Abstract
Cardiac angiosarcoma is a rare, diagnostically elusive disease with a poor prognosis. Herein, we describe the case of a 61-year-old man who presented with cardiac tamponade caused by perforation of the right atrial wall resulting from an invasive angiosarcoma. The tumour, which had spread throughout the entire right atrial free wall, was resected under cardiopulmonary bypass. Subsequent reconstruction of the right atrial wall involved the use of both autologous and bovine pericardial patches. Cardiac wall rupture due to invasive cardiac angiosarcoma is an extremely rare complication, with only a few cases reported. Therefore, we also review the relevant literature herein.
Keywords: angiosarcoma, right atrial wall perforation, cardiac tamponade
Cardiac angiosarcomas usually affect middle-aged men and are located in the right atrium in the two-thirds of affected patients.
INTRODUCTION
Cardiac angiosarcomas usually affect middle-aged men and are located in the right atrium in the two-thirds of affected patients. The growth is aggressive, with rapid progression and poor prognosis. Obtaining a diagnosis can be difficult and is usually delayed because there are no specific symptoms [1–3]. We present a rare case of the right atrial (RA) perforation due to the tumour invasion into the RA wall.
CASE REPORT
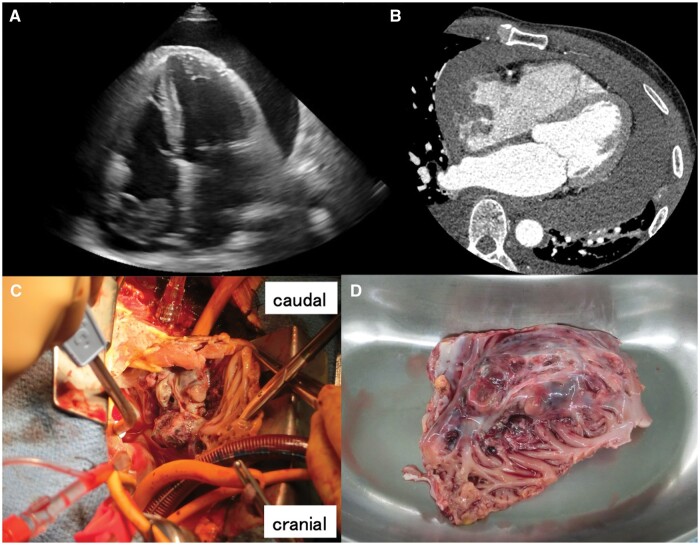
A 61-year-old man was emergently transferred to our clinic after developing cardiogenic shock at home. Echocardiography revealed significant pericardial effusion (Fig. 1A), suggestive of cardiac tamponade. Computed tomography revealed an irregularly formed mass in the right atrium, spreading throughout the entire RA free wall (Fig. 1B) and close to the orifice of the inferior vena cava (IVC). No remote metastases were observed. Approximately 1000 cc of haematogenous discharge was removed during an emergency pericardiocentesis, and as the fluid drained out, the patient’s systemic blood pressure decreased. The level of haemoglobin in the fluid was the same as that in the venous blood level. The patient was diagnosed with a perforated RA wall due to an invasive tumour, and emergent surgery was planned.
Figure 1:
(A) Echocardiograph displaying a large pericardial effusion and a cardiac mass in the right atrium. (B) Computed tomography image showing tumour spread throughout the right atrial free wall. (C) Nodular tumour extending into the RA wall. (D) Excised specimen, including the tumour and almost the entire RA free wall. RA: right atrial.
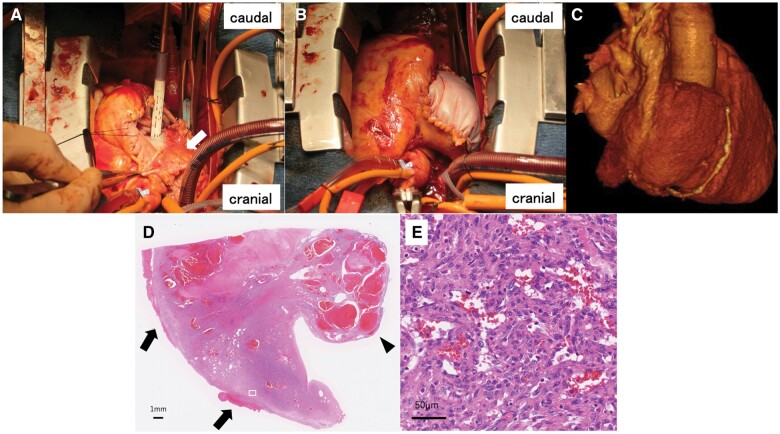
The pericardium was opened, and haematogenous discharge was observed to appear from the perforated RA free wall. The tumour had spread throughout the RA free wall, invading near the orifice of the IVC (Fig. 1C). Due to tumour invasion, there was difficulty inserting the IVC drainage tube from the right atrium to establish cardiopulmonary bypass; therefore, the tube was inserted from the femoral vein without a guidewire in consideration of tumour injury and possible embolism. The IVC was clamped with forceps at the distal portion of the orifice in the tumour-free space. The superior vena cava was directly cannulated, and cardiopulmonary bypass was subsequently established. The tumour was completely resected, including almost all of the RA free wall (Fig. 1D). The defective RA wall was reconstructed using autologous and bovine pericardial patches, 1st longitudinally from the superior vena cava to the IVC with an autologous pericardial patch (Fig. 2A), then the remaining free wall was reconstructed using a bovine pericardial patch while creating a bulge similar to the right atrium (Fig. 2B). The RA wall morphology was recreated by dividing the patch into 2 parts (Fig. 2C). Postoperative pathological findings revealed the tumour with indistinct borders and infiltrative growth in the total layer of myocardium (Fig. 2D). Tumour cells forming large and small malformed vascular cavities were also observed (Fig. 2E). Immunohistochemistry showed vimentin (+), S-100 (+, partial), CD34 (+++), α-smooth muscle actin (++) and Ki-67 (+++), leading to a diagnosis of angiosarcoma. The patient was discharged 9 days after surgery without any complications.
Figure 2:
(A) Reconstructed defective RA wall, initially longitudinally from the superior to inferior vena cava using an autologous pericardial patch (arrow), (B) and subsequently the remaining free wall using a bovine pericardial patch, creating a bulge similar to the right atrium. (C) Computed tomography image of the reconstructed RA wall. (D) Tumour with indistinct borders and infiltrative growth through the entire layer of myocardium and a polypoid protrusion into the cardiac cavity (arrowhead) associated with haemopericardium (arrow). (E) Large, strongly atypical epithelioid tumour cells, forming irregular vessels. RA: right atrial.
DISCUSSION
Primary cardiac tumours are exceedingly rare, detected in only 0.1% of a series of autopsies [1]. Cardiac malignancies are uncommon, accounting for 25% of cardiac tumours [3]. Angiosarcomas are the most common malignant tumour of the heart and show rapid growth, local invasion and distant metastasis in middle-aged patients. Signs and symptoms are usually nonspecific and depend on the location and extent of the tumour [1–3]. At the time of diagnosis, ∼80% of patients have metastases [2], and the survival rate is very low (12–30 months) despite multimodality treatment [1]. Our patient developed shortness of breath ∼2 weeks before presenting to the emergency department but had no prior symptoms. More than half of the patients diagnosed with cardiac angiosarcoma exhibit pericardial effusion; therefore, pericardial effusion and cardiac tamponade are the most common complications. There are sometimes found to be negative for malignancy on cytological review if the tumour has invaded the pericardium [3]. While many reports document exudative pericardial effusions caused by tumours, there are few reports of tamponade resulting from cardiac wall ruptures [1–4].
No guidelines have been established for the treatment of primary cardiac angiosarcoma owing to its rarity. Surgical resection is the preferred first-line treatment in the absence of metastasis, and complete tumour resection is required. Preoperative chemotherapy may decrease the bulk and eliminate micrometastases before local excision, which is beneficial for patients with metastatic disease [5]. Although there are reports of effective chemotherapy, there is no definitive evidence supporting chemotherapy due to the small number of cases and the very poor prognosis [3–5]. An early diagnosis is the most important aspect for improving patient outcomes; therefore, primary cardiac malignancy should be included in the differential diagnosis when younger patients present with an unknown cardiac tamponade, even if the cytologic evaluation of the pericardial effusion reveals a negative result.
ACKNOWLEDGEMENTS
The authors would like to thank Senba M.D. for the useful pathological diagnoses.
Conflict of interest: none declared.
Contributor Information
Yuichiro Yokoyama, Department of Cardiovascular Surgery, Yotsuba Circulation Clinic, Ehime, Japan.
Takeshi Emmoto, Department of Cardiovascular Surgery, Yotsuba Circulation Clinic, Ehime, Japan.
Yumiko Abe, Department of Cardiovascular Surgery, Yotsuba Circulation Clinic, Ehime, Japan.
Mitsunori Abe, Department of Cardiovascular Surgery, Yotsuba Circulation Clinic, Ehime, Japan.
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author, Yuichiro Yokoyama, upon reasonable request.
ETHICAL STATEMENT
The patient provided written informed consent for the publication of this case report using protected health information, including medical images, in accordance with the requirements of the Health Insurance Portability and Accountability Act (HIPAA) privacy regulations.
REFERENCES
- 1. Murthy JSN, Gorantla R, Periyasamy T. et al. Right atrial angiosarcoma presenting as giant pseudoaneurysm with impending rupture. IHJ Cardiovascular Case Reports (CVCR) 2017;1:165–8. [Google Scholar]
- 2. Yoshitake I, Hata M, Sezai A. et al. Cardiac angiosarcoma with cardiac tamponade diagnosed as a ruptured aneurysm of the sinus valsalva. Jpn J Clin Oncol 2009;39:612–5. [DOI] [PubMed] [Google Scholar]
- 3. Kim J, Da Nam BD, Hwang JH. et al. Primary cardiac angiosarcoma with right atrial wall rupture: a case report. Medicine (Baltimore) 2019;98:e15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jain G, Mukhopadhyay S, Kurien S, Yusuf J, Tyagi S, Jain R.. Ruptured cardiac angiosarcoma with pulmonary metastases: a rare disease with a common (mis)diagnosis! Indian Heart J 2012;64:603–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y, Liu Q, Wu H.. Primary cardiac tumor: a case report of right atrial angiosarcoma and review of the literature. Front Oncol 2023;13:1164153. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, Yuichiro Yokoyama, upon reasonable request.