Abstract
Uncontrollable worry is a hallmark of generalized anxiety disorder and a transdiagnostic feature of psychopathology. Mindfulness-based strategies show promise for treating worry, but it is unknown which specific strategies are most beneficial, and how these skills might operate on a neurobiological level. We recruited 40 participants with clinically significant worry to undergo functional magnetic resonance imaging while engaging in real-time, idiographic worry and instructed disengagement using two mindfulness strategies (focused attention, acceptance) and one comparison strategy (suppression). Hypotheses were preregistered and partially supported. All disengagement strategies downregulated default mode and upregulated frontoparietal and salience networks, suggesting some shared mechanisms. Focused attention was most effective for promoting disengagement and elicited decreased activity in cognitive control and sensorimotor regions. Successful disengagement was associated with increased activity in rostrolateral prefrontal cortex and functional connectivity between posterior cingulate and primary somatosensory cortex. Findings support the role of cognitive control and somatosensory networks in disengagement from worry and suggest common and distinct mechanisms of disengagement, with focused attention a particularly promising strategy.
Keywords: anxiety disorders, worry, mindfulness, fMRI
General Scientific Summary
Although everyone worries sometimes, uncontrollable worries play an outsized role in the development and maintenance of anxiety disorders, and there is growing interest in understanding how people disengage from worry. Mindfulness skills show promise in promoting worry disengagement, but it is unknown which skills might be most beneficial or what the mechanisms are. Here, we use functional magnetic resonance brain imaging of two commonly taught mindfulness practices and an active control to interrogate whether and how mindfulness practices promote worry disengagement, and differences between successful and unsuccessful disengagement.
Worry is a defining feature of generalized anxiety disorder (GAD), a hallmark of anxiety-related psychopathology more broadly, and among the most pervasive and impactful forms of psychiatric symptomatology worldwide (Baxter et al., 2013). Defined as a chain of uncontrollable, future-oriented, negative thoughts and images (Borkovec et al., 1983), worry plays an important mechanistic role in the development and maintenance of anxiety (McLaughlin et al., 2007). A growing literature suggests that it is the uncontrollability of worry and related forms of perseverative thought per se that accounts for much of their relationship to depression and anxiety (Ehring et al., 2011; Hallion et al., 2022; Hallion & Ruscio, 2013). The ability to improve the controllability of worry—in other words, to facilitate conscious disengagement—therefore emerges as a promising target for intervention.
In this milieu, mindfulness-based approaches have emerged as especially promising. Mindfulness entails present-moment awareness and acceptance of thoughts and emotions (Brewer et al., 2020). A growing empirical literature suggests promising effects for mindfulness-based interventions in reducing anxiety, worry, and related forms of perseverative thought (Creswell, 2017; Gaynor, 2014). Additionally, ad hoc mindfulness exercises proliferate on popular websites such as YouTube, and clinicians often recommend such exercises as part of routine clinical practice (Harrington & Dunne, 2015). Establishing which types of interventions best promote successful regulation of worry would therefore inform both research (e.g., identifying the cognitive mechanisms that play a causal role in maintaining worry) and clinical practice.
Mindfulness is not a unitary construct but refers to a family of related but distinct qualities and practices (K. C. R. Fox et al., 2016). Although different theoretical models of mindfulness practices exist, two qualities are consistently identified as central (Lindsay & Creswell, 2017). One is focused attention, or the ability to sustain focus on a stimulus without deviation. The second is acceptance, an umbrella term encapsulating the ability to notice experiences, thoughts, and emotions without judging or reacting to them. Different practices (or exercises) target these different qualities of mindfulness. Some exercises train the ability to sustain attention to a particular stimulus, such as sounds or breath, while others work to enhance compassion or awareness of all experience (K. C. R. Fox et al., 2016).
There are theoretical reasons that both focused attention and acceptance might facilitate worry disengagement, as prominent theories of worry highlight elements directly relevant to mindfulness. The first is an impairment of conscious, intentional (i.e., top-down) attentional control, leading to difficulty redirecting attention away from threatening thoughts or stimuli (Hirsch & Mathews, 2012). Such impairments of executive attentional processes are also thought to underlie other forms of repetitive negative thought, such as rumination (Koster et al., 2011). Focused attention exercises, on the contrary, involve consciously directing and sustaining one’s full attention onto a pre-selected stimulus, training and strengthening this capacity over time (Lutz et al., 2008). In this way, the technique bears similarity to distraction and thought-replacement techniques, which can be effective strategies for immediate worry disengagement (e.g., Eagleson et al., 2016) and have been related to decreased worry and increased positive affect in daily life (Boemo et al., 2022).
However, distraction can also be maladaptive when used for avoidance of aversive internal states (Wolgast & Lundh, 2017), which highlights the another key element of worry: experiential avoidance (Newman et al., 2013). Acceptance has been proposed as a necessary component of worry termination (Berenbaum, 2010), which counteracts avoidance tendencies (Hayes-Skelton & Eustis, 2020). Acceptance-based exercises promote the ability to observe negative thoughts without reacting to them, evaluating them, or trying to change them (Westbrook et al., 2013). These qualities (present-focused acceptance of experience) are in direct opposition to major qualities and functions of worry (e.g., future orientation, Thompson et al., 2022; experiential avoidance, Newman et al., 2013) and thus may serve to interrupt worry-maintenance processes. It has been proposed that focused attention requires an underlying stance of acceptance in order to effectively promote emotion regulation (Lindsay & Creswell, 2017); thus, these two strategies may represent two sides of the same coin in terms of psychological mechanisms of worry termination.
The purpose of the present study was to offer a proof-of-concept examination of the relative efficacy of these two forms of mindfulness practice for facilitating disengagement from worry and to interrogate similarities and differences in their neural circuitry. We were also interested in the extent to which the neural correlates of mindfulness strategies mirror those observed during successful disengagement from worry more generally, which in turn can provide insights for neurobiologically informed treatment development.
Proposed Neural Circuitry
Worrying has been linked to increased activity and connectivity within the default mode network (DMN, Makovac et al., 2020), a distributed network associated with spontaneous mentation (Raichle, 2015). Its two primary nodes are the posterior cingulate cortex (PCC) and medial prefrontal cortex (mPFC, M. D. Fox et al., 2005). Disengaging from worry, by contrast, more likely engages the frontoparietal (FPN) and salience networks (SN), which are both involved in top-down attention and executive processes. The FPN, comprising dorsolateral prefrontal (dlPFC) and posterior parietal cortices (PPC), supports effortful cognitive control (Vincent et al., 2008), while the SN comprises dorsal anterior cingulate (dACC) and anterior insula, and acts as the “switch” between the FPN and DMN, directing attention and resources as necessary (Goulden et al., 2014). The FPN and SN are reliably activated during emotion regulation (Morawetz et al., 2017), presumably due to the conscious and effortful cognitive processing involved.
A final area that is frequently implicated in severe worry is a local network rooted in ventrolateral prefrontal cortex (vlPFC). A recent meta-analysis showed a consistent area of activation extending from vlPFC to the adjacent anterior insula that was preferentially recruited by high trait worriers during emotion processing (Weber-Goericke & Muehlhan, 2019). Automated meta-analysis using NeuroSynth (Yarkoni et al., 2011) suggests an association of this region with language processing, perhaps reflecting the verbal-linguistic nature of worry.
Although the main corpus of research on this topic has focused on emotion regulation rather than worry disengagement per se, he few studies that have examined worry disengagement suggest that it broadly engages these same networks. Individuals with GAD show less functional connectivity both within and between the SN and FPN when disengaging from worry (Andreescu et al., 2016). GAD has also been linked to increased cerebral blood flow in FPN and vlPFC during attempted disengagement (Karim et al., 2017).
Mindfulness practices appear to engage this circuitry as well. A meta-analysis found that focused attention meditation increases activity in the SN and FPN and decreases activity in major nodes of the DMN (K. C. R. Fox et al., 2016); while open monitoring meditation (an acceptance-based practice) increases activity in the SN. Acceptance-based practices more broadly have been found to engage FPN and SN (Dixon et al., 2020) and to disengage DMN (Messina et al., 2021), while longer-term mindfulness interventions strengthen FPN recruitment as well as functional connectivity between DMN and major hubs of the SN and FPN. These changes in turn correlate with decreased anxiety, suggestive of a mechanistic role (Zhao et al., 2019). Mindfulness training has been associated with decreased activation in vlPFC and greater connectivity between vlPFC and FPN as well (Hölzel et al., 2013; Taren et al., 2017).
The Present Study: Design and Hypotheses
We recruited adults high in trait worry to complete a worry-disengagement imaging task based on idiographic worry cues that they self-identified previously via semi-structured interview. We used real-time thought sampling (Christoff et al., 2009) at the conclusion of each disengagement block to help identify brain activity specific to successful disengagement, as well as to compare and contrast brain activity associated with the two mindfulness and one control disengagement strategies.
We compared two well-characterized and widely used mindfulness exercises as disengagement strategies: focused attention (Dickenson et al., 2013) and acceptance (Westbrook et al., 2013). These strategies are core components of many mindfulness-based interventions and are well characterized in terms of their neural signatures (Lindsay & Creswell, 2017). Thought suppression (heretofore referred to as “suppression”) was selected as an active control condition because its neural signatures are also well characterized (e.g., Wyland et al., 2003), but it often paradoxically increases negative cognitions and worsens negative affect (e.g., Dalgleish & Yiend, 2006). A suppression condition allowed us to control for disengagement effort and task demands across conditions, while also allowing us to isolate the neural correlates of mindfulness (focused attention and acceptance) specifically. We did not include an additional passive control condition to conserve scanner time and because such a condition would likely simply reflect continued worry. All four inductions (cued worry, focused attention, acceptance, and suppression) have been validated and used successfully in previous research with similarly brief training (e.g., Paz et al., 2017; Westbrook et al., 2013).
Hypotheses
Primary hypotheses were preregistered on the Open Science Framework concurrent with data collection and prior to data analysis (https://osf.io/mygwc/). Functional connectivity hypotheses were added to preregistration after univariate analyses.
We anticipated that active worry would be associated with greater DMN and vlPFC activity, while mindfulness and successful disengagement from worry would have the opposite effect. Specifically, compared to suppression and the worry period, we expected both mindfulness conditions to be associated with decreased activity of PCC (a key DMN node) and decreased activity in vlPFC. Competing hypotheses (significant increases or decreases in activation) were seen as equally plausible and interesting for the SN (dACC and anterior insula) in light of conflicting findings for these regions in previous research (Ives-Deliperi et al., 2011). We expected successful (vs. failed) disengagement to likewise correspond with decreased activity in PCC and significantly altered (increased or decreased) activity in salience regions.
Worry has not been associated with frontoparietal activity in large studies, so we did not predict specific relationships between FPN activity and worry or disengagement. However, we did expect mindfulness and successful disengagement from worry to be associated with increased frontoparietal-default mode connectivity. Specifically, we predicted that mindfulness (vs. worry) would be associated with increased PCC-dlPFC and PCC-vlPFC connectivity (representing increased DMN-FPN connectivity) and with decreased PCC-mPFC connectivity (representing decreased intra-DMN connectivity). This same pattern of functional connectivity (increased PCC-vlPFC and PCC-dlPFC, decreased PCC-mPFC) was predicted for successful (vs. unsuccessful) disengagement trials.
Method
Participants
Participants were right-handed native English speakers between 20 and 43 years of age (Mage = 27) with normal or corrected-to-normal vision, recruited through posted fliers, a university-wide research registry, and recontacting from prior studies. Fifty individuals completed the first study visit (diagnostic interview and eligibility assessment), and 40 completed the second (functional magnetic resonance imaging [fMRI]) visit (see Table 1 for demographic and clinical characteristics). Two participants’ data were removed from fMRI analyses: one for sleeping during the task, and one for excessive motion (>25% volumes censored for framewise displacement 0.9 mm). Four participants were removed from analyses of successful versus unsuccessful disengagement because they reported all successful or unsuccessful trials, and therefore the successful–unsuccessful contrast could not be computed. Thus, final study had a sample size of N = 38, except for successful–unsuccessful contrasts, for which sample size was smaller (N = 34). A sample size of 34 has 81% power to detect an effect size of d = 0.5 (t = 1.41) in a within-subjects design, which is within the range of prior fMRI results (Mumford, 2012).
Table 1.
Demographic and Clinical Characteristics
| Variable | M | SD | Range | % |
|---|---|---|---|---|
| Age | 27 | 6.89 | 20–43 | |
| Gender | ||||
| Women | 80 | |||
| Men | 20 | |||
| Ethnicity | ||||
| Not Hispanic or Latino | 92.5 | |||
| Hispanic or Latino | 7.5 | |||
| Race | ||||
| White | 70 | |||
| Black/African American | 12.5 | |||
| Asian | 7.5 | |||
| Biracial | 5 | |||
| American Indian/Alaska Native | 2.5 | |||
| Other | 2.5 | |||
| Highest level of education | ||||
| High school diploma or GED | 7.5 | |||
| Some college | 25 | |||
| Bachelor’s degree | 47.5 | |||
| Master’s degree or greater | 20 | |||
| Current psychiatric medication | 57.5% | |||
| Self-report measures | ||||
| PSWQ | 67.51 | 12.62 | 55–78 | |
| PHQ-9 | 5.79 | 3.79 | 0–15 | |
| Diagnoses: ADIS-5 | ||||
| Generalized anxiety disorder | 62.5 | |||
| Social anxiety disorder | 62.5 | |||
| Specific phobia | 17.5 | |||
| Panic disorder | 12.5 | |||
| Posttraumatic stress disorder | 12.5 | |||
| Major depressive disorder | 12.5 | |||
| Obsessive-compulsive disorder | 10 | |||
| Agoraphobia | 5 | |||
| Persistent depressive disorder | 2.5 | |||
| Body dysmorphic disorder | 2.5 | |||
| Somatic symptom disorder | 2.5 | |||
| Diagnoses: DIAMOND | ||||
| Excoriation disorder | 7.5 | |||
| Bulimia nervosa | 2.5 |
Note. This table reflects assessments at the first (pre-scanner) study visit; thus, some scores are lower than the ≥65 PSWQ cutoff imposed at screening. Final eligibility was determined via diagnostic interview. GED = Generalized Educational Development certificate; ADIS-5 = Anxiety Disorder Interview Schedule for DSM-5; DIAMOND = Diagnostic Interview for Anxiety, Mood, and Obsessive-Compulsive and Related Neuropsychiatric Disorders; PHQ-9 = Patient Health Questionnaire–9-item version; PSWQ = Penn State Worry Questionnaire.
Participants were prescreened for a score ≥65 on the Penn State Worry Questionnaire (PSWQ, Meyer et al., 1990) and a response of “often” or “always” on questions 1–3 of the “worry over past week” scale. Participants were also required to have a PHQ-9 (Patient Health Questionnaire-9) score ≤13 at screening, reflecting no greater than moderately severe depression. Although the majority of participants (80%) met DSM-5 (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition) diagnostic criteria for one or more anxiety-related disorders using the Anxiety and Related Disorders Interview Schedule for DSM-5 (ADIS-5; Brown & Barlow, 2014), a GAD diagnosis was not required, and a major depressive disorder (MDD) diagnosis was not an exclusion. This allowed us to balance internal validity (findings are unlikely to be attributable to comorbid depression) with external validity (we focused on transdiagnostic worry and did not artificially select for “pure” GAD).
Exclusion criteria included MRI contraindications; history of traumatic brain injury; lifetime diagnoses (assessed via ADIS-5) of psychotic, bipolar, neurological, learning, or attentional disorder; current substance use disorder (ADIS-5); psychotropic medication except selective serotonin reuptake inhibitors (SSRIs); unstable doses of SSRI (<6 weeks) or medications affecting cardiovascular function; and history of formal mindfulness training or practice (>10 hr).
Study Procedure
All study procedures were reviewed and approved by the University of Pittsburgh and Carnegie Mellon Institutional Review Boards. After an initial phone or email screen to determine preliminary eligibility, participants completed a laboratory visit that lasted approximately 4 hr. During this visit, they completed formal consent, diagnostic interviews, and self-report measures. Eligibility was finalized at this study visit. Eligible participants then completed a structured interview to identify personal worry topics for use in the fMRI task.
The fMRI visit occurred approximately 1 week later and lasted 2 hr. This visit comprised training in the primary study task, including instructions in the cued worry, mindfulness, and suppression task conditions (see the supplemental materials for complete text), further screening for MRI compatibility, a mock scanner session to acclimatize participants to the scanner environment, and the 60-min fMRI scan itself, followed by post-scan questionnaires and a verbal debriefing.
Participants also completed additional self-report measures 24 hr prior to the scan, the day of the scan, 24-hr postscan, and 7-day postscan (see the supplemental materials). Those data were not analyzed.
Study Materials
Screening Measures
Trait worry.
The PSWQ (Meyer et al., 1990) is a widely used, 16-item questionnaire of trait worry with strong psychometric properties in unselected and clinical samples (Fresco et al., 2003). Cronbach’s α = 0.79 in the present sample.
Depression.
The PHQ-9 (Kroenke et al., 2001) is a nine-item questionnaire measuring depressive symptoms over the past 2 weeks. It has strong psychometric properties in clinical and non-clinical samples (Beard et al., 2016). Cronbach’s α = 0.75 in the present sample.
Semistructured Interviews
Diagnostic Interviews.
Diagnostic interviews were conducted during the first study visit by trained postbaccalaureate and graduate student diagnosticians.
The ADIS-5 (Brown & Barlow, 2014) is a semistructured interview that is considered a “gold standard” for the diagnosis of anxiety and related forms of psychopathology (including MDD and persistent depressive disorder).
The Diagnostic Interview for DSM-5 Anxiety, Mood, and Obsessive-Compulsive and Related Disorders (DIAMOND, Tolin et al., 2018) is a semistructured diagnostic interview for the diagnosis of DSM-5 disorders. Selected modules of the DIAMOND assessing eating disorders, hoarding, trichotillomania, skin picking, attention-deficite hyperactivity disorder (ADHD), and tic disorders were administered as a supplement to the ADIS-5.
Idiographic Worry Topics.
The Worry and Rumination Interview (WARI, Ruscio et al., 2011) is an unpublished structured interview in which participants are provided with a definition of worry and work with an experimenter to identify personally relevant (idiographic) worry topics. Participants then create a brief (2–5 word) reminder phrase to be used as a worry cue during the scanning session. We used a modified version of the worry module (see the supplemental materials for complete text).
Other Self-Report Measures
Thought Characteristics.
Prior to the scanning session, participants rated each of their worry topics on a variety of characteristics, including dyscontrol (uncontrollability, intrusiveness, repetitiveness) and valence (happy, sad, angry, etc.), using a Likert scale of 0 (not at all) to 4 (very much) (Hallion et al., 2022). Participants also reported time spent thinking about each topic in the previous 24 hr. Cronbach’s α = 0.83 for dyscontrol and 0.78 for valence in the present sample.
Thought Sampling.
At the end of each of the 12 disengagement periods during the scanner task, participants answered a series of six yes/no prompts phrased as statements: “At the signal, I was ___.” Participants responded via button press to indicate “yes” or “no” to each probe: “worrying,” “focusing on my current sights/sounds/physical sensations,” “mindfully listening,” “mind wandering,” “trying to push thoughts out of my mind,” and “having negative thoughts.” Successful disengagement from worry was operationalized as a “no” response to both the “worrying” and “having negative thoughts” probes. These were followed by meta-awareness probes, which we did not analyze.
Manipulation Check.
After the scan, participants self-reported on the effectiveness, intensity, and ecological validity of their worry and mindfulness during the scanning session.
fMRI Task and Instructions
The scanner task comprised two runs of approximately 12.5 min each. Each run comprised six complete (worry-disengagement-probe) trials with a 5s rest between each trial and a 10s rest at the beginning and end of each run. Trial order was pseudorandomized with the constraint to not repeat disengagement strategies on subsequent trials and counterbalanced between runs. Each trial began with an idiographic worry cue displayed on the screen for 35s (worry period). This period was chosen based on pilot testing and feedback by participants who indicated that they needed at least 30s to fully engage with worrying, balanced against a need for more trials to maximize power. Following the worry period was a 3s rest. An instruction for one of the disengagement strategies (acceptance, focused attention, or suppression) was then displayed on the screen for a jittered interval varying from 31 to 35s duration (disengagement period). A 1s cue signaled the end of the trial, after which the participant completed thought probes for a maximum of 45s. Trial structure is visualized in Figure 1a.
Figure 1.
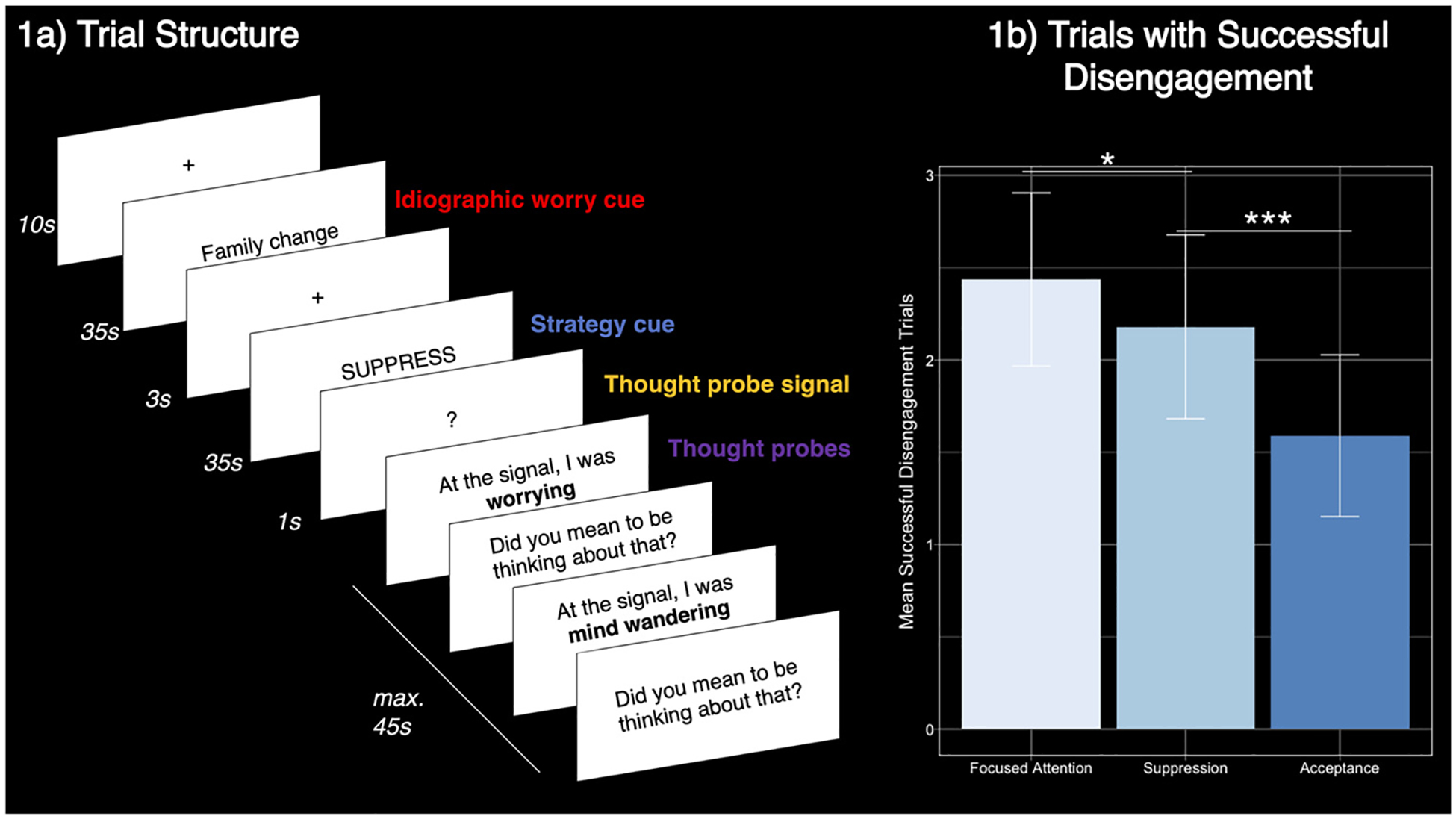
Trial Structure and Disengagement Success by Strategy
Note. Error bars in 1b represent 95% CI of one-sample t-test for the mean of that bar. See the online article for the color version of this figure.
*p < .05. ***p < .001.
fMRI Data Collection and Analysis
fMRI Data Analysis
See the supplemental materials for details of image acquisition, preprocessing, and analysis.
Univariate Task Analysis.
fMRI data were analyzed using a general linear model. All task periods were modeled. The disengagement period was divided into two blocks: the first 25s to capture disengagement effort, and the final 10s immediately preceding the thought probes (following Christoff et al., 2009) to capture disengagement success/failure.
Contrasts were constructed at the first level by subtracting regressors between conditions, for example, acceptance–suppression. The approach of paired t tests between conditions was chosen over ANOVA because only the planned contrasts and not the omnibus hypothesis were of interest. Participants who endorsed “yes” to worry or negative thought on every trial (all unsuccessful disengagement and no successful disengagement) or “no” on every trial (all successful disengagement and no unsuccessful) were excluded from successful–unsuccessful contrasts only. There were 10 participants for whom one run was excluded, and four participants for whom both runs were excluded for this reason.
Connectivity.
Connectivity analyses were performed using a generalized psychophysiological interaction (gPPI) approach (McLaren et al., 2012). This approach enables the identification of clusters of functional connectivity across the whole brain without requiring causal hypotheses or the identification of target regions of interest (ROIs) within large territories (such as vlPFC). An a priori region of interest was identified in the precuneus/posterior cingulate area as a hub of the DMN (Raichle, 2015). A 4-mm sphere was placed in a region of precuneus overlapping with task activation in the Worry–Rest and Disengagement–Worry contrasts (Montreal Neurological Institute [MNI] coordinates: 0, −54, 29). The blood oxygen-level dependent timecourse was extracted from this ROI, deconvolved (Gitelman et al., 2003) using Statistical Parametric Mapping (SPM12, Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK), and multiplied by task boxcar regressors to make PPI regressors. These regressors, along with the seed timeseries, were then added to the univariate models described above. Contrasts were constructed between these new PPI regressors (e.g., PPI acceptance–PPI suppression), averaged, and modeled at the group level as above.
Both univariate and connectivity analyses were repeated using masks of specific ROIs (e.g., vlPFC) but none of these produced significant clusters of activation, so only whole-brain results are reported.
Behavioral Data.
Behavioral data from the task were analyzed using R (https://cran.r-project.org/). Successful disengagement trials were averaged across both task runs for acceptance, suppression, and focused attention separately and analyzed using paired t tests.
Preregistration
This study is preregistered on the Open Science Framework (original preregistration: https://osf.io/tsd74 updates to preregistration: https://osf.io/b8exs/). Data and code are available publicly or upon request; details are included in the supplemental materials.
Results Manipulation Checks
Detailed manipulation checks are provided in the supplemental materials. Briefly, worry topics were rated as highly uncontrollable and negative in prescan ratings, and participants endorsed spending a significant amount of time spontaneously thinking about them in daily life. Most participants reported being able to worry with at least moderate intensity during the scanning session. Only two participants reported having significant difficulty worrying on cue. Most participants also reported achieving at least “moderate” or greater mindfulness during the mindfulness tasks.
Behavioral Results
Participants reported significantly more successful disengagement trials in the focused attention (M = 2.44 out of four possible trials, SD = 1.44) versus acceptance condition (M = 1.59, SD = 1.35; t(38) = 3.92, p < .001, CI = [0.24, 1.46]), and in the suppression (M = 2.18, SD = 1.53) versus acceptance condition (t(38) = 2.37, p < .05, [−1.29, −0.01]). Focused attention and suppression counts did not significantly differ (t(38) = 1.06, p = .30, [−0.46, 0.86]).
Imaging Results: Task Activity
Worry Disengagement–Overall and Common Effects
In partial confirmation of our hypotheses, all three strategies were associated with deactivations in large territories, corresponding to DMN and activations in large FPN territories, compared to worry. Mindfulness-based strategies also engaged right-sided anterior insula, as hypothesized, and disengaged nonhypothesized brain regions, including hippocampus, temporal, and angular gyri. No strategy was associated with vlPFC deactivation. Thus, worry disengagement across all three strategies deactivated DMN and engaged FPN and some associated SN regions relative to active worry. Peak activation coordinates are reported in Table 2; see Figure 2a for cluster activation.
Table 2.
Significant fMRI Activations for Disengagement–Worry
| Associated network | Peak voxel location | BA | Side | Cluster size (k) | Peak activation (MNI) | t | ||
|---|---|---|---|---|---|---|---|---|
| Focused attention > worry | ||||||||
| FPN | dlPFC | 9 | R | 4056 | 48 | 28 | 26 | 5.36 |
| FPN | Lateral parietal lobule | 7 | L | 2649 | −34 | −46 | 42 | 6.15 |
| Supramarginal gyrus | 40 | R | 1963 | 36 | −48 | 44 | 5.84 | |
| FPN | dlPFC | 9 | L | 1821 | −36 | 34 | 38 | 5.10 |
| Supplementary motor area | 6 | L | 232 | −28 | −6 | 48 | 4.15 | |
| Worry > focused attention | ||||||||
| DMN | mPFC (extends to PCC) | 32 | L | 21788 | −8 | 48 | −4 | 6.61 |
| Medial temporal gyrus | 21 | L | 1521 | −66 | −10 | −16 | 5.45 | |
| Secondary visual cortex | 18 | R | 441 | 24 | −88 | 2 | 4.93 | |
| Primary motor cortex | 4 | L | 336 | −18 | −30 | 78 | 4.11 | |
| Accept > worry | ||||||||
| FPN | Lateral parietal lobule | 7 | R | 15938 | 40 | −44 | 44 | 6.95 |
| FPN | dlPFC | 9 | R | 7246 | 40 | 30 | 36 | 6.01 |
| FPN | dlPFC | 9 | L | 4930 | −36 | 22 | 28 | 5.60 |
| Fusiform gyrus | 37 | R | 674 | 62 | −46 | −2 | 5.30 | |
| Thalamus | — | L | 587 | −12 | −14 | 12 | 5.28 | |
| Posterior corpus callosum | — | midline | 312 | 0 | −34 | 24 | 4.78 | |
| Thalamus | — | R | 263 | 8 | −16 | 14 | 4.43 | |
| Brain stem | — | R | 242 | 4 | −24 | −28 | 4.08 | |
| Worry > accept | ||||||||
| DMN | mPFC | 32 | L | 4563 | −6 | 28 | −8 | 6.03 |
| DMN | PCC | 23 | L | 1553 | −4 | −54 | 24 | 6.60 |
| Primary motor cortex | 4 | R | 827 | 34 | −22 | 50 | 4.98 | |
| Supplementary motor cortex | 6 | R | 635 | 8 | −20 | 60 | 4.51 | |
| DMN | Parahippocampal gyrus | 36 | L | 507 | −22 | −34 | −10 | 5.19 |
| DMN | Angular gyrus | 39 | L | 459 | −40 | −76 | 40 | 4.92 |
| 219 | 66 | 8 | 14 | 4.81 | ||||
| Suppress > worry | ||||||||
| FPN | Orbitofrontal cortex | 11 | L | 17653 | −20 | 36 | −20 | 5.71 |
| FPN | Lateral parietal lobule | 7 | L | 16831 | −24 | −58 | 44 | 6.00 |
| Cerebellum | — | R | 1610 | 34 | −36 | −32 | 5.01 | |
| Brain stem | — | R | 208 | 6 | −18 | −38 | 4.33 | |
| Corpus callosum | — | L | 199 | −8 | −26 | 30 | 4.80 | |
| Worry > suppress | ||||||||
| DMN | mPFC | 11 | L | 3772 | −10 | 32 | −12 | −6.07 |
| DMN | PCC | 23 | L | 701 | −4 | −54 | 20 | −5.69 |
| All strategies—conjunction analysis | ||||||||
| Disengagement > worry | ||||||||
| FPN | Frontal pole | 10 | R | 2597 | 46 | 48 | −2 | N/A |
| FPN | Angular gyrus | 39 | L | 2386 | −34 | −49 | 43 | N/A |
| FPN | dlPFC | 46 | L | 1593 | −42 | 42 | 10 | N/A |
| FPN | Angular gyrus | 39 | R | 1330 | 58 | −46 | 24 | N/A |
| Supplementary motor area | 6 | L | 225 | −28 | −8 | 44 | N/A | |
| SN | Insula | 13 | R | 176 | 34 | 20 | −6 | N/A |
| Worry > disengagement | ||||||||
| DMN | mPFC | 32 | L | 2967 | −2 | 38 | −8 | N/A |
| DMN | PCC | 23 | L | 697 | −6 | −53 | 22 | N/A |
| Corpus callosum | — | R | 14 | 6 | 22 | 10 | N/A | |
Note. All clusters reported passed multiple comparison correction using cluster-based random field theory with a cluster forming threshold of z = 3.1 (Eklund et al., 2016) and a corrected cluster p-value threshold of .05. BA = Brodmann Area; MNI = Montreal Neurological Institute coordinates; dlPFC = dorsolateral prefrontal cortex; DMN = default mode network; fMRI = functional magnetic resonance imaging; FPN = frontoparietal network; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; SN = salience network.
Figure 2.

Common and Differential Activity for Different Disengagement Strategies
Note. (a) Significant activations for each disengagement strategy versus worry, thresholded and cluster-corrected across the whole brain. Red/orange denotes activations, blue/white denotes deactivations. “DMN” refers to the two major default mode network nodes (PCC and mPFC), and “FPN” refers to the two major frontoparietal nodes (dlPFC and posterior parietal cortex). Other pertinent activations are also noted. (b) Significant deactivations in the contrasts between focused attention and acceptance/suppression, thresholded and cluster-corrected across the whole brain. There were no significant differences between acceptance and suppression, so this contrast is not visualized. dACC = dorsal anterior cingulate cortex; dlPFC = dorsolateral prefrontal cortex; dmPFC = dorsomedial prefrontal cortex; mPFC = medial prefrontal cortex; PCC = posterior cingulate cortex; SMA = supplementary motor area; vlPFC = ventrolateral prefrontal cortex. See the online article for the color version of this figure.
Worry Disengagement—Differences Between Strategies
Contrary to predictions, there were no significant differences in brain activity between acceptance and suppression. Focused attention, however, showed significant deactivations compared to both acceptance and suppression in vlPFC, as well as nonhypothesized regions, including dorsomedial PFC, supplementary motor area, and caudate (regions associated with emotion regulation). Thus, focused attention engaged distinct neural circuits from acceptance and suppression, which did not differ from one another. See Figure 2b for visualization and Table 3 for a list of peak activations.
Table 3.
Significant fMRI Activations for Pairwise Contrasts Between Disengagement Strategies
| Peak voxel location | BA | Side | Cluster size (k) | Peak activation (MNI) | t | ||
|---|---|---|---|---|---|---|---|
| Focused attention > accept | |||||||
| None | |||||||
| Accept > focused attention | |||||||
| Cerebellum | — | R | 6578 | 0 | −64 | −20 | −4.99 |
| dlPFC | 9 | L | 1276 | −12 | 56 | 36 | −5.63 |
| White matter (extends into occipital cortex) | — | R | 1005 | 20 | −16 | 36 | −4.75 |
| Cerebellum | — | L | 865 | −28 | 88 | −32 | −5.02 |
| Frontal eye fields (dlPFC) | 8 | R | 732 | 10 | 34 | 58 | −4.63 |
| Temporal pole | 38 | L | 654 | −50 | 20 | −16 | −4.68 |
| Pars triangularis (vlPFC) | 45 | L | 267 | −56 | 24 | 22 | −4.24 |
| Caudate | — | R | 249 | 16 | 22 | 4 | −4.05 |
| Temporal pole | 38 | R | 241 | 54 | 16 | −34 | −4.55 |
| Caudate | — | L | 230 | −18 | 26 | 0 | −4.99 |
| Focused attention > suppress | |||||||
| None | |||||||
| Suppress > focused attention | |||||||
| Secondary visual cortex | 18 | L | 8058 | −6 | −90 | −2 | −5.20 |
| Fusiform gyrus | 37 | L | 2741 | −42 | −44 | −16 | −4.65 |
| Pars orbitalis (vlPFC) | 47 | L | 2109 | −50 | 36 | −8 | −5.30 |
| Supplementary motor area | 6 | L | 932 | −8 | 6 | 72 | −4.81 |
| Posterior insula | 13 | R | 896 | 36 | −12 | 20 | −4.69 |
| Pars orbitalis (vlPFC) | 47 | R | 688 | 48 | 32 | −16 | −4.31 |
| Cerebellum | — | L | 311 | −8 | −54 | −50 | −4.55 |
| Frontal pole | 10 | L | 234 | −16 | 62 | 26 | −4.19 |
| Accept > suppress | |||||||
| None | |||||||
| Suppress > accept | |||||||
| None | |||||||
Note. All clusters reported passed multiple comparison correction using cluster-based random field theory with a cluster forming threshold of z = 3.1 (Eklund et al., 2016) and a corrected cluster p-value threshold of .05. BA = Brodmann area; MNI = Montreal Neurological Institute coordinates; dlPFC = dorsolateral prefrontal cortex; fMRI = functional magnetic resonance imaging; vlPFC = ventrolateral prefrontal cortex.
Successful Versus Unsuccessful Disengagement
Contrary to our hypotheses, no significant differences were seen in PCC, dACC, or anterior insula between successful and unsuccessful disengagement. However, one cluster of significant activation was observed for successful disengagement trials in the right rostrolateral PFC (peak MNI coordinates: 44, 58, −6; K = 209, t = 4.74). Thus, successful disengagement did not alter DMN and SN but was associated with activity in an area associated with FPN and cognitive control.
Imaging Results: Connectivity
Differences Between Disengagement Strategies
Pairwise contrasts between PCC connectivity during each disengagement strategy (focused attention, acceptance, and suppression) found no significant differences across the whole brain.
Successful Versus Unsuccessful Disengagement
We did not find significant differences in connectivity when the PPI was conducted on the last 10 s of the disengagement period. However, when we conducted the PPI across the full disengagement period, PCC showed increased connectivity to a large cluster in primary somatosensory cortex (peak MNI coordinates: −10, 36, 74; K = 295, t = 4.58). Thus, our hypotheses of decreased connectivity within DMN and increased DMN-FPN connectivity were not confirmed. Instead, successful disengagement was characterized by increased connectivity between DMN and somatosensory cortex (Figure 3).
Figure 3.

Successful Versus Unsuccessful Disengagement: Activity and Connectivity
Note. (a) Significant activation from the contrast successful–unsuccessful disengagement, thresholded and cluster-corrected across the whole brain. (b) Significant cluster from a psychophysiological interaction (PPI) analysis of functional connectivity to a posterior cingulate seed (green) during successful–unsuccessful disengagement, thresholded and cluster-corrected across the whole brain. Red/orange denotes increased functional connectivity (successful > unsuccessful) to PCC seed. See the online article for the color version of this figure.
Discussion
The present study used fMRI to examine the neural correlates and relative efficacy of two widely used mindfulness practices (acceptance and focused attention) and a thought suppression control. The key results of the present study fall into two broad areas. First, contrasts between mindfulness and control disengagement techniques revealed that focused attention differed from both acceptance and suppression in disengagement efficacy and brain activity, while acceptance and suppression did not differ from one another in brain activity. Second, successful worry disengagement differed from unsuccessful disengagement in both brain activation and brain connectivity. Our work both supports and contradicts prevailing theories of both mindfulness and worry, in some ways that were quite surprising.
Drawing on prior research, we anticipated that both mindfulness techniques, but not suppression, would engage the cognitive control regions (FPN and SN) and disengage the DMN. However, our analyses revealed that all disengagement strategies corresponded to deactivation of the DMN (mPFC, PCC) and activation of the FPN (posterior parietal cortex, dlPFC, and frontal pole) and SN (anterior insula for all conditions and dACC for acceptance and suppression). FPN recruitment typically accompanies increasing cognitive demands (Berry et al., 2017) and may simply reflect task effort. Thus, this pattern of results indicates that engagement of cognitive control regions is common to worry disengagement regardless of attempted strategy, which aligns with theory and prior research on effortful emotion regulation (Braunstein et al., 2017) as well as worry generation and maintenance (Hirsch & Mathews, 2012).
Some surprising differences emerged in pairwise contrasts between disengagement strategies. First, the three strategies differed in disengagement success (as assessed by real-time thought sampling methods). Focused attention was the most effective, followed by suppression, and finally acceptance. Focused attention also differed from both acceptance and suppression in large networks across the whole brain, including regions implicated in cognitive reappraisal of worry and emotions (Karim et al., 2017; Morawetz et al., 2017; Picó-Pérez et al., 2017), such as dlPFC, temporal pole, and caudate (vs. acceptance); and insula, supplementary motor area, and a medial area of frontal pole (vs. suppression); and finally, vlPFC, cerebellum, and visual cortex (vs. both). Cerebellum and visual cortex were not identified as a priori ROI, but both have been linked to emotion regulation and reappraisal (e.g., Dixon et al., 2020).
This pattern of results was somewhat surprising. First of all, focused attention would seem to represent simple, top-down attentional control, and as such, would be expected to engage FPN and SN the most out of all three strategies. Second, instructions for acceptance and suppression could reasonably be described as opposing, with one involving active and deliberate alteration of cognitive-emotional experience, and the other, accepting cognitive-emotional experience as-is. This would seem to contradict the role of acceptance in termination of worry (Berenbaum, 2010) as well as the relatively worse performance of suppression as a regulation technique (Dalgleish & Yiend, 2006).
One possible explanation is that focused attention differs not in the degree to which it recruits top-down attentional control, but rather the cognitive and neural architecture for maintenance and manipulation of representations. These functions are known to be subserved by FPN brain regions, particularly under high task demands (Berry et al., 2017). Focused attention involves monitoring and shifting of attention without explicit re-evaluation of its contents, while both acceptance and suppression require continued representation of worries in mind (paradoxically in the case of suppression), which could account for greater FPN recruitment compared to focused attention. In this way, they would more closely resemble cognitive emotion regulation strategies such as reappraisal, which has been well studied (Braunstein et al., 2017).
In addition, these latter two strategies may be using much of the same infrastructure (e.g., working memory) to subserve very different aims, accounting for similar patterns of brain activity despite very different goals. We note that this fact may also help to explain the apparent lack of efficacy for the acceptance condition, which explicitly instructed participants not to alter their experience but may have engendered less subjective distress in regard to them. Thus, they may have continued to report thinking about worries while experiencing them in subjectively less-negative ways. Future research should include thought probes for subjective distress to help shed light on this issue.
Nevertheless, the observation that our two mindfulness strategies differed from each other in both neural correlates and efficacy has some potentially important implications. Few studies within the clinical science literature have examined which facets of mindfulness are necessary and sufficient to facilitate disengagement from perseverative thought, and which exercises are best suited to strengthen these skills (Wielgosz et al., 2019). Our results highlight focused attention as an especially promising strategy that may operate with mechanisms very different from well-studied emotion regulation strategies such as reappraisal.
Another aim of this study was to identify neural correlates of successful compared to unsuccessful disengagement. Our results did not confirm our hypotheses that successful disengagement would correspond to altered PCC, vlPFC, and SN activity. Instead, the only area of significant activation was a cluster in right rlPFC, which is a large region thought to be necessary for complex, higher-order cognitive functions, such as abstract cognition, planning, and metacognition (e.g., Dixon et al., 2017). Among the functions that seem particularly applicable are the rlPFC’s relationship to metacognitive awareness (Christoff et al., 2009), that is, being aware of the content of one’s own thoughts. This is a capacity that would seem critical to both top-down attention and acceptance processes proposed in theories of worry (Berenbaum, 2010; Hirsch & Mathews, 2012; Newman et al., 2013) and has been remarked on in relation to emotion regulation more broadly (Creswell, 2017).
Findings from the functional connectivity analyses add nuance to our understanding of successful disengagement. We calculated functional connectivity to the PCC, a central hub of the DMN known to support worry and other forms of abstract and internally directed cognition (Brewer & Garrison, 2014). Contrary to hypotheses, successful disengagement corresponded only with increased functional connectivity between PCC and primary somatosensory cortex (S1). Although S1 was not a hypothesized brain region, it is an area that can be modulated by mindfulness meditation (Kerr et al., 2013; Zeidan et al., 2019) and can play a role in regulation of emotion (Kropf et al., 2018). Our results are thus more in line with theories wherein mindfulness facilitates body-focused awareness rather than explicit emotional regulation (Vago & Silbersweig 2012). However, it is possible that alternative methods for assessing functional connectivity could provide different insights or results, such as effective (causal) connectivity or graph-theoretical methods (Farahani et al., 2019). For example, the directionality of connectivity to regions such as vlPFC or PCC could provide insights into their function in worry or disengagement, while graph-theoretical techniques could indicate whether such networks reconfigure during different types of disengagement. Unfortunately, we lacked highly specific ROIs with strong hypothesized network relationships, and the computational complexity in calculating multivariate outcomes across large territories (vlPFC, dlPFC, and MPFC) was prohibitive, especially in light of our moderate sample size.
The present findings should be interpreted in light of the strengths and limitations of the study. Analyses were preregistered prior to analysis, and data are available upon request. The sample is medium-sized at 38, with sufficient power to detect moderately sized effects (d = 0.5), although smaller effects do occur in fMRI and could have been missed (Mumford, 2012). Our participants were recruited to represent an ecologically valid sample of uncontrollable worriers; participants had a clinically relevant burden of worry but were not excluded for common comorbidities such as moderate levels of depression or SSRI usage. A limitation is the relative overrepresentation of women and White participants in the sample, which may limit the broad applicability of the findings.
We also note inherent challenges to studying uncontrollable worry in the scanner. The majority of participants experienced some difficulty worrying naturally, citing the instructed nature of the task and the artificial nature of the scanning environment. However, worry topics were identified in advance to be highly uncontrollable and ecologically valid, and participants did report moderate levels of worry success. Thought probes occurred only once during the worry epoch, such that the participant’s thoughts during the rest of the epoch were not continually assessed. This methodological decision was intentional, as ongoing metacognitive monitoring would risk altering our key neural and psychological processes of interest, but it nevertheless limits the precision of our assessment. We attempted to address this using extensive pilot testing to optimize the length of the worry epoch, and modeled our study on prior well-established paradigms (e.g., Christoff et al., 2009). Along these lines, challenges to confirming the validity of self-report measures of internal subjective states have been discussed extensively in the literature (e.g., Seli et al., 2015), which in turn raises challenges for precision of measurement. Converging approaches to studying worry and its disengagement are ultimately needed, and the present study offers one such perspective.
In conclusion, our results shed light onto both general and specific mechanisms of disengagement from worry and lend support to the growing body of literature demonstrating the clinical promise of mindfulness-based practices. We demonstrate that different mindfulness-based disengagement strategies engage some common and some distinct brain networks, and allude to complex relationships with circuitry involved in successful worry disengagement. Future research on this topic will likely be surprising and fruitful in equal measures.
Supplementary Material
Acknowledgments
Cecilia A. Westbrook served as lead for formal analysis, software, visualization, writing—original draft, writing—review and editing. Janine Dutcher served in a supporting role for methodology. Susan Kusmierski served as lead for data curation and served in a supporting role for investigation, software. J. David Creswell served in a supporting role for conceptualization and methodology. Essang Akpan contributed equally to data curation. Lauren S. Hallion served as lead for conceptualization, funding acquisition, methodology, resources, supervision, and contributed equally to writing—review and editing.
Footnotes
This study is preregistered on the Open Science Framework (original preregistration: https://osf.io/tsd74 updates to preregistration: https://osf.io/b8exs/). A full preprint is posted (https://psyarxiv.com/3he2b). Data and code are available publicly or upon request; details are included in the supplemental materials. Study procedures were approved by the University of Pittsburgh (IRB# PRO17040137) and Carnegie Mellon (IRB# STUDY2017_00000522) Institutional Review Boards.
Portions of this work were presented at the 2021 Meetings of the Society of Biological Psychiatry and the Anxiety Depression and Association of America. The authors have no conflicts of interest to disclose.
Supplemental materials: https://doi.org/10.1037/abn0000804.supp
References
- Andreescu C, Sheu LK, Tudorascu D, Gross JJ, Walker S, Banihashemi L, & Aizenstein H (2016). Emotion reactivity and regulation in late-life generalized anxiety disorder: Functional connectivity at baseline and post-treatment. The American Journal of Geriatric Psychiatry, 23(2), 200–214. 10.1016/j.jagp.2014.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter AJ, Scott KM, Vos T, & Whiteford HA (2013). Global prevalence of anxiety disorders: A systematic review and meta-regression. Psychological Medicine, 43(5), 897–910. 10.1017/S003329171200147X [DOI] [PubMed] [Google Scholar]
- Beard C, Hsu KJ, Rifkin LS, Busch AB, & Björgvinsson T (2016). Validation of the PHQ-9 in a psychiatric sample. Journal of Affective Disorders, 193, 267–273. 10.1016/j.jad.2015.12.075 [DOI] [PubMed] [Google Scholar]
- Berenbaum H (2010). An initiation–termination two-phase model of worrying. Clinical Psychology Review, 30(8), 962–975. 10.1016/j.cpr.2010.06.011 [DOI] [PubMed] [Google Scholar]
- Berry AS, Sarter M, & Lustig C (2017). Distinct frontoparietal networks underlying attentional effort and cognitive control. Journal of Cognitive Neuroscience, 29(7), 1212–1225. 10.1162/jocn_a_01112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boemo T, Nieto I, Vazquez C, & Sanchez-Lopez A (2022). Relations between emotion regulation strategies and affect in daily life: A systematic review and meta-analysis of studies using ecological momentary assessments. Neuroscience & Biobehavioral Reviews, 139, Article 104747. 10.1016/j.neubiorev.2022.104747 [DOI] [PubMed] [Google Scholar]
- Borkovec TD, Robinson E, Pruzinsky T, & DePree JA (1983). Preliminary exploration of worry: Some characteristics and processes. Behaviour Research and Therapy, 21(1), 9–16. 10.1016/0005-7967(83)90121-3 [DOI] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, & Ochsner KN (2017). Explicit and implicit emotion regulation: A multi-level framework. Social Cognitive and Affective Neuroscience, 12(10), 1545–1557. 10.1093/scan/nsx096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, & Garrison KA (2014). The posterior cingulate cortex as a plausible mechanistic target of meditation: Findings from neuroimaging. Annals of the New York Academy of Sciences, 1307(1), 19–27. 10.1111/nyas.12246 [DOI] [PubMed] [Google Scholar]
- Brewer JA, Roy A, Deluty A, Liu T, & Hoge EA (2020). Can mindfulness mechanistically target worry to improve sleep disturbances? Theory and study protocol for app-based anxiety program. Health Psychology, 39(9), 776–784. 10.1037/hea0000874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown TA, & Barlow DH (2014). Anxiety and Related Disorders Interview Schedule for DSM-5 (ADIS-5): Adult Version. Client Interview Schedule. Oxford University Press. [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, & Schooler JW (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America, 106(21), 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell JD (2017). Mindfulness interventions. Annual Review of Psychology, 68(1), 491–516. 10.1146/annurev-psych-042716-051139 [DOI] [PubMed] [Google Scholar]
- Dalgleish T, & Yiend J (2006). The effects of suppressing a negative autobiographical memory on concurrent intrusions and subsequent autobiographical recall in dysphoria. Journal of Abnormal Psychology, 115(3), 467–473. 10.1037/0021-843X.115.3.467 [DOI] [PubMed] [Google Scholar]
- Dickenson J, Berkman ET, Arch J, & Lieberman MD (2013). Neural correlates of focused attention during a brief mindfulness induction. Social Cognitive and Affective Neuroscience, 8(1), 40–47. 10.1093/scan/nss030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon ML, Girn M, & Christoff K (2017). Hierarchical organization of frontoparietal control networks underlying goal-directed behavior. In Watanabe M (Ed.), The prefrontal cortex as an executive, emotional, and social brain (pp. 133–148). Springer. 10.1007/978-4-431-56508-6_7 [DOI] [Google Scholar]
- Dixon ML, Moodie CA, Goldin PR, Farb N, Heimberg RG, & Gross JJ (2020). Emotion regulation in social anxiety disorder: Reappraisal and acceptance of negative self-beliefs. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 5(1), 119–129. 10.1016/j.bpsc.2019.07.009 [DOI] [PubMed] [Google Scholar]
- Eagleson C, Hayes S, Mathews A, Perman G, & Hirsch CR (2016). The power of positive thinking: Pathological worry is reduced by thought replacement in generalized anxiety disorder. Behaviour Research and Therapy, 78, 13–18. 10.1016/j.brat.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T, Zetsche U, Weidacker K, Wahl K, Schönfeld S, & Ehlers A (2011). The Perseverative Thinking Questionnaire (PTQ): Validation of a content-independent measure of repetitive negative thinking. Journal of Behavior Therapy and Experimental Psychiatry, 42(2), 225–232. 10.1016/j.jbtep.2010.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences of the United States of America, 113(28), 7900–7905. 10.1073/pnas.1602413113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farahani FV, Karwowski W, & Lighthall NR (2019). Application of graph theory for identifying connectivity patterns in human brain networks: A systematic review. Frontiers in Neuroscience, 13, Article 585. 10.3389/fnins.2019.00585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox KCR, Dixon ML, Nijeboer S, Girn M, Floman JL, Lifshitz M, Ellamil M, Sedlmeier P, & Christoff K (2016). Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neuroscience & Biobehavioral Reviews, 65, 208–228. 10.1016/j.neubiorev.2016.03.021 [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, & Raichle ME (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America, 102(27), 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fresco DM, Mennin DS, Heimberg RG, & Turk CL (2003). Using the Penn State Worry Questionnaire to identify individuals with generalized anxiety disorder: A receiver operating characteristic analysis. Journal of Behavior Therapy and Experimental Psychiatry, 34(3–4), 283–291. 10.1016/j.jbtep.2003.09.001 [DOI] [PubMed] [Google Scholar]
- Gaynor K (2014). A critical review of mindfulness-based psychological treatments for worry and rumination. OA Behavioural Medicine, 2(1), Article 2. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, & Friston KJ (2003). Modeling regional and psychophysiologic interactions in fMRI: The importance of hemodynamic deconvolution. NeuroImage, 19(1), 200–207. 10.1016/S1053-8119(03)00058-2 [DOI] [PubMed] [Google Scholar]
- Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, & Mullins PG (2014). The salience network is responsible for switching between the default mode network and the central executive network: Replication from DCM. NeuroImage, 99, 180–190. 10.1016/j.neuroimage.2014.05.052 [DOI] [PubMed] [Google Scholar]
- Hallion LS, & Ruscio AM (2013). Should uncontrollable worry be removed from the definition of GAD? A test of incremental validity. Journal of Abnormal Psychology, 122(2), 369–375. 10.1037/a0031731 [DOI] [PubMed] [Google Scholar]
- Hallion LS, Wright AGC, Joormann J, Kusmierski SN, Coutanche MN, & Caulfield MK (2022). A five-factor model of perseverative thought. Journal of Psychopathology and Clinical Science, 131(3), 235–252. 10.1037/abn0000737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington A, & Dunne JD (2015). When mindfulness is therapy: Ethical qualms, historical perspectives. American Psychologist, 70(7), 621–631. 10.1037/a0039460 [DOI] [PubMed] [Google Scholar]
- Hayes-Skelton SA, & Eustis EH (2020). Experiential avoidance. In Abramowitz JS & Blakey SM (Eds.), Clinical handbook of fear and anxiety: Maintenance processes and treatment mechanisms (pp. 115–131). American Psychological Association. 10.1037/0000150-007 [DOI] [Google Scholar]
- Hirsch CR, & Mathews A (2012). A cognitive model of pathological worry. Behaviour Research and Therapy, 50(10), 636–646. 10.1016/j.brat.2012.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzel BK, Hoge EA, Greve DN, Gard T, Creswell JD, Brown KW, Barrett LF, Schwartz C, Vaitl D, & Lazar SW (2013). Neural mechanisms of symptom improvements in generalized anxiety disorder following mindfulness training. NeuroImage: Clinical, 2, 448–458. 10.1016/j.nicl.2013.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ives-Deliperi VL, Solms M, & Meintjes EM (2011). The neural substrates of mindfulness: An fMRI investigation. Social Neuroscience, 6(3), 231–242. 10.1080/17470919.2010.513495 [DOI] [PubMed] [Google Scholar]
- Karim HT, Tudorascu DL, Butters MA, Walker S, Aizenstein HJ, & Andreescu C (2017). In the grip of worry: Cerebral blood flow changes during worry induction and reappraisal in late-life generalized anxiety disorder. Translational Psychiatry, 7(8), Article e1204. 10.1038/tp.2017.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr CE, Sacchet MD, Lazar SW, Moore CI, & Jones SR (2013). Mindfulness starts with the body: Somatosensory attention and top-down modulation of cortical alpha rhythms in mindfulness meditation. Frontiers in Human Neuroscience, 7, Article 12. 10.3389/fnhum.2013.00012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster EHW, De Lissnyder E, Derakshan N, & De Raedt R (2011). Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review, 31(1), 138–145. 10.1016/j.cpr.2010.08.005 [DOI] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JBW (2001). The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropf E, Syan SK, Minuzzi L, & Frey BN (2018). From anatomy to function: The role of the somatosensory cortex in emotional regulation. Brazilian Journal of Psychiatry, 41(3), 261–269. 10.1590/1516-4446-2018-0183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay EK, & Creswell JD (2017). Mechanisms of mindfulness training: Monitor and acceptance theory (MAT). Clinical Psychology Review, 51, 48–59. 10.1016/j.cpr.2016.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Slagter HA, Dunne JD, & Davidson RJ (2008). Attention regulation and monitoring in meditation. Trends in Cognitive Sciences, 12(4), 163–169. 10.1016/j.tics.2008.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makovac E, Fagioli S, Rae CL, Critchley HD, & Ottaviani C (2020). Can’t get it off my brain: Meta-analysis of neuroimaging studies on perseverative cognition. Psychiatry Research: Neuroimaging, 295, Article 111020. 10.1016/j.pscychresns.2019.111020 [DOI] [PubMed] [Google Scholar]
- McLaren DG, Ries ML, Xu G, & Johnson SC (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage, 61(4), 1277–1286. 10.1016/j.neuroimage.2012.03.068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Mennin DS, & Farach FJ (2007). The contributory role of worry in emotion generation and dysregulation in generalized anxiety disorder. Behaviour Research and Therapy, 45(8), 1735–1752. 10.1016/j.brat.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Messina I, Grecucci A, & Viviani R (2021). Neurobiological models of emotion regulation: A meta-analysis of neuroimaging studies of acceptance as an emotion regulation strategy. Social Cognitive and Affective Neuroscience, 16(3), 257–267. 10.1093/scan/nsab007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer TJ, Miller ML, Metzger RL, & Borkovec TD (1990). Development and validation of the Penn State Worry Questionnaire. Behaviour Research and Therapy, 28(6), 487–495. 10.1016/0005-7967(90)90135-6 [DOI] [PubMed] [Google Scholar]
- Morawetz C, Bode S, Derntl B, & Heekeren HR (2017). The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: A meta-analysis of fMRI studies. Neuroscience & Biobehavioral Reviews, 72, 111–128. 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- Mumford JA (2012). A power calculation guide for fMRI studies. Social Cognitive and Affective Neuroscience, 7(6), 738–742. 10.1093/scan/nss059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MG, Llera SJ, Erickson TM, Przeworski A, & Castonguay LG (2013). Worry and generalized anxiety disorder: A review and theoretical synthesis of evidence on nature, etiology, mechanisms, and treatment. Annual Review of Clinical Psychology, 9(1), 275–297. 10.1146/annurev-clinpsy-050212-185544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Zvielli A, Goldstein P, & Bernstein A (2017). Brief mindfulness training de-couples the anxiogenic effects of distress intolerance on reactivity to and recovery from stress among deprived smokers. Behaviour Research and Therapy, 95, 117–127. 10.1016/j.brat.2017.05.017 [DOI] [PubMed] [Google Scholar]
- Picó-Pérez M, Radua J, Steward T, Menchón JM, & Soriano-Mas C (2017). Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 79, 96–104. 10.1016/j.pnpbp.2017.06.001 [DOI] [PubMed] [Google Scholar]
- Raichle ME (2015). The brain’s default mode network. Annual Review of Neuroscience, 38(1), 433–447. 10.1146/annurev-neuro-071013-014030 [DOI] [PubMed] [Google Scholar]
- Ruscio AM, Hallion LS, & Coleman ES (2011). Worry and rumination interview [Unpublished measure]. Department of Psychology, University of Pennsylvania. [Google Scholar]
- Seli P, Jonker TR, Cheyne JA, Cortes K, & Smilek D (2015). Can research participants comment authoritatively on the validity of their self-reports of mind wandering and task engagement? Journal of Experimental Psychology: Human Perception and Performance, 41(3), 703–709. 10.1037/xhp0000029 [DOI] [PubMed] [Google Scholar]
- Taren AA, Gianaros PJ, Greco CM, Lindsay EK, Fairgrieve A, Brown KW, Rosen RK, Ferris JL, Julson E, Marsland AL, & Creswell JD (2017). Mindfulness meditation training and executive control network resting state functional connectivity: A randomized controlled trial. Psychosomatic Medicine, 79(6), 674–683. 10.1097/PSY.0000000000000466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JS, Jamal-Orozco N, & Hallion LS (2022). Dissociable associations of facets of mindfulness with worry, rumination, and transdiagnostic perseverative thought. Mindfulness, 13(1), 80–91. 10.1007/s12671-021-01747-w [DOI] [Google Scholar]
- Tolin DF, Gilliam C, Wootton BM, Bowe W, Bragdon LB, Davis E, Hannan SE, Steinman SA, Worden B, & Hallion LS (2018). Psychometric properties of a structured diagnostic interview for DSM-5 anxiety, mood, and obsessive–compulsive and related disorders. Assessment, 25(1), 3–13. 10.1177/1073191116638410 [DOI] [PubMed] [Google Scholar]
- Vago DR, & Silbersweig DA (2012). Self-awareness, self-regulation, and self-transcendence (S-ART): A framework for understanding the neurobiological mechanisms of mindfulness. Frontiers in Human Neuroscience, 6, Article 296. 10.3389/fnhum.2012.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, & Buckner RL (2008). Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. Journal of Neurophysiology, 100(6), 3328–3342. 10.1152/jn.90355.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber-Goericke F, & Muehlhan M (2019). A quantitative meta-analysis of fMRI studies investigating emotional processing in excessive worriers: Application of activation likelihood estimation analysis. Journal of Affective Disorders, 243, 348–359. 10.1016/j.jad.2018.09.049 [DOI] [PubMed] [Google Scholar]
- Westbrook C, Creswell JD, Tabibnia G, Julson E, Kober H, & Tindle HA (2013). Mindful attention reduces neural and self-reported cue-induced craving in smokers. Social Cognitive and Affective Neuroscience, 8(1), 73–84. 10.1093/scan/nsr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielgosz J, Goldberg SB, Kral TRA, Dunne JD, & Davidson RJ (2019). Mindfulness meditation and psychopathology. Annual Review of Clinical Psychology, 15(1), 285–316. 10.1146/annurev-clinpsy-021815-093423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgast M, & Lundh L-G (2017). Is distraction an adaptive or maladaptive strategy for emotion regulation? A person-oriented approach. Journal of Psychopathology and Behavioral Assessment, 39(1), 117–127. 10.1007/s10862-016-9570-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyland CL, Kelley WM, Macrae CN, Gordon HL, & Heatherton TF (2003). Neural correlates of thought suppression. Neuropsychologia, 41(14), 1863–1867. 10.1016/j.neuropsychologia.2003.08.001 [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, & Wager TD (2011). Large-scale automated synthesis of human functional neuroimaging data. Nature Methods, 8(8), 665–670. 10.1038/nmeth.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F, Baumgartner JN, & Coghill RC (2019). The neural mechanisms of mindfulness-based pain relief: A functional magnetic resonance imaging-based review and primer. Pain Reports, 4(4), Article e759. 10.1097/PR9.0000000000000759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X-R, Chen Z-F, Kang C-Y, Liu R-X, Bai J-Y, Cao Y-P, Cheng Y-Q, Xu X-F, & Zhang Y-L (2019). Mindfulness-based cognitive therapy is associated with distinct resting-state neural patterns in patients with generalized anxiety disorder. Asia-Pacific Psychiatry, 11(4), Article e12368. 10.1111/appy.12368 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


