Abstract
Background
Adolescent females have a high prevalence of temporomandibular joint (TMJ) anterior disc displacement (ADD), which can lead to condylar resorption and dentofacial deformity. Polycystic ovarian syndrome (PCOS) is a common endocrine disorder that disrupts bone metabolism. However, the effects of PCOS on bone remodeling especially after disc repositioning (DR) surgery are not well understood.
Materials and methods
This was a retrospective study. Patients aged 12 to 20 years diagnosed with ADD were reviewed and matched into 3 groups: A (ADD without PCOS), B (ADD with untreated PCOS), and C (ADD with treated PCOS). Each group was divided into 2 subgroups according to ADD observation (A1, B1, C1) and DR (A2, B2, C2). Condylar height (CH) was measured by MRI at the start (T0) and after more than 6 months follow-up (T1). ∆CH (T1-T0) were compared within and between groups.
Results
93 patients (157 joints) with an average age of 15.17 ± 2.35 years and follow-up period of 14.04 ± 9.11 months were selected in the study. ∆CH in Group B1 was significantly larger than that in Groups A1 and C1 (p = 0.048, p = 0.018). While in Group B2, it was significant smaller than Groups A2 and C2 (p < 0.001, p = 0.023). There was no significant difference of ∆CH between Groups C2 and A2. DR acquired larger ∆CH than observation within each A, B, C Groups (p < 0.05). Multiple linear regression analysis showed that ∆CH was related to the presence of PCOS (p = 0.003), PCOS treatment (p < 0.001), and DR (p < 0.001).
Conclusions
Adolescent ADD with untreated PCOS can aggravate condylar degeneration and affect bone remodeling after DR. PCOS treatment can improve bone remodeling.
Keywords: Temporomandibular joint, Anterior disc displacement, Disc repositioning, Polycystic ovarian syndrome, Adolescent
Introduction
Anterior disc displacement (ADD) of the temporomandibular joint (TMJ) is common in adolescents [1] with a prevalence of 18-25% [2]. It is much higher in females than males, with females accounting for 80% of cases [3]. Research has shown that adolescent females have a threefold increased risk of developing ADD compared with other stages of life [4]. ADD is characterized by symptoms such as pain, popping, and restricted mouth opening [1, 5]. Specifically, individuals with ADD without reduction (ADDwoR) are at a higher risk of experiencing condylar resorption, which can contribute to the development or exacerbation of dentofacial deformities [6–8], including mandibular deviation, retrusion, and open bite during growth period [6–14]. Our previous studies have found that disc repositioning surgery (DR) can effectively promote condylar bone regeneration in adolescents and thus reduce dentofacial deformities [15–21], but the effect of bone regeneration may be affected by systemic factors.
Polycystic ovarian syndrome (PCOS) is one of the most common endocrine disorders in women, typically manifesting in early adolescence with a prevalence rate of 6-14% [22]. The primary symptoms include hyperandrogenism, oligomenorrhea/amenorrhea, or polycystic ovarian morphology, which can affect fertility [23]. Sidika et al. [24] and Hasmet et al. [25] have reported that the prevalence and intensity of TMJ disorders among adult patients with PCOS are notably elevated in comparison to the general female population (86% vs. 24%, P = 0.001). The pathogenesis of PCOS is hormonal imbalance, which consequently affects bone metabolism [26]. However, there is a lack of research on the effects of PCOS on condylar bone regeneration in adolescent ADD, especially after disc repositioning surgery.
The aim of the study was to examine the impact of PCOS on adolescent ADD bone remodeling by magnetic resonance imaging (MRI) measurement.
Materials and methods
Participants
This was a retrospective study which was designed following the Declaration of Helsinki and approved by the Ethics Committee of the Shanghai Ninth People’s Hospital (SH9H-2023-T294-1). Patients diagnosed with ADDwoR via MRI, who were treated at the TMJ specialist clinic of the hospital from February 2018 to February 2022 were reviewed. The inclusion criteria were as follows: [1] females aged 12–20 at initial diagnosis; [2] Wilkes stages III-V which are disc displacement with and without bone degeneration [27]; [3] duration of ADD (onset of ADD symptoms) ≥ 6 months; [4] patients who had not responded well to nonsurgical therapy for at least 6 months [28]; [5] complete MRI data; [6] normal menstruation or diagnosed with PCOS. The diagnosis of adolescent PCOS requires the following 2 criteria [23]: (a) oligomenorrhea persisting for at least 2 years after menarche or amenorrhea; (b) clinical manifestations of hyperandrogenism such as acne, hirsutism, and obesity [29] (Fig. 1) and/or laboratory hyperandrogenemia. Exclusion criteria included: [1] pregnancy; [2] history of TMJ surgery; [3] autoimmune and systemic diseases such as hyperprolactinemia, thyroid disease, malignancy and end-stage disease, etc.
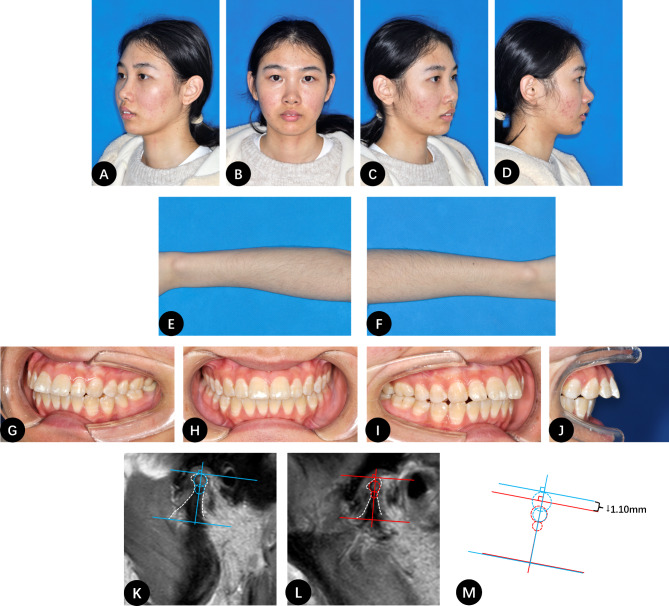
Fig. 1.
A 20-year-old female patient with untreated ADD and PCOS. A-D, Frontal and profile photographs of the patient showing the upper front teeth biting the lower lip and noticeable facial acne; E-F, Photographs of the patient’s forearms showing significant hair growth; G-J, Intraoral photographs showing a pronounced anterior open bite; K, MRI image of the right condyle with a 3-year history of ADD; L, Right condyle height decreased by 1.10 mm after 18 months of observation; M, Comparison of the condylar height between K and L. The blue line indicates the contour of the condyle at the initial visit, and the red line indicates the contour at follow-up
Patients were categorized into 3 groups: Group A, ADD with normal menstruation; Group B, ADD with untreated PCOS; Group C, ADD with treated PCOS. Each group was further divided into 2 subgroups as observation (A1, B1, and C1) or DR surgery (A2, B2, and C2). Group A was as a control group and patients were selected after matching the age, follow-up period and initial condylar height (CH) with the study Groups B and C by propensity match.
Sample selection
This was a retrospective study as a pilot study for the future prospective one. We selected ADD patients without PCOS as the control group by matching the age, follow-up period and the initial condylar height with the PCOS patients in the study group. Post-hoc calculation was used to estimate the sample size and analyze the power of the study.
Surgical procedures
Disc repositioning by open suturing as we previously reported [20] was performed under general anesthesia by a senior surgeon (D.H.). After complete release of the anterior disc attachment, the disc was repositioned and sutured to the posterior joint capsule by 3 − 0 nonabsorbable thread through the external auricular canal.
Treatment of PCOS
Patients were administered oral short-acting combined contraceptives (such as Diane-35) for 21 days, followed by a 5-day drug-free period. The treatment was continued for more than 3 months until the patient’s menstrual cycles returned to normal.
CH measurement
TMJ MRI (1.5-Tesla imager, Signa; General Electric, Milwaukee, WI, USA) was obtained for all the patients at their initial visit or before DR (T0) and more than 6 months follow-up (T1). Three-circle method was used to measure CH in the sagittal plane with the largest sectional area of condyle [20]. In order to avoid subjective bias, we randomly assigned MRI images to two doctors (C.W. and C.L.) without patient information for evaluation. Measurements were repeated after two weeks, and the average values of the two clinicians were used if their evaluations were consistent. If a paired t-test revealed a statistical difference (P < 0.05) between the two sets of measurements and if evaluations were inconsistent, a TMJ specialist was consulted for repeated measurement. After data collection was completed, the data analyst (J.S.) unblinded the patient information and conducted the statistical analysis.
Statistical analysis
SPSS 25.0 software (IBM Corp., Armonk, NY, USA) was used for data analysis. Continuous variables with normal distribution were expressed as mean ± standard deviation; non-normally distributed variables were expressed as median (interquartile range). Baseline data including age, ADD duration, follow-up period and CH at T0 among the 6 groups were matched without significantly difference. Intra-group differences of CH were compared by Paired t-tests for normally distributed variables and Wilcoxon tests for non-normally distributed variables. Also ΔCH was compared by Student t-tests for normally distributed variables and Mann-Whitney U tests for non-normally distributed variables between A1 and B1, A2 and B2, B1 and C1, B2 and C2 to assess the effect of PCOS and PCOS after treatment on observation and DR. Collinearity analysis was conducted to identify influencing factors including age, ADD treatment method, presence and treatment of PCOS, duration of follow-up, ADD duration, and Wilkes stage of ΔCH. Multiple linear regression analysis was used to clarify the correlation between these influencing factors and ΔCH. P < 0.05 was considered statistically significant. At the end of the study, we conducted post-hoc power and sample size calculations by G*Power 3 software (Version 3.1.9.7) to guide interpretation of our findings [30].
Results
Sample selection and group description
During 2018 to 2022, there were 262 female patients (393 joints) diagnosed with ADDwoR via MRI after screening. Among them, 36 patients (59 joints) who had PCOS were selected as a study group. There were 20 patients (35 joints) in Group B and 16 (24 joints) in Group C. From the other 126 patients without PCOS, we chose 57 patients (98 joints) after matching the age, follow-up period and initial condylar height as the control Group A. A total of 93 patients with 157 joints were included in the study. Their average age was 15.17 ± 2.35 years (12–20 years) and the average follow-up period was 14.04 ± 9.11 months (6–54 months, Table 1).
Table 1.
Subgroup Information
| Group | A | B | C | P1 | |||
|---|---|---|---|---|---|---|---|
| A1 | A2 | B1 | B2 | C1 | C2 | ||
| Number of patients (joints) | 20 (34) | 37 (64) | 11 (19) | 9 (16) | 7 (9) | 9 (15) | |
| Age (years) | 14.70 ± 2.32 | 15.11 ± 2.21 | 16.00 ± 3.23 | 16.33 ± 2.55 | 14.29 ± 1.60 | 15.00 ± 1.87 | 0.371 |
| Wilkes stage III (%) | 21 (61.76) | 55 (85.94) | 8 (42.11) | 11 (68.75) | 4 (44.44) | 14 (93.33) | <0.001 |
| Wilkes stage IV (%) | 13 (38.24) | 9 (14.06) | 11 (57.89) | 5 (31.25) | 5 (55.56) | 1 (6.67) | |
| ADD duration (months) | 22.59 ± 18.81 | 21.98 ± 19.35 | 27.32 ± 18.99 | 28.13 ± 16.02 | 26.00 ± 22.18 | 22.40 ± 15.79 | 0.953 |
| FU (months) | 11.71 ± 7.31 | 14.63 ± 5.61 | 11.79 ± 6.24 | 20.00 ± 17.17 | 14.89 ± 15.36 | 13.00 ± 5.48 | 0.506 |
| T0 CH (mm) | 21.30 (16.08, 23.37) $ | 19.36 ± 3.74 | 18.89 ± 3.04 | 18.31 ± 3.15 | 21.34 ± 4.11 | 19.31 ± 4.09 | 0.286 |
| T1 CH (mm) | 19.13 ± 3.66$ | 21.87 ± 4.28 | 17.51 ± 3.00 | 18.65 ± 4.10 | 21.19 ± 3.84 | 21.97 ± 3.32 | <0.001 |
| ΔCH (mm) | -0.80 (-1.43, -0.18)!&a% | 2.51 ± 1.19!^c% | -1.31 ± 1.62@&b% | 1.40 (-0.13, 1.98)@^d% | -0.16 ± 0.76#ab% | 2.66 ± 1.27#cd% | <0.001 |
| P2 | <0.001$* | <0.001* | 0.002* | 0.709 | 0.556 | <0.001* | |
FU: Follow up. P1: comparison of 6 groups; P2: T0 vs. T1; !P<0.001; @P = 0.002; #P<0.001; &P = 0.048; aP = 0.032; bP = 0.018; ^P<0.001; cP = 0.665; dP = 0.023. $Wilcoxon tst; %Mann-Whitney U test. *P<0.05
Comparison between test and control groups
CH at T0 and T1 was compared within each group and ΔCH was compared among the 6 groups. At T1, CH decreased in observation groups (A1, B1 and C1, Figs. 1 and 2), but increased in DR groups (A2, B2 and C2, Fig. 2). The changes were significant in A1 (-0.80 mm, -1.43~-0.18, P < 0.001), B1 (-1.31 ± 1.62 mm, P < 0.002), A2 (2.51 ± 1.19 mm, P < 0.001) and C2 (2.66 ± 1.27 mm, P < 0.001) groups. Among the observation groups, ΔCH in B1 was significantly larger than A1 and C1 (P = 0.048, P = 0.018). The difference of ΔCH between A1 and C1 was significant (P = 0.032). Among the DR groups, ΔCH in B2 was significantly less than A2 and C2 (P < 0.001, P = 0.023, Fig. 2). There was no significant difference of ΔCH between A2 and C2 (P = 0.665, Tables 1 and 2).
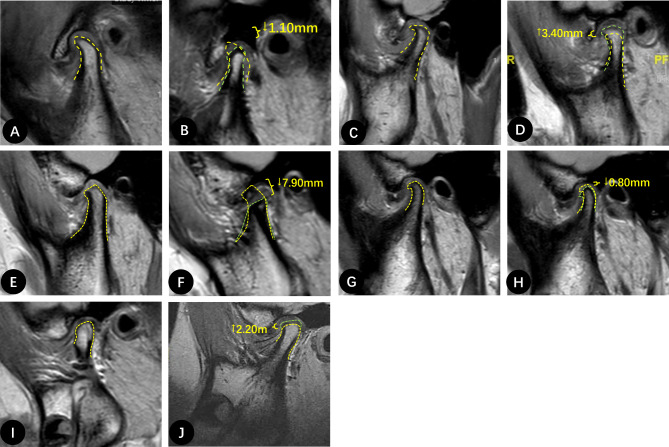
Fig. 2.
MRI measurements of typical participants in each group: A-B, a 14-year-old female from Group A1. A, MRI image of the left condyle with a 1-year history of ADD. B, 1.10 mm decrease in left CH observed 8 months later. C-D, a 14-year-old female from Group A2. C, MRI image of the condyle with a 1-year history of ADD. D, 3.40 mm increase in CH 13 months after DR. E-F, a 20-year-old female from Group B2. E, MRI image of the right condyle with a 1-year history of ADD. F, 7.90 mm decrease in right CH and signal intensity of the bone marrow decreased 40 months after DR. G-H, a 15-year-old female from Group C1. G, MRI image of the right condyle with a 1-year history of ADD. H, right CH decreased by 0.80 mm after 10 months of observation. I-J, a 12-year-old female from Group C2. I, MRI images of a condyle with a 1-year history of ADD. J, CH increased by 3.60 mm with significant new bone formation 14 months after DR. The yellow line indicates the contour of the condyle at the initial visit or pre-surgery, and the green line indicates the position of the new bone at follow-up
Table 2.
Univariate analysis of factors influencing ΔCH
| Age | ADD duration | FU | Wilkes stage | Disease# | DR | ||
|---|---|---|---|---|---|---|---|
| △CH | r | -0.017 | -0.027 | -0.192 | -0.245 | 0.230 | 0.778 |
| P | 0.938 | 0.736 | 0.016* | 0.002* | 0.004* | <0.001* |
FU: Follow up. #Disease:1 = group B, 2 = group C, 3 = group A. *P<0.05
Correlation analysis
Univariate analysis showed that ΔCH was correlated with Wilkes stage, follow-up period, disease grouping, and treatment method (observation vs. DR) (P < 0.05), while not with age and ADD duration (P > 0.05, Table 2). Among them, the Wilkes stage had a negative correlation with ΔCH (P = 0.002), indicating that the more severe the disease, the greater the difference in CH changes. The follow-up period (P = 0.016), disease grouping (P = 0.004), and treatment method (P < 0.001) were positively correlated with ΔCH, indicating that longer follow-up times, PCOS treatment, and DR were associated with increased ΔCH.
Multiple regression analysis
A multiple linear regression model was constructed, incorporating Wilkes stage, disease grouping, follow-up period, and treatment method. The model showed a significant correlation (R²=0.459, R²>0.1 indicating a meaningful correlation) between ΔCH and several factors, which were the presence of PCOS (B=-1.251, P = 0.003), PCOS treatment (B = 1.640, P < 0.001), DR (B = 3.069, P < 0.001), and follow-up period (B=-0.083, P < 0.001, Table 3).
Table 3.
Linear regression analyses of factors influencing ΔCH
| Factors | B | SE | β | P |
|---|---|---|---|---|
| Wilkes stage(IV) | -0.343 | 0.384 | -0.058 | 0.372 |
| Disease(ADD + PCOS treated) | 1.640 | 0.460 | 0.251 | <0.001* |
| Disease(ADD + PCOS untreated) | -1.251 | 0.409 | -3.062 | 0.003* |
| ADD treatment(DR) | 3.069 | 0.355 | 8.647 | <0.001* |
| Duration of FU | -0.083 | 0.019 | -0.273 | <0.001* |
FU: Follow up. SE, standard error
Power and sample size calculation
Post-hoc power analysis (including A, B, C three groups’ joint number and ΔCH) showed that the power of this study was 98.36%. The 157 study joints were more than the estimated 123 sample size (effect size f = 0.36 and power of 95%).
Discussion
In adolescents with ADDwoR, research indicated a significantly elevated prevalence of condylar resorption compared to the general population [14]. Condylar resorption may decrease mandibular ramus height, causing mandibular retrusion or asymmetry with malocclusion [31]. Previous measurement by MRI in adolescents showed that in unilateral ADD, the condylar height was reduced from 0.41 to 1.10 mm after a follow-up period exceeding 12 months, accompanied by a deterioration in mandibular deviation [11, 17, 32]. In bilateral ADD, condylar height was notably reduced from 0.71 to 0.81 mm, accompanied by an increase in mandibular retrusion and exacerbation of Class II malocclusion, as well as an increase in overbite of 0.85 ± 0.74 mm [15, 16]. After DR surgery, condylar height significantly increased with an average of 1.22 to 2.29 mm, so that the jaw deformity was minimized [16, 19, 31, 33, 34]. We had similar results in this study. A1 group had condylar height reduction during an average of 11.71 ± 7.31 months of observation, and A2 group had an average of 2.51 ± 1.19 mm condylar height increase after DR. So, DR had better results than observation, but there are some factors which may affect bone regeneration. Liu had reported 6 factors including age of ADD onset, nocturnal bruxism, disc morphology, bone mineral density, Wilkes’ classification, and postoperative splint therapy that affect postoperative bone remodeling [35].
As a systemic factor, PCOS is the most common endocrine disorder among women of reproductive age [36], characterized primarily by hyperandrogenemia, oligomenorrhea or amenorrhea, or polycystic ovaries [37]. Twenty-five years ago, PCOS was a relatively unknown condition that fell within the realms of pediatrics, gynecology, endocrinology, and dermatology, with limited scholarly resources available. In recent years, there has been a notable increase in the prevalence of adolescent PCOS, leading to a surge in academic interest and research on the topic [29]. Research conducted by Sidika [24] and Hasmet [25] et al. revealed a higher incidence and severity of TMJ disorders in patients with PCOS compared to those with normal menstrual cycles. However, these studies did not assess condylar height and changes after PCOS treatment. In this study, we utilized MRI to measure CH over time and compared the differences between ADD, ADD + PCOS untreated and ADD + PCOS treated patients. The results showed that: (1) in PCOS untreated patients, condylar height decreased significantly during observation and increased less after DR than ADD and PCOS treated patients; (2) in PCOS treated patients, the increase of condylar height after DR was similar with ADD patients; (3) DR had better condylar height than observation. Multiple linear analysis also exhibited that the condylar height changes were significantly associated with the presence and treatment of PCOS and ADD treatment (observation or DR). These findings suggest that in ADD adolescents, condylar remodeling was significantly poorer in PCOS without treatment, and improved after PCOS treatment. Additionally, DR improved condylar bone regeneration except for non-treated PCOS patients.
The literature reports that PCOS patients are affected by insulin resistance [38], hyperandrogenemia [39], and systemic low-grade inflammation [40], all of which have implications for bone metabolism, exacerbating condylar resorption and impeding new bone formation. Among them, insulin resistance weakens bone formation by reducing the expression of osteoprotegerin [41]. Elevated androgen levels contribute to disrupted bone metabolism and diminished bone density [42]. Androgen receptors play a direct role in bone health [43], with excessive androgens potentially leading to the inactivation of cortisol, elevated levels of pro-inflammatory cytokines such as TNF-α and IL-1β, and ultimately promoting an inflammatory environment that can result in decreased bone mass [39]. The presence of insulin resistance and hyperandrogenemia may also trigger the release of somatostatin, which can suppress growth hormone secretion and impair bone remodeling capacity [39]. Presently, oral contraceptives serve as the primary treatment for acne and menstrual irregularities in patients with PCOS [44]. Diane-35, containing 2 mg cyproterone acetate and 35 µg ethinyl estradiol, is commonly prescribed to address hyperandrogenemia in PCOS patients [45]. Its mechanism of action involves inhibiting androgenic effects, such as those of testosterone, while activating progesterone receptors. In the context of this investigation, all PCOS participants received oral Diane-35, leading to the restoration of regular menstrual cycles and the promotion of new bone growth in the condyle following disc repositioning, accompanied by a large increase in condylar height. Hence, it is advisable to actively treat PCOS in patients with concurrent ADD.
The shortcoming of this study is limited PCOS patients. As a retrospective study, we have to select patients without PCOS as a control. Fortunately, there are enough patients without PCOS who can be matched to the PCOS ones. A post-hoc power analysis showed that this was a highly powered study. The results can be used as a pilot study for future prospective one with a larger sample size to elucidate other variables such as age, duration of ADD, follow-up duration, and Wilkes stage, which may impact the change in condylar height.
Conclusion
ADD combined with untreated PCOS in adolescents may aggravate condylar degeneration and impact bone regeneration after DR surgery. PCOS treatment facilitates bone remodeling especially regeneration after DR.
Acknowledgements
Not applicable.
Abbreviations
- TMJ
Temporomandibular joint
- ADD
Anterior disc displacement
- ADDwoR
ADD without reduction
- DR
Disc repositioning
- PCOS
Polycystic ovarian syndrome
- CH
Condylar height
- MRI
Magnetic resonance imaging
Author contributions
Conceptualization: Xiang Ye and Dongmei He; Methodology: Jiali Sun, Chuyao Wang, Jieyun Zhao, Xin Nie, Chuan Lu, Xiang Ye and Dongmei He; Formal analysis and investigation: Jiali Sun; Writing - original draft preparation: Jiali Sun and Chuyao Wang; Writing - review and editing: Xiang Ye and Dongmei He; Funding acquisition: Dongmei He; Resources: Xiang Ye and Dongmei He; Supervision: Xiang Ye and Dongmei He. All authors read and approved the final manuscript.
Funding
This study was supported by grants from the National Natural Science Foundation of China (32071313, 82270996); Cross-disciplinary Research Fund, Rare diseases registration project, Fund of Department of Oral and Maxillofacial Surgery of Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine (JYJC202203, JYHJB202304, 2023-03), Shanghai’s Top Priority Research Center (2022ZZ01017), CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-037).
Data availability
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Shanghai Ninth People’s Hospital affiliated with Shanghai Jiao Tong University, School of Medicine (Number: SH9H-2023-T294-1; Registry Time: 09/15/2023), and the need for consent to participate was waived by the ethics committee. This study was conducted in accordance with the Helsinki Declaration of 1975, as revised in 2013.
Consent for publication
The patient provided written informed consent for publishing this case report and accompanying photographs. The written consent is available on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jiali Sun and Chuyao Wang these authors are co-first authors.
Contributor Information
Chuan Lu, Email: 1041123231@qq.com.
Xiang Ye, Email: xiangnan312@163.com.
Dongmei He, Email: lucyhe119@163.com.
References
- 1.Oğütcen-Toller M, Taşkaya-Yilmaz N, Yilmaz F. The evaluation of temporomandibular joint disc position in TMJ disorders using MRI. Int J Oral Maxillofac Surg. 2002;31(6):603–7. [DOI] [PubMed] [Google Scholar]
- 2.Naeije M, Te Veldhuis AH, Te Veldhuis EC, Visscher CM, Lobbezoo F. Disc displacement within the human temporomandibular joint: a systematic review of a noisy annoyance. J Oral Rehabil. 2013;40(2):139–58. [DOI] [PubMed] [Google Scholar]
- 3.LeResche L. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 1997;8(3):291–305. [DOI] [PubMed] [Google Scholar]
- 4.Isberg A, Hägglund M, Paesani D. The effect of age and gender on the onset of symptomatic temporomandibular joint disk displacement. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(3):252–7. [DOI] [PubMed] [Google Scholar]
- 5.Ribeiro RF, Tallents RH, Katzberg RW, Murphy WC, Moss ME, Magalhaes AC, et al. The prevalence of disc displacement in symptomatic and asymptomatic volunteers aged 6 to 25 years. J Orofac Pain. 1997;11(1):37–47. [PubMed] [Google Scholar]
- 6.Nebbe B, Major PW. Prevalence of TMJ disc displacement in a pre-orthodontic adolescent sample. Angle Orthod. 2000;70(6):454–63. [DOI] [PubMed] [Google Scholar]
- 7.Nebbe B, Major PW, Prasad NG. Adolescent female craniofacial morphology associated with advanced bilateral TMJ disc displacement. Eur J Orthod. 1998;20(6):701–12. [DOI] [PubMed] [Google Scholar]
- 8.Nebbe B, Major PW, Prasad NG, Hatcher D. Quantitative assessment of temporomandibular joint disk status. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(5):598–607. [DOI] [PubMed] [Google Scholar]
- 9.Schellhas KP, Pollei SR, Wilkes CH. Pediatric internal derangements of the temporomandibular joint: effect on facial development. Am J Orthod Dentofac Orthop. 1993;104(1):51–9. [DOI] [PubMed] [Google Scholar]
- 10.Xie Q, Yang C, He D, Cai X, Ma Z. Is mandibular asymmetry more frequent and severe with unilateral disc displacement? J Craniomaxillofac Surg. 2015;43(1):81–6. [DOI] [PubMed] [Google Scholar]
- 11.Xie Q, Yang C, He D, Cai X, Ma Z, Shen Y, et al. Will unilateral temporomandibular joint anterior disc displacement in teenagers lead to asymmetry of condyle and mandible? A longitudinal study. J Craniomaxillofac Surg. 2016;44(5):590–6. [DOI] [PubMed] [Google Scholar]
- 12.Zhuo Z, Cai X. Results of radiological follow-up of untreated anterior disc displacement without reduction in adolescents. Br J Oral Maxillofac Surg. 2016;54(2):203–7. [DOI] [PubMed] [Google Scholar]
- 13.Hu YK, Yang C, Cai XY, Xie QY. Does condylar height decrease more in temporomandibular joint nonreducing disc displacement than reducing disc displacement? A magnetic resonance imaging retrospective study. Med (Baltim). 2016;95(35):e4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roh HS, Kim W, Kim YK, Lee JY. Relationships between disk displacement, joint effusion, and degenerative changes of the TMJ in TMD patients based on MRI findings. J Craniomaxillofac Surg. 2012;40(3):283–6. [DOI] [PubMed] [Google Scholar]
- 15.Zhu H, He D, Yang Z, Song X, Ellis E. The effect of disc repositioning and post-operative functional splint for the treatment of anterior disc displacement in juvenile patients with Class II malocclusion. J Craniomaxillofac Surg. 2019;47(1):66–72. [DOI] [PubMed] [Google Scholar]
- 16.Zhu H, He D, Yang Z, Lu C, Zhao J, Yang C. The Effect of Condylar Regeneration After Different Disc Repositioning Surgeries in Adolescents with Skeletal Class II Malocclusion. J Oral Maxillofac Surg. 2021;79(9):1851–61. [DOI] [PubMed] [Google Scholar]
- 17.Zhiyang Liu, Xie Q, Yang C, Chen M, Bai G, Abdelrehem A. The effect of arthroscopic disc repositioning on facial growth in juvenile patients with unilateral anterior disc displacement. J Craniomaxillofac Surg. 2020;48(8):765–71. [DOI] [PubMed] [Google Scholar]
- 18.Cai XY, Yang C, Jin JM, Song H. Condylar remodelling following arthroscopic disc repositioning of TMJ in teenage patients: a longitudinal magnetic resonance imaging study. China J Oral Maxillofac Surg. 2012;10(4):322–7. [Google Scholar]
- 19.Zhu H, Yang Z, He D, Hu N, Cheng Z. The effect of TMJ disk repositioning by suturing through open incision on adolescent mandibular asymmetry with and without a functional orthodontic appliance. Oral Surg Oral Med Oral Pathol Oral Radiol. 2021;131(4):405–14. [DOI] [PubMed] [Google Scholar]
- 20.Sun J, Zhu H, Lu C, Zhao J, Nie X, Yang Z, et al. Temporomandibular joint disc repositioning and occlusal splint for adolescents with skeletal class II malocclusion: a single-center, randomized, open-label trial. BMC Oral Health. 2023;23(1):694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Xie Q, Yang C, Chen M, Bai G, Shen P, et al. Functional orthodontics after arthroscopic disk repositioning in adolescent anterior disk displacement with mandibular retrusion. Oral Surg Oral Med Oral Pathol Oral Radiol. 2020;130(4):357–62. [DOI] [PubMed] [Google Scholar]
- 22.Young HE, Ward WE. The Relationship Between Polycystic Ovarian Syndrome, Periodontal Disease, and Osteoporosis. Reprod Sci. 2021;28(4):950–62. [DOI] [PubMed] [Google Scholar]
- 23.Stener-Victorin E, Teede H, Norman RJ, Legro R, Goodarzi MO, Dokras A, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2024;10(1):27. [DOI] [PubMed] [Google Scholar]
- 24.Soydan SS, Deniz K, Uckan S. Aslı Dogruk Unal, Neslihan Bascıl Tutuncu. Is the incidence of temporomandibular disorder increased in polycystic ovary syndrome? Br J Oral Maxillofac Surg. 2014;52(9):822–6. [DOI] [PubMed] [Google Scholar]
- 25.Hasmet Yazici MI, Taskin G, Guney AA, Hismiogullari E, Arslan, Kamil Gokce Tulaci. The novel relationship between polycystic ovary syndrome and temporomandibular joint disorders. J Stomatol Oral Maxillofac Surg. 2021;122(6):544–8. [DOI] [PubMed] [Google Scholar]
- 26.Sudhakaran G, Priya PS, Jagan K, Haridevamuthu B, Meenatchi R, Arockiaraj J. Osteoporosis in polycystic ovary syndrome (PCOS) and involved mechanisms. Life Sci. 2023;335:122280. [DOI] [PubMed] [Google Scholar]
- 27.Dimitroulis G. The prevalence of osteoarthrosis in cases of advanced internal derangement of the temporomandibular joint: a clinical, surgical and histological study. International journal of oral and maxillofacial surgery [Internet]. 2005;34(4). https://pubmed.ncbi.nlm.nih.gov/16053840/ [DOI] [PubMed]
- 28.Yang C, Cai XY, Chen MJ, Zhang SY. New arthroscopic disc repositioning and suturing technique for treating an anteriorly displaced disc of the temporomandibular joint: part I–technique introduction. Int J Oral Maxillofac Surg. 2012;41(9):1058–63. [DOI] [PubMed] [Google Scholar]
- 29.Ibáñez L, de Zegher F. Adolescent PCOS: a postpubertal central obesity syndrome. Trends Mol Med. 2023;29(5):354–63. [DOI] [PubMed] [Google Scholar]
- 30.Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–91. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Sun J, He D. Review of the studies on the relationship and treatment of anterior disk displacement and dentofacial deformity in adolescents. Oral Surg Oral Med Oral Pathol Oral Radiol. 2023;135(4):470–4. [DOI] [PubMed] [Google Scholar]
- 32.Zhuo Z, Cai X, Xie Q. Is anterior disc displacement without reduction associated with temporomandibular joint condylar height in juvenile patients younger than 20 years? J Oral Maxillofac Surg. 2015;73(5):843–9. [DOI] [PubMed] [Google Scholar]
- 33.Schmitter M, Zahran M, Duc JMP, Henschel V, Rammelsberg P. Conservative therapy in patients with anterior disc displacement without reduction using 2 common splints: a randomized clinical trial. J Oral Maxillofac Surg. 2005;63(9):1295–303. [DOI] [PubMed] [Google Scholar]
- 34.Goncalves JR, Wolford LM, Cassano DS, da Porciuncula G, Paniagua B, Cevidanes LH. Temporomandibular joint condylar changes following maxillomandibular advancement and articular disc repositioning. J Oral Maxillofac Surg. 2013;71(10):e17591–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X, Shen P, Wang X, Zhang S, Zheng J, Yang C. A Prognostic Nomogram for Postoperative Bone Remodeling in Patients with ADDWoR. Sci Rep. 2018;8:4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rotterdam ESHRE, ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 37.Fauser B, Tarlatzis B, Chang J, Azziz R, Legro R, Dewailly D, et al. The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41–7. [DOI] [PubMed] [Google Scholar]
- 38.Shieh A, Greendale GA, Cauley JA, Srikanthan P, Karlamangla AS. Longitudinal associations of insulin resistance with change in bone mineral density in midlife women. JCI Insight. 2022;7(20):e162085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krishnan A, Muthusami S. Hormonal alterations in PCOS and its influence on bone metabolism. J Endocrinol. 2017;232(2):R99–113. [DOI] [PubMed] [Google Scholar]
- 40.Cao JJ, Gregoire BR, Shen CL. A High-Fat Diet Decreases Bone Mass in Growing Mice with Systemic Chronic Inflammation Induced by Low-Dose, Slow-Release Lipopolysaccharide Pellets. J Nutr. 2017;147(10):1909–16. [DOI] [PubMed] [Google Scholar]
- 41.Clemens TL, Karsenty G. The osteoblast: An insulin target cell controlling glucose homeostasis. J Bone Miner Res. 2011;26(4):677–80. [DOI] [PubMed] [Google Scholar]
- 42.Piovezan JM, Premaor MO, Comim FV. Negative impact of polycystic ovary syndrome on bone health: a systematic review and meta-analysis. Hum Reprod Update. 2019;25(5):633–45. [DOI] [PubMed] [Google Scholar]
- 43.Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of androgen receptors in human bone. J Clin Endocrinol Metab. 1997;82(10):3493–7. [DOI] [PubMed] [Google Scholar]
- 44.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Buzney E, Sheu J, Buzney C, Reynolds RV. Polycystic ovary syndrome: a review for dermatologists: Part II. Treatment. J Am Acad Dermatol. 2014;71(5):859.e1-859.e15; quiz 873–4. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.