Spontaneous intracerebral hemorrhage (ICH) accounts for 10–30% of all strokes worldwide and is more prevalent in Asians, Hispanics, and African Americans.[1–3] Uncontrolled hypertension and anticoagulant use are the common causes of spontaneous ICH.[4–6] Non-contrast head computed tomography (CT) is very sensitive for the diagnosis of ICH. CT angiography (CTA), magnetic resonance angiography (MRA), or catheter angiography are indicated to rule out vascular abnormalities and other underlying etiologies, particularly in younger patients.[3] Depressed level of consciousness, large ICH volume, intraventricular extension, and hematoma expansion are the predictors of poor outcomes after ICH.[7–11] Although early pioneering studies of surgical treatment showed promising results,[12,13] subsequent landmark randomized controlled trials (RCTs) including surgical trials in intracerebral hemorrhage (STICH), STICH II, and Minimally Invasive Surgery plus Alteplase for Intracerebral Hemorrhage Evacuation Phase III (MISTIE III) failed to show definitive outcome benefit from surgical evacuation.[14–17] Medical therapies targeting hematoma expansion, such as the intensive lowering of systolic blood pressure (SBP) to less than 140 mmHg, reversal of anticoagulation or hemostatic therapy with Andexanet alfa, recombinant activated factor VII (rFVIIa), or tranexamic acid, showed a reduction of hematoma expansion without significant outcome benefit.[18–23]
Recent Advances in Medical Management
In patients with spontaneous ICH, the major determinants of functional outcome include hematoma location, size, and hematoma expansion. Hematoma expansion occurs in approximately 30% of patients.[9,10] It occurs mostly during the first 3 h after ICH.[24–26] Several imaging markers, including spot signs, may predict early hematoma expansion with good accuracy on a contrast CT or CTA.[25,26]
Intensive BP control
Two phase III, multicenter, and prospective RCTs evaluated the effect of intensive blood pressure (BP) control after ICH.[19,20] The rapid blood-pressure lowering in patients with acute intracerebral hemorrhage (INTERACT-2) trial compared early lowering of SBP to <140 mmHg vs. <180 mmHg within 6 h of ICH onset. It showed no significant differences in adverse events, mortality, or severe disability at 90 days.[19] An ordinal analysis of modified Rankin Scale (mRS) scores indicated improved functional outcomes with intensive BP control. The antihypertensive treatment of acute cerebral hemorrhage (ATACH-2) trial used intravenous nicardipine within 3 h of ICH onset to target SBP <140 mmHg vs. <180 mmHg.[20] There was no significant difference in hematoma expansion, mortality, or disability at 90 days. The intensive BP control group, however, had a higher rate of adverse renal events than the standard treatment group (9.0% vs. 4.0%, P = 0.002). The contradictory results were likely attributable to differences in study design and overly aggressive BP control in the ATACH-2 trial (i.e., a mean minimum SBP of 128.9 mmHg vs. 141.1 mmHg during the first 2 h, as compared to a mean SBP of 150 mmHg vs. 164 mmHg at 1 h in the INTERACT-2 trial).
A pooled analysis of individual patient data from INTERACT-2 and ATACH-2 trials showed that achieving early and stable SBP to 120–130 mmHg was associated with favorable outcomes in patients with mild-to-moderate severe ICH, whereas drops >60 mmHg within the first hour were harmful.[27] An analysis of pooled data from all the RCTs registered in the Blood Pressure in Acute Stroke Collaboration showed that earlier SBP control to 120–140 mmHg over 24 h was associated with a significant lower risk of hematoma expansion and better functional outcomes, especially in patients with hematoma volume >10 mL.[28]
The INTERACT-3 trial evaluated the effectiveness of a care bundle of early intensive SBP lowering to <140 mmHg, strict glucose control, fever control, and reversal of anticoagulation in 7036 ICH patients presenting within 6 h of symptom onset.[29] The likelihood of a poor functional outcome at 6 months was lower in the care bundle group (odds ratio [OR], 0.86; 95% CI, 0.76–0.97; P = 0.015).
INTERACT-4 trial evaluated the effect of ultra-early BP control in the ambulance.[30] Suspected acute stroke patients with a motor deficit and elevated SBP ≥150 mmHg were randomized within 2 h of symptom onset to receive immediate treatment to lower the SBP to 130–140 mmHg (intervention group) or usual BP management (usual-care group). At hospital arrival, the mean SBP was 159 mmHg in the intervention group and 170 mmHg in the usual-care group. Prehospital reduction of SBP was associated with a decrease in the odds of a poor functional outcome among patients with hemorrhagic stroke (common OR, 0.75; 95% CI, 0.60–0.92). In summary, early SBP reduction to 120–140 mmHg within the first 2–6 h may reduce the risk of hematoma expansion and improve the functional outcomes after ICH.
Reversal of oral anticoagulation
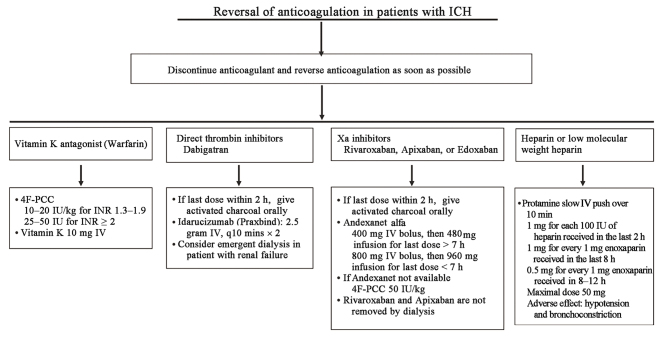
Anticoagulant-associated ICH increases the risk of hematoma expansion, poor outcome, and death.[5,6,31] Early reversal of anticoagulation is crucial to prevent hematoma expansion. The current guidelines on reversal of anticoagulation are summarized in Figure 1.[11,32] In patients with anticoagulant-associated ICH, the anticoagulant should be discontinued immediately. A dose of activated charcoal is recommended to prevent the absorption of direct oral anticoagulants (DOAC) if the agent was taken within the previous 2 h. Specific reversal agents should be administered as soon as possible with respect to the different anticoagulants.[24,33]
Figure 1.
Current approaches for reversal of anticoagulation. 4F-PCC: 4-factor prothrombin complex concentrate; ICH: Intracerebral hemorrhage; INR: International normalized ratio; IV: Intravenous injection.
4-factor prothrombin complex concentrate (4F-PCC)
The management of vitamin K antagonist (VKA)-related ICH includes the quick reversal of its effect by 4F-PCC and vitamin K, with a goal of international normalized ratio (INR) <1.3 within 4 h.[32] 4F-PCC was superior to fresh frozen plasma (FFP) with respect to normalizing the INR. Faster INR normalization was associated with smaller hematoma expansion in these studies.[33,34] Intravenous vitamin K should be administered after PCC to prevent later increase in INR and subsequent hematoma expansion.[32] In a multicenter, retrospective study of 67 patients with intracranial hemorrhage, activated PCC (aPCC) or 4F-PCC were found to be equally effective for the reversal of apixaban or rivaroxaban effect.[35] There were no significant differences in hemostatic efficacy (P = 0.362) or mortality (P = 0.838) between the study groups.
Idarucizumab
Dabigatran (Pradaxa) is a direct thrombin inhibitor. Idarucizumab is a monoclonal antibody fragment developed to reverse the dabigatran effect.[36] In a multicenter, prospective, open-label study, 235 patients (137 with gastrointestinal bleeding and 98 with intracranial hemorrhage) received intravenous idarucizumab (administered in a two 2.5 g dose) with rapid and complete reversal of dabigatran effect. At 90 days, thrombotic events occurred in 6.3% of the patients. The 30-day mortality rate was 16.4% among patients with intracranial hemorrhage. There was no available data on hematoma expansion. Of note, dabigatran is excreted by the kidneys. When idarucizumab is not available, dialysis can be considered to reduce the plasma dabigatran concentration in patients with renal impairment.[37]
Andexanet
Andexanet is a recombinant human factor Xa decoy protein designed to reverse the effect of factor Xa inhibitors.[38] In a multicenter, prospective, single-group study of 479 patients with acute major bleeding (69% intracranial and 23% gastrointestinal), an Andexanet bolus (400 mg or 800 mg) over 15–30 min followed by a 2-h infusion (480 mg or 960 mg) resulted in good or excellent hemostatic efficacy in 80% of the patients treated with four different factor Xa inhibitors.[39] The Andexanet for FXa inhibitor-associated ICH (ANNEXA-1) trial was a randomized, multicenter clinical trial evaluating the safety and efficacy of Andexanet vs. usual care for ICH patients within 6 h of symptom onset and within 15 h of taking an oral FXa inhibitor.[23] The primary endpoint was hemostatic efficacy, defined by hematoma expansion by 35% or less at 12 h after baseline, an increase in National Institutes of Health Stroke Scale (NIHSS) score of less than seven points at 12 h, and no rescue therapy between 3 h and 12 h after randomization. The Andexanet group was associated with higher rates of hemostatic efficacy (67.0% vs. 53.1%; P = 0.003), thrombotic events (10.3% vs. 5.6%; P = 0.048), and ischemic stroke (6.5% vs. 1.5%). There was no significant difference in functional outcomes at 30 days between the two groups. Given the higher rate of thrombotic events in the Andexanet group and the lack of functional outcome benefit at 30 days, additional studies are warranted to prove the outcome benefit of Andexanet.
Hemostatic therapy
Hemostatic therapy is a treatment that uses medications to stop bleeding when the body’s natural hemostatic system cannot. In patients with intracranial hemorrhage, hemostatic efficacy is defined as stable hematoma volume or increase by less than 35% as compared to baseline volume.[23]
Factor VIIa
rFVIIa directly activates factor X on the surface of activated platelets, resulting in acceleration of coagulation. In the factor VII for acute intracerebral hemorrhage (FAST) trial, 841 patients with non-coagulopathic ICH were randomly assigned to receive placebo, 20 μg/kg rFVIIa, or 80 μg/kg rFVIIa within 4 h of symptom onset. The study showed that the use of rFVIIa reduced hematoma expansion without survival or functional outcome benefit at 90 days after ICH.[18]A secondary analysis of the FAST data suggested that patients with age ≤70 years, ICH volume <60 mL, intraventricular hemorrhage (IVH) volume <5 mL, and onset-to-treatment time ≤2.5 h had almost 50% reduction in hematoma growth (7.3 ± 3.2 vs. 3.8 ± 1.5; P = 0.02) and a trend toward better functional outcome (adjusted OR, 0.28; 95% CI, 0.08–1.06).[40] The rFVIIa for hemorrhagic stroke trial (FASTEST) is currently enrolling patients for earliest treatment with rFVIIa 80 μg/kg or placebo within 2 h of symptom onset (https://classic.clinicaltrials.gov/ct2/show/NCT03496883). The primary outcome will be determined at 180 days.
Tranexamic acid
Tranexamic acid is a synthetic lysine analog that competes with lysine residues on fibrin and effectively inhibits the interaction between fibrinolytic enzymes and fibrin. It prevents the dissolution of fibrin clots and hematoma expansion. In the tranexamic acid for hyperacute primary intracerebral hemorrhage (TICH-2) trial, 2325 ICH patients were randomized to receive 1 g intravenous tranexamic acid bolus followed by an 8-h infusion of 1 g tranexamic acid or placebo within 8 h of symptom onset. The study showed no significant difference in functional outcomes at 90 days (adjusted OR, 0.88; 95% CI, 0.76–1.03; P = 0.11) between the two treatment groups.[21] In the subgroup analysis of TICH-2 data, there was also no significant difference in hematoma growth between the two groups with spot-sign positive (OR, 0.85; 95% CI, 0.29–2.46) or negative (OR, 0.77; 95% CI, 0.41–1.45) participants (Pheterogenity = 0.88).[41] Recently, tranexamic acid for acute ICH growth based on imaging assessment (TRAIGE) trial and the tranexamic acid for intracerebral hemorrhage in patients on non-vitamin K antagonist oral anticoagulants (TICH-NOAC) trial also showed no effect of intravenous tranexamic acid on hematoma expansion.[22,42]
Platelet transfusion
Antiplatelet drugs interfere with platelet aggregation and increase the risk of hematoma expansion and poor outcomes after ICH. The platelet transfusion vs. standard care for spontaneous ICH associated with antiplatelet therapy (PATCH) trial randomized 190 patients with prior antiplatelet use to standard care or standard care plus platelet transfusion within 90 min of diagnostic brain imaging.[43] The odds of death or dependence at 3 months were higher in the platelet transfusion group than in the standard care group (adjusted OR, 2.05; 95% CI, 1.18–3.56; P = 0.0114). In addition, platelet transfusion was also associated with more serious adverse events (42% vs. 29%). Therefore, platelet transfusions are potentially harmful and should not be recommended unless the patient requires emergency surgery.[11]
Desmopressin (DDAVP)
DDAVP induces the synthesis of the von Willebrand factor (VWF) by endothelial cells and is commonly used as a pro-hemostatic drug for the treatment of inherited bleeding disorders. In a recent multicenter retrospective study comparing patients with (n = 118) or without treatment with DDAVP (n = 91), there were no significant differences in hematoma expansion (16.1% vs. 17.6%; P = 0.78) or secondary outcomes between the two groups.[44]
Recent Advances in Surgical Treatment
Early surgical hematoma evacuation may reduce the mass effect, intracranial pressure (ICP), midline shift, or herniation. Additionally, it may also reduce secondary brain injury. The common surgical approaches include (1) craniotomy for hematoma evacuation, (2) minimally invasive surgery (MIS) for hematoma evacuation, and (3) external ventricular drain (EVD) for IVH and hydrocephalus.
Craniotomy for hematoma evacuation
The STICH was the first multicenter trial to investigate the effect of surgical hematoma evacuation.[14] A total of 1033 patients with supratentorial lobar or basal ganglia hemorrhage were randomized to receive conservative management or hematoma evacuation within 24 h of randomization. The main inclusion criteria were a spontaneous supratentorial ICH within 72 h of ictus, a hematoma diameter ≥2 cm, and a Glasgow Coma Scale (GCS) ≥5. The study showed no significant difference in favorable functional outcome (26% vs. 24%, P = 0.414) or mortality (36% vs. 37%, P = 0.707) at 6 months between the two groups.
The STICH II trial was performed by the same group of investigators to test early surgery vs. conservative treatment in patients with spontaneous supratentorial lobar ICH.[15] A total of 601 patients with hematoma volume 10–100 mL, a best GCS motor score of 5 or 6, and a best eye score of 2 or more within 48 h of ictus were randomized to surgical evacuation or conservative management. There was also no significant difference in unfavorable outcome (62% vs. 59%, P = 0.367) or mortality (18% vs. 24%, P = 0.095) at 6 months between the two groups. The subgroup analysis revealed a small survival benefit in patients with superficial lobar hemorrhage without IVH. The major limitations of the two landmark trials were delayed median ictus to surgery time (30 h and 26 h, respectively) and inclusion of patients with small or massive ICH (a hematoma diameter ≥2 cm or 10–100 mL).
MIS
Craniotomy for hematoma evacuation requires a large bone flap, brain parenchyma dissection, and retraction of normal brain tissue to reach the hematoma. MIS has the advantage of causing less injury to the normal brain tissue through smaller openings and peri-fascicular tract formation.
Auer et al[12] were the first to report a clinical study comparing endoscopic hematoma evacuation with medical treatment in 1989. A total of 100 spontaneous ICH patients with focal deficits with or without altered level of consciousness, and hematoma volume ≥10 mL were treated within 48 h of ictus. In the surgical group, the hematomas were evacuated through a burr hole with a neuro-endoscope. The SBP goal was 140–160 mmHg in both surgical and medical groups. At 6 months after hemorrhage, the surgical group was associated with a significantly lower mortality (42% vs. 70%, P <0.01) and better outcome with no or only a minimal deficit (40% vs. 25%, P <0.05). The surgical benefit was significant for patients with hematomas less than 50 mL.
In 2009, Wang et al[13] published the results of an RCT comparing minimally invasive craniopuncture with conservative treatment for patients with spontaneous basal ganglia ICH. The inclusion criteria were hematoma volume of 25–40 mL, GCS score ≥9, and symptom onset within 72 h of ictus. 377 patients were randomly assigned to receive craniopuncture (n = 195) or conservative treatment (n = 182). Craniopuncture was associated with a significantly lower rate of dependent survivors (mRS >2) than conservative treatment at 3 months (40.9% vs. 63.0%, P <0.01). There was no significant difference in mortality (6.7% vs. 8.8%) between the two groups.
MIS with thrombolysis in intracerebral hemorrhage evacuation (MISTIE III) trial was an open-label, phase 3 RCT conducted at 78 hospitals in North America, Europe, Australia, and Asia.[17] The procedure included stereotactic or image-guided placement of a catheter inside the hematoma, followed by the intra-hemorrhage aspiration and thrombolysis with 1.0 mg recombinant tissue-type plasminogen activator (r-tPA) every 8 h to a maximum of nine doses. The 506 supratentorial ICH patients with hematoma volume ≥30 mL, GCS ≤14 or NIHSS ≥6, and hematoma expansion <5 mL for at least 6 h after diagnostic CT scan were randomized to either MISTIE group or conservative management. Although MISTIE led to a mean reduction in hematoma size by 69% compared with 3% in the conservative treatment, there was no significant difference in favorable functional outcome (mRS 0–3) at 12 months (45% vs. 41%, P = 0.33). A meta-analysis of outcome data at 180 days showed a significantly higher rate of favorable functional outcome in patients with residual hemorrhage volume was <15 mL.
Xu et al[45] investigated the outcome of MIS with endoscopic surgery or stereotactic aspiration and small-bone flap craniotomy in hypertensive supratentorial ICH (MISICH trial). In the study conducted at 16 centers in China, 733 patients with ICH volume ≥25 mL were randomized to receive endoscopic surgery, stereotactic aspiration, or craniotomy at a 1:1:1 ratio. MIS was associated with better functional outcomes at 6 months (33.3% in the endoscopy group and 32.7% in the aspiration group) than craniotomy (22.2%, P = 0.017). In subgroup analysis, MIS was associated with better functional outcomes in patients with basal ganglia and thalamic hemorrhage than craniotomy. There was no significant difference in patients with lobar hemorrhage between the three groups. The limitation of the study was the lack of a medical treatment group.
In the recently published early minimally invasive removal of intracerebral hemorrhage (ENRICH) trial,[46] 300 patients with anterior basal ganglia or lobar hemorrhage of 30–80 mL were randomized within 24 h of last-known normal to either minimally invasive surgical removal of the hematoma plus medical management (surgery group) or medical management alone (control group). The primary efficacy endpoint was the mean score on the utility-weighted mRS at 180 days. The study demonstrated better functional outcomes in the surgery group at 180 days (the mean utility-weighted mRS was 0.458 vs. 0.374; difference, 0.084; 95% Bayesian credible interval, 0.005–0.163; posterior probability of superiority of surgery, 0.981). The percentage of patients who had died by 30 days was 9.3% in the surgery group and 18.0% in the control group.
While ENRICH trial showed better outcomes at 180 days after MIS, the beneficial effect of MIS appeared to be mostly attributable to intervention for lobar hemorrhages (+0.1418). The basal ganglia patients fared worse (−0.0406). These results are different from the findings from other RCTs, which showed better functional outcomes with MIS for basal ganglia hemorrhage.[13,45] The inconsistent results are likely due to different inclusion criteria, surgical techniques, efficacy endpoint, and statistical analysis. Additional studies are warranted to investigate the effect of MIS on both lobar and deep subcortical hematomas.
EVD for IVH
IVH is seen in up to 45% of patients with ICH.[47] It is associated with lower GCS and worse outcomes. In addition to clotting the cerebral aqueduct and causing obstructive hydrocephalus, intraventricular blood and its breakdown products cause significant inflammation of the ependymal and subependymal tissue.[48] The IVH may also cause inflammation and fibrosis of the arachnoid granulations, resulting in delayed communicating hydrocephalus.
Clot lysis: evaluating accelerated resolution of intraventricular hemorrhage phase III (CLEAR III) trial randomized 500 patients with ICH volume ≤30 mL, IVH obstructing the 3rd or 4th ventricles, and no underlying pathology to receive intraventricular r-tPA 1 mg every 8 h for up to 12 doses (n = 249) or 0.9% saline (n = 251) via EVD.[16] At 6 months after hemorrhage, there was no significant difference in good functional outcome (mRS ≤3) between the two groups (48% vs. 45%; P = 0.554). In the subgroup analysis, patients with large IVH (20–50 mL) appear to have better functional outcomes with r-tPA treatment.
Critical Thinking on Future Clinical Trial Design
Timing of medical therapy
In a post hoc analysis of the combined data from INTERACT-2 and ATACH-2 trials, intensive BP control within 2 h of ICH onset and reaching the target within one hour were found to be associated with reduced hematoma growth and improved functional outcome.[49,50] INTERACT-4 results supported the finding.[30] INTERACT-3 results suggested functional outcome benefit from early comprehensive medical therapy within 6-h of symptom onset.[29] These data strongly support early comprehensive medical therapy, including intensive BP lowering and reversal of anticoagulation, within 2–6 h of ICH onset in all future clinical trial designs.
Hematoma volume and timing of hematoma evacuation
Patients with small hematomas may not benefit from surgical evacuation due to brain tissue injury from the surgical procedure, especially in patients with basal ganglia and thalamic hemorrhage. In contrast, surgery could be futile for patients with massive ICH (hematoma volumes >90 mL) due to irreversible early brain damage.[7] The inclusion criteria, intervention, outcome, and limitations of recent landmark surgical trials for ICH are summarized in Table 1. Among these trials, MISTIE III and CLEAR III had the most significant delay from onset to randomization (47 h and 51.8 h, respectively).[17,18] The STICH and STICH II trials included patients with small and large hematomas (minimal hematoma diameters ≥2 cm, or hematoma volume 10–100 mL) and median ictus to surgery time of 26–30 h.[15,16] The study by Wang et al[13] and ENRICH trial[46] showed significant functional outcome benefit from MIS for ICH. Both studies had better patient selection (with hematoma volume 25–40 mL and 30–80 mL, respectively) and earlier surgery. In a meta-analysis of pooled data (n = 2186) from eight studies on surgical treatment of ICH, randomization within 8 h of ictus was associated with better outcomes than those randomized more than 8 h of ictus.[51] Therefore, future surgical trials should select patients with hematoma volumes 25–80 mL for early surgery within 2–8 h of ictus.
Table 1.
Summary of the landmark RCTs on surgical treatment of ICH or IVH.
| Study | Inclusion criteria | Sample size: surgery vs. control (n) | Median or mean time from ictus to randomization or surgery (h) | Primary outcome | Favorable outcome | Mortality: surgery vs. control (%) | Limitation |
|---|---|---|---|---|---|---|---|
| Mendelow et al[14] 2005 STICH | Minimal hematoma diameters ≥2 cm GCS ≥5, within 72 h of ictus |
503 vs. 530 | 30 (16–49) | GOS at 6 months | 26% vs. 24%, P = 0.414 | 36% vs. 37%, P = 0.707 | Patients with small and large hematoma volume were included, delayed surgery |
| Wang et al[13] 2009 | Motor strength 0–3, GCS >9, basal ganglion hemorrhage 25–40 mL, within 72 h of ictus | 195 vs. 182 | 21.1 (4–72) | Death or dependency (mRS >2) at 90 days | 59.1% vs. 37.0%, P <0.0001 | 6.7% vs. 8.8%, P = 0.44 | Relatively small sample size, unable to compare the outcome of early vs. late surgery |
| Mendelow et al[15] 2013 STICH II | Conscious, superficial ICH, volume 10–100 mL, no IVH, within 48 h of ictal | 305 vs. 292 | 26 (15.3–25.3) | GOSE at 6 months | 41% vs. 38%, P = 0.367 | 18% vs. 24%, P = 0.095 | Patients with small and large hematomas were included, 47% surgery ≥21 h after ICH |
| Hanley et al[16] 2017 CLEAR III | ICH volume ≤30 mL, IVH obstructing the 3rd or 4th ventricles | 246 vs. 245 | 51.8 (36.4–65.8) | mRS 0–3 at 180 days | 48% vs. 45%, P = 0.554 | 18% vs. 29%, P = 0.007 | Most significantly delayed treatment |
| Hanley et al[17] 2019 MISTIE III | ICH volume ≥30 mL, GCS ≤14 or NIHSS ≥6, and hematoma expansion <5 mL for at least 6 h | 255 vs. 251 | 47 (33–60) | mRS 0–3 at 12 months | 45% vs. 41%, P = 0.33 | 19% vs. 26%, P = 0.08 | Very significantly delayed treatment |
| Pradilla et al[46] 2024 ENRICH | Hematoma volume 30–80 mL, GCS 5–14, NIHSS >5, within 24 h of ictus | 150 vs. 150 | 16.75 (10.7–21.3) | Utility weighted-mRS at 180 days | 0.458 vs. 0.374. Significant for superiority | 20% vs. 23%, P = 0.669 | Utility weighted-mRS used as primary endpoint |
ENRICH: Early minimally invasive removal of intracerebral hemorrhage; GCS: Glasgow Coma Scale; GOS: Glasgow Outcome Scale; GOSE: Extended Glasgow Outcome Scale; ICH: Intracerebral hemorrhage; IVH: Intraventricular hemorrhage; mRS: Modified Rankin Scale; NIHSS: National Institutes of Health Stroke Scale; RCTs: Randomized controlled trials; STICH: Surgical trial in intracerebral hemorrhage.
Validated functional outcome measurement as primary endpoint
In most clinical trials on the reversal of anticoagulation, hemostatic efficacy was used as the primary efficacy endpoint.[23,36,39] As shown in Annexa-I trial, effective reversal of oral anticoagulation is not necessarily associated with better functional outcomes.[23] Therefore, in all future clinical trials, long-term functional outcomes should be used as the primary efficacy endpoint.
The ENRICH trial used utility-weighted mRS score as the primary efficacy endpoint,[46] which has not been validated for assessment of functional outcome in stroke trials.[46] A lower mortality rate in the MIS group likely contributed to a higher mean utility-weighted mRS score. Finally, recovery from severe ICH can be slow and gradual. In a recent post hoc analysis of 715 survivors with severe disability (mRS 4–5) at 30 days from the CLEAR-III and MISTIE III trials, 43% had achieved mRS 0–3 at 12 months.[52] Therefore, the optimal timing for the primary efficacy endpoint in future clinical trials should be at least 3 months.
“Time is brain” should also apply to ICH. Early comprehensive therapy and surgery within 2–8 hours, optimal hematoma volumes for surgical evacuation, and the use of validated functional outcome assessment are critical for future clinical trial design.
Conflicts of interest
None.
Footnotes
How to cite this article: Yu WG, Alexander MJ. Spontaneous intracerebral hemorrhage: Recent advances and critical thinking on future clinical trial design. Chin Med J 2024;137:2899–2906. doi: 10.1097/CM9.0000000000003408
References
- 1.Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: A systematic review. Lancet Neurol 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- 2.Wang W Jiang B Sun H Ru X Sun D Wang L, et al. Prevalence, incidence, and mortality of stroke in China: Results from a nationwide population-based survey of 480 687 adults. Circulation 2017;135:759–771. doi: 10.1161/CIRCULATIONAHA.116.025250. [DOI] [PubMed] [Google Scholar]
- 3.Dastur CK, Yu W. Current management of spontaneous intracerebral haemorrhage. Stroke Vasc Neurol 2017;2:21–29. doi: 10.1136/svn-2016-000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qureshi AI Ezzeddine MA Nasar A Suri MF Kirmani JF Hussein HM, et al. Prevalence of elevated blood pressure in 563,704 adult patients with stroke presenting to the ED in the United States. Am J Emerg Med 2007;25:32–38. doi: 10.1016/j.ajem.2006.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke 1995;26:1471–1477. doi: 10.1161/01.str.26.8.1471. [DOI] [PubMed] [Google Scholar]
- 6.Huhtakangas J, Tetri S, Juvela S, Saloheimo P, Bode MK, Hillbom M. Effect of increased warfarin use on warfarin-related cerebral hemorrhage: A longitudinal population-based study. Stroke 2011;42:2431–2435. doi: 10.1161/STROKEAHA.111.615260. [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Brott TG, Duldner JE, Tomsick T, Huster G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993;24:987–993. doi: 10.1161/01.str.24.7.987. [DOI] [PubMed] [Google Scholar]
- 8.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001;32:891–897. doi: 10.1161/01.str.32.4.891. [DOI] [PubMed] [Google Scholar]
- 9.Davis SM Broderick J Hennerici M Brun NC Diringer MN Mayer SA, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006;66:1175–1181. doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 10.Dowlatshahi D Demchuk AM Flaherty ML Ali M Lyden PL Smith EE, et al. Defining hematoma expansion in intracerebral hemorrhage: Relationship with patient outcomes. Neurology 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM Ziai WC Cordonnier C Dowlatshahi D Francis B Goldstein JN, et al. 2022 guideline for the management of patients with spontaneous intracerebral hemorrhage: A guideline from the American Heart Association/American Stroke Association. Stroke 2022;53:e282–e361. doi: 10.1161/STR.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 12.Auer LM Deinsberger W Niederkorn K Gell G Kleinert R Schneider G, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: A randomized study. J Neurosurg 1989;70:530–535. doi: 10.3171/jns.1989.70.4.0530. [DOI] [PubMed] [Google Scholar]
- 13.Wang WZ Jiang B Liu HM Li D Lu CZ Zhao YD, et al. Minimally invasive craniopuncture therapy vs. conservative treatment for spontaneous intracerebral hemorrhage: Results from a randomized clinical trial in China. Int J Stroke 2009;4:11–16. doi: 10.1111/j.1747-4949.2009.00239.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendelow AD Gregson BA Fernandes HM Murray GD Teasdale GM Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International surgical trial in intracerebral haemorrhage (STICH): A randomised trial. Lancet 2005;365:387–397. doi: 10.1016/S0140-6736(05)17826-X. [DOI] [PubMed] [Google Scholar]
- 15.Mendelow AD Gregson BA Rowan EN Murray GD Gholkar A Mitchell PM, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): A randomised trial. Lancet 2013;382:397–408. doi: 10.1016/S0140-6736(13)60986-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hanley DF Lane K McBee N Ziai W Tuhrim S Lees KR, et al. Thrombolytic removal of intraventricular haemorrhage in treatment of severe stroke: Results of the randomised, multicentre, multiregion, placebo-controlled clear III trial. Lancet 2017;389:603–611. doi: 10.1016/S0140-6736(16)32410-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hanley DF Thompson RE Rosenblum M Yenokyan G Lane K McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): A randomised, controlled, open-label, blinded endpoint phase 3 trial [published correction appears in Lancet. 2019 Apr 20;393(10181):1596]. Lancet 2019;393:1021–1032. doi: 10.1016/S0140-6736(19)30195-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer SA Brun NC Begtrup K Broderick J Davis S Diringer MN, et al. Efficacy and safety of recombinant activated factor VII for acute intracerebral hemorrhage. N Engl J Med 2008;358:2127–2137. doi: 10.1056/NEJMoa0707534. [DOI] [PubMed] [Google Scholar]
- 19.Anderson CS Heeley E Huang Y Wang J Stapf C Delcourt C, et al. Rapid blood-pressure lowering in patients with acute intracerebral hemorrhage. N Engl J Med 2013;368:2355–2365. doi: 10.1056/NEJMoa1214609. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI Palesch YY Barsan WG Hanley DF Hsu CY Martin RL, et al. Intensive blood-pressure lowering in patients with acute cerebral hemorrhage. N Engl J Med 2016;375:1033–1043. doi: 10.1056/NEJMoa1603460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sprigg N Flaherty K Appleton JP Al-Shahi Salman R Bereczki D Beridze M, et al. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): An international randomised, placebo-controlled, phase 3 superiority trial. Lancet 2018;391:2107–2115. doi: 10.1016/S0140-6736(18)31033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J Nie X Gu H Zhou Q Sun H Tan Y, et al. Tranexamic acid for acute intracerebral haemorrhage growth based on imaging assessment (TRAIGE): A multicentre, randomised, placebo-controlled trial. Stroke Vasc Neurol 2021;6:160–169. doi: 10.1136/svn-2021-000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Connolly SJ Sharma M Cohen AT Demchuk AM Członkowska A Lindgren AG, et al. Andexanet for factor Xa inhibitor-associated acute intracerebral hemorrhage. N Engl J Med 2024;390:1745–1755. doi: 10.1056/NEJMoa2313040. [DOI] [PubMed] [Google Scholar]
- 24.Brott T Broderick J Kothari R Barsan W Tomsick T Sauerbeck L, et al. Early hemorrhage growth in patients with intracerebral hemorrhage. Stroke 1997;28:1–5. doi: 10.1161/01.str.28.1.1. [DOI] [PubMed] [Google Scholar]
- 25.Demchuk AM Dowlatshahi D Rodriguez-Luna D Molina CA Blas YS Dzialowski I, et al. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): A prospective observational study. Lancet Neurol 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 26.Morotti A Boulouis G Dowlatshahi D Li Q Shamy M Al-Shahi Salman R, et al. Intracerebral haemorrhage expansion: Definitions, predictors, and prevention. Lancet Neurol 2023;22:159–171. doi: 10.1016/S1474-4422(22)00338-6. [DOI] [PubMed] [Google Scholar]
- 27.Moullaali TJ Wang X Martin RH Shipes VB Robinson TG Chalmers J, et al. Blood pressure control and clinical outcomes in acute intracerebral haemorrhage: A preplanned pooled analysis of individual participant data. Lancet Neurol 2019;18:857–864. doi: 10.1016/s1474-4422(19)30196-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang X Yang J Moullaali TJ Sandset EC Woodhouse LJ Law ZK, et al. Influence of time to achieve target systolic blood pressure on outcome after intracerebral hemorrhage: The Blood Pressure in Acute Stroke Collaboration. Stroke 2024;55:849–855. doi: 10.1161/STROKEAHA.123.044358. [DOI] [PubMed] [Google Scholar]
- 29.Ma L Hu X Song L Chen X Ouyang M Billot L, et al. The third intensive care bundle with blood pressure reduction in acute cerebral haemorrhage trial (INTERACT-3): An international stepped-wedge cluster-randomized controlled trial. Lancet 2023;402:27–40. doi: 10.1016/S0140-6736(23)00806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li G Lin Y Yang J Anderson CS Chen C Liu F, et al. Intensive ambulance-delivered blood-pressure reduction in hyperacute stroke. N Engl J Med 2024;390:1862–1872. doi: 10.1056/NEJMoa2314741. [DOI] [PubMed] [Google Scholar]
- 31.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 32.Bower MM, Sweidan AJ, Shafie M, Atallah S, Groysman LI, Yu W. Contemporary reversal of oral anticoagulation in intracerebral hemorrhage. Stroke 2019;50:529–536. doi: 10.1161/STROKEAHA.118.023840. [DOI] [PubMed] [Google Scholar]
- 33.Kuramatsu JB Gerner ST Schellinger PD Glahn J Endres M Sobesky J, et al. Anticoagulant reversal, blood pressure levels, and anticoagulant resumption in patients with anticoagulation-related intracerebral hemorrhage. JAMA 2015;313:824–836. doi: 10.1001/jama.2015.0846. [DOI] [PubMed] [Google Scholar]
- 34.Steiner T Poli S Griebe M Hüsing J Hajda J Freiberger A, et al. Fresh frozen plasma versus prothrombin complex concentrate in patients with intracranial haemorrhage related to vitamin K antagonists (INCH): A randomised trial. Lancet Neurol 2016;15:566–573. doi: 10.1016/S1474-4422(16)00110-1. [DOI] [PubMed] [Google Scholar]
- 35.Castillo R Chan A Atallah S Derry K Baje M Zimmermann LL, et al. Treatment of adults with intracranial hemorrhage on apixaban or rivaroxaban with prothrombin complex concentrate products [published correction appears in J Thromb Thrombolysis. 2020 Jun 23]. J Thromb Thrombolysis 2021;51:151–158. doi: 10.1007/s11239-020-02154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pollack CV Jr. Reilly PA van Ryn J Eikelboom JW Glund S Bernstein RA, et al. Idarucizumab for dabigatran reversal – Full cohort analysis. N Engl J Med 2017;377:431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 37.Chai-Adisaksopha C, Hillis C, Lim W, Boonyawat K, Moffat K, Crowther M. Hemodialysis for the treatment of dabigatran-associated bleeding: A case report and systematic review. J Thromb Haemost 2015;13:1790–1798. doi: 10.1111/jth.13117. [DOI] [PubMed] [Google Scholar]
- 38.Lu G DeGuzman FR Hollenbach SJ Karbarz MJ Abe K Lee G, et al. A specific antidote for reversal of anticoagulation by direct and indirect inhibitors of coagulation factor Xa. Nat Med 2013;19:446–451. doi: 10.1038/nm.3102. [DOI] [PubMed] [Google Scholar]
- 39.Milling TJ Jr. Middeldorp S Xu L Koch B Demchuk A Eikelboom JW, et al. Final study report of andexanet alfa for major bleeding with factor Xa inhibitors. Circulation 2023;147:1026–1038. doi: 10.1161/CIRCULATIONAHA.121.057844. [DOI] [PubMed] [Google Scholar]
- 40.Mayer SA Davis SM Skolnick BE Brun NC Begtrup K Broderick JP, et al. Can a subset of intracerebral hemorrhage patients benefit from hemostatic therapy with recombinant activated factor VII? Stroke 2009;40:833–840. doi: 10.1161/STROKEAHA.108.524470. [DOI] [PubMed] [Google Scholar]
- 41.Ovesen C Jakobsen JC Gluud C Steiner T Law Z Flaherty K, et al. Tranexamic acid for prevention of hematoma expansion in intracerebral hemorrhage patients with or without spot sign. Stroke 2021;52:2629–2636. doi: 10.1161/STROKEAHA.120.032426. [DOI] [PubMed] [Google Scholar]
- 42.Polymeris AA Karwacki GM Siepen BM Schaedelin S Tsakiris DA Stippich C, et al. Tranexamic acid for intracerebral hemorrhage in patients on non-vitamin K antagonist oral anticoagulants (TICH-NOAC): A multicenter, randomized, placebo-controlled, phase 2 trial. Stroke 2023;54:2223–2234. doi: 10.1161/STROKEAHA.123.042866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baharoglu MI Cordonnier C Al-Shahi Salman R de Gans K Koopman MM Brand A, et al. Platelet transfusion versus standard care after acute stroke due to spontaneous cerebral haemorrhage associated with antiplatelet therapy (PATCH): A randomised, open-label, phase 3 trial. Lancet 2016;387:2605–2613. doi: 10.1016/S0140-6736(16)30392-0. [DOI] [PubMed] [Google Scholar]
- 44.Summers A Singh J Lai M Schomer KJ Martin R Vitt JR, et al. A multicenter retrospective study evaluating the impact of desmopressin on hematoma expansion in patients with antiplatelet-associated intracranial hemorrhage. Thromb Res 2023;222:96–101. doi: 10.1016/j.thromres.2022.12.016. [DOI] [PubMed] [Google Scholar]
- 45.Xu X Zhang H Zhang J Luo M Wang Q Zhao Y, et al. Minimally invasive surgeries for spontaneous hypertensive intracerebral hemorrhage (MISICH): A multicenter randomized controlled trial. BMC Med 2024;22:244. doi: 10.1186/s12916-024-03468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pradilla G Ratcliff JJ Hall AJ Saville BR Allen JW Paulon G, et al. Trial of early minimally invasive removal of intracerebral hemorrhage. N Engl J Med 2024;390:1277–1289. doi: 10.1056/NEJMoa2308440. [DOI] [PubMed] [Google Scholar]
- 47.Hallevi H Albright KC Aronowski J Barreto AD Martin-Schild S Khaja AM, et al. Intraventricular hemorrhage: Anatomic relationships and clinical implications. Neurology 2008;70:848–852. doi: 10.1212/01.wnl.0000304930.47751.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Holste KG, Xia F, Ye F, Keep RF, Xi G. Mechanisms of neuroinflammation in hydrocephalus after intraventricular hemorrhage: A review. Fluids Barriers CNS 2022;19:28. doi: 10.1186/s12987-022-00324-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Q Warren AD Qureshi AI Morotti A Falcone GJ Sheth KN, et al. Ultra-early blood pressure reduction attenuates hematoma growth and improves outcome in intracerebral hemorrhage. Ann Neurol 2020;88:388–395. doi: 10.1002/ana.25793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang X Arima H Heeley E Delcourt C Huang Y Wang J, et al. Magnitude of blood pressure reduction and clinical outcomes in acute intracerebral hemorrhage: Intensive blood pressure reduction in acute cerebral hemorrhage trial study. Hypertension 2015;65:1026–1032. doi: 10.1161/HYPERTENSIONAHA.114.05044. [DOI] [PubMed] [Google Scholar]
- 51.Gregson BA Broderick JP Auer LM Batjer H Chen XC Juvela S, et al. Individual patient data subgroup meta-analysis of surgery for spontaneous supratentorial intracerebral hemorrhage [published correction appears in Stroke. 2013 Jul;44(7):e82]. Stroke 2012;43:1496–1504. doi: 10.1161/STROKEAHA.111.640284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shah VA Thompson RE Yenokyan G Acosta JN Avadhani R Dlugash R, et al. One-year outcome trajectories and factors associated with functional recovery among survivors of intracerebral and intraventricular hemorrhage with initial severe disability. JAMA Neurol 2022;79:856–868. doi: 10.1001/jamaneurol.2022.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]