Abstract
Ovarian cancer remains the most lethal gynecological malignancy. Despite the approval of promising targeted therapy such as bevacizumab and PARP inhibitors, 5-year survival has not improved significantly. Thus, there is an urgent need for new therapeutics. New advancements in therapeutic strategies target the pivotal hallmarks of cancer. This review is giving an updated overview of innovative and upcoming therapies for the treatment of ovarian cancer that focuses specific on the hallmarks of cancer. The hallmarks of cancer constitute a broad concept to reenact complexity of malignancies and furthermore identify possible targets for new treatment strategies. For this purpose, we analyzed approvals and current clinical phase III studies (registered at ClinicalTrials.gov (National Library of Medicine, National Institutes of Health; U.S. Department of Health and Human Services, 2024)) for new drugs on the basis of their mechanisms of action and identified new target approaches. A broad spectrum of new promising drugs is currently under investigation in clinical phase III studies targeting mainly the hallmarks “self-sufficiency in growth signals,” “genomic instability,” and “angiogenesis.” The benefit of immune checkpoint inhibitors in ovarian cancer has been demonstrated for the first time. Besides, targeting the tumor microenvironment is of growing interest. Replicative immortality, energy metabolism, tumor promoting inflammation, and the microbiome of ovarian cancer are still barely targeted by drugs. Nevertheless, precision medicine, which focuses on specific disease characteristics, is becoming increasingly important in cancer treatment.
Graphical Abstract
Keywords: Ovarian cancer, Therapy, Hallmarks of cancer
Background
Ovarian cancer (OC) represents the 8th most common cancer death worldwide among women. In 2022, 325,000 women were newly diagnosed, and 207,000 women died from OC. With regard to the prediction of a 40.4% increase in incidence by 2045, we are facing a global problem [1–3]. Limited treatment options and late diagnosis of OC pose the major challenges. Besides the lack of biomarkers, the relapse rate is high due to natural and acquired resistance mechanisms of the cancer cells and the tumor microenvironment (TME) [4–6]. Despite innovations in treatment such as bevacizumab, poly ADP-ribose polymerase inhibitors (PARPi), and antibody drug conjugates (ADC) such as mirvetuximab soravtansine (MIRV), further therapeutic approaches are needed to significantly improve the situation.
First-line therapy for OC includes surgery followed by a chemotherapy combining platinum- and taxane-based treatment [4]. For a long time, this scheme did not undergo major changes, until bevacizumab and PARPi were supplemented for maintenance therapy. According to the SOLO1 study, olaparib was introduced as maintenance treatment of BRCA mutated (BRCAm) OC. Based on the results of PAOLA-1 study, approval of olaparib maintenance therapy was extended to combination with bevacizumab for BRCAm and BRCA-wild type (BRCAwt) but homologous recombination deficient (HRD) women [7, 8]. Niraparib is further approved as first line maintenance therapy for BRCA-wild type (BRCAwt) and homologous recombination proficient (HRP) patients. Treatment of recurrent OC (ROC) depends on platinum-responsiveness and includes combinational and single therapies with gemcitabine, liposomal doxorubicin, or topotecan. In ROC, PARPi are approved regardless of mutational status [9, 10]. Interestingly a newly developed ADC, MIRV, is approved on folate receptor alpha (FRα) overexpressing platinum resistant OC [11].
OC shows a vast tumor heterogeneity. Based on histopathological and molecular patterns, five different types of epithelial OC can be differentiated: high-grade serous cancer (HGSC), low-grade serous cancer (LGSC), mucinous cancer, endometrioid cancer, and clear cell carcinoma. So far, these differences are concomitant with slight changes in therapy algorithm. However, in order to achieve a successful response to therapy, subgroup categorization is of crucial importance. New clinical trials are increasingly focusing on the heterogenic characteristics of the tumor and using subgroups or specific biomarkers as selection criteria for inclusion in a trial, as the patient stratification influences the response to therapy and thus the success of a trial. Currently, homologous recombination deficiency (HRD) testing and next generation sequencing (NGS) is used. Four major gene mutations have been identified that are highly correlated with OC, including TP53, BRCA1/2, KRAS, and PIK3CA, resulting in abnormal DNA repair, impaired tumor suppression, gain of oncogene function, and epigenetic changes [12]. These specific characteristics can form the basis for the development of new, targeted therapies and can be classified by the hallmarks of cancer.
The introduction of the “Hallmarks of Cancer” in 2000 by Hanahan and Weinberg provides a logical framework for a better understanding of the complexity of malignant diseases and enables more systematic cancer research. The initial six hallmarks were “sustaining proliferative signaling,” “evading growth suppressors,” “resisting cell death,” “enabling replicative immortality,” “inducing angiogenesis,” and “activating invasion and metastasis,” supported by the enabling characteristics “genome instability and mutation,” and “tumor-promoting inflammation” [13]. In 2011, the hallmarks were expanded by “reprogramming energy metabolism” and “evading immune destruction.” Besides, the crucial role of TME regarding tumorigenesis and treatment response was underlined [14]. The latest update was published in January 2022 introducing two emerging hallmarks, “unlocking phenotypic plasticity,” and “senescent cells,” as well as two further enabling characteristics “nonmutational epigenetic reprogramming” and “polymorphic microbiomes” [15]. Since the hallmarks are supposed to be essential for development of cancer, described alterations in signal pathways and protein expression represent excellent targets to impair tumor growth and cancer progression, thereby not only making cancer research more logical, but also systematic drug development. This could lead to a modular system in drug development.
While classic cytostatic drugs in general inhibit increased cell proliferation and thus cause increased side effects, targeted therapy more specifically inhibits specific altered pathways of cancer cells and the microenvironment. Drug development currently focuses primarily on single receptors, while each hallmark is regulated by semi-redundant signaling pathways that allow tumor adaptation and chemoresistance through mutation. However, challenging regarding OC is the absence of a druggable driver oncogene [16, 17]. Nevertheless, many promising molecules such as kinase inhibitors, PARPi, proteasome inhibitors, or immune checkpoint inhibitors which affect altered pathways are currently investigated for OC treatment. Several other auspicious techniques, targeting among others the highly immunosuppressive TME of OC, include adoptive cell therapy, chimeric-antigen receptor T cells, cancer vaccines, and gene therapy [18, 19]. In order to prevent tumor adaptation, therapies that broadly target the hallmarks of cancer are beneficial [20].
The hallmarks of cancer describe the complexity of tumor diseases and identify potential targets for new treatment strategies. On the occasion of the last update of the hallmarks in 2022 [15] and following the review by Petrillo et al. [20] and the book chapter by El Bairi et al. [21], we would like to provide an update on the latest developments and upcoming therapeutics for the treatment of OC according to the hallmarks. This review compiles therapeutic strategies for OC based on the hallmarks of cancer and the cellular signaling pathways involved. Furthermore, promising new drugs and mechanisms of action that are investigated in actual ongoing and recently completed phase III trials are presented.
Targeting hallmarks of cancer
Sustaining proliferative signaling
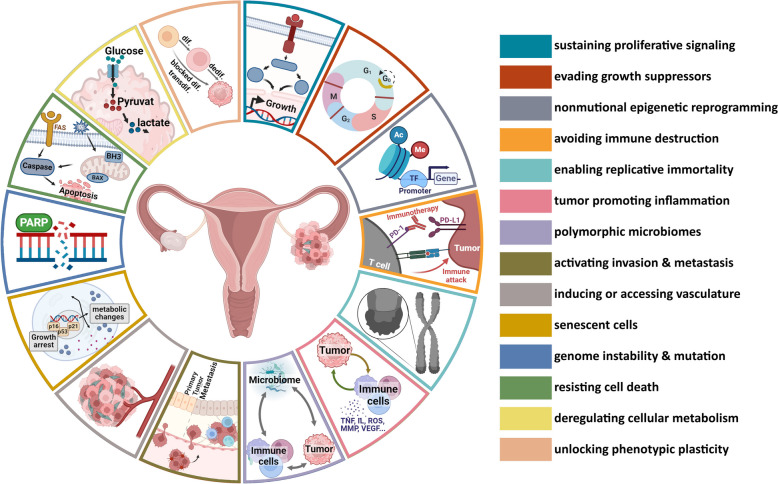
Deregulated cell proliferation plays a pivotal role in cancer development. Alterations in growth factors and their receptor expressions, intracellular signaling pathways, and disrupted negative-feedback mechanisms lead to constitutively activated cell proliferation [14]. Interestingly, it has been shown that extensive cell proliferation, reflected in a high expression of the oncoprotein RAS, can cause cell senescence [22]. This might play an important role regarding chemotherapy-resistance, promotion of tumor heterogeneity, and adaptive strategies becoming a more aggressive cancer [23]. Since many of the involved signal molecules are protein kinases, especially small-molecule kinase inhibitors are intensively investigated targeting this hallmark (Fig. 1).
Fig. 1.
Sustaining proliferative signaling. Alterations in growth factors such as EGFR, FRα, ALK, AXL, and IGFR lead to constitutive activity in downstream signaling pathways. Most frequently affected are RAS/RAF/MAPK, PI3K/AKT/mTOR, and JAK/STAT pathway. Constitutive activity leads to dysregulated cell proliferation, cell survival, and cell cycle progression. Currently ongoing and recently completed phase III trials targeting those molecules are displayed in the table. This figure was created using Biorender.com [26, 29, 30, 34, 35, 38–52]
Epidermal growth factor receptors (EGFR), such as EGFR/HER1, ErbB2/HER2, ErbB3, and ErbB4, activate multiple signaling pathways including RAS/RAF/MAPK, PI3K/AKT/mTOR, and JAK/STAT. Increased expression of EGFR was determined in 48% of OC [24, 25]. No improvement in progression-free survival (PFS) or overall survival (OS) has been demonstrated in phase III clinical trials with erlotinib (EGFR inhibitor) as maintenance therapy in OC [26]. Utility of EGFR localization and expression patterns as prognostic biomarker, determined by immunohistochemistry (IHC), has also been denied [27]. Trastuzumab and pertuzumab, monoclonal antibodies directed against HER2, are already successfully approved for breast cancer. HER2 expression is increased among 40% of OC [28]. Unfortunately, combination of pertuzumab and chemotherapy for OC in a phase III trial did not show benefits in PFS or OS [29]. Nevertheless, the ongoing trial DETERMINE examines the combinational treatment with trastuzumab and pertuzumab in patients with HER2 amplification and rare cancer subtype [30]. ErbB3, initially overexpressed in 41.3–67.5% of OC, concomitant with inferior prognosis and increased expression in case of recurrence, is not targeted in advanced clinical trials so far [31]. The same does apply for ErbB4, which expression is suspected to be negatively correlated with OS [32].
The RAS/RAF/MAPK pathway regulates the expression of the transcription factors, crucial for cell proliferation, survival, and cell cycle progression. Mutation of either K-RAS or BRAF is frequent in LGSC [28, 33]. Thus, two phase III trials, MILO and LOGS, have proven the great benefit of the MEK inhibitors binimetinib and trametinib as treatment for recurrent LGSC [34, 35]. Upcoming biomarker analyses should identify a subgroup of patients who selectively benefit from binimetinib [34]. To overcome resistance, treatments with either intra-pathway or inter-pathway combination are used [36]. Recently, the FDA has approved combinational treatment of dabrafenib (RAF inhibitor) and trametinib (MEK inhibitor) for all unresectable metastatic solid tumors with BRAF V600E mutation [37]. Based on this, combination of vemurafenib (BRAF inhibitor) and cobimetinib (MEK inhibitor) is currently investigated in the DETERMINE study for OC in case of BRAF V600 mutation [30].
Seventy percent of OC present mutations in PI3K/AKT/mTOR pathway. Hyperactive signaling either due to activating mutations in PI3K, AKT, and mTOR itself or due to loss of negative regulators as PTEN leads to constitutive activity of cell proliferation, motility, and survival [14, 28, 53]. High copy number variations of PIK3CA in 40% and mutation in 12% of OC, and encouraging results of a phase Ib trial, initiated the currently ongoing EPIK-O phase III study investigating alpelisib (PI3K inhibitor) and olaparib combination in platinum-resistant or -refractory recurrent OC (PRROC) without germline BRCAm (gBRCAm) [42]. Biomarker analyses will investigate the PI3K pathway, HRR status, and DNA damage/repair pathways to identify favored subgroups [42]. Besides, focal adhesion kinase (FAK) and its receptor anaplastic lymphoma kinase (ALK) are part of oncogenic signaling in OC. Whereas monotherapy with FAK inhibitor defactinib has not been successful, it is currently investigated in recurrent LGSC in combination with multikinase inhibitor avutometinib (RAMP301 trial) [17, 54]. It is designed as confirmatory trial aiming full approval by FDA. PROTAC (FAK proteolysis targeting chimeric molecule) degraders are promising in preclinical research for OC treatment [55]. Inhibition of Src, being part of the FAK signaling pathway, with saracatinib in a clinical study was not efficient [44].
Further interesting targets regarding cell proliferation have been the FRα and the insulin-like growth factor receptor (IGFR). Although IGF1R signaling is often dysregulated in OC, inhibition with monoclonal antibodies as ganitumab and kinase inhibitors as linsitinib have not been successful in clinical trials [56, 57]. This might be due to difficulties in maintaining insulin receptor signaling [17]. More than 90% of OC overexpress FRα, concomitant with especially increased JAK-STAT downstream signaling [58, 59]. Unfortunately, monoclonal antibodies as farletuzumab (MORAb-300) failed to show benefits in a phase III study in platinum-sensitive recurrent OC (PSROC) [39]. Nevertheless, FRα remains an interesting target for targeted transport systems. Disruption of AXL-axis in platinum-refractory women by batiraxcept also did not improve PFS [40].
Growing importance of biomarker-guided patient stratification is reflected in clinical trials as STAPOVER. Regardless of histological subtype and based on a signal transduction pathway assay, women with either estrogen receptor (ER), androgen receptor (AR), PI3K, or Hedgehog signaling pathway (HH) alterations are either treated with letrozole (aromatase inhibitor), bicalutamide (antiandrogen), or itraconazole (mTOR inhibitor) [43]. The expression of ERα is increased in ~ 80% of OC [60]. Clinical studies have proven that predominantly LGSC and endometrioid cancer show good responses to endocrine therapy [6, 61]. Ongoing MATAO study analyzes maintenance therapy with letrozole in OC [48]. Further studies include NRG-GY019 comparing letrozole monotherapy with carboplatin-paclitaxel followed by letrozole as maintenance therapy for LGSC [47].
Inducing or accessing vasculature
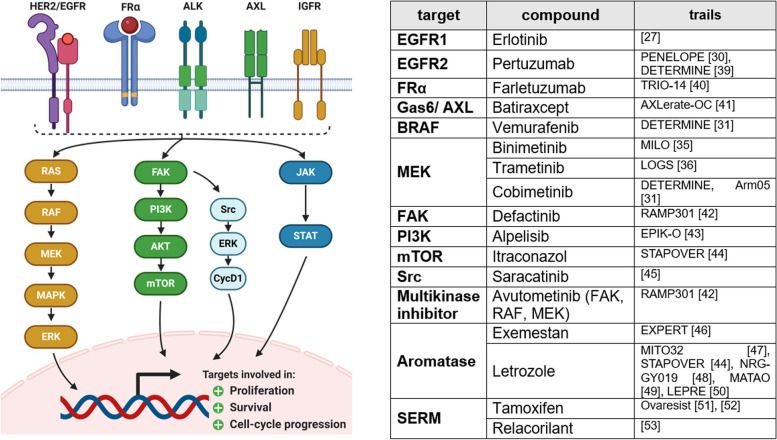
To ensure sufficient nutrients, oxygen, and evacuation of waste or metabolites, endothelial cells are reactivated in cancer (Fig. 2). Angiogenesis stimulatory molecules, such as vascular endothelial growth factor A (VEGFA), tumor growth factor β (TGF β), and fibroblast growth factor (FGF), interact with its receptors VEGFR, TGFR, and FGFR [14, 62]. In contrast, by binding to the Tie receptor, angiopoietin (Ang) inhibits vasculature maturation [58]. Furthermore, platelet-derived growth factor β (PDGFβ) is involved in proangiogenic signaling, promoting proliferation of pericytes. Tumor neovasculature is characterized by leakiness, excessive vessel branching, and increased levels of apoptosis [14].
Fig. 2.
Inducing or accessing vasculature. In cancer, angiogenesis is stimulated by proangiogenic factors such as vascular endothelial growth factor (VEGF), tumor growth factor β (TGF β), and fibroblast growth factor (FGF), interacting with their receptors VEGFR, TGFR, and FGFR. Binding of angiopoietin to Tie2 receptor inhibits vascular maturation and platelet-derived growth factor (PDGF) promotes proliferation of pericytes. Increased activity of depictured signaling pathways leads to leaky neovasculature with high levels of apoptosis and excessive vessel branching. Currently ongoing and recently completed phase III trials targeting those pathways are listed in the table. This figure was created using Biorender.com [63–85]
Bevacizumab, inhibitor of VEGF, was the first targeted and antiangiogenic therapy approved in front-line treatment and treatment of relapsed OC. Bevacizumab shows improvement in PFS but does not affect the OS rate. Clinically, it is of great value for patients with extensive ascites [63, 64]. Analyses based on the World Pharmacovigilance Database (FDA) and randomized controlled trials assess the long-term safety profile of bevacizumab as relatively positive [86]. Next to bevacizumab, several other VEGF-inhibitors have been investigated clinically. Aflibercept, a recombinant fusion protein trapping VEGF, was investigated in advanced chemoresistant OC with recurrent malignant ascites and led to less rapid ascites formation. Due to increased risk of fatal bowel perforation, aflibercept was not approved [68]. Navicixizumab is a fist-in-class bispecific antibody targeting delta-like ligand 4 (DLL4) and VEGF. Tumors responding to anti-VEGF therapy present low levels of DLL4, but unfortunately DLL4 is overexpressed in 72% of OC [87]. The high overall response rate (ORR) of 43.2% to navicixizumab in a phase Ib study in PRROC led to the initiation of a phase III trial with an estimated primary completion date in November 2023, which includes a further 12 months survival follow-up [88, 70].
BD0801 is a monoclonal antibody blocking VEGF/VEGFR interaction, which is investigated in phase III trial with supposed primary completion in December 2023. Results are still pending [69]. Following the promising AEROC study, which investigated the VEGFR2 inhibitor apatinib, apatinib is further investigated in AMELIE trial for OC therapy and as maintenance therapy in combination with unapproved PARPi fluzoparib after first-line treatment [71, 72, 89]. Again, no results are available so far. Trebananib is a peptibody trapping Ang1/2. TRINOVA1-3 trials have investigated trebananib in combination with paclitaxel in ROC proving significant prolonging of PFS compared to placebo, in combination with pegylated liposomal doxorubicin (PLD) showing improved ORR but without PFS benefit and as first-line treatment in combination with carboplatin and paclitaxel unfortunately demonstrating only minimal benefits for patients [77–79]. With the shift in patient stratification from recurrent epithelial OC (TRINOVA1-2) to epithelial OC, primary peritoneal or fallopian tube carcinoma (TRINOVA 3), the benefit has decreased.
Several multikinase inhibitors are part of treatment strategy investigations for OC. Cediranib is a multikinase inhibitor targeting VEGFR1-3. ICON6 and NRG-GY004 study failed to show significant OS benefits of cediranib given concurrently to standard of care therapy, given as maintenance therapy and given in combination with olaparib [73, 74]. However, worthwhile activity was suspected, featuring ICON9 study, which investigates maintenance therapy of olaparib with cediranib or placebo. Results are expected for 2025 [75]. Another study (phase II/III) evaluating cediranib and olaparib combination for recurrent or metastatic OC, is ongoing [76]. Likewise, no results of masitinib, a multikinase inhibitor recently approved for amyotrophic lateral sclerosis, in combination with gemcitabine in PSROC have been published so far [80]. Multikinase inhibitor nintedanib, investigated in 2009 in combination with carboplatin and paclitaxel, has not been approved due to absence of OS benefits although realizing PFS benefits [81]. Sadly, AGO-OVAR16 study, which investigated the multikinase inhibitor pazopanib (Votrient), did not confirm suspected OS benefit of MITO11 study [82, 90]. Furthermore, the combination of TQB2450, a programmed death-ligand inhibitor, and anlotinib is currently investigated [83, 91]. Anlotinib is further investigated in a phase I/IIa/III study in ROC concurrently to standard of care and as maintenance therapy [84]. CHIPRO is an ongoing phase III trial investigating the multikinase inhibitor chiauranib targeting VEGFR1-3, PDGFRα, cKIT, Aurora B, and CSF-1R, in combination with weekly paclitaxel in patient with PRROC [85].
Evading growth suppressors
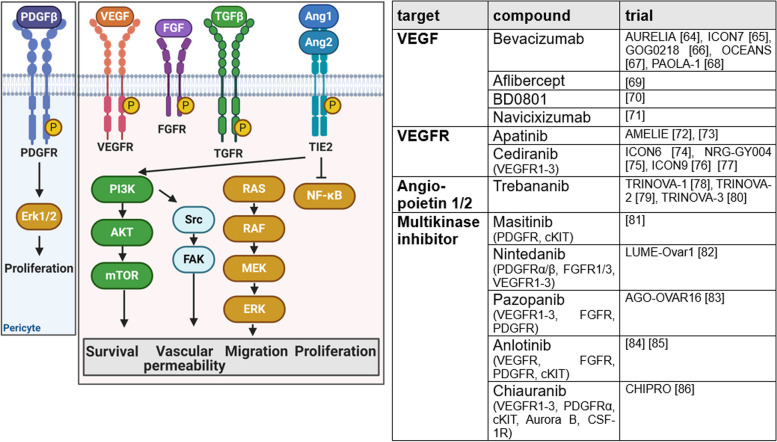
Processes that circumvent growth-inhibiting signals are a main characteristic of cancer cells. Absence of critical gatekeeper of cell cycle proliferation, in particular p53 (tumor protein p53) and RB (retinoblastoma-associated), leads to uncontrolled cell growth. RB mainly regulates extracellular signals; TP53 mostly processes intracellular signals and can induce cell cycle arrest to repair DNA damage or start apoptosis [14]. Both tumor suppressors are commonly altered in OC (e.g., TP53 in over 90%) [92]. Cell cycle progression is tightly regulated by cyclin-dependent kinases (CDKs) interacting with cyclins (Fig. 3). The frequent dysregulation in cancer makes them promising targets for therapy.
Fig. 3.
Evading growth suppressors. Cell cycle progression is strictly regulated by checkpoints controlled by cyclin-dependent kinases (CDKs)/cyclin-complexes. Due to frequent aberrations in checkpoint control, as well as alterations in critical gatekeepers, uncontrolled cell cycle progression is a common feature in OC. Current strategies in phase III studies targeting evasion of growth suppressors are listed in the table. This figure was created using Biorender.com [85, 98]
G1-S-phase transition is controlled by cyclin D/CDK4/6 and cyclin E/CDK2 complexes. A positive feedback loop, wherein mitogenic stimuli such as c-myc increase cyclin D expression which inactivates together with CDK4/6 RB and results in release of transcription factor E2F, leads to G1/S-transition and cyclin E expression, which can further promote its own expression independent of other stimuli [93]. G2/M transition is regulated by CDK1/2 building a complex with cyclin A/B, which formation is under control of DNA damage response ATM-CHK2 and ATR-CHK1 axis. Activated ATR causes phosphorylation of CHK1, which leads to reduced CDK activity through proteasomal degradation of CDC25 and causes a delay in the cell cycle [94, 95]. Further, Wee1 kinase is activated by CHK1, subsequently inhibiting CDK1/2. Activated ATM/ATR further activates p53-signaling, among others leading to upregulation of p21, and thereby stabilizes the RB-E2F complexes and prevents apoptosis [96]. To support cell cycle progression after DNA repair, Aurora kinase A activates polo-like kinase 1 (PLK1), which in turn inhibits Wee1 activity, whereas Aurora kinase B accelerates degradation of p53. Defects in G1/2 transition are common in cancer cells, making them more reliant on intra S and G2/M checkpoints for survival [95, 97].
CCNE1 (cyclin E1) amplification is a common copy number variation (> 20%) in HGSC. CCNE1 amplification leads to facilitated cell cycle progression and replicational stress accompanied by genomic instability [6]. Thus, treatment strategies focus on CDK inhibitors, inhibition of ATR/CHK1/WEE1 axis and restoration of p53.
Preclinical studies have demonstrated a benefit of CDK4/6 inhibitors, such as palbociclib and ribociclib, already approved for breast cancer, in estrogen receptor-positive cancer. A clinical phase II study investigating ribociclib in combination with letrozole in ROC has proven high response rates in LGSC [99]. Recently, the ALEPRO study started investigating another CDK4/6i, abemaciclib, together with letrozole in patients with estrogen receptor-positive rare OCs as an international, multicentre, open-label, single-arm phase II study [100]. Patient-derived organoids (PDOs) have responded well to flavopiridol, a multiple CDKi, which was clinically confirmed (phase II) in cisplatin-resistant recurrent OC [17, 101, 102].
Inhibition of ATR/CHK1/WEE1 axis enhances sensitivity of cancer cells to treatment due to uncontrolled cell cycle progression and high replicational stress, therefore being interesting for combinational treatments [6, 95, 103, 104]. Ceralasertib, an ATR inhibitor, combined with PARPi has proven a clinical benefit rate of 62.5% in HRD and/or BRCAm PARPi-resistant ROC [105]. ATR inhibitor prexasertib was granted FDA Fast Track designation due to promising interim phase II study results [106, 107]. Unfortunately, other CHK inhibitors caused severe side effects [17]. Wee1, upregulated in OC, can be inhibited by adavosertib, thereby increasing sensitivity towards chemotherapy in TP53 mutant HGSC [108]. Inhibition of cell cycle progression by Aurora kinase A inhibitor, alisertib, and the PLK1 inhibitor, volasertib, has shown in phase II studies to be beneficial and merit further investigation [109, 110].
Apart from this, restoration of p53 is a promising anticancer approach. Gene therapy with recombinant adenovirus p53 (SCH-58500) had been shown to be safe and favorable in phase I/II trials and progressed to a phase II/III trial in 1999. However, results have not been published. Further efforts aim to re-engineer p53, with adenoviruses or nanoparticles as carrier systems (e.g., Au-C225). Peptide-based p53 therapy, such as the p53-SLP vaccine, failed to show benefits in phase II trial. Promising small molecules reactivating mutant p53 are APR-246 and zinc metallochaperones [111]. HSP90i, ganetespib, which promotes degradation of mutant p53 by MDM2 machinery, represents another approach [20]. In addition, the degradation of p53 can be influenced by the multikinase inhibitor chiauranib, which inhibits AURORA kinase B and is being investigated in the ongoing CHIPRO study together with paclitaxel [85].
Resisting cell death
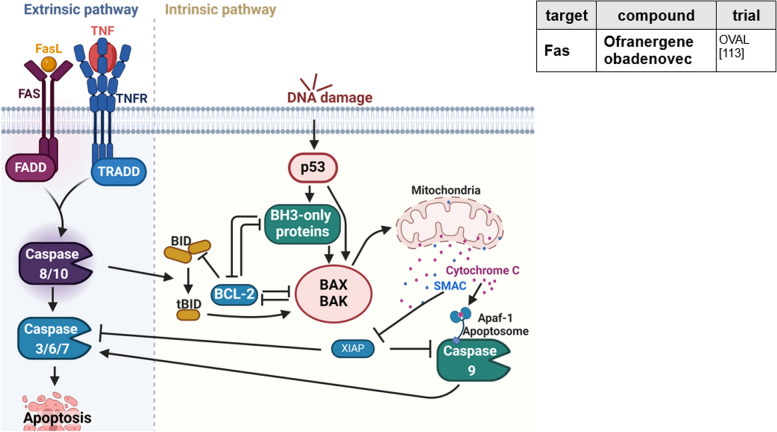
Apoptosis is a pathway to eliminate cells harboring mutations (Fig. 4) [14]. In the extrinsic pathway, Fas-ligand-receptor interaction activates caspase 8 and, in the following, stimulates effector caspases triggering apoptosis. The intrinsic pathway is activated consequently to DNA damage and excessive oncogenic signaling. While regulators such as BCL-2 inhibit proapoptotic proteins as BAX and BAK, p53 promotes apoptosis by upregulation of BH-3-only proteins Noxa and Puma, which in contrast activate BAX and BAK. Subsequently, release of cytochrome c out of the outer mitochondrial membrane is promoted, featuring activation of caspase cascades. Cleavage of Bid to tBid displays cross activation of intrinsic pathway in case of extrinsic induced apoptosis. Inhibitors of apoptosis (IAP), e.g., XIAP and survivin, are important regulators of apoptosis, which suppress activity of intrinsic and extrinsic pathway by caspase inhibition [113].
Fig. 4.
Resisting cell death. With regard to apoptosis induction, extrinsic and intrinsic pathways are differentiated. Several mechanisms of cancer cells are known to circumvent apoptosis. Treatment strategies to inhibit resistance to cell death are listed in the table. TNF, tumor necrosis factor; TRADD, TNFR1-associated death domain protein; FADD, Fas-associating protein with death domain. This figure was created using Biorender.com [112]
OC is known for increased expression of antiapoptotic signals and survival signals, as well as decreased expression of proapoptotic signals [4, 14, 21]. Thus, these represent valuable targets for therapy. BH3 mimetics, such as ABT-737 and WEHI-539, which antagonize BCL-XL, have shown to synergize with carboplatin in cell growth assays, as does ABT-263 (navitoclax) with PARPi in vitro [114, 115]. Clinically, monotherapy of navitoclax, investigated in a phase II trial, was only marginally effective [116]. Another approach seems to be upregulation of BH3-only proteins with naftopidil [21].
Ofranergene obadenovec (VB-111) is a viral-based therapy, delivering a Fas-TNFR1 chimeric pro-apoptotic protein. It is supposed to drive endothelium specific expression and induction of apoptosis, leading to vascular disruption and activation of immune system. A phase III trial completed in July 2022 investigated VB-111 in combination with paclitaxel. Further analyses included subgroup analyses, quality of life, histopathology, and biomarkers. No improvement in PFS or OS was observed for PRROC [112, 117].
Avoiding immune destruction
Since the approval of ipilimumab in 2011, immune checkpoint inhibitors (ICI) have revolutionized the treatment of many solid cancer types, except for OC [118]. Long-term follow-ups (≥ 3 years) of patients treated with ipilimumab indicate a consistent quality of life and an improvement in OS [119]. ICI prevent interaction of receptor and corresponding ligands such as CTLA-4/CD80/86, PD-1/PD-L1/2, PD-L1/2/CD80, and LAG-3/MHC-II, and thus override tumor’s survival mechanisms, especially the inhibition of T cell activity (Fig. 5) [18, 120].
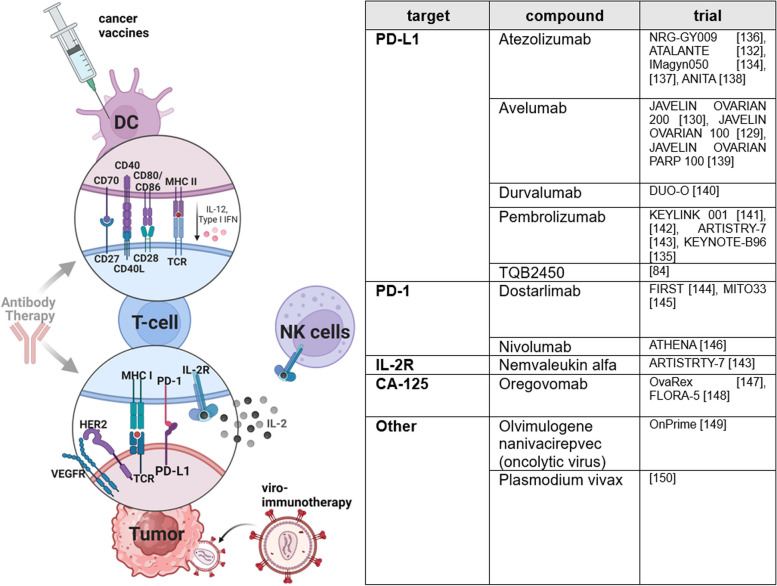
Fig. 5.
Avoiding immune destruction. Expression of immune checkpoint molecules and programmed cell death ligands compromise immunostimulatory interaction of tumor, T, and dendritic cells. In addition, OC is characterized by highly immunosuppressive tumor microenvironment, which further attenuates antitumoral immune response by secretion of cyto- and chemokines. Current endeavors fighting immune evasion encompass immune checkpoint inhibitors, cancer vaccines, viro-immunotherapy, and interleukin application. Recently completed and currently ongoing phase III trials targeting immune evasion of OC are listed in the table. This figure was created using Biorender.com [83, 128, 129, 131, 133–149]
For single-agent therapy with ICI, such as anti-PD-L1 pembrolizumab and anti-PD-1 nivolumab, accordingly to studies as KEYNOTE-100 and NINJA, limited efficacy had been announced and no biomarkers have been identified [121, 122]. Only for clear cell carcinoma partial responses were detected. Causes of failure to improve patients’ outcome include the comparatively poorer ability of the immune system to show antitumoral response. Efficacy of ICI depends on high PD-L1 expression, low prevalence of somatic copy number variations, and the immunosuppressive properties of the TME [6, 17]. Although BRCA-1/2 deficient cells show the highest PD-L1 expressions and immunogenicity due to mutational burden, therapy response to ICI was not major in BRCA-1/2-deficient cells compared to BRCA-1/2-proficient cells [17, 122, 123]. TME of OC is highly immunosuppressive and contains various types of immune cells. High amount of tumor-infiltrating lymphocytes (TIL) as well as high ratio of CD8 + TILs/CD4 + regulatory T cells (Treg) have established positive prognostic properties in OC [124–126]. Due to changed expression of surface molecules and secretion of immunosuppressive chemokines and cytokines by tumor cells and innate immune cells, as myeloid-derived suppressor cells, immature dendritic cells, and tumor-associated macrophages of M2 phenotype, immunosuppressive Tregs get activated and effector T cells and natural killer (NK) cells get inhibited [6, 124]. Non-immune cell TME is mainly built by cancer-associated fibroblasts, cancer-associated adipocytes, endothelial cells, and pericytes and supports immunosuppression by growth factors and cytokine production [18, 124, 127]. Increasing knowledge about functions of TME exhibits further strategies to improve treatment. With identification of predictive biomarkers, immunotherapy can either target inhibition of immunosuppression or support stimulation of the immune system.
In view of the complexity of the mechanisms, it seems sensible to address different signaling pathways by combination treatment. Since combination of ICI avelumab and standard of care treatment (carboplatin-paclitaxel) likewise did not prove benefits, nor in first-line, nor in second-line (JAVELIN 100/200), and also the combination of PLD with ICI pembrolizumab or durvalumab did not improve efficacy significantly despite a pro-antigen-presenting effect, current research focuses on combination with other agents as PARPi and bevacizumab [128–130]. In the ATALANTE-trial, which investigated atezolizumab in combination with bevacizumab and chemotherapy in ROC, the coprimary PFS in intention to treat population and PD-L1 positive populations was not reached [131]. AGO-OVAR 2.29/ENGOT-ov34 study results, recently presented at the ASCO Annual Meeting 2024, similarly did not show OS or PFS benefits for atezolizumab combined with bevacizumab in non-platinum-based chemotherapy in ROC compared to placebo [132]. Likewise, IMagyn050 study did not verify a PFS benefit in newly diagnosed OC [133]. Results of NRG-GY009 study, investigating atezolizumab-PLD-bevacizumab combinations in ROC, are still pending, and KEYNOTE-B96, investigating pembrolizumab in addition to weekly paclitaxel with or without bevacizumab in PRROC, is ongoing [134, 135].
Preclinically determined immunomodulatory properties of PARPi, such as increased neoantigen formation, increased PD-L1 expression and increased immune cell infiltration, built rationale to combine them with ICI [150]. Following phase I/II studies proving benefit of ICI-PARPi combinations regardless of BRCA-/HRD/PD-L1 status and providing proof of advanced OS rates by therapy triplet including bevacizumab, several phase III studies are currently ongoing [151, 152]. JAVELIN Ovarian PARP 100 trial evaluated treatment efficacy of avelumab in combination with chemotherapy followed by maintenance therapy with avelumab and talazoparib in advanced OC but was stopped in 2019 due to missing benefits of avelumab for unselected patients in front-line setting observed in interim-analysis of JAVELIN Ovarian 100 trial [153]. The results of the ANITA study, which investigated application of chemotherapy in combination with atezolizumab and niraparib in patients with ROC, have been recently presented at the ESMO Annual Congress 2023. They indicate a PFS benefit for only non-BRCAm OC [137]. Large ongoing studies further investigating PARPi-ICI combination in first-line and recurrent situations include KEYLINK-001, FIRST, MITO-33, and the COMBO arm within ATHENA-trial [140, 143–145].
The DUO-O study investigated the benefits of durvalumab therapy in combination with chemotherapy and bevacizumab, followed by maintenance therapy with durvalumab (anti-PD-L1), bevacizumab, and olaparib, in newly diagnosed advanced OC without BRCA mutation. For the first time, benefits of PARPi and ICI were seen. Durvalumab and olaparib combination led to a significant improvement in PFS, from 19.3 months to 24.2 months (HR 0.63). Considering only HRD-positive patients, a median PFS of 37.3 months (vs. 23 months) was reached (HR 0.49) [139]. This gives rise to hope for further advances in ICI-PARPi-bevacizumab combination. Nevertheless, critics fault the lack of an olaparib maintenance control arm in DUO-O study. Follow-up studies will provide further insights into the long-term benefits. So far, durvalumab is not approved in OC.
Other combinational strategies include testing TQB2450 (PD-L1 inhibitor) in combination with anlotinib [83]. Backed on preclinical and phase Ib data for PRROC showing ORR of 47.1%, a phase III trial is currently conducted in China [91]. Nemvaleukin alfa, a novel engineered IL-2 cytokine fusion protein, is presently investigated in ARTISTRY-7 trial in combination with ICI pembrolizumab in PRROC and has already gained fast track designation by FDA [142]. Due to sterical occlusion, it only stimulates IL-2 receptors (IL2-R) and activity of TEff and NK-cells and not those of Tregs, thereby preventing capillary leak syndromes as often noticed in case of simple IL-2 administration [142, 154]. ARTISTRY-1 trial provided evidence for the activity and safety of nemvaleukin alfa in PRROC [155].
Cancer vaccines are a growing field of research in OC. Peptide-based vaccines consist of known or predicted tumor-associated antigens (TAAs) administered with adjuvants to enhance immunogenicity. Presentation of processed antigens by antigen presenting cells and dendritic cells (DC) leads to activation of TEff cells and cytotoxicity by B cells [156]. Common TAAs to target in OC are FRα, HER2, CA125 (MUC16), MAGE-A4, NY-ESO 1, and mesothelin [4, 6, 18]. Oregovomab, a CA-125-specific murine monoclonal antibody, is already investigated as cancer vaccine in phase III trials since 2002. Despite missing improvement of clinical outcome using oregovomab as maintenance therapy in advanced OC, it is currently investigated as front-line therapy in newly diagnosed advanced epithelial OC in combination with paclitaxel and carboplatin in FLORA-5 study [146, 147].
Oncolytic viruses (OV) act by direct oncolysis of infected cells and contribute to indirect activation of the host immune system due to release of danger associated molecular patterns, viral antigens, and TAAs [18, 157]. Genetic engineering enables expression of transgenes, increases tumor specificity, and grows oncolytic potency [156]. OV therapy also affects the TME [18]. Following the success in phase II trial, GL-ONC1 (olvimulogenic nanivacirepvec) is currently investigated in the phase III OnPrime trial as front-line treatment in combination with platinum-based chemotherapy with or without bevacizumab [148, 158]. Further approaches but still in preclinical research include infected cell vaccines (ICVs), considering autologous tumor cells as vehicles to tumor niche, thereby turning immunologically “cold” tumors into “hot” tumors [4, 18].
Another line of attack is adoptive cell therapy (ACT), a transfer of autologous or allogeneic immune cells. Besides successful use of TIL for ACT after platinum-based chemotherapy in 1995, the utilization of dendritic cell vaccines (DCV) pulsed with TAAs is likewise interesting [159, 160]. Also, chimeric antigen receptor T cells (CAR-T) are actively investigated in OC, allowing an antigen-specific recognition of cancer cells and major histocompatibility complex (MHC)-independent activation of T cells [161]. However, CAR-T cell therapy still faces many issues, as off-target effects and tumor heterogeneity [162]. Bispecific antibodies (e.g., ubamatamab and REGN5668), which activate the T cell response by simultaneous binding to tumor and T cells, are currently in phase I/II trials [163–165]. In addition, in 2024, an unconventional phase II/III study is expected to test the effect of Plasmodium vivax on OC [149].
Genome instability and mutation
Germline, somatic, and epigenetic mutations compromising DNA damage-detection and -repair lead to genome instability, a fundamental feature of cancer [14]. Genome instability is associated with deficiency in homologous recombination (HR), which is present in 41–50% of OC and is utilized by therapies targeting DNA repair [181]. In addition to BRCA1 and BRCA2 mutations, various other genetic mutations and amplifications, e.g., in RAD51C, ATM/ATR, PTEN and CHEK2, have an impact on HRD in OC [92]. Since recent clinical research has proven predictive potential of HRD regarding response to platinum-based and PARPi therapy, HRD tests were introduced to diagnostic algorithm of OC [182, 183]. Germline and somatic mutations are screened by next generation sequencing [181]. Further HRD tests focus on identification of loss of heterozygosity, telomeric allelic imbalances, and large-scale transitions, the “scars” of genomic instability [184]. So far, two commercially FDA approved tests are available: FoundationOne by Foundation Medicine and myChoice HRD test by Myriad Genetics. Since mutagenesis during tumor evolution can compromise accuracy of HRD tests, much effort is put into development of functional HRD assays, as quantification of nuclear RAD51, to display current HRD status [184].
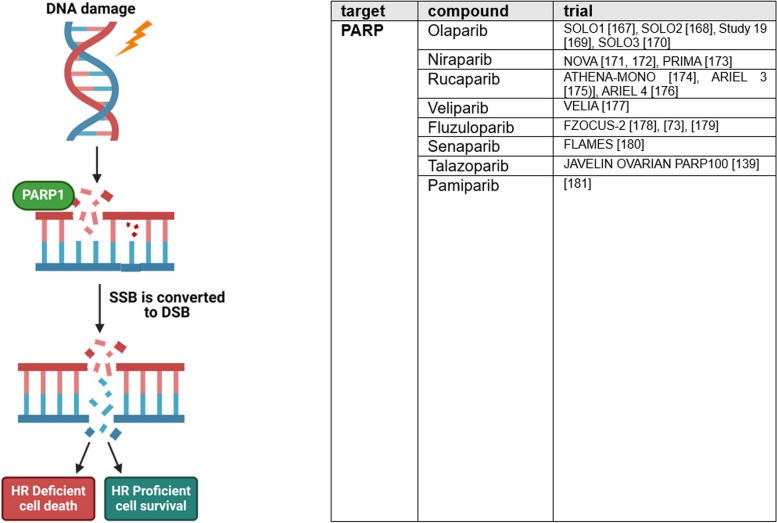
Based on synthetic lethality, PARPi are highly efficient in OC (Fig. 6). Among others, PARPi inhibit repair of DNA single-strand breaks and thereby cause accumulation of DNA double-strand breaks. Deficiency of high-quality HR and concurrent inhibition of alternative end joining (alt-EJ) by PARPi, as well as dependency on more error-prone non-homologous end joining (NHEJ) to repair DSBs, leads to accumulation of mutations, unregulated cell division, and apoptosis [95, 181, 184, 185].
Fig. 6.
Genome instability and mutation. High quality repair of DNA damage is crucial to maintain genomic stability. In case of DNA damage repair defects, as homologous repair deficiency or artificially induced defects by PARP inhibition, DNA damage leads to genomic instability or cell death due to synthetic lethality. Recently completed and currently ongoing phase III trials targeting genome instability of OC are listed in the table. SSB, single-strand breaks; DSB, double-strand breaks. This figure was created using Biorender.com [72, 138, 166–180]
Thus, today PARPi are considered as first-line standard of care maintenance therapy of OC after response to platinum-based therapy. Based on several phase III studies, investigating olaparib, niraparib, and rucaparib in first-line setting, including SOLO-1, PRIMA, and ATHENA trial which have been reviewed in detail elsewhere, olaparib is approved for first-line maintenance treatment of advanced OC with BRCAm; niraparib is approved regardless of HRD status [186, 187]. Olaparib approval was extended by its use in combination with bevacizumab as first-line maintenance therapy in HRD positive OC due to PAOLA-1 study [186].
Based on SOLO2 and study 19, olaparib is also approved as maintenance treatment for ROC [188]. Caused by new results of ARIEL-3 and NOVA study, approval of rucaparib and niraparib has recently been restricted to tumors with BRCAm in recurrent situations [10,108,171,190-195].
PARPi as monotherapy in late line treatment have been discouraging so far. In SOLO 3 study, which treated gBRCA1/2 mutated HGSC PSROC with olaparib monotherapy, no significant difference in OS and PFS2 compared to placebo group was seen [195, 196]. Likewise, third-line monotherapy with rucaparib for BRCAm OC was withdrawn in June 2022. New OS results of ARIEL 4 study, contrary to initially encouraging PFS results, favored chemotherapy over rucaparib [175, 196]. QUADRA study, a single-arm phase II study, investigated niraparib as fourth line or later treatment in HRD positive ROC. In September 2022, approval for this indication was voluntarily retrieved [197, 198]. Based on this long-term follow-up, the FDA withdrew approval for the PARP inhibitors olaparib, rucaparib, and niraparib for single-agent treatment. Long-term profile (> 2 years) proved olaparib to be safe and well tolerated [168].
Other PARPi, as for example veliparib, did not reach clinical approval despite phase III study VELIA proving a longer PFS compared to carboplatin-paclitaxel alone [176]. New PARPi as fluzuloparib and pamiparib are currently evaluated in clinical trials in China [72, 177]. In China, fluzuloparib is already approved for treatment of gBRCAm PSROC since 2020 [199]. The new PARPi senaparib also offers promising PFS benefits as maintenance therapy in first-line treatment, regardless of biomarkers [179]. However, PARPi treatment is associated with increased risk of myeloid-neoplasia, due to PARP2 inhibition [6]. Therefore, selective inhibition of PARP1 becomes a new strategy and promising agents as the selective PARP1 inhibitor AZD5305 are already under clinical evaluation [200, 201].
Since PARPi are chemosensitizing, they are popular agents for combination studies [202]. Combinational designs can be useful to overcome resistance mechanisms to PARPi and platinum-based therapy, including restoration of HR, upregulation of multidrug-resistance channels, or replication fork stabilization [4, 200, 203, 204]. Furthermore, cytotoxic effects of chemotherapeutics can be enhanced by combination with PARPi, be it through accumulation of topo I-DNA complexes or by induction of replication stress thereby sensitizing to cell cycle checkpoint inhibitors (ATRi/CHKi/WEE1i) [205].
Next to high sensitivity towards PARPi, genome instability in OC offers a broad range of targets to inhibit. Due to common deficiencies in HR, OC are more reliant on alternative repair mechanisms as NHEJ and alt-EJ [206]. Inhibition of alt-EJ regulating Pol-θ with agents as novobiocin and ART558, as well as inhibition of NHEJ regulating DNA-dependent protein-kinase catalytic subunits via peposertib, is already under early clinical investigation [6, 107, 207–209].
Other strategies targeting genome instability, include stabilization of G-quadruplex structures, for example by pidnarulex [204, 210]. Ubiquitin-specific protease 1 (USP-1) inhibitors as KSQ-4279 promote degradation of DNA repair proteins and are already part of clinical studies [201, 211]. Also interesting is AsiDNA™, which mimics DNA double-strand breaks and subsequently induces apoptosis of cancer cells [107, 212].
Tissue invasion and metastasis
Invasion and metastasis formation builds another basis of cancer progressing to higher malignancy and comprises cell detachment, dissemination, and implantation [124]. Epithelial-mesenchymal transition (EMT) represents a comprehensive model, how cancer cells acquire ability to detach from primary tumor and increase migratory capacity [4, 14]. Contrary to other epithelial cancers, EMT seems to be subsidiary for metastasis formation in OC, being more reliant on passive exfoliation of tumor cells by fluid current to peritoneal cavity [20, 124, 213]. Several mechanisms are described to overcome anoikis, a specific form of apoptosis usually induced upon loss of cell–matrix contact, including FAK activation and overexpression of RAB25, BCL-2 family proteins, and EGFR [124]. Ascites, with its unique TME, promotes cell metastasis. Surface markers expressed on OC as MUC16/CA125 and mesothelin, support adhesion to mesothelial cells [124].
However, typical changes associated with EMT, such as low expression of E-cadherin, are correlated with a poor outcome, making it an interesting target for OC treatment [214]. EMT is driven by transcription factors (EMT-TF) such as Slug, Snail, Zeb-1, and Twist, which increase expression of mesenchymal adhesive and cytoskeletal proteins (N-cadherin, Vimentin, Fibronectin, ß1-ß3-integrins, matrix metalloproteinases) and decrease epithelial state proteins (occludin, claudin, α6ß4 integrins, cytokeratin) [215]. Thus, current research focuses on inhibition of abovementioned features enabling invasion and metastasis and targeting signaling pathways that activate EMT-TF expression, including TGF ß signaling, Wnt pathway and mitogenic growth factor receptors triggering PI3K-AKT, RAS/RAF/MAPK, p38MAPK, and JNK pathways resulting in NFkB expression [215].
FANG vaccine (gemogenovatucel-T, vigil) is a tumor cell vaccine that stimulates dendritic cells and promotes downregulation of TGF ß1/ß2 [216]. TGF ß has multiple functions. It increases EMT-TF expression via SMAD, ERK, and PI3K/AKT signaling [215, 217]. Besides, TGF ß acts strongly immunosuppressive by inhibition of antitumoral T cell responses [218]. In phase II studies, the vaccine prolonged the time to recurrence in the first-line treatment of epithelial OC after standard therapy [219]. The VITAL study (IIb) analyzed vigil in stage IIIb-IV OC after complete clinical response to debulking surgery and primary chemotherapy [220]. Despite good toleration of treatment, primary endpoint was not reached [216]. This highlights the importance of both efficacy and safety profiles. Subgroup analysis has proven OS benefit for HR proficient women (HR 0.342) [221].
MET tyrosine kinase receptor and its ligand hepatocyte growth factor are involved in EMT activation by upregulation of SNAIL [215]. Cabozantinib is a multikinase inhibitor targeting Met, VEGFR2, Ret, Flt3, Kit, and Tie 2 and has been investigated in two phase II trials in ROC [222, 223]. Unfortunately, monotherapy failed to reach good response [224, 225]. Ongoing studies focus on application in germ line cell tumors and in combination with atezolizumab [226, 227]. Since FAK and AXL are also involved in metastasis formation, defactinib (FAKi) and batiraxcept (AXL decoy protein) are currently investigated in ROC phase III trials (Fig. 7) [40, 41, 228, 229].
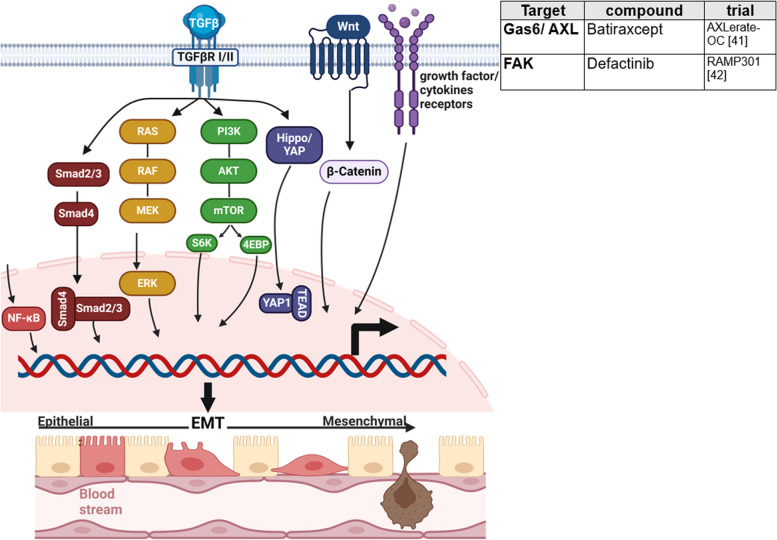
Fig. 7.
Tissue invasion and metastasis. Tissue invasion and metastasis are complex processes, modulated by various signaling pathways. Epithelial-mesenchymal transition and expression of its regulating transcription factors, which are controlled by TGF β, Wnt, and growth factor signaling, is pivotal. Recently completed and currently ongoing phase III studies targeting tissue invasion and metastasis are listed in the table. EMT, epithelial-mesenchymal transition. This figure was created using Biorender.com [40, 41]
Downstream activation of NFκB, e.g., by. PI3K-AKT signaling promotes inflammation and invasion among others by expression of MMPs [230]. Belinostat, a histone deacetylase complex (HDAC) inhibitor, reduces NFκB gene transcription by hypoacetylation. Despite initial encouraging results of a phase Ib/II study proving an ORR of 43% in ROC to treatment with belinostat, carboplatin, and paclitaxel, a phase II clinical trial in PRROC had to be stopped due to lack of drug activity [231–233].
Catumaxomab, approved in 2009 but withdrawn in 2014 due to insolvency, is a trifunctional bispecific antibody, targeting EpCAM [234]. Phase II/III trials, investigating catumaxomab and paracentesis, have shown slight improvement in puncture-free survival [235, 236]. Since August 2022, catumaxomab is once again under evaluation by CHMP (Committee for Medicinal Products for Human Use, EMA) for approval [237].
Further hallmarks to target/perspectives
Since, to our knowledge, no phase III clinical trials have evaluated compounds targeting the hallmarks “enabling replicative immortality,” “deregulating cellular metabolism,” “senescent cells,” and “unlocking phenotypic plasticity,” nor targeting the enabling characteristics “tumor-promoting inflammation,” “nonmutational epigenetic reprogramming,” and “polymorphic microbiomes,” we will discuss current preclinical strategies and initial ongoing clinical trials that indicate possible future directions (Figs. 8 and 9).
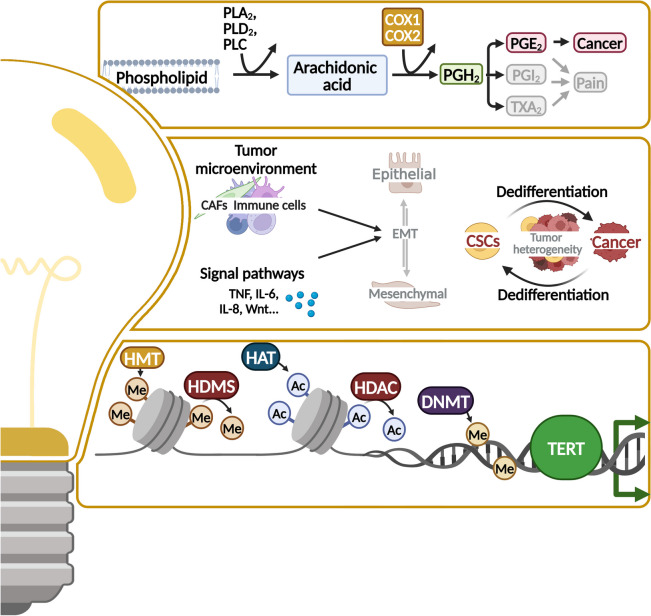
Fig. 8.
Further hallmarks to target. The hallmarks deregulating cellular metabolism, unlocking phenotypic plasticity and the enabling characteristics tumor-promoting inflammation offer broad possibilities of altered pathways to target by cancer treatment. Auspicious targets are displayed in the boxes. CSC, cancer stem cells; CAF, cancer-associated fibroblasts; TCA, tricarboxylic acid cycle; TERT, telomerase reverse transcriptase; HMT, histone methylases; HDMS, histone demethylases; HAT, histone acetyltransferases; HDAC, histone deacetylase; DNMT, DNA methyltransferase. This figure was created using Biorender.com
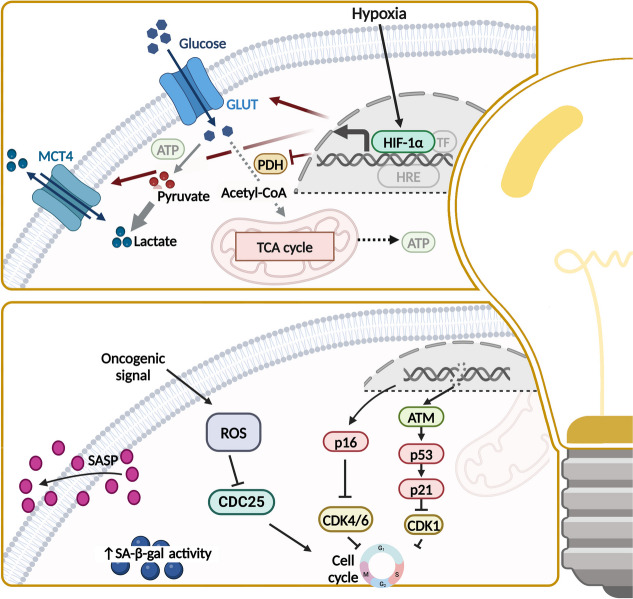
Fig. 9.
Further hallmarks to target. Other promising targets for treatment are the reprogramming of glucose metabolism in cancer cells and the cellular senescence of cancer by stimulating the senescence-associated secretory phenotype, which consists of proinflammatory cytokines, chemokines and matrix-reforming factors. MCT4, monocarboxylate transporter 4; ATP, adenosine triphosphate; GLUT, glucose transporter; TCA, tricarboxylic acid cycle; PDH, pyruvate dehydrogenase; HIF, hypoxia-inducible factor; ROS, reactive oxygen species; SASP, senescence-associated secretory phenotype; SA-β-gal, senescence-associated beta-galactosidase. This figure was created using Biorender.com
Infiltration of TME by inflammatory cells promotes neoplastic progression by the supply of growth factors, survival factors, extracellular matrix-modifying enzymes, and angiogenic molecules (Fig. 8) [14]. Cyclooxygenases (COX), especially COX-2, are fundamental in induction of inflammatory state, which has been proven to come along with poor outcome in OC patients [20, 238]. Preclinical studies have demonstrated that high COX-2 activity increases cell migration and cisplatin resistance in OC cells, explaining promising activity (ORR 28.9%) of celecoxib (COX-2 inhibitor) and carboplatin combination in a phase II study [239]. Unfortunately, further studies investigating COX-inhibitors in combination with cytostatic drugs did not show differences in OS [240–242]. High COX-2 levels also correlate with a low response to immunotherapy and COX-2 inhibition reduced Treg infiltration of the tumor [243]. Thus, acetylsalicylic acid (COX-1/2i) was combined with atezolizumab and bevacizumab in phase II study (EORTC 1508), but no efficacy benefit was observed [244]. Synergistic activity of TLR3 ligands, IFNα, and COX-2 inhibitors enhancing cytotoxic T-lymphocytes meanwhile suppressing Tregs was investigated in a phase I trial in recurrent PSROC [245]. Good safety and tolerability, as well as chemoattraction of cytotoxic T-lymphocytes by the triplet composed of cisplatin, rintalomid (TLR3 ligand), and celecoxib (+ in some cases IFNα as adjunct), led to a phase II trial investigating this triplet together with autologous tumor-loaded αDC1 vaccine. Unfortunately, PFS improvement did not meet predefined thresholds [246].
Whereas non-cancerous cells mainly process glucose to pyruvate and subsequently to carbon dioxide in mitochondria using the tricarboxylic acid cycle, cancer cells are mainly restricted to glycolysis. Since the “Warburg effect” is way less efficient in energy supply, upregulation of glucose transporters as GLUT1 and enhanced glutaminolysis can be seen in OC [14, 247–249]. Upregulation of GLUT1 is associated with poor prognosis in OC [250]. Key regulators of cancer cell metabolism are hypoxia-inducible factor 1α (HIF-1α) and AMP-activated protein kinase (AMPK). Oncogenes, tumor suppressors, and other signaling pathways as c-myc, RAS, p53, and AKT/PI3K/mTOR signaling further modulate energy metabolism (Fig. 9) [14, 20, 248]. CRLX101 is a HIF-1α directed nanoparticle-drug conjugate transporting camptothecin (topo I-inhibitor) to cancer cells. Encouraging results in phase II trials as monotherapy and in combination with bevacizumab or paclitaxel in ROC merit future investigation [21, 251–253]. Besides, pan-AKT inhibitor capivasertib (AZD5363) reached clinical studies and has shown good tolerability and safety in phase I [254]. Recently capivasertib has been approved in metastatic hormone receptor positive breast cancer in combination with fulvestrant [255]. Combination of capivasertib and olaparib has shown great antitumor activity in phase I study including OC, likewise did preliminary results of a study investigating mTORC1/2 inhibitor vistusertib (AZD2014) in combination with olaparib evidence durable anti-tumor activity [256–258]. Other preclinical strategies inhibiting aerobic glycolysis include BH3 mimetics, ivermectin, berberine, and ginsenoside and are reviewed elsewhere [21, 249].
Telomer shortening arises with each cell division and is a natural barrier of replicative immortality (Fig. 8). Critical telomere attrition promotes extensive genomic instability, which leads to apoptosis via p53 and RB pathway or to replicative senescence. Cancer cell alterations such as loss of TP53 and restoration of telomerase activity enable survival of incipient malignancies [14, 259]. Ninety percent of cancers are characterized by overexpression of telomerase, which counteracts telomere attrition by its telomerase reverse transcriptase (TERT) [260, 259]. Cancers with TERT promoter mutations and high expression of TERT are associated with poor outcome, making them and the telomer shortening, an interesting drug target [259, 261]. Current approaches consider small-molecule telomerase inhibitors, oligonucleotide inhibitors, telomerase-directed gene therapy, immunotherapeutic approaches, and alternative splicing as treatment [261]. Further approaches include the attack of shelterin complex and targeting of alternative lengthening of telomeres [261]. However, only imetelstat (GRN163L), an inhibitor of telomerase activity, has been tested in advanced clinical studies for myelodysplastic syndrome and non-small cell lung cancer [262]. Other preclinical hopefuls include BIBR 1532 (telomerase inhibitor) and pyridostatin (G-quadruplex stabilizer), which have shown promise in tumor spheroids [261, 263, 264].
“Senescent cells” were described as a new hallmark of cancer in 2022. Senescence is a non-proliferative but viable state of cells, concomitant with changes in cell morphology and activation of senescence-associated secretory phenotype (SASP) releasing chemokines and cytokines supporting proliferative signaling, angiogenesis, and metastasis (Fig. 9) [14, 15, 265]. Induction of senescence varies by DNA damage, imbalances in cell signaling, and cellular stress [15, 265]. SA-β gal, p16, and p21 represent several biomarkers of cellular senescence, but only a few markers have high specificity and sensitivity [266]. Some senescent cells can regain replicative abilities by cellular plasticity, considering senescence as a mechanism of therapy resistance [267]. Therefore, senescence has a key importance not only for tumor development but also for the response to cancer therapy and is correlated with poor prognosis [268, 269]. Thus, targeting senescence with senolytic or senomorphic drugs, as well as stem cell therapies, was able to extend lifespan and to minimize tissue damage in various animal models [266]. Combination with other anticancer drugs contributes to overcome resistance to apoptosis and reduce side effects [266]. For example, high expression of Bcl-x(L) induces senescence-mediated chemoresistance, which can be reduced by BCL-2 inhibitors as navitoclax, which was proven in phase II MONA VI-1 trial [116, 270]. According to epidemiological data, metformin is protective in OC and modulates the SASP, inhibits endothelial senescence, and enhances efficacy of CDK4 and CDK6 inhibitors [266, 271]. Furthermore, the inhibition of DYRK1A/B and DREAM complex, which are involved in cellular senescence in OC, is promising [271, 272]. Since hyperactivation of AKT/PI3K/mTOR signaling in OC is common, its inhibition is broadly investigated for OC treatment. Interestingly, it has been shown that AKT inhibition promotes senescence of cancer cells. Therefore, combination of AKT inhibitors and downstream blockage of autophagy and senescence could help to overcome therapy resistance [271]. Inversely, maintenance therapy with AKT inhibitors could keep tumor cells in senescent state, thereby preventing tumor recurrence [271]. However, in general, it remains controversial whether cellular senescence impedes cancer growth or supports tumor progress via SASP [15, 273]. In this context also, cancer stem cell (CSC)-related cell senescence displays an interesting approach to target [274].
Unlocking phenotypic plasticity covers another newly introduced hallmark of cancer, the ability to escape or evade terminal differentiation (Fig. 8). Cancer can acquire new molecular properties through dedifferentiation, blocked differentiation, or transdifferentiation, which facilitate metastasis and evasion of systemic therapy [15]. Influencing mechanisms are EMT, the formation of cancer stem cells, the activation or suppression of important signaling pathways, epigenetic changes, and changes in the tumor environment [275]. There is proof that EMT serves as a protective mechanism for cancer cells to survive. By inhibition of EMT, cisplatin resistance was successfully overcome in OC [275–277]. IL (interleukin)−8 contributes to tumor cell remodeling and is taking part in the regulation of tumor cell stemness, EMT, and resistance to therapy [275]. Treatment with SB225002 (CXCR2 inhibitor) attenuates IL-8-induced resistance in OC cells [278]. As therapy-related resistance is still a major obstacle to a complete cure, it is crucial to understand the mechanisms involved in plasticity, to develop targeted therapies [275].
Epigenetic alterations, such as histone modifications, DNA methylation, and post-transcriptional modifications of RNA, influence gene expression and promote tumor development [15, 279, 280]. Several epigenetic alterations can be used as predictive markers in molecular cancer screening and to derive treatment recommendations [281]. Dynamic epigenetic changes in cancer are related to unlimited self-renewal and multi-lineage differentiation as well as tumor heterogeneity and display possible escape mechanisms to therapy which are druggable [15]. MicroRNAs (miRNA), small non-coding RNAs regulating gene expression organized in tumor suppressive or oncogenic clusters, are described in pathogenesis of OC and correlate with therapy response as well as patients’ outcome and serve as biomarkers [282, 283]. For example, Let-7 family of miRNA has a tumor suppressor function and is downregulated in many cancers. Let-7 g overexpression induces a significant reduction in OC cell growth [283]. Aberrant CpG island methylation in OC influences apoptosis, drug sensitivity, and cell cycle regulation. Prime example of aberrant methylation in OC is BRCA1 silencing by promoter hypermethylation [281]. Other epigenetic mechanisms, as shown by ep-100 which targets gonadotropin-releasing hormone receptor and combined with olaparib increases histone H2A.X phosphorylation or the gain of platinum sensitivity due to USP-1 inhibitors, which stop deubiquitination of SNAIL, need to be further explored [281]. DNA methyltransferase (DNMT), histone deacetylase (HDAC), histone demethylase (HDT), and histone methyltransferase EZH2 are the main targets of so far marketed epidrugs [281]. Among DNMTis, ginsenoside Rg3 have shown to promote apoptosis; guadecitabine (SG-110) have increased PARPi sensitivity regardless of BRCA status [281, 284]. Current limitations of DNMTis are mainly due to toxic side effects [281]. HDACi, such as romidepsin, vorinostat, valproate, and PDX101, induce acetylation in OC and thereby promote transcriptional activation and synergism with platinum-based therapies [281, 285, 286]. Roxyl-ZHC-84 is a new HDACi, impeding JAK1-STAT3-BCL-2 provided resistance mechanism [287].
Evidence is growing that microbiota which are symbiotically associated with multiple barrier tissues impact cancer phenotype by either cancer-protective or tumor-promoting microbiome [15, 288, 289]. Mutagenesis due to bacterial toxins, epithelial proliferation caused by ligand mimetics and altered immune response and barrier function are ways how the microbiome can affect cancer. In addition, microbes can trigger DNA damage and apoptosis by releasing genotoxic metabolites or by formation of reactive oxygen species. In OC patients, cytokine levels of tumor necrosis factor α (TNFα) and IL-6, which are involved in regulation of tumor progression via JAK/STAT3 pathway and are regulated by gut microbiome, were increased [290, 291]. Also, a link between chlamydia infections and the risk of OC has already been established. Further endeavors employ microbiome alterations as biomarkers for OC [292]. Recently, Choi and Choi have described the role of the gut and cervicovaginal microbiota in OC concomitant with new therapeutic approaches, including, among others, fecal microbiome transplantation and vaginal microbiome transplantation to improve patients’ outcome [292–294].
Targeted transport
Targeted drug delivery systems that bind antibody, peptide, polymer, small molecules, and single-stranded oligonucleotides via linker enable selective delivery of high potent cytostatic drugs, thus increasing efficacy while decreasing systemic toxicity [295, 296]. A range of overexpressed surface markers is known for OC, including FRα, trophoblast antigen 2 (TROP2), cadherin 6 (CDH6), and type II sodium-phosphate cotransporter (NaPi2b) (Fig. 10). Preclinical research works at terrific rate on systematic identification and validation of further markers [295, 297]. After binding, the system usually passes the cell membrane by endocytosis, receptor-mediated uptake, or non-endocytic translocation pathways and thus reaches its target [298].
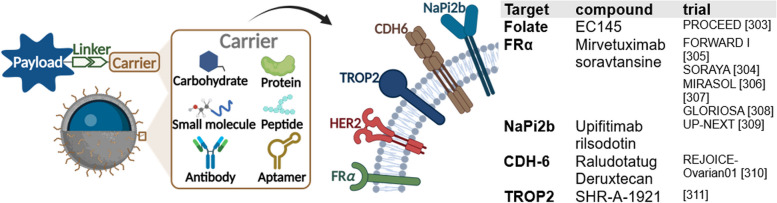
Fig. 10.
Targeted transport—overexpressed cell surface markers. Antibody drug conjugates (ADC) are composed by a carrier, a payload, and a linker. Common carrier molecules include carbohydrates, proteins, small molecules, peptides, and aptamers. FRα, HER2, TROP2, CDH6, and NaPi2b represent overexpressed surface molecules of OC, which are possible targets for ADCs. Recently completed and currently ongoing studies investigating ADC for OC are listed in the table. This figure was created using Biorender.com [302–310]
So far (03/24), 13 antibody drug conjugates (ADCs) have been approved worldwide [299]. Mirvetuximab soravtansine (MIRV), an FRα-directed antibody linked to maytansinoid DM4, is the first and only ADC approved by the FDA for OC [300]. FRα is overexpressed in about 90% of OC and expression increases with progress of disease [301]. A folate, conjugated with a vinca alkaloid, vintafolide, was already investigated in the phase III PROCEED trial in 2011 but did not meet the futility threshold [302]. In contrast, MIRV has proven high efficacy in clinical trials, such as SORAYA study (phase II/III) with an ORR of 32.4% [303].
MIRV is the first treatment demonstrating a benefit in PFS (HR 0.65) and OS (HR 0.67) in PRROC, which has been shown in MIRASOL study, confirming the good therapy response seen in SORAYA [305]. In November 2022, FDA granted accelerated approval for patients with FRα positive PRROC, who have previously received one to three systemic therapies [11]. A global study, GLORIOSA, is currently investigating the maintenance therapy in combination with bevacizumab in PSROC [307].
NaPi2b overexpression is reported in 95% of OC [295]. Upifitamab rilsodotin (UpRi) is a promising ADC targeting NaPi2b, linked to an anti-mitotic drug [311]. However, the phase III trial UP-NEXT was stopped in 2023 due to severe bleeding [308, 312]. Raludotatug deruxtecan is a potential first-in-class ADC, targeting CDH6. CDH6 is overexpressed in approximately 65% of OC [313]. Linked to the topoisomerase (topo) I-inhibitor deruxtecan, it has shown acceptable tolerability and early signals of efficacy in heavily pretreated women with OC as shown in interim-analysis of phase I study in 2023 [314]. Based on this, phase II/III REJOICE-Ovarian01 trial was initiated in February 2024 [309]. TROP2, a transmembrane glycoprotein, is overexpressed in 47–89% of OC and its overexpression is associated with poor prognosis [315]. Sacituzumab govitecan (Trodelvy), a TROP2-directed ADC, is already approved for triple negative breast cancer [316]. In SHR-A-1921, a topo I-inhibitor is connected to a TROP2 antibody with a cleavable linker [317]. Based on good safety and efficacy profile in a phase I trial, a phase II/III trial started in February 2024, evaluating the benefit in combination with carboplatin in ROC [310]. In addition, the TROP2 directed ADC BNT325/DB-1305 is clinically promising and received FDA fast track designation for PRROC [318].
Further endeavors to optimize targeted drug delivery encompass bispecific ADCs [298]. One representative in preclinical research is the novel SORT1xHER2 bispecific ADC, which is directed against HER2 and sortilin-1, which are co-overexpressed in OC [319]. Nevertheless, challenges regarding ADC, such as limited drug-to-antibody ratio and antibody-induced immunogenicity, remain [295].
Furthermore, there are drug-loaded nanoparticles that diffuse to the tumor tissue due to enhanced permeabilization and retention effect [295, 320, 321]. An example of this is liposomal doxorubicin (Doxil), a non-targeted nanoparticle approved by the FDA in 1995 [322]. Nanoparticle drug systems in clinical trials for OC include EP0057, camptothecin bound to a cyclodextrin-based polymer scaffold, and ELU001, exatecan combined with folic acid analogs [251, 323].
Further approaches
Intra- and intertumoral heterogeneity (morphological, prognostic, etiopathogenetic, and molecular heterogeneity) as well as growing knowledge about the impact of TME on cancer emphasize the importance of prognostic screening methods for treatment response, e.g., patient-derived ex vivo tumor organoid cultures, patient-derived xenografts, or “tumor/organ on a chip” models [324, 325]. Future dream would be to predict therapy response solely based on tumor sequencing. Due to this heterogeneity, biomarkers, patient stratification or rather precision oncology and adequate monitoring are crucial to select the appropriate therapy for each patient and thus contribute to the success of the drug, as shown in the work by Skorda et al. [17]. Therefore, not only tests are needed to identify subgroups but also markers with higher specificity and sensitivity. Furthermore, the individualized therapy approach is progressively represented in molecular tumor boards in the clinics.
Besides the development of new drugs, the investigation of tumor-treating field (TTF), an upcoming new cancer treatment modality, using alternating electric fields of intermediate frequency that are intended to disrupt tumor cell growth, is interesting [326]. INNOVATE-3, a phase III study, recently investigated TTF in combination with paclitaxel for PSROC. Although the primary endpoint of OS was not met, survival benefits among exploratory subgroups could be seen and merit further subgroup analyzes [327]. Taking into account major prognostic importance of complete debulking of OC for patient outcome, upcoming imaging agents as Gleolan (5-ALA) are another important tool to improve surgery [328]. Recently, a phase III study (OVA-302) was designed to investigate whether Gleolan can improve debulking surgery of OC [329, 330].
An appropriate study design is essential in order to be able to identify effects and side effects in clinical studies. This includes the choice of suitable biomarkers, the appropriate selection of in-process and follow-up controls, and the perfectly responsive subgroup, due to the large heterogeneity in OC. An incorrect selection of subgroups can lead to a reduction in the effect or even the absence of an effect. Another challenge in clinical trials is long-term follow-up, included in both phase III and phase IV trials. With the increase in patient cohort size and observation time, rare or slowly developing adverse effects are more likely to be detected. Pharmacovigilance is the monitoring of safety and/or efficacy over a longer-term period. Combination therapy is often used to reduce the risk of a poor safety profile. However, the challenge continues even after the clinical trials. In clinical trials, optimally suited patients are initially included in the study. Subgroups are optimally selected and intensively monitored. In the clinic, the drug is then used in a larger cohort of patients with poorer general health, different ages, and increased heterogeneity. For this reason, biomarkers, prognostic tests for treatment response (precision oncology), and appropriate monitoring are essential, even after approval, in order to be able to select the appropriate therapy for each patient and thus contribute to the success of the drug.
Conclusions
OC remains the most lethal gynecological cancer. Challenges faced in OC regarding drug therapy remain to be drug resistance mechanisms and CSCs leading to relapse situations. Many new strategies to improve patient’s outcome appear upon the horizon. Using tools as targeted therapy, immunotherapy, gene therapy, and drug-conjugates, a variety of new techniques and compounds has been developed within the last years to target the hallmarks of cancer.
For example, kinase inhibitors have been broadly investigated in OC treatment. To face challenges such as intratumoral heterogeneity and alterations of multiple pathways, mainly combinational treatments are currently under clinical evaluation. Especially, regarding immunotherapy, huge improvement was made within the last years. Numerous new compounds have evolved and have been investigated, finally showing OS benefits in treatment with ICI and PARPi in the DUO-O trial.
Newly emerged hallmarks of cancer and enabling characteristics as phenotypic plasticity, epigenetic reprogramming, and the microbiome display interesting targets to treat. Here, development and investigation of new compounds is still at the very beginning and merits future research. The information gaps in clinical studies that exist due to general absence of a mandatory international uniformly study register and due to the lack of an obligation to publish results should also be closed in the future.
Following the breakthrough of bevacizumab and PARPi within the recent years, the ADC MIRV appears to be the next drug with great potential in the pipeline. However, we are eagerly awaiting the pending study results and are curious to see which strategies to improve OC therapy will prevail.
Abbreviations
- ATP
Adenosine triphosphate
- ACT
Adoptive cell therapy
- alt-EJ
Alternative end joining
- AMPK
AMP-activated protein kinase
- ALK
Anaplastic lymphoma kinase
- AR
Androgen receptor
- Ang
Angiopoietin
- ADC
Antibody drug conjugates
- BRCA
Breast cancer gene
- CDH6
Cadherin 6
- CAF
Cancer-associated fibroblasts
- CSC
Cancer stem cell
- CAR-T
Chimeric antigen receptor T cells
- CHMP
Committee for Medicinal Products
- CDKs
Cyclin-dependent kinases
- COX
Cyclooxygenases
- DLL4
Delta-like ligand 4
- DCV
Dendritic cell vaccines
- DC
Dendritic cells
- DNMT
DNA methyltransferase
- DSB
Double-strand breaks
- TEff
Effector T cells
- EGFR
Epidermal growth factor receptors
- EMT
Epithelial-mesenchymal transition
- EMT-TF
Epithelial-mesenchymal transition transcription factors
- ER
Estrogen receptor
- FADD
Fas-associating protein with death domain
- FGF
Fibroblast growth factor
- FAK
Focal adhesion kinase
- FRα
Folate receptor alpha
- gBRCAm
Germline BRCAm
- GLUT
Glucose transporter
- HH
Hedgehog signaling pathway
- HGSC
High-grade serous cancer
- HAT
Histone acetyltransferases
- HDAC
Histone deacetylase
- HDT
Histone demethylase
- HDMS
Histone demethylases
- HMT
Histone methylases
- HR
Homologous recombination
- HRD
Homologous recombination deficient
- HRP
Homologous recombination proficient
- HIF
Hypoxia-inducible factor
- IHC
Immunohistochemistry
- ICVs
Infected cell vaccines
- IAP
Inhibitors of apoptosis
- IL
Interleukin
- LGSC
Low-grade serous cancer
- MHC
Major histocompatibility complex
- miRNA
MicroRNAs
- MIRV
Mirvetuximab soravtansine
- MCT4
Monocarboxylate transporter 4
- NK
Natural killer
- NHEJ
Non-homologous end joining
- OV
Oncolytic viruses
- OC
Ovarian cancer
- ORR
Overall response rate
- OS
Overall survival
- PDOs
Patient-derived organoids
- PLD
Pegylated liposomal doxorubicin
- PDGFβ
Platelet-derived growth factor β
- PSROC
Platinum-sensitive recurrent OC
- PRROC
Platinum-resistant or -refractory recurrent OC
- PLK1
Polo-like kinase 1
- PARPi
Poly ADP-ribose polymerase inhibitors
- PD-L1
Programmed cell death ligand-1
- PFS
Progression-free survival
- PDH
Pyruvate dehydrogenase
- ROS
Reactive oxygen species
- ROC
Recurrent OC
- Treg
Regulatory T cells
- RB
Retinoblastoma-associated
- SA-β-gal
Senescence-associated beta-galactosidase
- SASP
Senescence-associated secretory phenotype
- SSB
Single-strand breaks
- TERT
Telomerase reverse transcriptase
- TRADD
TNFR1-associated death domain protein
- topo
Topoisomerase
- TCA
Tricarboxylic acid cycle
- TROP2
Trophoblast-antigen-2
- TAAs
Tumor-associated antigens
- TGF β
Tumor growth factor β
- TIL
Tumor-infiltrating lymphocytes
- TME
Tumor microenvironment
- TNF
Tumor necrosis factor
- TTF
Tumor-treating field
- NaPi2b
Type II sodium-phosphate cotransporter
- USP-1
Ubiquitin-specific protease 1
- UpRi
Upifitamab rilsodotin
- VEGFA
Vascular endothelial growth factor A
Authors’ contributions
JH conceived the manuscript, conducted the bibliographic research, wrote the initial and final drafts of the manuscript. IF conceived the manuscript, created the images and participated in writing the initial draft and manuscript review. DB conceived the manuscript reviewed the manuscript and contributed to writing the final version. NM reviewed the manuscript and contributed to writing the final version. JH and IF had full access to all the data. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Data availability
Data for this review were identified by searching ClinicalTrials.gov (1) using keywords “ovarian cancer” “female” accessed: 03/19/2024 and PubMed and references from relevant articles using the search terms like “ovarian cancer,” “hallmarks of cancer,” “treatment,” “targeted therapy,” “chemotherapy,” “PARP inhibition,” “immunotherapy,” and/or “antibody drug conjugates.”
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dirk O. Bauerschlag, Email: Dirk.bauerschlag@med.uni-jena.de
Inken Flörkemeier, Email: inken.floerkemeier@uksh.de.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: CA. 2021; 10.3322/caac.21660. [DOI] [PubMed]
- 2.Ferlay J, Ervik M, Lam F, Laversanne M, Colombet M, Mery L et al. Global Cancer Observatory: Cancer Today. In: International Agency for Research on Cancer. 2024. https://gco.iarc.fr/en. Accessed 01 Jun 24.
- 3.Webb PM. Jordan SJ. Global epidemiology of epithelial ovarian cancer: Nat Rev Clin Oncol. 2024. 10.1038/s41571-024-00881-3. [DOI] [PubMed] [Google Scholar]
- 4.Chandra A, Pius C, Nabeel M, Nair M, Vishwanatha JK,Ahmad S, et al. Ovarian cancer: current status and strategies for improving therapeutic outcomes: Cancer Med. 2019. 10.1002/cam4.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain KR, Escobar Bermeo JD, Warton K, Valenzuela SM. New approaches and biomarker candidates for the early detection of ovarian cancer: Front. Bioeng Biotechnol. 2022. 10.3389/fbioe.2022.819183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Konstantinopoulos PA. Matulonis UA. Clinical and translational advances in ovarian cancer therapy: Nat Cancer. 2023. 10.1038/s43018-023-00617-9. [DOI] [PubMed] [Google Scholar]
- 7.Moore K, Colombo N, Scambia G, Kim B-G, Oaknin A,Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer: NEJM. 2018. 10.1056/NEJMoa1810858. [DOI] [PubMed] [Google Scholar]
- 8.Ray-Coquard I, Pautier P, Pignata S, Pérol D, González-Martín A,Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer: NEJM. 2019. 10.1056/NEJMoa1911361. [DOI] [PubMed] [Google Scholar]
- 9.O’Malley DM, Krivak TC, Kabil N, Munley J, Moore KN. PARP inhibitors in ovarian cancer: a review: Target Oncol. 2023. 10.1007/s11523-023-00970-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pignata S, Pisano C, Di Napoli M, Cecere SC, Tambaro R, Attademo L. Treatment of recurrent epithelial ovarian cancer: Cancer. 2019. 10.1002/cncr.32500. [DOI] [PubMed] [Google Scholar]
- 11.FDA. FDA grants accelerated approval to mirvetuximab soravtansine-gynx for FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or peritoneal cancer. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-mirvetuximab-soravtansine-gynx-fra-positive-platinum-resistant. Accessed 15 Apr 24.
- 12.Murawski M, Jagodziński A, Bielawska-Pohl A, Klimczak A. Complexity of the genetic background of oncogenesis in ovarian cancer-genetic instability and clinical implications: Cells. 2024. 10.3390/cells13040345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanahan D. Weinberg RA. The hallmarks of cancer: Cell. 2000. 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 14.Hanahan D. Weinberg RA. Hallmarks of cancer: the next generation: Cell. 2011. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 15.Hanahan D. Hallmarks of cancer: new dimensions: Cancer Discov. 2022; 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed]
- 16.Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I,Arnedo-Pac C, et al. A compendium of mutational cancer driver genes: Nat Rev Cancer. 2020. 10.1038/s41568-020-0290-x. [DOI] [PubMed] [Google Scholar]
- 17.Skorda A, Bay ML, Hautaniemi S, Lahtinen A, Kallunki T. Kinase inhibitors in the treatment of ovarian cancer: current state and future promises: Cancers. 2022. 10.3390/cancers14246257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curtis W. McCloskey, Galaxia M. Rodriguez, Kristianne J. C. Galpin, Barbara C. Vanderhyden. Ovarian cancer immunotherapy: preclinical models and emerging therapeutics: Cancers. 2018; 10.3390/cancers10080244. [DOI] [PMC free article] [PubMed]
- 19.Rodriguez GM, Galpin KJC, McCloskey CW, Vanderhyden BC. The tumor microenvironment of epithelial ovarian cancer and its influence on response to immunotherapy: Cancers. 2018. 10.3390/cancers10080242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrillo M, Nero C, Amadio G, Gallo D, Fagotti A, Scambia G. Targeting the hallmarks of ovarian cancer: the big picture: Gynecol Oncol. 2016. 10.1016/j.ygyno.2016.03.037. [DOI] [PubMed] [Google Scholar]
- 21.El Bairi K, Al Jarroudi O, Afqir S. Ovarian Cancer Biomarkers: The hallmarks of ovarian cancer: actionable genetics, targetable pathways, and predictive biomarkers. 1st ed. Singapore: Springer; 2021. [Google Scholar]
- 22.Collado M. Serrano M. Senescence in tumours: evidence from mice and humans: Nat Rev Cancer. 2010. 10.1038/nrc2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guillon J, Petit C, Toutain B, Guette C, Lelièvre E, Coqueret O. Chemotherapy-induced senescence, an adaptive mechanism driving resistance and tumor heterogeneity: Cell cycle. 2019; 10.1080/15384101.2019.1652047. [DOI] [PMC free article] [PubMed]
- 24.Lafky JM, Wilken JA, Baron AT, Maihle NJ. Clinical implications of the ErbB/epidermal growth factor (EGF) receptor family and its ligands in ovarian cancer: Biochim Biophys Acta. 2008; 10.1016/j.bbcan.2008.01.001. [DOI] [PubMed]
- 25.Hudson LG, Zeineldin R, Silberberg M, Stack MS. Activated epidermal growth factor receptor in ovarian cancer: Cancer Treat Res. 2009. 10.1007/978-0-387-98094-2_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vergote IB, Jimeno A, Joly F, Katsaros D, Coens C, Despierre E et al. Randomized phase III study of erlotinib versus observation in patients with no evidence of disease progression after first-line platin-based chemotherapy for ovarian carcinoma: a European Organisation for Research and Treatment of Cancer-Gynaecological Cancer Group, and Gynecologic Cancer Intergroup study: J Clin Oncol. 2014; 10.1200/JCO.2013.50.5669. [DOI] [PubMed]
- 27.Mehner C, Oberg AL, Goergen KM, Kalli KR, Maurer MJ,Nassar A, et al. EGFR as a prognostic biomarker and therapeutic target in ovarian cancer: evaluation of patient cohort and literature review: Genes Cancer. 2017. 10.18632/genesandcancer.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teplinsky E, Muggia F. EGFR and HER2: is there a role in ovarian cancer?: Transl Cancer Res. 2015; 10.3978/j.issn.2218-676X.2015.01.01.
- 29.Lorusso D, Hilpert F, González Martin A, Rau J, Ottevanger P, Greimel E et al. Patient-reported outcomes and final overall survival results from the randomized phase 3 PENELOPE trial evaluating pertuzumab in low tumor human epidermal growth factor receptor 3 (HER3) mRNA-expressing platinum-resistant ovarian cancer: IJGC. 2019; 10.1136/ijgc-2019-000370. [DOI] [PubMed]
- 30.Kreb M. DETERMINE (determining extended therapeutic indications for existing drugs in rare molecularly defined indications using a national evaluation platform trial). 2023. https://clinicaltrials.gov/study/NCT05722886. Accessed 06.2024.
- 31.Kojima Y, Sudo K, Yoshida H, Yazaki S, Tokura M, Mizoguchi C et al. Changes in HER3 expression profiles between primary and recurrent gynecological cancers: Cancer Cell Int. 2023; 10.1186/s12935-022-02844-z. [DOI] [PMC free article] [PubMed]
- 32.Saglam O, Xiong Y, Marchion DC, Strosberg C, Wenham RM, Johnson JJ et al. ERBB4 expression in ovarian serous carcinoma resistant to platinum-based therapy: Cancer control. 2017; 10.1177/107327481702400115. [DOI] [PMC free article] [PubMed]
- 33.Burotto M, Chiou VL, Lee J, Kohn EC. The MAPK pathway across different malignancies: a new perspective: Cancer. 2014. 10.1002/cncr.28864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monk BJ, Grisham RN, Banerjee S, Kalbacher E, Mirza MR, Romero I et al. MILO/ENGOT-ov11: binimetinib versus physician’s choice chemotherapy in recurrent or persistent low-grade serous carcinomas of the ovary, fallopian tube, or primary peritoneum: J Clin Oncol. 2020; 10.1200/JCO.20.01164. [DOI] [PMC free article] [PubMed]
- 35.Gershenson DM, Miller A, Brady WE, Paul J, Carty K, Rodgers W et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial: Lancet (The Lancet). 2022; 10.1016/S0140-6736(21)02175-9. [DOI] [PMC free article] [PubMed]
- 36.Hendrikse CSE, Theelen PMM, van der Ploeg P, Westgeest HM, Boere IA, Thijs AMJ et al. The potential of RAS/RAF/MEK/ERK (MAPK) signaling pathway inhibitors in ovarian cancer: a systematic review and meta-analysis: Gynecol Oncol. 2023; 10.1016/j.ygyno.2023.01.038. [DOI] [PubMed]
- 37.FDA. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid. Accessed 01 Jun 24.
- 38.Coyle V, Middleton G. DETERMINE Trial Treatment Arm 04: Trastuzumab in Combination With Pertuzumab in Adult, Teenage/Young Adult and Paediatric Patients With Cancers With HER2 Amplification or Activating Mutations. 2023. https://clinicaltrials.gov/study/NCT05786716. Accessed 01 Jun 24.
- 39.Vergote I, Armstrong D, Scambia G, Teneriello M, Sehouli J, Schweizer C et al. A randomized, double-blind, placebo-controlled, phase III study to assess efficacy and safety of weekly farletuzumab in combination with carboplatin and taxane in patients with ovarian cancer in first platinum-sensitive relapse: J Clin Oncol. 2016; 10.1200/JCO.2015.63.2596. [DOI] [PubMed]
- 40.Aravive, Inc. Batiraxcept (AVB-S6-500)/placebo in combination with paclitaxel in patients with platinum-resistant recurrent ovarian cancer (AXLerate-OC). 2021. https://clinicaltrials.gov/study/NCT04729608. Accessed 01 Jun 24.
- 41.Grisham R, Banerjee S. A study of avutometinib (VS-6766) + defactinib (VS-6063) in recurrent low-grade serous ovarian cancer (RAMP 301). 2024. https://clinicaltrials.gov/study/NCT06072781. Accessed 07 Apr 24.
- 42.Konstantinopoulos PA, Gonzalez-Martin A, Cruz FM, Friedlander M, Glasspool R, Lorusso D et al. EPIK-O/ENGOT-OV61: alpelisib plus olaparib vs cytotoxic chemotherapy in high-grade serous ovarian cancer (phase III study): Future Oncol. 2022; 10.2217/fon-2022-0666. [DOI] [PubMed]
- 43.van der Ploeg P, Hendrikse CS, Thijs AM, Westgeest HM, Smedts HP,Vos MC, et al. Phenotype-guided targeted therapy based on functional signal transduction pathway activity in recurrent ovarian cancer patients: The STAPOVER study protocol: Heliyon. 2024. 10.1016/j.heliyon.2023.e23170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNeish IA, Ledermann JA, Webber L, James L, Kaye SB, Hall M et al. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer†: Ann Oncol. 2014; 10.1093/annonc/mdu363. [DOI] [PubMed]
- 45.DeCensi A. Exemestane in hormone receptor positive high grade ovarian cancer (EXPERT). 2020. https://clinicaltrials.gov/study/NCT04460807. Accessed 07 Apr 24.
- 46.Fondazione Policlinico Universitario Agostino Gemelli IRCCS. Efficacy of letrozole in recurrent ovarian cancer (MITO32). 2020. https://clinicaltrials.gov/study/NCT04421547. Accessed 07 Apr 24.
- 47.Nickles Fader A, Gien LT, Miller A, Covens A, Gershenson DM. A randomized phase III, two-arm trial of paclitaxel, carboplatin, and maintenance letrozole versus letrozole monotherapy in patients with stage II-IV, primary low-grade serous carcinoma of the ovary or peritoneum: J Clin Oncol. 2021; 10.1200/JCO.2021.39.15_suppl.TPS5601.
- 48.McLaughlin PMJ, Klar M, Zwimpfer TA, Dutilh G, Vetter M, Marth C et al. Maintenance therapy with aromatase inhibitor in epithelial ovarian cancer (MATAO): study protocol of a randomized double-blinded placebo-controlled multi-center phase III trial: BMC cancer. 2022; 10.1186/s12885-022-09555-8. [DOI] [PMC free article] [PubMed]
- 49.DeCensi A. Letrozole for estrogen/progesterone receptor positive low-grade serous epithelial ovarian cancer (LEPRE trial) (LEPRE). 2022. https://clinicaltrials.gov/study/NCT05601700. Accessed 07 Apr 24.
- 50.Lindemann K, Gibbs E, Åvall-Lundqvist E, dePont Christensen R, Woie K, Kalling M et al. Chemotherapy vs tamoxifen in platinum-resistant ovarian cancer: a phase III, randomised, multicentre trial (Ovaresist): Br J Cancer. 2017; 10.1038/bjc.2016.435. [DOI] [PMC free article] [PubMed]
- 51.Hurteau J. Tamoxifen compared with thalidomide in treating women with ovarian epithelial cancer, fallopian tube cancer, or primary peritoneal cancer. 2003. https://clinicaltrials.gov/study/NCT00041080. Accessed 07 Apr 24.
- 52.Dreiling L. Relacorilant in combination with nab-paclitaxel in advanced, platinum-resistant, high-grade epithelial ovarian, primary peritoneal, or fallopian-tube cancer. 2022. https://clinicaltrials.gov/study/NCT05257408. Accessed 08 Apr 24.
- 53.Li H, Zeng J, Shen K. PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer: Arch Gynecol Obstet. 2014; 10.1007/s00404-014-3377-3. [DOI] [PubMed]
- 54.Sava J. Phase 3 Confirmatory Study of Avutometinib/Defactinib in LGSOC Finalizes Design In: Targeted Oncology. 2023. https://www.targetedonc.com/view/phase-3-confirmatory-study-of-avutometinib-defactinib-in-lgsoc-finalizes-design. Accessed 01 Jun 24.
- 55.Huo X, Zhang W, Zhao G, Chen Z, Dong P,Watari H, et al. FAK PROTAC inhibits ovarian tumor growth and metastasis by disrupting kinase dependent and independent pathways: Front Oncol. 2022. 10.3389/fonc.2022.851065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oza A, Kaye S, van Tornout J, Sessa C, Gore M, Naumann RW et al. Phase 2 study evaluating intermittent and continuous linsitinib and weekly paclitaxel in patients with recurrent platinum resistant ovarian epithelial cancer: Gynecol Oncol. 2018; 10.1016/j.ygyno.2018.01.019. [DOI] [PubMed]
- 57.Konecny GE, Haluska P, Janicke F, Sehouli J, Beckmann MW, Feisel G et al. A phase II, multicenter, randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with carboplatin/paclitaxel as front-line therapy for optimally debulked primary ovarian cancer: The TRIO14 trial: J Clin Oncol. 2014; 10.1200/jco.2014.32.15_suppl.5529.
- 58.Banerjee S. Kaye SB. New strategies in the treatment of ovarian cancer: current clinical perspectives and future potential: Clin Cancer Res. 2013. 10.1158/1078-0432.CCR-12-2243. [DOI] [PubMed] [Google Scholar]
- 59.Nawaz FZ, Kipreos ET. Emerging roles for folate receptor FOLR1 in signaling and cancer: trends in endocrinology and metabolism: TEM. 2022; 10.1016/j.tem.2021.12.003. [DOI] [PMC free article] [PubMed]
- 60.Sieh W, Köbel M, Longacre TA, Bowtell DD, deFazio A,Goodman MT, et al. Hormone-receptor expression and ovarian cancer survival: an Ovarian Tumor Tissue Analysis consortium study: Lancet Oncol. 2013. 10.1016/S1470-2045(13)70253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gershenson DM, Bodurka DC, Coleman RL, Lu KH, Malpica A, Sun CC. Hormonal maintenance therapy for women with low-grade serous cancer of the ovary or peritoneum: J Clin Oncol. 2017. 10.1200/JCO.2016.71.0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities: Cell Mol Life Sci. 2020; 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed]
- 63.Pujade-Lauraine E, Hilpert F, Weber B, Reuss A, Poveda A,Kristensen G, et al. Bevacizumab combined with chemotherapy for platinum-resistant recurrent ovarian cancer: the AURELIA open-label randomized phase III trial: J Clin Oncol. 2014. 10.1200/JCO.2013.51.4489. [DOI] [PubMed] [Google Scholar]
- 64.Oza AM, Cook AD, Pfisterer J, Embleton A, Ledermann JA, Pujade-Lauraine E et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial: Lancet Oncol. 2015; 10.1016/S1470-2045(15)00086-8. [DOI] [PMC free article] [PubMed]
- 65.Tewari KS, Burger RA, Enserro D, Norquist BM, Swisher EM,Brady MF, et al. Final overall survival of a randomized trial of bevacizumab for primary treatment of ovarian cancer: J Clin Oncol. 2019. 10.1200/JCO.19.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Aghajanian C, Blank SV, Goff BA, Judson PL, Teneriello MG, Husain A et al. OCEANS: a randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer: J Clin Oncol. 2012; 10.1200/JCO.2012.42.0505. [DOI] [PMC free article] [PubMed]
- 67.Ray-Coquard I, Leary A, Pignata S, Cropet C, González-Martín A, Marth C et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: final overall survival results from the PAOLA-1/ENGOT-ov25 trial: Ann Oncol. 2023; 10.1016/j.annonc.2023.05.005. [DOI] [PubMed]
- 68.Gotlieb WH, Amant F, Advani S, Goswami C, Hirte H, Provencher D et al. Intravenous aflibercept for treatment of recurrent symptomatic malignant ascites in patients with advanced ovarian cancer: a phase 2, randomised, double-blind, placebo-controlled study: Lancet Oncol. 2012; 10.1016/S1470-2045(11)70338-2. [DOI] [PubMed]
- 69.Jiangsu Simcere Pharmaceutical Co., Ltd. A phase III study of BD0801 combined with chemotherapy in recurrent, platinum-resistant epithelial ovarian cancer. 2021. https://clinicaltrials.gov/study/NCT04908787. Accessed 19 Jun 24.
- 70.Moore KN. A study of navicixizumab in patients with platinum resistant ovarian cancer. 2022. https://clinicaltrials.gov/study/NCT05043402. Accessed 01 Jun 24.
- 71.Jiangsu HengRui Medicine Co., Ltd. Apatinib and etoposide capsule versus weekly paclitaxel in patients with platinum resistant ovarian cancer. 2019. https://clinicaltrials.gov/study/NCT04000295. Accessed 07 Apr 24.
- 72.Jiangsu HengRui Medicine Co., Ltd. A study of fluzoparib±apatinib versus placebo maintenance treatment in patients with advanced ovarian cancer following response on first-line platinum-based chemotherapy. 2020. https://clinicaltrials.gov/study/NCT04229615. Accessed 07 Apr 24.
- 73.Ledermann JA, Embleton-Thirsk AC, Perren TJ, Jayson GC, Rustin GJS, Kaye SB et al. Cediranib in addition to chemotherapy for women with relapsed platinum-sensitive ovarian cancer (ICON6): overall survival results of a phase III randomised trial: ESMO open. 2021; 10.1016/j.esmoop.2020.100043. [DOI] [PMC free article] [PubMed]
- 74.Liu JF, Brady MF, Matulonis UA, Miller A, Kohn EC, Swisher EM et al. Olaparib with or without cediranib versus platinum-based chemotherapy in recurrent platinum-sensitive ovarian cancer (NRG-GY004): a randomized, open-label, phase III trial: J Clin Oncol. 2022; 10.1200/JCO.21.02011. [DOI] [PMC free article] [PubMed]
- 75.Elyashiv O, Ledermann J, Parmar G, Farrelly L, Counsell N, Feeney A et al. ICON 9-an international phase III randomized study to evaluate the efficacy of maintenance therapy with olaparib and cediranib or olaparib alone in patients with relapsed platinum-sensitive ovarian cancer following a response to platinum-based chemotherapy: Int J Gynecol Cancer. 2021; 10.1136/ijgc-2020-002073. [DOI] [PubMed]
- 76.Lee J. Testing the combination of cediranib and olaparib in comparison to each drug alone or other chemotherapy in recurrent platinum-resistant ovarian cancer. 2016. https://clinicaltrials.gov/study/NCT02502266. Accessed 21 May 24.
- 77.Monk BJ, Poveda A, Vergote I, Raspagliesi F, Fujiwara K, Bae D-S et al. Anti-angiopoietin therapy with trebananib for recurrent ovarian cancer (TRINOVA-1): a randomised, multicentre, double-blind, placebo-controlled phase 3 trial: Lancet Oncol. 2014; 10.1016/S1470-2045(14)70244-X. [DOI] [PubMed]
- 78.Marth C, Vergote I, Scambia G, Oberaigner W, Clamp A, Berger R et al. ENGOT-ov-6/TRINOVA-2: randomised, double-blind, phase 3 study of pegylated liposomal doxorubicin plus trebananib or placebo in women with recurrent partially platinum-sensitive or resistant ovarian cancer: Eur J Cancer. 2017; 10.1016/j.ejca.2016.09.004. [DOI] [PubMed]
- 79.Vergote I, Scambia G, O’Malley DM, van Calster B, Park S-Y, Del Campo JM et al. Trebananib or placebo plus carboplatin and paclitaxel as first-line treatment for advanced ovarian cancer (TRINOVA-3/ENGOT-ov2/GOG-3001): a randomised, double-blind, phase 3 trial: Lancet Oncol. 2019; 10.1016/S1470-2045(19)30178-0. [DOI] [PubMed]
- 80.Grenier J. Masitinib in combination with gemcitabine in advanced/metastatic epithelial ovarian cancer patients. 2014. https://clinicaltrials.gov/study/NCT02490488. Accessed 07 Apr 24.
- 81.Ray-Coquard I, Cibula D, Mirza MR, Reuss A, Ricci C, Colombo N et al. Final results from GCIG/ENGOT/AGO-OVAR 12, a randomised placebo-controlled phase III trial of nintedanib combined with chemotherapy for newly diagnosed advanced ovarian cancer: Int J Cancer. 2020;10.1002/ijc.32606. [DOI] [PubMed]
- 82.Vergote I, Du Bois A, Floquet A, Rau J, Kim J-W, Del Campo JM et al. Overall survival results of AGO-OVAR16: A phase 3 study of maintenance pazopanib versus placebo in women who have not progressed after first-line chemotherapy for advanced ovarian cancer: Gynecol Oncol. 2019; 10.1016/j.ygyno.2019.08.024. [DOI] [PubMed]
- 83.Huang X. A clinical study of TQB2450 injection combined with anlotinib hydrochloride capsules versus paclitaxel as weekly treatment of relapsed platinum-resistant ovarian cancer. 2021. https://clinicaltrials.gov/study/NCT05145218. Accessed 07 Apr 24.
- 84.Advenchen Laboratories LL. Phase 1/2a/3 evaluation of adding AL3818 to standard platinum-based chemotherapy in subjects with recurrent or metastatic endometrial, ovarian, fallopian, primary peritoneal or cervical carcinoma (AL3818-US-002) (AL3818). 2015. https://clinicaltrials.gov/study/NCT02584478. Accessed 07 Apr 24.
- 85.Wu X. Chiauranib plus weekly paclitaxel in patients with platinum-refractory or platinum-resistant recurrent ovarian cancer (CHIPRO). 2021. https://clinicaltrials.gov/study/NCT04921527. Accessed 07 Apr 24.
- 86.Wang L, Fei Y, Qu H, Zhang H, Wang Y,Wu Z, et al. Five years of safety profile of bevacizumab: an analysis of real-world pharmacovigilance and randomized clinical trials: J Pharm Health Care Sci. 2024. 10.1186/s40780-023-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu W, Lu C, Dong HH, Huang J, Shen D, Stone RL et al. Biological roles of the Delta family Notch ligand Dll4 in tumor and endothelial cells in ovarian cancer: Cancer Res. 2011; 10.1158/0008-5472.CAN-10-2719. [DOI] [PMC free article] [PubMed]
- 88.Fu S, Corr BR, Culm-Merdek K, Mockbee C, Youssoufian H, Stagg R et al. Phase Ib study of navicixizumab plus paclitaxel in patients with platinum-resistant ovarian, primary peritoneal, or fallopian tube cancer: J Clin Oncol. 2022; 10.1200/JCO.21.01801. [DOI] [PMC free article] [PubMed]
- 89.Pan Z, Luo Z, He H, Chen Y, Zhao B,Yang Z, et al. Observation of the therapeutic effect of apatinib in advanced platinum-resistant recurrent epithelial ovarian cancer: J Ovarian Res. 2023. 10.1186/s13048-022-01055-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pignata S, Lorusso D, Scambia G, Sambataro D, Tamberi S, Cinieri S et al. MITO-11: a randomized multicenter phase II trial testing the addition of pazopanib to weekly paclitaxel in platinum-resistant or -refractory advanced ovarian cancer (AOC): J Clin Oncol. 2014; 10.1200/jco.2014.32.15_suppl.5503.
- 91.Lan C-Y, Zhao J, Yang F, Xiong Y, Li R, Huang Y et al. Anlotinib combined with TQB2450 in patients with platinum-resistant or -refractory ovarian cancer: a multi-center, single-arm, phase 1b trial: Cell Rep Med. 2022; 10.1016/j.xcrm.2022.100689. [DOI] [PMC free article] [PubMed]
- 92.The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma: Nature. 2011; 10.1038/nature10166. [DOI] [PMC free article] [PubMed]
- 93.Gorski JW, Ueland FR, Kolesar JM. CCNE1 amplification as a predictive biomarker of chemotherapy resistance in epithelial ovarian cancer: Diagnostics. 2020; 10.3390/diagnostics10050279. [DOI] [PMC free article] [PubMed]
- 94.Mei L, Zhang J, He K, Zhang J. Ataxia telangiectasia and Rad3-related inhibitors and cancer therapy: where we stand: J Hematol Oncol. 2019; 10.1186/s13045-019-0733-6. [DOI] [PMC free article] [PubMed]
- 95.Gorecki L, Andrs M, Korabecny J. Clinical candidates targeting the ATR-CHK1-WEE1 axis in cancer: Cancers. 2021; 10.3390/cancers13040795. [DOI] [PMC free article] [PubMed]
- 96.Wang Y, Ji P, Liu J, Broaddus RR, Xue F, Zhang W. Centrosome-associated regulators of the G(2)/M checkpoint as targets for cancer therapy: Mol Cancer. 2009; 10.1186/1476-4598-8-8. [DOI] [PMC free article] [PubMed]
- 97.Gully CP, Velazquez-Torres G, Shin J-H, Fuentes-Mattei E, Wang E, Carlock C et al. Aurora B kinase phosphorylates and instigates degradation of p53: PNAS. 2012; 10.1073/pnas.1110287109. [DOI] [PMC free article] [PubMed]
- 98.Horowitz JA. Paclitaxel plus carboplatin with or without SCH-58500 in treating patients with newly diagnosed stage III ovarian or stage III primary peritoneal cancer. 1999. https://clinicaltrials.gov/study/NCT00003880. Accessed 13 Apr 24.
- 99.Colon-Otero G, Zanfagnin V, Hou X, Foster NR, Asmus EJ,Wahner Hendrickson A, et al. Phase II trial of ribociclib and letrozole in patients with relapsed oestrogen receptor-positive ovarian or endometrial cancers: ESMO open. 2020. 10.1136/esmoopen-2020-000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ottenbourgs T, van Gorp T, Kridelka F, Baert T, Denys H, Selle F et al. A phase II, multicenter, open-label study of abemaciclib and letrozole in patients with estrogen receptor-positive rare ovarian cancer: ALEPRO trial: Int J Gynecol Cancer. 2024; 10.1136/ijgc-2023-005189. [DOI] [PMC free article] [PubMed]
- 101.Bible KC, Peethambaram PP, Oberg AL, Maples W, Groteluschen DL, Boente M et al. A phase 2 trial of flavopiridol (Alvocidib) and cisplatin in platin-resistant ovarian and primary peritoneal carcinoma: MC0261: Gynecol Oncol. 2012; 10.1016/j.ygyno.2012.05.030. [DOI] [PMC free article] [PubMed]
- 102.Joshi H, Tuli HS, Ranjan A, Chauhan A, Haque S,Ramniwas S, et al. The pharmacological implications of flavopiridol: an updated overview: Molecules. 2023. 10.3390/molecules28227530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Da Costa AABA, Chowdhury D, Shapiro GI, D’Andrea AD, Konstantinopoulos PA. Targeting replication stress in cancer therapy: Nat Rev Drug Discov. 2023. 10.1038/s41573-022-00558-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nghiem P, Park PK, Kim Y, Vaziri C, Schreiber SL. ATR inhibition selectively sensitizes G1 checkpoint-deficient cells to lethal premature chromatin condensation: PNAS. 2001; 10.1073/pnas.161281798. [DOI] [PMC free article] [PubMed]
- 105.Mahdi H, Hafez N, Doroshow D, Sohal D, Keedy V, Do KT et al. Ceralasertib-mediated ATR inhibition combined with olaparib in advanced cancers harboring DNA damage response and repair alterations (olaparib combinations): JCO Precis Oncol. 2021; 10.1200/PO.20.00439. [DOI] [PMC free article] [PubMed]
- 106.Konstantinopoulos PA, Lee J, Gao B, Miller R, Lee J-Y, Colombo N et al. A phase 2 study of prexasertib (LY2606368) in platinum resistant or refractory recurrent ovarian cancer: Gynecol Oncol. 2022; 10.1016/j.ygyno.2022.09.019. [DOI] [PMC free article] [PubMed]
- 107.Moufarrij S. O’Cearbhaill RE. Novel therapeutics in ovarian cancer: expanding the toolbox: Curr Oncol. 2023. 10.3390/curroncol31010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oza AM, Estevez-Diz M, Grischke E-M, Hall M, Marmé F, Provencher D et al. A biomarker-enriched, randomized phase II trial of adavosertib (AZD1775) plus paclitaxel and carboplatin for women with platinum-sensitive TP53-mutant ovarian cancer: Clin Cancer Res. 2020; 10.1158/1078-0432.CCR-20-0219. [DOI] [PubMed]
- 109.Pujade-Lauraine E, Selle F, Weber B, Ray-Coquard I-L, Vergote I,Sufliarsky J, et al. Volasertib versus chemotherapy in platinum-resistant or -refractory ovarian cancer: a randomized phase II Groupe des Investigateurs Nationaux pour l’Etude des Cancers de l’Ovaire study: J Clin Oncol. 2016. 10.1200/JCO.2015.62.1474. [DOI] [PubMed] [Google Scholar]
- 110.Falchook G, Coleman RL, Roszak A, Behbakht K, Matulonis U,Ray-Coquard I, et al. Alisertib in combination with weekly paclitaxel in patients with advanced breast cancer or recurrent ovarian cancer: a randomized clinical trial: JAMA Oncol. 2019. 10.1001/jamaoncol.2018.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wallis B, Bowman KR, Lu P, Lim CS. The challenges and prospects of p53-based therapies in ovarian cancer: Biomolecules. 2023; 10.3390/biom13010159. [DOI] [PMC free article] [PubMed]
- 112.Arend RC, Monk BJ, Shapira-Frommer R, Haggerty AF, Alvarez EA, Amit A et al. Ofranergene obadenovec (Ofra-Vec, VB-111) with weekly paclitaxel for platinum-resistant ovarian cancer: randomized controlled phase III trial (OVAL Study/GOG 3018): J Clin Oncol. 2024; 10.1200/JCO.22.02915. [DOI] [PubMed]
- 113.Elmore S. Apoptosis: a review of programmed cell death: Toxicol Pathol. 2007; 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed]
- 114.Abed MN, Abdullah MI, Richardson A. Antagonism of Bcl-XL is necessary for synergy between carboplatin and BH3 mimetics in ovarian cancer cells: J Ovarian Res. 2016; 10.1186/s13048-016-0234-y. [DOI] [PMC free article] [PubMed]
- 115.Yokoyama T, Kohn EC, Brill E, Lee J. Apoptosis is augmented in high-grade serous ovarian cancer by the combined inhibition of Bcl-2/Bcl-xL and PARP: Int J Oncol. 2017; 10.3892/ijo.2017.3914. [DOI] [PMC free article] [PubMed]
- 116.Joly F, Fabbro M, Follana P, Lequesne J, Medioni J, Lesoin A et al. A phase II study of Navitoclax (ABT-263) as single agent in women heavily pretreated for recurrent epithelial ovarian cancer: The MONAVI - GINECO study: Gynecol Oncol. 2022; 10.1016/j.ygyno.2022.01.021. [DOI] [PubMed]
- 117.Triozzi PL, Borden EC. VB-111 for cancer: Expert Opin Biol Ther. 2011; 10.1517/14712598.2011.618122. [DOI] [PubMed]
- 118.Schardt J. Der Einsatz von Immuncheckpoint-Inhibitoren im onkologischen Alltag: Z Rheumatol. 2020; 10.1007/s00393-020-00876-2. [DOI] [PMC free article] [PubMed]
- 119.Dalle S, Mortier L, Corrie P, Lotem M, Board R,Arance AM, et al. Long-term real-world experience with ipilimumab and non-ipilimumab therapies in advanced melanoma: the IMAGE study: BMC cancer. 2021. 10.1186/s12885-021-08032-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy: Cancer cell. 2015. 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hamanishi J, Takeshima N, Katsumata N, Ushijima K, Kimura T, Takeuchi S et al. Nivolumab versus gemcitabine or pegylated liposomal doxorubicin for patients with platinum-resistant ovarian cancer: open-label, randomized trial in Japan (NINJA): J Clin Oncol. 2021; 10.1200/JCO.21.00334. [DOI] [PMC free article] [PubMed]
- 122.Matulonis UA, Shapira-Frommer R, Santin AD, Lisyanskaya AS, Pignata S, Vergote I et al. Antitumor activity and safety of pembrolizumab in patients with advanced recurrent ovarian cancer: results from the phase II KEYNOTE-100 study: Annals of Oncology. 2019; 10.1093/annonc/mdz135. [DOI] [PubMed]
- 123.Palaia I, Tomao F, Sassu CM, Musacchio L. Benedetti Panici P. Immunotherapy for ovarian cancer: recent advances and combination therapeutic approaches: Onco Targets Ther. 2020. 10.2147/OTT.S205950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schoutrop E, Moyano-Galceran L, Lheureux S, Mattsson J, Lehti K, Dahlstrand H et al. Molecular, cellular and systemic aspects of epithelial ovarian cancer and its tumor microenvironment: Semin Cancer Biol. 2022; 10.1016/j.semcancer.2022.03.027. [DOI] [PubMed]
- 125.Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer: PNAS. 2005; 10.1073/pnas.0509182102 [DOI] [PMC free article] [PubMed]
- 126.Stumpf M, Hasenburg A, Riener M-O, Jütting U, Wang C, Shen Y et al. Intraepithelial CD8-positive T lymphocytes predict survival for patients with serous stage III ovarian carcinomas: relevance of clonal selection of T lymphocytes: Br J Cancer. 2009; 10.1038/sj.bjc.6605274. [DOI] [PMC free article] [PubMed]
- 127.Kandalaft LE, Odunsi K, Coukos G. Immune therapy opportunities in ovarian cancer: Am Soc Clin Oncol Educ Book. 2020. 10.1200/EDBK_280539. [DOI] [PubMed] [Google Scholar]
- 128.Monk BJ, Colombo N, Oza AM, Fujiwara K, Birrer MJ, Randall L et al. Chemotherapy with or without avelumab followed by avelumab maintenance versus chemotherapy alone in patients with previously untreated epithelial ovarian cancer (JAVELIN Ovarian 100): an open-label, randomised, phase 3 trial: Lancet Oncol. 2021; 10.1016/S1470-2045(21)00342-9. [DOI] [PubMed]
- 129.Pujade-Lauraine E, Fujiwara K, Ledermann JA, Oza AM, Kristeleit R, Ray-Coquard I-L et al. Avelumab alone or in combination with chemotherapy versus chemotherapy alone in platinum-resistant or platinum-refractory ovarian cancer (JAVELIN Ovarian 200): an open-label, three-arm, randomised, phase 3 study: Lancet Oncol. 2021; 10.1016/S1470-2045(21)00216-3. [DOI] [PubMed]
- 130.Colombo I, Karakasis K, Suku S, Oza AM. Chasing immune checkpoint inhibitors in ovarian cancer: novel combinations and biomarker discovery: Cancers. 2023. 10.3390/cancers15123220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kurtz J-E, Pujade-Lauraine E, Oaknin A, Belin L, Leitner K, Cibula D et al. Atezolizumab combined with bevacizumab and platinum-based therapy for platinum-sensitive ovarian cancer: placebo-controlled randomized phase III ATALANTE/ENGOT-ov29 trial: J Clin Oncol. 2023; 10.1200/JCO.23.00529. [DOI] [PMC free article] [PubMed]
- 132.Marmé F, Harter P, Redondo A, Reuss A, Ray-Coquard IL, Lindemann K et al. Atezolizumab versus placebo in combination with bevacizumab and non-platinum-based chemotherapy in recurrent ovarian cancer: final overall and progression-free survival results from the AGO-OVAR 2.29/ENGOT-ov34 study: J Clin Oncol. 2024; 10.1200/JCO.2024.42.17_suppl.LBA5501.
- 133.Moore KN, Bookman M, Sehouli J, Miller A, Anderson C, Scambia G et al. Atezolizumab, bevacizumab, and chemotherapy for newly diagnosed stage III or IV ovarian cancer: placebo-controlled randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39): J Clin Oncol. 2021; 10.1200/JCO.21.00306. [DOI] [PMC free article] [PubMed]
- 134.Colombo N, Coleman RL, Wu X, Kose F, Wenham R, Sebastianelli A et al. 44TiP ENGOT-ov65/KEYNOTE-B96: phase III, randomized, double-blind study of pembrolizumab vs placebo + paclitaxel with optional bevacizumab for platinum-resistant recurrent ovarian cancer: Ann Oncol. 2022; 10.1016/j.annonc.2022.04.062.
- 135.O’Cearbhaill RE. Pegylated liposomal doxorubicin hydrochloride with atezolizumab and/or bevacizumab in treating patients with recurrent ovarian, fallopian tube, or primary peritoneal cancer. 2017. https://clinicaltrials.gov/study/NCT02839707. Accessed 10 Apr 24.
- 136.Harter P, Pautier P, van Nieuwenhuysen E, Reuss A, Redondo A, Lindemann K et al. Atezolizumab in combination with bevacizumab and chemotherapy versus bevacizumab and chemotherapy in recurrent ovarian cancer - a randomized phase III trial (AGO-OVAR 2.29/ENGOT-ov34): Int J Gynecol Cancer. 2020; 10.1136/ijgc-2020-001572. [DOI] [PubMed]
- 137.Doherty K. Atezolizumab plus chemotherapy fails to provide clinical benefit in recurrent ovarian cancer. 2023. https://www.onclive.com/view/atezolizumab-plus-chemotherapy-fails-to-provide-clinical-benefit-in-roc. Accessed 10 Apr 24.
- 138.Eskander RN, Ledermann JA, Birrer MJ, Fujiwara K, Gaillard S, Richardson GE et al. JAVELIN ovarian PARP 100 study design: phase III trial of avelumab + chemotherapy followed by avelumab + talazoparib maintenance in previously untreated epithelial ovarian cancer: J Clin Oncol. 2019; 10.1200/JCO.2019.37.8_suppl.TPS9.
- 139.Helwick C. DUO-O: benefit shown for durvalumab plus olaparib in advanced ovarian cancer. 2023. https://ascopost.com/issues/june-25-2023/benefit-shown-for-durvalumab-plus-olaparib-in-advanced-ovarian-cancer/. Accessed 10 Apr 24.
- 140.Vergote I, Sehouli J, Salutari V, Zola P, Madry R, Wenham RM et al. ENGOT-OV43/KEYLYNK-001: a phase III, randomized, double-blind, active- and placebo-controlled study of pembrolizumab plus chemotherapy with olaparib maintenance for first-line treatment of BRCA-nonmutated advanced epithelial ovarian cancer: J Clin Oncol. 2019; 10.1200/JCO.2019.37.15_suppl.TPS5603.
- 141.Zhang Z. Trans-artery/intra-tumor infusion of checkpoint inhibitors plus chemodrug for immunotherapy of advanced solid tumors. 2018. https://clinicaltrials.gov/study/NCT03755739. Accessed 10 Apr 24.
- 142.Herzog TJ, Hays JL, Barlin JN, Buscema J, Cloven NG, Kong LR et al. ARTISTRY-7: phase III trial of nemvaleukin alfa plus pembrolizumab vs chemotherapy for platinum-resistant ovarian cancer: Future Oncol. 2023; 10.2217/fon-2023-0246. [DOI] [PubMed]
- 143.Hardy-Bessard A-C, Moore KN, Mirza MR, Asselain B, Redondo A, Pfisterer J et al. ENGOT-OV44/FIRST study: a randomized, double-blind, adaptive, phase III study of standard of care (SOC) platinum-based therapy ± dostarlimab followed by niraparib ± dostarlimab maintenance as first-line (1L) treatment of stage 3 or 4 ovarian cancer (OC): J Clin Oncol. 2020; 10.1200/JCO.2020.38.15_suppl.TPS6101.
- 144.Musacchio L, Salutari V, Pignata S, Braicu E, Cibula D, Colombo N et al. Randomized phase III trial on niraparib-TSR-042 (dostarlimab) versus physician’s choice chemotherapy in recurrent ovarian, fallopian tube, or primary peritoneal cancer patients not candidate for platinum retreatment: NItCHE trial (MITO 33): Int J Gynecol Cancer. 2021; 10.1136/ijgc-2021-002593. [DOI] [PubMed]
- 145.Monk BJ, Coleman RL, Fujiwara K, Wilson MK, Oza AM, Oaknin A et al. ATHENA (GOG-3020/ENGOT-ov45): a randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA-MONO) and rucaparib in combination with nivolumab (ATHENA-COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer: Int J Gynecol Cancer. 2021; 10.1136/ijgc-2021-002933. [DOI] [PMC free article] [PubMed]
- 146.Berek J, Taylor P, McGuire W, Smith LM, Schultes B, Nicodemus CF. Oregovomab maintenance monoimmunotherapy does not improve outcomes in advanced ovarian cancer: J Clin Oncol. 2009. 10.1200/JCO.2008.17.8400. [DOI] [PubMed] [Google Scholar]
- 147.Secord AA, Barroilhet LM, Lim MC, Gupta S, Oosman S, Rao JS et al. FLORA-5/GOG3035: frontline chemo-immunotherapy (paclitaxel-carboplatin-oregovomab [PCO] versus chemotherapy (paclitaxel-carboplatin-placebo [PCP]) in patients with advanced epithelial ovarian cancer (EOC)—Phase III, double-blind, placebo-controlled, global, multinational study: J Clin Oncol. 2022; 10.1200/JCO.2022.40.16_suppl.TPS5619.
- 148.Holloway R, Thaker P, Mendivil A, Ahmad S, Bell M, Chambers S et al. TP026/#1435 phase 3 study of efficacy & safety of Olvi-Vec and platinum-doublet + bevacizumab compared to platinum-doublet + bevacizumab in platinum-resistant/refractory ovarian cancer (ONPRIME; GOG-3076) [NCT05281471]: Int J Gynecol Cancer. 2022; 10.1136/ijgc-2022-igcs.535.
- 149.Shuiqun G. Plasmodium immunotherapy for advanced ovarian cancer. 2024. https://clinicaltrials.gov/study/NCT05924776. Accessed 12 Apr 24.
- 150.Musacchio L, Cicala CM, Camarda F, Ghizzoni V, Giudice E, Carbone MV et al. Combining PARP inhibition and immune checkpoint blockade in ovarian cancer patients: a new perspective on the horizon?: ESMO open. 2022; 10.1016/j.esmoop.2022.100536. [DOI] [PMC free article] [PubMed]
- 151.Domchek SM, Postel-Vinay S, Im S-A, Park YH, Delord J-P, Italiano A et al. Olaparib and durvalumab in patients with germline BRCA-mutated metastatic breast cancer (MEDIOLA): an open-label, multicentre, phase 1/2, basket study: Lancet Oncol. 2020; 10.1016/S1470-2045(20)30324-7. [DOI] [PubMed]
- 152.Konstantinopoulos PA, Waggoner S, Vidal GA, Mita M, Moroney JW, Holloway R et al. Single-arm phases 1 and 2 trial of niraparib in combination with pembrolizumab in patients with recurrent platinum-resistant ovarian carcinoma: JAMA oncology. 2019; 10.1001/jamaoncol.2019.1048. [DOI] [PMC free article] [PubMed]
- 153.Segeberg F. Merck and Pfizer announce discontinuation of phase III JAVELIN ovarian PARP 100 trial in previously untreated advanced ovarian cancer. 2019. https://www.merckgroup.com/en/news/javelin-ovarian-parp-19-03-2019.html. Accessed 11 Apr 24.
- 154.Deng Y, Reyes RM, Zhang C, Conejo-Garcia J, Curiel TJ. Targeting ovarian cancer with IL-2 cytokine/antibody complexes: a summary and recent advances: J Cell Immunol. 2021; 10.33696/immunology.3.122. [DOI] [PMC free article] [PubMed]
- 155.Winer I, Gilbert L, Vaishampayan U, Rosen S, Hoimes C, Muzaffar J et al. 347 Clinical outcomes of ovarian cancer patients treated with ALKS 4230, a novel engineered cytokine, in combination with pembrolizumab: ARTISTRY-1 trial: J Immunother Cancer. 2020; 10.1136/jitc-2020-SITC2020.0347.
- 156.Liu J, Fu M, Wang M, Wan D, Wei Y, Wei X. Cancer vaccines as promising immuno-therapeutics: platforms and current progress: J Hematol Oncol. 2022. 10.1186/s13045-022-01247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Russell SJ. Peng K-W. Oncolytic virotherapy: a contest between apples and oranges: Mol Ther. 2017. 10.1016/j.ymthe.2017.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Holloway RW, Mendivil AA, Kendrick JE, Abaid LN, Brown JV, LeBlanc J et al. Clinical activity of olvimulogene nanivacirepvec-primed immunochemotherapy in heavily pretreated patients with platinum-resistant or platinum-refractory ovarian cancer: the nonrandomized phase 2 VIRO-15 clinical trial: JAMA oncology. 2023; 10.1001/jamaoncol.2023.1007. [DOI] [PMC free article] [PubMed]
- 159.Lee K-W, Yam JWP, Mao X. Dendritic cell vaccines: a shift from conventional approach to new generations: Cells. 2023. 10.3390/cells12172147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fujita K, Ikarashi H, Takakuwa K, Kodama S, Tokunaga A, Takahashi T et al. Prolonged disease-free period in patients with advanced epithelial ovarian cancer after adoptive transfer of tumor-infiltrating lymphocytes: Clin Cancer Res. 1995. [PubMed]
- 161.Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors: Curr Opin Immunol. 2009. 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang X-W, Wu Y-S, Xu T-M. Cui M-H. CAR-T cells in the treatment of ovarian cancer: a promising cell therapy: Biomolecules. 2023. 10.3390/biom13030465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Moore KN, Bouberhan S, Hamilton EP, Liu JF, O’Cearbhaill RE, O’Malley DM et al. First-in-human phase 1/2 study of ubamatamab, a MUC16xCD3 bispecific antibody, administered alone or in combination with cemiplimab in patients with recurrent ovarian cancer: J Clin Oncol. 2023; 10.1200/JCO.2023.41.16_suppl.TPS5624.
- 164.Winer IS, Shields AF, Yeku OO, Liu JF, Peterman MJ, Yoo SY et al. A phase I/II, multicenter, open-label study of REGN5668 (mucin [MUC]16 x CD28 bispecific antibody [bsAb]) with cemiplimab (programmed death [PD]-1 Ab) or REGN4018 (MUC16 x CD3 bsAb) in recurrent ovarian cancer (rOVCA): J Clin Oncol. 2021; 10.1200/JCO.2021.39.15_suppl.TPS5602.
- 165.Klein C, Brinkmann U, Reichert JM, Kontermann RE. The present and future of bispecific antibodies for cancer therapy: Nat Rev Drug Discov. 2024. 10.1038/s41573-024-00896-6. [DOI] [PubMed] [Google Scholar]
- 166.DiSilvestro P, Banerjee S, Colombo N, Scambia G, Kim B-G, Oaknin A et al. Overall survival with maintenance olaparib at a 7-year follow-up in patients with newly diagnosed advanced ovarian cancer and a BRCA mutation: the SOLO1/GOG 3004 trial: J Clin Oncol. 2023; 10.1200/JCO.22.01549. [DOI] [PMC free article] [PubMed]
- 167.Poveda A, Floquet A, Ledermann JA, Asher R, Penson RT, Oza AM et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial: Lancet Oncol. 2021; 10.1016/S1470-2045(21)00073-5. [DOI] [PubMed]
- 168.Friedlander M, Matulonis U, Gourley C, Du Bois A, Vergote I, Rustin G et al. Long-term efficacy, tolerability and overall survival in patients with platinum-sensitive, recurrent high-grade serous ovarian cancer treated with maintenance olaparib capsules following response to chemotherapy: Br J Cancer. 2018; 10.1038/s41416-018-0271-y. [DOI] [PMC free article] [PubMed]
- 169.Penson RT, Valencia RV, Cibula D, Colombo N, Leath CA, Bidziński M et al. Olaparib versus nonplatinum chemotherapy in patients with platinum-sensitive relapsed ovarian cancer and a germline BRCA1/2 mutation (SOLO3): a randomized phase III trial: J Clin Oncol. 2020; 10.1200/JCO.19.02745. [DOI] [PMC free article] [PubMed]
- 170.The ASCO Post Staff. NOVA: final analysis confirms no significant overall survival benefit for maintenance niraparib in recurrent ovarian cancer. 2023. https://ascopost.com/issues/may-25-2023/nova-final-analysis-confirms-no-significant-overall-survival-benefit-for-maintenance-niraparib-in-recurrent-ovarian-cancer/. Accessed 15 Apr 24.
- 171.Mirza MR, Monk BJ, Herrstedt J, Oza AM, Mahner S, Redondo A et al. Niraparib maintenance therapy in platinum-sensitive, recurrent ovarian cancer: N Engl J Med. 2016; 10.1056/NEJMoa1611310. [DOI] [PubMed]
- 172.González-Martín A, Pothuri B, Vergote I, Graybill W, Lorusso D, McCormick CC et al. Progression-free survival and safety at 3.5years of follow-up: results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer: Eur J Cancer. 2023; 10.1016/j.ejca.2023.04.024. [DOI] [PubMed]
- 173.Monk BJ, Parkinson C, Lim MC, O’Malley DM, Oaknin A, Wilson MK et al. A randomized, phase III Trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45): J Clin Oncol. 2022; 10.1200/JCO.22.01003. [DOI] [PMC free article] [PubMed]
- 174.Oza AM, Lorusso D, Aghajanian C, Oaknin A, Dean A, Colombo N et al. Patient-centered outcomes in ARIEL3, a phase III, randomized, placebo-controlled trial of rucaparib maintenance treatment in patients with recurrent ovarian carcinoma: J Clin Oncol. 2020; 10.1200/JCO.19.03107. [DOI] [PMC free article] [PubMed]
- 175.Kristeleit R, Lisyanskaya A, Fedenko A, Dvorkin M, Melo AC de, Shparyk Y et al. Rucaparib versus standard-of-care chemotherapy in patients with relapsed ovarian cancer and a deleterious BRCA1 or BRCA2 mutation (ARIEL4): an international, open-label, randomised, phase 3 trial: Lancet Oncol. 2022; 10.1016/S1470-2045(22)00122-X. [DOI] [PubMed]
- 176.Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD,Friedlander M, et al. Veliparib with first-line chemotherapy and as maintenance therapy in ovarian cancer: N Engl J Med. 2019. 10.1056/NEJMoa1909707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Li N, Zhang Y, Wang J, Zhu J, Wang L, Wu X et al. Fuzuloparib maintenance therapy in patients with platinum-sensitive, recurrent ovarian carcinoma (FZOCUS-2): a multicenter, randomized, double-blind, placebo-controlled, phase III trial: J Clin Oncol. 2022; 10.1200/JCO.21.01511. [DOI] [PubMed]
- 178.The First Affiliated Hospital of Xiamen University. Maintenance therapy after platinum-containing chemotherapy in patients with recurrent ovarian cancer - Full Text View - ClinicalTrials.gov. 2024. https://clinicaltrials.gov/study/NCT06188455. Accessed 15 Apr 24.
- 179.Wu X, Liu J, Wang X, Wang J, Wang L, Zhu J et al. LBA36 Efficacy and safety of senaparib as maintenance treatment in patients with newly diagnosed advanced ovarian cancer (FLAMES study): a randomized, double-blind, placebo-controlled, phase III trial: Ann Oncol. 2023; 10.1016/j.annonc.2023.10.030.
- 180.Ma D. Maintenance treatment with BGB-290 versus placebo in participants with platinum-sensitive recurrent ovarian cancer. 2018. https://clinicaltrials.gov/study/NCT03519230. Accessed 15 Apr 24.
- 181.Da Cunha Colombo Bonadio RR, Fogace RN, Miranda VC, Del Diz MPE. Homologous recombination deficiency in ovarian cancer: a review of its epidemiology and management: Clinics (Sao Paulo, Brazil). 2018; 10.6061/clinics/2018/e450s. [DOI] [PMC free article] [PubMed]
- 182.Hoppe MM, Sundar R, Tan DSP, Jeyasekharan AD. Biomarkers for homologous recombination deficiency in cancer: J Natl Cancer Inst. 2018. 10.1093/jnci/djy085. [DOI] [PubMed] [Google Scholar]
- 183.Rempel E, Kluck K, Beck S, Ourailidis I, Kazdal D, Neumann O et al. Pan-cancer analysis of genomic scar patterns caused by homologous repair deficiency (HRD): NPJ Precis Oncol. 2022; 10.1038/s41698-022-00276-6. [DOI] [PMC free article] [PubMed]
- 184.Mangogna A, Munari G, Pepe F, Maffii E, Giampaolino P,Ricci G, et al. Homologous recombination deficiency in ovarian cancer: from the biological rationale to current diagnostic approaches: J Pers Med. 2023. 10.3390/jpm13020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Biau J, Chautard E, Verrelle P, Dutreix M. Altering DNA repair to improve radiation therapy: specific and multiple pathway targeting: Front Oncol. 2019. 10.3389/fonc.2019.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Arora S, Balasubramaniam S, Zhang H, Berman T, Narayan P,Suzman D, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer: Oncologist. 2021. 10.1002/onco.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.FDA. FDA approves niraparib for first-line maintenance of advanced ovarian cancer. 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-first-line-maintenance-advanced-ovarian-cancer. Accessed 16 Apr 24.
- 188.FDA. FDA approves olaparib tablets for maintenance treatment in ovarian cancer. 2017. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-tablets-maintenance-treatment-ovarian-cancer. Accessed 16 Apr 24.
- 189.FDA. FDA approves rucaparib for maintenance treatment of recurrent ovarian, fallopian tube, or primary peritoneal cancer. 2018. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-rucaparib-maintenance-treatment-recurrent-ovarian-fallopian-tube-or-primary-peritoneal. Accessed 16 Apr 24.
- 190.Coleman RL, Oza A, Lorusso D, Aghajanian C, Oaknin A, Dean A et al. O003/#557 Overall survival results from ARIEL3: a phase 3 randomized, double-blind study of rucaparib vs placebo following response to platinum-based chemotherapy for recurrent ovarian carcinoma: Int J Gynecol Cancer. 2022; 10.1136/ijgc-2022-igcs.5.
- 191.Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the ENGOT-OV16/NOVA trial: J Clin Oncol. 2019;10.1200/JCO.18.02238. [DOI] [PMC free article] [PubMed]
- 192.Flaherty C. Restricted indication for maintenance niraparib therapy in recurrent ovarian cancer further supported by final survival analysis of NOVA trial. 2023. https://www.onclive.com/view/restricted-indication-for-maintenance-niraparib-therapy-in-recurrent-ovarian-cancer-further-supported-by-final-survival-analysis-of-nova-trial. Accessed 16 Apr 24.
- 193.Mirza MR, Herrstedt J, Oza A, Mahner S, Redondo A, Berton D et al. #161 Final overall survival and long-term safety in the ENGOT-OV16/NOVA phase 3 trial of niraparib in patients with recurrent ovarian cancer. In: A15-A16.
- 194.Matulonis U, Herrstedt J, Oza A, Mahner S, Redondo A, Berton D et al. Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer: Gynecol Oncol. 2021; 10.1016/S0090-8258(21)00693-4.
- 195.Penson R, Valencia RV, Colombo N, Leath C, Bidzinski M, Kim J-W et al. Final overall survival results from SOLO3: phase III trial assessing olaparib monotherapy versus non-platinum chemotherapy in heavily pretreated patients with germline BRCA1 - and/or BRCA2-mutated platinum-sensitive relapsed ovarian cancer (026): Gynecol Oncol. 2022; 10.1016/S0090-8258(22)01244-6.
- 196.Lee J-Y, Lee Y-Y, Park J-Y, Shim S-H, Kim SI, Kong T-W et al. Major clinical research advances in gynecologic cancer in 2022: highlight on late-line PARP inhibitor withdrawal in ovarian cancer, the impact of ARIEL-4, and SOLO-3: J Clin Oncol. 2023; 10.3802/jgo.2023.34.e51. [DOI] [PMC free article] [PubMed]
- 197.Moore KN, Secord AA, Geller MA, Miller DS, Cloven N, Fleming GF et al. Niraparib monotherapy for late-line treatment of ovarian cancer (QUADRA): a multicentre, open-label, single-arm, phase 2 trial: Lancet Oncol. 2019; 10.1016/S1470-2045(19)30029-4. [DOI] [PubMed]
- 198.Luik S. Dear Health Care Provider Letter (Niraparib) In: GSK. 2022. https://www.zejulahcp.com/content/dam/cf-pharma/hcp-zejulahcp-v2/en_US/pdf/ZEJULA%20(niraparib)%20Dear%20HCP%20Letter%20November%202022.pd. Accessed 03 Jul 24.
- 199.Lee A. Fuzuloparib: first approval: Drugs. 2021; 10.1007/s40265-021-01541-x. [DOI] [PMC free article] [PubMed]
- 200.Zheng J, Li Z, Min W. Current status and future promise of next-generation poly (ADP-ribose) polymerase 1-selective inhibitor AZD5305: Front Pharmacol. 2022; 10.3389/fphar.2022.979873. [DOI] [PMC free article] [PubMed]
- 201.Ngoi NYL, Leo E, O’Connor MJ, Yap TA. Development of next-generation poly(ADP-ribose) polymerase 1-selective inhibitors: Cancer J. 2021; 10.1097/PPO.0000000000000556. [DOI] [PubMed]
- 202.Curtin NJ, Szabo C. Poly(ADP-ribose) polymerase inhibition: past, present and future: Nat Rev Drug Discov. 2020; 10.1038/s41573-020-0076-6. [DOI] [PubMed]
- 203.Jones BA, Varambally S, Arend RC. Histone methyltransferase EZH2: a therapeutic target for ovarian cancer: Mol Cancer Ther. 2018; 10.1158/1535-7163.MCT-17-0437. [DOI] [PMC free article] [PubMed]
- 204.Garg V. Oza AM. Treatment of ovarian cancer beyond PARP inhibition: current and future options: Drugs. 2023. 10.1007/s40265-023-01934-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Murai J, Pommier Y. PARP trapping beyond homologous recombination and platinum sensitivity in cancers: Annu. Rev Cancer Biol. 2019. 10.1146/annurev-cancerbio-030518-055914. [Google Scholar]
- 206.Csizmar CM, Saliba AN, Swisher EM, Kaufmann SH. PARP inhibitors and myeloid neoplasms: a double-edged sword: Cancers. 2021. 10.3390/cancers13246385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Zatreanu D, Robinson HMR, Alkhatib O, Boursier M, Finch H,Geo L, et al. Polθ inhibitors elicit BRCA-gene synthetic lethality and target PARP inhibitor resistance: Nat Commun. 2021. 10.1038/s41467-021-23463-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Zhou J, Gelot C, Pantelidou C, Li A, Yücel H, Davis RE et al. A first-in-class polymerase theta inhibitor selectively targets homologous-recombination-deficient tumors: Nature cancer. 2021; 10.1038/s43018-021-00203-x. [DOI] [PMC free article] [PubMed]
- 209.Grisham RN. A study combining the peposertib (M3814) pill with standard chemotherapy in patients with ovarian cancer with an expansion in high grade serous ovarian cancer and low grade serous ovarian cancer. 2020. https://clinicaltrials.gov/study/NCT04092270. Accessed 16 Apr 24.
- 210.Zimmer J, Tacconi EMC, Folio C, Badie S, Porru M, Klare K et al. Targeting BRCA1 and BRCA2 deficiencies with G-quadruplex-interacting compounds: Mol Cell. 2016; 10.1016/j.molcel.2015.12.004. [DOI] [PMC free article] [PubMed]
- 211.Cadzow L, Gokhale PC, Ganapathy S, Sullivan P, Nayak S, Shenker S et al. KSQ-4279, a first-in-class USP1 inhibitor shows strong combination activity in BRCA mutant cancers with intrinsic or acquired resistance to PARP inhibitors: Eur J Cancer. 2022; 10.1016/S0959-8049(22)00900-5.
- 212.Chandrasekaran A. Elias KM. Synthetic lethality in ovarian cancer: Mol Cancer Ther. 2021. 10.1158/1535-7163.MCT-21-0500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Han Q, Huang B, Huang Z, Cai J, Gong L,Zhang Y, et al. Tumor cell-fibroblast heterotypic aggregates in malignant ascites of patients with ovarian cancer: Int J Mol Med. 2019. 10.3892/ijmm.2019.4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Faleiro-Rodrigues C, Macedo-Pinto I, Pereira D, Lopes CS. Prognostic value of E-cadherin immunoexpression in patients with primary ovarian carcinomas: Ann Oncol. 2004. 10.1093/annonc/mdh387. [DOI] [PubMed] [Google Scholar]
- 215.Dongre A. Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer: Nat Rev Mol Cell Biol. 2019. 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- 216.Rocconi RP, Grosen EA, Ghamande SA, Chan JK, Barve MA, Oh J et al. Gemogenovatucel-T (Vigil) immunotherapy as maintenance in frontline stage III/IV ovarian cancer (VITAL): a randomised, double-blind, placebo-controlled, phase 2b trial: Lancet Oncol. 2020; 10.1016/S1470-2045(20)30533-7. [DOI] [PubMed]
- 217.Pefani D-E, Pankova D, Abraham AG, Grawenda AM, Vlahov N, Scrace S et al. TGF-β targets the hippo pathway scaffold RASSF1A to facilitate YAP/SMAD2 nuclear translocation: Mol Cell. 2016; 10.1016/j.molcel.2016.05.012. [DOI] [PubMed]
- 218.Yoshimura A. Muto G. TGF-β function in immune suppression: Curr Top Microbiol Immunol. 2011. 10.1007/82_2010_87. [DOI] [PubMed] [Google Scholar]
- 219.Nemunaitis J. A trial of FANG™ vaccine for participants with ovarian cancer. 2011. https://clinicaltrials.gov/study/NCT01309230. Accessed 14 Apr 24.
- 220.Nemunaitis J. A trial of Vigil for participants with ovarian cancer (VITAL). 2015. https://clinicaltrials.gov/study/NCT02346747. Accessed 14 Apr 24.
- 221.Rocconi RP, Monk BJ, Walter A, Herzog TJ, Galanis E, Manning L et al. Gemogenovatucel-T (Vigil) immunotherapy demonstrates clinical benefit in homologous recombination proficient (HRP) ovarian cancer: Gynecol Oncol. 2021; 10.1016/j.ygyno.2021.03.009. [DOI] [PubMed]
- 222.Farley JH. Cabozantinib-S-malate in treating patients with recurrent or progressive ovarian, fallopian tube, or primary peritoneal cancer. 2015. https://www.clinicaltrials.gov/study/NCT02315430. Accessed 09 Apr 24.
- 223.Matulonis UA. Cabozantinib or paclitaxel in treating patients with persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cavity cancer. 2012. https://www.clinicaltrials.gov/study/NCT01716715. Accessed 17 Apr 24.
- 224.Konstantinopoulos PA, Brady WE, Farley J, Armstrong A, Uyar DS, Gershenson DM. Phase II study of single-agent cabozantinib in patients with recurrent clear cell ovarian, primary peritoneal or fallopian tube cancer (NRG-GY001): Gynecol Oncol. 2018; 10.1016/j.ygyno.2018.04.572. [DOI] [PMC free article] [PubMed]
- 225.Matulonis UA, Sill MW, Makker V, Mutch DG, Carlson JW, Darus CJ et al. A randomized phase II study of cabozantinib versus weekly paclitaxel in the treatment of persistent or recurrent epithelial ovarian, fallopian tube or primary peritoneal cancer: an NRG Oncology/Gynecologic Oncology Group study: Gynecol Oncol. 2019; 10.1016/j.ygyno.2018.12.008. [DOI] [PMC free article] [PubMed]
- 226.The First Affiliated Hospital of Xiamen University. Maintenance therapy after platinum-containing chemotherapy in patients with recurrent ovarian cancer. 2024. https://clinicaltrials.gov/study/NCT03170960. Accessed 02 Jul 24.
- 227.King J. A phase II trial of cabozantinib with patients with refractory GCTs. 2021. https://www.clinicaltrials.gov/study/NCT04876456. Accessed 01 Jun 24.
- 228.Chuang H-H, Zhen Y-Y, Tsai Y-C, Chuang C-H, Hsiao M,Huang M-S, et al. FAK in cancer: from mechanisms to therapeutic strategies: Int J Mol Sci. 2022. 10.3390/ijms23031726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications: Mol Cancer. 2019; 10.1186/s12943-019-1090-3. [DOI] [PMC free article] [PubMed]
- 230.Liu T, Zhang L, Joo D. Sun S-C. NF-κB signaling in inflammation: Sig Transduct Target Ther. 2017. 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dizon DS, Blessing JA, Penson RT, Drake RD, Walker JL, Johnston CM et al. A phase II evaluation of belinostat and carboplatin in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube, or primary peritoneal carcinoma: a Gynecologic Oncology Group study: Gynecol Oncol. 2012; 10.1016/j.ygyno.2012.02.019. [DOI] [PMC free article] [PubMed]
- 232.Dizon DS, Damstrup L, Finkler NJ, Lassen U, Celano P, Glasspool R et al. Phase II activity of belinostat (PXD-101), carboplatin, and paclitaxel in women with previously treated ovarian cancer: International Journal of Gynecologic Cancer. 2012; 10.1097/IGC.0b013e31825736fd. [DOI] [PubMed]
- 233.Guo F. Wang H. Potential of histone deacetylase inhibitors for the therapy of ovarian cancer: Front Oncol. 2022. 10.3389/fonc.2022.1057186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.European Medicines Agency. Removab | European Medicines Agency. 2024. https://www.ema.europa.eu/en/medicines/human/EPAR/removab. Accessed 14 Apr 24.
- 235.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO,Ivanchenko VV, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial: Int J Cancer. 2010. 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Baumann K, Pfisterer J, Wimberger P, Burchardi N, Kurzeder C,Du Bois A, et al. Intraperitoneal treatment with the trifunctional bispecific antibody Catumaxomab in patients with platinum-resistant epithelial ovarian cancer: a phase IIA study of the AGO Study Group: Gynecol Oncol. 2011. 10.1016/j.ygyno.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 237.European Medicines Agency. Medicines for human use under evaluation | European Medicines Agency. 2024. https://www.ema.europa.eu/en/medicines/medicines-human-use-under-evaluation. Accessed 14 Apr 24.
- 238.Sun H, Zhang X, Sun D, Jia X, Xu L, Qiao Y et al. COX-2 expression in ovarian cancer: an updated meta-analysis: Oncotarget. 2017; 10.18632/oncotarget.21538. [DOI] [PMC free article] [PubMed]
- 239.Legge F, Paglia A, D’Asta M, Fuoco G, Scambia G, Ferrandina G. Phase II study of the combination carboplatin plus celecoxib in heavily pre-treated recurrent ovarian cancer patients: BMC cancer. 2011. 10.1186/1471-2407-11-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Chung V. Cyclophosphamide with or without celecoxib in treating patients with recurrent or persistent ovarian epithelial, fallopian tube, or primary peritoneal cancer. 2003. https://clinicaltrials.gov/study/NCT00538031. Accessed 17 Apr 24.
- 241.Gupta R, Cristea M, Frankel P, Ruel C, Chen C, Wang Y et al. Randomized trial of oral cyclophosphamide versus oral cyclophosphamide with celecoxib for recurrent epithelial ovarian, fallopian tube, and primary peritoneal cancer: Cancer Treat Res Commun. 2019; 10.1016/j.ctarc.2019.100155. [DOI] [PMC free article] [PubMed]
- 242.Reyners AKL, Munck L de, Erdkamp FLG, Smit WM, Hoekman K, Lalisang RI et al. A randomized phase II study investigating the addition of the specific COX-2 inhibitor celecoxib to docetaxel plus carboplatin as first-line chemotherapy for stage IC to IV epithelial ovarian cancer, fallopian tube or primary peritoneal carcinomas: the DoCaCel study: Ann Oncol. 2012; 10.1093/annonc/mds107. [DOI] [PubMed]
- 243.Gómez-Valenzuela F, Wichmann I, Suárez F, Kato S, Ossandón E, Hermoso M et al. Cyclooxygenase-2 blockade is crucial to restore natural killer cell activity before anti-CTLA-4 therapy against high-grade serous ovarian cancer: Cancers. 2023; 10.3390/cancers16010080. [DOI] [PMC free article] [PubMed]
- 244.Banerjee S, Ottevanger PB, Sarivalasis A, Le Scodan R, Montes A, Kroep JR et al. LBA32 Principal results of the EORTC-1508 trial: a phase II randomised, multicentre study of bevacizumab vs atezolizumab and bevacizumab with acetylsalicylic acid or placebo in recurrent platinum-resistant ovarian, fallopian tube or primary peritoneal adenocarcinoma: Annals of Oncology. 2021; 10.1016/j.annonc.2021.08.2109.
- 245.Orr B, Mahdi H, Fang Y, Strange M, Uygun I, Rana M et al. Phase I trial combining chemokine-targeting with loco-regional chemoimmunotherapy for recurrent, platinum-sensitive ovarian cancer shows induction of CXCR3 ligands and markers of type 1 immunity: Clin Cancer Res. 2022; 10.1158/1078-0432.CCR-21-3659. [DOI] [PMC free article] [PubMed]
- 246.Ramanathan R, Choudry H, Jones H, Girgis M, Gooding W, Kalinski P et al. Phase II trial of adjuvant dendritic cell vaccine in combination with celecoxib, interferon-α, and rintatolimod in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal metastases: Ann Surg Oncol. 2021; 10.1245/s10434-020-09464-9. [DOI] [PMC free article] [PubMed]
- 247.Hosios AM. Manning BD. Cancer signaling drives cancer metabolism: AKT and the Warburg effect: Cancer Res. 2021. 10.1158/0008-5472.CAN-21-2647. [DOI] [PubMed] [Google Scholar]
- 248.Moldogazieva NT, Mokhosoev IM, Terentiev AA. Metabolic heterogeneity of cancer cells: an interplay between HIF-1, GLUTs, and AMPK: Cancers. 2020; 10.3390/cancers12040862. [DOI] [PMC free article] [PubMed]
- 249.Akins NS, Nielson TC, Le HV. Inhibition of glycolysis and glutaminolysis: an emerging drug discovery approach to combat cancer: Curr Top Med Chem. 2018. 10.2174/1568026618666180523111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cho H, Lee YS, Kim J, Chung J-Y, Kim J-H. Overexpression of glucose transporter-1 (GLUT-1) predicts poor prognosis in epithelial ovarian cancer: Cancer Invest. 2013; 10.3109/07357907.2013.849722. [DOI] [PubMed]
- 251.Duska LR, Krasner CN, O’Malley DM, Hays JL, Modesitt SC, Mathews CA et al. A phase Ib/II and pharmacokinetic study of EP0057 (formerly CRLX101) in combination with weekly paclitaxel in patients with recurrent or persistent epithelial ovarian, fallopian tube, or primary peritoneal cancer: Gynecol Oncol. 2021; 10.1016/j.ygyno.2020.12.025. [DOI] [PubMed]
- 252.Krasner CN, Birrer MJ, Berlin ST, Horowitz NS, Buss MK, Eliasof S et al. Phase II clinical trial evaluating CRLX101 in recurrent ovarian, tubal, and peritoneal cancer: J Clin Oncol. 2014; 10.1200/jco.2014.32.15_suppl.5581.
- 253.Penson R. CRLX101 in combination with bevacizumab for recurrent ovarian/tubal/peritoneal cancer. 2012. https://clinicaltrials.gov/study/NCT01652079. Accessed 17 Apr 24.
- 254.Banerji U, Dean EJ, Pérez-Fidalgo JA, Batist G, Bedard PL, You B et al. A phase I open-label study to identify a dosing regimen of the pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers: Clin Cancer Res. 2018; 10.1158/1078-0432.CCR-17-2260. [DOI] [PubMed]
- 255.Turner NC, Oliveira M, Howell SJ, Dalenc F, Cortes J,Gomez Moreno HL, et al. Capivasertib in hormone receptor-positive advanced breast cancer: N Engl J Med. 2023. 10.1056/NEJMoa2214131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Yap TA, Kristeleit R, Michalarea V, Pettitt SJ, Lim JSJ, Carreira S et al. Phase I trial of the PARP inhibitor olaparib and AKT inhibitor capivasertib in patients with BRCA1/2- and Non-BRCA1/2-mutant cancers: Cancer Discov. 2020; 10.1158/2159-8290.CD-20-0163. [DOI] [PMC free article] [PubMed]
- 257.Westin SN, Litton JK, Williams RA, Shepherd CJ, Brugger W, Pease EJ et al. Phase I trial of olaparib (PARP inhibitor) and vistusertib (mTORC1/2 inhibitor) in recurrent endometrial, ovarian and triple negative breast cancer: Journal of Clinical Oncology. 2018; 10.1200/JCO.2018.36.15_suppl.5504.
- 258.Westin SN. mTORC1/2 inhibitor AZD2014 or the oral AKT inhibitor AZD5363 for recurrent endometrial and ovarian. 2014. https://clinicaltrials.gov/study/NCT02208375. Accessed 17 Apr 24.
- 259.Guterres AN. Villanueva J Targeting telomerase for cancer therapy: Oncogene. 2020. 10.1038/s41388-020-01405-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Kim NW, Piatyszek MA, Prowse KR, Harley CB, West MD,Ho PL, et al. Specific association of human telomerase activity with immortal cells and cancer: Science. 1994. 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 261.Ali JH, Walter M. Combining old and new concepts in targeting telomerase for cancer therapy: transient, immediate, complete and combinatory attack (TICCA): Cancer Cell Int. 2023; 10.1186/s12935-023-03041-2. [DOI] [PMC free article] [PubMed]
- 262.Search for: imetelstat, Phase: 3 | List Results | ClinicalTrials.gov. 2024. Accessed 18 Apr 24. https://clinicaltrials.gov/study/.
- 263.Meng E, Taylor B, Ray A, Shevde LA, Rocconi RP. Targeted inhibition of telomerase activity combined with chemotherapy demonstrates synergy in eliminating ovarian cancer spheroid-forming cells: Gynecol Oncol. 2012. 10.1016/j.ygyno.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 264.Groelly FJ, Porru M, Zimmer J, Benainous H, Visser Y de, Kosova AA et al. Anti-tumoural activity of the G-quadruplex ligand pyridostatin against BRCA1/2-deficient tumours: EMBO Molecular Medicine. 2022; 10.15252/emmm.202114501. [DOI] [PMC free article] [PubMed]
- 265.Birch J. Gil J Senescence and the SASP: many therapeutic avenues: Genes & Development. 2020. 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Huang W, Hickson LJ, Eirin A, Kirkland JL, Lerman LO. Cellular senescence: the good, the bad and the unknown: Nat Rev Nephrol. 2022; 10.1038/s41581-022-00601-z. [DOI] [PMC free article] [PubMed]
- 267.de Blander H, Morel A-P, Senaratne AP, Ouzounova M, Puisieux A. Cellular plasticity: a route to senescence exit and tumorigenesis: Cancers. 2021. 10.3390/cancers13184561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Damen MPF, van Rheenen J, Scheele CLGJ. Targeting dormant tumor cells to prevent cancer recurrence: the FEBS Journal. 2021. 10.1111/febs.15626. [DOI] [PubMed] [Google Scholar]
- 269.Lam T, Aguirre-Ghiso JA, Geller MA, Aksan A, Azarin SM. Immobilization rapidly selects for chemoresistant ovarian cancer cells with enhanced ability to enter dormancy: Biotechnol Bioeng. 2020. 10.1002/bit.27479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Wong M, Tan N, Zha J, Peale FV, Yue P, Fairbrother WJ et al. Navitoclax (ABT-263) reduces Bcl-x(L)-mediated chemoresistance in ovarian cancer models: Mol Cancer Ther. 2012; 10.1158/1535-7163.MCT-11-0693. [DOI] [PubMed]
- 271.Shepherd TG. Dick FA. Principles of dormancy evident in high-grade serous ovarian cancer: Cell Division. 2022. 10.1186/s13008-022-00079-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.MacDonald J, Ramos-Valdes Y, Perampalam P, Litovchick L, DiMattia GE, Dick FA. A systematic analysis of negative growth control implicates the DREAM complex in cancer cell dormancy: Mol Cancer Res. 2017. 10.1158/1541-7786.MCR-16-0323-T. [DOI] [PubMed] [Google Scholar]
- 273.He S. Sharpless NE. Senescence in health and disease: Cell. 2017. 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Frąszczak K. Barczyński B. The role of cancer stem cell markers in ovarian cancer: Cancers. 2023. 10.3390/cancers16010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Shi Z-D, Pang K, Wu Z-X, Dong Y, Hao L,Qin J-X, et al. Tumor cell plasticity in targeted therapy-induced resistance: mechanisms and new strategies: Sig Transduct Target Ther. 2023. 10.1038/s41392-023-01383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Han X, Zhen S, Ye Z, Lu J, Wang L, Li P et al. A feedback loop between miR-30a/c-5p and DNMT1 mediates cisplatin resistance in ovarian cancer cells: Cell Physiol Biochem. 2017; 10.1159/000460618. [DOI] [PubMed]
- 277.Zhu X, Shen H, Yin X, Long L, Xie C, Liu Y et al. miR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin: Oncogene. 2016; 10.1038/onc.2015.84. [DOI] [PubMed]
- 278.Devapatla B, Sharma A, Woo S. CXCR2 inhibition combined with sorafenib improved antitumor and antiangiogenic response in preclinical models of ovarian cancer: PLOS ONE. 2015; 10.1371/journal.pone.0139237. [DOI] [PMC free article] [PubMed]
- 279.Da Costa PMS, Sales SLA, Pinheiro DP, Pontes LQ, Maranhão SS,Pessoa CdÓ, et al. Epigenetic reprogramming in cancer: from diagnosis to treatment: Front Cell Dev Biol. 2023. 10.3389/fcell.2023.1116805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Zhao H, Wu L, Yan G, Chen Y, Zhou M,Wu Y, et al. Inflammation and tumor progression: signaling pathways and targeted intervention: Sig Transduct Target Ther. 2021. 10.1038/s41392-021-00658-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Xie W, Sun H, Li X, Lin F, Wang Z, Wang X. Ovarian cancer: epigenetics, drug resistance, and progression: Cancer Cell Int. 2021; 10.1186/s12935-021-02136-y. [DOI] [PMC free article] [PubMed]
- 282.Kandettu A, Adiga D, Devi V, Suresh PS, Chakrabarty S,Radhakrishnan R, et al. Deregulated miRNA clusters in ovarian cancer: imperative implications in personalized medicine: Genes & Diseases. 2022. 10.1016/j.gendis.2021.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Petrillo M, Zannoni GF, Beltrame L, Martinelli E, DiFeo A,Paracchini L, et al. Identification of high-grade serous ovarian cancer miRNA species associated with survival and drug response in patients receiving neoadjuvant chemotherapy: a retrospective longitudinal analysis using matched tumor biopsies: Ann Oncol. 2016. 10.1093/annonc/mdw007. [DOI] [PubMed] [Google Scholar]
- 284.Pulliam N, Fang F, Ozes AR, Tang J, Adewuyi A,Keer H, et al. An effective epigenetic-PARP inhibitor combination therapy for breast and ovarian cancers independent of BRCA mutations: Clin Cancer Res. 2018. 10.1158/1078-0432.CCR-18-0204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Han Y, Wang Z, Sun S, Zhang Z, Liu J, Jin X et al. Decreased DHRS2 expression is associated with HDACi resistance and poor prognosis in ovarian cancer: Epigenetics. 2020; 10.1080/15592294.2019.1656155. [DOI] [PMC free article] [PubMed]
- 286.Lapinska K, Housman G, Byler S, Heerboth S, Willbanks A,Oza A, et al. The effects of histone deacetylase inhibitor and calpain inhibitor combination therapies on ovarian cancer cells: anticancer research. 2016. 10.21873/anticanres.11156. [DOI] [PubMed] [Google Scholar]
- 287.Huang Z, Zhou W, Li Y, Cao M, Wang T, Ma Y et al. Novel hybrid molecule overcomes the limited response of solid tumours to HDAC inhibitors via suppressing JAK1-STAT3-BCL2 signalling: Theranostics. 2018; 10.7150/thno.26627. [DOI] [PMC free article] [PubMed]
- 288.Helmink BA, Khan MAW, Hermann A, Gopalakrishnan V, Wargo JA. The microbiome, cancer, and cancer therapy: Nature medicine. 2019;10.1038/s41591-019-0377-7. [DOI] [PubMed]
- 289.Mehra Y, Chalif J, Mensah-Bonsu C, Spakowicz D, O’Malley DM, Chambers L. The microbiome and ovarian cancer: insights, implications, and therapeutic opportunities: J Cancer Metastasis Treat. 2023; 10.20517/2394-4722.2023.107.
- 290.Browning L, Patel MR, Horvath EB, Tawara K, Jorcyk CL. IL-6 and ovarian cancer: inflammatory cytokines in promotion of metastasis: CMAR. 2018; 10.2147/CMAR.S179189. [DOI] [PMC free article] [PubMed]
- 291.Hu X, Xu X, Zeng X, Jin R, Wang S,Jiang H, et al. Gut microbiota dysbiosis promotes the development of epithelial ovarian cancer via regulating Hedgehog signaling pathway: gut microbes. 2023. 10.1080/19490976.2023.2221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Choi S-Y. Choi J-H. Ovarian cancer and the microbiome: connecting the dots for early diagnosis and therapeutic innovations-a review: Medicina. 2024. 10.3390/medicina60030516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Chambers LM, Esakov Rhoades EL, Bharti R, Braley C, Tewari S,Trestan L, et al. Disruption of the gut microbiota confers cisplatin resistance in epithelial ovarian cancer: Cancer Res. 2022. 10.1158/0008-5472.CAN-22-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhao L-Y, Mei J-X, Yu G, Lei L, Zhang W-H,Liu K, et al. Role of the gut microbiota in anticancer therapy: from molecular mechanisms to clinical applications: Sig Transduct Target Ther. 2023. 10.1038/s41392-023-01406-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ogundipe OD, Olajubutu O, Adesina SK. Targeted drug conjugate systems for ovarian cancer chemotherapy: Biomed Pharmacother. 2023. 10.1016/j.biopha.2023.115151. [DOI] [PubMed] [Google Scholar]
- 296.Aloss K. Hamar P. Recent preclinical and clinical progress in liposomal doxorubicin: Pharmaceutics. 2023. 10.3390/pharmaceutics15030893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Onyido EK, James D, Garcia-Parra J, Sinfield J, Moberg A,Coombes Z, et al. Elucidating novel targets for ovarian cancer antibody-drug conjugate development: integrating in silico prediction and surface plasmon resonance to identify targets with enhanced antibody internalization capacity: Antibodies. 2023. 10.3390/antib12040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Dumontet C, Reichert JM, Senter PD, Lambert JM, Beck A. Antibody-drug conjugates come of age in oncology: Nat Rev Drug Discov. 2023. 10.1038/s41573-023-00709-2. [DOI] [PubMed] [Google Scholar]
- 299.Liu K, Li M, Li Y, Li Y, Chen Z, Tang Y et al. A review of the clinical efficacy of FDA-approved antibody–drug conjugates in human cancers: Mol Cancer. 2024; 10.1186/s12943-024-01963-7. [DOI] [PMC free article] [PubMed]
- 300.Ab O, Whiteman KR, Bartle LM, Sun X, Singh R, Tavares D et al. IMGN853, a folate receptor-α (FRα)-targeting antibody-drug conjugate, exhibits potent targeted antitumor activity against FRα-expressing tumors: Mol Cancer Ther. 2015; 10.1158/1535-7163.MCT-14-1095. [DOI] [PubMed]
- 301.Wang Z, Meng F, Zhong Z. Emerging targeted drug delivery strategies toward ovarian cancer: Adv Drug Deliv Rev. 2021. 10.1016/j.addr.2021.113969. [DOI] [PubMed] [Google Scholar]
- 302.Oza AM, Vergote IB, Gilbert LG, Lucy, Ghatage P, Lisyankaya A et al. A randomized double-blind phase III trial comparing vintafolide (EC145) and pegylated liposomal doxorubicin (PLD/Doxil®/Caelyx®) in combination versus PLD in participants with platinum-resistant ovarian cancer (PROCEED) (NCT01170650): Gynecol Oncol. 2015; 10.1016/j.ygyno.2015.01.010.
- 303.Matulonis UA, Lorusso D, Oaknin A, Pignata S, Dean A,Denys H, et al. Efficacy and safety of mirvetuximab soravtansine in patients with platinum-resistant ovarian cancer with high folate receptor alpha expression: results from the SORAYA study: J Clin Oncol. 2023.10.1200/JCO.22.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Moore KN, Oza AM, Colombo N, Oaknin A, Scambia G, Lorusso D et al. Phase III, randomized trial of mirvetuximab soravtansine versus chemotherapy in patients with platinum-resistant ovarian cancer: primary analysis of FORWARD I: Ann Oncol. 2021; 10.1016/j.annonc.2021.02.017. [DOI] [PubMed]
- 305.Moore KN, Angelergues A, Konecny GE, Banerjee SN, Pignata S, Colombo N et al. Phase III MIRASOL (GOG 3045/ENGOT-ov55) study: Initial report of mirvetuximab soravtansine vs. investigator’s choice of chemotherapy in platinum-resistant, advanced high-grade epithelial ovarian, primary peritoneal, or fallopian tube cancers with high folate receptor-alpha expression: J Clin Oncol. 2023; 10.1200/JCO.2023.41.17_suppl.LBA5507.
- 306.Hangzhou Zhongmei Huadong Pharmaceutical Co. A single-arm clinical trial of IMGN853 in Chinese adult patients with platinum-resistant, epithelial ovarian cancer. 2022. https://clinicaltrials.gov/study/NCT05622890. Accessed 14 Apr 24.
- 307.O’Malley DM, Myers TKN, Zamagni C, Diver E, Lorusso D. GLORIOSA: a randomized, open-label, phase 3 study of mirvetuximab soravtansine with bevacizumab vs. bevacizumab as maintenance in platinum-sensitive ovarian, fallopian tube, or primary peritoneal cancer: J Clin Oncol. 2023; 10.1200/JCO.2023.41.16_suppl.TPS5622.
- 308.Richardson DL, Harter P, O’Malley DM, Gonzalez Martin A, Herzog TJ, Rogalski C et al. UP-NEXT (GOG-3049/ENGOT-Ov71-NSGO-CTU): a study of upitifamab rilsodotin (UpRi), a NaPi2b-directed antibody drug conjugate (ADC), in platinum-sensitive recurrent ovarian cancer: J Clin Oncol. 2023; 10.1200/JCO.2023.41.16_suppl.TPS5614.
- 309.James D. Investigators initiate phase II/III REJOICE-Ovarian01 trial of raludotatug deruxtecan for platinum-resistant ovarian cancer. 2024. https://www.appliedclinicaltrialsonline.com/view/investigators-initiate-phase-ii-iii-rejoice-ovarian01-trial-of-raludotatug-deruxtecan-for-platinum-resistant-ovarian-cancer. Accessed 14 Apr 24.
- 310.Suzhou Suncadia Biopharmaceuticals Co. A study of SHR-A1921 with or without carboplatin in subjects with ovarian cancer. 2024. https://clinicaltrials.gov/study/NCT06211023. Accessed 14 Apr 24.
- 311.Banerjee S, Drapkin R, Richardson DL, Birrer M. Targeting NaPi2b in ovarian cancer: Cancer Treat Rev. 2023; 10.1016/j.ctrv.2022.102489. [DOI] [PubMed]
- 312.Conroy R. FDA pauses upifitamab rilsodotin trials in platinum-sensitive ovarian cancer. 2023. https://www.cancernetwork.com/view/fda-pauses-upifitamab-rilsodotin-trials-in-platinum-sensitive-ovarian-cancer. Accessed 07 Apr 24.
- 313.Shintani D, Hanaoka M, Sato S, Yano M, Ogasawara A, Kato T et al. Clinical significance of cadherin-6 expression in primary and recurrent epithelial ovarian cancer and its association with outcomes: a potential therapeutic target for epithelial ovarian cancer (206): Gynecol Oncol. 2022; 10.1016/S0090-8258(22)01432-9.
- 314.Hamilton EP, Jauhari S, Moore KN, Rini BI, McLeod R, Lin J et al. Phase I, two-part, multicenter, first-in-human (FIH) study of DS-6000a in subjects with advanced renal cell carcinoma (RCC) and ovarian tumors (OVC): J Clin Oncol. 2022; 10.1200/JCO.2022.40.16_suppl.3002.
- 315.Perrone E, Lopez S, Zeybek B, Bellone S, Bonazzoli E, Pelligra S et al. Preclinical activity of sacituzumab govitecan, an antibody-drug conjugate targeting trophoblast cell-surface antigen 2 (Trop-2) linked to the active metabolite of irinotecan (SN-38), in ovarian cancer: Front Oncol. 2020; 10.3389/fonc.2020.00118. [DOI] [PMC free article] [PubMed]
- 316.FDA. FDA grants regular approval to sacituzumab govitecan for triple-negative breast cancer. 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-sacituzumab-govitecan-triple-negative-breast-cancer. Accessed 15 Apr 24.
- 317.He N, Yang C, Yang Y, Xue Z, Xu J, Zhao L et al. Abstract LB030: SHR-A1921, a novel TROP-2 ADC with an optimized design and well-balanced profile between efficacy and safety: Cancer Res. 2023; 10.1158/1538-7445.AM2023-LB030.
- 318.Meissner V, Alatovic J. BioNTech and DualityBio receive FDA Fast Track designation for next-generation antibody-drug conjugate candidate BNT325/DB-1305. 2024. https://investors.biontech.de/news-releases/news-release-details/biontech-and-dualitybio-receive-fda-fast-track-designation-next. Accessed 15 Apr 24.
- 319.Zhuang W, Zhang W, Wang L, Xie L, Feng J, Zhang B et al. Generation of a novel SORT1×HER2 bispecific antibody-drug conjugate targeting HER2-low-expression tumor: Int J Mol Sci. 2023; 10.3390/ijms242216056. [DOI] [PMC free article] [PubMed]
- 320.Wu F, Gardinier TC, Turker MZ, Chen F, Chen P-M, Venkatesan AM, et al. Molecular engineering of surface functional groups enabling clinical translation of nanoparticle–drug conjugates: Chem. Mater. 2022. 10.1021/acs.chemmater.1c04447. [Google Scholar]
- 321.Johnston MC. Scott CJ. Antibody conjugated nanoparticles as a novel form of antibody drug conjugate chemotherapy: Drug Discov Today Technol. 2018. 10.1016/j.ddtec.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 322.Rodríguez F, Caruana P, La Fuente ND, Español P, Gámez M,Balart J, et al. Nano-based approved pharmaceuticals for cancer treatment: present and future challenges: Biomolecules. 2022. 10.3390/biom12060784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Ma WW, Tolcher AW, Strauss JF, Bekaii-Saab TS, Zhao Y, Perez CA et al. ELU-FRα-1: a study to evaluate ELU001 in patients with solid tumors that overexpress folate receptor alpha (FRα): J Clin Oncol. 2022; 10.1200/JCO.2022.40.16_suppl.TPS3158.
- 324.Spagnol G, Sensi F, Tommasi O de, Marchetti M, Bonaldo G, Xhindoli L et al. Patient derived organoids (PDOs), extracellular matrix (ECM), tumor microenvironment (TME) and drug screening: state of the art and clinical implications of ovarian cancer organoids in the era of precision medicine: Cancers. 2023; 10.3390/cancers15072059. [DOI] [PMC free article] [PubMed]
- 325.Liu X, Fang J, Huang S, Wu X, Xie X, Wang J et al. Tumor-on-a-chip: from bioinspired design to biomedical application: Microsyst Nanoeng. 2021; 10.1038/s41378-021-00277-8. [DOI] [PMC free article] [PubMed]
- 326.Wenger C, Miranda PC, Salvador R, Thielscher A, Bomzon Z, Giladi M et al. A review on tumor-treating fields (TTFields): clinical implications inferred from computational modeling: IEEE Rev Biomed Eng. 2018; 10.1109/RBME.2017.2765282. [DOI] [PubMed]
- 327.Sava J. Phase 3 Trial of TTFields and Paclitaxel Misses OS End Point in Ovarian Cancer. 2023. https://www.targetedonc.com/view/phase-3-trial-of-ttfields-and-paclitaxel-misses-os-end-point-in-ovarian-cancer. Accessed 19 May 24.
- 328.Du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO): Cancer. 2009; 10.1002/cncr.24149. [DOI] [PubMed]
- 329.Butler K, McBroom J. Gleolan for visualization of newly diagnosed or recurrent ovarian cancer (OVA-302). 2024. https://clinicaltrials.gov/study/NCT05804370. Accessed 19 Apr 24.
- 330.Hadjipanayis CG, Stummer W. 5-ALA and FDA approval for glioma surgery: J Neurooncol. 2019; 10.1007/s11060-019-03098-y. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data for this review were identified by searching ClinicalTrials.gov (1) using keywords “ovarian cancer” “female” accessed: 03/19/2024 and PubMed and references from relevant articles using the search terms like “ovarian cancer,” “hallmarks of cancer,” “treatment,” “targeted therapy,” “chemotherapy,” “PARP inhibition,” “immunotherapy,” and/or “antibody drug conjugates.”
No datasets were generated or analysed during the current study.