Abstract
Background
Various analgesic techniques have been applied, the pain after video assisted thoracic surgery (VATS) is still challenging for anesthesiologists. Paracetamol provide analgesic efficacy in many surgeries. However, clinical evidence in the lung surgery with regional block remain limited. This monocentric double-blind randomized controlled trial investigates the efficacy of paracetamol after VATS with regional block.
Methods
A total of 90 patients were randomized to receive paracetamol (1 g) or normal saline. Erector Spinae Plane Block and Intercostal Nerve block were applied during the surgery. The Visual Analogue Scales (VAS) pain score was measured in the PACU as well as 6, 12, 24, and 48 h postoperatively. And the total dose of rescue analgesics administered to patients in morphine milligram equivalents (MME), satisfaction score, length of hospital stays, and incidence of nausea and vomiting were also recorded.
Results
The VAS pain score at each time point, the primary endpoint, did not differ between the groups (3.09 ± 2.14 vs. 2.53 ± 1.67, p = 0.174 at PACU; 4.56 ± 2.80 vs. 4.06 ± 2.46, p = 0.368 at 6 h; 3.07 ± 1.98 vs. 3.44 ± 2.48, p = 0.427 at 12 h; 2.10 ± 2.00 vs. 2.49 ± 2.07, p = 0.368 at 24 h; and 1.93 ± 1.76 vs. 2.39 ± 1.97, p = 0.251 at 48 h postoperatively). Satisfaction scores (4.37 ± 0.76 vs. 4.14 ± 0.88, p = 0.201), nausea (35.6% vs. 37.8%, p = 0.827), hypotension (2.2% vs. 0.0%, p = 0.317), and bradycardia (6.7% vs. 2.2%, p = 0.309) were also reported at similar rates.
Conclusions
The analgesic efficacy of one gram of paracetamol with ESPB and ICNB after VATS was not proven. Thus, caution should be exercised when prescribing paracetamol for pain control during VATS.
Trial registration
this trial was registered on Clinical Research Information Service (CRIS), Republic of Korea (KCT0008710). Registration date: 17/08/2023.
Keywords: Paracetamol, Video assisted thoracic surgery, Erector spinae plane block, Intercostal nerve block, Analgesic efficacy, Visual analogue scales, Pain
Introduction
Various techniques have been proposed for the management of post-thoracotomy pain; however, thoracotomy remains a painful procedure that results in chronic pain [1, 2]. Video-assisted thoracic surgery (VATS) has facilitated minimally invasive lung surgery [3]. The Enhanced Recovery After Surgery guidelines for lung surgery recommend the use of regional techniques and non-opioid analgesics to reduce opioid use during the postoperative period [4]. Paravertebral block or erector spinae plane block (ESPB) with intercostal nerve block (ICNB) is performed after VATS; nevertheless, moderate to severe pain has been reported by up to 30% of patients, necessitating the use of high-dose opioids [5]. The paracetamol inhibits of the cyclooxygenase (COX) pathway in the central nervous system and reduce the pain mediating prostaglandin [6]. The use of paracetamol has reduced morphine consumption and facilitated adequate postoperative pain management [7, 8]. The paracetamol augments the analgesic efficacy of opioid, anti-inflammatory and anti-neuropathic drugs [9]. The result of previous studies suggest that the additional paracetamol has analgesic efficacy after VATS. We hypothesized that a supplementary paracetamol with regional analgesic technique may provide adequate pain relief following VATS. This prospective, randomized, double-blind study aimed to examine the efficacy of paracetamol as an adjunct analgesic after VATS with ESPB and ICNB.
Materials and methods
Study population
Present study was designed as a monocentric, double-blind, randomized controlled trial. The study protocol was approved by the Ethics Committee of Wonju Severance Christian Hospital (CR122093). The participants are listed at https://cris.nih.go.kr (accessed at KCT0008710; registration date: 17/08/2023). Present study was conducted in a tertiary university hospital in Wonju, Republic of Korea. Informed consent was obtained from all patients or their legal guardians before their participation. A total of 101 patients aged 19–80 years with an American Society of Anesthesiologists physical status of class 1–4 who had undergone VATS between March 2023 and September 2023 were enrolled in this study. Patients were excluded if they met at least one of the following criteria: cognitive impairment, P2Y12 inhibitor (e.g. clopidogrel, ticlopidine, ticagrelor, etc.) or anticoagulants (e.g. apixaban, dabigatran, edoxaban, rivaroxaban, warfarin, etc.) administration within the 48 h, taking dual anti-platelet drug defined as combination of aspirin and a P2Y12 inhibitor, infection at the surgical site, patient refusal, pregnancy, allergy to ropivacaine, sepsis, anatomical deformity of the thorax, and increased intracranial pressure.
Randomization and blinding
The participants were randomized and allocated to paracetamol or saline group using a computerized randomization table. A random allocation sequence was generated at 1:1 ratio by an anesthetist who was not involved study using a computer-generated table of random numbers sealed in opaque envelopes. Each envelope was opened by one of the authors (S.K.), and notified the group allocation to a nurse in preparation room immediately before surgery. Paracetamol (1 g with saline 100 mL) or saline (100 mL) were administered during the surgery. Patients and the investigator (H.L.) involved in the postoperative data collection were maintained blinding during the observational period. Therefore, design of the present study was double-blind.
Perioperative management
No premedication was given before the induction of anesthesia. On arrival in the operating room, patients were monitored with 3-lead electrocardiogra, pulse oximetry, noninvasive blood pressure, and Sedline® electroencephalography guidance (Masimo, Irvine, CA, USA). A bolus of propofol (1.5–2 mg/kg), remifentanil (1 µg/kg) and rocuronium (0.6 mg/kg) were used to induce anesthesia. Patients were intubated with a double-lumen endobronchial tube (DLT, Blue Line®, Smith Medical US, Minneapolis, 37Fr for men and 35 Fr for women) and arterial catheterization was performed. Anesthesia was maintained by administration 1.5-2.5% of sevoflurane or 4.0–6.0% of desflurane, 0.05–0.3 µg·kg− 1·min− 1 of remifentanil and 0.3 to 0.6 mg·kg− 1·min− 1 of rocuronium.The infusion rate of remifentanil was adjusted according to the overall hemodynamic data by the attending anesthesiologist, who also suggested the intensity of the surgical stimuli. The infusion rate of rocuronium was adjusted using a Train of Four watch value was less than 2. Inhalational anesthetics were administered as fractions under Sedline® value was 25 to 50. Acetphen premix® (HK Innoen Inc., Korea) or saline (100 mL) were administered intravenously over a 15-min at the end of the surgery in the paracetamol and control groups, respectively. ESPB was performed subsequently with the patient in the lateral position. The analgesic blocks were performed by the same anesthesiologist (S.K.). Fentanyl (18 mcg/kg) and ramosetron (0.3 mg) were dispensed via intravenous patient-controlled analgesia. The fentanyl dose was recorded as morphine milligram equivalents (MME). The patients were transported to the postanesthetic care unit (PACU) or surgical intensive care unit (ICU) following extubation in the operating room. Nefopam (20 mg) and fentanyl (50 ug) were administered intravenously to patients with visual analog scale (VAS) scores of 4–6 and > 6 in the PACU, respectively, according to the standard analgesic algorithm. The primary physician oversaw postoperative pain management after discharge of the PACU. The dosage of postoperative analgesics was recorded in MME. Intravenous tramadol (50 mg), intramuscular or subcutaneous meperidine (25 mg), oral ultracet (tramadol [37.5 mg]/acetaminophen [325 mg]), and transdermal fentanyl patches (25 mcg/h) were used postoperatively. Withdrawal from the study was considered in case of conversion to the open surgery, hypersensitivity reaction during anesthetic induction.
Surgical techniques and intercostal nerve block
The surgeon (C.S.B.) performed the conventional three-port VATS technique by making a utility incision 3–4 cm in size at the T4 level. Posterior instrument and camera ports 1 cm in size were placed at the T7 and T8 levels, respectively. Nerve-sparing techniques were not used. At the end of the surgical procedure, intercostal nerve block was performed from the T4–T9 levels using 2 mL of 0.375% ropivacaine for each level.
Erector spinae plane block
ESPB was performed by injecting 20 mL of 0.375% ropivacaine into the fascial plane between the tips of the transverse process and erector spinae muscle at the T5 level under ultrasound guidance. A 21-guage ultrasound-visible needle (Echoplex; Vygon, Ecouen, France) was inserted in the craniocaudal direction. The position of the needle tip was confirmed via hydro-dissection with 2–3 mL of saline, and the anesthesiologist aspirated every 5 mL to prevent intravascular injection.
Outcome measures
The primary outcome measure of the present study was the analgesic efficacy of adjuvant paracetamol after VATS with ESPB. The VAS pain score was measured in the PACU as well as 6, 12, 24, and 48 h postoperatively. The highest VAS scores were recorded at each timepoint. The secondary outcome measures included the total dose of analgesics administered to patients (in MME), satisfaction score, length of hospital stay, perioperative liver enzyme (AST, ALT) and incidence of nausea and vomiting.
Sample size calculation
The sample size was calculated based on the previous numeric rating scale (NRS) pain scores. The mean pain score after VATS with ESPB in a previous study was 5.96 ± 1.68 [10]. The number of patients enrolled in each group was estimated to be 45 if the administration of paracetamol enhanced the pain score by ≥ 1.0 points (type I error, 0.05; power, 0.90). Fifty patients were enrolled in each group, considering a dropout rate of 10%.
Statistical analysis
All statistical analyses were performed using IBM SPSS (IBM SPSS Statistics for Windows, version 25; IBM Corporation, Armonk, NY, USA). The Shapiro–Wilk test was used to assess the distribution of continuous variables. Independent t-test and the Mann–Whitney U test were used to analyze outcome variables with and without a normal distribution, respectively. The data are presented as the mean and standard deviation for normally distributed data and as median and interquartile range for non-normally distributed data. The Chi-square test was used to analyze the frequency. Repeated-measures analysis of pain scores was performed with a post-hoc Bonferroni correction.
Results
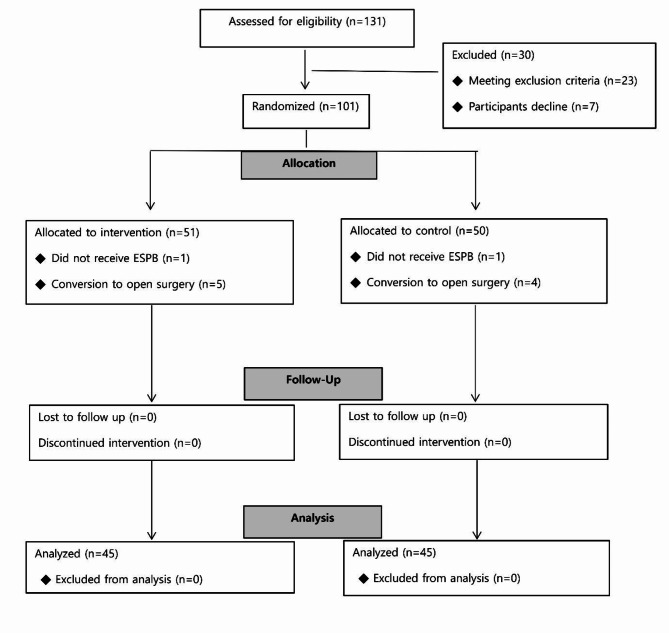
Among the 131 patients who had undergone VATS between March 2023 and September 2023 were assessed for eligibility and 101 enrolled in this study assigned to the two groups, 11 dropped out of the study. Two patients did not receive ESPB, and nine patients underwent open surgery (Fig. 1). The demographic characteristics of the two groups were similar (Table 1). A total of 90 patients were included in the final analysis.
Fig. 1.
CONSORT flow diagram
Table 1.
Demographic data
| Control (n = 45) | Paracetamol (n = 45) | p-Value | |
|---|---|---|---|
| Female (%) | 16(35.6) | 15(33.3) | 0.825 |
| Age | 59.87 ± 17.68 | 61.00 ± 15.04 | 0.744 |
| Height (cm) | 162.74 ± 9.70 | 163.24 ± 8.33 | 0.793 |
| Weight (kg) | 63.15 ± 10.14 | 67.64 ± 11.38 | 0.051 |
| Body Mass Index | 23.90 ± 3.40 | 25.42 ± 3.96 | 0.060 |
| Hypertension | 23(51.1) | 19(42.2) | 0.401 |
| Diabetes Mellitus | 11(24.4) | 11(24.4) | 1.000 |
| Chronic Kidney Disease | 0(0.0) | 1(2.2) | 0.317 |
| Cerebral Vasclular Disesase | 3(6.7) | 1(2.2) | 0.309 |
| ASA score | 0.812 | ||
| I | 2(4.4) | 0(0.0) | |
| II | 24(53.3) | 28(62.2) | |
| III | 18(40.0) | 17(37.8) | |
| IV | 1(2.2) | 0(0.0) |
Values are displayed as the mean ± SD or n(%). ASA: American Society of Anesthesia
The VAS pain score at each time point, the primary endpoint, and the number of patients having a moderate to severe pain did not differ between the groups. Nausea, and vomiting were also reported at similar rates. The length of stay at the hospital and ICU did not differ significantly between the groups (Table 2.).
Table 2.
Endpoint measures
| Control (n = 45) | Paracetamol (n = 45) | p -Value | Mean difference (95% CI)) | |
|---|---|---|---|---|
| Primary endpoint | ||||
| VAS_PACU | 3.09 ± 2.14 | 2.53 ± 1.67 | 0.174 | 0.56 (-0.25, 1.36) |
| VAS_6Hr | 4.56 ± 2.80 | 4.06 ± 2.46 | 0.368 | 0.50 (-0.60, 1.61) |
| VAS_12Hr | 3.07 ± 1.98 | 3.44 ± 2.48 | 0.427 | -0.38 (-1.32, 0.56) |
| VAS_24Hr | 2.10 ± 2.00 | 2.49 ± 2.07 | 0.368 | -0.39 (-1.24, 0.46) |
| VAS_48Hr | 1.93 ± 1.76 | 2.39 ± 1.97 | 0.251 | -0.46 (-1.24, 0.33) |
| Moderate to severe pain (VAS ≥ 4) | ||||
| VAS_PACU | 11(24.4) | 6(13.3) | 0.281 | |
| VAS_6Hr | 26(57.8) | 25(55.6) | 0.178 | |
| VAS_12Hr | 17(37.8) | 17(37.8) | 1.000 | |
| VAS_24Hr | 6(13.3) | 12(26.7) | 0.114 | |
| VAS_48Hr | 9(20.0) | 10(22.2) | 0.796 | |
| Secondary endpoints | ||||
| Total analgesics (MME) | 380.55 ± 88.95 | 410.02 ± 93.73 | 0.130 | -29.46 (-67.74, 8.82) |
| Rescue analgesic (MME) | 17.12 ± 22.51 | 18.83 ± 23.64 | 0.725 | -1.72 (-11.39, 7.95) |
| Satisfaction of patients | 4.37 ± 0.76 | 4.14 ± 0.88 | 0.201 | 0.22 (-0.12, 0.57) |
| Nausea | 16(35.6) | 17(37.8) | 0.827 | |
| Vomiting | 4(8.9) | 6(13.3) | 0.505 | |
| Hospital stays (day) | 4.31 ± 3.30 | 4.89 ± 5.29 | 0.536 | -0.58 (-2.42, 1.27) |
| ICU stays (day) | 0.24 ± 0.48 | 0.60 ± 2.25 | 0.303 | -0.36 (-1.03, 0.33) |
Values are displayed as the mean ± SD, n (%). The visual analogue scale was used to assess the pain scores (0 = no pain, 10 = worst imaginable pain). The analgesic doses are calculated in MME. The satisfaction score was assessed using a 5-point numerical scale (1 = extremely dissatisfied, 5 = extremely satisfied)
PACU, postanesthetic care unit; VAS, visual analog scale; ICU, intensive care unit; MME, morphine milligram equivalent
Furthermore, the preoperative and postoperative liver enzyme levels of the two groups were also similar (Table 3.).
Table 3.
Perioperative data
| Control (n = 45) | Paracetamol (n = 45) | p- Value | ||
|---|---|---|---|---|
| Operation type | 0.211 | |||
| Lobectomy | 9(20.0) | 7(15.6) | ||
| Segmentectomy | 10(22.2) | 12(26.7) | ||
| Wedge resection | 21(46.7) | 17(37.8) | ||
| Mediastinal mass | 5(11.1) | 9(20.0) | ||
| Operation time (minutes) | 72.44 ± 42.08 | 73.78 ± 37.18 | 0.853 | -1.33 (-17.97, 15.30) |
| Hypotension | 1(2.2) | 0(0.0) | 0.317 | |
| Bradycardia | 3(6.7) | 1(2.2) | 0.309 | |
| PreAST | 22.36 ± 10.55 | 25.20 ± 14.28 | 0.285 | -2.84( -8.10, 2.41) |
| PreALT | 21.53 ± 14.73 | 21.33 ± 11.65 | 0.943 | 0.20 (-5.36, 5.76) |
| PostAST | 24.20 ± 12.23 | 26.30 ± 17.66 | 0.650 | -2.10 (-8.48, 4.29) |
| PostALT | 20.56 ± 15.05 | 22.14 ± 17.64 | 0.721 | -1.58 (-8.49, 5.32) |
| (Post-pre) AST | 1.84 ± 7.90 | 1.09 ± 11.15 | 0.713 | 0.75 (-3.31, 4.82) |
| (Post-pre) ALT | -0.98 ± 7.60 | 0.89 ± 8.90 | 0.291 | -1.86 (-5.35, 1.62) |
Values are displayed as the mean ± SD, n (%)
ALT, alanine transaminase; AST, aspartate transaminase
No significant differences were observed between the control and acetaminophen groups in terms of the heart rate and mean arterial pressure (Fig. 2).
Fig. 2.
Hemodynamic data. (A) Heart rate (B) Mean arterial pressure. No significant difference was observed between the control and paracetamol group
Discussion
The present study demonstrated that the administration of paracetamol adjunctive to ESPB and ICNB did not increase the analgesic effect after VATS. Furthermore, the administration of paracetamol did not improve patient satisfaction or the requirement for rescue analgesics. No significant differences were observed in the total dose of opioids administered or the incidence of postoperative nausea and vomiting.
Although VATS is minimally invasive and has gained wide popularity, it is unclear whether it reduces acute postoperative pain or chronic pain compared to thoracotomy. A prospective study comparing pain and quality of life over 12 months reported similar after VATS and thoracotomy [11]. In other prospective study, there was no difference in pain intensity or incidence at 6 months after surgery [12]. Whether or not VATS reduces postoperative pain compared to thoracotomy, VATS still causes moderate to severe pain even after a regional block [13]. Therefore, pain management during the immediate postoperative period is an important concern among anesthesiologists.
The European Society of Thoracic Surgeons and the Enhanced Recovery After Surgery Society recommend the regional analgesia and use of acetaminophen and non-steroidal anti-inflammatory drugs unless contraindicated. However, the present study revealed that the addition of a single dose of paracetamol to the regional technique did not reduce the pain scores or the use of rescue analgesics. No differences were observed between the pain scores and satisfaction scores at any time point during the first 48 huors and moderate to severe pain was reported at a similar rate. The present result, contrary our primary hypothesis, may have been responsible for use in combination with ESPB and ICNB. In a previous study, the NRS pain scores of ESPB group and control group after VATS were 5.96 ± 1.68 and 7.59 ± 1.18 at PACU [10]. As our results indicate, the patients receiving both regional techniques were less painful after VATS regardless of paracetamol (3.09 ± 2.14 and 2.53 ± 1.67 at PACU). The analgesic effect of regional block may have masked the efficacy of paracetamol. In addition, despite paracetamol widely used, evidence regarding efficacy is insufficient [14]. As in acute lower back pain, for which there is high quality evidence [15], paracetamol may not relieve pain after VATS.
Compared with the placebo, a single intravenous dose of paracetamol or propacetamol reduced the pain intensity by > 50% in a significantly higher number of participants in a previous study [16]. Moreover, the use of additional analgesics was also reduced significantly. However, this systematic review did not include lung surgery and only collected data for up to 6 h. Consequently, there are insufficient data to guarantee the effectiveness of paracetamol in various surgeries [14]. A previous retrospective study reported a significant reduction in pain scores when 1 g of paracetamol was administered every 6 h for 2 days postoperatively with thoracic epidural analgesia after VATS. However, the participants received paracetamol four times a day, and the sample size was only 29 [17]. Only a few prospective randomized controlled trials have demonstrated the efficacy of paracetamol after thoracotomy or VATS.
In terms of paracetamol related adverse events, the single dose of paracetamol seems to be safe in blood pressure, heart rate and liver enzyme. Approximately 5% of paracetamol is converted to N-acetyl-p-benzoquinone imine, a toxic metabolite, by cytochrome P450. It is rapidly inactivated by glutathione sulfhydryl groups and excreted in urine. Hepatic function impairment may occur if the glutathione stores are depleted by the overuse of paracetamol or if cytochrome P450 is induced by drugs (rifampicin, barbiturates, etc.) [6]. Paracetamol is the most commonly available over-the-counter drug, and its overdose is reported frequently [18]. Paracetamol toxicity is the leading cause of acute liver failure in developed countries, accounting for approximately 50% of cases [19–21]. Paracetamol has a narrow therapeutic dose compared with that of other analgesics, and the risk of overdose is high [21]. Furthermore, it has adverse effects, and the dose prescribed during surgery is not equal to the total dose. Paracetamol (1 g) did not induce hepatic dysfunction in the present study. However, the Food and Drug Administration of the USA suggests that the dose of paracetamol should be limited to 650 mg based on data on liver failure associated with paracetamol [22]. Routine use of paracetamol should be avoided if the analgesic efficacy of paracetamol cannot be guaranteed, considering the adverse effects.
The present study had certain limitations. First, paracetamol was used in combination with regional techniques; it was not compared with the placebo alone. This may have resulted in the confounding of the efficacy of paracetamol. However, VATS results in moderate to severe pain; therefore, paracetamol is used as part of multimodal analgesia. It is more appropriate to compare the effects of combined use to estimate the efficacy in clinical situations. Second, the pain scores and rescue analgesic doses were only evaluated for up to 48 h postoperatively. The clinical influence can be better understood if the incidence of post-thoracotomy pain syndrome is investigated.
In conclusion, intravenous administration of one gram of paracetamol adjunctive to a regional block did not result in an improvement in analgesic efficacy after VATS or reduce the pain scores or the dose of rescue analgesics. Paracetamol is the most commonly used over-the-counter drug; however, it is not devoid of toxicity. Thus, caution should be exercised when prescribing paracetamol for pain control during VATS.
Author contributions
Sujin Kim (Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing). Seung Woo Song (Data curation; Investigation; Supervision; Visualiztion). Haesung Lee (Data curation; Investigation; Supervision; Visualization). Chun Sung Byun (Data curation; Investigation; Supervision; Visualization) Ji-Hyoung Park (Conceptualization; Data curation; Investigation; Methodology; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing).
Funding
This study was supported by HK Inno.N Corp. provided financial support, however no role in the design, conduct, data analysis, and interpretation of the study.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Maloney J, Wie C, Pew S, Covington S, Maita M, Kozinn R, Sabin M, Freeman J, Kraus M, Strand N. Post-thoracotomy Pain Syndrome. Curr Pain Headache Rep. 2022;26(9):677–81. [DOI] [PubMed] [Google Scholar]
- 2.Gupta R, Van de Ven T, Pyati S. Post-thoracotomy Pain: current strategies for Prevention and Treatment. Drugs. 2020;80(16):1677–84. [DOI] [PubMed] [Google Scholar]
- 3.Sihoe ADL. Video-assisted thoracoscopic surgery as the gold standard for lung cancer surgery. Respirology. 2020;25(Suppl 2):49–60. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, Brunelli A, Cerfolio RJ, Gonzalez M, Ljungqvist O, Petersen RH, Popescu WM, Slinger PD, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS®) Society and the European Society of thoracic surgeons (ESTS). Eur J Cardiothorac Surg. 2019;55(1):91–115. [DOI] [PubMed] [Google Scholar]
- 5.Kim S, Song SW, Do H, Hong J, Byun CS, Park JH. The analgesic efficacy of the single erector spinae plane block with intercostal nerve block is not inferior to that of the thoracic paravertebral block with intercostal nerve block in video-assisted thoracic surgery. J Clin Med. 2022;11(18):5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jóźwiak-Bebenista M, Nowak JZ. Paracetamol: mechanism of action, applications and safety concern. Acta Pol Pharm. 2014;71(1):11–23. [PubMed] [Google Scholar]
- 7.Hong JY, Kim WO, Chung WY, Yun JS, Kil HK. Paracetamol reduces postoperative pain and rescue analgesic demand after robot-assisted endoscopic thyroidectomy by the transaxillary approach. World J Surg. 2010;34(3):521–6. [DOI] [PubMed] [Google Scholar]
- 8.Lahtinen P, Kokki H, Hendolin H, Hakala T, Hynynen M. Propacetamol as adjunctive treatment for postoperative pain after cardiac surgery. Anesth Analg. 2002;95(4):813–9. [DOI] [PubMed] [Google Scholar]
- 9.Freo U. Paracetamol for multimodal analgesia. Pain Manag. 2022;12(6):737–50. [DOI] [PubMed] [Google Scholar]
- 10.Shim JG, Ryu KH, Kim PO, Cho EA, Ahn JH, Yeon JE, Lee SH, Kang DY. Evaluation of ultrasound-guided erector spinae plane block for postoperative management of video-assisted thoracoscopic surgery: a prospective, randomized, controlled clinical trial. J Thorac Dis. 2020;12(8):4174–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizk NP, Ghanie A, Hsu M, Bains MS, Downey RJ, Sarkaria IS, Finley DJ, Adusumilli PS, Huang J, Sima CS, et al. A prospective trial comparing pain and quality of life measures after anatomic lung resection using thoracoscopy or thoracotomy. Ann Thorac Surg. 2014;98(4):1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bayman EO, Parekh KR, Keech J, Selte A, Brennan TJ. A prospective study of Chronic Pain after thoracic surgery. Anesthesiology. 2017;126(5):938–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun K, Liu D, Chen J, Yu S, Bai Y, Chen C, Yao Y, Yu L, Yan M. Moderate-severe postoperative pain in patients undergoing video-assisted thoracoscopic surgery: a retrospective study. Sci Rep. 2020;10(1):795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdel Shaheed C, Ferreira GE, Dmitritchenko A, McLachlan AJ, Day RO, Saragiotto B, Lin C, Langendyk V, Stanaway F, Latimer J, et al. The efficacy and safety of Paracetamol for pain relief: an overview of systematic reviews. Med J Aust. 2021;214(7):324–31. [DOI] [PubMed] [Google Scholar]
- 15.Saragiotto BT, Machado GC, Ferreira ML, Pinheiro MB, Abdel Shaheed C, Maher CG. Paracetamol for low back pain. Cochrane Database Syst Rev. 2016;2016(6):Cd012230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNicol ED, Ferguson MC, Haroutounian S, Carr DB, Schumann R. Single dose intravenous Paracetamol or intravenous propacetamol for postoperative pain. Cochrane Database Syst Rev. 2016;2016(5):Cd007126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shikatani Y, Soh J, Shien K, Kurosaki T, Ohtani S, Yamamoto H, Taniguchi A, Okazaki M, Sugimoto S, Yamane M, et al. Effectiveness of scheduled intravenous acetaminophen in the postoperative pain management of video-assisted thoracic surgery. Surg Today. 2021;51(4):589–94. [DOI] [PubMed] [Google Scholar]
- 24.Park BK, Dear JW, Antoine DJ. Paracetamol (acetaminophen) poisoning. BMJ Clin Evid 2015, 2015:2101. [PMC free article] [PubMed]
- 19.Lee WM. Acetaminophen and the U.S. Acute Liver failure Study Group: lowering the risks of hepatic failure. Hepatology. 2004;40(1):6–9. [DOI] [PubMed] [Google Scholar]
- 20.Reuben A, Tillman H, Fontana RJ, Davern T, McGuire B, Stravitz RT, Durkalski V, Larson AM, Liou I, Fix O, et al. Outcomes in adults with Acute Liver failure between 1998 and 2013: an Observational Cohort Study. Ann Intern Med. 2016;164(11):724–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chidiac AS, Buckley NA, Noghrehchi F, Cairns R. Paracetamol (acetaminophen) overdose and hepatotoxicity: mechanism, treatment, prevention measures, and estimates of burden of disease. Expert Opin Drug Metab Toxicol. 2023;19(5):297–317. [DOI] [PubMed] [Google Scholar]
- 22.Graham GG, Day RO, Graudins A, Mohamudally A. FDA proposals to limit the hepatotoxicity of Paracetamol (acetaminophen): are they reasonable? Inflammopharmacology 2010, 18(2):47–55. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.