammation, metabolic disorders, and fibrosis (Fig. 1) [24]. The maintenance of joint cartilage primarily relies on cartilage cells and the extracellular matrix (ECM). An important factor in the development and progression of OA is the increased decomposition metabolism in the articular cartilage ECM [25]. The main proteins in cartilage ECM are type II collagen and aggregated proteoglycans, and the synthesis of ECM forms the foundation for maintaining joint cartilage function [26].
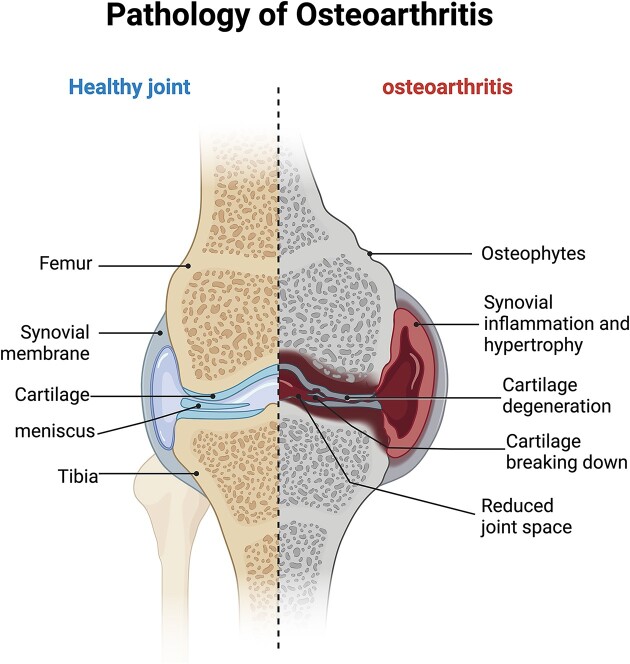
Figure 1.
OA is a multifactorial disease affecting the entire joint and its progression involves complex pathological mechanisms. The figure highlights the pathological changes occurring in the joint's cartilage, as well as the alterations in the synovial membrane, subchondral bone, ligaments, and surrounding muscles. (Created with BioRender.com).
The synovial membrane, which consists of synovial macrophages and synovial fibroblasts, is responsible for secreting joint fluids that lubricate cartilage [27, 28]. In patients with advanced knee OA, apoptotic cells accumulate in the synovial membrane, which can disrupt the important homeostatic function of synovial macrophages and result in the buildup of apoptotic cells [29]. Synovial inflammation in synovial cells can be mediated by mitochondrial dysfunction and damage-related molecular patterns [30]. A number of different tissues or cells communicate with exosomes thereby maintaining the homeostasis of the intra-articular environment or mediating the development of OA [31].
Several pro-inflammatory cytokines play a significant role in the development of OA. Cytokines that are found at elevated levels in OA joint tissues include interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, monocyte chemotactic protein (MCP)-1, vascular endothelial growth factor (VEGF), and interferon-inducible protein (IP)-10. These pro-inflammatory factors inhibit matrix synthesis by stimulating matrix degradation enzymes, thereby promoting cartilage degradation, which leads to progressive joint destruction and remodeling [32, 33]. Matrix degradation enzymes identified in OA joints include aggrecanase and collagenases, both of which are members of the matrix metalloproteinase (MMP) family, as well as various serine and cysteine proteases. The degradation of the matrix in early OA may be attributed to MMP-3 and aggrecanase, particularly the a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5). This degradation of aggregated proteoglycans is followed by increased collagen activity [34]. Additionally, certain growth factors typically stimulate the production of both matrix and pro-inflammatory factors. For instance, transforming growth factor-β (TGF-β) and bone morphogenetic protein 2 (BMP-2) can promote osteoblastic formation and lead to subchondral sclerosis. These pro-inflammatory mediators and anabolic factors are produced locally by cells within the affected tissues, including chondrocytes in the synovial membrane, synovial fibroblasts, immune cells, inflammatory cells in the perijoint fat, and cells in the bone [35]. Consequently, certain cytokines and growth factors are implicated in the pathogenesis of OA, suggesting that they may represent potential therapeutic targets for delaying the progression of the disease.
Exosomal therapy
Exosomes, as a therapeutic hotspot, have shown great potential in recent years in the field of joint disease progression. They participate in disease physiology and pathology processes by regulating intercellular communication [36]. The specific role and mechanism of exosomes in OA differ from other biological treatments. Exosome treatment of OA aims to protect chondrocytes from excessive death, reduce inflammation, maintain cartilage matrix metabolism, and regulate angiogenesis and subchondral bone remodeling [37].
Characteristics, separation, and identification of exosomes
Exosomes are present in all biological fluids and are secreted by all cells. They have the potential to track disease progression [38, 39]. The study of exosomes initially focused on clotting. In the 1940s, Chargaff and West conducted research on blood clotting in New York and discovered a ‘granular part’ that settled at 31000 g with high clotting potential [40]. In the 1980s and 1990s, this substance was identified as a biological entity with enzymatic and functional potential [41]. The term ‘exosome’ was first mentioned in four biomedical articles published in PNAS between 1970 and 1973 [42–45]. These articles described the transfer of transformed DNA fragments between Drosophila or Phlebocytes cells. However, the association of DNA with the lipid bilayer was not mentioned in the literature, so they cannot be easily explained as an early description of EVs from the exosomes studied [46]. Since the late 1980s, exosomes have been consistently referred to as EVs originating in the intracellular system [47–49]. The field of exosomes has rapidly expanded since the earlier research on electron microscopy and biochemistry in the 1940s–80s. Numerous studies have confirmed the findings of the early pioneers in this field, indicating that exosomes play a crucial role in intercellular communication.
The potential for exosomal transformation is being explored for its ability to diagnose, provide prognosis, and treat diseases. Researchers are particularly interested in understanding the regulation of exosomes in various biological processes and diseases [50, 51]. Exosome-based therapy offers advantages such as anti-aging and anti-inflammatory effects, as well as a lower risk of tumor formation and immune rejection compared to stem cell-based therapy. This provides hope for ‘cell-free’ tissue regeneration. In recent years, there has been a significant increase in the study of osteopathic degenerative diseases, with exosomes expected to contribute new therapeutic ideas for the treatment of patients with OA in the future [52].
Exosomal characteristics
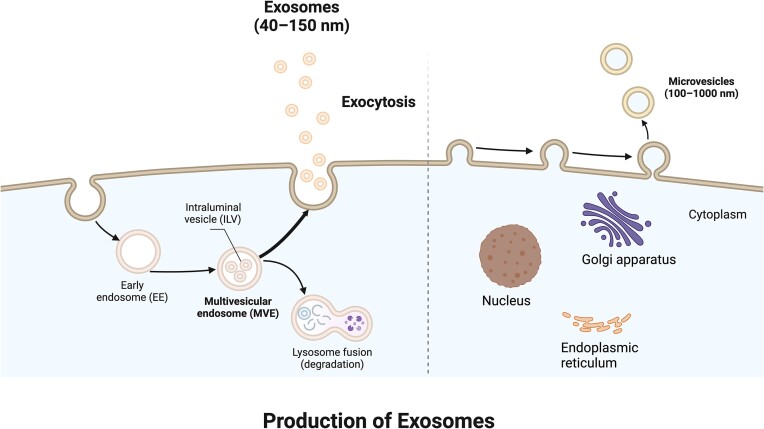
EVs are small vesicles that are released from cells into the extracellular space. They consist of various types of vesicles that differ in terms of their biogenesis pathway, size, and composition. EVs are characterized by lipid bilayers and cannot be replicated. They do not contain functional nuclei [53]. The current definition of an EV subtype, called an exosome, is a small EV 30–150 nm in size, produced by the multivesicular endosome pathway [54]. Multivesicular bodies undergo maturation from the endosome as their membranes bud inward in the cavity of the tube, forming vesicles. When these vesicles are released into the extracellular environment, they are referred to as exosomes (Fig. 2) [49, 55].
Figure 2.
The mechanism of exosome secretion. Exosomes are formed through inward budding of the cell membrane, resulting in the formation of endosomes. These endosomes are then produced through the MVE pathway and subsequently fuse with the cell membrane, leading to exocytosis. The term ‘exosome’ is used to describe these vesicles as they are released into the extracellular environment. (Created with BioRender.com).
Exosomes play a crucial role in various physiological and pathological processes, including antigen presentation, regulation of tumor growth and migration, and tissue repair. They also offer unique advantages in early disease diagnosis and targeted drug treatment [56, 57]. Intercellular communication through exosomes is observed in all organisms, ranging from bacteria to plants and animals [58–60]. These exosomes transport proteins, lipids, and RNA, facilitating communication between different cell types and influencing both normal and pathological conditions [61].
Exosome separation and identification
With the rapid development of biotechnology, exosomes have gained significant research value and prospects. Consequently, it becomes crucial to isolate high-purity exosomes from various biological fluids. Several methods have been developed for exosome separation, taking into account factors such as size, shape, density, and surface protein of the exosome [62]. These methods include high-speed centrifugation, ultrafiltration, immunophilic capture, charge-neutralized polymer precipitation, dimensional resistive chromatography, and microfluidic techniques [63]. Among these, ultracentrifugal separation is considered the classical method, which can be further categorized into density gradient ultracentrifugal separation and differential ultracentrifugal separation. Differential ultracentrifugation, also known as simple ultracentrifugation or precipitation, is one of the commonly employed techniques for exosome separation.
The principle of differential ultra-centrifugation is based on the separation of different extracellular components of fluid samples using centrifugal forces, which separates them sequentially according to their density, size, and shape [64, 65]. Previous studies have extensively used differential ultracentrifugation to isolate exosomes from various sources, including cell media, serum, saliva, urine, and cerebrospinal fluid [66–70]. However, these methods still have limitations such as being time-consuming, requiring immunomagnetic bead capture, and being costly [71]. Further refinement is needed to enhance the study and application of exosomes, considering the advantages and disadvantages of these methods.
Validation of exosomes after separation is commonly assessed using various identification techniques. These techniques include transmission electron microscopy (TEM), nanoparticle tracking analysis (NTA), and size distribution and shape characterization of isolated exosomes [72, 73]. TEM technology is well-established and has been extensively used in exosome studies, providing evidence for the presence of vesicular structures [74]. While standard light scattering NTA does not provide information about the biochemical composition or cellular origin of vesicles, fluorescent labeling can be employed for vesicle analysis [75]. Furthermore, western blot (WB) identification of exosome surface markers such as CD9, CD63, CD81, HSP70, and TSG101 is also a commonly used method for exosome identification [76, 77].
However, these methods possess both advantages and disadvantages. For instance, TEM can effectively observe the morphological structure of exosomes and provide information on particle size distribution. However, due to the complexity of TEM operations and the high demands of sample preparation, it is not suitable for the rapid measurement of large numbers of samples. NTA can detect the size and concentration of exosomes, offering high detection speed and resolution. Nevertheless, its operation is intricate, making it challenging to distinguish between contaminated proteins and exosomes, and its results may be influenced by the quality of the camera used. WB is a commonly employed method for exosome detection, with the advantage of mature technology that allows for qualitative and quantitative analysis of marked proteins. However, WB has notable drawbacks, including a complex and time-consuming identification process, variability in the detection of marker proteins based on cell type, and its unsuitability for detecting exosome marker proteins in biofluids. Flow cytometry can analyze the size of exosomes by targeting them with specific antibodies or fluorescent dyes. Additionally, other techniques such as scanning electron microscopy, atomic force microscopy, adjustable resistance pulse sensing, dynamic light scattering, resistance pulse sensing, enzyme-linked Immunosorbent assay, fluorescence activated cell sorting, as well as microfluidic and electrochemical biosensors, can also be employed to detect exosomes [78]. To ensure the reliability of exosome test results, it is often necessary to utilize multiple methods in conjunction with each other to evaluate the extracted exosomes.
The mechanism of OA treatment with exosomes
The disease process of OA is a complex one, involving various factors such as cartilage damage, inflammation of the synovial membrane, degeneration of ligaments and synovial membranes, remodeling of the inferior cartilage bone, and changes in the structure of the joint capsule, surrounding muscles, nerves, and local fat pads [79]. Researchers are continuously exploring the underlying pathological mechanisms of OA and developing new strategies to inhibit its progression. Hyperchondrocyte death, ECM degradation, synovial inflammation, neovascularization, nerve invasion, and subchondral remodeling are common pathological manifestations observed during the development of OA [80–82].
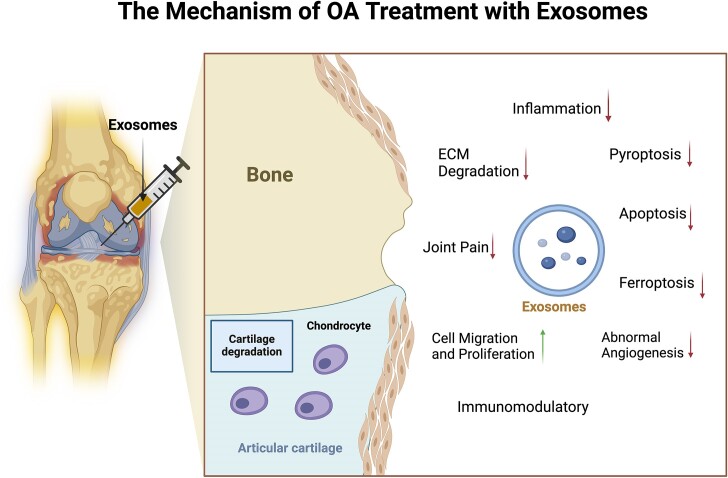
These pathological changes interact to promote the occurrence of OA. While some exosomes of cellular origin (stem cells, chondrocytes, etc.) may have a positive effect on OA, there are others (immune cells, inflamed synoviocytes, diseased subchondral osteoblasts, etc.) that have a negative effect and contribute to the persistent pathogenesis of OA. The exact mechanism of action of exosomes of regenerative or reparative cell origin in the treatment of OA is not yet fully understood. However, numerous studies have demonstrated that exosomes can slow down the progression of OA by inhibiting pathological responses through molecular communication between chondrocytes. This includes reducing the inflammatory response, regulating ECM, protecting chondrocytes from excessive cell death, immune regulation, inhibiting abnormal angiogenesis, promoting cell migration and proliferation, and improving symptoms of OA pain (Fig. 3).
Figure 3.
Exosomes play a crucial role in the physiological and pathological processes of OA by regulating intercellular communication. They achieve this by reducing the inflammatory response, influencing the ECM, safeguarding chondrocytes against excessive cell death, regulating the immune system, inhibiting abnormal blood vessel growth, promoting cell migration and proliferation, and alleviating OA pain symptoms. (Created with BioRender.com).
Reducing the inflammatory response
During the progression of OA, cartilage degenerates as a result of inflammation. This inflammation leads to pathological changes in the cartilage of the joints, triggering the secretion of inflammatory factors. These factors then worsen the damage to the cartilage tissue through signaling pathways, ultimately impacting the OA process. Exosomes have been found to play a crucial role in the biological progression of OA disease [83]. Researchers have identified T lymphocytes and macrophages as the primary inflammatory cells involved in OA [84]. Activation of macrophages results in the release of pro-inflammatory factors, while fibroblast-like synoviocytes and chondrocytes, stimulated by these pro-inflammatory factors, contribute to the degradation of the ECM [85]. Synovial macrophages release pro-inflammatory cytokines, growth factors, and enzymes in response to irritation during inflammatory conditions. Macrophages play a role in stimulating angiogenesis, recruiting leukocytes and lymphocytes, promoting fibroblast proliferation, and secreting proteases. These pathological processes contribute to joint damage [86]. Additionally, oxidative stress activates pro-inflammatory pathways and triggers inflammatory responses and chondrocyte aging, which accelerate the degradation of cartilage ECM during the onset of OA [87–89]. Inflammatory molecules, such as pro-inflammatory cytokines, secreted by OA are crucial mediators in the disruptive processes involved in OA pathophysiology. Specifically, IL-1β and TNF play a significant role in controlling the degeneration of the cartilage matrix in the joints, leading to cartilage destruction and local joint inflammation. Therefore, they are the primary targets of treatment strategies [90, 91].
MicroRNA (miRNA) is a regulator of cellular processes, and miRNA (miR) in exosomes plays a crucial role in intercellular signaling. Kim et al. discovered that bone marrow-mesenchymal stem cell (BM-MSC) exosomes treated with IL-1β and TNF-α exhibited significantly enhanced anti-inflammatory activity in SW982 cells of OA. Moreover, the MSC exosome triggered by IL-1β operates through the inhibition of miRNA, such as miR-147b, via the NF-κB pathway [92]. Qiu et al. reported that exosomes derived from human synovial-MSCs (HS-MSCs) and carrying miR-129–5p effectively reduced the inflammatory response and apoptosis of chondrocytes after OA treatment. Conversely, the absence of miR-129–5p in HS-MSC-exosomes (HS-MSC-Exos) intensified IL-1β-mediated chondrocyte inflammatory response and apoptosis [93]. In another study, Chang et al. demonstrated that exosomes derived from low-oxygen cultured adipose-derived stem cells (ADSCs) alleviate inflammation and slow down the progression of OA by reducing inflammatory cytokines [94]. Jin et al. injected miR-9–5p-containing exosomes derived from BM-MSCs into rat OA models to evaluate the levels of inflammatory factors (IL-1, IL-6, TNF-α, and CRP) and the oxidative stress damage index. The results showed that exosomes containing miR-9–5p were able to reduce inflammation [95]. Furthermore, some researchers discovered that miRNA delivered by exosomes from rat synovial fibroblast cells (SFCs) inhibits the expression of IL-1β, IL-6, and TNF-α, thereby improving chondrocyte inflammation and cartilage tissue degeneration [96, 97]. Zhang et al. found that exosomes derived from synovial MSCs (SMSC) overexpressing miR-212–5p can effectively inhibit chondrocyte degeneration and inflammation by targeting ELF3 [98]. Thus, miRNAs in exosomes can reduce the inflammatory response of chondrocytes through intercellular signalling, thereby alleviating the progression of OA (Table 1).
Table 1.
Mechanism of inhibition of OA inflammatory response by exosomes.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| BM-MSC | miR-147b | Inhibition of inflammatory reaction by suppressing the NF-κB pathway | [92] |
| HS-MSC | miR-129–5p | Inhibition of IL- 1β mediated chondrocyte inflammatory response | [93] |
| AD-MSC | miR-147b | Inhibition of inflammatory reaction by suppressing the NF-κB pathway | [94] |
| BM-MSC | miR-9–5p | Reduced levels of inflammatory factors (IL-1, IL-6, TNF-α, and CRP) | [95] |
| SFC | miR-126–3p | Inhibition of the expression of IL-1β, IL-6 and TNF-α | [96] |
| SFC | miR-214–3p | Inhibition of the expression of IL-1β, IL-6 and TNF-α | [97] |
| SMSC | miR-212-5p | Inhibition of inflammation by targeting ELF3 | [98] |
Regulating breakdown and synthesis of ECM
ECM not only provides a physical scaffold for cell embedding, but also regulates various cellular processes, such as growth, migration, differentiation, survival, homeostasis, and morphogenesis [99]. In the context of OA, the destruction of the cartilage ECM and the disruption of homeostasis are of increasing importance. Under normal physiological conditions, the ECM and chondrocytes mutually nourish each other, with a balanced synthesis and degradation of ECM. However, in OA chondrocytes, there is an imbalance where ECM degradation exceeds synthesis, leading to significant reductions in collagen and protein polysaccharides [100]. This ECM damage directly contributes to the worsening of the disease. During the progression of OA, various molecular pathways in the joints malfunction, affecting the metabolic homeostasis of bone ECM and causing structural disruption and deterioration of its biomechanical properties [101].
Xia et al. found that miR-125a-5p-enriched BMSCs-Exo could promote the migration of chondrocytes and remodelled ECM through the upregulation of COL II, aggregated proteoglycans, and SOX 9, as well as the downregulation of MMP-13 [102]. Exosomes derived from human embryonic stem cell-induced MSCs (ESC-MSCs) injected into joints reduced cartilage damage and matrix degradation in DMM models by increasing COL II expression and reducing ADAMTS 5 expression [103]. These exosomes play a therapeutic role in OA by balancing the synthesis and degradation of cartilage ECM. Wang et al. found that SMSC has the potential to mitigate damage induced by OA. In mouse models, miR-155–5p-Exos promoted the proliferation and migration of OA chondrocytes and enhanced ECM secretion by targeting runt-related transcription factor 2 (Runx 2) [104]. Tao et al. found that exosomes secreted by SMSC after miR-140-5p overexpression can activate Yes-related proteins(YAP) via alternative Wnt signaling pathways, which enhances the proliferation and migration of joint chondrocytes in vitro without compromising ECM secretion. In in vivo experiments, SMSC-140-Exos successfully prevented OA in rat models [105]. Thus, miRNAs in exosomes can exert a therapeutic effect on OA through intercellular signalling, which in turn balances the synthesis and degradation of cartilage ECM (Table 2).
Table 2.
Regulating breakdown and synthesis of ECM in OA.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| BM-MSC | miR-125a-5p | Upregulated COL II; aggregated proteoglycans and SOX 9 and downregulated MMP-13; reshaped ECM migration | [102] |
| ESC-MSC | Unknown | Increased COL II and reduced ADAMTS 5 expression; reduced matrix degradation | [103] |
| SMSC | miR-155-5p | ECM secretion is promoted by targeting Runx 2 | [104] |
| SMSC | miR-140-5p | Enhances the proliferation and migration of chondrocytes without compromising ECM secretion | [105] |
Protection of chondrocytes from excessive death
Inhibiting apoptosis
During OA, there is a significant increase in cartilage cell apoptosis, which scontributes to the further degradation of cartilage tissue [106]. Wang et al. conducted a study showing that exosomes derived from adipose tissue-derived MSCs (AD-MSCs) can aid in cartilage regeneration by reducing apoptosis and regulating inflammatory activity. Additionally, they found that miR-486–5p-modified exosomes can inhibit endoplasmic reticulum (ER) stress, decrease cartilage cell apoptosis, and promote matrix regeneration [107]. Xu et al. demonstrated that curcumin-sensitized AD-MSC-derived exosomes can effectively reduce oxidative stress and chondrocyte apoptosis in OA cartilage. These findings indicate great potential in the recovery of joint cartilage damage in OA patients [108]. Autophagy and apoptosis are closely related, with autophagy being able to inhibit apoptosis and help maintain cellular stability. In most cases, autophagy inhibits apoptosis or increases the stress threshold required to induce apoptosis [109]. Wang et al. discovered that activating transcription factor 4 (ATF4) is crucial for chondrocyte proliferation and bone formation. Intra-articular injection of ATF4-OA-Exo partially restores autophagy and inhibits chondrocyte apoptosis [110]. Wu et al. found that exosomes derived from the infrapatellar fat pad (IPFP) MSCs significantly enhance autophagy in cartilage cells by inhibiting mTOR, leading to reduced apoptosis and enhanced matrix synthesis to regulate the progression of OA [111].
Inhibiting cell pyroptosis and ferroptosis
Pyroptosis is a regulated form of cell death that may be associated with risk factors for OA. It is involved in cartilage degeneration, synovial changes, and OA-induced pain [112]. Pyroptosis is mediated by NLRP3 inflammators and caspase-1 signaling. In a study in rats, inhibiting NLRP3-mediated inflammation relieved the OA process [113, 114]. Xu et al. discovered that BMSC-Exos can deliver miR-326 to chondrocytes and cartilage. Targeting HDAC3 and STAT1/NF-κB p65 to inhibit cartilage and pyroptosis improved OA [115].
Ferroptosis is a form of non-apoptotic cell death that relies on iron. It is characterized by the inactivation of glutathione peroxidase 4 and the accumulation of reactive oxygen species [116]. Recent studies have shown that chondrocytes undergo ferroptosis in the presence of inflammation and iron overload, which promotes the development of OA. Furthermore, chondrocyte ferroptosis promoted articular cartilage MMP-13 expression and inhibited collagen II expression [117–119]. Kong et al. discovered that exosomes from OA fibroblast-like synoviocytes containing miR-19b-3p promote cartilage ferroptosis and injury by targeting ferroptosis-related factors such as SLC 7A11 [120]. Cheng et al. conducted a study using BMSC-Exos and an iron apoptosis inhibitor to intervene in an OA rat model. They found that BMSC-Exos reduced chondrocyte ferroptosis and prevented the progression of OA by disrupting the METTL3–m6A–ACSL4 axis [121]. Thus, exosomes can protect chondrocytes from excessive death by inhibiting forms of cell death such as apoptosis, pyroptosis, and iron death, thereby alleviating the process of OA (Table 3).
Table 3.
Protection of chondrocytes from different forms of death in OA.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| AD-MSC | miR-486-5p | Suppression of ER stress can reduce IL-1β-induced apoptosis | [107] |
| AD-MSC | Unknown | Inhibition of apoptosis of articular cartilage cells in mice | [108] |
| OA mouse serum | Unknown | ATF4 overexpression in OA-Exo promotes autophagy of chondrocytes and inhibits their apoptosis | [110] |
| IPFP-MSC | miR100-5p | Inhibition of mTOR enhances autophagy levels in chondrocytes to regulate apoptosis | [111] |
| BM-MSC | miR-326 | Targeting of HDAC3 and STAT1//NF-κB p65 to inhibit pyroptosis of chondrocytes and cartilage | [115] |
| BM-MSC | Unknown | Reduced chondrocyte ferroptosis and prevented OA progression via disruption of the METTL3–m6A–ACSL4 axis | [121] |
Immunomodulation
A growing body of research indicates that the immune system plays a role in the progression of OA. Various factors, such as genetics, metabolism, and mechanical stress, can cause damage to the cartilage. This damage results in the release of specific autoantigens, which then trigger an immune response [122]. Studies have shown that there is infiltration of monocytes in the OA synovial tissue, primarily consisting of CD3+ T cells [123, 124]. Macrophages, T cells, and B cells are the key immune cells involved in controlling the inflammatory process and immune response in OA [125]. These immune cells infiltrate the joint tissues and release cytokines and chemokines from different types of cells present in the joints. This leads to the activation of complement systems and the release of cartilage-degradation factors, such as MMP and prostaglandin E2, which further contribute to the damage of joint cartilage [126–130].
Ragni et al. discovered that BMSCs secrete several leukocyte chemokines. BMSC interacts with the abundance of activated immune cells in OA tissues and reduces the pro-inflammatory state through the action of different leukocyte subpopulations. [131]. Additionally, exosomes derived from BMSCs regulate the immune response by controlling the activation and differentiation of T cells, inhibiting B cell function, and reducing the release of inflammatory mediators [132]. Zhang et al. demonstrated that EVs from BMSC have an immunomodulatory effect on the regenerative immune phenotype. These EVs can decrease the infiltration of M1 macrophages by attracting M2 macrophages to infiltrate OA cartilage defects and synovial membranes, thereby reducing the expression of IL-1β and TNF-α [133]. Similarly, Zheng et al. found that exosomes derived from primary chondrocytes contain more immune-related proteins and can prevent the development of OA by restoring mitochondrial dysfunction and promoting a shift in macrophage response towards the M2 phenotype [134]. Li et al. found that EVs from human umbilical cord-MSCs (HU-MSCs) may promote M2 macrophage polarisation by delivering key proteins and modulating the miRNA-mediated PI3K-Akt signalling pathway, which improves immunomodulation [135]. Exosomes play a significant role in the immune response through various pathways, particularly in the regulation of synovial macrophages. In OA, macrophages may polarize to a pro-inflammatory M1 phenotype. Conversely, M2 macrophages can inhibit inflammation in OA and promote cartilage repair by secreting arginase-1 (Arg-1), IL-10 and TGF-β. Exosomes derived from stem cells of diverse origins may alleviate synovitis and mitigate cartilage degeneration by initiating the transcription of functional genes associated with M2 macrophages, thereby promoting their polarization and inhibiting the infiltration of M1 macrophages into the synovium. Thus, targeting macrophage polarization may represent an effective strategy for modulating inflammatory processes to prevent and reduce the progression of OA. Despite these findings, there are still many uncertainties regarding the interaction between MSC-derived EVs and immune cells related to OA, which require further exploration (Table 4).
Table 4.
Inhibition of OA by immunomodulation.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| BM-MSC | Unknown | Regenerating the immune phenotype reduces the infiltration of M1 macrophages | [133] |
| Primary chondrocyte | Unknown | Restore mitochondrial dysfunction and polarize macrophage response toward an M2 phenotype | [134] |
| UC-MSC | miR-122–5p, miR-148a-3p, miR-486–5p, miR-let-7a-5p, miR-100–5p | The polarization of M2 macrophages is facilitated by the PI3K-Akt signaling pathway | [135] |
Inhibiting abnormal angiogenesis
Angiogenesis and adequate blood supply are crucial for bone formation, and OA has the potential to disrupt normal angiogenesis [136, 137]. Hypoxia in chondrocytes plays a key role in gene expression related to angiogenesis in cartilage models. Given that hypoxia promotes angiogenesis in various contexts, hypoxia, in the absence of mesenchymal condensation, is generally considered the primary regulatory factor for angiogenesis associated with intramembrane osteogenesis [138]. Inhibition of cartilage osteogenesis is also linked to a significant decrease in vascular invasion [139, 140]. Wang et al. conducted a study where they isolated BMSCs and their exosomes from mice. They discovered that modifying exosomes derived from an MSC source with TGF-β1 helps maintain the microstructure of the subchondral bone in OA mice, inhibits abnormal angiogenesis, and provides protection against OA-induced pain and bone loss [141]. Similarly, Zhao et al. found that hypoxia-treated exosomes derived from ADSCs (Hypo-ADSC-Exos) can normalize non-coupling bone remodeling and abnormal H-type angiogenesis in the subchondral bone, thereby improving the progression of lumbar facet joint osteoarthritis (LFJOA) [142]. Therefore, inhibiting abnormal angiogenesis in the cartilage membrane may be one of the strategies to relieve the development of OA (Table 5).
Table 5.
Inhibition of abnormal angiogenesis in OA.
Promoting cell migration and proliferation
During the early stages of OA, researchers initially targeted cartilage cells for therapeutic intervention due to increased cell proliferation, matrix protein synthesis, and the presence of proteases and cytokines [143]. Exosomes containing growth factors and ECM-related proteins play a crucial role in promoting chondrocyte proliferation and differentiation, as well as regulating collagen fibrosis and matrix synthesis. As OA progresses, chondrocyte migration and proliferation are hindered, but exosomes can still facilitate cell migration and proliferation [144].
Liu et al. discovered that lncRNA-KLF 3-AS 1, derived from exosomes of human MSCs (hMSCs), facilitates the proliferation of OA chondrocytes and the expression of cartilage-forming genes through the miR-206/GIT 1 axis. Furthermore, the expression of MMP13 and its upstream regulator, RUNX 2, is suppressed [145]. Additionally, in rat OA models, researchers observed that exosomal KLF 3-AS 1 from hMSCs promotes cartilage repair and chondrocyte proliferation, while inhibiting IL-1β can induce apoptosis of cartilage cells [146].
One study demonstrated that EVs secreted by BMSCs not only promote cartilage formation but also inhibit hypertrophic differentiation of cartilage cells. Other factors in the BMSC secretion group also aid in controlling the proliferation of OA chondrocytes [147]. Proliferation occurred more rapidly with increasing doses of EV, and when a certain dose was reached it was sufficient to induce chondrocyte migration [148]. Nguyen et al. discovered that TGF-β-sensitized umbilical cord MSCs (UC-MSCs) can enhance the effect of EVs on OA chondrocyte migration. Furthermore, the distribution of miRNA in EVs is an important factor that may influence chondrocyte proliferation and migration [149]. Additionally, some researchers have found that EVs produced by MSCs can regulate proteins involved in chondrocyte adhesion, migration, and proliferation [150, 151]. Shao et al. found that BMSC-derived exosomes pre-treated with parathyroid hormone enhance their therapeutic effect on repairing OA chondrocytes by inhibiting the expression of pro-inflammatory cytokines. Furthermore, they can promote the migration, proliferation, and formation of cartilage matrix in OA chondrocytes [152]. Thus, exosomes derived from stem cells may promote chondrocyte proliferation and differentiation through their secretion of growth factors, ECM-related proteins, and thus OA chondrocytes proliferation and cartilage formation, thereby relieving OA (Table 6).
Table 6.
Promoting cell migration and proliferation.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| HM-MSC | LncRNA-KLF 3-AS 1 | Chondrocyte proliferation is facilitated by the miR-206/GIT 1 axis | [145] |
| HM-MSC | LncRNA-KLF 3-AS 1 | Proliferation of chondrocytes was promoted in a rat OA model by KLF 3-AS 1 | [146] |
| BM-MSC | Unknown | Secreted fibroblast growth factor 1 can promote the proliferation of chondrocytes | [147] |
| UC-MSC | Unknown | TGF-β sensitization promoted cartilage cell migration | [149] |
| BM-MSC | Unknown | Enhancement of IL-1β induced proliferation and migration of OA chondrocytes. | [152] |
Improvement of OA joint pain symptoms
The symptoms of joint pain in OA are typically caused by damage to the cartilage [153, 154]. While the primary changes in OA occur in the articular cartilage, there are various structural changes observed in the cartilage, bone, and synovial tissue of OA joints [155, 156]. The bone and synovial membranes may stimulate neuronal sensitization, leading to painful sensations during normal activities [157]. Additionally, inflammatory mediators activate and sensitize nociceptors, which is the main cause of pain in OA. In the advanced stages of the disease, chronic pain resulting from bone friction can lead to mobility loss and psychological impairment [158, 159].
Exosomes play a role in the pain process of OA, and therapy with MSC exosomes can alleviate joint pain symptoms and improve joint function [160]. Calcitonin gene-related peptide (CGRP) is typically involved in transmitting nociceptive signals and sensitizing pain in the peripheral nerve and spinal cord. Neuronal injury in the dorsal root ganglia (DRG) is a significant cause of neuropathic pain and pain sensitization [161]. By controlling inflammation and improving cartilage function, it may be possible to relieve pain in OA patients. He et al. discovered that therapy with BM-MSC-Exos significantly reduced the increased expression of CGRP and iNOS in DRG tissue of OA rats, leading to a reduction in joint pain. Furthermore, exosomes decrease anabolic factors such as COL2A1 and ACAN, while increasing the breakdown metabolic factors such as MMP13 and ADAMTS5. These mechanisms promote the repair of cartilage matrix in the OA model, thereby alleviating pain [162].
Li et al. investigated the use of BMSC exosomes in treating LFJOA mice. They discovered that these exosomes could alleviate joint pain by eliminating abnormal CGRP-positive nerves and abnormal H-type angiogenesis in LFJ cartilage [163]. Similarly, Yang et al. demonstrated that UC-MSCs could relieve OA joint pain in rats by utilizing exosome LncRNA H19 to regulate advanced OA pain through the miRNA-29a-3p/FOS axis [164]. Furthermore, MSC-derived exosomes were found to restore damaged tissue in temporomandibular joint osteoarthritis (TMJOA) cartilage, effectively treating dysfunction and pain associated with TMJOA [165]. Zhang et al. also observed that MSC exosomes repaired TMJOA in rat models, resulting in pain suppression, degeneration reduction, inflammation reduction, enhanced matrix expression, and improved structure of the lower cartilage bone, ultimately aiding in joint restoration and regeneration [166]. Therefore, exosomes secreted by stem cells may relieve joint pain caused by OA by regulating neurons and nociceptors. However, the molecular mechanism of exosome involvement in pain has not yet been fully clarified and further research is needed to fully understand the value of exosomes (Table 7).
Table 7.
Mechanisms to reduce joint pain symptoms.
| Source of exosomes | Main target molecule or pathway | Effect | Reference |
|---|---|---|---|
| BM-MSC | Unknown | Regulating the upregulation of CGRP and iNOS in DRG tissue to reduce inflammatory pain and neuropathological pain in OA rats. | [162] |
| BM-MSC | Unknown | Elimination of abnormal CGRP-positive nerves and abnormal H-angiogenesis in LFJ subchondral bone to relieve pain. | [163] |
| UC-MSC | miRNA-29a-3p | Improve the pain and central sensitization of advanced OA through LncRNA H19/microRNA-29a-3p/FOS axis. | [164] |
Other mechanisms
In addition to the aforementioned mechanisms, studies have demonstrated that Wnt 3 is an upregulated molecule following acute cartilage injury, contributing to the long-term regeneration of cartilage [167]. Moreover, exosomes can deliver functional active molecules to joint tissue, providing long-lasting protection. The transforming growth factor TGF-β and BMP are critical regulatory factors in cartilage formation [168, 169]. BMP plays a significant role in protecting cartilage from inflammation or trauma by engaging various receptor combinations to activate distinct intracellular signaling pathways. Furthermore, the loss of BMP-related receptor function leads to diminished internal repair of damaged cartilage. Given that TGF-β is essential for cartilage homeostasis, targeting it may represent a viable treatment option. Studies have indicated that TGF-β3 and BMP-6 can induce pluripotent stem cells to enhance cartilage formation, potentially mitigating the progression of OA. Pre-activating MSCs with TGF-β enhances the therapeutic potential of exosomes in OA. By leveraging the benefits of stem cells, TGF-β3, and BMP-6, it is possible to maintain cartilage homeostasis and delay OA progression. Therefore, administering MSC-derived exosomes as combined vectors of TGF-β3 and BMP-6 may offer a novel approach to preventing OA progression [170]. Additionally, to enhance the targeting and functionality of stem cell-derived exosomes, engineering these exosomes for OA treatment is a promising strategy. The engineering preparation methods for exosomes primarily include parent cell pretreatment, drug carrier optimization, and surface modification. Genetic or phenotypic modifications of parent cells can enhance the exosome's function as a highly targeted drug carrier, thereby improving drug efficacy [171]. Furthermore, the pathophysiological changes in hypochondral bone represent a critical process in the progression of OA, with exosome-mediated hypochondral bone remodeling identified as one of the key therapeutic mechanisms. Some researchers have demonstrated that groups treated with BM-MSC-Exos exhibit higher epiphyseal and subchondral bone volume, along with reduced bone degradation. This effect may be linked to the ability of stem cell exosomes to induce the expression of mature joint cartilage cell markers, such as type II collagen and aggregated proteoglycans, while concurrently decreasing the expression of catabolic markers associated with decomposition metabolism (MMP-13, ADAMTS 5) and inflammation (iNOS) [172]. In another study, researchers utilized an exosome injection model derived from dental pulp stem cells in mouse knee joint OA. Their findings indicated that exosomes from dental pulp stem cells effectively improved abnormal hypochondrial reconstruction, inhibited the development of bone sclerosis and osteoderms, and reduced cartilage degradation and synovial inflammation [173].
Others treatments for OA
Traditional treatment of OA
OA is a prevalent degenerative joint disease, with traditional treatment options encompassing both conservative and surgical approaches. Physiotherapy and medication are common conservative treatment modalities that may be effective when applied in the early stages of the disease [174–177]. When conservative treatments prove ineffective, surgical intervention is typically considered. For many patients, surgical treatment can significantly alleviate knee pain, improve joint mobility, and enhance overall quality of life [178]. The most prevalent surgical modalities include minimally invasive arthroscopic surgery and joint replacement, with the choice of procedure tailored to the patient's specific condition [179]. Although arthroscopy is advancing rapidly and becoming increasingly common, some patients may require joint replacement surgery within 2 years following arthroscopic procedures [180, 181]. Furthermore, intraoperative malpractice can potentially damage articular cartilage, contributing to the early onset of OA [182]. Intra-articular injections of medications such as corticosteroids and anesthetics can effectively alleviate symptoms in patients with early OA, providing pain relief and restoring transient joint function; however, the efficacy and safety of long-term use remain uncertain. Additionally, intra-articular drug injections do not reverse the progression of OA by eliminating pathogenic factors or promoting cartilage regenerative repair [183, 184]. Consequently, there is an urgent need to identify and implement more direct and effective treatments to address the growing number of patients affected by OA.
Biotherapy of OA
The current state of OA research indicates a growing interest in regenerative medicine for treating cartilage regeneration in joints. This approach aims to remodel the joint structure and effectively treat OA [185]. Regenerative methods are designed to address the loss of cartilage matrix by stimulating cartilage formation in endogenous stem cells and matrix metabolism in cartilage cells. This restoration process aims to restore the normal structure and function of damaged joints [186]. In recent years, various biotherapies, such as cell therapy, gene therapy, and biomaterials, have emerged in preclinical or clinical trials. These biotherapies primarily focus on regulating the microenvironment and cellular activity within the joint [187, 188]. Additionally, current OA research emphasizes disease prevention and early treatment, making biologics increasingly attractive due to their ability to intervene in OA diseases and promote cartilage regeneration. Inflammation is now recognized as a key pathophysiological process in OA, and the use of biological agents to address the inflammatory response is a crucial aspect to consider [189].
Cell therapy
Cell therapy has emerged as a prominent topic in the medical field in recent years as an alternative treatment for tissue damage. By utilizing various types of cells, it aims to alleviate the pathogenesis of diseases [190]. This therapeutic approach holds great potential in regenerating lost cartilage, combating cartilage degradation, relieving pain, and improving patient mobility [191]. Among the different sources of cells for regenerative medicine, MSCs have shown promise. These cells can be derived from adipose tissue, bone marrow, synovial tissue, and other sources. Currently, the MSCs being studied include BM-MSCs, AD-MSCs, and UC-MSCs [192–195]. Another highly potent tissue engineering therapy for joint cartilage injury is induced pluripotent stem cells (iPSCs). These cells have the ability to differentiate into chondrocytes and can be reprogrammed from somatic cells, offering a wider range of sources and avoiding ethical concerns [196]. iPSCs have the potential to overcome limitations associated with current cellular sources, as they allow for the generation of large numbers of autocytes from small starting groups. Moreover, iPSCs show promise as a viable source of cells for treating cartilage defects and could be directly utilized in clinical applications [197]. Researchers have also observed self-renewal activity in iPSCs, enabling the production of homogeneous iPSC-derived cartilage that can be transplanted to an unlimited number of patients. This approach addresses issues related to allogeneic cartilage, such as donor scarcity, risk of disease transmission, and variations in cartilage quality among donors [198]. These cells have the ability to proliferate, differentiate, metabolize, and replenish lost cartilage cells, as well as the ECM, thereby facilitating the repair of damaged joint cartilage and restoration of joint function.
It is important to acknowledge the existing limitations in the current application of cell therapy for the treatment of OA [199]. One significant challenge is the unsustainable cellular and hyaline cartilage phenotype of differentiated chondrocytes [200]. Additionally, the onset of senescence in MSCs adversely impacts their differentiation potential, immunomodulatory capacity, and migratory ability. Even with cryopreservation, studies have shown a decline in viability, colony-forming units, and integrin expression following thawing [201, 202]. With the rapid increase in the number of stem cell studies, there are concerns regarding the safety of stem cell therapy. For example, stem cell injections can potentially lead to cell transformation or premature cell differentiation [203, 204]. Currently, stem cell therapy remains a topic of controversy due to the immune regulatory function of stem cells, which may contribute to the development of tumors [205]. Additionally, there are still unresolved issues related to the risks of infection transmission during cell transplantation, ethical concerns surrounding embryonic stem cells, limitations on the potential of adult stem cell differentiation, and changes in the characteristics of stem cells after in vitro culture [206, 207]. Consequently, the development of cell therapy for the treatment of OA will require significant time and effort.
Growth factor therapy
Growth factors are believed to have the potential to enhance the healing of cartilage injuries and modify the progression of degenerative OA [208]. These growth factors can stimulate the differentiation of MSCs to produce phenotypes that resemble normal joint cartilage and possess similar biomechanical properties [209]. Platelet-rich plasma (PRP) is an autologous concentrate derived from human platelets, containing a substantial amount of growth factors alongside elevated concentrations of platelets, leukocytes, and fibrin [210]. By directly injecting these growth factors into the affected joint cavity, it is possible to stimulate chondrocyte proliferation and ECM synthesis, potentially delaying or even reversing the progression of degenerative joint disease. Moreover, the growth factors and other bioactive molecules present in PRP may facilitate critical tissue healing and alleviate pain by modulating inflammation, inhibiting chondrocyte apoptosis, and promoting collagen synthesis. This has been shown to result in pain relief and functional improvement in patients with mild-to-moderate knee OA [211–214]. Park et al. evaluated the efficacy of intra-articular PRP injections in comparison to hyaluronic acid (HA) injections for the treatment of OA. They observed that patients receiving PRP exhibited high concentrations of growth factors, suggesting that these concentrations could serve as important indicators in future studies investigating the role of PRP in OA treatment [215].
Gene therapy
Gene therapy corrects or treats diseases caused by abnormal genes by introducing exogenous genes into target cells. It also provides new insights for the treatment of OA [216]. Advances in OA genetics, genomics, and epigenetics have enhanced our understanding of the complexity of the disease and guided the evaluation of genetic/epigenetic findings for translation and clinical application [217]. In recent years, genome-wide association studies have identified numerous novel genetic risk loci for OA. Epigenetic traits and mediators serve as a mechanistic link between genetic risk factors for OA and the onset or progression of the disease. Furthermore, since epigenetic traits are relatively easy to modulate, they present potential therapeutic targets [218, 219]. Some researchers have demonstrated significant differences between different OA patient populations, for example based on disease severity, affected joint sites, and gender, and have highlighted attractive drug targets [220].
Attur et al. conducted a study on the relationship between the single nucleotide polymorphism of the interleukin-1 receptor antagonist (IL-1RN) gene and the radiological severity of symptomatic OA, as well as the risk of developing OA. The study revealed that IL-1RN gene variation can predict the radiological severity and risk of OA in the knee [221]. In recent times, the application of OA genetics in large-scale genome-wide association scans has made significant progress, identifying >100 polymorphic DNA variants associated with OA. These genetic risk variants account for >20% of OA heritability and are primarily located in non-protein coding regions of the genome, suggesting that they function by regulating the expression of the target gene. Although the data from OA genetics studies have not directly led to new treatments, it is worth noting that some OA-related genes encode proteins for which treatments are already available [222]. Further research in osteogenetics has the potential to provide valuable insights into the pathogenesis of OA, and by identifying common molecular mechanisms, genetic understanding may help uncover causal pathways [223]. Consequently, a deeper understanding of the molecular basis of OA subtypes will enhance our knowledge of the molecular processes involved in the development of OA and contribute to improved patient diagnosis, management, and treatment.
Conclusion and perspectives
The pathology of OA is complex and can be influenced by a variety of factors such as environmental, genetic, metabolic, or mechanical injury. Exosomes are involved in the physiological and pathological processes of OA by regulating intercellular communication and show strong potential for application in the treatment of OA.
Despite all these promising preclinical results, there are currently several issues that hinder the practical application of exosomes as regenerative therapies in clinical OA treatment. Current studies on stem cell-derived exosomes as therapeutic agents for OA are primarily conducted using in vitro cellular models or in vivo models involving small animals. Their efficacy has been evaluated in fewer studies involving large animals, which may not yield significant clinical effects. Furthermore, the optimal dose and frequency of exosomes required for clinical application remain inadequately defined. Many studies have utilized higher doses or more frequent injections to demonstrate significant improvement in animal models of OA; however, the translation of these findings to human treatment requires further investigation. There is a notable lack of standardization in the methodologies employed across studies regarding the classification of exosomes, isolation techniques, characterization, stem cell sources and growth conditions, selection of OA models, outcome measurement types, and study durations. Consequently, results from different studies often lack reproducibility. Additionally, exosomes used in clinical trials must adhere to good manufacturing practices. Therefore, the development of pharmaceutical products with stable quality, clear and enhanced efficacy, and scalable production of stem cell-derived exosomes, as well as the enhancement of their druggability, are critical clinical translational issues that warrant emphasis and further exploration. Given that the biological effects of exosomes are mediated through their uptake by target cells, it is essential to clarify and control the biodistribution of exosomes for effective clinical therapeutic applications.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (Grant No. 82302758), China Postdoctoral Science Foundation (Grant No. 2023M731419), the Jiangsu Funding Program for Excellent Postdoctoral Talent (Grant No. 2022ZB896), and the Yangzhou Key Laboratory of Orthopedics (Grant No. YZ2023249). We have obtained the relevant permissions for BioRender (www.biorender.com) and have used the correct permission text as required by the copyright holders.
Contributor Information
Xiaofei Wang, The Graduate School, Dalian Medical University, Dalian 116044, China; Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Lei Xu, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Zhimin Wu, The Graduate School, Dalian Medical University, Dalian 116044, China; Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Linbing Lou, The Graduate School, Dalian Medical University, Dalian 116044, China; Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Cunyi Xia, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Haixiang Miao, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Jihang Dai, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Wenyong Fei, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Jingcheng Wang, Department of Orthopedics, Northern Jiangsu People's Hospital Affiliated to Yangzhou University, Yangzhou 225001, China.
Author contributions
Xiaofei Wang (Writing—original draft), Lei Xu (Data curation), Zhimin Wu (Data curation), Linbing Lou (Data curation), Cunyi Xia (Data curation), Haixiang Miao (Data curation), Jihang Dai (Writing—original draft, Writing—review & editing), Wenyong Fei (Conceptualization, Investigation) and Jingcheng Wang (Conceptualization, Resources, Supervision).
Conflict of interest
None declared.
References
- 1. Hunter DJ, Bierma-Zeinstra S Osteoarthritis. The Lancet. 2019;393:1745–59. 10.1016/S0140-6736(19)30417-9. [DOI] [PubMed] [Google Scholar]
- 2. Yue L, Berman J What is osteoarthritis?. JAMA. 2022;327:1300. 10.1001/jama.2022.1980. [DOI] [PubMed] [Google Scholar]
- 3. Martel-Pelletier J, Barr AJ, Cicuttini FM et al. Osteoarthritis. Nat Rev Dis Primers. 2016;2:16072. 10.1038/nrdp.2016.72. [DOI] [PubMed] [Google Scholar]
- 4. Lane N, Felson D A promising treatment for osteoarthritis?. Ann Intern Med. 2020;173:580–1. 10.7326/M20-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhao X, Shah D, Gandhi K et al. Clinical, humanistic, and economic burden of osteoarthritis among noninstitutionalized adults in the United States. Osteoarthritis Cartilage. 2019;27:1618–26. 10.1016/j.joca.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 6. Largo R, Herrero-Beaumont G Joint obesity as a pathogenic factor in osteoarthritis. Osteoarthritis Cartilage. 2021;29:1239–41. 10.1016/j.joca.2021.05.062. [DOI] [PubMed] [Google Scholar]
- 7. Wang XF, Ma ZH, Teng XR Isokinetic strength test of muscle strength and motor function in total knee arthroplasty. Orthopaedic Surgery. 2020;12:878–89. 10.1111/os.12699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kloppenburg M Inflammation is a relevant treatment target in osteoarthritis. The Lancet. 2023;402:1725–6. 10.1016/S0140-6736(23)01726-9. [DOI] [PubMed] [Google Scholar]
- 9. Hodgkinson T, Kelly DC, Curtin CM et al. Mechanosignalling in cartilage: an emerging target for the treatment of osteoarthritis. Nat Rev Rheumatol. 2022;18:67–84. 10.1038/s41584-021-00724-w. [DOI] [PubMed] [Google Scholar]
- 10. Wei Y, Luo L, Gui T et al. Targeting cartilage EGFR pathway for osteoarthritis treatment. Sci Transl Med. 2021;13:eabb3946. 10.1126/scitranslmed.abb3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Martin R, Wang G, Brandão BB et al. MicroRNA sequence codes for small extracellular vesicle release and cellular retention. Nature. 2022;601:446–51. 10.1038/s41586-021-04234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalluri R, LeBleu VS The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng R, Zhang K, Tan S et al. Exosomal circLPAR1 functions in colorectal cancer diagnosis and tumorigenesis through suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer. 2022;21:49. 10.1186/s12943-021-01471-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao Y, Wang Z, Yan Y et al. Enterotoxigenic bacteroidesfragilis promotes intestinal inflammation and malignancy by inhibiting exosome-packaged miR-149-3p. Gastroenterology. 2021;161:1552–66. 10.1053/j.gastro.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 15. Sahoo S, Adamiak M, Mathiyalagan P et al. Therapeutic and diagnostic translation of extracellular vesicles in cardiovascular diseases: roadmap to the clinic. Circulation. 2021;143:1426–49. 10.1161/CIRCULATIONAHA.120.049254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin S, Wang Y, Wu X et al. Young exosome bio-nanoparticles restore aging-impaired tendon stem/progenitor cell function and reparative capacity. Adv Mater. 2023;35:e2211602. 10.1002/adma.202211602. [DOI] [PubMed] [Google Scholar]
- 17. Jeppesen DK, Fenix AM, Franklin JL et al. Reassessment of exosome composition. Cell. 2019;177:428–45. 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy C, Withrow J, Hunter M et al. Emerging role of extracellular vesicles in musculoskeletal diseases. Mol Aspects Med. 2018;60:123–8. 10.1016/j.mam.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wu Y, Li J, Zeng Y et al. Exosomes rewire the cartilage microenvironment in osteoarthritis: from intercellular communication to therapeutic strategies. Int J Oral Sci. 2022;14:40. 10.1038/s41368-022-00187-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ni Z, Zhou S, Li S et al. Exosomes: roles and therapeutic potential in osteoarthritis. Bone Res. 2020;8:25. 10.1038/s41413-020-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhou QF, Cai YZ, Lin XJ The dual character of exosomes in osteoarthritis: antagonists and therapeutic agents. Acta Biomater. 2020;105:15–25. 10.1016/j.actbio.2020.01.040. [DOI] [PubMed] [Google Scholar]
- 22. Loeser RF, Collins JA, Diekman BO Ageing and the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2016;12:412–20. 10.1038/nrrheum.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berenbaum F, Wallace IJ, Lieberman DE et al. Modern-day environmental factors in the pathogenesis of osteoarthritis. Nat Rev Rheumatol. 2018;14:674–81. 10.1038/s41584-018-0073-x. [DOI] [PubMed] [Google Scholar]
- 24. Rezuş E, Burlui A, Cardoneanu A et al. From pathogenesis to therapy in knee osteoarthritis: bench-to-bedside. Int J Mol Sci. 2021;22:2697. 10.3390/ijms22052697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rahmati M, Nalesso G, Mobasheri A et al. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res Rev. 2017;40:20–30. 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 26. Luo Y, Sinkeviciute D, He Y et al. The minor collagens in articular cartilage. Protein Cell. 2017;8:560–72. 10.1007/s13238-017-0377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang CH, Gao Y, Hung HH et al. Creb5 coordinates synovial joint formation with the genesis of articular cartilage. Nat Commun. 2022;13:7295. 10.1038/s41467-022-35010-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen D, Shen J, Zhao W et al. Osteoarthritis: toward a comprehensive understanding of pathological mechanism. Bone Res. 2017;5:16044. 10.1038/boneres.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Del Sordo L, Blackler GB, Philpott HT et al. Impaired efferocytosis by synovial macrophages in patients with knee osteoarthritis. Arthritis & Rheumatology. 2023;75:685–96. 10.1002/art.42412. [DOI] [PubMed] [Google Scholar]
- 30. Sanchez-Lopez E, Coras R, Torres A et al. Synovial inflammation in osteoarthritis progression. Nat Rev Rheumatol. 2022;18:258–75. 10.1038/s41584-022-00749-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asghar S, Litherland GJ, Lockhart JC et al. Exosomes in intercellular communication and implications for osteoarthritis. Rheumatology (Oxford). 2020;59:57–68. 10.1093/rheumatology/kez462. [DOI] [PubMed] [Google Scholar]
- 32. Sohn DH, Sokolove J, Sharpe O et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lieberthal J, Sambamurthy N, Scanzello CR Inflammation in joint injury and post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1825–34. 10.1016/j.joca.2015.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Loeser RF, Goldring SR, Scanzello CR et al. Osteoarthritis: a disease of the joint as an organ. Arthritis & Rheumatism. 2012;64:1697–707. 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katz JN, Arant KR, Loeser RF Diagnosis and treatment of hip and knee osteoarthritis: A review. JAMA. 2021;325:568–78. 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li Y, Huang P, Nasser MI et al. Role of exosomes in bone and joint disease metabolism, diagnosis, and therapy. Eur J Pharm Sci. 2022;176:106262. 10.1016/j.ejps.2022.106262. [DOI] [PubMed] [Google Scholar]
- 37. Wu C, He Y, Yao Y et al. Exosomes treating osteoarthritis: hope with challenge. Heliyon. 2023;9:e13152. 10.1016/j.heliyon.2023.e13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhou Q, Cai Y, Jiang Y et al. Exosomes in osteoarthritis and cartilage injury: advanced development and potential therapeutic strategies. Int. J. Biol. Sci. 2020;16:1811–20. 10.7150/ijbs.41637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mathieu M, Martin-Jaular L, Lavieu G et al. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 40. CHARGAFF E, WEST R The biological significance of the thromboplastic protein of blood. J Biol Chem. 1946;166:189–97. [PubMed] [Google Scholar]
- 41. Couch Y, Buzàs EI, Di Vizio D et al. A brief history of nearly EV-erything—the rise and rise of extracellular vesicles. J Extracellular Vesicle. 2021;10:e12144. 10.1002/jev2.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fox AS, Duggleby WF, Gelbart WM, et al. DNA-induced transformation in Drosophila: evidence for transmission without integration. Proc Natl Acad Sci USA. 1970;67:1834–8. 10.1073/pnas.67.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fox AS, Yoon SB DNA-induced transformation in Drosophila: locus-specificity and the establishment of transformed stocks. Proc Natl Acad Sci USA. 1970;67:1608–15. 10.1073/pnas.67.3.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fox AS, Yoon SB, Gelbart WM DNA-induced transformation in Drosophila: genetic analysis of transformed stocks. Proc Natl Acad Sci USA. 1971;68:342–6. 10.1073/pnas.68.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mishra NC, Tatum EL Non-Mendelian inheritance of DNA-induced inositol independence in Neurospora. Proc Natl Acad Sci USA. 1973;70:3875–9. 10.1073/pnas.70.12.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Witwer KW, Théry C Extracellular vesicles or exosomes? On primacy, precision, and popularity influencing a choice of nomenclature. J of Extracellular Vesicle. 2019;8:1648167. 10.1080/20013078.2019.1648167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Johnstone RM, Adam M, Hammond JR et al. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J Biol Chem. 1987;262:9412–20. 10.1016/S0021-9258(18)48095-7. [DOI] [PubMed] [Google Scholar]
- 48. Johnstone RM, Bianchini A, Teng K Reticulocyte maturation and exosome release: transferrin receptor containing exosomes shows multiple plasma membrane functions. Blood. 1989;74:1844–51. 10.1182/blood.V74.5.1844.1844. [DOI] [PubMed] [Google Scholar]
- 49. Raposo G, Nijman HW, Stoorvogel W et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–72. 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Liu Y, Wang Y, Lv Q et al. Exosomes: from garbage bins to translational medicine. Int J Pharm. 2020;583:119333. 10.1016/j.ijpharm.2020.119333. [DOI] [PubMed] [Google Scholar]
- 51. Nikfarjam S, Rezaie J, Zolbanin NM et al. Mesenchymal stem cell derived-exosomes: a modern approach in translational medicine. J Transl Med. 2020;18:449. 10.1186/s12967-020-02622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wu R, Li H, Sun C et al. Exosome-based strategy for degenerative disease in orthopedics: recent progress and perspectives. Journal of Orthopaedic Translation. 2022;36:8–17. 10.1016/j.jot.2022.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Théry C, Witwer KW, Aikawa E et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J of Extracellular Vesicle. 2018;7:1535750. 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pluchino S, Smith JA Explicating exosomes: reclassifying the rising stars of intercellular communication. Cell. 2019;177:225–7. 10.1016/j.cell.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 55. Ostrowski M, Carmo NB, Krumeich S et al. Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat Cell Biol. 2010;12:19–30. 10.1038/ncb2000. [DOI] [PubMed] [Google Scholar]
- 56. Withrow J, Murphy C, Liu Y et al. Extracellular vesicles in the pathogenesis of rheumatoid arthritis and osteoarthritis. Arthritis Res Ther. 2016;18:286. 10.1186/s13075-016-1178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bjørge IM, Kim SY, Mano JF et al. Extracellular vesicles, exosomes and shedding vesicles in regenerative medicine—a new paradigm for tissue repair. Biomater. Sci. 2017;6:60–78. 10.1039/C7BM00479F. [DOI] [PubMed] [Google Scholar]
- 58. Brown L, Wolf JM, Prados-Rosales R et al. Through the wall: extracellular vesicles in gram-positive bacteria, mycobacteria and fungi. Nat Rev Micro. 2015;13:620–30. 10.1038/nrmicro3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. An Q, van Bel AJ, Hückelhoven R Do plant cells secrete exosomes derived from multivesicular bodies?. Plant Signal Behav. 2007;2:4–7. 10.4161/psb.2.1.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Man F, Wang J, Lu R Techniques and applications of animal- and plant-derived exosome-based drug delivery system. J Biomed Nanotechnol. 2020;16:1543–69. 10.1166/jbn.2020.2993. [DOI] [PubMed] [Google Scholar]
- 61. Colombo M, Raposo G, Théry C Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 62. Yang XX, Sun C, Wang L et al. New insight into isolation, identification techniques and medical applications of exosomes. J Controlled Release. 2019;308:119–29. 10.1016/j.jconrel.2019.07.021. [DOI] [PubMed] [Google Scholar]
- 63. Yang D, Zhang W, Zhang H et al. Progress, opportunity, and perspective on exosome isolation—efforts for efficient exosome-based theranostics. Theranostics. 2020;10:3684–707. 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Cao F, Gao Y, Chu Q et al. Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis. 2019;40:3092–8. 10.1002/elps.201900295. [DOI] [PubMed] [Google Scholar]
- 65. Sidhom K, Obi PO, Saleem A A review of exosomal isolation methods: is size exclusion chromatography the best option?. Int J Mol Sci. 2020;21:6466. 10.3390/ijms21186466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tran PHL, Wang T, Yin W et al. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int J Pharm. 2019;566:697–707. 10.1016/j.ijpharm.2019.06.028. [DOI] [PubMed] [Google Scholar]
- 67. Muller L, Hong CS, Stolz DB et al. Isolation of biologically-active exosomes from human plasma. J Immunol Methods. 2014;411:55–65. 10.1016/j.jim.2014.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mi B, Chen L, Xiong Y et al. Saliva exosomes-derived UBE2O mRNA promotes angiogenesis in cutaneous wounds by targeting SMAD6. J Nanobiotechnol. 2020;18:68. 10.1186/s12951-020-00624-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hiemstra TF, Charles PD, Gracia T et al. Human urinary exosomes as innate immune effectors. J Am Soc Nephrol. 2014;25:2017–27. 10.1681/ASN.2013101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu H, Li M, Pan Z et al. miR-3184-3p enriched in cerebrospinal fluid exosomes contributes to progression of glioma and promotes M2-like macrophage polarization. Cancer Sci. 2022;113:2668–80. 10.1111/cas.15372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Xu WM, Li A, Chen JJ et al. Research development on exosome separation technology. J Membrane Biol. 2023;256:25–34. 10.1007/s00232-022-00260-y. [DOI] [PubMed] [Google Scholar]
- 72. Wu Y, Deng W, Klinke DJ 2nd Exosomes: improved methods to characterize their morphology, RNA content, and surface protein biomarkers. Analyst. 2015;140:6631–42. 10.1039/C5AN00688K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Dragovic RA, Gardiner C, Brooks AS et al. Sizing and phenotyping of cellular vesicles using nanoparticle tracking Analysis. Nanomed Nanotechnol Biol Med. 2011;7:780–8. 10.1016/j.nano.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Witwer KW, Buzás EI, Bemis LT et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracellular Vesicle. 2013;2: 20360. 10.3402/jev.v2i0.20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gercel-Taylor C, Atay S, Tullis RH et al. Nanoparticle analysis of circulating cell-derived vesicles in ovarian cancer patients. Anal Biochem. 2012;428:44–53. 10.1016/j.ab.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 76. Qazi REM, Sajid Z, Zhao C et al. Lyophilization based isolation of exosomes. Int J Mol Sci. 2023;24:10477. 10.3390/ijms241310477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen J, Chen J, Cheng Y et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020;11:97. 10.1186/s13287-020-01610-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gurunathan S, Kang MH, Jeyaraj M et al. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8:307. 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kloppenburg M, Berenbaum F Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthritis Cartilage, 2020;28:242–8. 10.1016/j.joca.2020.01.002. [DOI] [PubMed] [Google Scholar]
- 80. Mapp PI, Walsh DA Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8:390–8. 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 81. Wei G, Lu K, Umar M et al. Risk of metabolic abnormalities in osteoarthritis: a new perspective to understand its pathological mechanisms. Bone Res. 2023;11:63. 10.1038/s41413-023-00301-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Sandhu A, Rockel JS, Lively S et al. Emerging molecular biomarkers in osteoarthritis pathology. Therapeutic Advances in Musculoskeletal. 2023;15:1759720X231177116. 10.1177/1759720X231177116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Fan WJ, Liu D, Pan LY et al. Exosomes in osteoarthritis: updated insights on pathogenesis, diagnosis, and treatment. Front Cell Dev Biol. 2022;10:949690. 10.3389/fcell.2022.949690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. de Lange-Brokaar BJ, Ioan-Facsinay A, van Osch GJ et al. Synovial inflammation, immune cells and their cytokines in osteoarthritis: a review. Osteoarthritis Cartilage. 2012;20:1484–99. 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 85. Han D, Fang Y, Tan X et al. The emerging role of fibroblast-like synoviocytes-mediated synovitis in osteoarthritis: an update. J Cellular Molecular Medi. 2020;24:9518–32. 10.1111/jcmm.15669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Griffin TM, Scanzello CR Innate inflammation and synovial macrophages in osteoarthritis pathophysiology. Clin Exp Rheumatol. 2019;37((Suppl 120):):57–63. [PMC free article] [PubMed] [Google Scholar]
- 87. Lepetsos P, Papavassiliou KA, Papavassiliou AG Redox and NF-κb signaling in osteoarthritis. Free Radical Biol Med. 2019;132:90–100. 10.1016/j.freeradbiomed.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 88. Brandl A, Hartmann A, Bechmann V et al. Oxidative stress induces senescence in chondrocytes. Journal Orthopaedic Research. 2011;29:1114–20. 10.1002/jor.21348. [DOI] [PubMed] [Google Scholar]
- 89. Yu H, Ye WB, Zhong ZM et al. Effect of advanced oxidation protein products on articular cartilage and synovium in a rabbit osteoarthritis model. Orthopaedic Surgery. 2015;7:161–7. 10.1111/os.12179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kapoor M, Martel-Pelletier J, Lajeunesse D et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 91. Domenis R, Zanutel R, Caponnetto F et al. Characterization of the proinflammatory profile of synovial fluid-derived exosomes of patients with osteoarthritis. Mediators Inflamm. 2017;2017:1. 10.1155/2017/4814987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kim M, Shin DI, Choi BH et al. Exosomes from IL-1β-primed mesenchymal stem cells inhibited IL-1β- and TNF-α-mediated inflammatory responses in osteoarthritic SW982 cells. Tissue Eng Regen Med. 2021;18:525–36. 10.1007/s13770-020-00324-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Qiu M, Liu D, Fu Q MiR-129-5p shuttled by human synovial mesenchymal stem cell-derived exosomes relieves IL-1β induced osteoarthritis via targeting HMGB1. Life Sci. 2021;269:118987. 10.1016/j.lfs.2020.118987. [DOI] [PubMed] [Google Scholar]
- 94. Chang LH, Wu SC, Chen CH et al. Exosomes derived from hypoxia-cultured Human adipose stem cells alleviate articular chondrocyte inflammaging and post-traumatic osteoarthritis progression. Int J Mol Sci. 2023;24:13414. 10.3390/ijms241713414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Jin Z, Ren J, Qi S Exosomal miR-9-5p secreted by bone marrow-derived mesenchymal stem cells alleviates osteoarthritis by inhibiting syndecan-1. Cell Tissue Res. 2020;381:99–114. 10.1007/s00441-020-03193-x. [DOI] [PubMed] [Google Scholar]
- 96. Zhou Y, Ming J, Li Y et al. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021;7:37. 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lai CC, Liao B, Peng S et al. Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol Cell Biochem. 2023;478:637–49. 10.1007/s11010-022-04535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Zheng T, Li Y, Zhang X et al. Exosomes derived from miR-212-5p overexpressed Human synovial mesenchymal stem cells suppress chondrocyte degeneration and inflammation by targeting ELF3. Front Bioeng Biotechnol. 2022;10:816209. 10.3389/fbioe.2022.816209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Theocharis AD, Skandalis SS, Gialeli C et al. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 100. Peng Z, Sun H, Bunpetch V et al. The regulation of cartilage extracellular matrix homeostasis in joint cartilage degeneration and regeneration. Biomaterials. 2021;268:120555. 10.1016/j.biomaterials.2020.120555. [DOI] [PubMed] [Google Scholar]
- 101. Huang S, Liu Y, Wang C et al. Strategies for cartilage repair in osteoarthritis based on diverse mesenchymal stem cells-derived extracellular vesicles. Orthopaedic Surgery. 2023;15:2749–65. 10.1111/os.13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Xia Q, Wang Q, Lin F et al. miR-125a-5p-abundant exosomes derived from mesenchymal stem cells suppress chondrocyte degeneration via targeting E2F2 in traumatic osteoarthritis. Bioengineered. 2021;12:11225–38. 10.1080/21655979.2021.1995580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang Y, Yu D, Liu Z et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res Ther. 2017;8:189. 10.1186/s13287-017-0632-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wang Z, Yan K, Ge G et al. Exosomes derived from miR-155-5p-overexpressing synovial mesenchymal stem cells prevent osteoarthritis via enhancing proliferation and migration, attenuating apoptosis, and modulating extracellular matrix secretion in chondrocytes. Cell Biol Toxicol. 2021;37:85–96. 10.1007/s10565-020-09559-9. [DOI] [PubMed] [Google Scholar]
- 105. Tao SC, Yuan T, Zhang YL et al. Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 2017;7:180–95. 10.7150/thno.17133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Yu E, Zhang M, Xu G et al. Consensus cluster analysis of apoptosis-related genes in patients with osteoarthritis and their correlation with immune cell infiltration. Front Immunol. 2023;14:1202758. 10.3389/fimmu.2023.1202758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Wang Y, Fan A, Lu L et al. Exosome modification to better alleviates endoplasmic reticulum stress induced chondrocyte apoptosis and osteoarthritis. Biochem Pharmacol. 2022;206:115343. 10.1016/j.bcp.2022.115343. [DOI] [PubMed] [Google Scholar]
- 108. Xu C, Zhai Z, Ying H et al. Curcumin primed ADMSCs derived small extracellular vesicle exert enhanced protective effects on osteoarthritis by inhibiting oxidative stress and chondrocyte apoptosis. J Nanobiotechnol. 2022;20:123. 10.1186/s12951-022-01339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Mariño G, Niso-Santano M, Baehrecke EH et al. Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol. 2014;15:81–94. 10.1038/nrm3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Wang Y, He SH, Liang X et al. ATF4-modified serum exosomes derived from osteoarthritic mice inhibit osteoarthritis by inducing autophagy. IUBMB Life. 2021;73:146–58. 10.1002/iub.2414. [DOI] [PubMed] [Google Scholar]
- 111. Wu J, Kuang L, Chen C et al. miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 2019;206:87–100. 10.1016/j.biomaterials.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 112. An S, Hu H, Li Y et al. Pyroptosis plays a role in osteoarthritis. Aging and disease. 2020;11:1146–57. 10.14336/AD.2019.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhao LR, Xing RL, Wang PM et al. NLRP1 and NLRP3 inflammasomes mediate LPS/ATPinduced pyroptosis in knee osteoarthritis. Mol Med Rep. 2018;17:5463–9. 10.3892/mmr.2018.8520. [DOI] [PubMed] [Google Scholar]
- 114. Zu Y, Mu Y, Li Q et al. Icariin alleviates osteoarthritis by inhibiting NLRP3-mediated pyroptosis. J Orthop Surg Res. 2019;14:307. 10.1186/s13018-019-1307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xu H, Xu B BMSC-derived exosomes ameliorate osteoarthritis by inhibiting pyroptosis of cartilage via delivering miR-326 targeting HDAC3 and STAT1//NF-κb p65 to chondrocytes. Mediators Inflamm. 2021;2021:1. 10.1155/2021/9972805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Seibt TM, Proneth B, Conrad M Role of GPX4 in ferroptosis and its pharmacological implication. Free Radical Biol Med. 2019;133:144–52. 10.1016/j.freeradbiomed.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 117. Yao X, Sun K, Yu S et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. Journal of Orthopaedic Translation. 2021;27:33–43. 10.1016/j.jot.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Zhang S, Xu J, Si H et al. The role played by ferroptosis in osteoarthritis: evidence based on iron dyshomeostasis and lipid peroxidation. Antioxidants. 2022;11:1668. 10.3390/antiox11091668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Miao Y, Chen Y, Xue F et al. Contribution of ferroptosis and GPX4’s dual functions to osteoarthritis progression. EBioMedicine. 2022;76:103847. 10.1016/j.ebiom.2022.103847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Kong R, Ji L, Pang Y et al. Exosomes from osteoarthritic fibroblast-like synoviocytes promote cartilage ferroptosis and damage via delivering microRNA-19b-3p to target SLC7A11 in osteoarthritis. Front Immunol. 2023;14:1181156. 10.3389/fimmu.2023.1181156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Cheng S, Xu X, Wang R et al. Chondroprotective effects of bone marrow mesenchymal stem cell-derived exosomes in osteoarthritis. J Bioenerg Biomembr. 2024;56:31–44. 10.1007/s10863-023-09991-6. [DOI] [PubMed] [Google Scholar]
- 122. Haseeb A, Haqqi TM Immunopathogenesis of osteoarthritis. Clin Immunol. 2013;146:185–96. 10.1016/j.clim.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Smith MD, Triantafillou S, Parker A et al. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J Rheumatol. 1997;24:365–71. [PubMed] [Google Scholar]
- 124. Ishii H, Tanaka H, Katoh K et al. Characterization of infiltrating T cells and Th1/Th2-type cytokines in the synovium of patients with osteoarthritis. Osteoarthritis Cartilage. 2002;10:277–81. 10.1053/joca.2001.0509. [DOI] [PubMed] [Google Scholar]
- 125. Zhao X, Zhao Y, Sun X et al. Immunomodulation of MSCs and MSC-derived extracellular vesicles in osteoarthritis. Front Bioeng Biotechnol. 2020;8:575057. 10.3389/fbioe.2020.575057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Revell PA, Mayston V, Lalor P et al. The synovial membrane in osteoarthritis: a histological study including the characterisation of the cellular infiltrate present in inflammatory osteoarthritis using monoclonal antibodies. Ann Rheum Dis. 1988;47:300–7. 10.1136/ard.47.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shen PC, Wu CL, Jou IM et al. T helper cells promote disease progression of osteoarthritis by inducing macrophage inflammatory protein-1γ. Osteoarthritis Cartilage. 2011;19:728–36. 10.1016/j.joca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 128. Haringman JJ, Smeets TJ, Reinders-Blankert P et al. Chemokine and chemokine receptor expression in paired peripheral blood mononuclear cells and synovial tissue of patients with rheumatoid arthritis, osteoarthritis, and reactive arthritis. Ann Rheum Dis. 2006;65:294–300. 10.1136/ard.2005.037176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Nakamura H, Tanaka M, Masuko-Hongo K et al. Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol Int. 2006;26:984–90. 10.1007/s00296-006-0116-5. [DOI] [PubMed] [Google Scholar]
- 130. Nedunchezhiyan U, Varughese I, Sun AR et al. Obesity, inflammation, and immune system in osteoarthritis. Front Immunol. 2022;13:907750. 10.3389/fimmu.2022.907750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Ragni E, Perucca Orfei C, de Girolamo L Secreted factors and extracellular vesicles account for the immunomodulatory and tissue regenerative properties of bone-marrow-derived mesenchymal stromal cells for osteoarthritis. Cells. 2022;11:3501. 10.3390/cells11213501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yin B, Ni J, Witherel CE et al. Harnessing tissue-derived extracellular vesicles for osteoarthritis theranostics. Theranostics. 2022;12:207–31. 10.7150/thno.62708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Zhang S, Chuah SJ, Lai RC et al. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials. 2018;156:16–27. 10.1016/j.biomaterials.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 134. Zheng L, Wang Y, Qiu P et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine (Lond.). 2019;14:3193–212. 10.2217/nnm-2018-0498. [DOI] [PubMed] [Google Scholar]
- 135. Li K, Yan G, Huang H et al. Anti-inflammatory and immunomodulatory effects of the extracellular vesicles derived from human umbilical cord mesenchymal stem cells on osteoarthritis via M2 macrophages. J Nanobiotechnol. 2022;20:38. 10.1186/s12951-021-01236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Goldring SR Alterations in periarticular bone and cross talk between subchondral bone and articular cartilage in osteoarthritis. Therapeutic Advances in Musculoskeletal. 2012;4:249–58. 10.1177/1759720X12437353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Portal-Núñez S, Lozano D, Esbrit P Role of angiogenesis on bone formation. Histol Histopathol. 2012;27:559–66. 10.14670/HH-27.559. [DOI] [PubMed] [Google Scholar]
- 138. Percival CJ, Richtsmeier JT Angiogenesis and intramembranous osteogenesis. Dev Dyn. 2013;242:909–22. 10.1002/dvdy.23992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Komori T, Yagi H, Nomura S et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–64. 10.1016/S0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 140. Colnot C, de la Fuente L, Huang S et al. Indian hedgehog synchronizes skeletal angiogenesis and perichondrial maturation with cartilage development. Development. 2005;132:1057–67. 10.1242/dev.01649. [DOI] [PubMed] [Google Scholar]
- 141. Wang R, Xu B TGFβ1-modified MSC-derived exosome attenuates osteoarthritis by inhibiting PDGF-BB secretion and H-type vessel activity in the subchondral bone. Acta Histochem. 2022;124:151933. 10.1016/j.acthis.2022.151933. [DOI] [PubMed] [Google Scholar]
- 142. Zhao J, Sun Y, Sheng X et al. Hypoxia-treated adipose mesenchymal stem cell-derived exosomes attenuate lumbar facet joint osteoarthritis. Mol Med. 2023;29:120. 10.1186/s10020-023-00709-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Goldring MB Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Practice & Research Clinical Rheumatology. 2006;20:1003–25. 10.1016/j.berh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 144. Riazifar M, Pone EJ, Lötvall J et al. Stem cell extracellular vesicles: extended messages of regeneration. Annu Rev Pharmacol Toxicol. 2017;57:125–54. 10.1146/annurev-pharmtox-061616-030146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Liu Y, Lin L, Zou R et al. MSC-derived exosomes promote proliferation and inhibit apoptosis of chondrocytes via lncRNA-KLF3-AS1/miR-206/GIT1 axis in osteoarthritis. Cell Cycle. 2018;17:2411–22. 10.1080/15384101.2018.1526603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Liu Y, Zou R, Wang Z et al. Exosomal KLF3-AS1 from hMSCs promoted cartilage repair and chondrocyte proliferation in osteoarthritis. Biochem J. 2018;475:3629–38. 10.1042/BCJ20180675. [DOI] [PubMed] [Google Scholar]
- 147. Vonk LA, van Dooremalen SFJ, Liv N et al. Mesenchymal stromal/stem cell-derived extracellular vesicles promote Human cartilage regeneration In vitro. Theranostics. 2018;8:906–20. 10.7150/thno.20746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Yin H, Li M, Tian G et al. The role of extracellular vesicles in osteoarthritis treatment via microenvironment regulation. Biomater Res. 2022;26:52. 10.1186/s40824-022-00300-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Nguyen TH, Dao HH, Duong CM et al. Cytokine-primed umbilical cord mesenchymal stem cells enhanced therapeutic effects of extracellular vesicles on osteoarthritic chondrocytes. Front Immunol. 2022;13:1041592. 10.3389/fimmu.2022.1041592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chen P, Zheng L, Wang Y et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439–59. 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ragni E, Perucca Orfei C, De Luca P et al. Secreted factors and EV-miRNAs orchestrate the healing capacity of adipose mesenchymal stem cells for the treatment of knee osteoarthritis. Int J Mol Sci. 2020;21:1582. 10.3390/ijms21051582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Shao LT, Luo L, Qiu JH et al. PTH (1-34) enhances the therapeutic effect of bone marrow mesenchymal stem cell-derived exosomes by inhibiting proinflammatory cytokines expression on OA chondrocyte repair in vitro. Arthritis Res Ther. 2022;24:96. 10.1186/s13075-022-02778-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Silver FH, Bradica G, Tria A Do changes in the mechanical properties of articular cartilage promote catabolic destruction of cartilage and osteoarthritis? Matrix Biol. 2004;23:467–76. 10.1016/j.matbio.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 154. Fujii Y, Liu L, Yagasaki L et al. Cartilage homeostasis and osteoarthritis. Int J Mol Sci. 2022;23:6316. 10.3390/ijms23116316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Sellam J, Berenbaum F The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol. 2010;6:625–35. 10.1038/nrrheum.2010.159. [DOI] [PubMed] [Google Scholar]
- 156. Eitner A, Hofmann GO, Schaible HG Mechanisms of osteoarthritic pain. Studies in humans and experimental models. Front. Mol. Neurosci. 2017;10:349. 10.3389/fnmol.2017.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Dieppe PA, Lohmander LS Pathogenesis and management of pain in osteoarthritis. The Lancet. 2005;365:965–73. 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 158. Duong V, Oo WM, Ding C et al. Evaluation and treatment of knee pain: A review. JAMA. 2023;330:1568–80. 10.1001/jama.2023.19675. [DOI] [PubMed] [Google Scholar]
- 159. Nguyen TH, Duong CM, Nguyen XH et al. Mesenchymal stem cell-derived extracellular vesicles for osteoarthritis treatment: extracellular matrix protection, chondrocyte and osteocyte physiology, pain and inflammation management. Cells. 2021;10:2887. 10.3390/cells10112887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. D'Agnelli S, Gerra MC, Bignami E et al. Exosomes as a new pain biomarker opportunity. Mol Pain. 2020;16:1744806920957800. 10.1177/1744806920957800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Iyengar S, Ossipov MH, Johnson KW The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain. 2017;158:543–59. 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. He L, He T, Xing J et al. Bone marrow mesenchymal stem cell-derived exosomes protect cartilage damage and relieve knee osteoarthritis pain in a rat model of osteoarthritis. Stem Cell Res Ther. 2020;11:276. 10.1186/s13287-020-01781-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Li J, Ding Z, Li Y et al. BMSCs-derived exosomes ameliorate pain via abrogation of aberrant nerve invasion in subchondral bone in lumbar facet joint osteoarthritis. Journal Orthopaedic Research. 2020;38:670–9. 10.1002/jor.24497. [DOI] [PubMed] [Google Scholar]
- 164. Yang Q, Yao Y, Zhao D et al. LncRNA H19 secreted by umbilical cord blood mesenchymal stem cells through microRNA-29a-3p/FOS axis for central sensitization of pain in advanced osteoarthritis. Am J Transl Res. 2021;13:1245–56. [PMC free article] [PubMed] [Google Scholar]
- 165. Lee YH, Park HK, Auh QS et al. Emerging potential of exosomes in regenerative medicine for temporomandibular joint osteoarthritis. Int J Mol Sci. 2020;21:1541. 10.3390/ijms21041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Zhang S, Teo KYW, Chuah SJ et al. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials. 2019;200:35–47. 10.1016/j.biomaterials.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 167. Thomas BL, Eldridge SE, Nosrati B et al. WNT3A-loaded exosomes enable cartilage repair. J Extracellular Vesicle. 2021;10:e12088. 10.1002/jev2.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Freyria AM, Mallein-Gerin F Chondrocytes or adult stem cells for cartilage repair: the indisputable role of growth factors. Injury. 2012;43:259–65. 10.1016/j.injury.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 169. Deng ZH, Li YS, Gao X et al. Bone morphogenetic proteins for articular cartilage regeneration. Osteoarthritis Cartilage. 2018;26:1153–61. 10.1016/j.joca.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 170. Ude CC, Shamsul BS, Ng MH et al. Long-term evaluation of osteoarthritis sheep knee, treated with TGF-β3 and BMP-6 induced multipotent stem cells. Exp Gerontol. 2018;104:43–51. 10.1016/j.exger.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 171. Cheng J, Sun Y, Ma Y et al. Engineering of MSC-derived exosomes: A promising cell-free therapy for osteoarthritis. Membranes. 2022;12:739. 10.3390/membranes12080739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Cosenza S, Ruiz M, Toupet K et al. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 2017;7:16214. 10.1038/s41598-017-15376-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Fu Y, Cui S, Zhou Y et al. Dental pulp stem cell-derived exosomes alleviate mice knee osteoarthritis by inhibiting TRPV4-mediated osteoclast activation. Int J Mol Sci. 2023;24:4926. 10.3390/ijms24054926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Mouamnia A, Desvages A AB0807 Toast-study: Treatment and osteoarthritis, what are people saying on Twitter? Ann Rheum Dis. 2021;80:1427.3–1428. 10.1136/annrheumdis-2021-eular.3299. [DOI] [Google Scholar]
- 175. Kloek CJJ, van Dongen JM, de Bakker DH et al. Cost-effectiveness of a blended physiotherapy intervention compared to usual physiotherapy in patients with hip and/or knee osteoarthritis: a cluster randomized controlled trial. BMC Public Health. 2018;18:1082. 10.1186/s12889-018-5975-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Jones SE, Campbell PK, Kimp AJ et al. Evaluation of a novel e-learning program for physiotherapists to manage knee osteoarthritis via telehealth: qualitative study nested in the PEAK (Physiotherapy Exercise and Physical Activity for Knee Osteoarthritis) randomized controlled trial. J Med Internet Res. 2021;23:e25872. 10.2196/25872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Perry TA, Wang X, Nevitt M et al. Association between current medication use and progression of radiographic knee osteoarthritis: data from the osteoarthritis initiative. Rheumatology (Oxford). 2021;60:4624–32. 10.1093/rheumatology/keab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Madry H Surgical therapy in osteoarthritis. Osteoarthritis Cartilage. 2022;30:1019–34. 10.1016/j.joca.2022.01.012. [DOI] [PubMed] [Google Scholar]
- 179. Abram SGF, Judge A, Beard DJ et al. Long-term rates of knee arthroplasty in a cohort of 834 393 patients with a history of arthroscopic partial meniscectomy. The Bone & Joint Journal. 2019;101-B:1071–80. 10.1302/0301-620X.101B9.BJJ-2019-0335.R1. [DOI] [PubMed] [Google Scholar]
- 180. Bryan AJ, Krych AJ, Pareek A et al. Are short-term outcomes of hip arthroscopy in patients 55 years and older inferior to those in younger patients?. Am J Sports Med. 2016;44:2526–30. 10.1177/0363546516652114. [DOI] [PubMed] [Google Scholar]
- 181. Hevesi M, Leland DP, Rosinsky PJ et al. Risk of conversion to arthroplasty after hip arthroscopy: validation of a published Risk score using an independent, prospectively collected database. Am J Sports Med. 2021;49:1192–8. 10.1177/0363546521993829. [DOI] [PubMed] [Google Scholar]
- 182. Compton J, Slattery M, Coleman M et al. Iatrogenic articular cartilage injury in arthroscopic hip and knee videos and the potential for cartilage cell death when simulated in a bovine model. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2020;36:2114–21. 10.1016/j.arthro.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Slomski A Corticosteroid and lidocaine injection for hip osteoarthritis. JAMA. 2022;327:1950. 10.1001/jama.2022.8170. [DOI] [PubMed] [Google Scholar]
- 184. Deyle GD, Allen CS, Allison SC et al. Physical therapy versus glucocorticoid injection for osteoarthritis of the knee. N Engl J Med. 2020;382:1420–9. 10.1056/NEJMoa1905877. [DOI] [PubMed] [Google Scholar]
- 185. Zhang W, Ouyang H, Dass CR et al. Current research on pharmacologic and regenerative therapies for osteoarthritis. Bone Res. 2016;4:15040. 10.1038/boneres.2015.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Cho Y, Jeong S, Kim H et al. Disease-modifying therapeutic strategies in osteoarthritis: current status and future directions. Exp Mol Med. 2021;53:1689–96. 10.1038/s12276-021-00710-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Deng J, Zong Z, Su Z et al. Recent advances in pharmacological intervention of osteoarthritis: A biological aspect. Front Pharmacol. 2021;12:772678. 10.3389/fphar.2021.772678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Whitney KE, Liebowitz A, Bolia IK et al. Current perspectives on biological approaches for osteoarthritis. Ann NY Acad Sci. 2017;1410:26–43. 10.1111/nyas.13554. [DOI] [PubMed] [Google Scholar]
- 189. Knights AJ, Redding SJ, Maerz T Inflammation in osteoarthritis: the latest progress and ongoing challenges. Curr Opin Rheumatol. 2023;35:128–34. 10.1097/BOR.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Xiang XN, Zhu SY, He HC et al. Mesenchymal stromal cell-based therapy for cartilage regeneration in knee osteoarthritis. Stem Cell Res Ther. 2022;13:14. 10.1186/s13287-021-02689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Burke J, Hunter M, Kolhe R et al. Therapeutic potential of mesenchymal stem cell based therapy for osteoarthritis. Clinical & Translational Med. 2016;5:27. 10.1186/s40169-016-0112-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Copp G, Robb KP, Viswanathan S Culture-expanded mesenchymal stromal cell therapy: does it work in knee osteoarthritis? A pathway to clinical success. Cell Mol Immunol. 2023;20:626–50. 10.1038/s41423-023-01020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Tevlin R, desJardins-Park H, Huber J et al. Musculoskeletal tissue engineering: adipose derived stromal cell implementation for the treatment of osteoarthritis. Biomaterials. 2022;286:121544. 10.1016/j.biomaterials.2022.121544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Mak CCH, To K, Fekir K et al. Infrapatellar fat pad adipose-derived stem cells co-cultured with articular chondrocytes from osteoarthritis patients exhibit increased chondrogenic gene expression. Cell Commun Signal. 2022;20:17. 10.1186/s12964-021-00815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Liang H, Suo H, Wang Z et al. Progress in the treatment of osteoarthritis with umbilical cord stem cells. Hum Cell. 2020;33:470–5. 10.1007/s13577-020-00377-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yamashita A, Yoshitomi H, Kihara S et al. Culture substrate-associated YAP inactivation underlies chondrogenic differentiation of human induced pluripotent stem cells. Stem Cells Transl Med. 2021;10:115–27. 10.1002/sctm.20-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Castro-Viñuelas R, Sanjurjo-Rodríguez C, Piñeiro-Ramil M et al. Induced pluripotent stem cells for cartilage repair: current status and future perspectives. eCM. 2018;36:96–109. 10.22203/eCM.v036a08. [DOI] [PubMed] [Google Scholar]
- 198. Abe K, Yamashita A, Morioka M et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat Commun. 2023;14:804. 10.1038/s41467-023-36408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Tatebe M, Nakamura R, Kagami H et al. Differentiation of transplanted mesenchymal stem cells in a large osteochondral defect in rabbit. Cytotherapy. 2005;7:520–30. 10.1080/14653240500361350. [DOI] [PubMed] [Google Scholar]
- 200. Loo SJQ, Wong NK Advantages and challenges of stem cell therapy for osteoarthritis (Review). Biomed Rep. 2021;15:67. 10.3892/br.2021.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Turinetto V, Vitale E, Giachino C Senescence in Human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci. 2016;17:1164. 10.3390/ijms17071164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Irioda AC, Cassilha R, Zocche L et al. Human adipose-derived mesenchymal stem cells cryopreservation and thawing decrease α4-integrin expression. Stem Cells International. 2016;2016:2562718. 10.1155/2016/2562718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Shang Z, Wanyan P, Zhang B et al. A systematic review, umbrella review, and quality assessment on clinical translation of stem cell therapy for knee osteoarthritis: are we there yet?. Stem Cell Res Ther. 2023;14:91. 10.1186/s13287-023-03332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Filardo G, Madry H, Jelic M et al. Mesenchymal stem cells for the treatment of cartilage lesions: from preclinical findings to clinical application in orthopaedics. Knee Surg Sports Traumatol Arthrosc. 2013;21:1717–29. 10.1007/s00167-012-2329-3. [DOI] [PubMed] [Google Scholar]
- 205. Tasso R, Augello A, Carida′ M et al. Development of sarcomas in mice implanted with mesenchymal stem cells seeded onto bioscaffolds. Carcinogenesis. 2009;30:150–7. 10.1093/carcin/bgn234. [DOI] [PubMed] [Google Scholar]
- 206. Kim SY, Lee DG, Kim MS et al. The influence of infection early after allogeneic stem cell transplantation on the risk of leukemic relapse and graft-versus-host disease. American J Hematol. 2008;83:784–8. 10.1002/ajh.21227. [DOI] [PubMed] [Google Scholar]
- 207. Shapiro RS Future issues in transplantation ethics: ethical and legal controversies in xenotransplantation, stem cell, and cloning research. Transplant Rev (Orlando). 2008;22:210–4. 10.1016/j.trre.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 208. Shah SS, Mithoefer K Current applications of growth factors for knee cartilage repair and osteoarthritis treatment. Curr Rev Musculoskelet Med. 2020;13:641–50. 10.1007/s12178-020-09664-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Strauss EJ, Barker JU, Kercher JS et al. Augmentation strategies following the microfracture technique for repair of focal chondral defects. Cartil. 2010;1:145–52. 10.1177/1947603510366718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Everts P, Onishi K, Jayaram P et al. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21:7794. 10.3390/ijms21207794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Cao Y, Luo J, Han S et al. A model-based quantitative analysis of efficacy and associated factors of platelet rich plasma treatment for osteoarthritis. Int J Surg. 2023;109:1742–52. 10.1097/JS9.0000000000000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Le ADK, Enweze L, DeBaun MR et al. Platelet-rich plasma. Clin Sports Med. 2019;38:17–44. 10.1016/j.csm.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 213. Zhao D, Pan JK, Yang WY et al. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: A systematic review and network meta-analysis. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2021;37:2298–314. 10.1016/j.arthro.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 214. Louis ML, Magalon J, Jouve E et al. Growth factors levels determine efficacy of platelets rich plasma injection in knee osteoarthritis: A randomized double blind noninferiority trial compared with viscosupplementation. Arthroscopy: The Journal of Arthroscopic & Related Surgery. 2018;34:1530–40. 10.1016/j.arthro.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 215. Park YB, Kim JH, Ha CW et al. Clinical efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: A randomized double-blind controlled Clinical trial As compared with hyaluronic acid. Am J Sports Med. 2021;49:487–96. 10.1177/0363546520986867. [DOI] [PubMed] [Google Scholar]
- 216. Ratneswaran A, Kapoor M Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2021;29:151–60. 10.1016/j.joca.2020.11.003. [DOI] [PubMed] [Google Scholar]
- 217. Young DA, Barter MJ, Soul J Osteoarthritis year in review: genetics, genomics, epigenetics. Osteoarthritis Cartilage. 2022;30:216–25. 10.1016/j.joca.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Rice SJ, Beier F, Young DA et al. Interplay between genetics and epigenetics in osteoarthritis. Nat Rev Rheumatol. 2020;16:268–81. 10.1038/s41584-020-0407-3. [DOI] [PubMed] [Google Scholar]
- 219. Loughlin J Translating osteoarthritis genetics research: challenging times ahead. Trends Mol Med. 2022;28:176–82. 10.1016/j.molmed.2021.12.007. [DOI] [PubMed] [Google Scholar]
- 220. Boer CG, Hatzikotoulas K, Southam L et al. Deciphering osteoarthritis genetics across 826,690 individuals from 9 populations. Cell. 2021;184:4784–818. 10.1016/j.cell.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Attur M, Zhou H, Samuels J et al. Interleukin 1 receptor antagonist (IL1RN) gene variants predict radiographic severity of knee osteoarthritis and risk of incident disease. Ann Rheum Dis. 2020;79:400–7. 10.1136/annrheumdis-2019-216055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Aubourg G, Rice SJ, Bruce-Wootton P et al. Genetics of osteoarthritis. Osteoarthritis Cartilage. 2022;30:636–49. 10.1016/j.joca.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Hartley A, Gregson CL, Paternoster L et al. Osteoarthritis: insights offered by the study of bone mass genetics. Curr Osteoporos Rep. 2021;19:115–22. 10.1007/s11914-021-00655-1. [DOI] [PMC free article] [PubMed] [Google Scholar]