Abstract
Background
Scarce evidence is available on the epidemiology of microbiologically proven clinical infections in patients admitted to the intensive care unit (ICU) after a great earthquake. The main aim of this study was to assess clinical infections and microbiological features in patients admitted to the ICU following the 2023 earthquake in the southeastern region of Türkiye with a focus on the timing of culture positivity during their ICU stay. The secondary objectives included determining antibiotic susceptibility patterns, identifying the types of antibiotics administered upon ICU admission, evaluating the appropriateness of antibiotic usage, assessing patient outcomes, and identifying factors that influence microbiologically confirmed clinical infections.
Methods
A retrospective, multicenter, observational study was conducted on adult earthquake victims admitted to the ICU after the 2023 earthquake in southeastern Türkiye. Patients were categorized into four groups on the basis of culture positivity timing at the 72-hour breakpoint and clinical characteristics were compared among these groups. Factors influencing microbiologically proven clinical infections were also analysed.
Results
A total of 107 earthquake-affected adults (58 females and 49 males, median [IQR] age: 37 [27–57] years) were analysed. Infection was present in 50.5% of the patients, predominantly with multidrug-resistant pathogens. Amputation (OR 5.30) and intermittent hemodialysis (OR 2.98) before ICU admission were independent predictors of infection.
Conclusions
This study demonstrated that half of the patients admitted to the ICU with earthquake-related injuries had microbiologically proven clinical infections, highlighting the early presence of multidrug-resistant pathogens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12879-024-10416-x.
Keywords: Crush syndrome, Infection, Antimicrobial resistance, Ineffective antibiotic treatment, Critical care, Critically-ill
Background
Earthquakes are natural disasters associated with profound and multifaceted effects on human health. On the 6th of February 2023, two devastating earthquakes (7.7 and 7.6 on the Richter scale) at the Kahramanmaras epicenter hit the southeast of Türkiye and Syria which occurred 9 h apart. The effects shatter and represent the strongest earthquake recorded in the last century of Türkiye. More than 50,000 people passed away and more than 100,000 people were injured. In addition, approximately half a million buildings were damaged because these earthquakes hit 11 provinces [1].
After an earthquake, a considerable number of deaths occur due to direct trauma to major organs. Crush syndrome and acute kidney injury (AKI) are significant causes of morbidity and mortality. However, among rescued earthquake victims, infections especially those complicated with sepsis appear to be important reasons for morbidity and mortality [2]. Bacteria tend to aggregate within necrotic muscle tissues through open wounds, subsequent fasciotomy procedures, or inadequate wound debridement, thereby increasing the risk of wound infection [3]. Additionally, catheter utilization, especially if inserted as emergency procedures, disrupts the usual anatomical barriers contributing to infection. After the earthquake, rescue operations were hindered by several factors, including a high volume of patients, transportation infrastructure damage, disrupted communication networks, and a shortage of healthcare personnel. As a result, the healthcare service infrastructure deteriorated, and emergency healthcare services were provided under suboptimal conditions, which increased the susceptibility of earthquake victims to healthcare-associated infections [4, 5]. In addition, immunological changes following multi-trauma, also known as ‘immune depression’ and ‘immuno-paralysis,’ may increase the risk of infection in these patients. Several mechanisms contribute to this phenomenon, including the release of stress hormones, auto-oxidative receptor injury, the activation of inflammatory pathways, an imbalance between inflammatory and anti-inflammatory responses, and immune cell dysfunction, as well as changes in natural killer cell activity [6].
Owing to the nature and location of disasters, a wide variety of infectious complications have caused various healthcare problems following previous earthquakes, and managing community and healthcare-acquired infections from different sources is critical and poses serious challenges [7–10]. Moreover, infections caused by multi-drug-resistant microorganisms are of concern; however, little is known regarding their importance in these patient groups [11]. Such devastating and powerful earthquakes are rare and can occur in different parts of the world, creating a research gap in studies that include only critically ill patients from different parts of the world. In addition, previous studies have identified different predominant microbiological agents in this patient population [7, 11].
The primary aim of this study was to determine the clinical infections and microbiological characteristics of patients admitted to the ICU after the earthquake in the southeastern part of Türkiye in 2023 concerning the timing of culture positivity during the ICU stay. The secondary aims were to determine, antibiotic susceptibility patterns, types of antibiotics administered at ICU admission, appropriateness of antibiotic use, and patient outcomes, and to identify factors influencing microbiologically proven clinical infections.
Methods
Study design and patients
This is a retrospective, multi-center, observational study conducted between February 6th and March 1st, 2023, in six ICUs of three tertiary referral hospitals that admitted earthquake victims. All adult earthquake victims (> 18 years old) who admitted to these ICUs from the emergency department, wards, or transferred from other hospitals were enrolled in the study. Patients admitted to ICUs for reasons other than earthquake-related injuries and those under 18 years of age were excluded from the study. Ethical approval was obtained with reference number AEŞH-EKI-2023-396 on 26 July 2023. Owing to the retrospective observational study design, informed consent from the patients was waived.
Patients were stratified into four groups based on the timing of culture positivity: no culture positivity, culture positivity only within the first 72 h, only after 72 h, and both within and after 72 h of ICU admission (Fig. 1). Patients in Groups 2, 3, and 4 were considered to have microbiologically proven clinical infections.
Fig. 1.
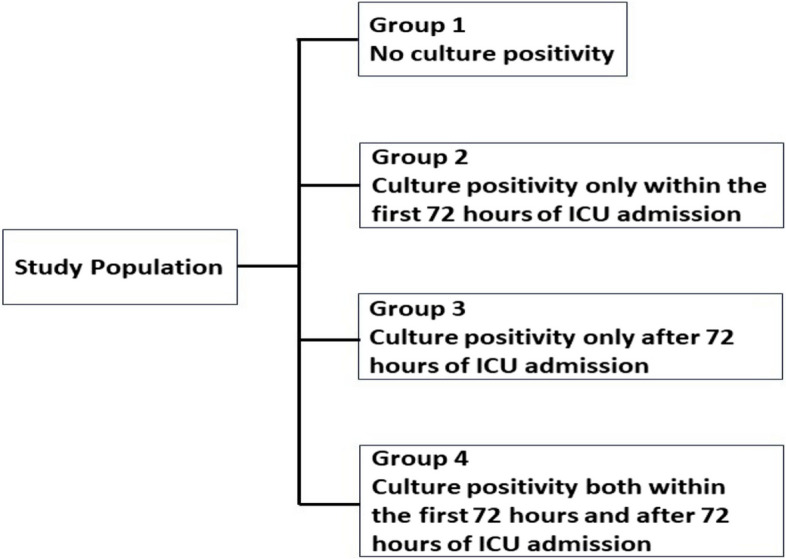
Categorization of patients based on the timing of culture positivity
Variables
A standardized data collection process was implemented using an Excel form. Demographic data, comorbidities, injury types, microbiological culture results, antibiotic susceptibility results, antibiotic usage, and patient outcomes were all entered by the responsible investigators for each hospital. After data entry, all information was anonymized and compiled for analysis. Variables were collected from the hospitals’ electronic health record systems and patient charts. The collected variables and microbiological sample collection procedures for culture analysis are outlined in the Supplementary File.
Microbiologically proven clinical infections were reported as (i) culture positivity within the first 72 h of ICU admission, and (ii) only the first microorganism isolated from the first infection episode for each distinct source after the first 72 h of ICU admission, antibiotic susceptibility results of isolated microorganisms within the first 72 h of ICU admission, the presence of antibiotic use before ICU admission, and the choice of the first antibiotic (initiated within the first 72 h) in the ICU were recorded.
Definitions
Crush syndrome was diagnosed when crush injuries and edema were accompanied by AKI and/or multiple organ failure involving organs other than the kidney [12, 13].
Compartment syndrome was diagnosed through clinical examination. AKI was assessed by the Kidney Disease Improving Global Outcomes (KDIGO) criteria [14].
Sepsis and septic shock were diagnosed according to the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [15].
Microbiologically proven clinical infections were defined as cases in which a patient exhibited symptoms such as fever or signs of sepsis, along with elevated inflammatory markers (e.g., CRP, procalcitonin) or radiological findings suggestive of an infectious condition. These findings were further supported by microbiological evidence (e.g., culture positivity), as assessed and confirmed by the consulting physician. Definitions of specific infections are given in the Supplementary File.
ICU-acquired infections were defined according to the European Center for Disease Prevention and Control (ECDC) criteria [16] and were considered when they occurred 72 h after admission to the ICU and were not present or in the incubation phase at the time of admission. Microbiologically proven clinical infections within the first 72 h of ICU admission were defined as early-onset infections.
Polymicrobial growth was defined as the isolation of more than one different species of pathogenic microorganism in a single sample.
Multidrug-resistance (MDR) was defined as non-susceptibility to at least one agent in three or more antimicrobial categories, extensively drug-resistance (XDR) was defined as non-susceptibility to at least one agent in all but two or fewer antimicrobial categories (i.e. bacterial isolates remained susceptible to only one or two categories) and, pandrug-resistance (PDR) was defined as non-susceptibility to all agents in all antimicrobial categories (i.e., no agents tested as susceptible for that organism) according to the Centers for Disease Control and Prevention (CDC) criteria [17].
Statistical analysis
Demographic and clinical characteristics before and during ICU admission were compared among these groups and the outcomes were observed throughout the ICU stay. The effects of factors present at the time of ICU admission on microbiologically proven clinical infections were analysed.
The results are presented as medians and interquartile ranges (IQRs) for continuous variables and frequencies and percentages for categorical variables. The predictive value of the time stuck under the rubble for microbiologically proven clinical infections was assessed via receiver-operating characteristics (ROC) curves. The cut-off value was determined on the basis of the Youden index for the determination of optimal sensitivity, specificity, and positive and negative predictive values. Chi-square, Fisher’s exact test, Mann-Whitney U test, or Kruskal-Wallis test were used for comparisons between groups, depending on the appropriateness of the data. Significant variables identified from the univariate analysis were included in the binary logistic regression model to identify independent predictors of microbiologically proven clinical infections. Two-tailed p-values less than 0.05 were regarded as statistically significant. All analyses were conducted via the SPSS 22 IBM® statistics program (IBM Inc., Armonk, NY, USA).
Results
A total of 107 earthquake victims, consisting of 58 females and 49 males, with a median age of 37 [27–57] years, were included in this study. Time stuck under rubble and length of stay at the initial healthcare facility were 12 [7–32] and 40 [17–76] hours respectively. The demographic and clinical characteristics of the patients before and at the time of ICU admission are shown in Table 1.
Table 1.
Clinical characteristics of the patients before and at admission to the intensive care unit
| Variables | n=107 |
|---|---|
| Age (yrs)a | 37 [27-57] |
| Female sex, n (%) | 58 (54.2) |
| Comorbidities, n (%) | 31 (28.9) |
| Diabetes mellitus | 16 |
| Hypertension | 15 |
| Hypothyroidism | 7 |
| Cardiovascular diseases | 7 |
| Respiratory diseases | 3 |
| Others | 3 |
| Time stuck under rubble (hrs),a (n=96) | 12 [7-32] |
| Length of stay at the initial healthcare facility (hrs),a (n=85) | 40 [17-76] |
| Admission reason, n (%) | |
| Crush syndrome | 70 (65.4) |
| Postoperative | 27 (25.2) |
| Others | 10 (9.4) |
| APACHE II Scorea | 15 [12-20] |
| SOFA Scorea | 3 [2-5] |
| GCS Scorea | 15 [15-15] |
| Revised trauma scorea | 12 [12-12] |
| Fasciotomy before admission, n (%) | 40 (37.4) |
| Amputation before admission, n (%) | 16 (15.0) |
| Acute kidney injury at admission, n (%) | 65 (60.7) |
| Intermittent hemodialysis before admission, n (%) | 29 (27.1) |
| Antibiotherapy before admission, n (%) (n=58) | 15 (25.9) |
| Leukocyte count (103/µL)a | 13.7 [10.2-19.2] |
| CRP (mg/dl)a | 10.5 [6.1-16.7] |
| Procalcitonin (ng/ml)a | 1.78 [0.32-6.22] |
APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, GCS Glasgow Coma Scale, CRP C-Reactive Protein
amedian [IQR], others n (%)
Fifty-four patients (50.5%) had microbiologically proven clinical infections. The number and type of all the isolated microorganisms throughout the ICU stay are shown in Supplementary Table 1. There were 171 isolated microorganisms 24.6% of which were Acinetobacter baumanii, 16.4% were Klebsiella pneumoniae, 16.4% were Enterococcus spp., 10.5% were Escherichia coli, and 9.9% were Pseudomonas aeruginosa. Among the 54 patients with positive culture results, 46.2% had polymicrobial growth. There was a significant difference between the patient groups in terms of the frequency of polymicrobial growth (0% in Group 2, 38.2% in Group 3, and 100% in Group 4, respectively; p < 0.001). Among the microorganisms isolated from Group 2 and Group 3, gram-negative pathogens were predominant (87.5% and 73.6%, respectively). Conversely, in Group 4, the frequency of both gram-negative and gram-positive microorganisms was greater (83.3% for both).
Twenty patients had 23 culture positivity within 72 h of ICU admission. On the other hand, considering the first positive culture results from all sources, 34 patients had 53 positive cultures after 72 h of ICU admission. Sources and isolated microorganisms within and after 72 h of ICU admission are shown in Supplementary Table 2.
The antibiotic susceptibility results of the microorganisms isolated within the first 72 h of ICU admission are shown in Table 2. All 8 Acinetobacter baumannii isolates were XDR; however, colistin susceptibility was tested in only 4 of them, and these isolates were found to be colistin-sensitive. However, since colistin susceptibility was not tested in the other 4 isolates, it is unknown whether they had PDR. Among the 4 Klebsiella pneumoniae isolates, 1 was MDR, 2 were XDR, and 1 was PDR. Additionally, 3 of the K. pneumonia isolates were carbapenem-resistant. All Enterococcus spp. isolates were resistant to ampicillin but susceptible to vancomycin. Two of the Enterobacter cloacae isolates were MDR. Two of the three Escherichia coli isolates were MDR, and one was XDR.
Table 2.
Antibiotic susceptibility results of microorganisms isolated in the first 72 h of ICU admission according to the source of infection
| Sources | Ampicillin/Methicillin | Ampicillin-Sulbactam | Vancomycin | Sulfamethoxazole-trimethoprim | Ceftriaxone | Cefepime | Ceftazidime | Piperacillin-Tazobactam | Amikacin | Gentamicin | Ciprofloxacin | Carbapenem | Colistin |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wound | |||||||||||||
| A. baumannii 1 | R | R | R | R | R | R | |||||||
| A. baumannii 2 | R | R | R | R | R | R | R | R | R | ||||
| A. baumannii 3 | R | R | R | R | R | R | R | R | R | R | S | ||
| A. baumannii 4 | R | R | R | R | R | R | R | R | R | R | |||
| A. baumannii 5 | R | R | R | R | R | R | R | R | R | R | |||
| Enterococcus spp. 1a | R | S | |||||||||||
| Enterococcus spp. 2a | R | S | |||||||||||
| Enterococcus spp. 3a | R | S | |||||||||||
| Enterococcus spp. 4a | R | S | |||||||||||
| E. cloacae 1 | R | S | R | S | R | R | S | R | |||||
| E. cloacae 2 | R | S | S | S | S | S | S | ||||||
| E. cloacae 3 | R | S | R | S | R | S | S | ||||||
| K. pneumonia | R | R | R | R | R | R | R | R | R | R | S | ||
| P. aureginosa | S | R | S | R | R | ||||||||
| Proteus penneri | R | S | R | S | |||||||||
| MRSA | R | S | |||||||||||
| Lower respiratory tract | |||||||||||||
| A. baumannii 1 | R | R | R | R | R | R | R | R | R | S | |||
| A. baumannii 2 | R | R | R | R | R | R | R | R | R | S | |||
| S. pneumoniae | S | S | |||||||||||
| H. influenzae | S | S | |||||||||||
| Citrobacter freundii | S | S | S | S | |||||||||
| S. maltophilia | R | S | S | S | S | ||||||||
| Aspergillus nigerb | |||||||||||||
| Urine | |||||||||||||
| K.pneumonia 1 | R | R | R | R | R | R | S | R | S | ||||
| K.pneumonia 2 | R | R | R | R | R | R | R | R | R | R | S | ||
| E. coli 1 | R | S | R | R | S | S | R | S | |||||
| E. coli 2 | R | R | S | S | R | S | |||||||
| C. glabratab | |||||||||||||
| Central venous linec | |||||||||||||
| K.pneumonia | R | R | R | R | R | R | R | R | R | R | R | R | |
| E. coli | R | R | R | R | R | R | R | R | R | ||||
| MRSA | R | S | S | R | |||||||||
| Blood | |||||||||||||
| A. baumannii | R | R | R | R | R | R | R | R | S | ||||
| MRSA | R | S | S | S | R | ||||||||
MRSA Methicillin-resistant Staphylococcus aureus
aThree of the four Enterococcus spp. isolated in the cultures were Enterococcus faecium, and one was Enterococcus faecalis
bCandida glabrata, and Aspergillus niger were sensitive to all antifungal agents including the azole group
cBlood obtained through the central venous line denotes CLABSI which was defined in the definition section of the supplementary file
Within 72 h of ICU admission, antibiotics were administered to 99 patients (92.5%), with 79.8% receiving coverage for anaerobic infections. The most prescribed antibiotics were Ciprofloxacin + Clindamycin, Ceftriaxone + Metronidazole, and Cefazoline + Metronidazole. The types of antibiotics administered are shown in detail in Supplementary Table 3. Among these patients, 51.5% had positive culture results, and only 6 (11.7%) received appropriate antibiotic therapy targeted at the initially isolated pathogen.
The comparison of patients’ clinical variables and outcomes among the four groups, according to the presence and timing of culture positivity is summarized in Table 3. The time stuck under rubble significantly differed among the groups (p = 0.025). Compared with patients in Groups 1 and 2, patients in Groups 3 and 4 remained stuck under the rubble for a longer duration. The presence of compartment syndrome before ICU admission significantly differed among the groups (p = 0.033). Patients in Groups 3 and 4 had higher rates of compartment syndrome than Group 1 and 2 patients did. There was a slight difference in the incidence of fasciotomy before ICU admission among the groups (p = 0.051); patients in Groups 3 and 4 had a higher rate of fasciotomy performed before ICU admission than those in Groups 1 and 2. However, there was a significant difference in the frequency of amputation before ICU admission among the patient groups (p = 0.007). In Group 3, 32.4% of patients underwent amputation before ICU admission, which was higher than the rates observed in Groups 1, 2, and 4. While there were no statistically significant differences between patient groups regarding the presence of crush syndrome (p = 0.21) and AKI (p = 0.24) upon ICU admission, there was a notable distinction between the groups concerning the application of intermittent hemodialysis before ICU admission (p = 0.007). Intermittent hemodialysis was administered to more than half of the patients in Group 4 (58.3%) before ICU admission. The incidence of sepsis was greater among patients in Groups 3 and 4 than in Groups 1 and 2 (p < 0.001). However, there was no statistically significant difference in the development of septic shock between the groups (p = 0.09). There was a significant difference in the ICU and hospital length of stay (LOS) among the four groups (p = 0.001 and p < 0.001, respectively). Patients in Groups 3 and 4 had longer ICU stays than those in Groups 1 and 2. ICU and hospital mortality rates were 9.3% and 10.3%, respectively. No significant difference was noted among the groups in terms of ICU and hospital mortality.
Table 3.
Comparison of patients’ clinical variables and outcomes according to the presence and timing of culture positivity
| Variables | All patients n = 107 |
Group 1 n = 53 |
Group 2 n = 8 |
Group 3 n = 34 |
Group 4 n = 12 |
p value |
|---|---|---|---|---|---|---|
| Age (yrs)a | 37 [27–57] | 39 [27.5–57.5] | 56 [26.5–66.5] | 32 [26.7–56.2] | 38 [28.2–43.5] | 0.60 |
| Female sex, n (%) | 58 (54.2) | 31 (58.5) | 5 (62.5) | 16 (47.1) | 6 (50) | 0.70 |
| Comorbidity, n (%) | 14 (26.4) | 14 (26.4) | 4 (50) | 10 (29.4) | 3 (25) | 0.58 |
| Time stuck under rubble (hrs),a (n = 96) | 12 [7–32] | 9 [5–23] | 8 [3–15] | 15 [8–36] | 25 [8–39] | 0.025 |
| LOS at the initial healthcare facility (hrs),a (n = 85) | 40 [17–76] | 24 [15–63] | 48 [29.5–87.5] | 39 [21.7–72] | 72 [24–144] | 0.35 |
| APACHE II Scorea | 15 [12–20] | 14 [12–18] | 16 [11–19] | 17 [12–22] | 20 [9.5–25.7] | 0.45 |
| SOFA Scorea | 3 [2–5] | 2 [1–4] | 3 [1–5] | 4 [2–5] | 5 [1–6] | 0.31 |
| GCS Scorea | 15 [15–15] | 15 [15–15] | 15 [14.25–15] | 15 [14.7–15] | 15 [15–15] | 0.67 |
| Revised trauma scorea | 12 [12–12] | 12 [12–12] | 12 [12–12] | 12 [12–12] | 12 [11.2–12] | 0.36 |
| Compartment syndrome before admission, n (%) | 49 (45.8) | 18 (34) | 3 (37.5) | 9 (55.9) | 9 (75) | 0.033 |
| Fasciotomy before admission, n (%) | 40 (37.4) | 14 (26.4) | 3 (37.5) | 15 (44.1) | 8 (66.7) | 0.051 |
| Amputation before admission, n (%) | 16 (15.0) | 3 (5.7) | 1 (12.5) | 11 (32.4) | 1 (8.3) | 0.007 |
| Crush syndrome, n (%) | 70 (65.4) | 30 (69.8) | 7 (87.5) | 25 (73.5) | 8 (66.7) | 0.21 |
| Acute kidney injury, n (%) | 65 (60.7) | 31 (58.5) | 6 (75) | 18 (52.9) | 10 (83.3) | 0.24 |
| IHD before admission, n (%) | 29 (27.1) | 8 (15.1) | 4 (50) | 10 (29.4) | 7 (58.3) | 0.007 |
| Sepsis during ICU stay, n (%) | 39 (36.4) | 9 (17) | 3 (37.5) | 19 (55.9) | 8 (66.7) | < 0.001 |
| Septic shock during ICU stay, n (%) | 19 (17.8) | 5 (9.4) | 1 (12.5) | 9 (26.5) | 4 (33.3) | 0.09 |
| IMV during ICU stay, n (%) | 34 (31.8) | 13 (24.5) | 2 (25) | 14 (41.2) | 5 (41.7) | 0.34 |
| Vasopressor therapy during ICU stay, n (%) | 20 (18.7) | 6 (11.3) | 1 (12.5) | 9 (26.5) | 4 (33.3) | 0.16 |
| RRT during ICU, n (%) | 42 (39.3) | 14 (26.4) | 4 (50) | 17 (50) | 7 (58.3) | 0.06 |
| IHD | 32 | 10 | 3 | 15 | 4 | |
| IHD/CRRTb | 8 | 3 | 1 | 2 | 2 | |
| CRRT | 2 | 1 | 0 | 0 | 1 | |
| LOS in ICU (days) | 5 [2–9] | 4 [1.5–6] | 3 [2–7] | 8.5 [5–17] | 6.5 [2–19] | 0.001 |
| LOS in hospital (days) | 22 [11–46] | 15 [8–28] | 15 [7.5–34.5] | 37.5 [20–52] | 50 [29.5–91.5] | < 0.001 |
| ICU mortality, n (%) | 10 (9.3) | 5 (9.4) | 1 (12.5) | 2 (5.9) | 2 (16.7) | 0.72 |
| Hospital mortality, n (%) | 11 (10.3) | 5 (9.4) | 1 (12.5) | 3 (8.8) | 2 (16.7) | 0.87 |
LOS Length of stay, APACHE Acute Physiology and Chronic Health Evaluation, SOFA Sequential Organ Failure Assessment, GCS Glasgow Coma Scale, IHD Intermittent hemodialysis, IMV Invasive mechanical ventilation, RRT Renal replacement therapy, CRRT Continuous renal replacement therapy
amedian [IQR], others n (%), bBoth intermittent hemodialysis and CRRT were administered to 8 patients during ICU follow-up
A comparison of patients’ leukocyte counts, and acute phase reactants is presented in Supplementary Table 4.
Multivariate logistic regression analysis for microbiologically proven clinical infections is presented in Table 4 which revealed that amputation (OR 5.30 [1.03–27.36], p = 0.046) and intermittent hemodialysis before ICU admission (OR 2.98 [1.04–8.52], p = 0.043) were found to be independent variables predicting infection. The ROC curve for microbiologically proven clinical infections revealed that patients who were stuck under the rubble for ≥ 11.5 h had an area under the curve (AUC) of 0.64 [0.53–0.75] (p = 0.019).
Table 4.
Multivariate logistic regression model for the development of microbiologically proven clinical infectiona
| Variable | OR [95% CI] | p-value |
|---|---|---|
| Time stuck under the rubble (for each hour) | 1.01 [0.99–1.03] | 0.28 |
| Compartment syndrome at admission | 1.24 [0.37–4.19 | 0.73 |
| Fasciotomy before ICU admission | 1.74 [0.50–6.07] | 0.39 |
| Amputation before ICU admission | 5.30 [1.03–27.36] | 0.046 |
| Intermittent hemodialysis before ICU admission | 2.98 [1.04–8.52] | 0.043 |
OR Odds Ratio, CI Confidence Interval
aGroup 2, 3, and 4 patients versus Group 1
Discussion
The present study provides valuable insights into potential pathogens and resistance patterns for future large-scale disasters by examining clinical findings and analyses related to infection risk in victims of earthquake-related injuries. In our cohort, half of the patients who were admitted to the ICU after the earthquake had microbiologically proven clinical infections. The top five identified microorganisms within 72 h of ICU admission were Acinetobacter baumannii, Klebsiella pneumoniae, Enterococcus spp, Escherichia coli, and Enterobacter cloacae. Nearly all of these isolates (except for one Enterobacter cloacae isolate) were MDR. Only 11.7% of patients received appropriate antibiotic therapy within 72 h of ICU admission that initially targeted isolated pathogens. Patients who experienced longer periods of sticking under rubble, who had compartment syndrome, and who underwent fasciotomy before ICU admission were more likely to have infections. Amputation history and the administration of intermittent hemodialysis before ICU admission were identified as independent variables for predicting infections.
The incidence of infections varies according to not only where the study was derived from as the place of the earthquake but also the reason for admission to the hospital and the definitions of infection. In studies conducted after the Wenchuan earthquake, the culture positivity rates varied between 54 and 67.2% [4, 18, 19]. In another multicenter retrospective study following the Wenchuan earthquake, the infection rate at the time of admission for 533 hospitalized patients due to earthquake-related injuries was reported to be 13% [20]. After the Marmara earthquake; Kazancioglu et al. reported that the culture positivity rate was 60% [21], whereas the infection rate was reported to be 23.6–34.9% in other studies [22, 23]. In this study, the microbiologically proven clinical infection rate was determined to be 50.5%. Notably, while previous studies included hospitalized earthquake victims, this study focused on critically ill patients with earthquake-related injuries admitted to the ICU.
Due to the inherent nature of earthquake disasters, an increase in the frequency of infections is inevitable. Major earthquakes have consistently resulted in significantly high rates of infections associated with Acinetobacter baumannii [11]. Acinetobacter baumannii was also the most frequently isolated microorganism in this study, followed by Klebsiella pneumoniae, Enterococcus spp., Escherichia coli, and Pseudomonas aeruginosa during the ICU stay. In the study conducted by Keven et al. [23], the most common pathogens causing sepsis in crush syndrome patients were Acinetobacter spp., Pseudomonas spp., Klebsiella spp., Enterobacter spp., and Staphylococcus aureus. In our study, Staphylococcus aureus was detected only in 4 isolates. Enterococcus spp. were the predominant gram-positive microorganisms in this study. This data was consistent with the results of the study conducted by Xiaolei et al. [19]. According to data from the Türkiye National Health Service-Associated Infection Surveillance Network (2022), the distribution of pathogens associated with healthcare-associated infections in Türkiye is 40.3% for non-fermentative gram-negative bacteria (23.7% Pseudomonas spp., 16% Acinetobacter spp.), 36.7% for Enterobacterales (16.1% Klebsiella spp., 13.3% E. coli), and 21.2% for gram-positive cocci (8% Staphylococcus aureus, 6.4% Enterococcus spp., and 6.3% coagulase-negative Staphylococcus) [24]. This data does not exclusively represent ICU infections and therefore limits a direct comparison. Our study results indicate a relatively high prevalence of Acinetobacter spp. and Enterococcus spp. However, as our patient population consists exclusively of individuals admitted to intensive care due to earthquake-related injuries, factors such as soil contact, emergency interventions, and/or invasive procedures performed under less-than-optimal infection control conditions, and the presence of open wounds may explain this discrepancy in microbial distribution.
The results of previous studies involving patients with earthquake-related injuries revealed that gram-negative pathogens were isolated in a range of 73.2–89% of cases, whereas gram-positive pathogens were isolated in a range of 4.5–24.4% [5, 18, 21–23, 25, 26]. In the present study, 77.9% of the bacterial isolates obtained from various samples were gram-negative microorganisms, whereas 22.1% were gram-positive. Notably, no anaerobic pathogens were isolated in this study, which may be attributed to the meticulous nature of anaerobes, making them difficult to culture and isolate and often resulting in their absence from clinical samples. The frequency of polymicrobial growth reported in various studies ranged from 41 to 59.6% [18, 19, 26]. Polymicrobial growth was observed in 46.2% of the patients in our cohort.
Previous studies on earthquake-related patients have shown that the majority of isolated microorganisms exhibit multidrug resistance, although their resistance profiles may vary [11]. In a study conducted by Oncul et al. [22] after the 1999 Marmara earthquake, two strains of Acinetobacter baumannii and one strain of Pseudomonas aeruginosa were resistant to all antibiotics including carbapenems. In another study reported by Tao et al. [25] after the Wenchuan earthquake, > 65% of Acinetobacter isolates were resistant to a wide range of antimicrobial drugs, except imipenem and 24.6% of isolates were PDR. In this study, the predominant microorganisms cultured within the first 72 h after admission to the ICU were Acinetobacter baumannii, Klebsiella pneumoniae, Enterococcus spp., Enterobacter cloacae, and Escherichia coli. According to the antibiotic susceptibility results, all the cultured Acinetobacter baumannii isolates were XDR, one Klebsiella pneumonia isolate was MDR, two were XDR, and one was PDR. All the Enterococcus spp. isolates were ampicillin-resistant but vancomycin-sensitive. Although Staphylococcus aureus was not the dominant gram-positive pathogen in this study, all strains isolated within the first 72 h of ICU admission were methicillin-resistant. Two Enterobacter cloacae isolates were MDR, two Escherichia coli isolates were MDR, and one was XDR. There could be several explanations for the early detection of MDR pathogens in patients following admission to the ICU. A significant portion of patients had sought care at another healthcare facility before being admitted to the ICU. Additionally, in Türkiye, the widespread emergence of antibiotic resistance, especially among gram-negative pathogens such as Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa, may have contributed to this situation [27].
In 2013, the CDC Emergency Wound Management guidelines recommended the use of beta-lactam antibiotics with anti-staphylococcal activity (such as cephalexin, dicloxacillin, ampicillin/sulbactam, etc.) and clindamycin as initial antimicrobial treatments for infected wounds [28]. Furthermore, it was stated in the 2013 guidance for wound infection management by the World Health Organization (WHO) that Penicillin G and metronidazole are recommended as initial antimicrobial treatments [29]. The European Renal Best Practice (ERBP) and the Renal Disaster Relief Task Force (RDRTF) of the International Society of Nephrology (ISN) recommend preemptively using cefazolin and ciprofloxacin antibiotherapy in patients with fasciotomy and/or open fractures in their “Recommendations for the Management of Crush Victims in Mass Disasters” guidelines published in 2012 [30]. This study revealed that antibiotherapy was initiated within 72 h of ICU admission in 92.5% of patients, and the most commonly administered antibiotic regimens were ciprofloxacin + clindamycin, ceftriaxone + metronidazole, and cefazoline + metronidazole. Nonetheless, when the antibiotic susceptibilities of the initially isolated pathogens were considered appropriate antibiotherapy was initiated in only 11.7% of the patients. One potential explanation is that most of these patients probably received antibiotics at the start of treatment, which may increase the risk of drug resistance in subsequent infections. Another factor may be that many patients with early infections likely acquired these infections from the hospital environment, where antimicrobial resistance is more common. Obviously, environmental antibiotic-resistant pathogens may also play a role in this context [31]. These wounds are likely to be contaminated with dust and water, which may harbor drug-resistant pathogens, potentially offering another explanation. The local ICU ecology remains a significant risk factor for acquiring MDR infections regardless of the time of admission and it may be linked to the high incidence of MDR organisms. In practical clinical settings, when the duration of being stuck under the rubble is prolonged and in the presence of risk factors such as a history of amputation or the requirement for IHD before hospital admission, antibiotherapy could be individualized, and broad-spectrum agents, including those that provide coverage for MDR pathogens, may be initiated. Our findings support the need to consider local susceptibility patterns in empiric antibiotic choices, prescribing appropriate antibiotics also taking into consideration the patient’s age and comorbidities [32]. Moreover, basic infection control strategies are essential in disaster management. Disaster response plans must be well established in advance.
Infection in earthquake-related patients can be attributed to various factors, including traumatic injuries, the time stuck under rubble, contact with soil and sand, invasive procedures such as fasciotomy and amputation, and immunological changes following trauma. Studies conducted in patients with earthquake-related injuries have shown that the time stuck under the rubble increases the risk of infection [4, 19, 22]. In addition, Zhang et al., as well as Xiaolei et al., demonstrated that prolonged time stuck under the rubble is an independent risk factor for infection, increasing the risk of infection by 2.25 and 1.06 fold, respectively [4, 19]. Consistent with these findings, our results demonstrated that patients with microbiologically proven clinical infections had a longer duration of time stuck under the rubble than those without culture positivity (p = 0.018). Additionally, our findings revealed that a duration of time stuck under the rubble exceedingly approximately 12 h may predict microbiologically proven clinical infections.
Fasciotomy is a common procedure in the management of compartment syndrome, but its necessity and timing remain topics of ongoing debate. In studies conducted after earthquakes, fasciotomy and amputation increased the risk of sepsis and infections [4, 20, 23, 33]. Furthermore, in the study conducted by Xiaolei et al., fasciotomy was found to be an independent risk factor for infection, leading to a 5.47-fold increase in infection risk [19]. In line with these results, we found that the frequency of fasciotomy and amputation before ICU admission was significantly greater in patients with microbiologically proven clinical infections than in those without culture positivity (p = 0.028 and p = 0.013 respectively). Moreover, in this study, amputation before ICU admission was found to be an independent risk factor that increased the rate of microbiologically proven clinical infections by 5.3-fold.
As a patient’s clinical condition deteriorates, the risks of both kidney injury and infection can increase. After the Marmara earthquake, it was demonstrated that in patients with earthquake-related injuries, RRT increased the risk of infection [23]. In a study conducted by Xiaolei et al. involving patients with crush syndrome after the Wenchuan earthquake, RRT was identified as a risk factor for the development of infection. Furthermore, in the same study, the duration of renal impairment was identified as an independent risk factor for infection development and was shown to increase the risk of infection by 1.09 times [19]. In this study, the administration of IHD before ICU admission independently increased the number of microbiologically proven clinical infections by 3-fold. Patients undergoing intermittent IHD may be at an increased risk of infection due to central venous catheterization. This risk is particularly significant in the aftermath of a major earthquake, when healthcare resources are constrained, and patient volumes are exceptionally high.
This study has several limitations to consider. First, the ability to generalize the results is a major concern due to the retrospective study design and the inclusion of a limited number of patients from three centers. Additionally, the lack of a control group, which could introduce selection bias during data collection, is another major limitation of this study. Without a control group, it is challenging to establish a baseline for comparison, potentially impacting the validity of our findings. This limitation may affect the accuracy and reliability of the data, as the study’s design does not account for variables that a control group could help to neutralize. However, due to the inclusion of patients without culture positivity (Group 1) in our study groups, this group could also be considered as a control group. Second, it is also important to note that there may be discrepancies or missing information in the data recorded in the patient files. The challenging circumstances and large influx of victims at the epicenter of the earthquake, from which patients were transferred, may have resulted in incomplete or inconsistent patient records and prohibited us from analysing all infections and antibiotic utilization throughout the ICU stay. Third, we lack detailed information about the healthcare facilities where the patients were treated first, which may have led to the occurrence of healthcare-associated infections upon ICU admission. Therefore, caution should be exercised when these findings are applied to populations with different demographics, healthcare settings, or regions. Finally, in the study design, although culture positivity was assessed alongside clinical and laboratory findings to define microbiologically proven clinical infections, a complete distinction between colonization and true infection may not have been fully achievable in all cases due to the complex clinical conditions of the patients. Additionally, although we have considered a 72-hour period as the cut-off time for discriminating early versus late ICU-acquired infections, variations in the length of stay at the previous facility may also influence the results.
Conclusion
In conclusion, half of the patients admitted to the ICU following the earthquake had microbiologically proven clinical infections. MDR pathogens were isolated from patients, even in the early stages of ICU admission. There is a high likelihood of microbiologically proven clinical infections among patients who have been stuck under rubble for more than 12 h, who have undergone invasive procedures such as fasciotomy and amputation, and who have required hemodialysis before admission to the ICU. To mitigate these heightened risks, early detection of potential infections and prompt initiation of appropriate antimicrobial therapy might be important to enhance patient outcomes and potentially save lives in the face of future massive disasters.
Supplementary Information
Acknowledgements
Not applicable.
Abbreviations
- AKI
Acute Kidney Injury
- APACHE
Acute Physiology and Chronic Health Evaluation
- CDC
Center for Disease Prevention and Control
- CI
Confidence Interval
- CRP
C-Reactive Protein
- CRRT
Continuous Renal Replacement Therapy
- ECDC
European Center for Disease Prevention and Control
- ERBP
The European Renal Best Practice
- GCS
Glasgow Coma Scale
- ICU
Intensive Care Unit
- IHD
Intermittent Hemodialysis
- IMV
Invasive Mechanical Ventilation
- IQR
Interquartile Range
- ISN
International Society of Nephrology
- KDIGO
Kidney Disease Improving Global Outcomes
- LOS
Length of Stay
- MDR
Multidrug-Resistant
- MRSA
Methicillin-Resistant Staphylococcus Aureus
- OR
Odds Ratio
- PDR
Pandrug-Resistant
- RDRTF
The Renal Disaster Relief Task Force
- ROC
Receiver-operator Characteristics Curve
- RRT
Renal Replacement Therapy
- Sepsis-3
Third International Consensus Definitions for Sepsis and Septic Shock
- SOFA
Sequential Organ Failure Assessment
- WHO
World Health Organization
- XDR
Extensively Drug-Resistant
Authors’ contributions
All authors contributed to the study conception and design. EKK, BH, GG, and AT conceptualized and supervised the study. EKK, BH, GG, KG, and AT designed the methodology. EKK, BH, GG, MY, AES, EG, MS, BES, RCY, ASK, AEH, BK, SBA, EOE, KG, and AT were responsible for the administration and acquired the data. EKK and BH curated the data. EKK, BH, and AT conducted the statistical analyses. EKK, BH, GG, and AT interpreted the data. EKK, BH, and AT wrote the original draft of the manuscript. JR critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Funding
The authors have no relevant financial or non-financial interests to disclose.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
In this retrospective study, individual informed consent from participants was not required as patient data were anonymized and analyzed without direct identifiers. The need for informed consent was waived by the Non-Interventional Ethics Committee of Etlik City Hospital, Ankara, Türkiye (approval number: AEŞH-EKI-2023-396). The study was conducted in accordance with the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.https://www.sbb.gov.tr/wp-content/uploads/2023/03/2023-Kahramanmaras-ve-Hatay-Depremleri-Raporu.pdf.
- 2.Najafi S, Rezayat AA, Beyzaei SF, Shahriari Z, Nour MG, Mosaed R, et al. Incidence of infectious diseases after earthquakes: a systematic review and meta-analysis. Public Health. 2022;202:131–8. [DOI] [PubMed] [Google Scholar]
- 3.Brook I, Frazier EH. Aerobic and anaerobic microbiology of infection after trauma. Am J Emerg Med. 1998;16(6):585–91. [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Zeng JW, Wang Gl TuCQ, Huang FG, Pei FX. Infectious complications in patients with crush syndrome following the Wenchuan earthquake. Chin J Traumatol. 2013;16(01):10–5. [PubMed] [Google Scholar]
- 5.Wang T, Li D, Xie Y, Kang M, Chen Z, Chen H, et al. The microbiological characteristics of patients with crush syndrome after the Wenchuan earthquake. Scand J Infect Dis. 2010;42(6–7):479–83. [DOI] [PubMed] [Google Scholar]
- 6.Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury. 2007;38(12):1346–57. [DOI] [PubMed] [Google Scholar]
- 7.Ergönül Ö, Keske Ş, Ksinzik A, Güldan M, Özbek L, Azap A, et al. The challenges in the monitoring of infectious diseases after the earthquake in Türkiye in 2023. The Lancet Infectious Diseases; 2023. [DOI] [PubMed] [Google Scholar]
- 8.Aoyagi T, Yamada M, Kunishima H, Tokuda K, Yano H, Ishibashi N, et al. Characteristics of infectious diseases in hospitalized patients during the early phase after the 2011 great East Japan earthquake: pneumonia as a significant reason for hospital care. Chest. 2013;143(2):349–56. [DOI] [PubMed] [Google Scholar]
- 9.Daito H, Suzuki M, Shiihara J, Kilgore PE, Ohtomo H, Morimoto K, et al. Impact of the Tohoku earthquake and tsunami on pneumonia hospitalisations and mortality among adults in northern Miyagi, Japan: a multicentre observational study. Thorax. 2013;68(6):544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shrestha AC, Flower RL, Seed CR, Rajkarnikar M, Shrestha SK, Thapa U, et al. Hepatitis E virus seroepidemiology: a post-earthquake study among blood donors in Nepal. BMC Infect Dis. 2016;16:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizk A, Abou Fayad A, Haraoui L-P. Antimicrobial-resistant infections after Turkey/Syria earthquakes, 2023. Emerg Infect Dis. 2023;29(6):1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slater MS, Mullins RJ. Rhabdomyolysis and myoglobinuric renal failure in trauma and surgical patients: a review. J Am Coll Surg. 1998;186(6):693–716. [DOI] [PubMed] [Google Scholar]
- 13.Bywaters E, Beall D. Crush injuries with impairment of renal function. Br Med J. 1941;1(4185):427–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-84. [DOI] [PubMed] [Google Scholar]
- 15.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.(2017) Surveillance of healthcare-associated infections and prevention indicators in European intensive care units. ECDC, European Centre for Disease Prevention and Control, Stockholm. https://ecdc.europa.eu/sites/portal/fles/documents/HAI-Net-ICU-protocol-v2.2_0.pdf. Accessed 12 Feb 2018.
- 17.Magiorakos A-P, Srinivasan A, Carey RB, Carmeli Y, Falagas M, Giske C, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Hao P, Lu B, Yu H, Huang W, Hou H, et al. Causes of infection after earthquake, China, 2008. Emerg Infect Dis. 2010;16(6):974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiaolei C, Hui Z, Ping F, Zhangxue H, Wei Q, Ye T. Infections in crush syndrome: a retrospective observational study after the Wenchuan earthquake. Emerg Med J. 2011;28(1):14–7. [DOI] [PubMed] [Google Scholar]
- 20.Yang C, Wang H-y, Zhong H-j, Zhou L, Jiang D-m, Du D-y, et al. The epidemiological analyses of trauma patients in Chongqing teaching hospitals following the Wenchuan earthquake. Injury. 2009;40(5):488–92. [DOI] [PubMed] [Google Scholar]
- 21.Kazancioglu R, Cagatay A, Calangu S, Korular D, Turkmen A, Aysuna N, et al. The characteristics of infections in crush syndrome. Clin Microbiol Infect. 2002;8(4):202–6. [DOI] [PubMed] [Google Scholar]
- 22.Öncül O, Keskin Ö, Acar H, Küçükardalı Y, Evrenkaya R, Atasoyu E, et al. Hospital-acquired infections following the 1999 Marmara earthquake. J Hosp Infect. 2002;51(1):47–51. [DOI] [PubMed] [Google Scholar]
- 23.Keven K, Ates K, Sever MS, Yenicesu M, Canbakan B, Arinsoy T, et al. Infectious complications after mass disasters: the Marmara earthquake experience. Scand J Infect Dis. 2003;35(2):110–3. [DOI] [PubMed] [Google Scholar]
- 24.https://hsgm.saglik.gov.tr/depo/birimler/bulasici-hastaliklar-ve-erken-uyari-db/Dokumanlar/Raporlar/ETKEN_DAGILIM_VE_DIRENC_2022_RAPOR-v2.pdf.
- 25.Tao C, Kang M, Chen Z, Xie Y, Fan H, Qin L, et al. Microbiologic study of the pathogens isolated from wound culture among Wenchuan earthquake survivors. Diagn Microbiol Infect Dis. 2009;63(3):268–70. [DOI] [PubMed] [Google Scholar]
- 26.Kiani Q, Amir M, Ghazanfar M, Iqbal M. Microbiology of wound infections among hospitalised patients following the 2005 Pakistan earthquake. J Hosp Infect. 2009;73(1):71–8. [DOI] [PubMed] [Google Scholar]
- 27.Aydın M, Azak E, Bilgin H, Menekse S, Asan A, Mert HTE, et al. Changes in antimicrobial resistance and outcomes of health care–associated infections. Eur J Clin Microbiol Infect Dis. 2021;40:1737–42. [DOI] [PubMed] [Google Scholar]
- 28.https://www.cdc.gov/disasters/emergwoundhcp.html
- 29.https://cdn.who.int/media/docs/default-source/documents/publications/prevention-and-management-of-wound-infectiond8f2d781-b842-418b-8b52-80284339b955.pdf?sfvrsn=fc237543_1&download=true.
- 30.Sever MS, Vanholder R, Disasters RoIWGoRftMoCViM. Recommendations for the management of crush victims in mass disasters. Nephrology dialysis transplantation. 2012;27(Suppl_1):i1–67. [DOI] [PubMed] [Google Scholar]
- 31.Larsson D, Flach C-F. Antibiotic resistance in the environment. Nat Rev Microbiol. 2022;20(5):257–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alves J, Prendki V, Chedid M, Yahav D, Bosetti D, Rello J. Challenges of antimicrobial stewardship among older adults. Eur J Intern Med. 2024. [DOI] [PubMed]
- 33.Sever MS, Erek E, Vanholder R, Akoglu E, Yavuz M, Ergin H, et al. Clinical findings in the renal victims of a catastrophic disaster: the Marmara earthquake. Nephrol Dial Transplant. 2002;17(11):1942–9. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


