Abstract
Background
The benefit of cytoreduction with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) for epithelial ovarian cancer (EOC) remains uncertain. This study investigated the relationship between serum cytokines, particularly monocyte chemoattractant protein-1 (MCP-1), a key inflammatory mediator, and recurrence risk in EOC patients undergoing CRS/HIPEC.
Methods
From January 2018 to January 2023, serum cytokine levels were analyzed in 34 EOC patients (17 primary, 17 recurrent) before and after CRS/HIPEC using MILLIPLEX Magnetic Bead Panels. Cox proportional hazards regression calculated adjusted hazard ratios (HRs) after controlling for clinical variables. Immunohistochemical (IHC) staining was performed on tissue microarrays from 19 patients.
Results
Higher 1-unit increment of MCP-1_Baseline were associated with increased recurrence risk within the first year post-CRS/HIPEC (HR: 1.010, 95% CI: 1.000-1.021). After one year, higher 1-unit increments of MCP-1_Post and MCP-1_Change were associated with increased recurrence risk. Lower IL-13 change and higher GROα change were associated with better progression-free survival (PFS) (p = 0.002 and p = 0.025, respectively). IHC analysis showed a trend towards worse PFS within the first year for patients with MCP-1 expression in tumor tissue (HR: 3.252, p = 0.264).
Conclusion
Cytokines, particularly MCP-1, may help predict PFS following CRS/HIPEC in EOC patients and could inform postoperative treatment decisions. Further research is needed to confirm these findings and explore clinical applications.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13048-024-01586-y.
Keywords: Ovarian cancer, Cytokine, Progression-free survival, Hyperthermia, Chemotherapy, Biomarkers, Cytoreductive surgery
Introduction
Epithelial ovarian cancer (EOC) is a leading cause of death in gynecological malignancies, with over 75% of patients diagnosed at an advanced stage due to its subtle onset and lack of early symptoms, leading to high morbidity and mortality [1]. Notably, circulating pro- and anti-inflammatory cytokine levels during cancer treatment may serve as valuable prognostic indicators [2]. Patients with EOC in a remission after chemotherapy have been reported to have lower serum levels of IL-8 and IL-10 compared to those with recurrence and metastasis after treatment [3]. Cytokines within the tumor microenvironment exhibit paracrine activity, with fibroblasts secreting elevated levels of IL-6, IL-8, MCP-1, and GROα. These cytokines can influence the proliferation of ovarian cancer cells [4].
One treatment for peritoneal carcinomatosis is cytoreductive surgery (CRS) combined with hyperthermic intraperitoneal chemotherapy (HIPEC), where chemotherapeutic agents are heated to 41–43 °C and applied directly to residual cancer cells, enhancing their effect through hyperthermia [5]. However, the effectiveness of CRS/HIPEC in managing advanced EOC, with its diffuse peritoneal spread and recurrence, remains debate [6–8]. Clinical factors like platinum resistance, multiple relapses, and ascites, may predict poorer outcomes in recurrent EOC, but accurate prognostication remains challenging [9]. Identifying effective biomarkers is crucial for better predicting prognosis in EOC patients undergoing CRS/HIPEC.
The relationship between circulating cytokines and CRS/HIPEC is significant because heat and surgery can trigger an inflammatory response. A study investigating circulating cytokines found that CRS/HIPEC was associated with the release of profound circulating danger-associated molecular patterns, which could potentially elicit an immune response and induce an immune-suppressed state [10]. The increase in temperature has been shown to lead to increased expressions of pro-inflammatory Th1 cytokines (IFN- and TNF-) and anti-inflammatory Th2 cytokines (IL-4 and IL-5), as well as IL-10 [11].
While CRS/HIPEC has shown promise in treating peritoneal carcinomatosis compared to conventional chemotherapy alone, particularly in improving local disease control, there is limited literature discussing the association between circulating immunoprofiles in EOC and CRS/HIPEC. Previous observational studies have demonstrated that CRS/HIPEC can trigger significant cytokine responses [10], but research has been limited to measurements of both baseline and post-CRS/HIPEC markers and correlating clinical characteristics. Notably, there is a critical gap in identifying reliable prognostic biomarkers for EOC patients undergoing CRS/HIPEC, which could help optimize patient selection and treatment strategies. We hypothesized that CRS/HIPEC may affect the levels of inflammatory cytokines, and that this could be used to predict progression-free survival (PFS) after CRS/HIPEC. Therefore, we conducted this observational study to analyze expressions of circulating cytokines in patients with ovarian cancer who were treated with HIPEC and had complete clinicopathologic data and adequate follow-up.
Patients and methods
Study design and population
This study was conducted at Chang Gung Memorial Hospital, Chiayi, Taiwan, from January 2018 to January 2023. Serum specimens and clinical data were prospectively collected and retrospectively examined for serum cytokines, and their correlations with clinical information were analyzed. We enrolled patients diagnosed with primary or recurrent EOC who were scheduled to undergo CRS/HIPEC for indications including: [1] neoadjuvant chemotherapy followed by interval debulking surgery and HIPEC, and [2] recurrent ovarian cancer with planned secondary CRS and HIPEC.
The exclusion criteria were: [1] age below 20 or above 75 years; [2] preoperative Eastern Cooperative Oncology Group (ECOG) performance status > 2; [3] patients undergoing palliative HIPEC for ascites control without curative intent; and [4] patients in whom optimal debulking surgery could not be achieved.
This study received approval from the Institutional Review Board of Chang Gung Memorial Hospital (approval code 202001607A3), and it met the guidelines set by the Helsinki Declaration. Written informed consent was obtained from all enrolled patients.
CRS/HIPEC procedure
All participants underwent a standardized CRS/HIPEC procedure performed by a multidisciplinary team (MDT) [12]. CRS was performed via a midline laparotomy. Post CRS, HIPEC was administered using the closed method with a Performer™ HT intraperitoneal hyperthermia system (RanD Biotech, Medolla, Italy). The perfusate comprised a mixture of normal saline and pentastarch (Haes-steril, 60 mg/mL, Meda, Sweden) at a concentration of 10% (3:1). The perfusate was administered at a dose of 2 L/m2 of body surface to achieve effective distribution within the peritoneal cavity. Chemotherapy infusion began once an intra-abdominal temperature of 41–43 °C had been reached, and HIPEC lasted for 60 min. After completion of HIPEC, the intra-abdominal chemotherapy drugs were drained.
The HIPEC regimen was chosen based on the patient’s clinical status. For individuals with primary or platinum-sensitive recurrence, a HIPEC regimen based on cisplatin was used. For those with platinum-resistant recurrence, a HIPEC regimen not based on cisplatin was used.
Study protocol and circulating cytokine measurements
The patients were recruited into the study within 1 month before their scheduled surgery after undergoing a comprehensive review by the MDT. Preoperative recruitment ensured timely enrollment, and pretreatment blood samples were routinely collected either during the preoperative evaluation or at the induction of anesthesia. A second blood sample was obtained on postoperative day 7. After collection, the samples were centrifuged at 2,600 x g for 8 min at 22 °C, and then immediately frozen and stored at -80 °C until further analysis.
CA-125 was routinely determined in all patients. In addition, with informed consent, extra serum was obtained for future research purposes. Customized MILLIPLEX MAP Human Cytokines/Chemokines/Growth Factor Magnetic Bead Panels (Millipore Corp., Billerica, MA) were used to assess a panel of candidate cytokine and chemokine concentrations. Cytokines that exhibited very low or undetectable concentrations in the patients were excluded from subsequent analysis. This selection process ensured a focus on cytokines with concentrations that were relevant when analyzing their association with the studied outcomes. The change in cytokine expression (cytokine_Change) represented the difference between post-CRS/HIPEC and baseline data, calculated as the post-CRS/HIPEC value minus the baseline value.
Clinical data collection
The case manager meticulously documented comprehensive patient-related information, operative details, postoperative outcomes, and pathology, all of which were thoroughly evaluated by the MDT committee. The patient data collected included demographics, pre-existing comorbidities such as diabetes, hypertension, and hepatitis, ECOG performance status, cancer type and disease status (primary or recurrence, histological type and grade, and peritoneal carcinomatosis index (PCI) [13]), and CRS/HIPEC parameters (chemotherapy regimen, perfusate, cytoreduction time, duration, blood loss, intraoperative blood transfusion, completeness cytoreduction (CC) score [13], and perioperative temperature).
Immunohistochemical (IHC) staining and tissue microarray (TMA)
Tissue specimens were obtained from individuals enrolled under the IRB-approved protocol (202202169A3C601) and provided by the Research Specimen Processing Laboratory, Chang Gung Memorial Hospital, Chiayi. TMAs were prepared from these specimens by the hospital’s histology lab, with each TMA containing two independent 1.5 mm cores per tumor.
IHC was performed on the TMAs using the Leica Bond MAX automated immunostainer to evaluate GROα, CXCR2, MCP-1, and CCR2α expression. Sections were first treated with 3% hydrogen peroxide to block endogenous peroxidase activity. They were then incubated with the following primary antibodies: anti-GROα (Abcam ab86436, 1:200 dilution); anti-CXCR2 (Proteintech Cat no: 20634-1-AP, 1:200 dilution); anti-MCP-1 (Abcam ab9669, 1:200 dilution); and anti-CCR2α (Proteintech Cat no: 16153-1-AP, 1:400 dilution). Subsequently, sections were labeled with horseradish peroxidase-conjugated secondary antibodies. Protein visualization was achieved using 3,3′-diaminobenzidine as a chromogen.
TMA slides were scanned using the Hamamatsu NanoZoomer S360 MD Digital Slide Scanner System at 40x magnification, with a resolution of 0.23 μm per pixel. The expression levels of the studied markers were evaluated based on staining intensity (scoring with 0, 1, 2, and 3).
Statistical analysis
The patients’ characteristics were presented as mean with standard deviation (SD) and median with interquartile range (IQR) for continuous variables, and count with percentage for categorical variables. Survival curves for overall survival (OS) and PFS were estimated using the Kaplan-Meier method, and log-rank tests were used for comparisons based on disease status before CRS/HIPEC or expression levels of biomarkers.
Adjusted hazard ratios (HRs) were calculated using Cox proportional hazards regression, along with their corresponding 95% confidence intervals (CIs), to assess the association between each pre-/post-treatment or change in cytokine concentration and disease progression. Time to progression was defined as the number of days between the date of CRS/HIPEC and the date of recurrence, death, or the end of follow-up.
The regression models were adjusted for predetermined confounders, including age at CRS/HIPEC, pre-CRS/HIPEC body mass index (BMI), PCI, CC score, disease status before CRS/HIPEC (primary or recurrence), and FIGO stage at diagnosis. Associations between biomarkers and recurrence within 1 year and beyond 1 year post CRS/HIPEC were examined using interval-specific HRs (95% CIs). IHC expression was calculated by Wilcoxon Rank-Sum Test. Statistical analyses were performed using SAS 9.4 (SAS Inc., Cary, NC) and R 4.2.3 (R Foundation for Statistical Computing, Vienna, Austria) software. Given our sample size, we acknowledge potential biases that might affect our findings: selection bias due to the single-center nature of the study, and possible confounding bias despite our adjustment for known prognostic factors. These biases could impact our study conclusions by limiting the generalizability of our findings to broader patient populations. To minimize these potential biases, we utilized standardized laboratory procedures, and multivariate analyses adjusting for key clinical variables. All statistical tests were two-sided, and a P value < 0.05 was considered statistically significant.
Results
Study population and measurements of circulating cytokines
The analysis included 34 patients diagnosed with EOC who successfully completed the study. Table 1 and 2 shows their demographic characteristics, baseline clinical data, and parameters related to CRS/HIPEC. The median age of the participants upon study entry was 54.3 years (IQR: 47.5 to 63.4 years). Of the 34 patients, 17 had primary disease and 17 had recurrent disease. Among the recurrent cases, 10 were classified as platinum-resistant and 7 as platinum-sensitive prior to CRS/HIPEC. Most patients (91.2%) maintained good performance status (ECOG 0–1). Most of the cases (23/34, 67.6%) had serous carcinoma histology, with 76.5% being high-grade (G3) tumors and 73.5% FIGO stage 3. Furthermore, 38.2% of the patients had undergone more than two lines of chemotherapy before CRS/HIPEC. The median CA-125 level was 35.8 U/mL (IQR: 13.8 to 117.2 U/mL), and there was no significant difference between the primary and recurrent patients (16.7 U/mL vs. 69.5 U/mL, p = 0.06). Details regarding the CRS/HIPEC parameters are provided in Table 2. The median PCI was 12 (IQR: 5 to 20), and 25 of the 34 patients (73.5%) achieved CC0, indicating successful optimal cytoreduction despite substantial tumor burden. In addition, 3 patients (8.8%) had retroperitoneal lymph node metastasis, while 6 patients (17.6%) had liver metastasis and underwent liver tumor resection.
Table 1.
Patients’ characteristics
| Characteristics | All patients (N = 34) |
|---|---|
| Age, years | |
| Mean (SD) | 54.4 (10.6) |
| Median (IQR) | 54.3 (47.5–63.4) |
| BMI, | |
| Mean (SD) | 22.9 (3.6) |
| Median (IQR) | 22.5 (19.7–25.3) |
| ECOG, N (%) | |
| 0 | 9 (26.5%) |
| 1 | 22 (64.7%) |
| 2 | 3 (8.8%) |
| HTN, N (%) | |
| No | 30 (88.2%) |
| Yes | 4 (11.8%) |
| DM, N (%) | |
| No | 30 (88.2%) |
| Yes | 4 (11.8%) |
| FIGO, N (%) | |
| 1 | 5 (14.7%) |
| 3 | 25 (73.5%) |
| 4 | 4 (11.8%) |
| Histology, N (%) | |
| Serous | 23 (67.6%) |
| Endometrioid | 3 (8.8%) |
| Clear cell | 3 (8.8%) |
| Othera | 5 (14.7%) |
| Tumor Grade, N (%) | |
| Grade 1 | 5 (14.7%) |
| Grade 2 | 3 (8.8%) |
| Grade 3 | 26 (76.5%) |
| Disease status, N (%) | |
| Primary | 17 (50.0%) |
| Recurrent | 17 (50.0%) |
| Previous chemotherapy (lines), N (%) | |
| 1 | 21 (61.8%) |
| ≥ 2 | 13 (38.2%) |
| CA-125(U/mL) | |
| Mean (SD) | 88.9 (113.6) |
| Median (IQR) | 35.8 (13.8- 117.2) |
a. other: mucinous, undifferentiated carcinoma
SD, standard deviation; IQR, interquartile range; BMI, body mass index; ECOG, Eastern Cooperative Oncology Group performance status; HTN, hypertension; DM, diabetes mellitus; FIGO, International Federation of Gynecology and Obstetrics
Table 2.
CRS/HIPEC parameters and oncologic outcome
| Characteristics | All patients (N = 34) |
|---|---|
| HIPEC regimen- Cisplatin use, N (%) | |
| No | 10 (29.4%) |
| Yes | 24 (70.6%) |
| PCI score | |
| Mean (SD) | 12.5 (8.9) |
| Median (IQR) | 12 (5–20) |
| CC Score, N (%) | |
| 0 | 25 (73.5%) |
| 1 | 9 (26.5%) |
| Retroperitoneal LN metastasis, N (%) | |
| No | 31 (91.2%) |
| Yes | 3 (8.8%) |
| Liver metastasis, N (%) | |
| No | 28 (82.4%) |
| Yes | 6 (17.6%) |
| Recurrence, N (%) | |
| No | 11 (32.4%) |
| Yes | 23 (67.6%) |
| Recurrent site, N (%) | |
| No recurrence | 11 (32.4%) |
| Intraperitoneal | 16 (47.1%) |
| Extraperitoneal | 1 (2.9%) |
| Both | 6 (17.6%) |
HIPEC, hyperthermic intraperitoneal chemotherapy; PCI, peritoneal carcinomatosis index; CC, completeness cytoreduction; LN, lymph node
Table 3 shows the measurements of circulating cytokines before (cytokine_Baseline), after (cytokine_Post) CRS/HIPEC and cytokine_Change. Among the measured cytokines, MCP-1 showed an increase (median change: 12.1 pg/mL), IL-13 demonstrated elevation (median change: 3.5 pg/mL), GROα exhibited minimal change (median change: 1.1 pg/mL), while MDC displayed a marked decrease (median change: -105.1 pg/mL) after CRS/HIPEC. No significant differences in cytokine levels were observed between the primary and recurrent cases (Supplementary Table 1).
Table 3.
Measurement of biomarkers before and after CRS/HIPEC
| Cytokines | Mean (SD) | Median (IQR) |
|---|---|---|
| GROα | ||
| Baseline | 20.2 (28.7) | 13.7 (5.0, 18.0) |
| Post | 21.5 (37.7) | 11.2 (5.9, 18.0) |
| Change | 1.2 (18.0) | 1.1 (-3.3, 3.0) |
| IL-6 | ||
| Baseline | 6.5 (19.8) | 1.5 (0.6, 2.7) |
| Post | 7.5 (9.4) | 3.3 (1.4, 9.4) |
| Change | 1.0 (18.2) | 2.2 (0.1, 6.3) |
| IL-8 | ||
| Baseline | 2.0 (1.8) | 1.6 (0.9, 2.4) |
| Post | 2.2 (1.6) | 1.8 (1.1, 2.6) |
| Change | 0.2 (2.1) | 0.5 (-0.3, 1.0) |
| IL-12 | ||
| Baseline | 36.0 (19.6) | 33.9 (21.2, 52.9) |
| Post | 36.7 (19.7) | 32.0 (22.7, 47.6) |
| Change | 0.7 (14.9) | 0.9 (-5.4, 7.9) |
| IL-13 | ||
| Baseline | 47.7 (36.3) | 40.8 (25.5, 59.8) |
| Post | 56.8 (35.6) | 50.7 (31.7, 79.4) |
| Change | 9.1 (32.5) | 3.5 (-7.7, 14.1) |
| IL-27 | ||
| Baseline | 1426.3 (914.8) | 1194.2 (739.4, 1804.9) |
| Post | 1646.7 (806.2) | 1313.4 (1020.6, 2069.7) |
| Change | 220.4 (669.1) | 288.2 (-261.3, 681.0) |
| IP-10 | ||
| Baseline | 221.4 (169.2) | 181.0 (140.2, 265.8) |
| Post | 160.5 (72.7) | 149.3 (103.5, 220.0) |
| Change | -60.9 (169.2) | -51.4 (-99.4, 6.1) |
| MCP-1 | ||
| Baseline | 205.3 (95.2) | 185.8 (119.9, 286.1) |
| Post | 250.4 (193.4) | 223.5 (133.0, 271.3) |
| Change | 45.1 (177.6) | 12.1 (-14.1, 65.7) |
| MDC | ||
| Baseline | 311.5 (156.7) | 281.9 (206.1, 403.8) |
| Post | 190.7 (108.2) | 160.3 (123.9, 231.6) |
| Change | -120.8 (174.4) | -105.1 (-200.3, -43.0) |
SD, standard deviation; IQR, interquartile range; CRS/HIPEC, cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy
Baseline: the measurements of cytokines before CRS/HIPEC; Post: the measurements of circulating cytokines after CRS/HIPEC; Change: the change in cytokines represents the difference between post-CRS/HIPEC and baseline data, calculated as the post-CRS/HIPEC value minus the baseline value
Oncologic outcomes, and survival analysis of recurrence, death, and biomarkers
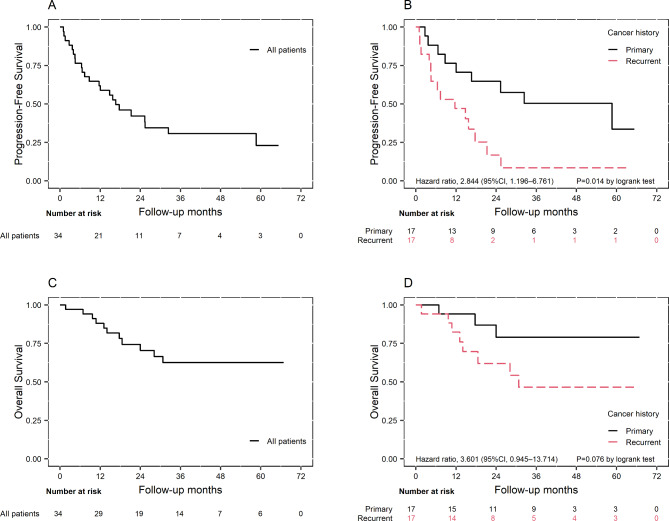
The median follow-up duration was 29.4 months (IQR: 16.0 to 44.1 months). In the entire cohort, the median PFS was 16.7 months (95% CI: 7.4–44.1 months), showing a more rapid decline in the recurrent group compared to the primary group (Fig. 1A). The median OS was not reached, indicating that more than 50% of patients were still alive at the last follow-up (Fig. 1C). The patients with primary EOC had a significantly longer median PFS compared to those with recurrent EOC (58.7 months [8.7-∞] vs. 11.7 months [95% CI: 4.0-17.7], p = 0.014), with a hazard ratio of 2.844 (95% CI: 1.196–6.761). As of October 31, 2023, 23 patients had experienced disease recurrence, of whom 11 died due to the disease. The recurrence pattern included 16 cases of intraperitoneal recurrence, 1 case of extraperitoneal recurrence, and others exhibiting both intraperitoneal and extraperitoneal recurrence (Table 2). Fourteen patients experienced recurrence within 1 year, of whom 5 had primary EOC and 9 had recurrent EOC.
Fig. 1.
Kaplan-Meier curves for progression-free survival of the entire study cohort (A) and the primary and recurrent groups (B), as well as the survival curves for overall survival of the entire study cohort (C) and the primary and recurrent groups (D)
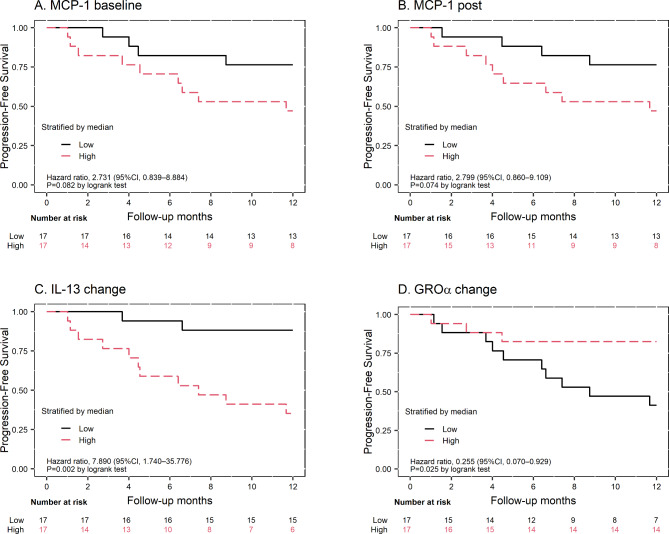
Patients were classified as having high or low cytokine levels based on whether their values were above or below the median, respectively. Higher IL-13_Change was associated with worse PFS (HR: 7.890, p = 0.002, Fig. 2C), while higher GROα_Change was associated with better PFS (HR: 0.255, p = 0.025, Fig. 2D). Additionally, patients with lower baseline and post-treatment levels of MCP-1 showed a trend towards improved PFS (HR: 2.731, p = 0.082 and HR: 2.799, p = 0.074, respectively) (Fig. 2A and B), though these differences approached but did not reach statistical significance. These findings suggested the potential prognostic value of these cytokines in predicting disease progression and outcomes in EOC patients undergoing CRS/HIPEC.
Fig. 2.
Kaplan-Meier curves for progression-free survival of the patients, stratified based on their median cytokine levels. (A) and (B) represent patients with higher and lower levels of MCP-1_Baseline and MCP-1_Post, respectively. (C) and (D) show patients with higher and lower levels of IL-13_Change and GROα_Change, respectively. The patients were stratified as high if their cytokine values were above the median, and as low if their values were below the median
We further performed subgroup analysis in supplementary. When examining platinum sensitivity, platinum-resistant patients showing higher IL-13_Post levels (65.9 vs. 37.5 pg/mL, p = 0.063) and greater IL-13 Change (21.4 vs. 2.3 pg/mL, p = 0.079) (Supplementary Table 3). In the platinum-resistant group, patients with high IL-13_Change had significantly shorter 1-year PFS compared to those with low IL-13_Change (median PFS: 4.2 vs. 11.3 months, p = 0.024) (Supplementary Fig. 1). For histological subtype analysis, non-serous carcinoma patients having high IL-13_Change demonstrate longer PFS compared to those with low levels (median PFS: 44.1 vs. 8.7 months, p = 0.031) (Supplementary Tables 4 and Supplementary Fig. 2). When stratifying by CA-125 levels (35 U/mL cutoff), patients with lower CA-125 showed significantly higher baseline IL-12 levels (45.0 vs. 27.9 pg/mL, p = 0.012), and persisted post-treatment (42.9 vs. 31.2 pg/mL, p = 0.081) (Supplementary Table 5). In patients with CA-125 ≥ 35 U/mL, high MDC_Post levels were associated with improved PFS (median PFS: 16.7 vs. 7.4 months, p = 0.042) (Supplementary Fig. 3).
Associations between 1-unit increments of cytokines and time to recurrence
We used extended Cox models with time-varying coefficients to analyze the relationship between changes in cytokine levels and the risk of recurrence. These analyses were conducted separately for two time periods: within 1 year after CRS/HIPEC and beyond 1 year. The results are presented as interval-specific HRs with corresponding 95% CIs in Table 4 and Supplementary Table 2.
Table 4.
Associations between 1-unit increment of cytokines and time to recurrence
| ≤ 1 year | > 1 year | |||
|---|---|---|---|---|
| Biomarkers (pg/mL) | aHR (95% CI) | P | aHR (95% CI) | P |
| GROα | ||||
| Baseline | 1.022 (0.987–1.058) | 0.219 | 0.997 (0.960–1.035) | 0.862 |
| Post | 1.005 (0.971–1.039) | 0.784 | 0.997 (0.970–1.026) | 0.850 |
| Change | 0.965 (0.917–1.015) | 0.165 | 0.992 (0.916–1.075) | 0.852 |
| IL-6 | ||||
| Baseline | 1.016 (0.987–1.046) | 0.283 | 1.654 (0.616–4.440) | 0.318 |
| Post | 0.982 (0.886–1.089) | 0.737 | 1.031 (0.936–1.136) | 0.531 |
| Change | 0.977 (0.945–1.010) | 0.177 | 0.884 (0.715–1.094) | 0.257 |
| IL-8 | ||||
| Baseline | 1.377 (0.948–1.999) | 0.093 | 0.781 (0.356–1.713) | 0.538 |
| Post | 1.334 (0.879–2.023) | 0.176 | 0.795 (0.406–1.560) | 0.506 |
| Change | 0.886 (0.621–1.263) | 0.503 | 0.908 (0.305–2.701) | 0.862 |
| IL-12 | ||||
| Baseline | 1.042 (0.988–1.100) | 0.132 | 0.984 (0.943–1.026) | 0.451 |
| Post | 1.058 (0.994–1.126) | 0.079 | 0.983 (0.936–1.033) | 0.503 |
| Change | 1.012 (0.955–1.072) | 0.690 | 1.013 (0.926–1.109) | 0.776 |
| IL-13 | ||||
| Baseline | 0.990 (0.950–1.031) | 0.615 | 1.029 (0.992–1.067) | 0.123 |
| Post | 1.021 (0.992–1.050) | 0.153 | 1.022 (0.989–1.056) | 0.199 |
| Change | 1.022 (0.997–1.047) | 0.090 | 0.995 (0.950–1.041) | 0.815 |
| IL-27 | ||||
| Baseline | 1.001 (1.000–1.002) | 0.201 | 1.000 (0.999–1.002) | 0.622 |
| Post | 1.001 (1.000–1.002) | 0.234 | 0.998 (0.996–1.000) | 0.137 |
| Change | 1.000 (0.998–1.001) | 0.856 | 0.999 (0.997–1.000) | 0.069 |
| IP-10 | ||||
| Baseline | 1.001 (0.998–1.005) | 0.392 | 0.993 (0.979–1.008) | 0.343 |
| Post | 0.999 (0.987–1.012) | 0.912 | 1.029 (0.991–1.068) | 0.139 |
| Change | 0.999 (0.996–1.002) | 0.414 | 1.013 (0.997–1.030) | 0.110 |
| MCP-1 | ||||
| Baseline | 1.010 (1.000–1.021) | 0.050 | 0.998 (0.984–1.013) | 0.827 |
| Post | 1.001 (0.998–1.004) | 0.534 | 1.013 (1.001–1.025) | 0.032 |
| Change | 1.000 (0.996–1.004) | 0.943 | 1.023 (1.003–1.043) | 0.025 |
| MDC | ||||
| Baseline | 1.003 (0.996–1.011) | 0.372 | 0.994 (0.987–1.001) | 0.114 |
| Post | 0.992 (0.979–1.004) | 0.188 | 0.995 (0.985–1.004) | 0.280 |
| Change | 0.978 (0.960–0.996) | 0.019 | 1.002 (0.996–1.007) | 0.576 |
CI, confidence interval;
aHR, adjusted hazard ratio. Hazard ratio was calculated using Cox’s proportional hazard model and adjusted for age, BMI, PCI score, CC score, disease status and FIGO. Bold for P value < 0.05
In the unadjusted model (Supplementary Table 2), several cytokines showed significant associations with recurrence risk within the first year post-CRS/HIPEC: each unit increase in IL-8_Post levels was associated with a 37.6% increase in recurrence risk (HR: 1.376, 95% CI: 1.007–1.881); each unit increase in IL-13_Change was associated with a 1.7% increase in recurrence risk (HR: 1.017, 95% CI: 1.002–1.033); and each unit increase in baseline MCP-1_Baseline levels was associated with a 0.6% increase in recurrence risk (HR: 1.006, 95% CI: 1.001–1.012).
After adjusting for potential confounders (age, BMI, PCI score, CC score, disease status, and FIGO stage), the multivariate model (Table 4) revealed: within the first year post-CRS/HIPEC: each unit increase in baseline MCP-1_Baseline levels was independently associated with a 1.0% increase in recurrence risk (adjusted HR: 1.010, 95% CI: 1.000-1.021); and each unit increase in MDC_Change was independently associated with a 2.2% decrease in recurrence risk (adjusted HR: 0.978, 95% CI: 0.960–0.996). Additionally, beyond the first year post-CRS/HIPEC: each unit increase in MCP-1_Post levels was independently associated with a 1.3% increase in recurrence risk (adjusted HR: 1.013, 95% CI: 1.001–1.025); and each unit increase in MCP-1_Change was independently associated with a 2.3% increase in recurrence risk (adjusted HR: 1.023, 95% CI: 1.003–1.043). Notably, the effects observed within the first year were not significant beyond 1 year, and vice versa. This time-dependent variation in prognostic value suggests different biological mechanisms may be dominant at different phases of follow-up.
Markers expressed on IHC of TMA
Among the 34 patients, 19 met the IRB criteria with informed consent and provided paraffin-fixed tissue for TMA preparation and IHC evaluation. These 19 cases comprised 10 primary ovarian cancer tumors and 9 tumors from peritoneal metastatic sites.
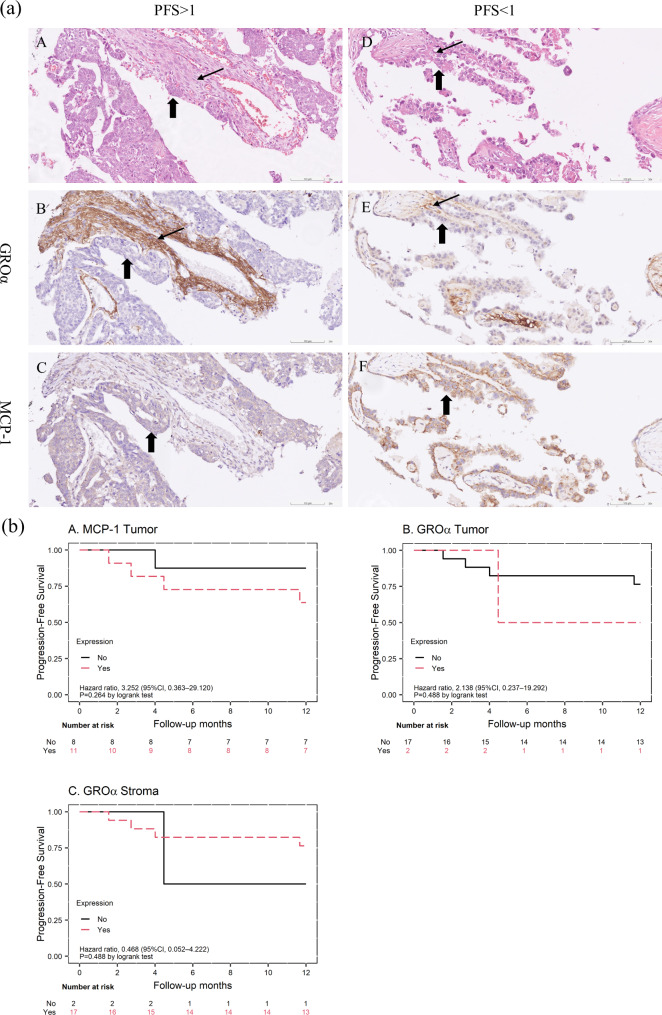
To examine the expression patterns of GROα and MCP-1, we performed IHC staining on sections from primary ovarian tumors (n = 10) and metastasized tumors (n = 9). GROα expression was significantly higher in fibroblasts surrounding the cancer cells compared to the cancer cells themselves (median [range]: 2 [0–3] vs. 0 [0–2], p < 0.001). Figure 3a illustrates two representative cases with contrasting PFS outcomes, clearly demonstrating the differential expression patterns in tumor and stromal compartments. H&E staining and IHC results for GROα and MCP-1 are shown for a patient with favorable PFS (> 3 years, high-grade serous carcinoma) and a patient with unfavorable PFS (< 1 year, clear cell carcinoma). GROα expression was predominantly observed in stromal fibroblasts (thin arrows), with minimal or absent staining in ovarian cancer cells (bold arrows) in both cases (Fig. 3a, panels B and E).
Fig. 3.
(a) Representative IHC staining for GROα and MCP-1 in ovarian cancer tissues. Panels A-C: Patient with favorable survival (high-grade serous carcinoma, PFS > 3 years). Panels D-F: Patient with unfavorable survival (clear cell carcinoma, PFS < 1 year). A and D: H&E staining. B and E: GROα staining, predominantly in stromal fibroblasts (thin arrows) with minimal expression in tumor cells (bold arrows). C and F: MCP-1 staining. (b) Kaplan-Meier curves for progression-free survival of the 19 TMA cases, stratified by IHC marker expression. (A) MCP-1 expression in tumor cells. (B) GROα expression in tumor cells. (C) GROα expression in fibroblasts
Of the 19 patients, 5 had PFS < 1 year, and 14 had PFS > 1 year. MCP-1 expression was categorized as absent (score 0) or present (score 1–3). We observed a trend towards lower PFS in the first year for patients with MCP-1 expression (HR: 3.252, p = 0.264). Interestingly, MCP-1 expression differed between the two representative cases, with no expression in the favorable PFS case (Fig. 3a, panel C) and strong expression (2 + in 80% of cancer cells) in the unfavorable PFS case (Fig. 3a, panel F). However, GROα expression in stroma or tumor did not show a significant effect on PFS in the first year (Fig. 3b).
Discussion
In this study, we observed a significant association between MCP-1 levels and an increased HR for recurrence, both within and beyond 1 year after CRS/ HIPEC. Lower baseline levels of MCP-1 showed a trend towards better PFS. In addition, the patients with lower IL-13_Change and higher GROα_Change had significantly improved PFS. These findings indicate that serum cytokines may play a role in predicting PFS following CRS/HIPEC in patients with EOC and help to discuss the postoperative maintenance treatment in selected high-risk patients.
Advances in immuno-oncology have improved our understanding of the immunological landscape of EOC. Cytokines, a diverse group of proteins including growth factors, interferons, and chemokines, are essential to various physiological processes, and they play critical roles in cancer development and the maintenance of malignant phenotypes. Serum cytokine levels may reflect the systemic immunological interactions in cancer patients [14], and previous cytokine studies in patients with EOC have aimed to identify diagnostic or prognostic markers.
In this study, we compared the levels of nine cytokines in patients with EOC associated with serum, and identified significant markers related to PFS after CRS/HIPEC. One such marker of interest was MCP-1, also known as chemokine (CC-motif) ligand 2 (CCL2), which belongs to the family of CC chemokines. Chemokines are key mediators orchestrating complex tumor-stromal interactions during metastasis through simultaneously recruiting immune cells and directly influencing tumor cell behavior [15]. MCP-1 plays a key role in inflammation as it attracts or enhances the expressions of other inflammatory factors or cells. In tumor biology, both tumor cells and stromal cells produce MCP-1, which recruits macrophages to the tumor microenvironment in various cancers, thereby contributing to invasion, migration, and metastasis [16]. Previous research has highlighted the role of MCP-1 in promoting ovarian cancer progression and metastasis by activating the mitogen-activated protein kinase (MEK)/extracellular signal-regulated kinase (ERK) pathway [17]. Initial unadjusted analyses identified associations between MCP-1, IL-8, and IL-13 with early recurrence after CRS/HIPEC, suggesting a coordinated inflammatory cascade. Through the MEK/ERK pathway, IL-8 works in concert with MCP-1 via interconnected signaling mechanisms [15], while IL-13 adds an immunomodulatory dimension to this network [18], potentially altering the post-surgical inflammatory environment. This integrated cytokine response to surgical stress and hyperthermia may identify patients at higher risk for early recurrence.
Despite its significance, clinical data regarding circulating MCP-1 levels in EOC show some contradictory results. While circulating MCP-1 has been shown to be elevated in ovarian cancer patients across all stages compared to healthy controls [19], some researchers have found no significant correlation between serum MCP-1 levels and survival outcomes. These discrepancies might be attributed to differences in study populations and treatment modalities. In our study, we found that increased MCP-1_Baseline levels correlated with a higher adjusted HR for PFS and a trend towards worse PFS with higher MCP-1_Baseline or MCP-1_Post levels, suggesting that higher MCP-1 levels may be associated with a higher risk of recurrence following CRS/HIPEC. Furthermore, few studies have reported an increase in MCP-1 levels following hyperthermia. In a series of six cases of primary ovarian cancer, elevated MCP-1 levels were detected in ascites samples after CRS/HIPEC [20]. Similarly, another study reported increased urinary MCP-1 levels after intravesical hyperthermia in patients with bladder cancer [21]. In the present study, our multivariate analysis demonstrated that MCP-1 levels at different timepoints (baseline for early recurrence, post-treatment for late recurrence) and MDC changes remained significant predictors of recurrence, even after adjusting for the common ovarian cancer survival factors (age, BMI, disease status, FIGO stage) [22, 23] and critical HIPEC prognostic indicators (CC score and PCI) [13]. These patterns suggest cytokines could serve as biomarkers for risk stratification. Further investigations into the response of MCP-1 to hyperthermia are warranted.
In addition to MCP-1, we also found that lower levels of GROα_Change were significantly associated with worse PFS. The observed changes in cytokine levels may reflect the inflammatory response elicited by CRS/HIPEC. The dissemination of peritoneal cancer cells is influenced by CXC chemokines in the progression of EOC [24]. C-X-C motif chemokine ligand 1 (CXCL1), also known as GROα, is a member of the CXC chemokine subfamily, and acts as a ligand for CXCR2. Higher serum GROα levels have been associated with resistance to carboplatin in patients with high-grade serous ovarian carcinoma [25]. However, it is important to note that the observed findings may also be related to the effects of heat. CXC chemokines are considered to belong to the family of heat shock proteins, and their expressions can be enhanced by febrile-range hyperthermia [26]. In addition, a mouse model reported a higher level of GROα after HIPEC [27]. During surgical procedures, hyperthermia and surgery can significantly affect levels of inflammatory cytokines, and these effects may persist for several days after the operation [28]. These changes may be due to physiological responses to major surgery and heat, as well as interventions such as blood transfusions. We collected baseline data and data on postoperative day 7, providing insights into the long-term dynamics of inflammatory cytokines. Our results suggest that a reduced GROα response to CRS/HIPEC may be linked to greater resistance to treatment, indicating a need for further research.
Our TMA and IHC findings provide preliminary insights into the local tumor microenvironment, complementing our serum cytokine data. The trend of lower PFS in the first year associated with MCP-1 expression in tumor tissues aligns with our serum analysis, reinforcing MCP-1’s potential prognostic value. Notably, we observed higher GROα expression in stromal fibroblasts compared to cancer cells. The distinct distribution of chemokines between stromal and tumor compartments highlights the complex interplay within the tumor microenvironment where stromal cells can serve as key sources of factors that promote cancer progression [15]. This observation is consistent with Park et al.‘s findings on the crucial role of epithelial-stromal communication via the CXCL1-CXCR2 axis in ovarian cancer progression [4]. While preliminary due to limited sample size, these tissue-based results offer promising directions for future research into cytokines’ roles in ovarian cancer progression and their potential as therapeutic targets.
Our analysis revealed that patients with recurrent EOC demonstrated significantly worse PFS compared to primary disease, aligning with previous observations of more aggressive tumor biology in recurrent cases [22]. Despite this marked difference in clinical outcomes, baseline cytokine levels showed no significant differences between primary and recurrent cases, leading us to adjust for disease status and other clinical variables in subsequent analyses. The differential expression patterns of cytokines across subgroups provided valuable prognostic insights, particularly IL-13’s association with worse outcomes in platinum-resistant disease and its varying prognostic significance across histological subtypes. The relationship between IL-12 levels and CA-125 status further suggests potential interactions between traditional tumor markers and the immune microenvironment. While these findings offer promising directions for personalized prognostication, validation in larger cohort studies is needed before clinical implementation.
This study has several strengths, including its novel focus on cytokine profiles in EOC patients undergoing CRS/HIPEC, longitudinal data collection enabling comprehensive assessment of cytokine dynamics, and integration of serum and tissue-based analyses. The meticulous collection of clinical parameters and maintenance of a prospective database likely minimized bias. However, limitations include the relatively small and heterogeneous sample size, which limits generalizability, and our single-institution design, which may not reflect practices and outcomes at other centers. The single postoperative cytokine measurement, which may not capture the full immune response dynamics. Despite efforts to control confounding factors, unmeasured variables may influence results. These findings provide valuable insights into potential prognostic biomarkers for EOC patients undergoing CRS/HIPEC, but larger, multi-center studies with more homogeneous populations and multiple postoperative measurements are needed to confirm and extend these results.
In conclusion, our findings suggest that MCP-1 may be a significant predictor of recurrence risk after CRS/HIPEC in patients with EOC. Furthermore, changes in GROα and IL-13 levels may indirectly reflect the biological behavior and PFS of ovarian cancer. Future studies could investigate cytokine profiles in ovarian cancer cell lines and explore the tumor microenvironment under heat shock conditions. Additionally, further research is needed to clarify the association between the cytokinome and responses in serum, ascites, and peritoneum to HIPEC treatment. Such investigations can improve our understanding of the underlying mechanisms and treatment strategies for patients with EOC undergoing CRS/HIPEC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the Health Information and Epidemiology Laboratory for their comments and assistance in data analysis. The authors are grateful to the members of the Peritoneal Malignancy Program of the Cancer Center Chang-Gung Memorial Hospital, Chiayi, the case manager, Tzu-Ting Liao, and the study assistant, Yen-Chun Lin.
Author contributions
Conception: Chen CY and Lung Jr. Interpretation or analysis of data: Lee CP, Liu JL and Chen CY. Preparation of the manuscript: Chen CY and Lee CP. Prepared figures: Lee CP, Liu JL and Chen CY. Revision for important intellectual content: Wang TY, Ou YC, Lee LW, Liu JL and Hung CH. Supervision: Ou YC, Lung Jr, and Hung CH. All authors reviewed the manuscript.
Funding
This research was funded by the Chang Gung Medical Foundation, grant numbers CMRPG6L0091, CMRPG6L0092, CMRPG6L0093, and CMRPG6P0091.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jrhau Lung and Chuan-Pin Lee contributed equally to this paper.
Contributor Information
Chuan-Pin Lee, Email: cblee@cgmh.org.tw.
Jrhau Lung, Email: jrhaulung@gmail.com.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Nolen BM, Marks JR, Ta’san S, Rand A, Luong TM, Wang Y, et al. Serum biomarker profiles and response to neoadjuvant chemotherapy for locally advanced breast cancer. Breast Cancer Res. 2008;10(3):R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang L, Liu W, Wang X, Wang X, Sun H. Prognostic value of serum IL-8 and IL-10 in patients with ovarian cancer undergoing chemotherapy. Oncol Lett. 2019;17(2):2365–2369. [DOI] [PMC free article] [PubMed]
- 4.Park GY, Pathak HB, Godwin AK, Kwon Y. Epithelial-stromal communication via CXCL1-CXCR2 interaction stimulates growth of ovarian cancer cells through p38 activation. Cell Oncol (Dordr). 2021;44(1):77–92. [DOI] [PubMed] [Google Scholar]
- 5.Bhatt A, de Hingh I, Van Der Speeten K, Hubner M, Deraco M, Bakrin N, et al. HIPEC Methodology and regimens: the need for an Expert Consensus. Ann Surg Oncol. 2021;28(13):9098–113. [DOI] [PubMed] [Google Scholar]
- 6.Spiliotis J, Halkia E, Lianos E, Kalantzi N, Grivas A, Efstathiou E, et al. Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol. 2015;22(5):1570–5. [DOI] [PubMed] [Google Scholar]
- 7.Filis P, Mauri D, Markozannes G, Tolia M, Filis N, Tsilidis K. Hyperthermic intraperitoneal chemotherapy (HIPEC) for the management of primary advanced and recurrent ovarian cancer: a systematic review and meta-analysis of randomized trials. ESMO open. 2022;7(5):100586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee JY, Lee YJ, Son JH, Kim S, Choi MC, Suh DH, et al. Hyperthermic intraperitoneal chemotherapy after interval cytoreductive surgery for patients with Advanced-Stage Ovarian Cancer who had received Neoadjuvant Chemotherapy. JAMA Surg. 2023;158(11):1133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayhan A, Akilli H, Abasiyanik MA, Taskiran C. Hyperthermic intraperitoneal chemotherapy in the treatment of recurrent ovarian cancer: when, and for whom? J Surg Oncol. 2023;127(3):457–64. [DOI] [PubMed] [Google Scholar]
- 10.Leijte GP, Custers H, Gerretsen J, Heijne A, Roth J, Vogl T, et al. Increased plasma levels of Danger-Associated molecular patterns are Associated with Immune suppression and postoperative infections in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Front Immunol. 2018;9:663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jolesch A, Elmer K, Bendz H, Issels RD, Noessner E. Hsp70, a messenger from hyperthermia for the immune system. Eur J Cell Biol. 2012;91(1):48–52. [DOI] [PubMed] [Google Scholar]
- 12.Wang TY, Chen CY, Lu CH, Chen MC, Lee LW, Huang TH, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for peritoneal malignancy: preliminary results of a multi-disciplinary teamwork model in Asia. Int J Hyperth. 2018;34(3):328–35. [DOI] [PubMed] [Google Scholar]
- 13.Harmon RL, Sugarbaker PH. Prognostic indicators in peritoneal carcinomatosis from gastrointestinal cancer. Int Semin Surg Oncol. 2005;2(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yabuno A, Matsushita H, Hamano T, Tan TZ, Shintani D, Fujieda N, et al. Identification of serum cytokine clusters associated with outcomes in ovarian clear cell carcinoma. Sci Rep. 2020;10(1):18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan SU, Fatima K, Malik F, Kalkavan H, Wani A. Cancer metastasis: molecular mechanisms and clinical perspectives. Pharmacol Ther. 2023;250:108522. [DOI] [PubMed] [Google Scholar]
- 16.Singh S, Anshita D, Ravichandiran V. MCP-1: function, regulation, and involvement in disease. Int Immunopharmacol. 2021;101(Pt B):107598. [DOI] [PMC free article] [PubMed]
- 17.Liu W, Wang L, Zhang J, Cheng K, Zheng W, Ma Z. CC chemokine 2 promotes ovarian Cancer Progression through the MEK/ERK/MAP3K19 Signaling Pathway. Int J Mol Sci. 2023;24(13). [DOI] [PMC free article] [PubMed]
- 18.Bernstein ZJ, Shenoy A, Chen A, Heller NM, Spangler JB. Engineering the IL-4/IL-13 axis for targeted immune modulation. Immunol Rev. 2023;320(1):29–57. [DOI] [PubMed] [Google Scholar]
- 19.Lambeck AJ, Crijns AP, Leffers N, Sluiter WJ, ten Hoor KA, Braid M, et al. Serum cytokine profiling as a diagnostic and prognostic tool in ovarian cancer: a potential role for interleukin 7. Clin Cancer Res. 2007;13(8):2385–91. [DOI] [PubMed] [Google Scholar]
- 20.Chen WC, Chang TC, Chou HH, Cheng MH, Hong JJ, Hsieh YS et al. Peritoneal Fluid Analysis of Advanced Ovarian Cancers after Hyperthermic Intraperitoneal Chemotherapy. Int J Mol Sci. 2023;24(11). [DOI] [PMC free article] [PubMed]
- 21.Arends TJ, Falke J, Lammers RJ, Somford DM, Hendriks JC, de Weijert MC, et al. Urinary cytokines in patients treated with intravesical mitomycin-C with and without hyperthermia. World J Urol. 2015;33(10):1411–7. [DOI] [PubMed] [Google Scholar]
- 22.Pignata S, Du Bois SCC, Harter A, Heitz P. Treatment of recurrent ovarian cancer. Ann Oncol. 2017;28(suppl8):viii51–6. [DOI] [PubMed] [Google Scholar]
- 23.Gaitskell K, Hermon C, Barnes I, Pirie K, Floud S, Green J, et al. Ovarian cancer survival by stage, histotype, and pre-diagnostic lifestyle factors, in the prospective UK million women study. Cancer Epidemiol. 2022;76:102074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Son DS, Parl AK, Rice VM, Khabele D. Keratinocyte chemoattractant (KC)/human growth-regulated oncogene (GRO) chemokines and pro-inflammatory chemokine networks in mouse and human ovarian epithelial cancer cells. Cancer Biol Ther. 2007;6(8):1302–12. [DOI] [PubMed] [Google Scholar]
- 25.Mlynska A, Salciuniene G, Zilionyte K, Garberyte S, Strioga M, Intaite B, et al. Chemokine profiling in serum from patients with ovarian cancer reveals candidate biomarkers for recurrence and immune infiltration. Oncol Rep. 2019;41(2):1238–52. [DOI] [PubMed] [Google Scholar]
- 26.Nagarsekar A, Hasday JD, Singh IS. CXC chemokines: a new family of heat-shock proteins? Immunol Invest. 2005;34(3):381–98. [DOI] [PubMed] [Google Scholar]
- 27.Huang WC, Wu CC, Hsu YT, Chang CL. Effect of hyperthermia on improving neutrophil restoration after intraperitoneal chemotherapy. Int J Hyperth. 2019;36(1):1255–63. [DOI] [PubMed] [Google Scholar]
- 28.Coccolini F, Corbella D, Finazzi P, Brambillasca P, Benigni A, Prussiani V, et al. Time course of cytokines, hemodynamic and metabolic parameters during hyperthermic intraperitoneal chemotherapy. Minerva Anestesiol. 2016;82(3):310–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.