Abstract
Aim
To compare trajectories of social functioning in peer problems and prosocial behavior from 5 to 13 years between individuals born very preterm (VPT) and full‐term (FT).
Methods
Participants were from the Victorian Infant Brain Study (VIBeS) longitudinal cohort, consisting of 224 individuals born VPT and 77 born FT recruited at birth. Social functioning was measured using the parent‐rated Strengths and Difficulties Questionnaire (SDQ) peer problems and prosocial behavior subscales at 5, 7, and 13 years' corrected age. Multilevel mixed effects models were fitted.
Results
Peer problems increased with age (adjusted mean difference per year = 0.04, 95% confidence interval [CI] = 0.01, 0.07, p = 0.02), with higher peer problems in the VPT compared with the FT group (adjusted mean difference between groups = 0.46, 95% CI = 0.06, 0.86, p = 0.02). Prosocial behavior increased from early to middle childhood and decreased approaching adolescence, but was similar between VPT and FT groups (adjusted mean difference between groups = −0.05, 95% CI = −0.50, 0.40, p = 0.82).
Conclusion
Children born VPT are at greater risk for peer problems than FT peers and may benefit from receiving greater early social support.
Keywords: adolescence, childhood, development, prematurity, social functioning
Abbreviations
- ASD
Autism Spectrum Disorder
- FT
Full Term
- SDQ
Strengths and Difficulties Questionnaire
- VPT
Very Preterm
Key notes.
Children born very preterm (VPT) display increased social difficulties compared with full‐term (FT) peers; however, how social functioning develops throughout childhood for individuals born VPT is unclear.
Peer problems were persistently higher among children born VPT relative to FT peers, but prosocial behavior was similar between groups.
It is important to monitor social functioning in children born VPT who may benefit from receiving early social support.
1. INTRODUCTION
Children born very preterm (VPT; <32 weeks' gestation) or very low birth weight (<1500 g) display greater vulnerability to a range of neurodevelopmental difficulties compared with their full‐term (FT) counterparts. 1 , 2 , 3 , 4 , 5 Social functioning is a key developmental outcome that is also affected, but less often reported. Social functioning refers to the ability to use social skills to adapt behavior and emotions to social contexts, facilitating interpersonal relationships.
Higher rates of social problems, which include greater interpersonal peer issues and reduced social skills, have been reported in children born VPT as young as 2 years through to adolescence compared with children born full‐term (FT). 6 , 7 , 8 , 9 , 10 , 11 Some have reported additional sex differences, showing that VPT boys compared with FT boys experience greater bullying, while VPT girls compared with FT girls face greater social isolation. 11 , 12 Findings are mixed for prosocial behavior, with most studies observing no differences between VPT and FT groups, 9 , 13 , 14 , 15 , 16 , 17 while a select few report worse prosocial behavior in VPT children. 1 , 15 , 18 , 19
VPT children exhibit different developmental trajectories compared with FT children. 12 , 20 , 21 , 22 Some studies have reported an increasing gap for social difficulties between VPT and FT groups into adolescence, e.g., for social withdrawal. 4 , 12 , 23 Nadeau et al. observed that VPT individuals have heightened rates of social isolation compared with FT individuals that remain stable during childhood. 12 Hosozawa et al. found that social competence difficulties (combined peer problems and prosocial behavior) followed u‐shaped trajectories from childhood to adolescence that were similar across VPT, moderate‐late preterm, early‐term, and FT groups, although the VPT group consistently exhibited the greatest difficulties at each timepoint. 20 Thus, to date, findings relating to longitudinal studies regarding the trajectories of social functioning in individuals born VPT during childhood have varied.
This study aimed to describe trajectories of social functioning in peer problems and prosocial behavior from 5 to 13 years in VPT and FT children. We hypothesized that VPT children would display poorer social functioning compared with FT children that would worsen into adolescence, particularly in the peer problems domain.
2. PATIENTS AND METHODS
2.1. Participants
Participants were from the Victorian Infant Brain Study (VIBeS) longitudinal cohort, initially comprising 224 individuals born <30 weeks' gestation or with a birthweight <1250 g. They were recruited shortly after birth between July 2001 and December 2003 from the Royal Women's Hospital, Melbourne. A comparison group of 77 individuals born FT (37–41 weeks' gestation) was recruited, of whom 46 were recruited at birth from the Royal Women's Hospital and 31 recruited at 2 years of age from Maternal and Child Health Centres in Victoria. One individual born FT was later excluded due to experiencing neonatal complications. Participants were assessed at 2 (219 VPT; 74 FT), 5 (194 VPT; 68 FT), 7 (197 VPT; 69 FT), and 13 (79 VPT; 61 FT) years' corrected age (Figure 1). Ethical approval was obtained from the Human Research and Ethics Commitees of the Royal Women's Hospital and Royal Children's Hospital, Melbourne.
FIGURE 1.
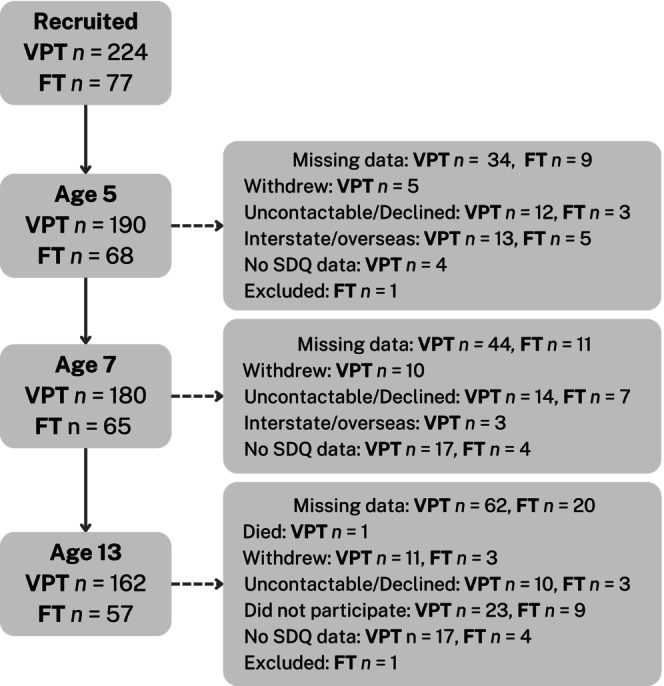
Participant flowchart of those with data at each time point where SDQ was measured. FT, Full term; SDQ, Strengths and Difficulties Questionnaire; VPT, Very preterm.
2.2. Procedures
Follow‐up assessments were administered at 2, 5, 7, and 13 years of age, corrected for prematurity, with informed consent obtained from primary caregivers at each follow‐up. Parents of participants completed demographic, emotional, behavioral, and social functioning questionnaires at 5, 7, and 13years
2.3. Measures
2.3.1. Social functioning
Social functioning was measured with the peer problems and prosocial behavior subscales from the Strengths and Difficulties Questionnaire (SDQ) 24 at ages 5, 7 and 13 years. The SDQ is a 25‐item parent‐rated questionnaire designed to screen behavioral, emotional, and social functioning in children and adolescents. The peer problems subscale includes items such as ‘has at least one good friend’ and ‘picked on or bullied by other children’, while the prosocial behavior subscale includes items such as ‘often volunteers to help others’ and ‘shares readily with other children’. Both subscales comprise 5‐items, each measured using a 3‐point Likert scale (0 = not true, 1 = somewhat true, 2 = certainly true), which are summed to produce a subscale score ranging from 0 to 10. Higher scores on the peer problems subscale indicate more problematic behavior, and for this study we classified ‘at‐risk’ as scores ≥3 (British categorisation: normal = 0–2, borderline = 3, abnormal = 4–10). Higher scores on the prosocial behavior subscale indicate higher positive adaptive social behaviors, and for this study we classified ‘at‐risk’ as scores ≤5 (normal = 6–10, borderline = 5, abnormal = 0–4). 25
2.3.2. Perinatal data
Perinatal data including sex, gestational age, birth weight, multiple births (singleton/multiple), neonatal brain injury, neonatal infection, and bronchopulmonary dysplasia were collected from hospital records. Family social risk was evaluated at age 2 years, using an index that included family structure, maternal age, primary carer education, primary income earner occupation and employment status, and primary language spoken at home. 26 Scores were dichotomised around the median, with ≥2 reflecting higher social risk compared with lower social risk (<2). 26
2.4. Statistical analyses
Data were analysed using Stata version 17.0. Mean trajectories of peer problems and prosocial behavior from 5 to 13 years in individuals born VPT and FT were described using multilevel mixed effects models that included fixed effects for birth group, age (as a continuous variable), sex and social risk, and random effects for individuals (slopes and intercepts). Individual trajectories over time for both outcomes were plotted and examined to inform model form (linear or quadratic term for age). Formal hypothesis testing was performed for the linear and quadratic terms for age to see whether these improved model specifications and for the inclusion of an interaction between birth group and age. For outcomes where there was little evidence for an interaction between group and age, results are reported from the model without the interaction term included. Results are presented as graphs of the marginal mean trajectories in outcomes as a function of age and group, with 95% confidence intervals (CIs).
Secondary analyses were undertaken examining differences in social functioning between birth groups in males and females. Additional analyses were also undertaken to examine differences in birth groups after excluding children with neurosensory impairments (moderate–severe cerebral palsy, blindness, or deafness) assessed at age 2, cognitive impairment (those with a full‐scale IQ <70 assessed according to the Wechsler Abbreviated Scale of Intelligence (WASI)) 27 or a diagnosis of autism spectrum disorder (ASD) on the developmental and wellbeing assessment (DAWBA) 26 , 28 assessed at age 7 (n = 23).
3. RESULTS
The number of participants with parent‐reported SDQ data available at each age is shown in Figure 1. Table 1 summarises the characteristics of those included in the analysis, i.e. who had SDQ data for at least one timepoint (282 total; 207 VPT and 75 FT). As expected, the VPT group had a higher rate of neonatal complications and multiple births than the FT group. Over half of the VPT group showed elevated social risk, compared with less than a third of the FT group. Parents of children in the VPT group tended to report more peer problems than the FT group at ages 5, 7, and 13 years, while similar prosocial behaviors were reported by parents in both groups. The percentage of participants in the ‘at‐risk’ range for peer problems was higher for the VPT group at each timepoint than the FT group. The percentage of participants in the ‘at‐risk’ range for prosocial behavior was similar at each time point for the two groups.
TABLE 1.
Sample characteristics for those with SDQ data for at least one time point.
| Characteristics | Participants with SDQ data | |
|---|---|---|
| VPT (n = 207) | FT (n = 75) | |
| Female, n (%) | 100.0 (48.0%) | 39.0 (52.0%) |
| Gestational age (weeks), M (SD) | 27.5 (1.9) | 39.2 (1.3) |
| Birth weight (grams), M (SD) | 968.3 (217.9) | 3313.4 (514.6) |
| Multiple birth, n (%) | 88.0 (42.5%) | 4.0 (5.4%) |
| Severe brain injury a , n (%) | 15.0 (7.2%) | – |
| Neonatal infection b , n (%) | 72.0 (34.8%) | – |
| Bronchopulmonary dysplasia c , n (%) | 70.0 (33.82%) | – |
| Higher social risk at 2 years, n (%) | 111.0 (53.6%) | 23.0 (30.7%) |
| Single parent household | 27.0 (11.1%) | 4.0 (5.3%) |
| Maternal age at birth <21 years | 11.0 (5.3%) | 2.0 (2.6%) |
| Primary income earner unemployed | 34.0 (16.4%) | 4.0 (5.3%) |
| Primary income earner unskilled occupation | 57.0 (27.5%) | 14.0 (18.7%) |
| Language other than English spoken at home | 22.0 (10.6%) | 6.0 (8.0%) |
| Primary carer highest education level completed less than Year 12 | 25.0 (12.1%) | 1.0 (1.3%) |
| Age at assessment, M (SD) range | ||
| Age 5 | 5.4 (0.4) 4.5–5.8 | 5.0 (0.2) 4.7–6.3 |
| Age 7 | 7.5 (0.3) 6.6–8.4 | 7.7 (0.3) 6.8–8.4 |
| Age 13 | 13.3 (0.4) 11.8–14.9 | 13.2 (0.5) 12–14.3 |
| Peer problems, M (SD), % at‐risk d | ||
| Age 5 | 1.8 (1.9), 44.4% | 1.0 (1.4), 21.3% |
| Age 7 | 1.8 (1.8), 47.8% | 1.3 (1.3), 32.0% |
| Age 13 | 2.0 (2.0), 58.0% | 1.3 (1.5), 37.3% |
| Prosocial behavior, M (SD), % at‐risk d | ||
| Age 5 | 8.0 (2.0), 12.1% | 8.0 (2.1), 13.3% |
| Age 7 | 8.4 (1.8), 8.2% | 8.7 (1.6), 5.3% |
| Age 13 | 8.4 (1.9), 8.7% | 8.4 (1.8), 9.3% |
Abbreviations: M, mean; n, number; SD, standard deviation; SDQ, Strengths and Difficulties Questionnaire.
Severe brain injury = presence of either grade III/IV intraventricular haemorrhage or cystic periventricular leukomalacia as detected by neonatal cranial ultrasound.
Neonatal infection = confirmed sepsis or necrotising enterocolitis.
Bronchopulmonary dysplasia = oxygen dependency at 36 weeks' postmenstrual age.
Percentage at‐risk (borderline and abnormal) categorised according to British SDQ norms (4–17). 25
Table S2 outlines the sample characteristics of those included and excluded from the analysis (i.e., with and without SDQ data) in the VPT group, which had missing data for 17 participants. There was only 1 FT participant with missing data (male, gestational age = 40 wks, birthweight = 2752 kg). VPT participants had slightly elevated rates of neonatal complications and were of higher social risk than VPT non‐participants.
3.1. Trajectories of social functioning
A linear model was specified for describing the mean trajectory of peer problems, while a model that included a quadratic term for age was specified for describing the mean trajectory of prosocial behavior (Figure S1A,B, Table S1).
3.1.1. Peer problems
Peer problems were greater in individuals born VPT compared with those born FT (adjusted mean difference = 0.46, 95% confidence interval (CI) = 0.06, 0.86, p = 0.02; Figure 2A), and increased with age (adjusted mean difference per year = 0.04, 95% CI = 0.01, 0.07, p = 0.02). Overall, the model was significant despite the overlapping confidence intervals illustrated in Figure 2A. There was little evidence that the effect of birth group on peer problems varied by age (adjusted mean difference = 0.01, 95% CI = −0.06, 0.08, p = 0.80).
FIGURE 2.
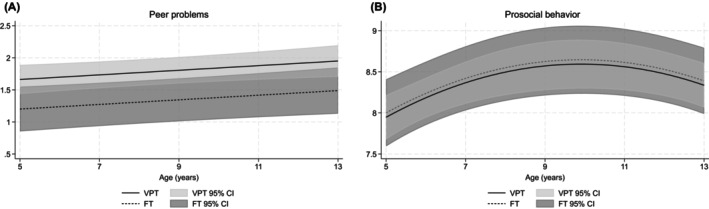
Mean trajectories in (A) peer problems and (B) prosocial behavior for individuals born very preterm (VPT) and full‐term (FT) between age 5 and 13 years, presented with 95% confidence intervals (CIs) while controlling for sex and social risk.
There was little evidence of an effect of birth group on peer problems for males (adjusted mean difference = 0.28, 95% CI = −0.33, 0.88, p = 0.37; Figure 3A). Peer problems were greater in females born VPT compared with those born FT (adjusted mean difference = 0.60, 95% CI = 0.07, 1.11, p = 0.03; Figure 3B). Mean peer problems increased at a similar rate for males (adjusted mean difference = 0.02, 95% CI = −0.02, 0.06, p = 0.27) and females (adjusted mean difference = 0.05, 95% CI = 0.01, 0.09, p = 0.02) over time, although mean peer problems were slightly greater in males than females between groups.
FIGURE 3.
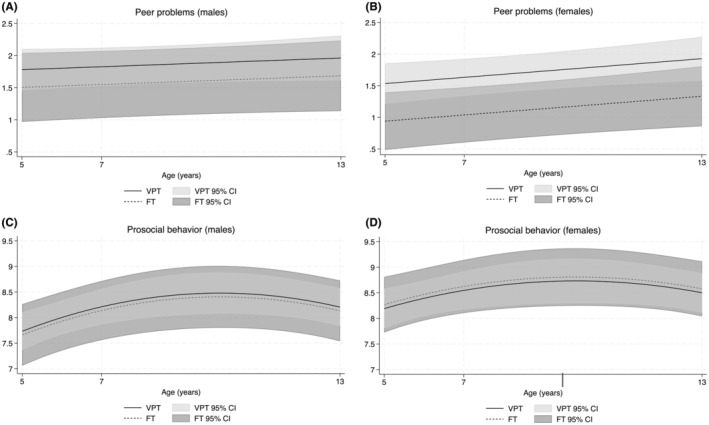
Mean trajectories in peer problems and prosocial behavior for (A,C) males and (B,D) females born very preterm (VPT) and full‐term (FT) between age 5 and 13 years, presented with 95% confidence intervals (CIs) while controlling for social risk.
Results of secondary analyses excluding children with a neurosensory impairment, cognitive impairment, or an ASD diagnosis found mean peer problems in individuals born VPT were higher compared with those born FT; however, confidence intervals were wide (adjusted mean difference = 0.46, 95% confidence interval (CI) = 0.06, 0.86; p = 0.02; Figure 4A). There was little evidence that the effect of birth group on peer problems varied by age (interaction p = 0.87).
FIGURE 4.
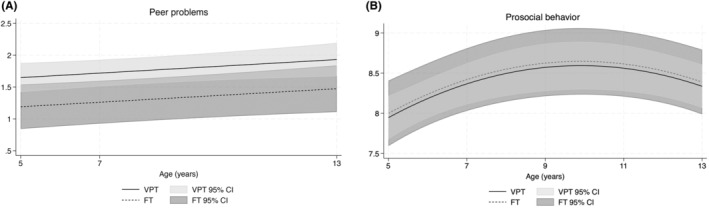
Mean trajectories in (A) peer problems and (B) prosocial behavior for individuals born very preterm (VPT) and full‐term (FT) between age 5 and 13 years excluding individuals with neurosensory impairment, cognitive impairment, or a diagnosis of Autism spectrum disorder (ASD), presented with 95% confidence intervals (CIs) while controlling for sex and social risk.
3.1.2. Prosocial behavior
Prosocial behavior was similar between the VPT and FT groups (adjusted mean difference = −0.05, 95% CI = −0.50, 0.40, p = 0.82; Figure 2B). Mean prosocial behavior increased from early to middle childhood and then stabilised before decreasing when approaching adolescence. There was little evidence to suggest that the effect of birth group on prosocial behavior varied by age (adjusted mean difference = −0.001, 95% CI = −0.07, 0.07, p = 0.97).
There was little evidence to suggest that the effect of birth group on prosocial behavior varied by sex for both males (adjusted mean difference = 0.07, 95% CI = −0.59, 0.73, p = 0.83; Figure 3C) and females (adjusted mean difference = −0.08, 95% CI = −0.68, 0.53, p = 0.80; Figure 3D). Prosocial behavior was similar between males and females over time, which increased from early to middle childhood and then stabilised before decreasing when approaching adolescence, although mean prosocial behavior was slightly greater in females than males.
Results of secondary analyses excluding children with a neurosensory impairment, cognitive impairment, or an ASD diagnosis found little evidence for differences in prosocial behavior in those born VPT compared with those born FT (adjusted mean difference = −0.05, 95% CI = −0.50, 0.40; p = 0.82; Figure 4B. Mean prosocial behavior increased from early to middle childhood and then stabilised before decreasing when approaching adolescence. There was little evidence to suggest that the effect of birth group on prosocial behavior varied by age (interaction p = 0.80).
4. DISCUSSION
This study examined trajectories of social functioning in peer problems and prosocial behavior from childhood to adolescence in VPT and FT born individuals. We found peer problems increased from early childhood to adolescence, although peer problems were consistently higher in the VPT group relative to FT controls at all ages. Prosocial behavior showed similar patterns for both groups, increasing in middle childhood before decreasing approaching adolescence.
Our finding of persistently higher peer problems in individuals born VPT compared with their FT peers is consistent with previous research. 4 , 12 , 20 , 23 Approximately one half (44%–58%) of the VPT group fell within the ‘at‐risk’ range for peer problems, reflecting a 1.5–2.1 times increase in risk compared with the FT group. The increase in peer problems with age regardless of birth group, aligns with past developmental studies. 4 , 23 Linsell et al. reported a similar upward trajectory until 16 years, wherein those born VPT showed consistently greater problems. 4 Johns et al. similarly noted an increase in peer problems until age 12, followed by a decrease in both groups thereafter. 23 Reyes et al. found peer problems remained consistently ‘low’ or ‘high’ from childhood to early adulthood, with VPT status associated with higher problems. 22 Research using relational measures has reported more variable patterns of social functioning over time. Some have reported inverse u‐shaped or dynamic patterns for both groups, similarly indicating adolescence to be a period of marked social difficulties, particularly for those born VPT. 20 , 22 In contrast, others have noted stability for children born FT but increased problems with victimisation for those born VPT. 12 Heterogeneity in study findings may be related to factors including variability in follow‐up age, different informants, gestational age of the VPT cohort, and outcome measurements. Despite mixed findings on how social behavior and relationships change across childhood, studies using teacher‐ and parent‐rated peer relationship measures provide evidence that overall VPT children and adolescents display greater social problems compared with their FT peers which persist and sometimes worsen with time. 4 , 6 , 8 , 10 , 23 , 29 , 30 This could have long‐lasting implications for a range of outcomes including mental health, academic and occupational achievement, and their overall quality of life.
We found little difference in prosocial behavior between groups from ages 5 to 13 years. The overall trajectory of prosocial behavior showed a marked increase from age 5 to 7 that preceded a period of stability in middle childhood, followed by a decrease. A relatively small proportion of children across both birth groups were classified in the ‘at‐risk’ range for prosocial behavior compared with peer problems, with a 4%–8% decrease in ‘at‐risk’ scores from ages 5 to 7 followed by a 0.5%–4% increase from ages 7 to 12. This inverse ‘u‐shaped’ trajectory aligns with previous developmental literature, which has reported an increase in prosocial behaviors between 6 and 11 years and a decrease approaching 16 years. 4 Young children typically demonstrate more positive and engaging interpersonal behaviors when adapting to school and fostering new relationships, whereas in later childhood, more self‐ and peer‐focused behaviors emerge during the transition to high school. Dissimilarly, other studies have reported an increase in mean prosocial behaviors for both groups until age 16. 23 Most studies have similarly observed little difference in prosocial behavior between birth groups. 9 , 13 , 14 , 15 , 16 , 17 , 31 In the few studies that reported poorer prosocial behavior in VPT children compared with FT peers, these differences were related to lower gestational age (<28 weeks) and/or neurodevelopmental delays, including intellectual disabilities. 1 , 15 , 18 , 19 Differences in social behavior primarily pertain to social withdrawal, where individuals born VPT have displayed persistently higher, 12 or worsening issues with age compared to those born FT. 23 Our finding of little evidence for group differences may reflect the relatively low number of children with moderate–severe neurodevelopmental impairments or the measure of social behavior used.
Our finding of greater peer problems among VPT compared to FT born females should be interpreted cautiously due to limited power in the subgroup analysis. Sex differences in social behavior between VPT and FT born females have been noted in past research, evidenced by greater social isolation among VPT‐born females compared to their FT peers. 12 Sex differences between groups have also been reported at particular time points, for instance, worse prosocial behavior and adaptive behavior in VPT females and worse maladaptive behavior in VPT males at age 8 but not age 12, relative to FT counterparts. 9 Differences in study findings pertaining to sex may be attributed to sample sizes, measures, and timepoints, warranting further exploration.
Our findings suggest that individuals born VPT display similar developmentally appropriate positive adaptive behaviors for social interaction compared with their FT peers. These behaviors are distinct from peer relationships at school and do not reflect closeness with family, siblings, or other adults. Past studies have shown greater closeness between VPT adolescents and parents compared with FT dyads and greater peer discord for VPT adolescents from lower socioeconomic backgrounds. 32 Increased peer problems among VPT children may be related to characteristics associated with VPT birth, such as underlying cognitive or developmental challenges. Studies have linked poorer general cognitive abilities and poorer peer relationships, 10 , 22 which may potentially contribute to misunderstandings between VPT children and their peers. Temperament may also explain discrepancies between social functioning outcomes. Personality studies of VPT adults indicate greater shyness and worry and less social engagement and risk‐taking behavior compared with those born FT. 33 , 34 These attributes suggest those born VPT may be more reserved, due to a potential preference to be alone or sensitivity to others' reactions, which could contribute to greater peer difficulties.
The longitudinal investigation of social functioning within a prospective cohort is a strength of our study. This design enabled us to examine social functioning trajectories during a crucial period of social skill development and social transition. The inclusion of a term‐born group enabled the comparison of birth group differences and assisted with generalisability of findings. However, our study has some limitations. Attrition and missing data may have introduced a potential risk for bias, where individuals with greater social problems may have dropped out of the study, meaning group trajectories may appear more similar than they really are. In addition, only 2 social functioning domains could be examined within the scope of the existing dataset; thus, future research into social functioning should consider a more comprehensive approach. While our discrete follow‐up assessments allowed some variability in assessment age, we were unable to precisely capture social functioning between assessments. Assessment of social functioning in later adolescence and later adulthood will be important to determine long‐term trajectories incorporating later developmental transitions. The inclusion of data from multiple sources (teachers and/or children) would be preferable to understand social functioning from different perspectives. However, in a longitudinal context, young children are unlikely to accurately perceive their own social functioning, and classroom teachers generally do not remain consistent from year to year. Parent reports provide important insights over time, which enable advocacy and engagement with early intervention, if necessary. Lastly, elucidating factors relating to social functioning over time is essential for understanding risk factors associated with poor long‐term social outcomes.
This study corroborates past research demonstrating VPT children are at greater, ongoing risk of poor peer relationships. While peer problems increase with age from early childhood to adolescence, individuals born VPT display persistently greater problems relative to FT controls. Supportive peer relationships promote optimal emotional and behavioural development, establish a positive self‐concept, and provide greater social support. 35 , 36 Social functioning is important to assess and monitor across development for children born VPT, given significant implications for identity, connectedness, school performance, career development, and general wellbeing. 37 , 38 , 39 These findings have implications for parents, schools, and clinicians, suggesting that children born VPT could benefit from early social support to establish and foster improved peer relationships, such as through peer mentoring or structured play. 40
Given little evidence for differences in prosocial behavior between VPT and FT children in this study, prosocial behavior may reflect an area of relative strength in VPT individuals that could be harnessed in interventions to support overall social functioning. Furthermore, a systems approach involving parents, educators, and healthcare providers would be ideal to tailor supports towards the individual's strengths and weaknesses, as informed by different perspectives. 41 Considering the multifaceted nature of social functioning, the benefits of providing early intervention for social issues may also extend to improving long‐term outcomes in other domains are affected for VPT individuals, such as cognitive development, academic achievement, and mental health.
AUTHOR CONTRIBUTIONS
Sarah C. H. Thompson: Conceptualization; methodology; funding acquisition; writing – original draft; writing – review and editing; formal analysis. Karli Treyvaud: Conceptualization; methodology; investigation; supervision; funding acquisition; writing – review and editing; data curation; formal analysis. Leona Pascoe: Methodology; conceptualization; investigation; supervision; data curation; formal analysis; writing – review and editing. Rheanna M. Mainzer: Methodology; data curation; formal analysis; resources; writing – review and editing; visualization; supervision; software. Thi‐Nhu‐Ngoc Nguyen: Investigation; data curation; writing – review and editing. Terrie Inder: Methodology; conceptualization; investigation; supervision; funding acquisition; writing – review and editing; data curation. Lex W. Doyle: Conceptualization; methodology; resources; writing – review and editing; supervision; funding acquisition. Peter J. Anderson: Conceptualization; methodology; investigation; data curation; supervision; funding acquisition; writing – review and editing; resources; project administration.
FUNDING INFORMATION
All phases of this study were supported by the Australian National Health and Medical Research Council (NHMRC; Centre for Research Excellence 1 060 733; Project Grants 237 117, 491 209, and 1 066 555; Investigator Grant 1 176 077 to PJA), Research Training Program (RTP) Stipend to ST, The Murdoch Children's Research Institute, Royal Children's Hospital, and Victorian Government's Operational Infrastructure Support Program.
CONFLICT OF INTEREST STATEMENT
The other authors have no conflicts of interest to disclose.
5. ETHICS STATEMENT
Ethical approval was obtained from the Human Research and Ethics Commitees of the Royal Women’s Hospital and Royal Children’s Hospital, Melbourne.
Supporting information
Figure S1.
Table S1.
Table S2.
ACKNOWLEDGEMENTS
We acknowledge the valuable contribution of Victorian Infant Brain Studies (VIBeS) team members, participants, and their families to the project over the years. Open access publishing facilitated by Monash University, as part of the Wiley ‐ Monash University agreement via the Council of Australian University Librarians.
Thompson SCH, Treyvaud K, Pascoe L, Mainzer RM, Nguyen T‐N‐N, Inder TE, et al. Trajectories of social outcomes in individuals born very preterm from childhood to adolescence. Acta Paediatr. 2025;114:355–363. 10.1111/apa.17434
REFERENCES
- 1. Anderson P, Doyle LW. Neurobehavioral outcomes of school‐age children born extremely low birth weight or very preterm in the 1990s. JAMA. 2003;289(24):3264‐3272. doi: 10.1001/jama.289.24.3264 [DOI] [PubMed] [Google Scholar]
- 2. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJS. Cognitive and behavioral outcomes of school‐aged children who were born preterm: a meta‐analysis. JAMA. 2002;288(6):728‐737. doi: 10.1001/jama.288.6.728 [DOI] [PubMed] [Google Scholar]
- 3. Anderson PJ, Doyle LW. Cognitive and educational deficits in children born extremely preterm. Semin Perinatol. 2008;32(1):51‐58. doi: 10.1053/j.semperi.2007.12.009 [DOI] [PubMed] [Google Scholar]
- 4. Linsell L, Johnson S, Wolke D, Morris J, Kurinczuk JJ, Marlow N. Trajectories of behavior, attention, social and emotional problems from childhood to early adulthood following extremely preterm birth: a prospective cohort study. Eur Child Adolesc Psychiatry. 2019;28(4):531‐542. doi: 10.1007/s00787-018-1219-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wolke D, Johnson S, Mendonça M. The life course consequences of very preterm birth. Annu Rev Dev Psychol. 2019;1(1):69‐92. doi: 10.1146/annurev-devpsych-121318-084804 [DOI] [Google Scholar]
- 6. Jones KM, Champion PR, Woodward LJ. Social competence of preschool children born very preterm. Early Hum Dev. 2013;89(10):795‐802. doi: 10.1016/j.earlhumdev.2013.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Reijneveld SA, De Kleine MJK, van Baar AL, et al. Behavioural and emotional problems in very preterm and very low birthweight infants at age 5 years. Arch Dis Child Fetal Neonatal Ed. 2006;91(6):423‐428. doi: 10.1136/adc.2006.093674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ritchie K, Bora S, Woodward LJ. Social development of children born very preterm: a systematic review. Dev Med Child Neurol. 2015;57(10):899‐918. doi: 10.1111/dmcn.12783 [DOI] [PubMed] [Google Scholar]
- 9. Spittle AJ, Treyvaud K, Doyle LW, et al. Early emergence of behavior and social‐emotional problems in very preterm infants. J Am Acad Child Adolesc Psychiatry. 2009;48(9):909‐918. doi: 10.1097/CHI.0b013e3181af8235 [DOI] [PubMed] [Google Scholar]
- 10. Twilhaar ES, de Kieviet JF, Bergwerff CE, Finken MJJ, van Elburg RM, Oosterlaan J. Social adjustment in adolescents born very preterm: evidence for a cognitive basis of social problems. J Pediatr. 2019;213:66‐73. doi: 10.1016/j.jpeds.2019.06.045 [DOI] [PubMed] [Google Scholar]
- 11. Yau G, Schluchter M, Taylor HG, et al. Bullying of extremely low birth weight children: associated risk factors during adolescence. Early Hum Dev. 2013;89(5):333‐338. doi: 10.1016/j.earlhumdev.2012.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nadeau L, Oslejskova E, Tessier R. Do social functioning problems of premature children persist between 7 and 11 years of age? Canadian J School Psychol. 2018;33(2):125‐135. doi: 10.1177/0829573516678705 [DOI] [Google Scholar]
- 13. Baron IS, Erickson K, Ahronovich MD, Baker R, Litman FR. Neuropsychological and behavioral outcomes of extremely low birth weight at age three. Dev Neuropsychol. 2011;36(1):5‐21. doi: 10.1080/87565641.2011.540526 [DOI] [PubMed] [Google Scholar]
- 14. Bora S, Pritchard VE, Moor S, Austin NC, Woodward LJ. Emotional and behavioural adjustment of children born very preterm at early school age: very preterm birth and behavioural sequelae. J Paediatr Child Health. 2011;47(12):863‐869. doi: 10.1111/j.1440-1754.2011.02105.x [DOI] [PubMed] [Google Scholar]
- 15. Delobel‐Ayoub M, Arnaud C, White‐Koning M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE study. Pediatrics. 2009;123(6):1485‐1492. doi: 10.1542/peds.2008-1216 [DOI] [PubMed] [Google Scholar]
- 16. Hutchinson EA, De Luca CR, Doyle LW, Roberts G, Anderson PJ. Victorian infant collaborative study group. School‐age outcomes of extremely preterm or extremely low birth weight children. Pediatrics. 2013;131(4):e1053‐e1061. doi:10.1542/peds.2012‐2311 [DOI] [PubMed] [Google Scholar]
- 17. Treyvaud K, Doyle LW, Lee KJ, et al. Social‐emotional difficulties in very preterm and term 2 year olds predict specific social‐emotional problems at the age of 5 years. J Pediatr Psychol. 2012;37(7):779‐785. doi: 10.1093/jpepsy/jss042 [DOI] [PubMed] [Google Scholar]
- 18. Elgen I, Sommerfelt K, Markestad T. Population based, controlled study of behavioural problems and psychiatric disorders in low birthweight children at 11 years of age. Arch Dis Child Fetal Neonatal Ed. 2002;87(2):128‐132. doi: 10.1136/fn.87.2.f128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Samara M, Marlow N, Wolke D. Pervasive behavior problems at 6 years of age in a total‐population sample of children born at ≤25 weeks of gestation. Pediatrics. 2008;122(3):562‐573. doi: 10.1542/peds.2007-3231 [DOI] [PubMed] [Google Scholar]
- 20. Hosozawa M, Cable N, Kelly Y, Sacker A. Gestational age on trajectories of social competence difficulties into adolescence. Arch Dis Child. 2021;106:1075‐1080. doi: 10.1136/archdischild-2020-321317 [DOI] [PubMed] [Google Scholar]
- 21. Johns CB, Lacadie C, Vohr B, Ment LR, Scheinost D. Amygdala functional connectivity is associated with social impairments in preterm born young adults. NeuroImage Clin. 2019;21:101626. doi: 10.1016/j.nicl.2018.101626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Reyes LM, Jaekel J, Bartmann P, Wolke D. Peer relationship trajectories in very preterm and term individuals from childhood to early adulthood. J Dev Behav Pediatr. 2021;42(8):621‐630. doi: 10.1097/dbp.0000000000000949 [DOI] [PubMed] [Google Scholar]
- 23. Johns C. The Worsening Trajectory of Social Impairment in Preterm Born Young Adults and its Association with Altered Amygdalar Functional Connectivity. Yale; 2019. Accessed January 23, 2024. https://elischolar.library.yale.edu/cgi/viewcontent.cgi?article=3506&context=ymtdl [Google Scholar]
- 24. Goodman A, Goodman R. Strengths and difficulties questionnaire as a dimensional measure of child mental health. J Am Acad Child Adolesc Psychiatry. 2009;48(4):400‐403. doi: 10.1097/CHI.0b013e3181985068 [DOI] [PubMed] [Google Scholar]
- 25. Meltzer H, Gatward R, Goodman R, Ford T. The mental health of children and adolescents in Great Britain: (622732007‐001). 2000. doi: 10.1037/e622732007-001 [DOI] [PubMed]
- 26. Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44(5):276‐280. doi: 10.1111/j.1440-1754.2007.01251.x [DOI] [PubMed] [Google Scholar]
- 27. Wechsler DA. Wechsler Abbreviated Scale of Intelligence (WASI). Psychological Corporation; 1999. [Google Scholar]
- 28. Goodman R, Ford T, Richards H, Gatward R, Meltzer H. The development and well‐being assessment: description and initial validation of an integrated assessement of child and adolescent psychopathology. J Child Psychol Psychiatry Allied Discip. 2000;41(5):645‐655. doi: 10.1017/S0021963099005909 [DOI] [PubMed] [Google Scholar]
- 29. Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (≤800 g) adolescents who are free of major impairment compared with term‐born control subjects. Pediatrics. 2004;114(6):e725‐e732. doi: 10.1542/peds.2004-0932 [DOI] [PubMed] [Google Scholar]
- 30. Healy E, Reichenberg A, Nam KW, et al. Preterm birth and adolescent social functioning‐alterations in emotion‐processing brain areas. J Pediatr. 2013;163(6):1596‐1604. doi: 10.1016/j.jpeds.2013.08.011 [DOI] [PubMed] [Google Scholar]
- 31. Larroque B, Ancel PY, Marchand‐Martin L, et al. Special care and school difficulties in 8‐year‐old very preterm children: the Epipage cohort study. PLoS One. 2011;6(7):e21361. doi: 10.1371/journal.pone.0021361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taylor HG, Hoskinson KR, Vrantsidis DM, et al. Quality of social relationships with parents and peers in adolescents born extremely preterm. J Dev Behav Pediatr. 2023;44(3):e218‐e224. doi: 10.1097/DBP.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 33. Eryigit‐Madzwamuse S, Strauss V, Baumann N, Bartmann P, Wolke D. Personality of adults who were born very preterm. Arch Dis Child Fetal Neonatal Ed. 2015;100(6):F524‐F529. doi: 10.1136/archdischild-2014-308007 [DOI] [PubMed] [Google Scholar]
- 34. Schmidt LA, Miskovic V, Boyle MH, Saigal S. Shyness and timidity in young adults who were born at extremely low birth weight. Pediatrics. 2008;122(1):e181‐e187. doi: 10.1542/peds.2007-3747 [DOI] [PubMed] [Google Scholar]
- 35. Thompson, RA , Flood, MF and Goodvin, R. . Social support and developmental psychopathology. In: Cicchetti D, Cohen DJ. Developmental Psychopathology. John Wiley & Sons; 2015. doi: 10.1002/9780470939406 [DOI] [Google Scholar]
- 36. Parker JG, Rubin KH, Erath SA, Wojslawowicz JC, Buskirk AA. Peer relationships, child development, and adjustment: a developmental psychopathology perspective. In: Cicchetti D, Cohen DJ, eds. Developmental Psychopathology. 1st ed. Wiley; 2015:419‐493. doi: 10.1002/9780470939383.ch12 [DOI] [Google Scholar]
- 37. Samuel R, Bergman MM, Hupka‐Brunner S. The interplay between educational achievement, occupational success, and well‐being. Soc Indic Res. 2013;111(1):75‐96. doi: 10.1007/s11205-011-9984-5 [DOI] [Google Scholar]
- 38. Burt KB, Obradović J, Long JD, Masten AS. The interplay of social competence and psychopathology over 20 years: testing transactional and Cascade models. Child Dev. 2008;79(2):359‐374. doi: 10.1111/j.1467‐8624.2007.01130.x [DOI] [PubMed] [Google Scholar]
- 39. Haslam C, Cruwys T, Haslam SA, Jetten J. Social connectedness and health. In: Pachana NA, ed. Encyclopedia of Geropsychology. Springer; 2015:1‐10. doi: 10.1007/978-981-287-080-3_46-2 [DOI] [Google Scholar]
- 40. Odom SL, McConnell SR, McEvoy MA, et al. Relative effects of interventions supporting the social competence of young children with disabilities. Top Early Child Spec Educ. 1999;19(2):75‐91. doi: 10.1177/027112149901900202 [DOI] [Google Scholar]
- 41. Guralnick MJ. Why early intervention works: a systems perspective. Infants Young Child. 2011;24(1):6‐28. doi: 10.1097/IYC.0b013e3182002cfe [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1.
Table S1.
Table S2.


