Abstract
In allergology, clinical registries fill knowledge gaps of epidemiology, mechanisms of allergic diseases, and real-world treatment outcomes. Considering the continuous rise of allergic diseases worldwide, registries become increasingly important for the optimization and harmonization of patient care. In the current review, we present four ongoing allergy-focused registries initiated in Germany. We conducted a focused literature search and discussed their structure, main purposes, and findings. Registries included are the “Information Network of Departments of Dermatology”, the “European Anaphylaxis Registry”, the “GAN Severe Asthma Registry”, and “TREATgermany”. Despite differences in scope and operation, all registries gather harmonized real-world data that is indispensable for evidence-based decision making in clinical practice and ultimately improves patient care in allergology.
Keywords: registry, allergology, anaphylaxis, severe asthma, contact allergy, atopic dermatitis, biologics, real-world data
Introduction
Allergic diseases have become a global health issue and heavily impact healthcare resources as they continue to increase worldwide [1, 2]. In Germany, almost one third of the adult population was diagnosed with at least one allergic disease in their lifetime [3]. Considering the high prevalence of allergies in the German population, but also alarming upward trend worldwide [1, 2, 4], registries play an increasingly important role in the surveillance and management of allergic diseases.
They provide comprehensive clinical data to different stakeholders (healthcare professionals, regulatory bodies, patients) [4, 5] and may cover the following important aspects:
First, registries enable the evaluation of real-world (long-term) effectiveness and safety of drugs that are implemented in routine care. Randomized controlled trials (RCT) consist of a homogenous, narrowly defined study cohort in accordance with predefined inclusion criteria. Registries include a broader spectrum of participants, leading to an increased generalizability of gathered data [5]. Such registries in allergology are “The GAN Severe Asthma Registry” for severe asthma and “TREATgermany” for atopic dermatitis (AD).
Second, registries generate important evidence for treatment guidelines where clinical trials are difficult to conduct. Additionally, they facilitate the surveillance of disease management in accordance with current guideline recommendations [5]. Considering the unpredictability and severity of anaphylaxis [6, 7, 8, 9] and lack of robust evidence for treatment guidelines [10, 11], findings of the “European Anaphylaxis Registry” (EAR) are essential.
Lastly, registries may also serve as a tool for active surveillance of allergies [5]. By continuously analyzing epidemiologic data, novel trends can be detected and used to implement preventive measures by regulatory bodies. The “Information Network of Departments of Dermatology” (“Informationsverbund Dermatologischer Kliniken” – IVDK) actively monitors contact allergies in the population of German-speaking countries [12].
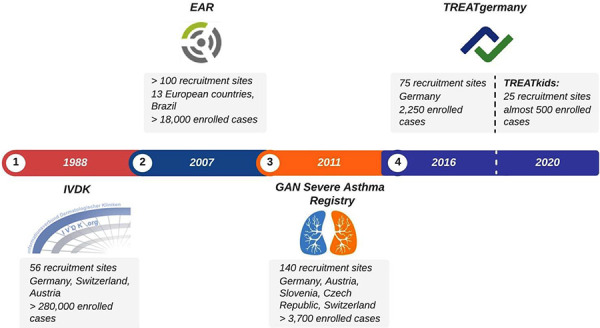
Here, we present the ongoing allergy-related registries initiated in Germany (Figure 1) and discuss their main purposes, findings, strengths, and limitations.
Figure 1. Presented registries and year of their establishment.
Materials and methods
We analyzed the structure and key findings of the different registries covering topics of allergology. A focused literature search was performed, and the obtained data were critically reviewed. Search was conducted using PubMed database as well as the registries’ websites.
Results
Structure, target groups, and recent data from the allergy registries
The Information Network of Departments of Dermatology (IVDK)
The IVDK is a network of 56 currently active dermatology departments across Germany, Austria, and Switzerland, initiated in 1988, that continuously monitors the prevalence and trends of contact allergies in the population [12]. Generation of data does not depend on the selective recruitment of patients, as all routinely patch-tested patients in cooperating centers for suspected contact allergy are included [12]. Patch testing is performed in a standardized manner according to national and international guidelines using test preparations of the “Deutsche Kontaktallergie-Gruppe”, to which all participating centers belong [42] (Table 1).
Table 1. Overview of the registries.
| IVDK | EAR | GAN Severe Asthma Registry |
TREATgermany
TREATkids |
|
|---|---|---|---|---|
| Year established | 1988 [12] | 2007 [13] | 2011 [41] | 2016 [41] 2020 [14] |
| Countries | Germany, Switzerland, Austria [15] | 13 European countries and Brazil [43] | Germany, Austria, Slovenia, Czech Republic, Switzerland [41] | Germany |
| Participating centers | Dermatology dpts [12] | Tertiary allergy centers | Pulmonology departments and practices with specialist pulmonologists [16] | Dermatology dpts and practices [17, 18] |
| Number of participating centers | 56 [15] | > 100 [13] Germany: 43 centers | 140, 73 % German centers [41] | Approximately 75 [44] Approximately 25 [44] |
| Inclusion criteria | Patch tested patients for suspected contact allergy [12] | Anaphylactic reaction ≤ 12 months prior to enrollment [13] | Physician-diagnosed severe asthma (according to the ERS/ATS2 criteria) [19] | Moderate-to-severe atopic dermatitis: oSCORAD > 20 or systemic treatment current or ≤ 24 months prior to enrollment [44] |
| Included age | All | All | ≥ 6 years [16] | ≥ 18 years [44] < 18 years [44] |
| Enrolled cases | > 280,000 [42] | Total: > 18,000 Germany: 10,140 [43] | > 3,700 [41] | 2,250 [44] almost 500 [44] |
| Follow-up visits | – | – | At least once a year ideally for 15 years [16] |
Every 3 – 6 months for ≥ 24 months [20] |
| Gathered data | Patient demographics, medical history | |||
| Results of patch tests, suspected allergen source and occupational context, anatomical site of the dermatosis, cofactors [12, 42] | Detailed description of anaphylactic reaction and management [13] |
Current asthma treatment Lung function, biomarkers, exacerbations, asthma control (GINA2 control status) Patient-reported outcomes (PROs) [16] |
Current AD treatment Objective parameters: IGA; oSCORAD, EASI3 Patient-reported outcomes (PROs) Biosamples (optional) [44] |
|
1European Respiratory Society/American Thoracic Society; 2Global Initiative for Asthma; 3Investigator global assessment, objective scoring atopic dermatitis, eczema area and severity index.
The IVDK has published various analyses of long-term surveillance periods, regarding the sensitization patterns in the overall population, but also considering specific allergens and distinct demographic subgroups. Assessment of current trends and sensitization pattern of contact allergies are presented in Table 2.
Table 2. Selected key findings of the presented registries.
| Registry & [Reference] | Key findings |
|---|---|
| IVDK [22] Study period: 2007 – 2018 Patients: n = 125,436 |
Time trends and current spectrum of CA to the most common allergens (DKG baseline series) (excerpt) |
| Most common allergens and overall percentage of positive test results: nickel (14.7%), fragrance mix I (8.1%), myroxylon pereirae resin (7.5%), cobalt (5.2%), no definite trend Preservatives: - MI epidemic: 2014 peak in Europe followed by a continuous decline - 2018: almost pre-epidemic level of MCI/MI CA reached Propolis: - Significant increase from 2.35% positive tests in 2007 – 2010 to 3.94% positive tests in 2015 – 2018 | |
| EAR [9] Study period: 2007 – 2017 Patients: n = 7,316 |
Risk factors of severe anaphylaxis: |
| - Higher age - Mastocytosis - Vigorous physical exercise (independent from elicitor) - Male sex - Psychological burden - Intake of beta-blockers or ACE inhibitors | |
| GAN Severe Asthma Registry [30] Registry data up to July 2022 Patients: n = 443 |
Treatment response to biologics according to BARS(-L) criteria (Milger et al. [19])1. Comparison of treatment with and without biologics. |
| One year of treatment with biologics: - Greater improvements regarding annual exacerbations, oral corticosteroid dose reduction, ACT score, FEV1 - Higher BARS response rates: “good” in 61.4 vs. 34.8% (biologic treatment vs. no biologics) - Higher remission rates: 37.6 vs. 17.2% (biologic treatment vs. no biologics) | |
| TREATgermany [32] Registry data up to July 2021 Patients: Dupilumab n = 369 CyA n = 41 |
Real-world effectiveness and drug safety of dupilumab and CyA |
| Dupilumab: favorable safety profile, robust long-term effectiveness CyA: higher discontinuation rates - After 12 months: 78% (CyA) vs. 5% (dupilumab) - Reasons for discontinuing CyA: side-effects (31%), insufficient efficacy (27%), no report (56%) Most frequent dupilumab-associated adverse events: - Ocular complaints (29.8%), predominantly conjunctivitis |
1Biologics Asthma Response Score [30]. CA = contact allergy; DKG = German Contact Dermatitis Research Group (Deutsche Kontaktallergie-Gruppe); MI = methylisothiazolinone; MCI = methylchloroisothiazolinone; ACE = angiotensin converting enzyme; BARS = Biologics Asthma Response Score; ACT = asthma control test; FEV1 = forced expiratory volume in 1 second; CyA = cyclosporin A.
Evaluation of regulatory actions
Continuous, long-term surveillance of sensitization patterns enables the detection of trends, emerging allergens, persisting problems and the evaluation of the success of implemented directives [12]. Epidemiological data gathered by the IVDK illustrated the success of regulatory measures counteracting the “epidemic” of contact allergies to the preservative methylisothiazolinone (MI) in Europe [21, 22]. As an example of detecting persistent problems, IVDK data revealed a consistently high prevalence of nickel allergy in young female patients, despite the first EU nickel directive [23]. Investigations initiated by the IVDK exposed non-compliance with the first nickel directive, which was revised in 2004 [24]. Consequently, sensitization to nickel in young females significantly decreased from 2005 to 2012 [24].
Occupational dermatitis
The IVDK also plays an essential role for the identification of occupations at risk for the development of occupational dermatitis due to an increased allergen exposure [12].
The European Anaphylaxis Registry (EAR)
The EAR was launched in 2007, started recruiting patients of German speaking countries and expanded to other European countries in 2011 [13]. To date, the registry encompasses over 150 specialized tertiary allergy centers, 43 of which are located in Germany [43]. Participating centers continuously collect real-life data from patients of all age groups who suffered from anaphylaxis ≤ 12 months prior to their visit [13] (Table 1).
The spectrum of eliciting allergens and clinical presentation differ significantly between children and adults [25, 26, 27]. Risk factors of severe anaphylaxis as observed by the EAR are presented in Table 2.
Discrepancy of guideline recommendations and real-life data regarding the use of epinephrine as the first line drug in anaphylaxis
Registry data repeatedly reveal an underuse of epinephrine as the first-line treatment in anaphylaxis (23.2% of cases between 2006 and 2017) [11, 25, 27]. Epinephrine administration by health professionals varied internationally, being lowest in Germany (19.6%), while overall increasing within the analyzed period, indicating an improved guidelines awareness and adherence [11]. Additionally, data on adrenaline autoinjector prescription practices reveal a low adherence to the current “European Academy of Allergy and Clinical Immunology” guidelines outside of specialized allergy centers, underpinning the relevance of allergy centers for ensuring adequate patient care [28].
The GAN Severe Asthma Registry
The GAN Severe Asthma Registry intends to provide data on the epidemiology, phenotypes, and treatment of severe asthma [29]. It aims to close important knowledge gaps regarding pathomechanisms, natural course, and prognosis of the disease [41]. The registry was established in 2011 by the “German Asthma Net e.V.” (GAN) [41] and started the recruitment of patients ≥ 6 years suffering from physician-diagnosed severe asthma. Currently, the registry encompasses 140 participating centers mainly from Germany [41] (Table 1).
Characterization of the severe asthma cohort
Analysis of baseline characteristics of patients enrolled in the GAN Severe Asthma Registry demonstrates the heterogeneity of severe asthma. Despite being treated in specialized clinics or practices and by specialized pulmonologists, data of the registry reveal a suboptimal disease control in severe asthma patients [16, 19]. Continuous measurement of functional parameters and biomarkers of patients aids the characterization and differentiation of asthma phenotypes, facilitating the identification of patient subgroups that will respond to a given treatment regimen [29] (Table 2).
Real-world practice, effectiveness, and safety of biologics in patients with severe asthma
Comparative assessment of clinical response in patients receiving biologics and those without targeted therapy, reveal higher response rates and more frequent disease remission in patients treated with biologics [30] (Table 2). Treatment with mepolizumab results in the improvement and long-term stability of asthma control and lung function [31].
The TREATgermany registry
TREATgermany is a multicenter, observational registry and currently encompasses ~ 75 participating German centers [44]. Data of patients with moderate-to-severe AD is prospectively collected in a real-world setting [20]. Considering the rapid progress of targeted systemic treatment for AD in the past years, TREATgermany independently and continuously evaluates systemic treatment in routine care [32, 33]. The registry not only examines drug safety and effectiveness of biologics (dupilumab, tralokinumab, lebrikizumab) but also of JAK inhibitors, such as baricitinib [34]. In 2020, the section TREATkids was launched [14] and currently comprises almost 500 children and adolescents under the age of 18 years [44].
Real-world effectiveness and safety of biologics
Treatment with dupilumab significantly improves objective parameters and PROs compared to baseline values. Dupilumab displays a favorable safety profile, and much lower discontinuation rates compared to cyclosporin A (CyA) [35]. However, analysis of TREATgermany data reveals more dupilumab-related ocular complaints (29.8% of patients) than preceding trials [35] (Table 2). Dupilumab induces a shift of the skin microbiome of patients toward the skin flora of healthy controls, largely independent of clinical response [36].
Discussion
Participating centers of all registries gather data in a standardized manner and transmit them to the registry centers for data analysis. In general, standardized data acquisition results in a large set of harmonized data with increased statistical power. This enables stratification, subgroup analysis, and even interregional and -national comparisons. Furthermore, continuous exchange with participating centers is fundamental for the harmonization of disease management in accordance with current guidelines. Because the registries focus on different allergic diseases, they vary in scope and operation. However, all the registries assess real-world data and therefore provide valuable insight into the current state of patient and disease management and draw attention to gaps in patient care. The IVDK serves as allergen surveillance systems and not only addresses clinicians but also regulatory bodies for the implementation of preventive measures and directives [12]. Data of the EAR repeatedly highlighted the need for an increased guideline awareness and patient education regarding the management of anaphylaxis [11]. Real-world data obtained by the GAN Severe Asthma Registry and TREATgermany regarding the (long-term) effectiveness and drug safety of novel targeted therapies is indispensable. The observed disparities of dupilumab-induced ocular complaints in real-world patients compared to participants in clinical trials underpin the relevance of registries in identifying and understanding product safety in the diverse cohort of routine care [5, 37]. The heterogeneity of patients with severe asthma limits the generalizability of drug effectiveness observed in well-defined study cohorts of clinical trials. The eligibility criteria for many RCTs would exclude 95% of asthma patients [38]. Registries like the GAN Severe Asthma Registry and TREATgermany will continue to provide crucial information to fill gaps for decision-makers by assessing real-world drug safety and effectiveness. Furthermore, assessment of biomarkers and research on underlying disease mechanisms and endotypes, will pave the way for an individualized treatment approach [39]. Findings of the presented registries are limited by only voluntary participation of recruitment centers and patients, which may introduce selection bias [25, 27, 31, 40]. Hence, apart from data of the IVDK, they may not be representable for the population as total and epidemiological studies are not possible.
Conclusion
In allergology, registries act as allergen surveillance systems by actively monitoring trends and emerging elicitors of contact allergies and anaphylaxis in the population. Moreover, registries give insight into real-world long-term safety and effectiveness of systemic therapies for AD and asthma and significantly contribute to patient-oriented research. Therefore, they should be established and interconnected for all allergic diseases.
Authors’ contributions
MF performed the literature search, drafted and wrote the manuscript. PG and AA revised the manuscript. MW was responsible for the concept and revising of the manuscript. All authors reviewed and approved the final version of the manuscript and its submission.
Funding
None.
Conflict of interest
Prof. Worm declares the receipt of honoraria or consultation fees by the following companies: Novartis Pharma GmbH, Sanofi-Aventis Deutschland GmbH, DBV Technologies S.A, Aimmune Therapeutics UK Limited, Leo Pharma GmbH, AstraZenceca GmbH, ALK-Abelló Arzneimittel GmbH, Lilly Deutschland GmbH, Kymab Limited, Amgen GmbH, Abbvie Deutschland GmbH & Co. KG, Pfizer Pharma GmbH, Mylan Germany GmbH (A Viatris Company), Boehringer Ingelheim Pharma GmbH & Co. KG, GlaxoSmithKline GmbH & Co. KG, Almirall S. A., Amgen GmbH, Pfizer Deutschland GmbH, Bristol-Myers Squibb GmbH & Co. KGaA and FomF GmbH.
All other authors declare to have no conflict of interest.
References
- 1. Zhang L Akdis CA The past, present, and future of allergic diseases in China. Allergy. 2022; 77: 354–356. [DOI] [PubMed] [Google Scholar]
- 2. Papadopoulos NG Agache I Bavbek S Bilo BM Braido F Cardona V Custovic A Demonchy J Demoly P Eigenmann P Gayraud J Grattan C Heffler E Hellings PW Jutel M Knol E Lötvall J Muraro A Poulsen LK Roberts G Research needs in allergy: an EAACI position paper, in collaboration with EFA. Clin Transl Allergy. 2012; 2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergmann KC Heinrich J Niemann H Current status of allergy prevalence in Germany: Position paper of the Environmental Medicine Commission of the Robert Koch Institute. Allergo J Int. 2016; 25: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stiles SL Roche I Said M Clifford RM Sanfilippo FM Loh R Salter SM Overview of registries for anaphylaxis: a scoping review protocol. JBI Evid Synth. 2021; 19: 1193–1201. [DOI] [PubMed] [Google Scholar]
- 5. Gliklich RE, Leavy MB, Dreyer NA. Registries for evaluating patient outcomes: a user’s guide. 4th edition. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Sep. Available from: https://www.ncbi.nlm.nih.gov/books/NBK562575 . [PubMed]
- 6. Dhami S Panesar SS Roberts G Muraro A Worm M Bilò MB Cardona V Dubois AE DunnGalvin A Eigenmann P Fernandez-Rivas M Halken S Lack G Niggemann B Rueff F Santos AF Vlieg-Boerstra B Zolkipli ZQ Sheikh A Management of anaphylaxis: a systematic review. Allergy. 2014; 69: 168–175. [DOI] [PubMed] [Google Scholar]
- 7. Francuzik W Ruëff F Bauer A Bilò MB Cardona V Christoff G Dölle-Bierke S Ensina L Fernández Rivas M Hawranek T O’B Hourihane J Jakob T Papadopoulos NG Pföhler C Poziomkowska-Gęsicka I Van der Brempt X Scherer Hofmeier K Treudler R Wagner N Wedi B Phenotype and risk factors of venom-induced anaphylaxis: A case-control study of the European Anaphylaxis Registry. J Allergy Clin Immunol. 2021; 147: 653–662. [DOI] [PubMed] [Google Scholar]
- 8. Worm M Eckermann O Dölle S Aberer W Beyer K Hawranek T Hompes S Koehli A Mahler V Nemat K Niggemann B Pföhler C Rabe U Reissig A Rietschel E Scherer K Treudler R Ruëff F Triggers and treatment of anaphylaxis: an analysis of 4,000 cases from Germany, Austria and Switzerland. Dtsch Arztebl Int. 2014; 111: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Worm M Francuzik W Renaudin JM Bilo MB Cardona V Scherer Hofmeier K Köhli A Bauer A Christoff G Cichocka-Jarosz E Hawranek T Hourihane JO Lange L Mahler V Muraro A Papadopoulos NG Pföhler C Poziomkowska-Gęsicka I Ruëff F Spindler T Factors increasing the risk for a severe reaction in anaphylaxis: An analysis of data from The European Anaphylaxis Registry. Allergy. 2018; 73: 1322–1330. [DOI] [PubMed] [Google Scholar]
- 10. Muraro A Worm M Alviani C Cardona V DunnGalvin A Garvey LH Riggioni C de Silva D Angier E Arasi S Bellou A Beyer K Bijlhout D Bilò MB Bindslev-Jensen C Brockow K Fernandez-Rivas M Halken S Jensen B Khaleva E EAACI guidelines: Anaphylaxis (2021 update). Allergy. 2022; 77: 357–377. [DOI] [PubMed] [Google Scholar]
- 11. Grabenhenrich LB Dölle S Ruëff F Renaudin JM Scherer K Pföhler C Treudler R Koehli A Mahler V Spindler T Lange L Bilò MB Papadopoulos NG Hourihane JOB Lang R Fernández-Rivas M Christoff G Cichocka-Jarosz E Worm M Epinephrine in Severe Allergic Reactions: The European Anaphylaxis Register. J Allergy Clin Immunol Pract. 2018; 6: 1898–1906. [DOI] [PubMed] [Google Scholar]
- 12. Schnuch A Geier J Lessmann H Arnold R Uter W Surveillance of contact allergies: methods and results of the Information Network of Departments of Dermatology (IVDK). Allergy. 2012; 67: 847–857. [DOI] [PubMed] [Google Scholar]
- 13. Worm M Höfer V Dölle-Bierke S Bilo MB Hartmann K Sabouraud-Leclerc D Treudler R Occupational anaphylaxis-Data from the anaphylaxis registry. Allergy. 2024; 79: 702–710. [DOI] [PubMed] [Google Scholar]
- 14. Ott H Abraham S Kind B Haufe E Weidinger S Werfel T Schmitt J Comment on: “Interim results from the PEDISTAD Real-World Registry by Paller et al”-First results from the German TREATkids registry for children and adolescents with moderate-to-severe atopic dermatitis. J Am Acad Dermatol. 2024; 90: e31–e33. [DOI] [PubMed] [Google Scholar]
- 15. Geier J Schubert S Rieker-Schwienbacher J Brans R Weisshaar E Kränke B Brockow K Ruёff F Recke A Uter W Declining frequency of sensitization to fragrance mixes I and II: IVDK-data of the years 2012-2021. Contact Dermatitis. 2024; 90: 470–478. [DOI] [PubMed] [Google Scholar]
- 16. Korn S Milger K Skowasch D Timmermann H Taube C Idzko M Voß HW Holtdirk A Hamelmann E Buhl R The German severe asthma patient: Baseline characteristics of patients in the German Severe Asthma Registry, and relationship with exacerbations and control. Respir Med. 2022; 195: 106793. [DOI] [PubMed] [Google Scholar]
- 17. Traidl S Hollstein MM Kroeger N Fischer S Heratizadeh A Heinrich L Kind B Siegels D Abraham S Schäfer T Augustin M Harder I Pinter A Schäkel K Wollenberg A Ertner K Ramaker-Brunke J Bong A Quist S Gorriahn-Maiterth H Obesity is linked to disease severity in moderate to severe atopic dermatitis-Data from the prospective observational TREATgermany registry. J Eur Acad Dermatol Venereol. 2024; jdv.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stölzl D Sander N Siegels D Harder I Kind B Fonfara M Heinrich L Ott H Abraham S Neustädter I Kleinheinz A Gerdes S Wollenberg A Lau S Nemat K Heratizadeh A Gellhaus I Werfel T Schmitt J Weidinger S Clinical and molecular response to dupilumab treatment in pediatric atopic dermatitis: Results of the German TREATkids registry. Allergy. 2024; 79: 2849–2852. [DOI] [PubMed] [Google Scholar]
- 19. Milger K Korn S Buhl R Hamelmann E Herth FJ Gappa M Drick N Fuge J Suhling H Age- and sex-dependent differences in patients with severe asthma included in the German Asthma Net cohort. Respir Med. 2020; 162: 105858. [DOI] [PubMed] [Google Scholar]
- 20. Heratizadeh A Haufe E Stölzl D Abraham S Heinrich L Kleinheinz A Wollenberg A Weisshaar E Augustin M Wiemers F Zink A von Kiedrowski R Hilgers M Worm M Pawlak M Sticherling M Fell I Handrick C Schäkel K Staubach-Renz P Bacharacteristics, disease severity and treatment history of patients with atopic dermatitis included in the German AD Registry TREATgermany. J Eur Acad Dermatol Venereol. 2020; 34: 1263–1272. [DOI] [PubMed] [Google Scholar]
- 21. Reeder MJ Warshaw E Aravamuthan S Belsito DV Geier J Wilkinson M Atwater AR White IR Silverberg JI Taylor JS Fowler JF Maibach HI DeKoven JG Buhl T Botto N Giménez-Arnau AM Gallo R Mowad C Lang CCV DeLeo VA Trends in the Prevalence of Methylchloroisothiazolinone/Methylisothiazolinone Contact Allergy in North America and Europe. JAMA Dermatol. 2023; 159: 267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uter W Gefeller O Mahler V Geier J Trends and current spectrum of contact allergy in Central Europe: results of the Information Network of Departments of Dermatology (IVDK) 2007-2018. Br J Dermatol. 2020; 183: 857–865. [DOI] [PubMed] [Google Scholar]
- 23. Schnuch A Wolter J Geier J Uter W Nickel allergy is still frequent in young German females - probably because of insufficient protection from nickel-releasing objects. Contact Dermatitis. 2011; 64: 142–150. [DOI] [PubMed] [Google Scholar]
- 24. Schnuch A Schwitulla J Decrease in nickel allergy in women after the second EU nickel directive. Contact Dermatitis. 2013; 69: 253–256. [DOI] [PubMed] [Google Scholar]
- 25. Grabenhenrich LB Dölle S Moneret-Vautrin A Köhli A Lange L Spindler T Ruëff F Nemat K Maris I Roumpedaki E Scherer K Ott H Reese T Mustakov T Lang R Fernandez-Rivas M Kowalski ML Bilò MB Hourihane JO Papadopoulos NG Anaphylaxis in children and adolescents: The European Anaphylaxis Registry. J Allergy Clin Immunol. 2016; 137: 1128–1137. [DOI] [PubMed] [Google Scholar]
- 26. Cichocka-Jarosz E Dölle-Bierke S Jedynak-Wąsowicz U Sabouraud-Leclerc D Köhli A Lange L Papadopoulos NG Hourihane J Nemat K Scherer Hofmeier K Hompes S Ott H Lopes de Oliveira L Spindler T Vogelberg C Worm M Cow’s milk and hen’s egg anaphylaxis: A comprehensive data analysis from the European Anaphylaxis Registry. Clin Transl Allergy. 2023; 13: e12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aurich S Dölle-Bierke S Francuzik W Bilo MB Christoff G Fernandez-Rivas M Hawranek T Pföhler C Poziomkowska-Gȩsicka I Renaudin JM Oppel E Scherer K Treudler R Worm M Anaphylaxis in Elderly Patients-Data From the European Anaphylaxis Registry. Front Immunol. 2019; 10: 750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kraft M Knop MP Renaudin JM Scherer Hofmeier K Pföhler C Bilò MB Lang R Treudler R Wagner N Spindler T Hourihane JO Maris I Koehli A Bauer A Lange L Müller S Papadopoulos NG Wedi B Moeser A Ensina LF Secondary prevention measures in anaphylaxis patients: Data from the anaphylaxis registry. Allergy. 2020; 75: 901–910. [DOI] [PubMed] [Google Scholar]
- 29. Korn S Hübner M Hamelmann E Buhl R [The German severe asthma registry]. Pneumologie. 2012; 66: 341–344. [DOI] [PubMed] [Google Scholar]
- 30. Milger K Suhling H Skowasch D Holtdirk A Kneidinger N Behr J Timmermann H Schulz C Schmidt O Ehmann R Hamelmann E Idzko M Taube C Lommatzsch M Buhl R Korn S Response to Biologics and Clinical Remission in the Adult German Asthma Net Severe Asthma Registry Cohort. J Allergy Clin Immunol Pract. 2023; 11: 2701–2712. [DOI] [PubMed] [Google Scholar]
- 31. Korn S Milger K Skowasch D Schulz C Mohrlang C Wernitz M Paulsson T Hennig M Buhl R Real-World Experience on the Use of Mepolizumab from the Severe Asthma Registry of the German Asthma Net (MepoGAN-Study). J Asthma Allergy. 2023; 16: 541–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stölzl D Sander N Heratizadeh A Haufe E Harder I Abraham S Heinrich L Kleinheinz A Wollenberg A Weisshaar E Schäkel K Ertner K Wiemers F Wildberger J Worm M von Kiedrowski R Effendy I Asmussen A Augustin M Pawlak M Real-world data on the effectiveness, safety and drug survival of dupilumab: an analysis from the TREATgermany registry. Br J Dermatol. 2022; 187: 1022–1024. [DOI] [PubMed] [Google Scholar]
- 33. Bosma AL Spuls PI Garcia-Doval I Naldi L Prieto-Merino D Tesch F Apfelbacher CJ Arents BWM Barbarot S Baselga E Deleuran M Eichenfield LF Gerbens LAA Irvine AD Manca A Mendes-Bastos P Middelkamp-Hup MA Roberts A Seneschal J Svensson Å TREatment of ATopic eczema (TREAT) Registry Taskforce: protocol for a European safety study of dupilumab and other systemic therapies in patients with atopic eczema. Br J Dermatol. 2020; 182: 1423–1429. [DOI] [PubMed] [Google Scholar]
- 34. Traidl S Heinrich L Siegels D Heratizadeh A Kind B Haufe E Abraham S Schäfer T Augustin M Harder I Pinter A Schäkel K Wollenberg A Ertner K Ramaker-Brunke J Bong A Quist S Gorriahn-Maiterth H Schenck F Sticherling M Treatment of moderate-to-severe atopic dermatitis with baricitinib: Results from an interim analysis of the TREATgermany registry. J Eur Acad Dermatol Venereol. 2024; 38: e887–e891. [DOI] [PubMed] [Google Scholar]
- 35. Abraham S Haufe E Harder I Heratizadeh A Kleinheinz A Wollenberg A Weisshaar E Augustin M Wiemers F Zink A Biedermann T von Kiedrowski R Hilgers M Worm M Pawlak M Sticherling M Fell I Handrick C Schäkel K Staubach P Implementation of dupilumab in routine care of atopic eczema: results from the German national registry TREATgermany. Br J Dermatol. 2020; 183: 382–384. [DOI] [PubMed] [Google Scholar]
- 36. Hartmann J Moitinho-Silva L Sander N Harder I Häsler R Rodriguez E Haufe E Kleinheinz A Abraham S Heratizadeh A Weisshaar E Schäkel K Handrick C Augustin M Wollenberg A Staubach-Renz P Ertner K Sticherling M Schwarz B Quist S Dupilumab but not cyclosporine treatment shifts the microbiome toward a healthy skin flora in patients with moderate-to-severe atopic dermatitis. Allergy. 2023; 78: 2290–2300. [DOI] [PubMed] [Google Scholar]
- 37. Musters AH van Lookeren FL van der Gang LF Middelkamp-Hup MA Bosma AL Jessurun NT Lapeere H Nguyen AL Ouwerkerk W de Schepper S Gerbens LAA Spuls PI Real-world reported adverse events related to systemic immunomodulating therapy in patients with atopic dermatitis: Results from the TREAT NL (TREatment of ATopic eczema, the Netherlands) registry. J Eur Acad Dermatol Venereol. 2024; 38: 530–542. [DOI] [PubMed] [Google Scholar]
- 38. Urdova V Rogers L Jesenak M Seys SF Real-life studies and registries of severe asthma: The advent of digital technology. Respir Med. 2023; 220: 107429. [DOI] [PubMed] [Google Scholar]
- 39. Möbus L Rodriguez E Harder I Boraczynski N Szymczak S Hübenthal M Stölzl D Gerdes S Kleinheinz A Abraham S Heratizadeh A Handrick C Haufe E Werfel T Schmitt J Weidinger S Blood transcriptome profiling identifies 2 candidate endotypes of atopic dermatitis. J Allergy Clin Immunol. 2022; 150: 385–395. [DOI] [PubMed] [Google Scholar]
- 40. Weisshaar E Bentz P Apfelbacher C Haufe E Heinrich L Heratizadeh A Abraham S Harder I Kleinheinz A Wollenberg A Schäkel K Wiemers F Ertner J Augustin M Wildberger J Von Kiedrowski R Worm M Zink A Effendy I Asmussen A Itching in Atopic Dermatitis: Patient- and Physician-reported Outcomes in the German Atopic Dermatitis Registry TREATgermany. Acta Derm Venereol. 2023; 103: adv00854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seefeldt M Buhl R Hamelmann E Idzko M Taube C Korn S German Asthma Net – Was haben wir bisher gelernt? Z Pneumol. 2023; 20: 88–93. [Google Scholar]
- 42. IVDK.org. 2020, Informationsbroschüre. https://www.ivdk.org/de/über-den-ivdk/informationsbroschüre/. Date of access July 26, 2024.
- 43. Anaphylaxie.net. 2024, Zentren. https://www.anaphylaxie.net/de/zentren/. Date of access July 24, 2024.
- 44. TREATgermany.org. 2024. https://treatgermany.org. Date of access October 7, 2024.


