Abstract
Hepatocellular carcinoma (HCC) is the most common and lethal type of primary liver cancer, frequently arising from chronic liver injury and inflammation. Despite treatment advancements, HCC prognosis remains poor, emphasizing the need for effective preventive and therapeutic strategies. This study investigates the hepatoprotective and anti-tumor effects of Hongjam, a steamed freeze-dried silkworm powder, in a diethylnitrosamine (DEN) and thioacetamide (TAA)-induced HCC mouse model. Mice were administered DEN intraperitoneally for 8 weeks, followed by TAA in drinking water for 9 weeks, with Hongjam supplementation (0.01, 0.1, and 1 g/kg) provided daily through food. Hongjam markedly reduced the tumor incidence, the size, and the histological lesions compared to the DEN/TAA group. Serum biochemical analysis revealed reduction in liver damage markers, including alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, and total bilirubin, with a notable decrease in total bilirubin surpassing. Immunohistochemical and Western blot analyses demonstrated that Hongjam downregulated expression of proliferation markers, including Ki67, phosphorylation of protein kinase B, and proliferating cell nuclear antigen, while upregulating the pro-apoptotic protein Bcl-2-associated X protein, indicating its dual role in suppressing proliferation and promoting apoptosis. Furthermore, Hongjam inhibited angiogenesis by suppressing the expression of key markers, including interleukin 6, VEGF, hypoxia-inducible factor-1 subunit alpha, platelet-derived growth factor subunit beta, matrix metalloproteinase-2, and cluster of differentiation 31, thereby disrupting the tumor microenvironment. These findings suggest that Hongjam exerts multifaceted protective effects against HCC by targeting proliferation, apoptosis, and angiogenesis pathways, while also mitigating liver damage. This study highlights the potential of Hongjam as a functional food or a complementary therapeutic agent for HCC prevention and management.
Keywords: Hepatocellular carcinoma, Hongjam, Diethylnitrosamine, Thioacetamide, Anti-proliferation, Angiogenesis
INTRODUCTION
Hepatocellular carcinoma (HCC) is the most common and lethal type of primary liver cancer worldwide, accounting for the majority of liver cancer cases [1,2]. Despite advancements in treatment, the prognosis for HCC remains poor due to late diagnosis, high recurrence rates, and limited efficacy of existing therapeutic options [3]. This highlights the urgent need for effective preventive strategies and alternative therapeutic agents to combat HCC [4].
Natural products are gaining attention for their cancer preventive and therapeutic potential due to their bioavailability, low toxicity, and multi-target capabilities [5,6]. Among these, silkworm-derived compounds have emerged as promising candidates due to their significant anti-inflammatory, antioxidant, and protective effects, as reported in various studies [7-9]. These properties make silkworm-derived compounds valuable for therapeutic and preventive applications in inflammation, oxidative stress-related diseases, and metabolic disorders [10,11]. Previous studies identified Hongjam (steamed freeze-dried mature silkworm powder) as a potential therapeutic agent [12,13]. Hongjam is derived from silkworms through a steaming and freeze-drying process, designed to retain its bioactive components, including fibroin peptides. It has protective effects against liver fat accumulation, inflammation, and oxidative stress by modulating AMP-activated protein kinase signaling pathways, reducing pro-inflammatory cytokines, and enhancing lipid metabolism. These findings suggest its potential role in preventing alcoholic liver diseases [12]. Moreover, Hongjam has been shown to reduce gastric mucosal ulceration, enhance antioxidant defenses, and suppress inflammation by improving mucus secretion and inhibiting pro-inflammatory pathways, highlighting its therapeutic potential for ethanol-induced gastric ulcers [13].
In this study, we evaluated the protective effects of Hongjam in a diethylnitrosamine (DEN) and thioacetamide (TAA)-induced mouse model, a system that mimics the progression of liver cancer in the context of chronic liver injury and fibrosis. A DEN/TAA-induced mouse model was also used to study HCC. Specifically, we investigated its impact on tumor growth, liver damage markers, cancer growth factors, apoptosis-related proteins, and angiogenesis-related factors. By understanding the multifaceted actions of Hongjam, this study seeks to may provide insights into its potential application as a functional food or therapeutic supplement for liver cancer prevention and management.
MATERIALS AND METHODS
Preparation of Hongjam and silymarin
Hongjam preparation followed a previous protocol [14], harvesting Bombyx mori larvae (white-jade cocoon strain) on the 7th day of the 5th instar. The larvae were cooked at 100°C for 130 minutes using an electric pressure-free cooker (KumSeong Ltd.) and subsequently freeze-dried for 24 hours (FDT-8612, Operon 105 Ltd.). The freeze-dried larvae were ground into a fine powder (particle size: 0.1 mm) using a disc mill (HM001, Korean Pulverizing Machinery Co. Ltd.). The powdered Hongjam was then formulated into food samples at concentrations of 0.01, 0.1, and 1 g/kg (Samin Science). Silymarin, a hepatoprotective agent purchased from Sigma-Aldrich, was prepared in the same food formulation as Hongjam to ensure consistency.
Animal experiment and reagents
Male C57BL/6 mice (5 weeks old) were obtained from Orient Bio and housed under specific pathogen-free conditions at 24°C with a 12-hour light/dark cycle. The experiment was conducted at the CHA University Animal Center following the Institutional Animal Care and Use Committee (IACUC) guidelines (approval number: IACUC240050). After a one-week acclimation period, mice were randomly assigned to experimental groups and monitored for 34 weeks. Food intake and body weight were measured weekly.
To induce liver cancer, DEN (Sigma-Aldrich) was administered intraperitoneally (i.p.) once a week for 8 consecutive weeks, followed by TAA (Sigma-Aldrich) in drinking water for 8 weeks (weeks 9-16). Hongjam and silymarin were administered daily through food starting from the initiation of cancer induction. At the end of the experiment, liver and blood samples were collected. Livers were fixed in paraffin, and blood samples collected in heparin-coated tubes were centrifuged at 845 × g for 10 minutes at 4°C for further analysis.
Serum analysis
Serum biochemical markers of liver injury, including alkaline phosphatase (ALP), alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin, were measured using a Hitachi automatic analyzer 7600-210 (Hitachi High-Technologies Corporation).
Histology and immunohistochemistry staining
Liver tissues were embedded in paraffin blocks, sectioned at a thickness of 5 μm, and mounted on slide glasses. Hematoxylin and eosin staining was performed using standard protocols. Tumor scoring was conducted by assigning points based on the size of each tumor [15]. We modified scoring the tumor size according to our experiment. Tumors with a diameter of less than 2 mm were given 1 point, while those larger than 2 mm but smaller than 5 mm were assigned 2 points. Tumors exceeding 5 mm in diameter were assigned 3 points. The total tumor score for each animal was calculated by summing the points of all tumors observed in that individual. Finally, the average tumor score for each group was determined by calculating the mean of the total scores across all animals within the group. For immunohistochemistry (IHC), slides were deparaffinized with xylene and rehydrated. Endogenous peroxidase activity was blocked using 3% H2O2 for 30 minutes. Antigen retrieval was performed by heating slides in 0.1 M citrate buffer (pH 6.0) at boiling temperature for 1 minute in a microwave. After cooling, slides were blocked with a Vectastain ABC kit blocking solution (Vector Laboratories) for 1 hour at room temperature and incubated overnight at 4°C with a primary antibody against Ki67 (1:150, ab15580, Abcam). The next day, slides were incubated with a secondary antibody for 1 hour at room temperature, treated with ABC-horseradish peroxidase (HRP) for 30 minutes, and visualized using 3,3’-diaminobenzidine (Quanto, Thermo Scientific) for 3 minutes. Counterstaining was performed with hematoxylin for 30 seconds. After dehydration, slides were mounted with cover glasses and observed using a LEICA ICC50 E microscope (Leica Microsystems). Staining intensity was scored by five independent observers blinded to the experimental conditions, using a scale of 1 to 10, with the normal control set as the baseline (score of 1).
Western blotting
Liver tissues were pulverized in liquid nitrogen and lysed in a buffer containing protease inhibitors. The lysates were centrifuged at 15,871 × g for 15 minutes at 4°C to obtain total protein. Protein concentrations were quantified, and 20 μg of each sample was separated by SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Membranes were blocked with 3% bovine serum albumin in PBS tween for 1 hour at room temperature and incubated overnight at 4°C with primary antibodies against phosphorylation of protein kinase B (p-AKT), total AKT, glutathione S-transferase π (GST-π), proliferating cell nuclear antigen (PCNA) and Bcl-2-associated X protein (BAX). Details of the antibodies used are provided in Table 1. After washing, membranes were incubated with HRP-conjugated secondary antibodies. Signals were detected using enhanced chemiluminescence reagents and visualized with the ImageQuant™ LAS 4000 Multi-Mode Imager (GE Healthcare).
Table 1.
Antibodies used for Western blot analysis
| Antibody | Dilution | Product NO. | Species of origin and supplier |
|---|---|---|---|
| p-AKT | 1:1,000 | #9271 | Rabbit polyclonal, Cell signaling technology, Inc. |
| AKT | 1:1,000 | #9272 | Rabbit polyclonal, Cell signaling technology, Inc. |
| GST-π | 1:4,000 | ADI-MSA-101-E | Rabbit polyclonal, ENZO life sciences, Inc. |
| PCNA | 1:4,000 | Ab29 | Mouse monoclonal, Abcam |
| BAX | 1:2,000 | Sc-7480 | Mouse monoclonal, Santa cruz Biotechnology, Inc. |
| β-actin | 1:4,000 | SC-47778 | Mouse monoclonal, Santa cruz Biotechnology, Inc. |
No., number; p-AKT, phosphorylation of protein kinase B; GST-π, glutathione S-transferase π; PCNA, proliferating cell nuclear antigen; BAX, Bcl-2-associated X protein.
RNA isolation and gene expression quantitative analysis
Total RNA was extracted from liver tissues homogenized in liquid nitrogen using TRIzol® reagent (Invitrogen). cDNA was synthesized using a Labopass cDNA synthesis kit (Cosmogenetech). Real-time quantitative PCR (qPCR) was performed using Luna universal qPCR master mix (New England Biolabs) with a ViiA™ 7 real-time PCR system (Applied Biosystems). The expression levels of interleukin 6 (IL6), VEGF, hypoxia-inducible factor-1 subunit alpha (HIF-1α), platelet-derived growth factor subunit beta (PDGF-β), matrix metalloproteinase-2 (MMP2) and cluster of differentiation 31 (CD31) were analyzed. Primer sequences are listed in Table 2.
Table 2.
Primer sequenced for qRT-PCR analysis in mouse
| Gene | Forward | Reverse | Size (bp) |
|---|---|---|---|
| 18s rRNA | GCAATTATTCCCCATGAACG | GGCCTCACTAAACCATCCAA | 123 |
| IL6 | TCCTTCCTACCCCAATTTCCA | GTCTTGGTCCTTAGCCACTCC | 76 |
| VEGF | CTGCTGTAACGATGAAGCCCTG | GCTGTAGGAAGCTCATCTCTCC | 119 |
| HIF-1α | CCTGCACTGAATCAAGAGGTTGC | CCATCAGAAGGACTTGCTGGCT | 111 |
| PDGF-β | AATGCTGAGCGACCACTCCATC | TCGGGTCATGTTCAAGTCCAGC | 106 |
| MMP2 | CAAGGATGGACTCCTGGCACAT | TACTCGCCATCAGCGTTCCCAT | 138 |
| CD31 | CCAAAGCCAGTAGCATCATGGTC | GGATGGTGAAGTTGGCTACAGG | 144 |
qRT-PCR, real-time quantitative PCR; IL6, interleukin 6; HIF-1α, hypoxia-inducible factor-1 subunit alpha; PDGF-β, platelet-derived growth factor subunit beta; MMP2, matrix metalloproteinase-2; CD31, cluster of differentiation 31.
Statistical analysis
All experiments were performed at least three times. Results are expressed as mean ± SD. Statistical analyses were conducted using GraphPad Prism 10 (GraphPad Software). Statistical significance was determined using two-way ANOVA followed by Tukey’s multiple comparison test, with a P-value of < 0.05 considered significant.
RESULTS
Hongjam supplementation reduces tumor burden in a DEN/TAA-induced HCC model
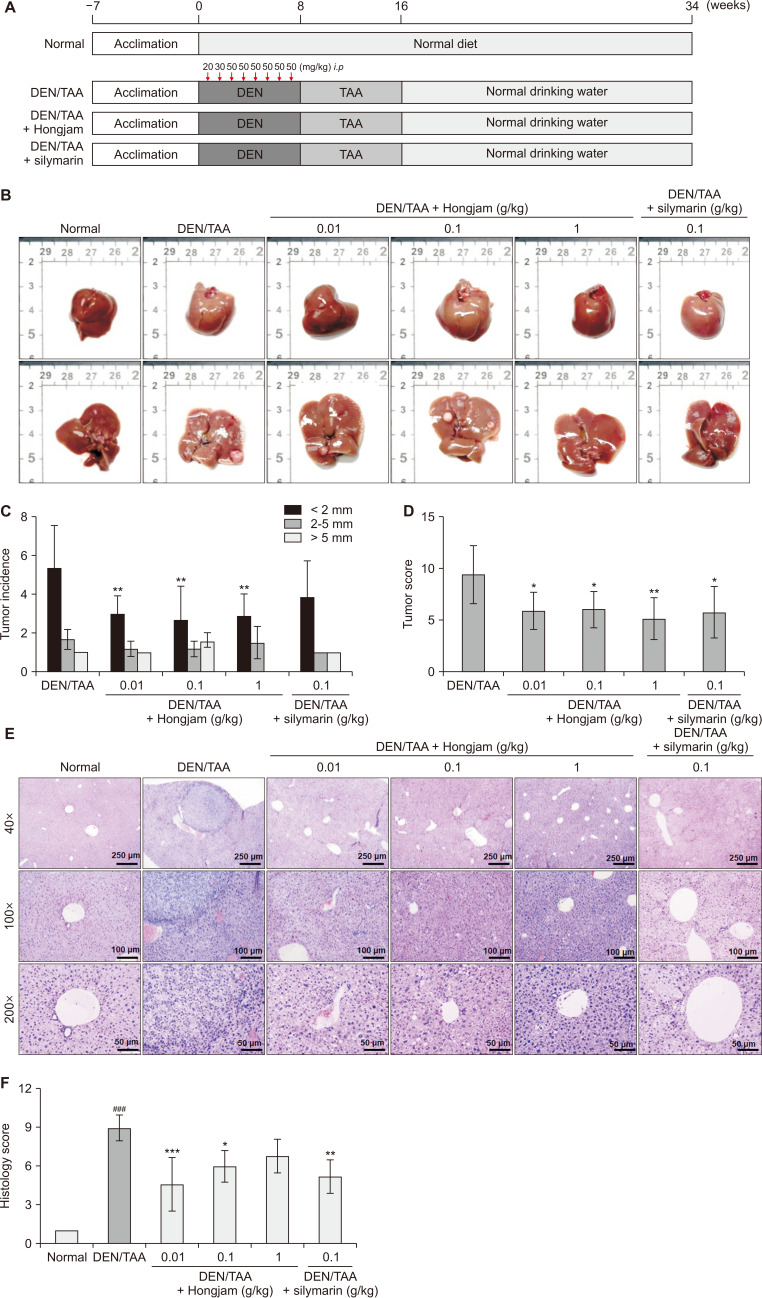
This study assessed the hepatoprotective effects of Hongjam using a DEN/TAA-induced HCC mouse model. In this model, 5-week-old C57BL6 mice received intraperitoneal DEN injections weekly for 8 weeks, followed by TAA continuously provided in drinking water for 9 weeks (Fig. 1A). Throughout the experimental period, mice were supplemented daily with Hongjam (0.01, 0.1, 1 g/kg) or silymarin (0.1 g/kg; Fig. 1A). Afterward, normal drinking water was provided, and the mice were fed daily diets supplemented with Hongjam or silymarin (Fig. 1A). Silymarin, a well-known hepatoprotective agent and widely used health functional food for liver function, was included as a positive control to validate the study’s outcomes [16]. The DEN/TAA group showed a significantly increased tumor incidence and a size, reflecting HCC characteristics (Fig. 1B). In contrast, the groups supplemented with Hongjam or silymarin exhibited a significant reduction in the tumor incidence and the size. Notably, the number of tumors smaller than 2 mm was markedly reduced in the Hongjam-treated groups, particularly in the 1 g/kg group, where no tumors larger than 5 mm were observed (Fig. 1C). The tumor size scoring number was also significantly decreased in both Hongjam and silymarin groups (Fig. 1D). According to the histopathological findings, several pathological characteristics were distinctly observed in the DEN/TAA-induced HCC group, reflecting the progression and severity of the disease. Dysplastic nodules, a precursor to malignancy, were frequently detected, indicating the onset of tumor formation. Additionally, both small and large cell changes were evident, suggesting an increased likelihood of malignancy. These alterations progressed to invasive carcinoma, where tumor cells disrupted the normal liver architecture and invaded surrounding tissues. Extensive infiltration of immune cells was observed around the tumors, further highlighting the aggressive nature of the disease. In contrast, in the Hongjam-treated group, these pathological changes were generally attenuated. Thus, dysplastic nodules were less frequent, and cellular atypia was reduced. Small and large cell changes were significantly less pronounced, with lower mitotic activity and fewer irregular nuclei. Furthermore, immune cell infiltration was also diminished, indicating an improvement in the immune microenvironment. These results suggest that Hongjam supplementation alleviates the progression of liver cancer and modulates the tumor microenvironment to a less aggressive state (Fig. 1E and 1F). Overall, the tumor incidence, the size, and histological lesions were significantly reduced in the Hongjam-treated groups compared to the DEN/TAA-only group. These results highlight Hongjam’s potential to mitigate tumor progression in HCC.
Figure 1. Effect of Hongjam supplementation on tumor incidence and tissue lesion in the DEN/TAA-induced HCC model.
(A) Schematic representation of the experimental protocol for the DEN/TAA-induced HCC model. DEN was administered intraperitoneally for 8 weeks, followed by TAA in drinking water for an additional 8 weeks. Animals were sacrificed at week 34. (B) Representative images of extracted livers. (C) Tumor count and size measurements. (D) Tumor scoring based on size: tumors < 2 mm were scored as 1 point, those 2-5 mm as 2 points, and tumors > 5 mm as 5 points. (E) H&E staining showing the effect of Hongjam on tissue lesions (magnification: ×40, ×100, ×200). (F) Quantification of H&E staining intensity using histopathological scoring. Statistical significance was determined using Tukey’s multiple comparison test, with data presented as mean ± SD (n = 8). ###P < 0.001 vs. Normal group; ***P < 0.001, **P < 0.01, *P < 0.05 vs. DEN/TAA group. DEN, diethylnitrosamine; TAA, thioacetamide; HCC, hepatocellular carcinoma; H&E, hematoxylin and eosin; i.p., intraperitoneally.
Serum biochemical analysis indicates reduced liver damage with Hongjam treatment
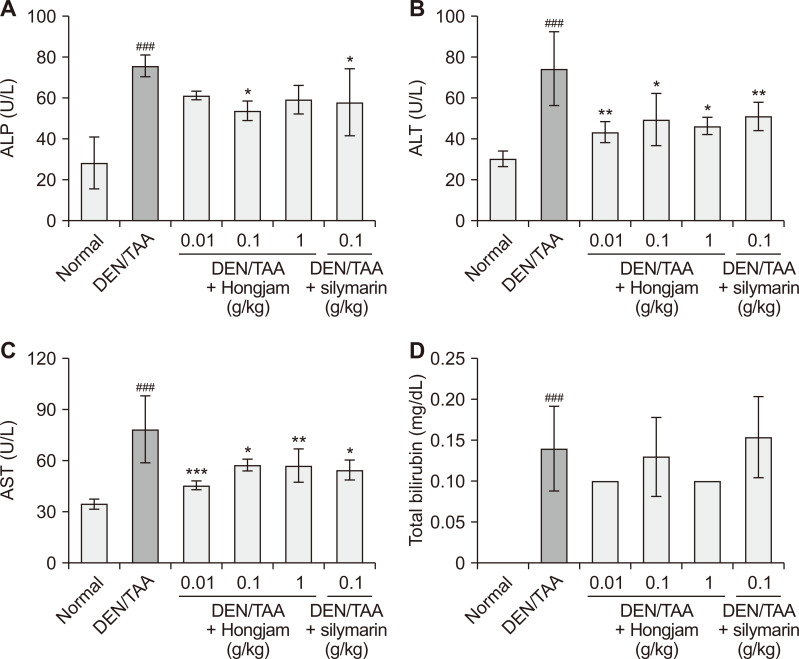
To evaluate the hepatoprotective effects of Hongjam, serum biochemical markers of liver injury, including ALP, ALT, AST, and total bilirubin, were analyzed (Fig. 2). Liver injury is typically associated with elevated levels of these biomarkers, which reflect hepatocyte damage, cholestasis, and impaired liver function [17,18]. The DEN/TAA-induced HCC group showed elevated levels of above markers, reflecting severe liver damage and tumorigenesis. Reductions in ALP, ALT, and AST levels in the Hongjam-treated groups were comparable to those observed in the silymarin-treated group (Fig. 2A-2C). Notably, total bilirubin levels were reduced more significantly in the Hongjam-treated group, particularly at 1 g/kg, than in the silymarin-treated group, suggesting a superior effect of Hongjam in alleviating cholestasis (Fig. 2D). These findings indicate that Hongjam effectively mitigates DEN/TAA-induced liver damage by reducing biochemical markers of liver injury.
Figure 2. Biochemical analysis of the DEN/TAA-induced model with Hongjam supplementation.
(A) ALP, (B) ALT (C) AST, and (D) total bilirubin levels. Statistical significance was determined using Tukey’s multiple comparison test. Data are presented as mean ± SD (n = 5-10). ###P < 0.001 vs. Normal group; ***P < 0.001, **P < 0.01, *P < 0.05 vs. DEN/TAA group. DEN, diethylnitrosamine; TAA, thioacetamide; ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Hongjam inhibits cellular proliferation and promotes apoptosis in a DEN/TAA-induced HCC model
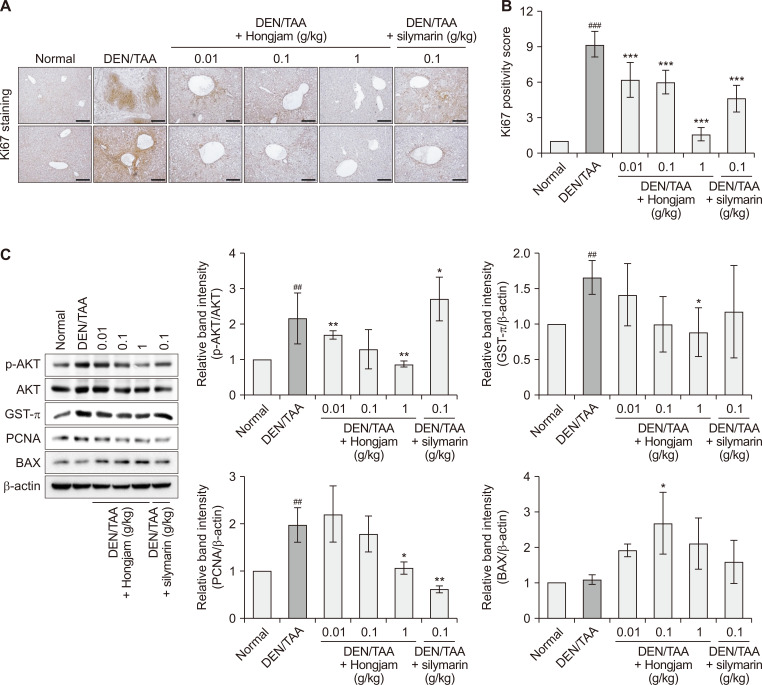
The effect of Hongjam on tumor proliferation and apoptosis in HCC was accessed using IHC and Western blot analyses. IHC analysis of Ki67, a marker of cellular proliferation, revealed significantly reduced expression in Hongjam-treated groups compared to that in the DEN/TAA-only group (Fig. 3A). Notably, the reduction in Ki67 expression was more pronounced in the Hongjam-treated groups, particularly at 1 g/kg, than in the silymarin-treated group, suggesting a superior inhibitory effect on proliferation (Fig. 3B). Western blot analysis further supported these findings, showing a dose-dependent reduction in p-AKT expression in the Hongjam-treated groups (Fig. 3C). Proliferation-related factors, such as GST-π and PCNA, were also significantly downregulated with Hongjam supplementation, indicating its ability to suppress key signaling pathway involved in tumor growth (Fig. 3C). On the other hand, the expression of BAX, a pro-apoptotic protein involved in mitochondrial-mediated apoptosis, was significantly increased in the Hongjam-treated groups (Fig. 3C). This observation suggests that Hongjam not only inhibits tumor proliferation but also promotes apoptosis in cancer cells. The concurrent downregulation of proliferation markers and upregulation of pro-apoptotic signals highlights a Hongjam’s dual role in reducing cancer cell survival and promoting tumor regression. Collectively, these results demonstrate that Hongjam exerts potential anti-proliferative and pro-apoptotic effects in the DEN/TAA-induced liver cancer model, underscoring its potential as a therapeutic agent for HCC.
Figure 3. Hongjam suppresses proliferation in the DEN/TAA-induced HCC model.
(A) IHC staining of Ki67 in liver tissue, indicating levels of cell proliferation (magnification: × 100). (B) Quantitative analysis of Ki67 positivity in IHC staining (magnification: ×100; scale bar: 100 μm). (C) Western blot analysis of liver-derived proteins showing the expression levels of p-AKT, AKT, GST-π, PCNA, and BAX. Statistical significance was determined using a Tukey’s multiple comparison test. Data are presented as mean ± SD (n = 3). ###P < 0.001, ##P < 0.01 vs. Normal group; ***P < 0.00, **P < 0.01, *P < 0.05 vs. DEN/TAA group. DEN, diethylnitrosamine; TAA, thioacetamide; HCC, hepatocellular carcinoma; IHC, immunohistochemistry; p-AKT, phosphorylation of protein kinase B; GST-π, glutathione S-transferase π; PCNA, proliferating cell nuclear antigen; BAX, Bcl-2-associated X protein.
Hongjam suppresses angiogenic factor expression to mitigate cancer progression
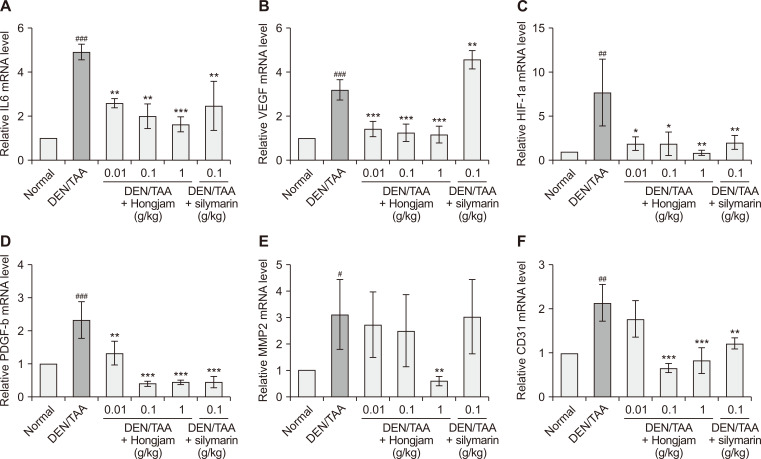
To evaluate the anti-angiogenic effects of Hongjam, the expression levels of angiogenic markers, including IL6, VEGF, HIF-1α, PDGF-β, MMP2, and CD31, were measured (Fig. 4). These markers, essential for tumor growth and nutrient supply, are often upregulated during cancer progression [19,20]. In the DEN/TAA-induced HCC group, the expression of all these markers was significantly increased, highlighting an angiogenic tumor microenvironment. Hongjam treatment resulted in a dose-dependent reduction in IL6 expression, a pro-inflammatory cytokine that promotes VEGF-mediated angiogenesis [21]. VEGF, a key regulator of angiogenesis, was significantly downregulated in the Hongjam-treated groups (Fig. 4B). Similarly, other angiogenic markers, including HIF-1α, PDGF-β, MMP2, and CD31, showed marked decreases in expression across the Hongjam-treated groups, particularly at higher doses (Fig. 4C-4F). These reductions were comparable to or exceeded the effects observed with silymarin. By downregulating angiogenic factors, Hongjam disrupted the tumor microenvironment, reducing the availability of nutrients and oxygen necessary for tumor growth. These findings suggest that Hongjam mitigates cancer progression and proliferation through multiple mechanisms. These findings underscore the potential of Hongjam as an effective therapeutic agent targeting angiogenesis in HCC.
Figure 4. Hongjam’s effect on angiogenesis-related mRNA expression.
(A) IL6, (B) VEGF, (C) HIF-1α, (D) PDGF-β, (E) MMP2, and (F) CD31 mRNA expression levels in liver tissues. Statistical significance was determined using Tukey’s multiple comparison test. Data are presented as mean ± SD (n = 3). ###P < 0.001, ##P < 0.01 vs. Normal group; ***P < 0.001, **P < 0.01, *P < 0.05 vs. DEN/TAA group. IL6, interleukin 6; HIF-1α, hypoxia-inducible factor-1 subunit alpha; PDGF-β, platelet-derived growth factor subunit beta; MMP2, matrix metalloproteinase-2; CD31, cluster of differentiation 31; DEN, diethylnitrosamine; TAA, thioacetamide.
DISCUSSION
The DEN/TAA-induced liver cancer model is a well-established system that replicates the progression of HCC under conditions of chronic liver injury, fibrosis, and inflammation [18,22]. Unlike the DEN-only model used in our previous study, which focuses on early carcinogenesis, the DEN/TAA model more closely mimics the complex tumor microenvironment in human HCC, particularly in cases associated with chronic liver disease [22,23]. Therefore, the latter model was employed to assess the therapeutic efficacy of Hongjam in mitigating HCC progression.
The findings of this study highlight Hongjam’s significant therapeutic potential for HCC prevention and treatment. Its ability to target multiple hallmarks of cancer progression—proliferation, apoptosis resistance, angiogenesis, and liver injury—suggests Hongjam as a promising candidate for addressing the complex pathology of HCC.
Compared to other natural products studied for HCC, such as curcumin, resveratrol, and silymarin, Hongjam demonstrates a unique combination of anti-proliferative, pro-apoptotic, and anti-angiogenic effects. For instance, silymarin, a well-known hepatoprotective agent, primarily exerts antioxidant and anti-inflammatory effects and has shown efficacy in improving liver function markers [24]. In this study, we confirmed that Hongjam effectively reduces tumor-associated markers, including total bilirubin, a key indicator of cholestasis, as well as major angiogenic markers such as VEGF and CD31. These findings demonstrate that Hongjam’s efficacy is not only comparable to but also exceeds that of silymarin, suggesting a stronger potential to disrupt the tumor microenvironment. Moreover, while compounds like resveratrol and curcumin have shown anti-proliferative effects via AKT signaling inhibition [25,26], Hongjam achieves a dual effect by simultaneously upregulating the pro-apoptotic protein BAX. This highlights its advantage as a multi-mechanistic agent, addressing both cancer cell survival and programmed cell death pathways, which are crucial for overcoming tumor progression and resistance mechanisms.
The dual role of Hongjam in inhibiting proliferation and angiogenesis is particularly valuable in the context of HCC therapy. Proliferation markers such as Ki67 and PCNA are often elevated in aggressive HCC and are indicative of poor prognosis [27]. The activation of the AKT signaling pathway in cancer has been shown to promote cell proliferation [28]. Various compounds targeting the inhibition of this pathway have been reported, and recent studies have highlighted the diverse bioactive properties of fibroin peptide in diabetes, cell proliferation, and cancer [29,30]. Notably, silk fibroin peptide has been reported to exert anti-inflammatory effects and inhibit cell proliferation in breast cancer by suppressing AKT phosphorylation [31]. Hongjam’s suppression of these markers, alongside its dose-dependent inhibition of p-AKT—a key oncogenic pathway—demonstrates its ability to impair critical survival and growth mechanisms of tumor cells [32].
Angiogenesis is another hallmark of HCC, driven by HIFs like HIF-1α and pro-angiogenic cytokines such as VEGF and PDGF-β [33,34]. These factors facilitate the formation of new blood vessels, ensuring a continuous supply of oxygen and nutrients to rapidly growing tumors [33,34]. Hongjam significantly reduces angiogenic factor expression, disrupting vascularization critical for tumor growth and metastasis. This dual-action approach is particularly critical in HCC, where angiogenesis is not only a driver of tumor progression but also a key target for systemic therapies, such as sorafenib [35,36]. However, unlike synthetic agents, Hongjam achieves this with a natural origin, suggesting potential for lower toxicity and better tolerability.
The findings of this study align with the growing body of evidence supporting the use of natural products as complementary or alternative therapies in oncology. Hongjam’s multi-faceted actions, combined with its favorable safety profile as a food-derived product, make it an attractive candidate for integration into HCC management strategies. Furthermore, its effects on angiogenesis and proliferation suggest potential synergy with existing systemic therapies like tyrosine kinase inhibitors, warranting further investigation. In this study, the actual Hongjam intake by experimental animals was calculated based on body weight and dietary intake. When converted to a human equivalent dose, the 0.1 g/kg Hongjam group corresponds to approximately 2.96 g/day for a 60 kg adult. These results suggest that a daily intake of approximately 3 g of Hongjam may provide protective effects against HCC.
Despite aforementioned promising findings, this study has some limitations. The precise molecular mechanisms underlying Hongjam’s effects require further elucidation. Advanced techniques such as transcriptomics, proteomics, and metabolomics could provide deeper insights into its multifaceted actions. Additionally, while the DEN/TAA model is highly relevant for preclinical studies, the translation of these findings to human applications necessitates validation through clinical trials to assess efficacy, safety, and optimal dosing in humans. In conclusion, Hongjam demonstrates significant potential for HCC prevention and therapy by targeting multiple hallmarks of cancer progression, including tumor growth, apoptosis resistance, and angiogenesis. These findings establish a basis for future clinical investigations to evaluate Hongjam’s efficacy and safety as a complementary therapy.
ACKNOWLEDGMENTS
This research was funded by the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ017032) of the Rural Development Administration, Republic of Korea.
Funding Statement
FUNDING None.
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
References
- 1.Blonski W, Kotlyar DS, Forde KA. Non-viral causes of hepatocellular carcinoma. World J Gastroenterol. 2010;16:3603–15. doi: 10.3748/wjg.v16.i29.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010;28:3994–4005. doi: 10.1200/JCO.2010.28.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang MD, Li C, Liang L, Xing H, Sun LY, Quan B, et al. Early and late recurrence of hepatitis B virus-associated hepatocellular carcinoma. Oncologist. 2020;25:e1541–51. doi: 10.1634/theoncologist.2019-0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoshida Y, Fuchs BC, Bardeesy N, Baumert TF, Chung RT. Pathogenesis and prevention of hepatitis C virus-induced hepatocellular carcinoma. J Hepatol. 2014;61(1 Suppl):S79–90. doi: 10.1016/j.jhep.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yarla NS, Bishayee A, Sethi G, Reddanna P, Kalle AM, Dhananjaya BL, et al. Targeting arachidonic acid pathway by natural products for cancer prevention and therapy. Semin Cancer Biol. 2016;40-41:48–81. doi: 10.1016/j.semcancer.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Sarkar FH, Li Y. Harnessing the fruits of nature for the development of multi-targeted cancer therapeutics. Cancer Treat Rev. 2009;35:597–607. doi: 10.1016/j.ctrv.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biganeh H, Kabiri M, Zeynalpourfattahi Y, Costa Brancalhão RM, Karimi M, Shams Ardekani MR, et al. Bombyx mori cocoon as a promising pharmacological agent: a review of ethnopharmacology, chemistry, and biological activities. Heliyon. 2022;8:e10496. doi: 10.1016/j.heliyon.2022.e10496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wu X, He K, Velickovic TC, Liu Z. Nutritional, functional, and allergenic properties of silkworm pupae. Food Sci Nutr. 2021;9:4655–65. doi: 10.1002/fsn3.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diez-Echave P, Ruiz-Malagón AJ, Molina-Tijeras JA, Hidalgo-García L, Vezza T, Cenis-Cifuentes L, et al. Silk fibroin nanoparticles enhance quercetin immunomodulatory properties in DSS-induced mouse colitis. Int J Pharm. 2021;606:120935. doi: 10.1016/j.ijpharm.2021.120935. [DOI] [PubMed] [Google Scholar]
- 10.Long X, Zhao X, Wang W, Zhang Y, Wang H, Liu X, et al. Protective effect of silkworm pupa oil on hydrochloric acid/ethanol-induced gastric ulcers. J Sci Food Agric. 2019;99:2974–86. doi: 10.1002/jsfa.9511. [DOI] [PubMed] [Google Scholar]
- 11.Baek SY, Li FY, Kim JH, Ahn C, Kim HJ, Kim MR. Protein hydrolysate of silkworm pupa prevents memory impairment induced by oxidative stress in scopolamine-induced mice via modulating the cholinergic nervous system and antioxidant defense system. Prev Nutr Food Sci. 2020;25:389–99. doi: 10.3746/pnf.2020.25.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DY, Hong KS, Song MY, Yun SM, Ji SD, Son JG, et al. Hepatoprotective effects of steamed and freeze-dried mature silkworm larval powder against ethanol-induced fatty liver disease in rats. Foods. 2020;9:285. doi: 10.3390/foods9030285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yun SM, Cho JM, Hong KS, Lee DY, Ji SD, Son JG, et al. Gastroprotective effect of mature silkworm, Bombyx mori against ethanol-induced gastric mucosal injuries in rats. J Funct Foods. 2017;39:279–86. doi: 10.1016/j.jff.2017.10.036. [DOI] [Google Scholar]
- 14.Ji SD, Kim NS, Lee JY, Kim MJ, Kweon H, Sung G, et al. Development of processing technology for edible mature silkworm. J Seric Entomol Sci. 2015;53:38–43. doi: 10.7852/jses.2015.53.1.38. [DOI] [Google Scholar]
- 15.Tokumitsu Y, Shindo Y, Matsui H, Matsukuma S, Nakajima M, Suzuki N, et al. Utility of scoring systems combining the product of tumor number and size with liver function for predicting the prognosis of patients with hepatocellular carcinoma after hepatectomy. Oncol Lett. 2019;18:3903–13. doi: 10.3892/ol.2019.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fraschini F, Demartini G, Esposti D. Pharmacology of silymarin. Clin Drug Investig. 2002;22:51–65. doi: 10.2165/00044011-200222010-00007. [DOI] [Google Scholar]
- 17.Megahed A, Gadalla H, Abdelhamid FM, Almehmadi SJ, Khan AA, Albukhari TA, et al. Vitamin D ameliorates the hepatic oxidative damage and fibrotic effect caused by thioacetamide in rats. Biomedicines. 2023;11:424. doi: 10.3390/biomedicines11020424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson JM, Polak N, Chen J, Roediger B, Weninger W, Kench JG, et al. Multiple liver insults synergize to accelerate experimental hepatocellular carcinoma. Sci Rep. 2018;8:10283. doi: 10.1038/s41598-018-28486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Li J. Research on the correlation between ultrasonographic features of breast cancer and expressions of ER, CD34 and p53. J BUON. 2018;23:372–7. [PubMed] [Google Scholar]
- 20.Passam FH, Alexandrakis MG, Kafousi M, Fotinou M, Darivianaki K, Tsirakis G, et al. Histological expression of angiogenic factors: VEGF, PDGFRalpha, and HIF-1alpha in Hodgkin lymphoma. Pathol Res Pract. 2009;205:11–20. doi: 10.1016/j.prp.2008.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Gopinathan G, Milagre C, Pearce OM, Reynolds LE, Hodivala-Dilke K, Leinster DA, et al. Interleukin-6 stimulates defective angiogenesis. Cancer Res. 2015;75:3098–107. doi: 10.1158/0008-5472.CAN-15-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kurma K, Manches O, Chuffart F, Sturm N, Gharzeddine K, Zhang J, et al. DEN-induced rat model reproduces key features of human hepatocellular carcinoma. Cancers (Basel) 2021;13:4981. doi: 10.3390/cancers13194981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulien I, Hasselblatt P. Diethylnitrosamine-induced liver tumorigenesis in mice. Methods Cell Biol. 2021;163:137–52. doi: 10.1016/bs.mcb.2020.08.006. [DOI] [PubMed] [Google Scholar]
- 24.Aghemo A, Alekseeva OP, Angelico F, Bakulin IG, Bakulina NV, Bordin D, et al. Role of silymarin as antioxidant in clinical management of chronic liver diseases: a narrative review. Ann Med. 2022;54:1548–60. doi: 10.1080/07853890.2022.2069854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu YZ, Wu K, Huang J, Liu Y, Wang X, Meng ZJ, et al. The PTEN/PI3K/Akt and Wnt/β-catenin signaling pathways are involved in the inhibitory effect of resveratrol on human colon cancer cell proliferation. Int J Oncol. 2014;45:104–12. doi: 10.3892/ijo.2014.2392. [DOI] [PubMed] [Google Scholar]
- 26.Muhanmode Y, Wen MK, Maitinuri A, Shen G. Curcumin and resveratrol inhibit chemoresistance in cisplatin-resistant epithelial ovarian cancer cells via targeting P13K pathway. Hum Exp Toxicol. 2022;41:9603271221095929. doi: 10.1177/09603271221095929. [DOI] [PubMed] [Google Scholar]
- 27.Juríková M, Danihel Ľ, Polák Š, Varga I. Ki67, PCNA, and MCM proteins: markers of proliferation in the diagnosis of breast cancer. Acta Histochem. 2016;118:544–52. doi: 10.1016/j.acthis.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 28.He Y, Sun MM, Zhang GG, Yang J, Chen KS, Xu WW, et al. Targeting PI3K/Akt signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:425. doi: 10.1038/s41392-021-00828-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma XY, Cui D, Wang Z, Liu B, Yu HL, Yuan H, et al. Silk fibroin/hydroxyapatite coating improved osseointegration of porous titanium implants under diabetic conditions via activation of the pi3k/akt signaling pathway. ACS Biomater Sci Eng. 2022;8:2908–19. doi: 10.1021/acsbiomaterials.2c00023. [DOI] [PubMed] [Google Scholar]
- 30.Wang MS, Du YB, Huang HM, Zhu ZL, Du SS, Chen SY, et al. Silk fibroin peptide suppresses proliferation and induces apoptosis and cell cycle arrest in human lung cancer cells. Acta Pharmacol Sin. 2019;40:522–9. doi: 10.1038/s41401-018-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheema SK, Gobin AS, Rhea R, Lopez-Berestein G, Newman RA, Mathur AB. Silk fibroin mediated delivery of liposomal emodin to breast cancer cells. Int J Pharm. 2007;341:221–9. doi: 10.1016/j.ijpharm.2007.03.043. [DOI] [PubMed] [Google Scholar]
- 32.Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi: 10.1038/s41568-019-0216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen H, Nio K, Tang H, Yamashita T, Okada H, Li Y, et al. BMP9-ID1 signaling activates HIF-1α and VEGFA expression to promote tumor angiogenesis in hepatocellular carcinoma. Int J Mol Sci. 2022;23:1475. doi: 10.3390/ijms23031475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faloppi L, Puzzoni M, Casadei Gardini A, Silvestris N, Masi G, Marisi G, et al. Angiogenesis genotyping and clinical outcomes in patients with advanced hepatocellular carcinoma receiving sorafenib: the ALICE-2 study. Target Oncol. 2020;15:115–26. doi: 10.1007/s11523-020-00698-x. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, Cao Y, Chen C, Zhang X, McNabola A, Wilkie D, et al. Sorafenib blocks the RAF/MEK/ERK pathway, inhibits tumor angiogenesis, and induces tumor cell apoptosis in hepatocellular carcinoma model PLC/PRF/5. Cancer Res. 2006;66:11851–8. doi: 10.1158/0008-5472.CAN-06-1377. [DOI] [PubMed] [Google Scholar]
- 36.Gutierrez JA, Gish RG. Efficacy of combination treatment modalities for intermediate and advanced hepatocellular carcinoma: intra-arterial therapies, sorafenib and novel small molecules. Transl Cancer Res. 2013;2:460–71. doi: 10.3978/j.issn.2218-676X.2013.10.01. [DOI] [PMC free article] [PubMed] [Google Scholar]