Abstract
Background and aim
Bile acid metabolism plays a crucial role in maintaining the delicate balance between coagulation and anticoagulation processes. However, there is a paucity of research exploring the relationship between serum total bile acids (TBA) levels and the risk of deep venous thrombosis (DVT) in Chinese individuals. The primary objective of this study is to investigate the association between TBA levels and DVT occurrence in this population.
Methods and results
A cross-sectional study was conducted involving 4522 patients with suspected DVT, recruited from June 2018 to October 2023, at the First Affiliated Hospital of Xi’an Jiaotong University. After rigorous screening, 3165 patients were included in the final analysis. Participants were categorized into three TBA level groups: low-level (TBA < 2.3 umol/L), moderate-level (2.3 ≤ TBA < 4.3 umol/L), and high-level (TBA ≥ 4.3 umol/L). Logistic regression analysis was utilized to assess the relationship between TBA levels and DVT risk, adjusting for potential confounders. The median age of the study population was 63 years, with a median TBA level of 3.2 (1.9–5.3) umol/L. The findings revealed that, compared to the low-level TBA group, the moderate and high-level TBA groups had significantly higher odds ratios (ORs) for DVT, with ORs of 1.47 (95% CI: 1.21 to 1.78, P < 0.001) and 1.91 (95% CI: 1.58 to 2.32, P < 0.001), respectively. Interestingly, higher TBA levels were also associated with reduced odds of bleeding risk, with ORs of 0.81 (95% CI: 0.63 to 1.03, P < 0.001) and 0.64 (95% CI: 0.5 to 0.81, P < 0.001), respectively.
Conclusions
Our study provides evidence that serum TBA levels may serve as a risk factor for DVT in Chinese individuals, with higher levels conferring an increased risk. Additionally, unexpectedly, higher TBA levels were found to be associated with a reduced risk of bleeding complications. These findings have significant implications for understanding the complex interplay between bile acid metabolism and deep venous thrombosis, and warrant further investigation.
Keywords: Deep venous thrombosis, Total bile acids, Bleeding risk
Introduction
Deep venous thrombosis (DVT) typically presents with pain and asymmetrical limb swelling, representing a life-threatening vascular condition. This condition often leads to severe complications, including pulmonary embolism (PE) and post-thrombotic syndrome (PTS) [1, 2]. While numerous risk factors, such as age, body mass index (BMI), and D-dimer levels, have been associated with DVT and serve as predictors of its occurrence, the diagnosis of DVT remains challenging prior to the manifestation of overt symptoms. Notably, anticoagulation management plays a pivotal role in minimizing the risks of thromboembolic and bleeding complications [1]. Hence, there is a pressing need to identify readily accessible clinical markers that are strongly correlated with the presence of DVT, as these can aid in earlier diagnosis and improved management of the disease.
Bile acids, as crucial physiological agents, function as signaling molecules and metabolic regulators that activate nuclear receptors to regulate hepatic lipid, glucose, and energy homeostasis. They are integral to intestinal nutrient absorption and biliary excretion of lipids, toxic metabolites, and xenobiotics [3–5]. Imbalances in bile acid metabolism have been implicated in various pathologies, including cholestatic liver disease, dyslipidemia, fatty liver, diabetes, and cardiovascular diseases [6, 7]. Additionally, contemporary research indicates that bile acids may foster coagulation by augmenting tissue factor (TF) activity, serving as cofactors to expedite the activation of the TF/FVIIa complex [8]. This phenomenon hints at a potential disruption in the delicate coagulation-anticoagulation balance, potentially contributing to the pathogenesis of deep venous thrombosis (DVT) and bleeding events. Nevertheless, the nexus between serum total bile acid (TBA) levels and DVT remains poorly understood. Therefore, exploring the correlation between serum TBA levels and DVT holds significant clinical value.
Methods
Study population
This study encompassed a total of 4522 patients suspected of having deep venous thrombosis (DVT), with Wells scores [1] of 2 or above, who underwent lower limb venous Doppler ultrasonography from June 2018 to October 2023, all admitted to the First Affiliated Hospital of Xi’an Jiaotong University. The exclusion criteria were stringent, including (1) patients under 18 years old; (2) pregnant women; (3) individuals with renal or liver dysfunction, defined as serum creatinine exceeding 133µmol/L or serum alanine transaminase levels more than three times the upper normal limit; (4) patients with malignant tumors; (5) those with obstructive biliary tract diseases; and (6) those prescribed bile acid sequestrants. After rigorous screening, 3165 patients were included in the final analysis (Fig. 1). Among this cohort, 1011 patients were definitively diagnosed with DVT (Table 1). Detailed clinical and demographic data were retrieved from medical records. The study adhered to the principles of the Declaration of Helsinki and received approval from the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. As this was a retrospective study, informed consent was not required.
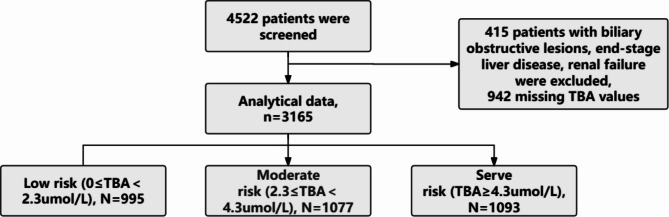
Fig. 1.
Study flow for the present analysis. A total of 4522 patients were screened initially. We excluded 415 patients with biliary obstructive lesions, end-stage liver disease, renal failure, 942 patients whose TBA values were missed, and finally 3165 patients were included in this study
Table 1.
Baseline characteristics of participants according to TBA values
| Variables | Total (n = 3165) | TBA < 2.3umol/L (n = 995) | TBA < 4.3umol/L (n = 1077) | TBA ≥ 4.3umol/L (n = 1093) | p |
|---|---|---|---|---|---|
| TBA (umol/L) | 3.2 (1.9, 5.3) | 1.5 (1, 1.9) | 3.1 (2.7, 3.6) | 6.4 (5.2, 8.7) | < 0.001 |
| Age (yr) | 63 (51, 75) | 62 (48, 72) | 63 (50, 75) | 65 (53.5, 76) | < 0.001 |
| Gender, male (%) | 1760 (56) | 529 (53) | 599 (56) | 632 (58) | 0.101 |
| DM (%) | 469 (15) | 152 (15) | 161 (15) | 156 (14) | 0.803 |
| Hypertension (%) | 1025 (32) | 355 (36) | 336 (31) | 334 (31) | 0.026 |
| CAD (%) | 1 (0) | 1 (0) | 0 (0) | 0 (0) | 0.314 |
| Liver.diseases (%) | 202 (6) | 58 (6) | 56 (5) | 88 (8) | 0.017 |
| DVT (%) | 1011 (32) | 250 (25) | 351 (33) | 410 (38) | < 0.001 |
| ALT (mmol/L) | 18 (12, 29) | 18 (12, 28) | 18 (12, 29) | 18 (12, 30.3) | 0.315 |
| TBILI (mmol/L) | 11.7 (8.5, 16.8) | 11.6(8.28, 16.33) | 11.8 (8.6, 16.9) | 11.9 (8.6, 17.1) | 0.708 |
| ALB (mmol/L) | 36.8 (33, 40) | 36.5(32.62, 39.9) | 36.3 (32.8, 39.5) | 37.4 (33.8, 40.4) | < 0.001 |
| MELD (mmol/L) | 2.7 (1.1, 5.09) | 2.71 (1.09, 5.09) | 2.72 (1.13, 5.17) | 2.67 (1.07, 5.03) | 0.934 |
| Crea (mmol/L) | 58 (47, 72.1) | 57 (46, 71) | 57 (46, 73) | 59 (48, 73) | 0.035 |
| TG (mmol/L) | 1.15(0.84, 1.63) | 1.2 (0.87, 1.63) | 1.1 (0.81, 1.57) | 1.16 (0.85, 1.65) | 0.078 |
| Cho (mmol/L) | 3.83 (3.2, 4.58) | 3.85 (3.26, 4.59) | 3.74 (3.15, 4.54) | 3.91 (3.21, 4.6) | 0.064 |
| Hokusai_score (%) | 0.003 | ||||
| 1 | 903 (29) | 252 (25) | 303 (28) | 348 (32) | |
| 2 | 1245 (39) | 385 (39) | 433 (40) | 427 (39) | |
| 3 | 1017 (32) | 358 (36) | 341 (32) | 318 (29) |
TBA: total bile acids; DM: diabetes mellitus; CAD: coronary artery disease; DVT: deep venous thrombosis; ALT: alanine aminotransferase; TBILI: total bilirubin; ALB: albumin; MELD: Model for End-Stage Liver Disease; Crea: creatinine; TG: triglycerides; Cho: cholesterol
Definitions
For the current investigation, the diagnosis of deep venous thrombosis (DVT) was established during the final evaluation in 2019, adhering to the guidelines outlined by the National Institute for Health and Care Excellence. In the Chinese context, the diagnosis of DVT necessitates a comprehensive approach encompassing clinical assessment, estimation of pre-test probability, and objective diagnostic testing. The typical manifestations of DVT encompass pain, swelling, erythema, and dilated veins in the affected limb. The pre-test probability of DVT can be categorized as “unlikely” or “likely” using a clinical decision rule. In cases where DVT is deemed “unlikely,” a D-dimer test is recommended. If the D-dimer level is within the normal range, DVT can be ruled out; however, if the D-dimer level is elevated, further evaluation with compression ultrasound is advisable. Conversely, when DVT is considered “likely,” compression ultrasound is the recommended diagnostic modality.
For ultrasonography, a high-resolution linear-array transducer operating at 5- or 7.5-MHz was utilized. The assessment focused on the compressibility of the deep veins, examining them at 1-cm intervals from the common femoral vein down to the point where the popliteal vein merges with the calf veins. In patients without a prior history of DVT, a non-compressible vein was indicative of DVT. Conversely, for patients with echographically identified old thrombosis, we would contact the patients to obtain their previous ultrasound examination records for comparison, the diagnosis was based on the presence of a new non-compressible site or an increase in clot diameter of at least 4 mm compared to previous measurements. A change in clot diameter of 1 mm or less excluded the possibility of recurrence. However, if the increase in clot diameter ranged from 1.1 to 3.9 mm, the ultrasound examination was repeated after a week or venography was performed [1]. Additionally, bleeding risk was assessed using the Hokusai-score, which incorporated several high-risk factors: female gender, APT (antiplatelet therapy), hemoglobin levels below 10 g/dL, a history of hypertension, and systolic blood pressure exceeding 160 mmHg [9]. Scores of 0, 1, and 2 were classified as low, intermediate, and high bleeding risk, respectively [10].
Measurements
Serum fasting TBA was measured with an enzymatic cycling method using reagents (Pureauto S TBA, SEKISUI MEDICAL CO., LTD.) on a Hitachi Chemistry Analyzer 7600 according to the protocols. Quality control procedures were conducted according to the protocols as well, and the coefficients of variation were all ≤ 5%.
Serum TBA, alanine aminotransferase (ALT), total bilirubin (TBILI), albumin (ALB), creatinine (Crea), triglycerides (TG) and cholesterol (Cho) were obtained from fasting venous blood samples at admission or the following morning. All of these laboratory tests were implemented using standard methods.
Statistics
All statistical computations were executed utilizing R studio version 3.5.3. For continuous variables, median values with interquartile ranges were reported, while categorical variables were summarized using frequencies and percentages. The analysis of variance (ANOVA) was employed to assess differences in parameters among groups for normally distributed variables. For non-normally distributed continuous variables, the Kruskal-Wallis test was applied, while the chi-square test was utilized for categorical variables. To explore the relationship between TBA levels and the occurrence of DVT, as well as its bleeding risk, considering potential confounders such as age, gender, liver diseases, and the MELD (model for end-stage liver disease) score, restricted cubic splines were applied. A logistic regression model was implemented to assess the association between serum TBA levels (categorized) and the presence of DVT, along with its bleeding risk, expressed as odds ratios (ORs) with 95% confidence intervals (CIs). This regression model was adjusted for factors including age, gender, liver diseases, and the MELD score. Statistical significance was defined as a two-sided P-value below 0.05.
Results
Baseline characteristics of the study population
Among the included participants, 56.0% were male, and the median age was 63 years, ranging from 51 to 75 years. We categorized all the patients into three groups as low-level (TBA < 2.3umol/L), moderate-level (2.3 ≤ TBA < 4.3umol/L), and high-level (TBA ≥ 4.3umol/L) according to the tertile of TBA concentrations.
As indicated in Table 1, participants in moderate-level and high-level groups exhibited consistently higher presence of DVT and lower risk of bleeding. In addition to having more elders, more patients with hypertension and liver diseases, participants in moderate-level and high-level groups also had higher ALB levels (all P < 0.001). Other factors were similar among the three groups.
Association between TBA and DVT and its bleeding risk
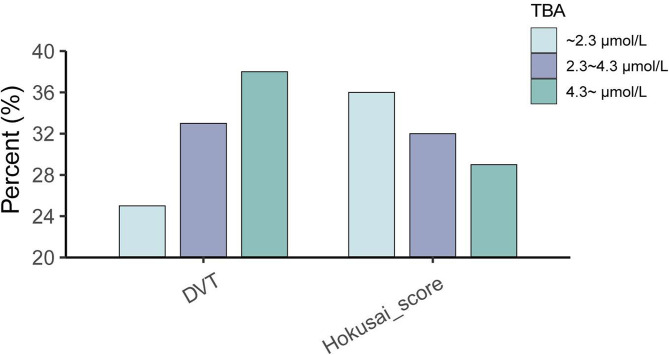
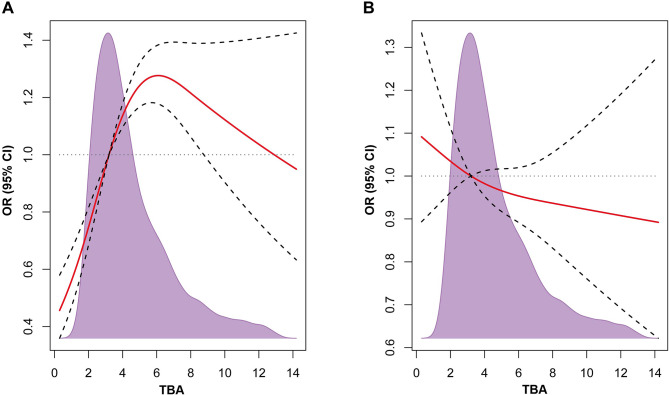
We conducted a comparison of DVT and bleeding outcomes based on different TBA levels. As shown in Fig. 2, we found that the moderate-level and high-level groups exhibited higher presence of DVT and lower bleeding risk when compared to the low-level group. Spline analyses showed that with the increase of TBA levels, the ORs of DVT rose sharply at first and then decrease slowly, and the breakpoint approximated 6 µmol/L. The association between TBA and DVT remained significant even after adjusting for age, gender, liver diseases and MELD score (Fig. 3). However, there was no relationship observed between TBA and bleeding risk (Fig. 3).
Fig. 2.
Histograms show the prevalence of DVT and bleeding risk (Hokusai-score ≥ 1). Group1: TBA < 2.3umol/L, Group2: 2.3 ≤ TBA < 4.3umol/L, Group3: TBA ≥ 4.3umol/L
Fig. 3.
Restricted spline curves for the associations between TBA and DVT or bleeding risk. Solid lines represent the odds ratio, dashed lines represent the 95% confidence intervals. (A) Association between TBA and DVT. (B) Association between TBA and bleeding risk (Hokusai-score ≥ 1). OR (95% CI) was adjusted by age, gender, liver diseases and MELD score. DVT: deep venous thrombosis; OR: odds ratio; TBA: total bile acids
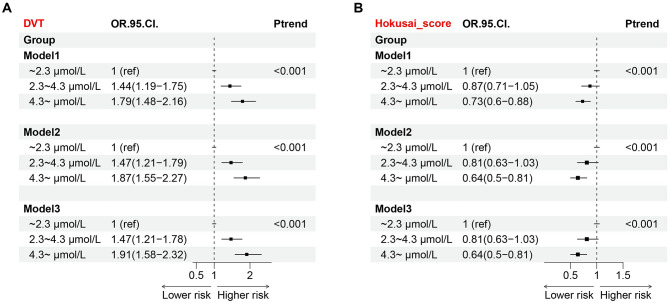
Compare with the low-level group, the ORs (95% CIs) of DVT in moderate-level and high-level groups were 1.44 [1.19–1.75] and 1.79 [1.48–2.16] (P<0.001), respectively, in model1. In model2 that further adjusted for age and sex, the ORs (95% CIs) of the latter two groups were 1.47 [1.21–1.79] and 1.87 [1.55–2.27] (P<0.001). In model3 that fully adjusted, the ORs (95% CIs) of the latter two groups were 1.47 [1.21–1.78] and 1.91 [1.58–2.32] (P<0.001) (Fig. 4).
Fig. 4.
Associations between TBA and DVT or bleeding risk. The adjusted odds ratio and 95% CI are displayed in the forest plot. (A) Association between TBA and DVT. (B) Association between TBA and bleeding risk (Hokusai-score ≥ 1). DVT: deep venous thrombosis; CI: confidence interval; OR: odds ratio. Model 1: unadjusted. Model 2: adjusted for age, sex. Model 3: adjusted for age, gender, liver diseases and MELD score
As shown in Fig. 4, the ORs of bleeding risk in moderate- and high-level groups decreased compared to the low-level group (OR = 0.87 [0.71–1.05], OR = 0.73 [0.6–0.88], respectively) in model1. The ORs (95% CIs) of the latter two groups were 0.81 [0.63–1.03] and 0.64 [0.5–0.81] (P<0.001) in model2. The ORs (95% CIs) of the latter two groups were 0.81 [0.63–1.03] and 0.64 [0.5–0.81] (P<0.001) in model3 (Fig. 4).
Discussion
Deep vein thrombosis (DVT) poses a significant threat, manifesting in localized swelling and pain, and posing a high risk for potentially fatal pulmonary embolism. As part of the diagnostic process, identifying risk factors is paramount. Although most DVT patients undergo anticoagulant therapy, the choice and duration of treatment vary significantly based on individual risk factors [11]. While oral anticoagulation has been established as an effective means to prevent future thromboembolic events, it is not without its risks, including the potential for major bleeding complications. Consequently, it becomes imperative to identify patients at high risk for DVT or bleeding events using readily accessible clinical characteristics. This identification is crucial in facilitating individualized management strategies to mitigate the patient’s risk. The decision to initiate and maintain anticoagulation therapy often hinges on a meticulous evaluation of both thromboembolism and bleeding risks [10].
Prior investigations have demonstrated a link between serum total bile acid (TBA) levels and various chronic illnesses, encompassing liver diseases, dyslipidemia, fatty liver diseases, diabetes, and cardiovascular conditions [6]. Despite this, the specific role of bile acid in the pathogenesis of DVT and its associated bleeding risk remains poorly understood. Our current study aimed to elucidate this role, revealing a strong association between elevated fasting serum TBA levels and a heightened prevalence of DVT, coupled with a reduced bleeding risk. This association persisted even after considering confounding factors such as age, gender, liver diseases, and the MELD (Model for End-Stage Liver Disease) score. Notably, our study is the first large-scale examination to unmask this significant correlation between serum TBA levels and both DVT presence and bleeding risk in patients under suspicion of DVT.
Past research has explored potential mechanisms underlying bile acids’ modulation of coagulation. Bile acids function as signaling molecules, regulating metabolism and inflammation via receptors like the nuclear farnesoid X receptor (FXR) and the Takeda G protein-coupled receptor 5 (TGR5) [12]. Alongside the classical Virchow’s triad factors (blood flow disturbance, hypercoagulability, and vessel wall changes), inflammation holds a pivotal role in DVT’s pathogenesis. Clinical conditions associated with inflammation, such as sepsis, systemic infections, cancer, trauma, and surgery, are known risk factors for venous thromboembolism (VTE) [13]. Furthermore, previous studies have linked TBA to elevated levels of tumor necrosis factor-α, a marker of inflammation, suggesting that inflammation may serve as a bridge between TBA and DVT. Additionally, immune dysregulation has been identified as a contributory factor in thrombosis, and bile acids have been shown to regulate immune functions. A range of clinical conditions associated with an increased VTE risk, including inflammatory bowel disease, systemic lupus erythematosus, obesity, surgery, cancer, and acute and chronic infections, exhibit deregulated immune networks that intersect with coagulation pathways [14]. Hence, immune dysregulation could potentially moderate the relationship between TBA and DVT.
Platelets are pivotal in the development of venous thrombosis, as recent evidence indicates that they can facilitate thrombus formation by directly activating coagulation pathways. This activation occurs through the release of polyphosphate, which triggers factor XII activation, and protein disulfide isomerase (PDI), which enhances tissue factor (TF) activation [15]. Given that inhibiting platelet activation can safeguard against DVT, which affects a vast population, the role of these cells is particularly significant. Prior studies have established the farnesoid X receptor (FXR) as a crucial factor in the formation of coated platelets, which are preactivated platelets exhibiting increased fibrinogen binding and a prothrombotic phenotype. Bile acids, being natural ligands of FXR, have the potential to induce platelet activation, thus contributing to thrombus formation [16]. Consequently, the correlations between serum total bile acids (TBA) and the presence of DVT as well as bleeding risk may be partially attributed to alterations in platelet function. However, the precise mechanisms underlying these associations remain to be elucidated.
The role of endothelial dysfunction in thrombus formation is pivotal [15]. Notably, bile acids have been linked to endothelial dysfunction [17, 18], which can exacerbate the progression of DVT. Bile acids, through their activation of the farnesoid X receptor (FXR), have been demonstrated to reduce endothelin-1 expression in lung endothelial cells. Additionally, TGR5, another bile acid receptor, is present in aortic endothelial cells that produce nitric oxide. Moreover, bile acids can modulate endothelial cell responses via S1P receptors and Ca2+-dependent K + currents [18]. Furthermore, bile acids’ association with vascular calcification and fibrosis is attributed to their regulation of various signaling pathways [19].
A recent investigation has revealed a novel mechanism in which the nutrient-sensing nuclear receptor FXR regulates coagulation factor synthesis. Malnutrition-induced coagulation dysfunction in mice could be attributed to diminished FXR activation and synthesis resulting from lower bile acid levels [20]. Furthermore, bile acids have the potential to enhance the procoagulant activity of hepatocytes and activate coagulation factors, indicating their relevance in TF-driven coagulation [8]. Consequently, bile acids may exert influence at various stages of thrombus formation, thereby affecting the risk of bleeding events.
Our research revealed an initial steep rise in the correlation between serum TBA levels and DVT events, followed by a gradual decline when TBA concentrations surpassed approximately 6 µmol/L. This pattern suggests a saturation point beyond which an increased risk of DVT events is not observed. The variations in serum TBA levels observed post-inflection point in our study may stem, in part, from potential mild liver irregularities and alterations in the serum TBA profiles among patients with higher TBA levels. To further elucidate the mechanisms underlying TBA’s role in DVT development, studies exploring various serum TBA concentrations and their dynamic changes are warranted.
Our study presents several limitations that merit consideration. Firstly, being a single-center study focused on Chinese patients with suspected DVT, our findings should be cautiously extrapolated to other ethnic groups. Additional studies encompassing diverse ethnic populations are required to validate our observations. Secondly, the cross-sectional nature of our study falls short of establishing a causal link between TBA and DVT. Thirdly, our analysis was confined to fasting TBA levels, without accounting for specific bile acid components. Future prospective investigations are warranted to further explore our preliminary findings.
In summary, our investigation of Chinese patients suspected of having DVT has revealed that fasting serum TBA levels are significantly elevated in those with DVT compared to their non-DVT counterparts. Notably, fasting serum TBA displays a robust and independent association with the presence of DVT and bleeding risk. Consequently, TBA emerges as a clinically valuable and cost-effective biomarker for identifying DVT and assessing bleeding risk. Our findings have the potential to significantly impact nutrition science and practice in the foreseeable future. By understanding this association, nutrition researchers and practitioners can explore dietary interventions or nutritional strategies that may help modulate TBA levels, ultimately contributing to the prevention and management of DVT. Furthermore, incorporating TBA levels into nutritional assessments could provide additional insights into individual bleeding risks, enabling personalized nutrition recommendations for patients.
Acknowledgements
We thank the Biobank of First Affiliated Hospital of Xi’an Jiaotong University for data collection.
Abbreviations
- TBA
Total bile acids
- DVT
Deep venous thrombosis
- PE
Pulmonary embolism
- PTS
Post-thrombotic syndrome
- BMI
Body mass index
- TF
Tissue factor
- ANOVA
Analysis of variance
- MELD
Model for end-stage liver disease
- ORs
Odds ratios
- CIs
Confidence intervals
- DM
Diabetes mellitus
- CAD
Coronary artery disease
- ALT
Alanine aminotransferase
- TBILI
Total bilirubin
- ALB
Albumin
- Crea
Creatinine
- TG
Triglycerides
- Cho
Cholesterol
- FXR
Farnesoid X receptor
- TGR5
Takeda G protein-coupled receptor 5
- VTE
Venous thromboembolism
- PDI
Protein disulfide isomerase
Author contributions
Bolin Li: Conceptualization, Project administration, Writing - original draft, Writing - review & editing. Xinxin Feng: Methodology, Project administration, Data analysis, Writing - review & editing. Xinping Kang: Investigation, Data curation. Yi Zhao: Investigation, Data curation. Miaomiao Cao: Resources, Data curation. Bao Xu: Writing - review & editing, Data curation. Hui Liu: Investigation, Data curation. Shuyi Deng: Writing - review & editing. Yue Wu: Project administration, Supervision, Writing - review & editing. Tao Zheng: Project administration, Supervision, Writing - review & editing.
Funding
This study was supported by the Research Fund of the First Affiliated Hospital of Xi’an Jiaotong University (2021ZXY-21).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Bolin Li and Xinxin Feng contributed equally to this work.
Contributor Information
Yue Wu, Email: yue.wu@xjtu.edu.cn.
Tao Zheng, Email: zhengtao900305@xjtufh.edu.cn.
References
- 1.Kruger PC, Eikelboom JW, Douketis JD, Hankey GJ. Deep vein thrombosis: update on diagnosis and management, Med J Aust, vol. 210, no. 11, pp. 516–524, Jun. 2019, 10.5694/mja2.50201 [DOI] [PubMed]
- 2.Watson C et al. Mar., Venous Thromboembolism: Review of Clinical Challenges, Biology, Assessment, Treatment, and Modeling, Ann Biomed Eng, vol. 52, no. 3, pp. 467–486, 2024, 10.1007/s10439-023-03390-z [DOI] [PubMed]
- 3.Liu Z, You C. The bile acid profile. Clin Chim Acta. Jan. 2025;565:120004. 10.1016/j.cca.2024.120004. [DOI] [PubMed]
- 4.Gillard J, Leclercq I. New discoveries in bile acids, gut microbiota and host interactions in health and diseases, Clinical Science, vol. 138, no. 23, pp. 1521–1523, Nov. 2024, 10.1042/CS20240940 [DOI] [PubMed]
- 5.Zhuang T et al. Nov., Biological functions and pharmacological behaviors of bile acids in metabolic diseases, J Adv Res, pp. S2090-1232(24)00495–8, 2024, 10.1016/j.jare.2024.11.003 [DOI] [PubMed]
- 6.Chiang JYL. Bile acid metabolism and signaling, Compr Physiol, vol. 3, no. 3, pp. 1191–1212, Jul. 2013, 10.1002/cphy.c120023 [DOI] [PMC free article] [PubMed]
- 7.Li W, et al. Fasting serum total bile acid level is associated with coronary artery disease, myocardial infarction and severity of coronary lesions. Atherosclerosis. Jan. 2020;292:193–200. 10.1016/j.atherosclerosis.2019.11.026. [DOI] [PubMed]
- 8.Baker KS et al. Oct., Direct Amplification of Tissue Factor:Factor VIIa Procoagulant Activity by Bile Acids Drives Intrahepatic Coagulation, Arterioscler Thromb Vasc Biol, vol. 39, no. 10, pp. 2038–2048, 2019, 10.1161/ATVBAHA.119.313215 [DOI] [PMC free article] [PubMed]
- 9.Nisio MD, et al. Prediction of major and clinically relevant bleeding in patients with VTE treated with edoxaban or vitamin K antagonists. Thromb Haemost. Apr. 2017;117(4):784–93. 10.1160/TH16-11-0830. [DOI] [PubMed]
- 10.Gorog DA et al. Nov., Assessment and mitigation of bleeding risk in atrial fibrillation and venous thromboembolism: A Position Paper from the ESC Working Group on Thrombosis, in collaboration with the European Heart Rhythm Association, the Association for Acute CardioVascular Care and the Asia-Pacific Heart Rhythm Society, Europace, vol. 24, no. 11, pp. 1844–1871, 2022, 10.1093/europace/euac020 [DOI] [PMC free article] [PubMed]
- 11.Olaf M, Cooney R, Thrombosis DV. Emergency Medicine Clinics of North America, vol. 35, no. 4, pp. 743–770, Nov. 2017, 10.1016/j.emc.2017.06.003 [DOI] [PubMed]
- 12.Chávez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, type 2 diabetes, Dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology. May 2017;152(7):1679–e16943. 10.1053/j.gastro.2017.01.055. [DOI] [PubMed]
- 13.Borgel D, Bianchini E, Lasne D, Pascreau T, Saller F. Inflammation in deep vein thrombosis: a therapeutic target? Hematology, vol. 24, no. 1, pp. 742–750, Dec. 2019, 10.1080/16078454.2019.1687144 [DOI] [PubMed]
- 14.Colling ME, Tourdot BE, Kanthi Y. Inflammation, infection and venous thromboembolism. Circ Res. Jun. 2021;128(12):2017–36. 10.1161/CIRCRESAHA.121.318225. [DOI] [PMC free article] [PubMed]
- 15.Navarrete S, Solar C, Tapia R, Pereira J, Fuentes E, Palomo I. Pathophysiology of deep vein thrombosis, Clin Exp Med, vol. 23, no. 3, pp. 645–654, Jul. 2023, 10.1007/s10238-022-00829-w [DOI] [PubMed]
- 16.Zobel J, et al. Bile acids induce platelet activation leading to Degranulation and a prothrombotic phenotype. Blood. Nov. 2019;134. 10.1182/blood-2019-126095.
- 17.Khurana S, Raufman J, Pallone TL. Bile acids regulate Cardiovascular function. Clin Transl Sci. Jun. 2011;4(3):210–8. 10.1111/j.1752-8062.2011.00272.x. [DOI] [PMC free article] [PubMed]
- 18.Zhang S, Zhou J, Wu W, Zhu Y, Liu X. The Role of Bile Acids in Cardiovascular Diseases: from Mechanisms to Clinical Implications, Aging Dis, vol. 14, no. 2, pp. 261–282, Apr. 2023, 10.14336/AD.2022.0817 [DOI] [PMC free article] [PubMed]
- 19.Yin L, Li X, Ghosh S, Xie C, Chen J, Huang H. Role of gut microbiota-derived metabolites on vascular calcification in CKD, J Cell Mol Med, vol. 25, no. 3, pp. 1332–1341, Feb. 2021, 10.1111/jcmm.16230 [DOI] [PMC free article] [PubMed]
- 20.Preidis GA et al. Dec., Coagulopathy in Malnourished Mice Is Sexually Dimorphic and Regulated by Nutrient-Sensing Nuclear Receptors, Hepatol Commun, vol. 4, no. 12, pp. 1835–1850, 2020, 10.1002/hep4.1622 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.