Abstract
Background
Intermittent fasting (IF) can be an effective dietary therapy for weight loss and improving cardiometabolic health. However, there is scant evidence regarding the role of IF on indicators of liver function, particularly in adults with metabolic disorders. Therefore, we performed a systematic review and meta-analysis to investigate the effects of IF on liver function in adults with metabolic disorders.
Methods
Three primary electronic databases including PubMed, Web of Science, and Scopus, were searched from inception to September 2024 to identify original studies that used IF interventions with or without control groups in adults with metabolic disorders. Inclusion criteria were (1) studies of human participants with metabolic diseases, (2) interventions that evaluated the effects of IF, (3) with or without a control group, and (4) measured liver fat, liver steatosis, liver fibrosis, or liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) as primary outcomes. Standardized mean differences (SMD) and 95% confidence intervals were calculated using random effects models. Heterogeneity was assessed using the Cochran’s Q statistic and I-squared statistic (I2). Publication bias was assessed using the visual inspection of funnel plots and Egger’s tests. The risk of bias was assessed using the PEDro scale and the NIH quality assessment tool.
Results
A total 21 studies involving 1,226 participants with metabolic disorders were included in the meta-analysis. Overall, IF effectively decreased liver fat with a large effect size [SMD: -1.22 (95% CI: -1.63 to -0.80), p = 0.001], liver steatosis with a medium effect size [SMD: -0.73 (95% CI: -1.12 to -0.35), p = 0.001], ALT with a small effect size [SMD: -0.44 (95% CI: -0.58 to -0.30), p = 0.001], and AST with a small effect size [SMD: -0.30 (95% CI: -0.49 to -0.11), p = 0.001], but not liver fibrosis [SMD: -0.28 (95% CI: -0.59 to 0.02), p = 0.07]. Subgroup analyses showed that IF decreased liver fat and ALT significantly, independent of IF mode, participant age, health status, weight status, and intervention duration. IF significantly decreased liver fibrosis in those with obesity; and decreased AST following 5:2 diets, in middle-aged adults, adults with obesity, and regardless of health status or intervention duration.
Conclusions
IF seems to be an effective dietary therapy for improving liver function in adults with metabolic disorders, and many of liver function-related benefits occur regardless of IF mode, intervention duration, or participant health status.
Limitations
Significant heterogeneity, small numbers of studies and inclusion of non-randomized trials or single-group pre-post trials were the main limitation of our meta-analysis. Further randomized clinical trials are needed to elucidate the effects of IF on liver function in adults with metabolic disorders.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12986-024-00885-x.
Keywords: Dietary strategy, Fatty liver, Metabolic syndrome, Obesity, Lifestyle intervention
Introduction
The worldwide prevalence of obesity and comorbid diseases has increased substantially in recent decades, leading to increased incident cardiometabolic diseases, mortality, and healthcare costs [1–3]. Recognized as a major public health pandemic, obesity is known as a primary and independent factor for the development of cardiometabolic diseases [4]. The link between obesity and cardiometabolic diseases is mediated by dyslipidemia, hypertension, systemic inflammation, and insulin resistance [4–6]. In addition, as a result of the obesity pandemic, nonalcoholic fatty liver disease (NAFLD), characterized by an increase in intrahepatic liver fat content with and without fibrosis, is the most common cause of chronic liver disease in the world [7, 8]. NAFLD is a spectrum of progressive liver disease including steatosis, steatohepatitis, fibrosis, and cirrhosis [9, 10]. NAFLD is related to cardiometabolic diseases via alterations in hepatic lipid content, lipoprotein and glucose metabolism, impaired vascular function, and systematic inflammation [11–15]. Additionally, metabolic diseases such as obesity and diabetes contribute to liver damage and fibrosis via increased oxidative stress and systemic inflammation [16, 17], and the rates of liver disease in patients with T2D are increasing [14]. Furthermore, NAFLD also occurs in individuals without obesity, though often goes undiagnosed [18]. Therefore, preventing of impaired liver function and progression of liver damage is important for patients with or without NAFLD. At present, nutritional interventions, such as reducing calorie intake or increasing energy expenditure are considered as effective non-pharmacological approaches to treat liver diseases [19–23].
Lifestyle interventions including dietary therapy and physical activity are the first-line therapeutic and preventive strategies for many metabolic disorders, and clinical guidelines have proposed weight loss as the cornerstone for the treatment and management of metabolic diseases including obesity, diabetes, and NAFLD [22, 24–26]. Previous research has indicated significant improvements are achieved in a dose-dependent manner in liver inflammation, ballooning, and resolution of NAFLD [23]. Caloric restriction (CR) is a nutritional intervention whereby energy intake is reduced by 15–60% of daily energy, and has been the most common approach for weight reduction described in the research literature. In this large body of evidence, clinical effects on several cardiometabolic risk factors have been reported to vary according to patient health status [27–31]. The potential beneficial role of CR for improving liver function and lowering liver fat content is also known [32–34]. In recent years, intermittent fasting (IF) has become popular and has been proposed as a promising alternate dietary therapy for the treatment and management of obesity and cardiometabolic diseases. Some evidence has suggested that IF may be effective due to high adherence levels and compliance with this change in eating timing [35–37]. IF encompasses eating patterns with periods of fasting with little or no energy intake (e.g., 16–48 h), followed by periods of feeding with usual food intake. While numerous types of IF have been described, they can be categorized into four primary forms, including alternate day fasting diets (ADF), periodic fasting, 5:2 diets, and time restricted feeding (TRF) [36, 38].
Regardless of IF mode, several meta-analyses have been conducted to determine whether IF is effective for improving cardiometabolic health outcomes and weight loss, with some promising results on body composition and cardiometabolic risk factor [36, 39–46]. Several clinical trials have been conducted to evaluate liver function following IF intervention [47–55]. Particularly, all modes of IF, including TRF [48], 5:2 diet [54] and ADF [50] reported to promote liver function in patients with NAFLD. Due to its clinical significance, IF is gaining popularity, but little is known about the effective mode of IF on liver function. A previous meta-analysis with a small number of studies (n = 6) reported beneficial effects of IF on weight management and liver enzymes in patients with NAFLD [56]. However, yet there is no comprehensive meta-analysis to address the influence of IF modes on liver function. Therefore, we performed an updated and more comprehensive systematic review and meta-analysis to investigate the effects IF on liver function parameters, including liver fat content, fibrosis, steatosis, and enzymes in adults with metabolic diseases.
Methods
The current systematic review and meta-analysis was conducted according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions, and the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist. The protocol was prospectively registered in the International Prospective Register for Systematic Reviews (PROSPERO) database with ID: CRD42024531043.
Search strategy
Three primary electronic databases including PubMed, Web of Science, and Scopus were searched through September 2024. The searches were conducted using the key words including “intermittent fasting”. Human, English and article were limited when studies were available in databases. In addition, hand searches of reference lists of the included studies and previous a meta-analysis [56] were conducted to identify potential additional studies. Furthermore, snowballing search through backward searching using article reference list and forward searching by looking at who cited it were also performed [57]. Additional search was also conducted from grey literature source; however, no extra studies were included due to lack of eligibility criteria or duplicates records. The complete search strategy is presented in the supplementary Table 1. The searches were conducted with two independent reviewers (M Kh and F G).
Study selection and inclusion/exclusion criteria
All records retrieved from the electronic database searches were imported into EndNote (version 21). After removing duplicate records, the study selection was conducted in two steps against inclusion and exclusion criteria, by two independent reviewers (F G and Sh Kh). Any disagreements were resolved via discussion between reviewers or with a third reviewer when needed (M Kh). In the first step, the titles and abstracts of all studies were screened to identify potentially relevant to the scope of our study. Subsequently in the second step, the full-texts of relevant studies were screened for inclusion based on the eligibility criteria. The original studies were considered to be eligible if they met the following PICOS criteria (1-Participants, 2-Intervention, 3-Comparator, 4-Outcome, and 5-Study design), including (1) studies of human participants with metabolic diseases, such as overweight, obesity, diabetes, metabolic syndrome, or NAFLD, regardless of age and biological sex; (2) interventions that evaluated the effects of IF with durations longer than 2 weeks, regardless of IF mode; (3) studies with or without a control group; (4) measured and reported the primary outcomes of liver fat, liver steatosis, liver fibrosis, or liver enzymes, including alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and (5) randomized and non-randomized trials or single-group pre–post trials with parallel or crossover designs. In addition, articles had to be published in English, peer-reviewed, and had the full-text available. The exclusion criteria were non-original studies, animal studies, published abstracts only, duplicate studies, and articles where data were not available to perform a meta-analysis. Also, studies involving participants without metabolic disorders, pregnant women or children, and studies involving Ramadan fasting were excluded.
Data extraction and synthesis
Data extraction was completed by two independent reviewers (F G and A H M) and any disagreements were resolved by discussion between reviewers or with a third reviewer (M Kh). Using Cohen’s kappa statistics inter-rater agreement [58] prior to correction disagreements was 0.83. The following data were extracted from individual studies: study information including first author name, publication year, study design; participant characteristics including participant ages, BMI, biological sex, and health status; intervention characteristics including mode, duration, and intermittent fasting protocols; outcome variables, and measurement methods. In addition, to calculate effect sizes, mean changes (post values - pre values), standard deviations (SD) and sample sizes for each group were extracted. For studies that reported means and SDs as pre- and post-intervention values, mean changes were calculated by subtracting the pre-exercise from the post-exercise values, and SD changes using the following formula:
In addition, when requires, these data were extracted from figures using GetData software, or were calculated from other data such as standard errors, medians and IQRs, or confidence intervals using relevant formulas [59–61]. When studies included more than one IF intervention arm, all interventions were included as separate intervention arms. If studies presented both ITT and post-pre protocol values with complete data, only the data from the ITT were included. However, when means and SDs were not presented in the published article, or when they could not be calculated from other data, the corresponding authors were contacted and asked to provide missing data.
Risk of bias assessment
The risk of b; ias of the included studies was assessed using the Physiotherapy Evidence Database (PEDro) scale, which is a valid measure of the methodological quality of clinical trials [62] and the NIH quality assessment tool for single-group trials [63]. Quality assessment were carried out by two independent reviewers (F G and A H M) and any conflicts were resolved by a third reviewer. The details of the risk of bias assessment are presented in supplementary Tables 2 and 3.
Meta-analysis
For each outcome, the IF versus either the control (CON) or the post-intervention values versus pre-intervention values, were compared according to the mean changes from post-intervention to baseline for each study. To calculate effect sizes, standardized mean difference (SMD), weighted mean difference (WMD), and 95% confidence intervals (CI) were calculated using random effects models. SMDs and WMDs were chosen based on whether the measurement units were the same or not. Random effects models were chosen due to assumed heterogeneity was likely from a clinical or methodologic perspective [64] and studies with greater potential for bias, such as uncontrolled or nonrandomized ones, show greater treatment effect as well as greater heterogeneity [65] which may have affected the results. When there were sufficient data, subgroup analyses were performed based on IF mode (ADF, 5:2 diets, or TRF), mean age (middle-aged: < 50 years vs. older adults ≥ 50 years), BMI (non-obese: < 30 kg.m2 vs. obese ≥ 30 kg.m2), health status (with NAFLD vs. without NAFLD), intervention duration (medium-term: <16 weeks vs. long-term: ≥16 weeks) and study design (with or without control groups). Heterogeneity among included studies was assessed using the Cochran’s Q statistic (significance level: < 0.05) and Tau Squared and the I-squared statistic (I2) where I was interpreted as low heterogeneity (25%), moderate heterogeneity (50%), and high heterogeneity (75%) [61]. Comprehensive meta-analysis (CMA) 3.0 statistical software was employed for the forest plots. Publication bias was examined through visual inspection of funnel plots and Egger’s tests as secondary outcomes (significant levels: < 0.10) [66]. Where there was bias indicated, the trim and fill method was used to determine bias-adjusted results [67]. In addition, when there were more than 10 intervention arms for any meta-analysis, sensitivity analyses were performed by omitting individual studies to insure that the results were not influenced by a single study.
Results
Search results
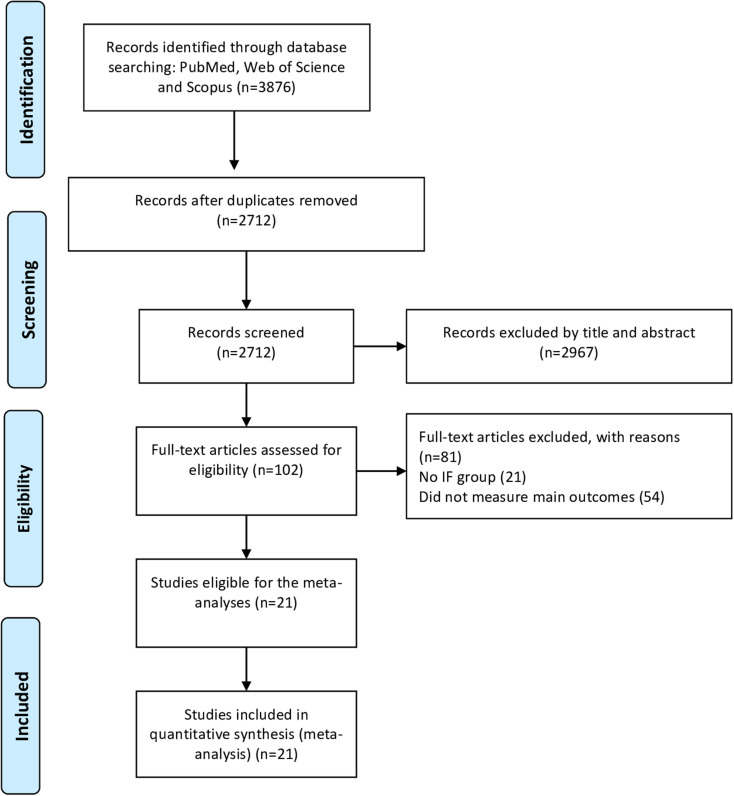
The database searches yielded 3876 records, of which 2712 records remained after removing the duplicates. Following the first step of evaluation, 2643 records were removed and 102 records remained for the full-text screening. In the second step screening, 81 articles were excluded for reasons presented in Fig. 1. Finally, 21 studies were included in the meta-analysis [47–55, 68–79]. Among the included studies, 14 studies [47, 50, 51, 53–55, 68–70, 72, 74–76, 79] consisted of IF and CON groups, and seven studies [48, 49, 52, 71, 73, 77, 78] consisted only IF without a CON trial. In addition, several studies had more than one intervention arm [47, 48, 52, 70, 79]. The detailed flow of the systematic search according to the PRISMA guidelines is presented in Fig. 1, and study characteristics are presented in Table 1.
Fig. 1.
Flow diagram of systematic literature search
Table 1.
Characteristics of participants and interventions
| Source, year | Participant characteristics | Intervention characteristics | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample size (sex) |
Health status | Age (years) |
BMI (kg/m2) |
Duration (weeks or months) |
IF mode | IF protocol | CON protocol | |||||
| Cai et al., 2019 [48] |
271 (F and M) |
NAFLD |
IF1: 35.50 ± 4.41 IF2: 33.56 ± 6.23 CON: 34.54 ± 6.96 |
IF1: 26.12 ± 2.21 IF2: 26.76 ± 1.59 CON: 26.34 ± 2.73 |
12 weeks |
IF1: ADF IF2: TRF |
IF1: Consummation diet with 25% of baseline energy needs for fasting days and eating ad libitum for feeding days IF2: Ad libitum feeding for a 8-h and refrain from eating for the remaining 16 h |
CON: consumption of 80% of subject energy needs every day | Liver stiffness | |||
| Deng et al., 2024 [49] |
40 (F and M) |
NAFLD |
IF1: 38.95 ± 7.98 IF2: 37.55 ± 8.20 |
IF1: 28.94 IF2: 27.69 |
8 weeks |
IF1: TRE IF2: TRE |
IF1: early time-restricted feeding between 8:00 a.m. and 4:00 p.m. IF2: late time-restricted feeding eating between 12:00 p.m. and 8:00 p.m. |
- | Liver stiffness, Liver fat, ALT, AST | |||
| Dokpuang et al., 2023 [50] | 10 (F and M) | Prediabetes | 53 ± 2.3 | 33.95 ± 0.96 | 12 weeks | 5:2 diet | 5 days of regular diet for feeding day and 2 inconsecutive days of 650 kcal/d for males and 600 kcal/d for females for fasting days | - | Liver fat | |||
| Ezpeleta et al., 2023 [51] |
39 (F and M) |
NAFLD |
IF: 44 ± 16 CON: 44 ± 12 |
IF: 36 ± 8 CON: 37 ± 5 |
3 months | ADF | Consummation diet with 600 kcal as a dinner (between 5–8 pm) for fasting days and eating ad libitum for feeding days | CON: habitual diet | Liver fat, liver fibrosis, AST, ALT | |||
| Feehan et al., 2023 [52] |
23 (F and M) |
NAFLD |
IF: 61.9 ± 8.7 CON: 54.7 ± 13.0 |
IF: 29.5 ± 3.0 CON: 29.9 ± 3.3 |
12 weeks | TRF | Ad libitum feeding for a 8-h and refrain from eating for the remaining 16 h | CON: Nutrition and exercise advice. | Liver stiffness, Liver steatosis, ALT | |||
| Hirsh et al., 2019 [70] |
22 (F and M) |
Overweight |
IF: 43.4 ± 13.0 CON: 39.0 ± 10.7 |
IF: 26.7 ± 1.9 CON: 27.7 ± 3.1 |
6 weeks | 5:2 diet | 5 days of regular diet for feeding days and 2 inconsecutive days of 730 kcal/d for fasting days | CON: habitual diet | AST, ALT | |||
| Holmer et al., 2021 [71] |
44 (F and M) |
NAFLD |
IF: 57 ± 10 CON: 56 ± 9 |
IF: 32.3 ± 2.7 CON: 32.9 ± 5.2 |
12 weeks | 5:2 diet | 5 days of intake limit of 2,000 kcal/day for female and 2,400 kcal/day for male for feeding days and 2 inconsecutive days of 600 kcal/d for males and 500 kcal/d for females for fasting days | CON: Nutrition advice | Liver fat, Liver steatosis, Liver stiffness, AST, ALT | |||
| Hutchison et al., 2019 [72] |
62 (F) |
Overweight |
IF1: 51 ± 2 IF2: 49 ± 2 CON: 49 ± 3 |
IF1: 31.2 ± 0.9 IF2: 32.4 ± 0.8 CON: 30.9 ± 1.5 |
8 weeks |
4:3 diet 4:3 diet |
IF1: 4 days of feeding with ~ 145% of energy requirements for feeding days and 3 days of fasting IF2: 4 days of feeding with ~ 100% of energy requirements for feeding days and 3 days of fasting |
CON: 100% of calculated baseline energy requirements daily. | AST, ALT | |||
| Ingersen et al., 2022 [53] | 23 (M) | T2DM and Obesity |
T2DM: 57 ± 6 Obesity: 55 ± 7 |
T2DM: 32.3 ± 2.6 Obesity: 32.9 ± 2.1 |
6 weeks | ADF | Consummation their diet or doubling their diet for feeding days and no caloric intake for fasting days | - | Liver fat, ALT, AST | |||
| Johari et al., 2019 [54] |
43 (F and M) |
NAFLD |
IF: 45.33 ± 10.77 CON: 52.60 ± 12.03 |
IF: 31.60 ± 5.19 CON: 28.21 ± 3.32 |
8 weeks | ADF | Restriction of 70% of their calorie requirement per day for fasting days and eating ad libitum for feeding days | CON: usual habitual diet | Liver steatosis, liver fibrosis, AST, ALT | |||
| Kahleova et al., 2014 [73] |
54 (F and M) |
T2D | 59.4 ± 7 | 32.6 ± 4.9 | 12 weeks | TRF | A restriction of 500 kcal/day with 2 meals/day including breakfast 6–10 AM; lunch 12 − 4 PM | - | Liver fat | |||
| Kord Varkaneh et al., 2022 [55] |
44 (F and M) |
NAFLD & Overweight/Obesity |
IF: 46.42 ± 13.35 CON: 44.17 ± 4.9 |
IF: 30.42 ± 2.27 CON: 30.60 ± 30.09 |
12 weeks | 5:2 diet | 5 days of regular diet for feeding days and 2 inconsecutive days of receiving 25% of the recommended calorie intake for fasting days | CON: continue usual habitual diet | Liver steatosis, liver fibrosis, AST, ALT | |||
| Lao et al., 2023 [74] |
27 (F and M) |
CKD & Overweight/Obesity |
IF: 51.8 ± 7.7 CON: 52.5 ± 11.3 |
IF: 29.3 ± 2.3 CON: 28.0 ± 2.4 |
12 weeks | TRF | Ad libitum feeding for a 8-h between 7:00 a.m. and noon and refrain from eating for the remaining 16 h | CON: continue usual habitual | AST, ALT | |||
| Liu et al., 2022 [75] |
69 (F and M) |
Obesity | IF: 31.6 ± 9.3 | IF: 31.8 ± 2.9 | 12 months | TRF | Consume the 75% of the participants’ daily caloric intake within an 8-hour period from 8:00 a.m. to 4:00 p.m | - | Liver fat | |||
| Schroder et al., 2021 [76] |
32 (F) |
Obese |
IF: 36.6 ± 1.6 CON: 42.3 ± 3.5 |
IF: 32.53 ± 1.13 CON: 34.55 ± 1.20 |
3 months | TRF | A fasting period (no energy intake whatsoever) of 16 h (8 pm to 12 pm) and ad libitum feeding for 8 h (12 pm to 8 pm) | CON: Maintain the same dietary and living habits | ALT, | |||
| Schübel et al., 2018 [77] |
101 (F and M) |
Overweight/Obesity |
IF: 49.4 ± 9.0 CON: 50.7 ± 7.1 |
IF: 32.0 ± 3.8 CON: 31.1 ± 3.6 |
12 weeks | 5:2 diet | 5 days of regular diet for feeding days and 2 days with 75% energy deficit | CON: No advice to restrict energy | Liver Fat, ALT, AST | |||
| Sulaj et al., 2022 [78] |
40 (F and M) |
T2D |
IF: 64.9 ± 1.6 CON: 66.8 ± 1.4 |
IF: 30.9 ± 0.9 CON: 30.2 ± 1.0 |
6 months | FMD | Comply for 5 consecutive days each month plant-based diet: day 1 supplied 4600 kJ, whereas days 2 to 5 provided 3000 kJ per day, and to return to their normal diet until the next diet cycle that was initiated about 25 days later | CON: Comply for 5 consecutive days each month with a Mediterranean diet (no change in caloric intake compared to participant’s diet before the study) | Liver stiffness, ALT, AST | |||
| Teong et al., 2023 [56] |
103 (F and M) |
T2D |
IF: 57 ± 10 CON: 59 ± 11 |
IF: 34.7 ± 4.6 CON: 33.8 ± 4.9 |
6 months | TRF | 30% energy requirements between 0800 and 1200 h and followed by a 20-h fasting period on three nonconsecutive days per week, and ad libitum eating on other days | CON: weight loss booklet | ALT, AST | |||
| Wei et al., 2023 [79] |
88 (F and M) |
NAFLD & Obesity | 32.3 ± 10.5 | 32.2 ± 3.4 | 12 months | TRF | Eating only between 8:00 AM and 4:00 PM with a diet of 1500 to 1800 kcal/d for men and 1200 to 1500 kcal/d for women | - | Liver fat, Liver stiffness | |||
| Xiao et al., 2022 [80] |
31 (F and M) |
T2D with NAFLD | 42.87 ± 6.84 | 30.44 ± 1.42 | 24 weeks | 5:2 diet | 5 days of regular diet and 2 inconsecutive days of 500 kcal/day diet | - | Liver fat, ALT, AST | |||
| Zhang et al., 2022 [81] |
60 (F and M) |
Overweight/Obesity |
IF1: 23.8 ± 0.6 IF2: 23.2 ± 0.5 CON: 22.1 ± 0.4 |
IF1: 27.1 ± 0.7 IF2: 28.5 ± 0.8 CON: 27.8 ± 0.8 |
8 weeks | TRF |
IF1: 6-h of eating ad libitum from 7 a.m. to 1 p.m. IF2: 6-h of eating ad libitum from 12 p.m. to 6 p.m. |
CON: Ad libitum intake in a day | ALT, AST | |||
Abbreviations: TRF time-restricted fasting ADF alternate-day fasting, RIF ramadan model of intermittent fasting, FMD fasting-mimicking diet, eTRC early time-restricted carbohydrate consumption, eTRE early time-restricted eating, lTRE late time-restricted eating, NAFLD nonalcoholic fatty disease, CKD chronic kidney disease, LStif liver stiffness, LStea liver steatosis, LFib liver fibrosis, IHTG Intrahepatic triglyceride content, ALT alanine aminotransferase, AST aspartate aminotransferase, ALP alkaline phosphatase, RCT Randomized controlled trials, h hours, LF liver fat, IF intermittent fasting, CR calorie restriction, CON control, BMI body mass index, F female, M male
Studies characteristics
The details of the participant and intervention characteristics, as well as the outcomes assessed in the studies, are presented in Table 1. Briefly, a total of 1226 participants with metabolic disorders were included. The age of participants was ranging from 23 [79] to 67 [76] years, and BMI was ranging from 27 [47] to 37 [50] kg.m2. Among included studies, nice studies involved patients with NAFLD [47, 48, 50, 51, 53, 54, 69, 77, 78], and other studies involved patients with overweight, obesity, T2D, or chronic kidney disease [49, 52, 55, 68, 70–76, 79]. Except for three studies that included males or females only [52, 70, 74], all other studies included both males and females. Regarding the IF characteristics, the modes, including ADF, 5:2 diets, and TRF, with intervention durations ranging from six weeks to 12 months. Four studies used two modes of IF in separate study arms [47, 48, 70, 79].
Meta-analysis results
Liver fat content
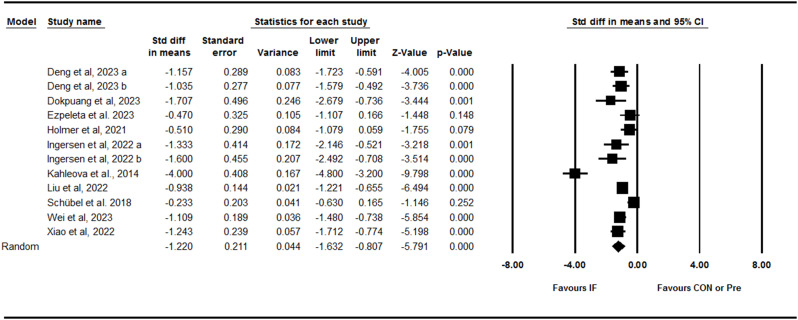
Pooling data from 12 trials including three between-group comparisons and nice within-group comparisons showed that IF effectively decreased liver fat content with a large effect size [SMD: -1.22 (95% CI: -1.63 to -0.80), p = 0.001] (Fig. 2). There was high and significant heterogeneity amongst studies (I2 = 86.26, Tau2 = 0.42, p = 0.001). Visual interpretation of the funnel plot suggested potential publication bias, but the Egger’s test did not confirm the bias (p = 0.14). When the missing studies were accounted using the trim and fill method, the overall effect size was increased [SMD: -1.61 (95% CI: -2.09 to -1.12)]. Several subgroup analyses were performed, indicating that liver fat content decreased with ADF [SMD: -1.08 (95% CI: -1.79 to -0.37), p = 0.003], 5:2 diets [SMD: -0.84 (95% CI: -1.46 to -0.23), p = 0.007], and TRF [SMD: -1.57 (95% CI: -2.31 to -0.84), p = 0.001]; in both young adults to middle-aged adults [SMD: -1.01 (95% CI: -1.18 to -0.83), p = 0.001] and middle-aged to older adults [SMD: -1.53 (95% CI: -2.62 to -0.45), p = 0.005]; in participants with obesity [SMD: -1.25 (95% CI: -1.75 to -0.76), p = 0.001] and without obesity [SMD: -1.09 (95% CI: -1.48 to -0.70), p = 0.001]; adults with NAFLD [SMD: -1.37 (95% CI: -2.18 to -0.56), p = 0.001] and without NAFLD [SMD: -1.03 (95% CI: -1.43 to -0.63), p = 0.001]; and adults with medium-term [SMD: -1.30 (95% CI: -1.96 to -0.63), p = 0.001] and longer-term [SMD: -1.04 (95% CI: -1.24 to -0.84), p = 0.001] interventions. In addition, subgroup analysis based on the study deign showed that liver fat content was decreased in both studies with control [SMD: -1.35 (95% CI: -0.64 to -0.06), p = 0.01] and studies without control [SMD: -1.50 (95% CI: -1.97 to -1.04), p = 0.001] groups.
Fig. 2.
Forest plot of the effects of intermittent fasting (IF) versus control diet (CON) or pre on liver fat content
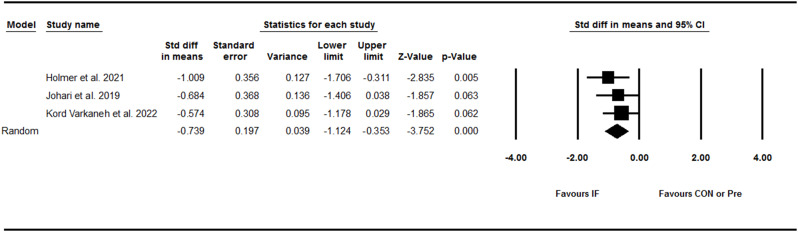
Liver fibrosis
Pooling data from 11 trials, including eight between-group comparisons and three within-group comparisons showed that IF did not significantly decrease liver fibrosis [SMD: -0.28 (95% CI: -0.59 to 0.02), p = 0.07] (Fig. 3). There was high and significant heterogeneity amongst studies (I2 = 75.56, Tau2 = 0.20, p = 0.001). Visual interpretation of the funnel plot suggested potential publication bias, however the Egger’s test did not confirm the bias (p = 0.85). When the missing studies were accounted using the trim and fill method, the overall effect size was [SMD: -0.36 (95% CI: -0.68 to -0.04)]. Several subgroup analyses were performed, and found that liver fibrosis decreased only in participants with obesity [SMD: -0.52 (95% CI: -0.98 to -0.05), p = 0.02]. Furthermore, subgroup analysis results for the study deign have shown that liver fibrosis did not decrease in both studies with control [SMD: -0.21 (95% CI: -0.44 to 0.01), p = 0.06] and without control [SMD: -0.33 (95% CI: -1.26 to 0.60), p = 0.48] groups.
Fig. 3.
Forest plot of the effects of intermittent fasting (IF) versus control diet (CON) or pre on liver fibrosis
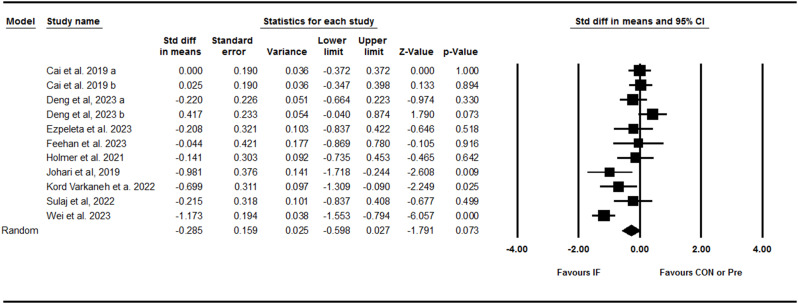
Liver steatosis
Pooling data from three trials including three between-group comparisons showed that IF effectively decreased liver steatosis with a medium effect size [SMD: -0.73 (95% CI: -1.12 to -0.35), p = 0.001] (Fig. 4). There was no significant heterogeneity amongst studies (I2 = 0.00, Tau2 = 0.00, p = 0.64). Visual interpretation of the funnel plot did not suggest publication bias and the Egger’s test confirmed no bias (p = 0.58). Subgroup analysis was not performed due to the small number of studies for this outcome.
Fig. 4.
Forest plot of the effects of intermittent fasting (IF) versus control diet (CON) or pre on liver steatosis
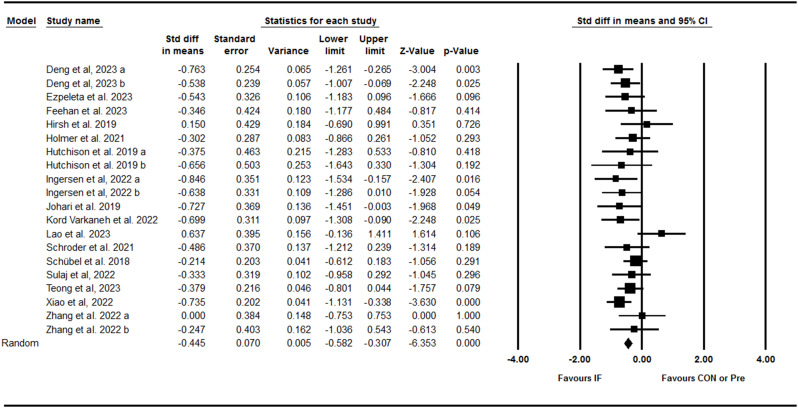
ALT
Pooling data from 20 trials including 15 between-group comparisons and five within-group comparisons showed that IF effectively decreased ALT with a small effect size [SMD: -0.44 (95% CI: -0.58 to -0.30), p = 0.001] (Fig. 5). There was no significant heterogeneity amongst studies (I2 = 4.05, Tau2 = 0.00, p = 0.40). Visual interpretation of the funnel plot did not suggest publication bias and the Egger’s test confirmed no bias (p = 0.29). Results from various subgroup analyses revealed that ALT decreased with ADF [SMD: -0.68 (95% CI: -1 S.01 to -0.34), p = 0.001], 5:2 diets [SMD: -0.41 (95% CI: -0.70 to -0.12), p = 0.005], and TRF [SMD: -0.33 (95% CI: -0.60 to -0.06), p = 0.01]; in both young adult to middle-aged adults [SMD: -0.55 (95% CI: -0.73 to -0.37), p = 0.001] and middle-aged to older adults [SMD: -0.31 (95% CI: -0.55 to -0.08), p = 0.009]; in adults with obesity [SMD: -1.25 (95% CI: -1.75 to -0.76), p = 0.001]; with NAFLD [SMD: -0.61 (95% CI: -0.80 to -0.41), p = 0.001] and without NAFLD [SMD: -0.29 (95% CI: -0.49 to -0.10), p = 0.003], and with medium-term [SMD: -0.41 (95% CI: -0.58 to -0.25), p = 0.001] and longer-term interventions duration [SMD: -0.52 (95% CI: -0.78 to -0.26), p = 0.001]. Subgroup analysis for the study deign showed that ALT was significantly decreased in both studies with control [SMD: -0.31 (95% CI: -0.48 to -0.15), p = 0.001] and studies without control [SMD: -0.69 (95% CI: -0.92 to -0.46), p = 0.001] group.
Fig. 5.
Forest plot of the effects of intermittent fasting (IF) versus control diet (CON) or pre on ALT
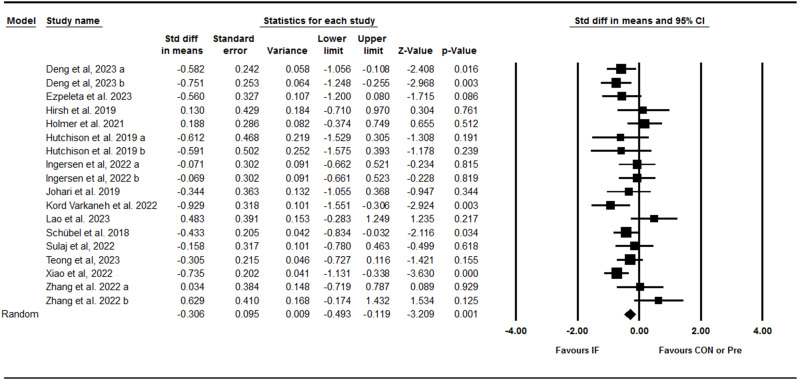
AST
Next we assessed the IF effect on changes in AST activity. Meta-analysis results from 18 trials including 13 between-group comparisons and five within-group comparisons showed that IF effectively decreased AST with a small effect size [SMD: -0.30 (95% CI: -0.49 to -0.11), p = 0.001] (Fig. 6). We found a significant heterogeneity amongst studies (I2 = 42.66, Tau2 = 0.06, p = 0.02). Both visual interpretation of the funnel plot and the Egger’s test suggested publication bias (p = 0.06). When the missing studies were accounted for using the trim and fill method, the overall effect size was [SMD: -0.44 (95% CI: -0.65 to -0.23)]. Subgroup analyses results indicating that AST decreased only following 5:2 diets [SMD: -0.40 (95% CI: -0.79 to -0.00), p = 0.04]; in young adults to middle-aged adults [SMD: -0.46 (95% CI: -0.71 to -0.21), p = 0.001]; adults with obesity [SMD: -0.38 (95% CI: -0.57 to -0.18), p = 0.001]; with NAFLD [SMD: -0.54 (95% CI: -0.81 to -0.28), p = 0.001] and without NAFLD [SMD: -0.29 (95% CI: -0.49 to -0.10), p = 0.003] NAFLD. Besides, both intervention durations, medium-term [SMD: -0.26 (95% CI: -0.48 to -0.04), p = 0.01] and longer-term [SMD: -0.44 (95% CI: -0.78 to -0.10), p = 0.01] significantly decreased AST. Subgroup analysis for the study deign showed that AST was decreased only in studies without control group [SMD: -0.40 (95% CI: -0.77 to -0.20), p = 0.001], not with control group [SMD: -0.21 (95% CI: -0.44 to 0.01), p = 0.06].
Fig. 6.
Forest plot of the effects of intermittent fasting (IF) versus control diet (CON) or pre on AST
Risk of bias judgements
The results of quality assessments of included studies are provided in the supplementary Tables 2 and 3, which were ranged from 10 to 12 for randomized trials and only one study was assessed using and the NIH quality assessment tool.
Discussion
Results from the current systematic review and meta-analysis showed that IF seems to have beneficial effects on liver function by reducing the liver fat content, liver steatosis, and liver enzymes, but not fibrosis. Findings also indicated that any mode of IF intervention could be effective in reducing the liver fat content and ALT, while only 5:2 diets reduced AST. Many of the beneficial effects of IF appear to occur in individuals with different metabolic statuses, ages, and different weight statuses, independent of the of the intervention duration. However, people with obesity may experience some additional beneficial effects following IF practice, especially in reduction of fibrosis and AST.
Meta-analytic evidence has previously shown that lifestyle interventions, including exercise training, dietary intervention, and combination of both are effective approaches in reducing the liver fat content [23, 80, 81]. In addition, a previous meta-analysis showed that dietary interventions, without any exercise training or physical activity also reduced intrahepatic lipid content [82]. Additionally, the macronutrient compositions of weight loss diets play a role on the effects on liver fat content, where low-carbohydrate diets have been shown to be more effective than low-fat diets [83]. With the increasing knowledge regarding the beneficial effects of dietary interventions, IF has gained significant attention as an alternate approach to the typical CR methods for weight loss and improving metabolic health outcomes. Although some meta-analyses have previously reported the effects of IF on reducing fat mass [84–86], the effects of IF on liver fat content have not been investigated in individuals with metabolic disorders. We found that IF interventions are effective in reducing the liver fat content. The potential mechanism by which IF may have reduced the liver fat content is not clearly understood. However mechanistically, an increase in circulating free fatty acid (FFA) and triglycerides (TG) input, increase TG de novolipogenesis and/or reduce FFA and TG output, lead to subsequent accumulation of fat in the liver [87, 88]. Some animal studies have shown that IF is able to decrease hepatic lipogenesis and increase β-oxidation markers [89–92].
Liver steatosis and fibrosis have been widely investigated in studies due to their role in the diagnosis and prognosis of liver diseases [93–95]. The current results indicated that IF effectively reduced liver steatosis, but not fibrosis. Consistent with our results, a previous meta-analysis involving individuals with NAFLD indicated that IF can reduce liver steatosis but not fibrosis [96]. The lack of beneficial effects on fibrosis may be due to short-term intervention durations or inclusion of participants without significant degrees of fibrosis. In addition, substantive evidence has shown that there is a dose-response relationship between weight loss and histological improvement of liver [93, 97, 98], indicating that greater weight loss are effective for histological improvement [93]. Further, improvement of fibrosis requires intensive and sustained weight loss (≥ 10%), while steatosis is improved even with moderate weight loss (7–10%) [93]. Therefore, longer and more intense IF intervention may be needed to induce greater weight loss and thereby decrease fibrosis in adults with metabolic disorders.
Liver enzymes, including ALT and AST are commonly used as biomarkers of liver function [99] which elevate in liver diseases, and are associated with the risk of progression of liver diseases [100]. The findings of the current meta-analysis showed that IF is able to decrease both AST and ALT, which reflect improvement of liver function and potential morphological changes in adults. These results are in agreement with a previous meta-analysis, which reported reductions in ALT and AST following IF in patients with NAFLD [101]. The potential mechanisms by which IF reduced liver AST and ALT may be related to weight loss, reduced visceral fat and steatosis, as well as decreased pro-inflammatory response and oxidative stress markers [53, 89, 90, 102, 103].
To better determine the reasons for heterogeneity, several subgroup analyses were performed in our study. We found that the IF mode and the health status of the participants moderated the effects of IF on liver function. We further noticed that liver fat content and ALT were decreased following IF, and these findings appear to be independent of IF mode, where all subgroups, including ADF, 5:2 diets, and TRF were associated with reductions in liver fat content, while AST was only reduced by 5:2 diets. Despite the previous clinical and meta-analytic studies, elucidating an optimal mode of IF still requires further investigation. In this regard, a recent network meta-analysis showed that ADF may be more effective as compared with other IF modes for adults with overweight and obesity [104]. While, another network meta-analysis reported that twice weekly fasting (5:2 diets) led to a larger combined effect on blood glucose and insulin resistance in patients with type 2 diabetes [105]. It seems that the effects of IF can differ depending on the outcome of the interest. Our results indicate that at least for liver fat content and ALT, any mode of IF is effective in adults with metabolic disorders. In addition, the health status and metabolic disorder type of participants may be one of the factors affecting metabolic responses to lifestyle interventions, where patients with favorable risk factors may achieve greater improvements in liver histological markers with moderate weight loss (7–10%), while patients with unfavorable risk factors may need greater weight loss (more than 10%) to produce beneficial effects on markers of liver health [93]. In the current meta-analysis, subgroup analyses based on BMI, age, and health status of participants (with NAFLD vs. without NAFLD) have shown that IF was associated with improvements in liver fat content regardless of these moderators. However, individuals with obesity may benefit more, since liver fibrosis and AST were significantly reduced only in people with obesity. Our findings are supported by the fact that obesity is closely associated with NAFLD, and liver disease of metabolic origin is now recognized as the most prevalent liver disease [106, 107]. The clinical significance of IF interventions for improving liver function may be particularly important, since research has shown that IF interventions can be effective for weight loss, and given that obesity has been suggested as a first-line strategy for the prevention and treatment of liver diseases [106].
Strength and limitations
Our study has several strengths and few limitations that should be considered. In this study, liver function was investigated by focusing on histological and enzyme biomarkers, which emphasized the clinical significance of the findings. Several subgroup analyses were performed to identify the role of moderating factors, especially the type of fasting. In our analyses, we included studies that were randomized and non-randomized trials or single-group pre-post trials. Due to potential biases and limitations within non-randomized and single-group trials, inclusion of such studies in meta-analyses is difficult to determine the clinical significance of the findings [108]. However, this approach allowed us to include more studies, and to perform important subgroup analyses. In addition, the majority of studies in our analysis had a high or moderate risk of bias, therefore, this issue should be considered when interpreting our meta-analysis results. The quality of the available evidence indicates that additional randomized clinical trials are needed to elucidate the effects of IF on liver function in adults with metabolic disorders. We further noticed significant heterogeneity amongst the studies for several outcomes, which may be due to the differences in study designs, participants’ characteristics, and outcome measurement methods. There were small numbers of studies for several outcomes, and therefore subgroup analyses should be interpreted with caution. Finally, there were small numbers of studies available that examined the role of IF as compared with continuous caloric restriction, and this type of analysis should be considered in future studies.
Conclusions
Findings from our meta-analysis demonstrated that IF is an effective dietary therapy for improving liver function in adults with metabolic disorders. This was confirmed by significant associations with reduced liver fat, liver steatosis, and liver enzymes with IF. Clinically, significant effects of IF on liver function seem to occur independent of IF mode, and age and health status of participants. Since lifestyle interventions are the first-line treatment strategy for obesity and related metabolic disorders, IF can be prescribed as a potential therapeutic approach to improve liver function in patients with metabolic diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
M Kh, F G, Sh Kh , A H M, S K R, M K and J T conceptualization of the systematic review and meta-analysis. M Kh, A H M, F G, S K R and Sh Kh, carried out the screenings and reviews. M Kh, M K and J T analyzed the data and performed meta-analyses. M Kh, M K and J T drafted the manuscript. M Kh and S K R revised the manuscript. All authors read and approved the final manuscript.
Funding
This research was supported by grant from the China Medical University (CMU112-N-13) of Taiwan.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mallikarjuna Korivi, Email: mallik.k5@gmail.com, Email: mallik@zjnu.edu.cn.
Jung-Piao Tsao, Email: fatbear110@gmail.com.
References
- 1.World H, Organization WHO. WHO Global report on diabetes. 2016.
- 2.Organization WH. Obesity: preventing and managing the global epidemic: report of a WHO consultation. 2000. [PubMed]
- 3.Cotter TG, Rinella M. Nonalcoholic fatty liver disease 2020: the state of the disease. Gastroenterology. 2020;158(7):1851–64. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela PL, et al. Obesity and the risk of cardiometabolic diseases. Nat Reviews Cardiol. 2023;20(7):475–94. [DOI] [PubMed] [Google Scholar]
- 5.Jung UJ, Choi M-S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kivimäki M, et al. Overweight, obesity, and risk of cardiometabolic multimorbidity: pooled analysis of individual-level data for 120 813 adults from 16 cohort studies from the USA and Europe. Lancet Public Health. 2017;2(6):e277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vernon G, Baranova A, Younossi Z. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non‐alcoholic steatohepatitis in adults. Volume 34. Alimentary pharmacology & therapeutics; 2011. pp. 274–85. 3. [DOI] [PubMed]
- 8.Estes C, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67(1):123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Reviews Gastroenterol Hepatol. 2013;10(6):330–44. [DOI] [PubMed] [Google Scholar]
- 10.Matteoni CA, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–9. [DOI] [PubMed] [Google Scholar]
- 11.Kasper P, et al. NAFLD and cardiovascular diseases: a clinical review. Clin Res Cardiol. 2021;110:921–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deprince A, Haas JT, Staels B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol Metabolism. 2020;42:101092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Targher G, Byrne CD, Tilg H. NAFLD and increased risk of cardiovascular disease: clinical associations, pathophysiological mechanisms and pharmacological implications. Gut. 2020;69(9):1691–705. [DOI] [PubMed] [Google Scholar]
- 14.Tilg H, Moschen AR, Roden M. NAFLD and Diabetes Mellitus. Nat Reviews Gastroenterol Hepatol. 2017;14(1):32–42. [DOI] [PubMed] [Google Scholar]
- 15.Targher G, et al. The complex link between NAFLD and type 2 diabetes mellitus—mechanisms and treatments. Nat Reviews Gastroenterol Hepatol. 2021;18(9):599–612. [DOI] [PubMed] [Google Scholar]
- 16.Mohamed J, et al. Mechanisms of diabetes-induced liver damage: the role of oxidative stress and inflammation. Sultan Qaboos Univ Med J. 2016;16(2):e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiang DJ, Pritchard MT, Nagy LE. Obesity, diabetes mellitus, and liver fibrosis. Am J Physiology-Gastrointestinal Liver Physiol. 2011;300(5):G697–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Albhaisi S, Chowdhury A, Sanyal AJ. Non-alcoholic fatty liver disease in lean individuals. JHEP Rep. 2019;1(4):329–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musazadeh V, et al. Omega-3 polyunsaturated fatty acids in the treatment of non‐alcoholic fatty liver disease: an umbrella systematic review and meta‐analysis. Clin Exp Pharmacol Physiol. 2023;50(5):327–34. [DOI] [PubMed] [Google Scholar]
- 20.Musazadeh V, et al. The effect of synbiotics on liver enzymes, obesity indices, blood pressure, lipid profile, and inflammation in patients with non-alcoholic fatty liver: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2024;107398:p. [DOI] [PubMed] [Google Scholar]
- 21.Farhangi MA, et al. Effectiveness of omega-3 and prebiotics on adiponectin, leptin, liver enzymes lipid profile and anthropometric indices in patients with non-alcoholic fatty liver disease: a randomized controlled trial. J Funct Foods. 2022;92:105074. [Google Scholar]
- 22.Leoni S, et al. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroenterol. 2018;24(30):3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koutoukidis DA, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: a systematic review and meta-analysis. Metabolism. 2021;115:154455. [DOI] [PubMed] [Google Scholar]
- 24.Hamman RF, et al. Effect of weight loss with lifestyle intervention on risk of diabetes. Diabetes Care. 2006;29(9):2102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kushner RF. Weight loss strategies for treatment of obesity. Prog Cardiovasc Dis. 2014;56(4):465–72. [DOI] [PubMed] [Google Scholar]
- 26.Bray GA, et al. Management of obesity. Lancet. 2016;387(10031):1947–56. [DOI] [PubMed] [Google Scholar]
- 27.Varady K. Intermittent versus daily calorie restriction: which diet regimen is more effective for weight loss? Obes Rev. 2011;12(7):e593–601. [DOI] [PubMed] [Google Scholar]
- 28.Hammer S, et al. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52(12):1006–12. [DOI] [PubMed] [Google Scholar]
- 29.Most J, et al. Calorie restriction in humans: an update. Ageing Res Rev. 2017;39:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MD, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice guidelines and the obesity society. Circulation. 2014;129(25suppl2):S102–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wing RR, et al. Caloric restriction per se is a significant factor in improvements in glycemic control and insulin sensitivity during weight loss in obese NIDDM patients. Diabetes Care. 1994;17(1):30–6. [DOI] [PubMed] [Google Scholar]
- 32.Larson-Meyer DE, et al. Effect of 6‐month calorie restriction and exercise on serum and liver lipids and markers of liver function. Obesity. 2008;16(6):1355–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Larson-Meyer DE, et al. Effect of calorie restriction with or without exercise on insulin sensitivity, β-cell function, fat cell size, and ectopic lipid in overweight subjects. Diabetes Care. 2006;29(6):1337–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura E et al. Lifestyle intervention involving calorie restriction with or without aerobic exercise training improves liver fat in adults with visceral adiposity. Journal of obesity, 2014. 2014. [DOI] [PMC free article] [PubMed]
- 35.De Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51. [DOI] [PubMed] [Google Scholar]
- 36.Mattson MP, Longo VD, Harvie M. Impact of intermittent fasting on health and disease processes. Ageing Res Rev. 2017;39:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Minciuna I, et al. Intermittent fasting—the future treatment in NASH patients? Hepatology. 2023;78(4):1290–305. [DOI] [PubMed] [Google Scholar]
- 38.Tinsley GM, La PM, Bounty. Effects of intermittent fasting on body composition and clinical health markers in humans. Nutr Rev. 2015;73(10):661–74. [DOI] [PubMed] [Google Scholar]
- 39.Meng H, et al. Effects of intermittent fasting and energy-restricted diets on lipid profile: a systematic review and meta-analysis. Nutrition. 2020;77:110801. [DOI] [PubMed] [Google Scholar]
- 40.Cho Y, et al. The effectiveness of intermittent fasting to reduce body mass index and glucose metabolism: a systematic review and meta-analysis. J Clin Med. 2019;8(10):1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harris L, et al. Intermittent fasting interventions for treatment of overweight and obesity in adults: a systematic review and meta-analysis. JBI Evid Synthesis. 2018;16(2):507–47. [DOI] [PubMed] [Google Scholar]
- 42.Obermayer A, et al. Efficacy and safety of intermittent fasting in people with insulin-treated type 2 diabetes (INTERFAST-2)—A Randomized Controlled Trial. Diabetes Care. 2023;46(2):463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grajower MM, Horne BD. Clinical management of intermittent fasting in patients with diabetes mellitus. Nutrients. 2019;11(4):873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Varady KA, et al. Clinical application of intermittent fasting for weight loss: progress and future directions. Nat Reviews Endocrinol. 2022;18(5):309–21. [DOI] [PubMed] [Google Scholar]
- 45.Santos HO. Intermittent fasting in the management of diabetes: a review of glycemic control and safety. Nutr Rev. 2024;82(10):1437–43. [DOI] [PubMed]
- 46.Khalafi M, et al. The effects of intermittent fasting on body composition and cardiometabolic health in adults with prediabetes or type 2 diabetes: a systematic review and meta-analysis. Diabetes Obes Metabolism. 2024;26(9):3830–41. [DOI] [PubMed] [Google Scholar]
- 47.Cai H, et al. Effects of alternate-day fasting on body weight and dyslipidaemia in patients with non-alcoholic fatty liver disease: a randomised controlled trial. BMC Gastroenterol. 2019;19:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deng Y, et al. Effects of time-restricted eating on intrahepatic fat and metabolic health among patients with nonalcoholic fatty liver disease. Obesity. 2024;32(3):494–505. [DOI] [PubMed] [Google Scholar]
- 49.Dokpuang D, et al. Magnetic resonance study of visceral, subcutaneous, liver and pancreas fat changes after 12 weeks intermittent fasting in obese participants with prediabetes. Diabetes Res Clin Pract. 2023;202:110775. [DOI] [PubMed] [Google Scholar]
- 50.Ezpeleta M, et al. Effect of alternate day fasting combined with aerobic exercise on non-alcoholic fatty liver disease: a randomized controlled trial. Cell Metabol. 2023;35(1):56–70. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feehan J, et al. Time-Restricted Fasting improves liver steatosis in non-alcoholic fatty liver Disease—A single blinded crossover trial. Nutrients. 2023;15(23):4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ingersen A, et al. Metabolic effects of alternate-day fasting in males with obesity with or without type 2 diabetes. Front Physiol. 2022;13:1061063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johari MI, et al. A randomised controlled trial on the effectiveness and adherence of modified alternate-day calorie restriction in improving activity of non-alcoholic fatty liver disease. Sci Rep. 2019;9(1):11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kord Varkaneh H, et al. Effects of the 5: 2 intermittent fasting diet on non-alcoholic fatty liver disease: a randomized controlled trial. Front Nutr. 2022;9:948655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teong XT, et al. Intermittent fasting plus early time-restricted eating versus calorie restriction and standard care in adults at risk of type 2 diabetes: a randomized controlled trial. Nat Med. 2023;29(4):963–72. [DOI] [PubMed] [Google Scholar]
- 56.Yin C et al. Effect of intermittent fasting on non-alcoholic fatty liver disease: systematic review and Meta-analysis. Front Nutr. 2021;8:709683. [DOI] [PMC free article] [PubMed]
- 57.Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. in Proceedings of the 18th international conference on evaluation and assessment in software engineering. 2014.
- 58.Cohen J. Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psychol Bull. 1968;70(4):213–20. [DOI] [PubMed] [Google Scholar]
- 59.Wan X, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions. 2008.
- 62.De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Australian J Physiotherapy. 2009;55(2):129–33. [DOI] [PubMed] [Google Scholar]
- 63.Ma L-L, et al. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Military Med Res. 2020;7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tufanaru C, et al. Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. JBI Evid Implement. 2015;13(3):196–207. [DOI] [PubMed] [Google Scholar]
- 65.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 66.Egger M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Duval S, Tweedie R. Trim and fill: a simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455–63. [DOI] [PubMed] [Google Scholar]
- 68.Hirsh SP, et al. Avoiding holiday seasonal weight gain with nutrient-supported intermittent energy restriction: a pilot study. J Nutritional Sci. 2019;8:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holmer M, et al. Treatment of NAFLD with intermittent calorie restriction or low-carb high-fat diet–a randomised controlled trial. JHEP Rep. 2021;3(3):100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hutchison AT, et al. Effects of intermittent versus continuous energy intakes on insulin sensitivity and metabolic risk in women with overweight. Obesity. 2019;27(1):50–8. [DOI] [PubMed] [Google Scholar]
- 71.Kahleova H, et al. Eating two larger meals a day (breakfast and lunch) is more effective than six smaller meals in a reduced-energy regimen for patients with type 2 diabetes: a randomised crossover study. Diabetologia. 2014;57:1552–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lao B-n, et al. Time-restricted feeding’s effect on overweight and obese patients with chronic kidney disease stages 3–4: a prospective non-randomized control pilot study. Front Endocrinol. 2023;14:1096093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu D, et al. Calorie restriction with or without time-restricted eating in weight loss. N Engl J Med. 2022;386(16):1495–504. [DOI] [PubMed] [Google Scholar]
- 74.Schroder JD, et al. Effects of time-restricted feeding in weight loss, metabolic syndrome and cardiovascular risk in obese women. J Translational Med. 2021;19:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schübel R, et al. Effects of intermittent and continuous calorie restriction on body weight and metabolism over 50 wk: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):933–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sulaj A, et al. Six-month periodic fasting in patients with type 2 diabetes and diabetic nephropathy: a proof-of-concept study. J Clin Endocrinol Metabolism. 2022;107(8):2167–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wei X, et al. Effects of time-restricted eating on nonalcoholic fatty liver disease: the TREATY-FLD randomized clinical trial. JAMA Netw Open. 2023;6(3):e233513–233513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiao Y, et al. Effect of 5: 2 fasting diet on liver fat content in patients with type 2 diabetic with nonalcoholic fatty liver disease. Metab Syndr Relat Disord. 2022;20(8):459–65. [DOI] [PubMed] [Google Scholar]
- 79.Zhang L-m et al. Randomized controlled trial for time-restricted eating in overweight and obese young adults. IScience, 2022. 25(9). [DOI] [PMC free article] [PubMed]
- 80.Medrano M, et al. Evidence-based exercise recommendations to reduce hepatic fat content in youth-a systematic review and meta-analysis. Prog Cardiovasc Dis. 2018;61(2):222–31. [DOI] [PubMed] [Google Scholar]
- 81.Smart N, et al. Effect of exercise training on liver function in adults who are overweight or exhibit fatty liver disease: a systematic review and meta-analysis. Br J Sports Med. 2018;52(13):834–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Houttu V, et al. Dietary interventions in patients with non-alcoholic fatty liver disease: a systematic review and meta-analysis. Front Nutr. 2021;8:716783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chai X-N, et al. Effects of lifestyle intervention on adults with metabolic associated fatty liver disease: a systematic review and meta-analysis. Front Endocrinol. 2023;14:1081096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gu L, et al. Effects of intermittent fasting in human compared to a non-intervention diet and caloric restriction: a meta-analysis of randomized controlled trials. Front Nutr. 2022;9:871682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Park J, et al. Effect of alternate-day fasting on obesity and cardiometabolic risk: a systematic review and meta-analysis. Metabolism. 2020;111:154336. [DOI] [PubMed] [Google Scholar]
- 86.Elortegui Pascual P, et al. A Meta-analysis comparing the effectiveness of alternate day fasting, the 5: 2 diet, and Time‐Restricted eating for weight loss. Obesity. 2023;31:9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332(6037):1519–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li G, et al. Metabolic adaptation to intermittent fasting is independent of peroxisome proliferator-activated receptor alpha. Mol Metabolism. 2018;7:80–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.de Souza Marinho T, et al. Beneficial effects of intermittent fasting on steatosis and inflammation of the liver in mice fed a high-fat or a high-fructose diet. Nutrition. 2019;65:103–12. [DOI] [PubMed] [Google Scholar]
- 90.de Castro-de-Paiva P, et al. Intermittent fasting, high-intensity interval training, or a combination of both have beneficial effects in obese mice with nonalcoholic fatty liver disease. J Nutr Biochem. 2022;104:108997. [DOI] [PubMed] [Google Scholar]
- 91.Baumeier C, et al. Caloric restriction and intermittent fasting alter hepatic lipid droplet proteome and diacylglycerol species and prevent diabetes in NZO mice. Biochim et Biophys Acta (BBA)-Molecular Cell Biology Lipids. 2015;1851(5):566–76. [DOI] [PubMed] [Google Scholar]
- 92.Deng J, et al. Efficacy and mechanism of intermittent fasting in metabolic associated fatty liver disease based on ultraperformance liquid chromatography-tandem mass spectrometry. Front Nutr. 2022;9:838091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Vilar-Gomez E, et al. Weight loss through lifestyle modification significantly reduces features of nonalcoholic steatohepatitis. Gastroenterology. 2015;149(2):367–78. e5. [DOI] [PubMed] [Google Scholar]
- 94.Lee J-H, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Disease. 2010;42(7):503–8. [DOI] [PubMed] [Google Scholar]
- 95.Siddiqui MS, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(1):156–63. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Saleh SA, et al. Effects of intermittent fasting regimens on glycemic, hepatic, anthropometric, and clinical markers in patients with non-alcoholic fatty liver disease: systematic review and meta-analysis of randomized controlled trials. Clinical Nutrition ESPEN; 2023. [DOI] [PubMed]
- 97.Promrat K, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology. 2010;51(1):121–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Petersen KF, et al. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54(3):603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kunutsor SK, Apekey TA, Khan H. Liver enzymes and risk of cardiovascular disease in the general population: a meta-analysis of prospective cohort studies. Atherosclerosis. 2014;236(1):7–17. [DOI] [PubMed] [Google Scholar]
- 100.Ekstedt M, et al. Long-term follow‐up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44(4):865–73. [DOI] [PubMed] [Google Scholar]
- 101.Yin C, et al. Effect of intermittent fasting on non-alcoholic fatty liver disease: systematic review and meta-analysis. Front Nutr. 2021;8:405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li D, et al. Intermittent fasting activates macrophage migration inhibitory factor and alleviates high-fat diet-induced nonalcoholic fatty liver disease. Sci Rep. 2023;13(1):13068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brandhorst S, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metabol. 2015;22(1):86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ma Y, Sun L, Mu Z. Network meta-analysis of three different forms of intermittent energy restrictions for overweight or obese adults. Int J Obes. 2024;48(1):55–64. [DOI] [PubMed] [Google Scholar]
- 105.Xiaoyu W, Yuxin X, Li L. The effects of different intermittent fasting regimens in people with type 2 diabetes: a network meta-analysis. Front Nutr. 2024;11:1325894. [DOI] [PMC free article] [PubMed]
- 106.Polyzos SA, Kountouras J, Mantzoros CS. Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 2019;92:82–97. [DOI] [PubMed] [Google Scholar]
- 107.Marchesini G, et al. Obesity-associated liver disease. J Clin Endocrinol Metabolism. 2008;93(11supplement1):s74–80. [DOI] [PubMed] [Google Scholar]
- 108.Kunz R, Oxman AD. The unpredictability paradox: review of empirical comparisons of randomised and non-randomised clinical trials. BMJ. 1998;317(7167):1185–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.