Abstract
Tooth extraction is physiologically followed by resorption of alveolar bone. Surgical method which aims to minimise this reduction in alveolar bone with a goal to provide enough bone volume for dental implant insertion is called socket preservation. The purpose of this article was to asses clinical, histomorphometric and histological results of socket preservation conducted with natural bovine bone substitute with hyaluronate. Three patients with one or more hopeless teeth in posterior region planned for extraction and implant placement were included in these case reports. After atraumatic extractions, empty sockets were filled with the bovine xenograft with hyaluronate, and then covered with collagen sponge. After 4–7.5 months the samples for biopsy were taken and then implants were inserted. The augmented sites healed uneventfully and without any complications. The histological specimens demonstrated new bone formation and osteoclastic activity around the biomaterial, as well as blood vessels in soft tissue. Histomorphometrically, formation of new bone averaged 24.8% ± 4.7% (mean ± standard deviation) in bone biopsies taken from the center of the augmented site, while the residual biomaterial averaged 52.7% ± 4.9% and the soft tissue averaged 22.6% ± 4%. In conclusion, the natural bovine bone substitute with hyaluronate demonstrated excellent osteoconductive potential for bone regeneration.
Graphical Abstract

Introduction
The goal of dental treatments is to maintain a healthy, functional tooth with preserved periodontium in the oral cavity for as long as possible. However, if the dental treatment fails, then a tooth extraction can be indicated [1]. The tooth extraction is followed by a physiological bone volume loss with significant structural, dimensional and dynamical changes [2, 3]. For example, a horizontal bone loss 29–63% and vertical bone loss 11–22% have been reported in the first 6 months after tooth extraction in humans [4]. Such rapid decrease in the first 3–6 months is then followed by a gradual dimensional decrease.
Dental implants are nowadays the most favorable option for oral rehabilitation of partial or complete edentulism [5]. Although the modern practice of immediate implantation shows certain advantages compared to delayed implantation, for example shorter treatment time and greater patient satisfaction, studies have demonstrated significantly higher survival rate with delayed implantation [6]. Since the placement of dental implants requires sufficient horizontal and vertical bone dimensions, various augmentation techniques have been developed to compensate the alveolar bone resorption which follows tooth extraction [7, 8]. In fact, the augmentation techniques can be performed before and during implant placement [9]. More specifically, the socket preservation is a preventive procedure that minimizes bone loss after extraction by filling the empty socket with bone substitutes material immediately after extraction [10–12].
Various biomaterials have been utilized as graft materials, including autologous bone, bone substitutes (allografts, xenografts, and alloplasts), autologous blood products, and bioactive substances [7, 13]. Autologous bone fulfills all physiochemical and biological properties: it is osteogenic (new bone is formed from osteogenic cells in the graft), osteoinductive (new bone is formed by the active recruitment of mesenchymal stem cells from the surrounding tissue), and osteocondutive (the biomaterial supports new bone formation on its surface). An ideal bone graft substitute should possess these properties [14]. The disadvantages of autologous bone are limited availability, increased morbidity at the harvest site, postoperative pain, and higher risk of infection and complications. The most common alternatives to autologous bone are human allografts and animal xenografts [15]. The natural bovine bone substitutes are most commonly used biomaterials in the daily practice [16]. Because of their naturally high crystalline and rigid structure, these xenogenic biomaterials have a slow resorption rate. Their regeneration process is dependent on penetration of blood vessels and newly formed bone into the graft porosity, which is followed by the graft’s cellular osseous integration. However, variations in their production process can cause differences in morphology, chemistry, cristallinity and impurity that can affect the clinical result. More precisely, when compared to low temperature or chemically treated bovine bone substitutes, the high temperature calcination procedure without the use of additives results in enhanced crystalline size, which provides improved long-term volume stability at the grafting site [17, 18].
Hyaluronic acid, a naturally occurring polymer with great potential, finds its application in various medical fields. As an extracellular matrix component, it performs numerous functions such as wound healing, cell migration and cell signal transduction [19]. For that reason, it has been used for development of new and improved bone grafting substitutes such as cerabone® plus, which comes with the bovine bone granules available in 2 granule sizes, pre-mixed with a water soluble salt form of the hyaluronic acid (sodium hyaluronate) [20]. It has been demonstrated that hyaluronic acid promotes angiogenesis by providing a cell-supporting matrix and directly stimulating the cells [21]. It also increases viability, migratory activity, and proliferation of human osteoblasts due to involvement of certain regulatory mechanisms [22, 23]. In vivo, the hyaluronic acid degrades in less than two weeks and the remaining osteoconductive bovine granules enable gradual bone regeneration [24]. Recent human studies showed promising results for the use of such natural bovine bone substitute with hyaluronate in the regenerative dentistry [20, 25].
The aim of these case reports was to retrospectively investigate the regenerative potential in three patients having socket preservation by using such natural bovine bone substitute with hyaluronate via histologic, histomorphometric, and radiologic analysis.
Materials and methods
Three patients are presented in these case reports. Patients included in the reports were randomly selected from the general population of oral surgery practice (Table 1). Including factors were age (older than 18 and younger than 65), no systemic diseases, no allergies, no substance abuse (smoking or alcohol), oral and written consent as well as understanding of protocol. Excluding factors were one or more relative or absolute contraindications for implant placement, pregnant and lactative women [26, 27].
Table 1.
Overview of patients and methodology
| Patient details, location of recipient beds and implant placement location | Site | Graft used | Barrier membrane used | Follow-up | |
|---|---|---|---|---|---|
| Patient 1 | 53 years old, female, non-smoker, socket preservation and implant placement at location 36* | Mandible | Bovine xenograft with hyaluronate | Porcine collagen sponge | 7,5 months |
| Patient 2 | 61 years old, male, non-smoker, socket preservation and implant placement at location 26* | Maxilla | Bovine xenograft with hyaluronate | Porcine collagen sponge | 5 months |
| Patient 3 | 57 years old, female, non-smoker, socket preservation at locations 44, 45 47, 48; implant placement at locations 44 and 47* | Mandible | Bovine xenograft with hyaluronate | Porcine collagen sponge | 4 months |
*FDI notation system
Approval for the case series was granted by the Ethics Committee of the Faculty of Dentistry and Health Osijek (Class: 602-01/23-12/03, No: 2158/97-97-10-23-22). Each patient was adequately informed about the purpose of the study, surgical procedure, histological evaluation, and possible complications. The surgery in all three cases was performed by one experienced surgeon (DJ), after each patient signed the informed consent form.
An oral antibiotic was administered 1 h before the procedure (Amoksicillin 1000 mg, Belupo, Koprivnica or Clindamycin-MIP 600 mg, Chem. pharm. Fabrik GmbH, Ingbert, Germany in patients allergic to penicillin). After mouth rinsing with a chlorhexidine digluconate solution (Parodontax 0.2%, Brentford, London, UK), a local anesthesia with 4% articaine and epinephrine 1:100,000 (Ubistesin Forte 40 mg/mL +0.01 mg/mL, 3 M Deutschland GmbH, Seefeld, Germany) was administered. Tooth extraction was carefully performed to preserve the alveolar walls and the surrounding soft tissue (Fig. 1A). If the soft tissue was damaged during extraction, the damaged area was secured with nonabsorbable 6–0 monofilament sutures (Sofsilk™, Covidien, Dublin, Ireland). After extraction, thorough curettage was performed to remove possible granulation tissue. The used graft was natural bovine bone substitute with unknown concetration of hyaluronate (cerabone® plus, botiss biomaterials GmbH, Zossen, Germany) in granular form with particles sized 0.5–1.0 mm. The graft was hydrated according to the manufacturer’s instructions (~0.5 ml saline solution per 1 ml cerabone® plus) (Fig. 1B, C). The hydrated graft material was then placed in the empty alveolus (Fig. 1D) and covered with a folded porcine dermis collagen sponge (collafleece®, botiss biomaterials GmbH, Zossen, Germany), type of collagen not specified. Finally, the wound was closed and sutured with nonabsorbable 6–0 monofilament sutures (Fig. 1E). The patients received detailed instructions on postoperative oral hygiene, as the sutures were removed 14 days after the procedure and consecutive follow-up visits were scheduled (Table 1). At the follow-up (Fig. 1F), an incision was made in the middle of the alveolar ridge and the mucoperiosteal flap was elevated to expose the surgical site (Fig. 1G). Finally, implants (Nobel Biocare AB, Gothenburg, Sweden) were placed (Fig. 1H) at the indicated locations (Table 1) and soft tissue was sutured around healing abutments (Fig. 1I). During preparation of each implant site (4 in total), a bone biopsy was harvested with a trephine drill (Komet Dental, Gebr. Brasseler GmbH & Co. KG, Lemgo, Germany). Few months after, healed soft tissue around healing abutments was presented and sites were ready for prosthetic restoration (Fig. 1J).
Fig. 1.
Surgical procedure in patient 1: (A) empty alveolus after atraumatic extraction of tooth 36 in patient 1; (B) granules of natural bovine bone substitute with hyaluronate, hydrated in the provided blister; (C) sticky and malleable texture, prior to use; (D) clean alveolus is filled with granules of natural bovine bone with hyaluronate; (E) alveolus filled with bovine bone granules with hyaluronate is covered with folded porcine collagen sponge, papillae margins were approximated and sutured with nonabsorbable 6–0 monofilament sutures; (F) healed site in patient 1 after 7.5 months; (G) healed bone is presented after mid-crestal incision and elevation of mucoperiostal flap; (H) after taking the bone biopsy with trephine bur, implant is placed; (I) primary wound closure through papilla around healing abutment; (J) healed peri-implant soft tissue transition zone, 12 months after augmentation
The samples were then fixed with 4% formaldehyde solution (BioGnostLtd., Zagreb, Croatia). After fixation in formaldehyde solution for two weeks, the preparation was decalcified using ethyldiaminetetraacetic acid (EDTA, Osteomoll®, Sigma-Aldrich, St. Louis, MO, USA). The preparation was then placed in a tissue processor and embedded in a paraffine wax. The embedding in the paraffine blocks was oriented in the longitudinal position with microtomy in the sagittal plane, in order to measure the long axis of the alveoli, thus covering the cervical, middle and apical thirds of the dental alveolus. The samples were cut to 5 µm thickness with 50 µm distance between each section with a microtome (SLEE, Mainz, Germany), ultimately obtaining 5 slides per block. These samples were then stained with hematoxylin-eosin and examined under a light microscope (Olympus Tokyo Japan) connected to a video camera (Sony, Tokyo Japan). Histomorphometric analysis was performed by digital image analysis of microphotographs stained with hematoxylin-eosin under ×10 and ×20 objective magnification. Each of 5 sections of total 4 biological samples collected was evaluated histomorphometrically –20 in total. ImageJ software (WayneRasband, National Institute of Health, Bethesda, MD, USA) was used to analyze the histomorphometric images. Measurements of total area, residual bone graft area, bone tissue area, and soft tissue area were taken and noted in a Microsoft Excel spreadsheet. Mean values were calculated and converted to percent volume of bone tissue, residual graft material, and soft tissue.
The cone beam computed tomography (CBCT) analysis in all three patients was done with the three-dimensional (3D) Promax (Planmeca OY, Helsinki, Finland) at three time points: before tooth extraction, immediately after the extraction and socket preservation and several months (Table 1) after the socket preservation (Fig. 2).
Fig. 2.
Cone beam computed tomography (CBCT) in patient 1, in sagittal plane (upper row) and coronal plane (lower row): (A, B) before tooth extraction, visible unsatisfactory endodontic treatment; C, D) immediately after the extraction and augmentation; (E, F) 7,5 months after the augmentation
Results
The cone beam computed tomography (CBCT) analysis in all three patients showed successful treatment and was done at three time points: before tooth extraction (Fig. 2A, B); immediately after the extraction and socket preservation (Fig. 2C, D) and several months (Table 1) after the socket preservation (Fig. 2E, F). Once the bovine xenograft granules were used for socket preservation, they were clearly differentiated from the surrounding bone due to density and radiopacity (Fig. 2C, D). At follow-up, the grafting materials was surrounded and partially replaced by new bone (Fig. 2E, F). The homogenous filling of the socket with the xenograft granules enables successful implant placement in prosthetically correct position, and optimal bone-to-biomaterial contact. Finally, the prosthetics superstructure (crown) was placed on the implant after approximately 6 months.
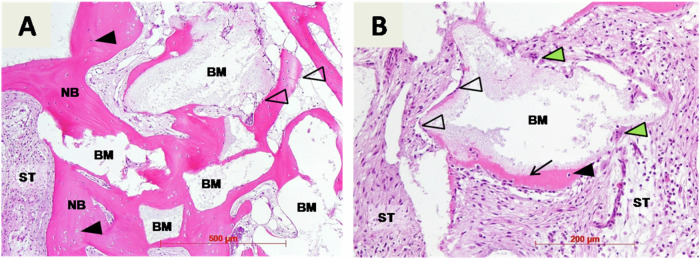
Biopsies were taken before implant placement and were used for histological examination (Figs. 3, 4, 5). Here the remaining biomaterial granules (BM) were surrounded and in close contact with newly formed cancellous bone (NB). New bone was filled with osteocytes (black fill triangles) trapped in lacunae and had a typical lamellar organization. Osteoblasts (no fill triangles) were visible at the margins of new bone and biomaterial granules. In addition, on the surface of the biomaterial granules early osteogenesis (arrows) was also observed (Figs. 3B, 4B, 5A). Here a thin layer of eosinophilic osteoid being produced by osteoblasts (no fill triangles) was located at the surface of the granules. Also, osteoclasts (green filled triangle) were detected around biomaterial granules, indicating at osteoclastic superficial resorption of the biomaterial remnants (Figs. 3B, 5B). Finally, blood vessels were clearly visible throughout soft tissue (Figs. 4B, 5B), and soft tissue was rich in cells, mainly fibroblasts.
Fig. 3.
Histological examination of bone biopsy harvested 7,5 months after alveolar ridge preservation in patient 1: (A) remaining biomaterial granules (BM), newly formed cancellous bone (NB), osteocytes (black fill triangles), osteoblasts (no fill triangles), soft tissue (ST), hematoxylin-eosin staining, magnification x10; (B) early osteogenesis (arrow), osteoclasts (green filled triangle), hematoxylin-eosin staining, magnification x20
Fig. 4.
Histological examination of bone biopsy harvested 5 months after alveolar ridge preservation in patient 2: (A) remaining biomaterial granules (BM), newly formed cancellous bone (NB), osteocytes (black fill triangles), osteoblasts (no fill triangles), soft tissue (ST), hematoxylin-eosin staining, magnification x10; (B) connective tissue vessels (yellow filled arrows), early osteogenesis (arrow), hematoxylin-eosin staining, magnification x20
Fig. 5.
Histological examination of bone biopsy harvested 5 months after alveolar ridge preservation in patient 3: (A) new bone (NB), osteocytes (black fill triangles), osteoblasts (no fill triangles), early osteogenesis characterized by osteoid (thin arrows), biomaterial granules (BM), soft tissue (ST) (hematoxylin-eosin staining, magnification x20; (B) connective tissue vessels (yellow filled arrows), osteoclasts (green filled triangles), hematoxylin-eosin staining, magnification x40
Discussion
The purpose of this case series was to perform histological and histomorphometric evaluation of natural bovine bone substitute with hyaluronate in a socket preservation.
The healing was uneventful and without complications in all three patients. The biomaterial osseointegrated in a newly formed bone that supported successful implants installation. No inflammatory reaction and the absence of fibrous encapsulation indicated optimal biocompatibility of the investigated bone substitute.
The surgical procedure in our cases included filling clean, empty alveolus after tooth extraction by natural bovine bone substitute with hyaluronate to preserve alveolar bone volume and to minimize bone resorption. In our 3 cases the alveolus was filled with the bone biomaterial and then secluded by a folded collagen sponge. Then the papillae margins were approximated and sutured as the wound healed with secondary intention. This approach is possible for smaller wounds, where the collagen sponge does not create a barrier but rather stabilizes the grafting material as it supports the natural healing and hemostasis [28–30].
During follow up after several months, it was recorded that the biomaterial was osseointegrated into newly formed bone and was followed by successful implants installation (Fig. 2).
The biopsies taken before implants insertion were evaluated by quantitative and qualitative histologic analysis. The quantitative histomorphometric results showed the volume ratio of newly formed bone (24.8 ± 4.7% (mean ± standard deviation)), residual biomaterial (52.7 ± 4.9%), and soft tissue (22.6 ± 4%) (Table 2). We can speculate that slight differences in the results between the 3 patients are due to the different follow-up time (Table 1). This is indicated by smaller amount of new bone and greater amount of biomaterial and connective tissue, as well as greater standard deviation in patient 3, whose follow-up time was shorter, compared to the others. Our results are comparable to those investigating dimensional changes and bone quality after socket preservation with bovine xenograft vs alloplast in 33 patients [11]. There was reported 22.50 ± 24.72% new bone formation, 40.18 ± 17.2% residual biomaterial, and 38% soft tissue structures for sockets filled with bovine xenograft. Similar study comparing bovine xenograft vs injectable biphasic calcium phosphate in 40 patients, confirmed 30.47 ± 16.39% new bone, 17.89 ± 11.81% residual biomaterial, and 51.64% ± 14.63 soft tissue structures for socket preservation by bovine xenograft [31]. Such slight result differences can be explained by different follow-up and healing times, different surgical procedures, as well as initial localization and morphology of the alveolar defect.
Table 2.
Histomorphometric results
| Newly formed bone (%) | Biomaterial (%) | Soft tissue (%) | |
|---|---|---|---|
| Patient 1 (mean ± st. deviation) | 33.5 ± 1.7 | 54.5 ± 4 | 12 ± 3 |
| Patient 2 (mean ± st. deviation) | 30.1 ± 5.5 | 53.1 ± 2.8 | 16.8 ± 3.6 |
| Patient 3, position 44 (mean ± st. deviation) | 17 ± 5.6 | 54.5 ± 8.2 | 28.5 ± 7.8 |
| Patient 3, position 45 (mean ± st. deviation) | 18.4 ± 5.9 | 48.5 ± 4.4 | 33.1 ± 1.6 |
| Total (mean ± st. deviation) | 24.8 ± 4.7 | 52.7 ± 4.9 | 22.6 ± 4 |
Qualitative histological analysis represents the biological interaction between the host tissue and the bone graft material. The evaluation of the histological specimens showed that newly formed bone with visible remodeling was in close contact with the remaining graft granules, suggesting successful bone regeneration and positive integration of the used biomaterial into the adjacent host tissue (Figs. 3, 4, 5). Osteoclasts as part of the monocyte-macrophage system were detected on the surface of the biomaterial granules (Figs. 3B, 5B). This suggests the slow biomaterial degradation process, as the bovine xenografts are known for their slow resorption rate [11].
It has been demonstrated that the addition of hyaluronate to bovine xenograft resulted in increased angiogenesis and it is well known that adequate blood supply is essential for successful bone regeneration [9, 21, 32]. The healing site requires oxygen, nutrients, immunomodulatory and osteoprogenitor cells, which are delivered through the blood network. In our histologic analysis, blood vessels are clearly visible throughout the soft tissue (Figs. 4B, 5B), which is consistent with the aforementioned findings [21]. Also, active osteoblasts can be found at the margin between the biomaterial and new bone, while osteocytes can be found trapped in the lacunae of the new bone (Figs. 3, 4, 5). Another study examined the in vitro effect of the same bovine bone substitute with hyaluronate on osteoblast viability, migration capacity and proliferation rate [22]. It was concluded that the hyaluronate positively affected these processes compared to the bovine bone substitute without hyaluronate [22]. Precise regulatory mechanisms have been investigated through which hyaluronic acid induces osteoblastic differentiation by enhancing bone morphogenetic protein-2 (BMP-2) [23]. These findings are accordant with the results of an animal study which concluded that hyaluronic acid enhances expression of osteogenic proteins BMP-2 and osteopontin (OPN) in a rat model [33].
Positive effects of hyaluronate on bone regeneration have been confirmed in several more animal studies. The effect of hyaluronate in combination with bovine xenograft on bone healing has been investigated in a calvarial bone defect in rabbits. The authors filled bicortical cranial defects with autograft in the first group and bovine xenograft, with and without hyaluronate, in the second and third groups. Histomorphometric evaluation showed that new bone formation was improved in the group with hyaluronate, while the percentage of remaining graft was reduced. However, the quality of the newly formed bone was not affected [34]. Successful bone formation has also been demonstrated in extraction sockets with chronic pathology in a canine model. The authors induced a periapical lesion of the mandibular third premolars in animals. After extraction, the extraction sockets of the test group were filled with hyaluronate, while the control group healed naturally. Histomorphometric analysis showed that mineralized bone was 63.29% ± 9.78% in the test group and 47.80% ± 6.60% in the control group, which was statistically significant. Histologically, reversal lines and abundant deposits of osteoblasts were observed [35]. Another canine model study investigated the effect of hyaluronate in combination with bovine xenograft and 10% collagen on the healing of impaired extraction sockets. The animals studied were divided into 4 groups: in two groups, the alveoli were filled with collagen sponges after extractions, with and without the addition of hyaluronate, and in the remaining two groups, the alveoli were preserved with bovine xenograft and 10% collagen, in one group with and one without hyaluronate. The results showed that the volume loss of the alveolar ridge after extraction was lower in the groups with socket preservation, while the percentage of mineralized bone was higher in the groups with hyaluronate addition [36].
The in vivo degradation of the hyaluronate is less than 2 weeks, which then allows the xenograft particles to support osteoconductive new bone formation [24]. The novel biomaterial composed of hyaluronate addition to bovine bone substitute enabled effective defect augmentation and lead to succesfull socket preservation, sinus lift and lateral augmentation in patients [20]. In addition, it has been demonstrated that biodegradable GBR membrane made from magnesium alloy can be safely utilized in combination with the aforementioned bovine bone substitute with hyaluronate in patients [37]. The effects of this grafting material has also been investigated in peri-implantitis reconstructive therapy [25]. Here the clinical and radiographic outcomes were improved six months after the treatment. More specifically, the implant stability and vertical marginal bone gain were significantly higher if compared to the bovine bone substitute without hyaluronate. Another human study investigated clinically and radiographically the effect of cross-linked hyaluronic acid in combination with bovine xenograft in socket preservation [38]. Seven patients with bilateral teeth planned for extraction were included. After extraction, the sockets were filled on one side with pure bovine xenograft and on the other side with bovine xenograft with added hyaluronate. CBCT scans were taken before extraction and 4 months after socket preservation, and volumetric bone changes were evaluated. The authors concluded that hyaluronic acid in combination with bovine xenograft can limit the dimensional changes that occur after tooth extraction [38].
Although the results of this case series provide valuable insight into the use of natural bovine bone graft substitute with hyaluronate in socket preservation, certain limitations associated with case studies should be considered. First, the small number of patients limits the generalizability of the results. Although histologic and histomorphometric data were collected, a larger sample size would be required to draw more definitive conclusions. In addition, the follow-up time varied between patients, which could influence the histomorphometric results due to differences in the stages of bone healing. Future studies with larger populations, standardized follow-up times, and control groups would be needed to validate the current results and better understand the long-term outcomes of using bovine bone graft substitute with hyaluronate in socket preservation.
Conclusions
Bovine bone substitutes are widely used and well researched in socket preservation. Also, the addition of hyaluronate to dental regenerative procedures to improve and accelerate soft and hard tissue regeneration has been intensively studied. Here we report histological, histomorphometric and radiological evaluation of natural bovine bone substitute pre-mixed with hyaluronate for socket preservation in three patients. The bone substitute osseointegrated in a newly formed bone that supported successful implants installation. Also, no inflammatory reaction and the absence of fibrous encapsulation showed its great biocompatibility. More research with larger patient’s population need to be performed to verify the current findings.
Acknowledgments
Author contributions
Conceptualization DJ, ŽPK; clinical work DJ; methodology MČ, ŽPK, OCP; histologic analysis OCP; writing—original draft preparation, KKM; writing—review and editing, MČ, IBP, BT; supervision, BT and ŽPK; project administration, KKM; All authors have read and agreed to the published version of the manuscript.
Data availability
The data presented in this article are available on request from the corresponding author.
Compliance with ethical standards
Conflict of interest
ŽPK and BT are part-time employees of botiss biomaterials GmbH.
Informed consent statement
Informed consent was obtained from all subjects involved in the study.
Institutional review board statement
Approval for the case series was granted by the Ethics Committee of the Faculty of Dentistry and Health Osijek (Class: 602-01/23-12/03, No: 2158/97-97-10-23-22).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Damir Jelušić, Katarina Komar Milas
References
- 1.Broers DLM, Dubois L, de Lange J, Su N, de Jongh A. Reasons for tooth removal in adults: a systematic review. Int Dent J. 2022;72:52–57. 10.1016/j.identj.2021.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cardaropoli G, Araújo M, Lindhe J. Dynamics of bone tissue formation in tooth extraction sites: an experimental study in dogs. J Clin Periodontol. 2003;30:809–18. 10.1034/j.1600-051X.2003.00366.x [DOI] [PubMed] [Google Scholar]
- 3.Araújo MG, Lindhe J. Dimensional ridge alterations following tooth extraction. An experimental study in the dog. J Clin Periodontol. 2005;32:212–8. 10.1111/j.1600-051X.2005.00642.x [DOI] [PubMed] [Google Scholar]
- 4.Tan WL, Wong TLT, Wong MCM, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23:1–21. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Bäumer D, Ozga AK, Körner G, Bäumer A. Patient satisfaction and oral health-related quality of life 10 years after implant placement. BMC Oral Health. 2021;21:1–14. 10.1186/s12903-020-01381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mello CC, Lemos CAA, Verri FR, dos Santos DM, Goiato MC, Pellizzer EP. Immediate implant placement into fresh extraction sockets versus delayed implants into healed sockets: a systematic review and meta-analysis. Int J Oral Maxillofac Surg. 2017;46:1162–77. 10.1016/j.ijom.2017.03.016 [DOI] [PubMed] [Google Scholar]
- 7.Darby I, Chen ST, Buser D. Ridge preservation techniques for implant therapy. Int J Oral Maxillofac Implants. 2009;24:260–71. [PubMed] [Google Scholar]
- 8.Morjaria KR, Wilson R, Palmer RM. Bone healing after tooth extraction with or without an intervention: a systematic review of randomized controlled trials. Clin Implant Dent Relat Res. 2014;16:1–20. 10.1111/j.1708-8208.2012.00450.x [DOI] [PubMed] [Google Scholar]
- 9.Liu J, Kerns DG. Mechanisms of guided bone regeneration: a review. Open Dent J. 2014;8:56–65. 10.2174/1874210601408010056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Avila-Ortiz G, Chambrone L, Vignoletti F. Effect of alveolar ridge preservation interventions following tooth extraction: a systematic review and meta-analysis. J Clin Periodontol. 2019;46:195–223. 10.1111/jcpe.13057 [DOI] [PubMed] [Google Scholar]
- 11.Machtei EE, Mayer Y, Horwitz J, Zigdon-Giladi H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: clinical and histological findings. Clin Implant Dent Relat Res. 2019;21:14–20. 10.1111/cid.12707 [DOI] [PubMed] [Google Scholar]
- 12.Willenbacher M, Al-Nawas B, Berres M, Kämmerer PW, Schiegnitz E. The effects of alveolar ridge preservation: a meta-analysis. Clin Implant Dent Relat Res. 2016;18:1248–68. 10.1111/cid.12364 [DOI] [PubMed] [Google Scholar]
- 13.Ponte J, Pérez-Guerrero J, Aragão F, Menezes Y, Melo M, Castro-Silva I. Histomorphometric evaluation of human extraction sockets treated with autologous fibrin, sticky bone or biphasic calcium phosphate. Acta Odontológica Latinoam. 2021;34:271–81. 10.54589/aol.34/3/271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barradas AMC, Yuan H, van Blitterswijk CA, Habibovic P. Osteoinductive biomaterials: current knowledge of properties, experimental models and biological mechanisms. Eur Cell Mater. 2011;21:407–29. 10.22203/eCM.v021a31 [DOI] [PubMed] [Google Scholar]
- 15.Shamsoddin E, Houshmand B, Golabgiran M. Biomaterial selection for bone augmentation in implant dentistry: a systematic review. J Adv Pharm Technol Res. 2019;10:46–50. 10.4103/japtr.JAPTR_327_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez AE, Nowzari H. The long-term risks and complications of bovine-derived xenografts: a case series. J Indian Soc Periodontol. 2019;23:487–92. 10.4103/jisp.jisp_656_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004;25:987–94. 10.1016/S0142-9612(03)00621-5 [DOI] [PubMed] [Google Scholar]
- 18.Riachi, F; Naaman, N; Tabarani, C; Aboelsaad, N; Aboushelib, MN; Berberi, A; Salameh, Z. Influence of material properties on rate of resorption of two bone graft materials after sinus lift using radiographic assessment. Int J Dent. 2012, 2012, 10.1155/2012/737262. [DOI] [PMC free article] [PubMed]
- 19.Garantziotis S, Savani RC. Hyaluronan biology: a complex balancing act of structure, function, location and context. Matrix Biol. 2019;78-79:1–10. 10.1016/J.MATBIO.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapanov K, Deliverska E, Zafiropoulos G, Trajkovski B. Lateral sinus augmentation by using natural bovine bone substitute with hyaluronate. Int J Dent Biomater Res. 2022;1:8–12. 10.56939/DBR22108CH [Google Scholar]
- 21.Kyyak S, Blatt S, Wiesmann N, Smeets R, Kaemmerer PW. Hyaluronic acid with bone substitutes enhance angiogenesis in vivo. Mater (Basel). 2022;15:1–11. 10.3390/ma15113839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kyyak, S; Pabst, A; Heimes, D; Kämmerer, PW; Weber, FE. Materials the influence of hyaluronic acid biofunctionalization of a bovine bone substitute on osteoblast activity in vitro. 2021, 10.3390/ma14112885. [DOI] [PMC free article] [PubMed]
- 23.Kawano M, Ariyoshi W, Iwanaga K, Okinaga T, Habu M, Yoshioka I, Tominaga K, Nishihara T. Mechanism involved in enhancement of osteoblast differentiation by hyaluronic acid. Biochem Biophys Res Commun. 2011;405:575–80. 10.1016/j.bbrc.2011.01.071 [DOI] [PubMed] [Google Scholar]
- 24.Pröhl A, Batinic M, Alkildani S, Hahn M, Radenkovic M, Najman S, Jung O, Barbeck M. In vivo analysis of the biocompatibility and bone healing capacity of a novel bone grafting material combined with hyaluronic acid. Int J Mol Sci. 2021;22:4818 10.3390/IJMS22094818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rakašević D, Šćepanović M, Mijailović I, Mišić T, Janjić B, Soldatović I, Marković A. Reconstructive peri-implantitis therapy by using bovine bone substitute with or without hyaluronic acid: a randomized clinical controlled pilot study. J Funct Biomater. 2023;14:149 10.3390/jfb14030149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang D, Wang HL. Medical contraindications to implant therapy: part i: absolute contraindications. Implant Dent. 2006;15:353–60. 10.1097/01.id.0000247855.75691.03 [DOI] [PubMed] [Google Scholar]
- 27.Hwang D, Wang HL. Medical contraindications to implant therapy: part ii: relative contraindications. Implant Dent. 2007;16:13–23. 10.1097/ID.0b013e31803276c8 [DOI] [PubMed] [Google Scholar]
- 28.Jelušić D, Siber S. The use of collagen fleece for healing purposes. Int J Dent Biomater Res 2022;1:20–27. 10.56939/dbr221020j [Google Scholar]
- 29.Sbricoli L, Guazzo R, Annunziata M, Gobbato L, Bressan E, Nastri L. Selection of collagen membranes for bone regeneration: a literature review. Mater (Basel). 2020;13:1–16. 10.3390/ma13030786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barone A, Ricci M, Tonelli P, Santini S, Covani U. Tissue changes of extraction sockets in humans: a comparison of spontaneous healing vs. ridge preservation with secondary soft tissue healing. Clin Oral Implants Res. 2013;24:1231–7. 10.1111/j.1600-0501.2012.02535.x [DOI] [PubMed] [Google Scholar]
- 31.Čandrlić, M; Tomas, M; Karl, M; Malešić, L; Včev, A; Perić Kačarević, Ž; Matijević, M. Comparison of injectable biphasic calcium phosphate and a bovine xenograft in socket preservation: qualitative and quantitative histologic study in humans. Int J Mol Sci. 2022, 23, 10.3390/ijms23052539. [DOI] [PMC free article] [PubMed]
- 32.Saghiri MA, Asatourian A, Garcia-Godoy F, Sheibani N. The role of angiogenesis in implant dentistry part ii: the effect of bone-grafting and barrier membrane materials on angiogenesis. Med Oral Patol Oral Cir Bucal. 2016;21:e526–e537. 10.4317/medoral.21200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mendes RM, Silva GAB, Lima MF, Calliari MV, Almeida AP, Alves JB, Ferreira AJ. Sodium hyaluronate accelerates the healing process in tooth sockets of rats. Arch Oral Biol. 2008;53:1155–62. 10.1016/j.archoralbio.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 34.Arpağ OF, Damlar İ, Altan A, Tatli U, Günay A. To what extent does hyaluronic acid affect healing of xenografts? a histomorphometric study in a rabbit model. J Appl Oral Sci. 2018;26:1–8. 10.1590/1678-7757-2017-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim J-J, Song HY, Ben Amara H, Kyung-Rim K, Koo K-T. Hyaluronic acid improves bone formation in extraction sockets with chronic pathology: a pilot study in dogs. J Periodontol. 2016;87:790–5. 10.1902/jop.2016.150707 [DOI] [PubMed] [Google Scholar]
- 36.Lee JB, Chu S, Ben Amara H, Song HY, Son MJ, Lee J, Kim HY, Koo KT, Rhyu IC. Effects of hyaluronic acid and deproteinized bovine bone mineral with 10% collagen for ridge preservation in compromised extraction sockets. J Periodontol. 2021;92:1564–175. 10.1002/JPER.20-0832 [DOI] [PubMed] [Google Scholar]
- 37.Blašković M, Blašković D, Hangyasi DB, Peloza OC, Tomas M, Čandrlić M, Rider P, Mang B, Kačarević ŽP, Trajkovski B. Evaluation between biodegradable magnesium metal GBR membrane and bovine graft with or without hyaluronate. Membr (Basel). 2023;13:1–13. 10.3390/membranes13080691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husseini B, Friedmann A, Wak R, Ghosn N, Khoury G, El Ghoul T, Abboud CK, Younes R. Clinical and radiographic assessment of cross-linked hyaluronic acid addition in demineralized bovine bone based alveolar ridge preservation: a human randomized split-mouth pilot study. J Stomatol Oral Maxillofac Surg. 2023, 124, 10.1016/j.jormas.2023.101426. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this article are available on request from the corresponding author.