Abstract
Background
The incidence of knee osteoarthritis after anterior cruciate ligament reconstruction (ACLR) is high to 57%, and the biomechanical abnormality during walking is one of the reasons. The purpose of this study was to investigate the effect of 12 weeks of knee extension constraint training on walking biomechanics during the stance phase of injured side after ACLR.
Methods
Forty-five patients were randomly assigned to three groups based on different brace conditions from 13 weeks to 24 weeks after ACLR: experimental (brace with knee extension constraint), placebo (brace without knee extension constraint), and control (no brace). Gait analysis was performed 3 and 6 months after ACLR. The peak for knee flexion angle (KFA), knee extension moment (KEM), and vertical ground reaction force (vGRF) were compared by 2 (time) x 3 (group) repeated-measures analysis of covariance (ANCOVA), and pairwise comparisons were conducted. .
Results
There was a significant time x group interaction for the peak KFA (p = 0.047), and there was no significant time x group interaction for the peak KEM and peak vGRF. The pairwise comparisons showed that there were no statistical differences among the groups both the pre-intervention and post-intervention in the peak KFA, peak KEM, and peak vGRF. Compared with pre-intervention, the peak vGRF in the experimental group was significantly greater (p = 0.009) and the peak KFA in the control group was significantly lower (p = 0.041) post-intervention. There were not significantly different in the placebo group between pre-intervention and post-intervention.
Conclusion
12 weeks of knee extension constraint training can increase lower extremity loading on the injured side, may be a potential therapeutic adjunct to improve abnormal gait after ACLR.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13018-024-05447-8.
Keywords: Anterior cruciate ligament reconstruction, Biomechanics, Rehabilitation, Walking
Introduction
Anterior cruciate ligament rupture is a common sports injury represent more than 50% of knee injuries, severely affecting the function of the knee joint and reducing the patient’s performance level and quality of life [1]. Anterior cruciate ligament reconstruction (ACLR) is a major treatment to restore knee stability and function [2, 3]. Rates of knee osteoarthritis (KOA) remain high after ACLR, with a 57% incidence of KOA, 3 times the rate than healthy knees [4]. Abnormal biomechanics of the lower extremity during walking was an important factor. Studies have shown that the presence of gait abnormalities at 6 months after ACLR is associated with lower knee function scores at 1 year postoperatively, and the persistence of gait abnormalities at 2 years may even influence knee function outcomes at 10 years postoperatively [5–8]. Lower peak vertical ground reaction force (vGRF) during walking 6 months after ACLR are associated with an increased T1ρ relaxation time of tibiofemoral articular cartilage and also result in increased concentrations of intra-articular inflammatory markers [9, 10]. The lower mechanical loading during walking may be associated with compositional changes in the knee cartilage, which may lead to cartilage degeneration and the development of KOA, it critical to improve the abnormal gait biomechanics that exists early after ACLR.
Resistance training can help to improve the knee strength weakness that persists after ACLR, but most of the training is done in a “non-functional” manner and is less effective in improving abnormal gait [11]. Functional resistance training is the sustained application of resistance during a specific task, which is effective in improving muscle function and correcting abnormal gait [12]. Functional resistance training has been shown to improve the symmetry of knee flexion angle (KFA) and knee extension moment (KEM) during walking and increase knee strength in post-ACLR patients [13, 14]. At the same time, functional resistance training also can improve the lack of loading of the lower extremity on the injured side in the early post-ACLR [15]. Functional knee brace is widely used after ACLR by constraining knee extension to protect the graft of ACLR [16–18]. A new type of knee extension constraint brace has been developed based on the traditional functional brace, which has a resistance mechanism that applies gradually increasing resistance during knee extension from 40 degrees to 10 degrees of flexion, with a stop device to limit further extension when 10 degrees of knee flexion is reached [19]. The peak vGRF at the time of landing was significantly reduced and the KFA at the time of peak vGRF was significantly increased when wearing a knee extension constraint brace during a stop-jump task [20, 21]. Training by wearing a knee extension constraint brace 4 weeks can reduce the risk of ACL injuries by improving the biomechanics of the lower extremity, and the improvement can be maintained in the following 4 weeks after training [19]. Knee extension constraint training is a form of functional resistance training, and it has been shown that wearing knee extension constraint braces early after ACLR significantly increases KFA in the stance phase and results in more symmetrical vGRF [22, 23]. However, the gait biomechanics after knee extension constraint training in ACLR patients have not been validated.
Therefore, the purpose of this study was to investigate the effect of 12 weeks knee extension constraint training on gait biomechanics during the stance phase after ACLR. Patients used different braces from 3 months to 6 months after operation, the experimental group wearing braces with knee extension constraint, the placebo group wearing braces without knee extension constraint, and the control group not wearing braces. We hypothesized that the peak KFA, peak KEM, and peak vGRF during the stance phase of the injured lower limb in the experimental group would be significantly greater than in the placebo group and the control group at 6 months after ACLR; The peak KFA, peak KEM, and peak vGRF during the stance phase of the injured lower limb post-intervention would be significantly greater than pre-intervention in the experimental group at 6 months after ACLR, and the placebo and control groups would not.
Materials and methods
Study design
This study was a randomized controlled double-blinded clinical trial (NCT04464902) designed to evaluate the effects of knee extension constraint training after ACLR to improve gait biomechanics (Fig. 1). This study was approved by Peking University Third Hospital Medical Science Research Ethics Committee (IRB00006761-2015243), and all participants read and signed an approved informed consent document before the experiment. Participants were randomly assigned to three groups using the randomized block group assignment method: experimental group, placebo group, and control group. Patients of the same surgeon are assigned within the same block group, the number of each block group is 3, and then the 3 patients within that block group are randomly assigned to groups. Variables were not controlled for gender, as gender has been shown to not affect the effectiveness of knee extension constraint training [21]. Participants were blinded to their group, and researchers were blinded to participants’ group information during testing and analysis. Participants had their first gait biomechanics test at 3 months postoperatively (baseline), followed by 12 weeks of various interventions, and again test at 6 months postoperatively. All data were collected and processed at the Exercise Biomechanics Laboratory of the Institute of Sports Medicine, Peking University Third Hospital. Participants were randomly assigned by a dedicated researcher (Yuanyuan Yu), detailed group information was stored in a confidential Excel file, and unblinding at the end of the experiment was performed by the same researcher.
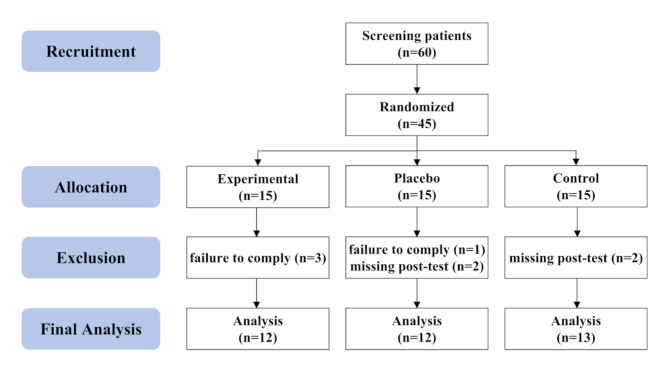
Fig. 1.
Study CONSORT (CONsolidated Standards of Reporting Trials) flow diagram, illustrating the screening, enrollment, and trial design of this clinical trial
Participants
Patients aged between 18 and 45 years who underwent ACLR surgery at Peking University Third Hospital between July 2016 and February 2019 were recruited (Table 1). Inclusion criteria were first-time unilateral ACLR surgery; isolated anterior cruciate ligament deficiency; anatomical single-bundle reconstruction; and hamstring tendon autograft. Exclusion criteria were a history of hip, knee or ankle surgery; postoperative infection or other complications; comorbidities with other conditions affecting gait; and pregnant, lactating or breastfeeding females. According to the results of the pre-test, the sample size was calculated using the mean difference test between the groups and it was concluded that each group needed at least 12 samples. Considering the loss rate of 20%, a total of 45 participants were enrolled in this study. There were 15 participants in the experimental group, placebo group, and control group respectively. Three participants in the experimental group failed to wear the brace as prescribed. Two participants in the placebo group failed to wear the brace as prescribed and one participant missed the second test. Two participants in the control group missed the second test. Ultimately, data were analyzed for 37 participants, including 12 experimental, 12 placebo, and 13 control (Fig. 1). No adverse events were reported by all participants in this study. At the time of the first test, all participants had recovered full knee range of motion and were able to walk with normal weight bearing without crutches and without pain.
Table 1.
Demographic information for participants
| Variables | Experimental (SD) | Placebo (SD) | Control (SD) | P Value |
|---|---|---|---|---|
| N | 12 (M = 10, F = 2) | 12 (M = 11, F = 1) | 13 (M = 13, F = 0) | |
| Age (years) | 26.08 (3.66) | 25.58 (5.27) | 28.46 (4.96) | 0.270 |
| Height (cm) | 170.92 (8.85) | 173.67 (6.93) | 175.85 (4.78) | 0.226 |
| Mass (kg) | 68.75 (9.90) | 79.83 (17.44) | 77.08 (10.47) | 0.108 |
| Time from injury to operation (days) | 75.50 (54.68) | 111.00 (63.21) | 70.92 (53.78) | 0.182 |
| Change in self-selected gait speed (%) | 6.26 (10.43) | 11.57 (18.41) | 5.60 (11.32) | 0.509 |
Training interventions
All participants underwent the same routine rehabilitation training from the first postoperative day, and were instructed by the same rehabilitation therapist. From the 13th postoperative week, the experimental group and the placebo group increased the training with braces. The reason for starting training with braces at 13 weeks post-operatively was to ensure that all participants had fully recovered knee range of motion [24]. In addition, 3 months after ACLR, the graft had passed the most dangerous and fragile stage and begun to ligamentate [25]. The routine rehabilitation program is as follows: Day 1–2: ankle pump training, quadriceps isometric contraction training, straight leg raising training, partial weight bearing walking; Day 3–7: range of motion training (0–90 degrees); Weeks 2–3: gradual transition to full weight bearing walking; Weeks 4–12: range of motion training (0–120 degrees), full weight bearing walking; Weeks 13–24: muscle strength training, stability training, endurance training, jogging. From week 1 to week 12, the frequency was training every day according to the rehabilitation plan. From week 13 to week 24, the frequency was 2–3 times per week according to the rehabilitation plan. Starting at week 13, participants in the experimental group conducted rehabilitation training while wearing the brace with knee extension constraint, and participants in the placebo group conducted rehabilitation training while wearing the brace without knee extension constraint. Participants wore braces for at least 2 h a week, which has been shown to be effective in previous research [19]. Each participant was given a logbook to record the time of wearing the brace, and the participants were informed that unannounced random checks would be made to ensure that they were wearing the brace as planned during rehabilitation training. We would eventually exclude participants with an average brace wearing time of less than 2 h per week from the data analysis. The resistance torque of the knee extension constraint brace (DJ Ortho) can be adjusted to different levels: no resistance, low resistance, medium resistance, and high resistance. In this study, the experimental group chose high resistance and the placebo group chose no resistance (Fig. 2).
Fig. 2.

The knee extension constraint brace (DJ Ortho)
Data collection
In each biomechanics test, reflective markers were placed bilaterally at acromions, anterior superior iliac spines, greater trochanters, lateral thighs, lateral femoral condyles, anterior superior shanks, anterior inferior shanks, lateral malleoli, heels, first and fifth metatarsophalangeal joints, medial femoral condyles, and medial malleoli. Another marker was placed on the intersection of L4-L5. A total of 27 reflective markers. Static and dynamic three-dimensional motion information of the subject was collected using an 8-camera infrared high-speed motion capture system at a sample rate of 100 Hz (Vicon MX, Oxford Metrics, UK). Ground-reaction forces were collected using two embedded force plates at a sample rate of 1000 Hz (AMTI, Advanced Mechanical Technology Inc., Watertown, Massachusetts, USA). A synchronization box (AMTI, GEN 5) was used to synchronize the high-speed motion capture system with the force plates to collect kinematic and kinetic data during the test. All subjects were asked to walk from a specified point so that one of his/her foot would unintentionally walk on the first force plate and the other would walk on the second force plate. Participants were instructed to walk wearing shoes. Before the actual test, practice tests were conducted to ensure that participants were familiar with the test. A successful trial was characterized as each foot stepping on the force plates at a selfselected speed. Once three successful gait trials were recorded, the data collection was completed.
Data processing
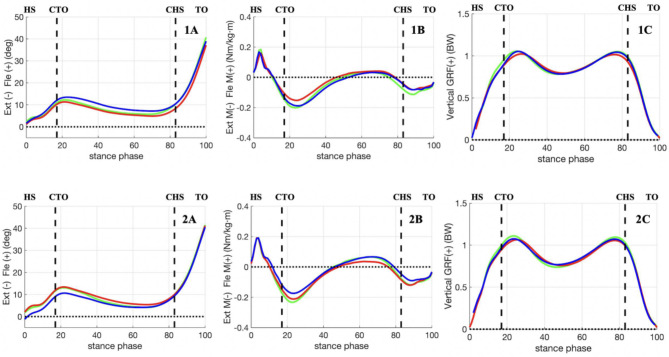
The 3D coordinates of all marker points collected in this study were smoothed using Butterworth low-pass filtering with a cutoff frequency of 12 Hz. A Butterworth low-pass filter with a cutoff frequency of 100 Hz was also used to smooth the ground reaction forces. The fourth order Butterworth filter was used. Time-series data for the kinematics and kinetics variables were calculated using Visual 3D software (C-motion, Germantown, MD). Biomechanical modeling of rigid bodies using static tests with the talonavicular joint in the neutral position. The Bell method was used to estimate the hip joint from the 3D coordinates of the reflective markers on the anterior superior iliac spines and L4-L5 joint [26]; The knee joint centers were estimated from the 3D coordinates of the reflective markers on the medial and lateral femoral condyles; The ankle joint centers were estimated from the 3D coordinates of the reflective markers on the medial and lateral malleoli. The knee joint angles were determined as Euler angles of the tibia’s reference frame relative to the femur’s reference frame rotated in an order of flexion-extension (x-axis), valgus-varus (y-axis), and internal-external rotation (z-axis) [27]. Determination of the onset of heel strike and toe-off by the force platform, thus determining the stance phase of the gait. The ground reaction force threshold to determine heelstrike and toe off was 20 N. The software was used to extract the kinematic data of the knee in the coronal, sagittal, and transverse planes during the stance phase and to obtain the internal knee moment by inverse dynamics calculations using the ground reaction force. Moments were normalized by the product of height and weight, and ground reaction forces were normalized by the product of weight and gravity acceleration. For the KFA, KEM, and vGRF, 101 discrete points corresponding to 0–100% stance phase at 1% interval were normalized using a cubic spline for statistical analysis (Fig. 3).
Fig. 3.
Waveforms of knee flexion angle (A), knee extension moment (B), and vertical ground reaction force (C) during the stance phase are shown for the involved limbs both at 3 (1) and 6 months (2). Green line: experimental group, Red line: placebo group, Blue line: control group. HS: heel strike, TO: toe off, CTO: contralateral toe off, CHS: contralateral heel strike
Statistical analysis
Before the main analysis, a one-way ANOVA was used to compare whether demographic variables were statistically different among the three groups. We first test the normal distribution of the data by Shapiro-Wilk test, and if it is not normal, we use nonparametric test. Considering that changes in self-selected gait speed may affect measures of both kinetic and kinematic outcomes during gait, it was included as a covariate. The calculation method of the changes in self-selected gait speed was (6 month value − 3 month value) / 3 month value x 100. For the primary analyses, 2 × 3 repeated-measures analysis of covariance (ANCOVA) were used to compare our dependent variables of time (3 and 6 months) and group (experimental, placebo, and control). For the secondary analyses, pairwise comparisons using least-significant differences were conducted to specifically compare whether there was a statistical difference between the groups on the main observational indicators, and whether there was a statistical difference of intra-group between pre-intervention and post-intervention on the main observational indicators. All statistical analyses were performed using SPSS 26.0 (SPSS, Chicago, IL, USA), and the significance level for statistical differences was set at a one-class error probability of no greater than 0.05.
Results
Age (p = 0.270), height (p = 0.226), weight (p = 0.108), time from injury to surgery (p = 0.182), and change in self-selected gait speed (p = 0.509) of participants did not differ statistically among the three groups (Table 1). The biomechanics data of pre-intervention and post-intervention in each group conform to the normal distribution (p = 0.082–989). The repeated-measures ANCOVA showed that there was a significant time x group interaction for the peak KFA (F = 3.359, p = 0.047), and there was no significant time x group interaction for the peak KEM (F = 1.341, p = 0.275) and peak vGRF (F = 0.325, p = 0.725) (Table 2). The least-significant differences pairwise comparisons showed that there were no statistical differences among the groups both the pre-intervention and post-intervention in the peak KFA, peak KEM, and peak vGRF (Table 3). According to the pairwise comparisons of the main observational indicators between pre-intervention and post-intervention, there were no statistical differences in the peak KFA (t = -0.579, p = 0.663) and peak KEM (t = -0.036, p = 0.209), and there were statistical difference in the peak vGRF (t = -0.054, p = 0.009) in the experimental group; The peak KFA (t = -1.992, p = 0.158), peak KEM (t = -0.053, p = 0.072), and peak vGRF (t = -0.039, p = 0.055) were not statistically different in the placebo group; There were statistical difference in the peak KFA (t = 2.704, p = 0.041), and there were no statistical differences in the peak KEM (t = 0.009, p = 0.736) and peak vGRF (t = -0.033, p = 0.085) in the control group (Table 4).
Table 2.
Comparison results of two-way repeated-measures ANCOVA
| Variables | Factors | F Value | P Value | Effect Size (η²p) |
|---|---|---|---|---|
| KFA (deg) | time | 0.037 | 0.584 | 0.009 |
| group | 0.130 | 0.879 | 0.008 | |
| time * group | 3.359 | 0.047* | 0.169 | |
| KEM (Nm/kg·m) | time | 0.174 | 0.679 | 0.005 |
| group | 0.534 | 0.591 | 0.031 | |
| time * group | 1.341 | 0.275 | 0.075 | |
| vGRF (BW) | time | 2.667 | 0.112 | 0.075 |
| group | 0.719 | 0.495 | 0.042 | |
| time * group | 0.325 | 0.725 | 0.019 |
KFA: knee flexion angle, KEM: knee extension moment, vGRF: vertical ground reaction force
Table 3.
Paired comparison results of two-way repeated-measures ANCOVA between groups
| Variables | Adjusted Mean (SD) | t Value | P Value | Cohen’s d | |
|---|---|---|---|---|---|
| Before intervention | |||||
| KFA (deg) | Experimental | Placebo | 0.747 | 0.764 | 0.430 |
| 13.30 (1.73) | 12.55 (1.75) | ||||
| Experimental | Control | −0.635 | 0.792 | 0.374 | |
| 13.30 (1.73) | 13.94 (1.67) | ||||
| Placebo | Control | −1.382 | 0.574 | 0.810 | |
| 12.55 (1.75) | 13.94 (1.67) | ||||
| KEM (Nm/kg·m) | Experimental | Placebo | 0.035 | 0.460 | 0.031 |
| 0.22 (0.03) | 0.19 (0.03) | ||||
| Experimental | Control | 0.021 | 0.652 | 0.019 | |
| 0.22 (0.03) | 0.20 (0.03) | ||||
| Placebo | Control | −0.014 | 0.757 | 0.431 | |
| 0.19 (0.03) | 0.20 (0.03) | ||||
| vGRF (BW) | Experimental | Placebo | 0.019 | 0.496 | 0.947 |
| 1.09 (0.02) | 1.07 (0.02) | ||||
| Experimental | Control | 0.021 | 0.435 | 1.081 | |
| 1.09 (0.02) | 1.07 (0.02) | ||||
| Placebo | Control | 0.002 | 0.938 | 0.108 | |
| 1.07 (0.02) | 1.07 (0.02) | ||||
| After intervention | |||||
| KFA (deg) | Experimental | Placebo | −0.595 | 0.810 | 0.344 |
| 13.88 (1.72) | 14.48 (1.74) | ||||
| Experimental | Control | 2.648 | 0.274 | 1.575 | |
| 13.88 (1.72) | 11.23 (1.66) | ||||
| Placebo | Control | 3.243 | 0.189 | 1.916 | |
| 14.48 (1.74) | 11.23 (1.66) | ||||
| KEM (Nm/kg·m) | Experimental | Placebo | 0.019 | 0.706 | 0.529 |
| 0.26 (0.03) | 0.24 (0.03) | ||||
| Experimental | Control | 0.066 | 0.172 | 1.970 | |
| 0.26 (0.03) | 0.19 (0.03) | ||||
| Placebo | Control | 0.047 | 0.331 | 1.433 | |
| 0.24 (0.03) | 0.19 (0.03) | ||||
| vGRF (BW) | Experimental | Placebo | 0.034 | 0.353 | 1.360 |
| 1.15 (0.03) | 1.11 (0.03) | ||||
| Experimental | Control | 0.042 | 0.237 | 1.714 | |
| 1.15 (0.03) | 1.10 (0.02) | ||||
| Placebo | Control | 0.008 | 0.822 | 0.326 | |
| 1.11 (0.03) | 1.10 (0.02) | ||||
KFA: knee flexion angle, KEM: knee extension moment, vGRF: vertical ground reaction force
Table 4.
Paired comparison results of two-way repeated-measures ANCOVA within the group
| Variables | Adjusted Mean (SD) | t Value | P Value | Cohen’s d | |
|---|---|---|---|---|---|
| Before intervention | After intervention | ||||
| Experimental | |||||
| KFA (deg) | 13.30 (1.73) | 13.88 (1.72) | −0.579 | 0.663 | 0.336 |
| KEM (Nm/kg·m) | 0.22 (0.03) | 0.26 (0.03) | −0.036 | 0.209 | 1.075 |
| vGRF (BW) | 1.09 (0.02) | 1.15 (0.03) | −0.054 | 0.009* | 2.432 |
| Placebo | |||||
| KFA (deg) | 12.55 (1.75) | 14.48 (1.74) | −1.922 | 0.158 | 1.102 |
| KEM (Nm/kg·m) | 0.19 (0.03) | 0.24 (0.03) | −0.053 | 0.072 | 1.582 |
| vGRF (BW) | 1.07 (0.02) | 1.11 (0.03) | −0.039 | 0.055 | 1.711 |
| Control | |||||
| KFA (deg) | 13.94 (1.67) | 11.23 (1.66) | 2.704 | 0.041* | 1.628 |
| KEM (Nm/kg·m) | 0.20 (0.03) | 0.19 (0.03) | 0.009 | 0.736 | 0.277 |
| vGRF (BW) | 1.07 (0.02) | 1.10 (0.02) | −0.033 | 0.085 | 1.508 |
KFA: knee flexion angle, KEM: knee extension moment, vGRF: vertical ground reaction force
Discussion
The purpose of this study was to evaluate the effect of 12 weeks of knee extension constraint training on gait biomechanics during the stance phase of walking after ACLR. Our first hypothesis was that following the intervention, the peak KFA, peak KEM, and peak vGRF in the experimental group were significantly greater than in the placebo and control groups. The first hypothesis of this study was not supported as the results of the study showed no significant differences in the peak KFA, peak KEM, and peak vGRF among the three groups following the intervention, which is inconsistent to the results of other previous studies. It has been shown that the KFA and KEM of the injured lower extremity of ACLR patients are significantly increased when wearing a knee extension constraint brace [23, 28]. A retrospective study conducted by Rocchi et al. showed that when braces were worn to rehabilitate the injured lower extremity from day 15 after ACLR, the peak KFA was significantly greater in the knee extension constraint brace group than in the conventional functional brace group at 2 months postoperatively [22]. In our study, there was no significant difference among the three groups following the intervention, which may be explained by the fact that in Rocchi’s study, the different interventions were started on the 15th postoperative day, whereas in our study, the different interventions were started on the 13th postoperative week. The reason for starting training with braces at 13 weeks post-operatively was to ensure that all participants had fully recovered knee range of motion [24]. In addition, 3 months after ACLR, the graft had passed the most dangerous and fragile stage and begun to ligamentate [25]. Therefore, knee functional braces should be chosen to protect the graft during the first three months after surgery, and knee extension constraint braces are not suitable for use [29]. Our second hypothesis was that the peak KFA, peak KEM, and peak vGRF were significantly increased following the intervention in the experimental group, with no significant difference in the placebo and control groups. The second hypothesis of this study was partially supported by the results of the study, which showed a significant increase in the peak vGRF in the experimental group, whereas there was no significant difference in the placebo and control groups between pre-intervention and post-intervention.
vGRF is a fundamental measure of lower extremity loading during walking, and vGRF on the operative side during walking at 6 months after ACLR was correlated with biochemical markers of knee cartilage metabolism [9, 30], patient-reported outcomes at 12 months [5], and changes in tibiofemoral cartilage composition at 24 months [31]. It has been reported that vGRF on the operative side during the first 12 months of walking was lower in patients after ACLR than in healthy controls, not significantly different from healthy controls between 12 and 24 months, and greater than healthy controls at 24 months [32]. The study by Davis-Wilson et al. also showed that patients after ACLR had lower peak vGRF during walking on the operated side than on the contralateral side and healthy controls at one year [33]. vGRF during walking on the operative side were lower than those on the uninjured side during early period of post-operation, suggesting that the uninjured side bears a greater load, possibly due to the patient’s fear of developing a sense of self-protection and thus a greater tendency to use the uninjured side of the lower limb for weight bearing [34]. Previous studies have reported that wearing a functional brace after ACLR is effective in improving patients’ confidence and reducing their concerns [35]. Therefore, we hypothesized that the peak vGRF in the experimental group following the intervention was significantly greater than in the placebo and control groups; and that the peak vGRF in the experimental group would increase significantly between pre-intervention and post-intervention, while there would be no significant difference in the placebo and control groups. The results showed that no significant difference was found in the peak vGRF of the injured lower limb among the three groups following the intervention, but the comparison of the results between pre-intervention and post-intervention showed a significant increase in the experimental group. This means that knee extension constraint training did not have the effect we expected, but there was some time-dependent effect.
Due to altered nerve excitability and decreased muscle strength after ACLR, patients develop quadriceps dysfunction, which leads to altered lower extremity loading during gait, resulting in an abnormal stiff knee gait [36–38]. Abnormal gait after ACLR occurs predominantly in the sagittal plane, as evidenced by altered peak KFA and peak KEM [39, 40]. Recent studies have shown that KFA and KEM were lower on the operative side of patients after ACLR than on the contralateral side and healthy controls during the stance phase in the first postoperative year [33]. Previous studies have shown that lower peak KFA and peak KEM that persist after ACLR are significantly associated with the development of post-traumatic KOA [41, 42]. Knee extension constraint training significantly increases the KFA and the improvement is sustained use of this training [19, 21]. In addition, knee extension constraint training can effectively increase the strength of the knee muscles [13]. Therefore, we hypothesized that the peak KFA and peak KEM of the experimental group would be significantly greater than those of the placebo and control groups following the intervention; and that the peak KFA and peak KEM of the experimental group would be significantly increased between pre-intervention and post-intervention. Our results showed no significant differences in the peak KFA and peak KEM among the three groups following the intervention; and there were no significant differences between pre-intervention and post-intervention in the experimental and placebo groups. Interestingly, post-intervention was significantly lower than pre-intervention in the peak KFA in the control group. During the stance phase, the peak KFA was lower on the operative side of the patients after ACLR, which is a typical stiff knee gait [43]. The stiff knee gait is thought to represent a strategy to mitigate episodic knee instability, and it is associated with the development of knee osteoarthritis [44]. The results of the study did not support our hypothesis for peak KFA and peak KEM. There were no significant differences among the three groups, and the experimental group did not significantly differ between pre-intervention and post-intervention. This suggests that knee extension constraint training has no significant effect on improving the knee stiff gait and knee extension strength during the stance phase of walking after ACLR. However, the peak KFA of patients without braces decreased significantly, suggesting that wearing braces can prevent further aggravation of knee stiff gait.
Reduced loads on the operated lower extremity and a stiff knee gait at 6 months after ACLR may lead to secondary KOA [9, 40]. In our study, the results of gait testing of patients at 6 months postoperatively following 12 weeks of different interventions showed that the experimental group that received knee extension constraint training did not show a significant benefit. Pre-post comparisons revealed a significant increase in peak vGRF following knee extension constraint training, but no significant change in the peak KFA and peak KEM, and the peak KFA of patients without braces decreased significantly. The results showed that knee extension constraint training early after ACLR increased lower extremity loading on the operated side to a certain extent and did not improve knee stiffness during walking, but it did prevent the aggravation of knee stiff gait. This suggests that knee extension constraint training may delay the development of knee cartilage degeneration to some extent. Therefore, knee extension constraint training has the potential to prevent knee osteoarthritis. The reason that our hypotheses were not fully supported could be: first, we only intervened for 12 weeks, but the abnormal gait biomechanics after ACLR will persist for a long time. Therefore, we suggest that the long-term effects of knee extension constraint trainin should be investigated. In addition, another limitation is that this study only focused on biomechanical characteristics during walking and did not analyze tasks such as jogging, cutting, and stop-jump; therefore, it is not clear how knee extension constraint training affects these high-risk tasks. In the future, higher intensity tasks should be conducted to have a more comprehensive validation of the clinical effects.
Conclusion
The results of this randomized controlled clinical trial suggest that 12 weeks of knee extension constraint training in ACLR patients did not significantly increase the peak KFA and peak KEM during the stance phase of walking, but may have played a role in increasing the peak vGRF. The knee extension constraint training can increase lower extremity loading on the injured side. Therefore, knee extension constraint training may be a potential therapeutic adjunct to improve abnormal gait after ACLR.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
Yingfang Ao conceived the idea for the study and then reviewed and edited. Tianyu Gao, Hongshi Huang, and Hui Liu designed the study. Tianyu Gao, Hongshi Huang, and Yuanyuan Yu collected the relevant data. Tianyu Gao performed the statistical analyses. Tianyu Gao drafted the manuscript. All authors reviewed and revised the manuscript critically read and approved the final manuscript.
Funding
The current study was funded by grants from: (1) Beijing Nova Program (20230484412); (2) Beijing Natural Science Foundation (L222138); (3) Tianjin Research Innovation Project for Postgraduate Students (2022SKY363); (4) Innovation and Transformation Fund Project of Peking University Third Hospital (BYSYZHKC2022119); (5) Clinical Key Projects of Peking University Third Hospital (BYSYZD2021012).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval
Ethics approval was obtained from Peking University Third Hospital Medical Science Research Ethics Committee (IRB00006761-2015243).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent for publication
All authors have approved the release of their work to the public.
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Tianyu Gao and Hongshi Huang are co-first authors of the article.
Contributor Information
Hui Liu, Email: liuhuibupe@163.com.
Yingfang Ao, Email: aoyingfang@163.com.
References
- 1.Musahl V, Karlsson J. Anterior cruciate ligament tear. N Engl J Med. 2019;380(24):2341–8. [DOI] [PubMed] [Google Scholar]
- 2.Mall NA, Chalmers PN, Moric M, Tanaka MJ, Cole BJ, Bach BR Jr., Paletta GA. Jr. Incidence and trends of anterior cruciate ligament reconstruction in the United States. Am J Sports Med. 2014;42(10):2363–70. [DOI] [PubMed] [Google Scholar]
- 3.Skvortsov D, Kaurkin S, Goncharov E, Akhpashev A. Knee joint function and walking biomechanics in patients in acute phase anterior cruciate ligament tear. Int Orthop. 2020;44(5):885–91. [DOI] [PubMed] [Google Scholar]
- 4.Barenius B, Ponzer S, Shalabi A, Bujak R, Norlen L, Eriksson K. Increased risk of osteoarthritis after anterior cruciate ligament reconstruction: a 14-year follow-up study of a randomized controlled trial. Am J Sports Med. 2014;42(5):1049–57. [DOI] [PubMed] [Google Scholar]
- 5.Pietrosimone B, Blackburn JT, Padua DA, Pfeiffer SJ, Davis HC, Luc-Harkey BA, Harkey MS, Stanley Pietrosimone L, Frank BS, Creighton RA, Kamath GM, Spang JT. Walking gait asymmetries 6 months following anterior cruciate ligament reconstruction predict 12-month patient-reported outcomes. J Orthop Res. 2018;36(11):2932–40. [DOI] [PubMed] [Google Scholar]
- 6.Azus A, Teng HL, Tufts L, Wu D, Ma CB, Souza RB, Li X. Biomechanical factors Associated with Pain and symptoms following anterior Cruciate Ligament Injury and Reconstruction. PM R. 2018;10(1):56–63. [DOI] [PubMed] [Google Scholar]
- 7.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi TP. Gait mechanics 2 years after anterior cruciate ligament reconstruction are associated with longer-term changes in patient-reported outcomes. J Orthop Res. 2017;35(3):634–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Erhart-Hledik JC, Chu CR, Asay JL, Andriacchi GBM. Vertical ground reaction force 2 years after anterior cruciate ligament reconstruction predicts 10-year patient-reported outcomes. J Orthop Res. 2022;40(1):129–37. [DOI] [PubMed] [Google Scholar]
- 9.Pfeiffer SJ, Spang J, Nissman D, Lalush D, Wallace K, Harkey MS, Pietrosimone LS, Schmitz R, Schwartz T, Blackburn T, Pietrosimone B. Gait mechanics and T1rho MRI of Tibiofemoral cartilage 6 months after ACL Reconstruction. Med Sci Sports Exerc. 2019;51(4):630–9. [DOI] [PubMed] [Google Scholar]
- 10.Pietrosimone B, Loeser RF, Blackburn JT, Padua DA, Harkey MS, Stanley LE, Luc-Harkey BA, Ulici V, Marshall SW, Jordan JM, Spang JT. Biochemical markers of cartilage metabolism are associated with walking biomechanics 6-months following anterior cruciate ligament reconstruction. J Orthop Res. 2017;35(10):2288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arhos EK, Capin JJ, Buchanan TS, Snyder-Mackler L. Quadriceps Strength Symmetry does not modify gait mechanics after Anterior Cruciate Ligament Reconstruction, Rehabilitation, and return-to-Sport Training. Am J Sports Med. 2021;49(2):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown SR, Washabaugh EP, Dutt-Mazumder A, Wojtys EM, Palmieri-Smith RM, Krishnan C. Functional resistance training to improve knee strength and function after Acute Anterior Cruciate Ligament Reconstruction: a Case Study. Sports Health. 2021;13(2):136–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmieri-Smith RM, Brown SR, Wojtys EM, Krishnan C. Functional resistance training improves thigh muscle strength after ACL Reconstruction: a Randomized Clinical Trial. Med Sci Sports Exerc. 2022;54(10):1729–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AK, Brown SR, Palmieri-Smith RM, Krishnan C. Functional Resistance Training after Anterior Cruciate Ligament Reconstruction improves knee Angle and Moment Symmetry during Gait: a Randomized Controlled Clinical Trial. Arthroscopy. 2022;38(11):3043–55. [DOI] [PubMed] [Google Scholar]
- 15.Washabaugh EP, Brown SR, Palmieri-Smith RM, Krishnan C. Functional Resistance Training differentially alters Gait kinetics after Anterior Cruciate Ligament Reconstruction: a pilot study. Sports Health. 2023;15(3):372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Theoret D, Lamontagne M. Study on three-dimensional kinematics and electromyography of ACL deficient knee participants wearing a functional knee brace during running. Knee Surg Sports Traumatol Arthrosc. 2006;14(6):555–63. [DOI] [PubMed] [Google Scholar]
- 17.Jalali M, Farahmand F, Esfandiarpour F, Golestanha SA, Akbar M, Eskandari A, Mousavi SE. The effect of functional bracing on the arthrokinematics of anterior cruciate ligament injured knees during lunge exercise. Gait Posture. 2018;63:52–7. [DOI] [PubMed] [Google Scholar]
- 18.Perrone GS, Webster KE, Imbriaco C, Portilla GM, Vairagade A, Murray MM, Kiapour AM. Risk of secondary ACL Injury in adolescents prescribed functional Bracing after ACL Reconstruction. Orthop J Sports Med. 2019;7(11):2325967119879880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H, Wu W, Yao W, Spang JT, Creighton RA, Garrett WE, Yu B. Effects of knee extension constraint training on knee flexion angle and peak impact ground-reaction force. Am J Sports Med. 2014;42(4):979–86. [DOI] [PubMed] [Google Scholar]
- 20.Yu B, Herman D, Preston J, Lu W, Kirkendall DT, Garrett WE. Immediate effects of a knee brace with a constraint to knee extension on knee kinematics and ground reaction forces in a stop-jump task. Am J Sports Med. 2004;32(5):1136–43. [DOI] [PubMed] [Google Scholar]
- 21.Lin CF, Liu H, Garrett WE, Yu B. Effects of a knee extension constraint brace on selected lower extremity motion patterns during a stop-jump task. J Appl Biomech. 2008;24(2):158–65. [DOI] [PubMed] [Google Scholar]
- 22.Rocchi JE, Labanca L, Luongo V, Rum L. Innovative rehabilitative bracing with applied resistance improves walking pattern recovery in the early stages of rehabilitation after ACL reconstruction: a preliminary investigation. BMC Musculoskelet Disord. 2020;21(1):644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanley CJ, Creighton RA, Gross MT, Garrett WE, Yu B. Effects of a knee extension constraint brace on lower extremity movements after ACL reconstruction. Clin Orthop Relat Res. 2011;469(6):1774–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piedade SR, Leite Arruda BP, de Vasconcelos RA, Parker DA, Maffulli N. Rehabilitation following surgical reconstruction for anterior cruciate ligament insufficiency: what has changed since the 1960s?-State of the art. J Isakos. 2023;8(3):153–62. [DOI] [PubMed] [Google Scholar]
- 25.Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):834–42. [DOI] [PubMed] [Google Scholar]
- 26.Bell AL, Pedersen DR, Brand RA. A comparison of the accuracy of several hip center location prediction methods. J Biomech. 1990;23(6):617–21. [DOI] [PubMed] [Google Scholar]
- 27.Bull AM, Amis AA. Knee joint motion: description and measurement. Proc Inst Mech Eng H. 1998;212(5):357–72. [DOI] [PubMed] [Google Scholar]
- 28.Lu TW, Lin HC, Hsu HC. Influence of functional bracing on the kinetics of anterior cruciate ligament-injured knees during level walking. Clin Biomech (Bristol). 2006;21(5):517–24. [DOI] [PubMed] [Google Scholar]
- 29.Lowe WR, Warth RJ, Davis EP, Bailey L. Functional Bracing after Anterior Cruciate Ligament Reconstruction: a systematic review. J Am Acad Orthop Surg. 2017;25(3):239–49. [DOI] [PubMed] [Google Scholar]
- 30.Pietrosimone B, Blackburn JT, Harkey MS, Luc BA, Hackney AC, Padua DA, Driban JB, Spang JT, Jordan JM. Greater Mechanical Loading during walking is Associated with Less collagen turnover in individuals with Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2016;44(2):425–32. [DOI] [PubMed] [Google Scholar]
- 31.Teng HL, Wu D, Su F, Pedoia V, Souza RB, Ma CB, Li X. Gait characteristics Associated with a Greater increase in medial knee cartilage T(1rho) and T(2) Relaxation Times in patients undergoing Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2017;45(14):3262–71. [DOI] [PubMed] [Google Scholar]
- 32.Pietrosimone B, Seeley MK, Johnston C, Pfeiffer SJ, Spang JT, Blackburn JT. Walking ground reaction Force Post-ACL Reconstruction: analysis of time and symptoms. Med Sci Sports Exerc. 2019;51(2):246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Davis-Wilson HC, Pfeiffer SJ, Johnston CD, Seeley MK, Harkey MS, Blackburn JT, Fockler RP, Spang JT, Pietrosimone B. Bilateral gait 6 and 12 months Post-anterior Cruciate Ligament Reconstruction compared with controls. Med Sci Sports Exerc. 2020;52(4):785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paterno MV, Flynn K, Thomas S, Schmitt LC. Self-reported fear predicts functional performance and second ACL Injury after ACL Reconstruction and Return to Sport: a pilot study. Sports Health. 2018;10(3):228–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDevitt ER, Taylor DC, Miller MD, Gerber JP, Ziemke G, Hinkin D, Uhorchak JM, Arciero RA, Pierre PS. Functional bracing after anterior cruciate ligament reconstruction: a prospective, randomized, multicenter study. Am J Sports Med. 2004;32(8):1887–92. [DOI] [PubMed] [Google Scholar]
- 36.Luc-Harkey BA, Harkey MS, Pamukoff DN, Kim RH, Royal TK, Blackburn JT, Spang JT, Pietrosimone B. Greater intracortical inhibition associates with lower quadriceps voluntary activation in individuals with ACL reconstruction. Exp Brain Res. 2017;235(4):1129–37. [DOI] [PubMed] [Google Scholar]
- 37.Pietrosimone BG, Lepley AS, Ericksen HM, Gribble PA, Levine J. Quadriceps strength and corticospinal excitability as predictors of disability after anterior cruciate ligament reconstruction. J Sport Rehabil. 2013;22(1):1–6. [DOI] [PubMed] [Google Scholar]
- 38.Blackburn JT, Pietrosimone B, Harkey MS, Luc BA, Pamukoff DN. Quadriceps function and Gait Kinetics after Anterior Cruciate Ligament Reconstruction. Med Sci Sports Exerc. 2016;48(9):1664–70. [DOI] [PubMed] [Google Scholar]
- 39.Slater LV, Hart JM, Kelly AR, Kuenze CM. Progressive changes in walking kinematics and kinetics after Anterior Cruciate Ligament Injury and Reconstruction: a review and Meta-analysis. J Athl Train. 2017;52(9):847–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart HF, Culvenor AG, Collins NJ, Ackland DC, Cowan SM, Machotka Z, Crossley KM. Knee kinematics and joint moments during gait following anterior cruciate ligament reconstruction: a systematic review and meta-analysis. Br J Sports Med. 2016;50(10):597–612. [DOI] [PubMed] [Google Scholar]
- 41.Capin JJ, Khandha A, Zarzycki R, Arundale AJH, Ziegler ML, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics and tibiofemoral loading in men of the ACL-SPORTS randomized control trial. J Orthop Res. 2018;36(9):2364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Capin JJ, Zarzycki R, Ito N, Khandha A, Dix C, Manal K, Buchanan TS, Snyder-Mackler L. Gait mechanics in women of the ACL-SPORTS Randomized Control Trial: Interlimb Symmetry improves over Time regardless of Treatment Group. J Orthop Res. 2019;37(8):1743–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garcia SA, Johnson AK, Brown SR, Washabaugh EP, Krishnan C, Palmieri-Smith RM. Dynamic knee stiffness during walking is increased in individuals with anterior cruciate ligament reconstruction. J Biomech. 2023;146:111400. [DOI] [PubMed] [Google Scholar]
- 44.Gustafson JA, Anderton W, Sowa GA, Piva SR, Farrokhi S. Dynamic knee joint stiffness and contralateral knee joint loading during prolonged walking in patients with unilateral knee osteoarthritis. Gait Posture. 2019;68:44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.