Abstract
Vascular dementia (VD) is a neurocognitive disorder resulting from cerebral vascular disorders, leading to the demise of neurons and cognitive deficits, posing significant health concerns globally. Derived from Ginkgo biloba leaves, EGb761 is a potent bioactive compound widely recognized for its benefits in treating cerebrovascular diseases. Previous studies have demonstrated that the administration of EGb761 to VD rats enhances the proliferation, differentiation, and migration of neurons, effectively alleviating cognitive dysfunction. However, the specific mechanisms by which EGb761 exerts its remedial influence on VD persist in ambiguity. This investigation utilized an integrated approach incorporating network pharmacology with experimental procedures on HT-22 mouse hippocampal neuronal cells amidst oxygen-glucose deprivation and reoxygenation (OGD/R) to delve into certain repercussions of EGb761 on cell proliferation and migration. Results revealed that ras homolog family member A (RHOA) and B-cell lymphoma 2 (BCL-2) are potential targets of Ginkgo biloba leaves. Target genes are mainly enriched in pathways including those involved in growth hormone synthesis, secretion and action and the neurotrophin signalling pathway. Cellular experiments further demonstrated that the application of EGb761 notably enhanced the viability, proliferation, and migration of HT22 cells subjected to OGD/R through RhoA-ROCK2 pathway. In conclusion, our findings indicated that EGb761 significantly enhances neuronal proliferation and migration following OGD/R injury by targeting the RhoA-ROCK2 signalling pathway, thus offering valuable insights into its potential as a treatment for VD.
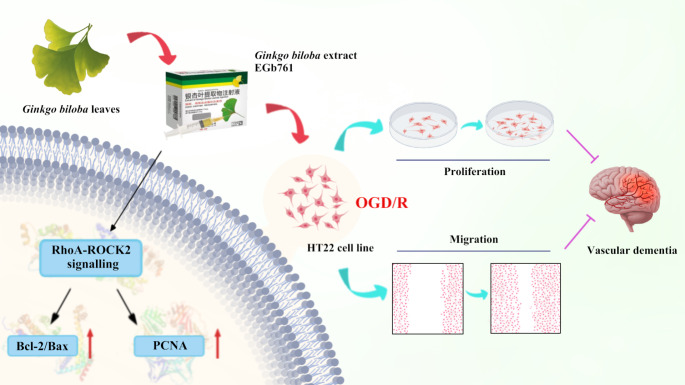
Graphical abstract
EGb761 mediates HT22 cell proliferation and migration after OGD/R via regulating RhoA-ROCK2 signalling pathway
Supplementary Information
The online version contains supplementary material available at 10.1007/s11011-024-01502-9.
Keywords: EGb761, Vascular dementia, Proliferation, Migration, RhoA-ROCK2 pathway
Introduction
Vascular dementia (VD) is a neurocognitive disorder characterised by cognitive decline resulting from vascular damage or small vessel disease within the brain (Bir et al. 2021). Culminating in the cessation of cellular vitality, these pathophysiological processes activate mechanisms linked to excitotoxicity, mitochondrial discordance, inflammatory reaction and oxidative stress (Li et al. 2021; Tian et al. 2022). Such a condition is prominently characterised by symptoms of memory loss and cognitive deficits, thereby exerting a severe and distressing impact on affected individuals (Bir et al. 2021). By constituting about 15% of cognitive decline cases worldwide, VD has attracted considerable attention as a noteworthy health issue (Torres-Simon et al. 2022).
The inability of mature neurons in the mammalian central nervous system to fully regenerate after trauma is broadly acknowledged. However, recent studies have shown that humans continue to generate new neurons from adulthood to advanced age (Tobin et al. 2019). These findings firmly conclude that the capacity for neurogenesis remains consistent across different ages of the brain. Another study indicates that the number of proliferating and developing neurons is markedly reduced within the brains of patients with cognitive impairment compared to healthy individuals. The observed decrease in developing neurons is believed to exacerbate cognitive deficits among affected patients. It has been further demonstrated that elderly individuals without cognitive impairment or psychiatric disorders have a greater number of proliferating neural cells in the brain. Following neurogenesis, these neurons continuously mature, differentiate, and migrate toward damaged areas of the brain, facilitating the timely formation of neural circuits, which shapes the brain structure and function, and enhances cognitive abilities (Boldrini et al. 2018). Furthermore, this reaffirms that the decline in hippocampal neurogenesis would be associated with impaired cognitive and emotional abilities. Consequently, the promotion of neurogenesis, proliferation and migration of neurons toward damaged areas may be a novel therapeutic approach for neurological disorders such as VD.
Rho family GTPases are indispensable in governing the cytoskeleton and are pivotal in cell proliferation, differentiation, and migration (Hall and Lalli 2010; Billuart et al. 2001). Serving as molecular toggles, they would typically function by oscillating between a dormant GDP-bound state and an active GTP-bound state. Upon activation, these GTPases interact with specific effectors (Yoon et al. 2006). Among them, GTP-bound RhoA has the capacity to stir Rho-associated kinase (ROCK), which includes ROCK1 and ROCK2. (Fujita and Yamashita 2014) Although similar, ROCK1 and ROCK2 are not always identical (Hartmann et al. 2015). In the domain of various neuronal activities like proliferation, migration, dendritic growth, and axonal expansion, the signalling cascade of RhoA/ROCK serves as a pivotal function (Fujita and Yamashita 2014; Rico et al. 2004). Research indicates that the signalling pathway of RhoA/ROCK, under the influence of IL-1β or Wnt5a, would promote neurite outgrowth and upregulate neuronal factors such as NT3 and Ngn1 (Park et al. 2018). Yet to be resolved is whether the RhoA-ROCK2 pathway promotes neuronal proliferation and migration, thereby serving as a crucial therapeutic target for VD.
The utilisation of Ginkgo biloba L. leaves for medicinal purposes can be dated back to as early as 1509 (Mostafa et al. 2016). Extracts of Ginkgo biloba leaves have demonstrated therapeutic efficacy in managing disorders including cognitive impairment, Alzheimer’s disease as well as cardiovascular disease (Chan et al. 2007). EGb761, a standardised extract, is derived from the foliage of Ginkgo biloba, formulated to contain 24% flavonol glycosides and 6% terpene lactones (National Toxicol Program 2013). These components were considered to have potential medical applications owing to their bioactive properties, particularly their antioxidant and anti-apoptotic effects, as well as their ability to enhance cognitive abilities, contributing to the treatment of cerebrovascular ailments (DeFeudis and Drieu 2000). For example, EGb761 can alleviate memory impairment by attenuating synaptic damage elicited by the scopolamine in mice (Zhang et al. 2022). Additionally, EGb761 has emerged in other studies as being capable of significantly stimulate hippocampal neuron cell proliferation while manifesting dose-dependent nuances (Müller et al. 2012). In young (8-week-old) mice, a 28-day treatment regimen involving EGb761 (40 mg/kg or 100 mg/kg) yielded a progressive increase specifically within the proliferative and differentiative abilities of the dentate gyrus, correlating with dosage (Yoo et al. 2011). In light of this, the central purpose was to ascertain whether EGb761 can exert neuroprotective effects by increasing neuronal proliferation and migration through the RhoA-ROCK2 pathway in VD.
Materials and methods
Materials and reagents
The standardized liquid formulation of EGb761 was procured from Yue Kang Pharmaceutical Group (Beijing, China). DMEM along with FBS were brought in from subsidiary of Thermo Fisher Scientific, Gibco (Waltham, MA, USA). Cell Counting Kit-8 (CCK-8) solution and 5-Ethynyl-2’-deoxyuridine (EdU) was sourced from Beyotime Biotechnology (Nanjing, China). The antibodies including rabbit anti-RhoA, rabbit anti-ROCK2 and rabbit anti-PCNA were procured by way through the company of Cell Signalling Technology (USA). Proteintech (Rosemont, IL, USA) provided rabbit anti-Bcl-2 and mouse anti-Bax antibodies. Fasudil hydrochloride (Fasu) was purchased from MedChemExpress (Princeton, NJ, USA).
Identification of bioactive components and potential targets of Ginkgo biloba leaves
The assay of high-performance liquid chromatography (HPLC) was employed with the aim of analysing the EGb761 sample. The outcomes are presented as Fig. S1 in the supplementary materials.
Bioactive components of Ginkgo biloba leaves were sourced from the Database of Traditional Chinese Systems Pharmacology (TCMSP) (https://tcmsp-e.com/tcmsp.php) by integrating the threshold of oral bioavailability (OB) ≥ 30% along with drug likeness (DL) ≥ 0.18%. Predicted biological counterparts corresponding to these active ingredients were anticipated and acquired using the data repository of Swiss Target Prediction (http://www.swisstargetprediction.ch), the data repository of Super Pred (https://prediction.charite.de) and by directly searching “BAI GUO YE” (Chinese term for Ginkgo biloba leaves) as keywords in the HERB (http://herb.ac.cn) Database, along with pertinent literature (Xuerui et al. 2016; DeFeudis et al. 2003; Peng et al. 2013; Chao and Chu 2004).
Prediction of therapeutic targets for VD
By utilizing ‘vascular dementia’ as a keyword, therapeutic targets related to VD were acquired by querying the data repository of GeneCards (https://www.genecards.org), PharmGKB (https://www.pharmgkb.org), as well as the data repository of OMIM (https://www.omim.org), Drugbank (https://go.drugbank.com) and TTD (http://db.idrblab.net/ttd), along with relevant literature (Lu et al. 2023; Zhang et al. 2021; Shi et al. 2016).
Construction of protein protein interaction (PPI) network and subsequent analysis
To visually illustrate the intersection between genes related to VD disease targets and those associated with Ginkgo biloba leaves, a Venn diagram was generated using the R tools. Subsequently, the intersected genes were employed, leveraging the STRING Database (https://string-db.org), to forge protein-protein (PPI) interaction network, with the search term ‘Homo sapiens’ specified. Cytoscape 3.9.1 was used to facilitate further explorations of protein interactions in the acquired network. Topological intricacies of the acquired network, specifically the betweenness centrality (BC), closeness centrality (CC), and degree centrality (DC) of each target, were computed through CytoNCA, and the core targets within the acquired PPI network were selected by values exceedingly twice the median of DC and surpassing the respective medians of BC and CC.
Analysis of functional enrichment
To further investigate the underlying mechanisms of Ginkgo biloba leaves on treating VD, gene ontology (GO) analysis and enrichment analysis of Kyoto Encyclopaedia of Genes and Genomes (KEGG) pathways were conducted by using the integrated data repository of DAVID (version 6.8) and subsequently visualised through the R tools. A network diagram of “compound-target-pathway” interactions was assembled utilizing the tool of Cytoscape 3.9.1 in order to visualise the intricate relationships.
Molecular docking
In order to assess binding affinity between bioactive compounds and target proteins, thereby creating an insight into their potential therapeutic efficacy and mechanisms of action, molecular docking was performed. Chemical architectures of the bioactive compounds of Ginkgo biloba leaves were retrieved from PubChem Database, and protein configurations of targets were obtained from the PDB Database. Conducting Schrödinger’s Ligand docking calculations through AutoDockTools 1.5.7 software to assess the docking score (affinity). A heatmap depicting binding energies resulting from molecular docking of main component compounds with 5 core targets is generated, higher absolute docking scores indicate greater binding affinity.
Induction of OGD/R injury and EGb761 administration in HT22 cells
We allocated HT22 cells into control, OGD/R, and EGb761 (at concentrations of 25, 50, and 100 µg/mL) groups. The control group was submerged in a 10% FBS fortified DMEM medium, as well as penicillin at a concentration of 100 units/mL accompanied with the preparation of 100 µg/mL streptomycin under optimized and standardized cultivation milieu within the incubator (37 °C, 5% CO2). In order to mimic ischaemia/reperfusion (I/R) injury, cells in the group of OGD/R and EGb761 group were exposed to OGD for the optimal duration, according to our previous experiment (Yin et al. 2024). Following OGD, the nutrient solution immediately substituted with DMEM including penicillin-streptomycin antibiotic and FBS, with EGb761 stood applied at the beginning of reoxygenation, and subsequently the cells underwent a 24 h-incubation to facilitate reoxygenation.
Cell counting kit-8 assay
Within the 96-well plates, cells from each group were seeded. Thereafter, CCK-8 solution was added following incubation for 2 h. Utilizing a microplate reader, the absorbance at 450 nm for each well would be accurately quantified.
EdU assay
Within 96-well plates, inoculations of cells in each group were conducted, where cells were cultured for 2 h with EdU medium, immersed for 30 min within cell fixative reagent, subsequently cultured with glycine (2 mg/mL) for 5 min, and incubated for 10 min with 100 µL penetrant. Cells were then subjected to staining at room temperature for 30 min, followed by 3 times of decolourisations and washes with 100 µL of penetrant in a shaker. Subsequent to this, a 30-min Hoechst staining protocol was applied to the cells and after which they were inspected under a fluorescent microscope.
Wound healing assay
The medium was drained and were given two PBS washes upon reaching approximately 80% cell density. A straight line was evenly marked in the centre of each well with pipette tips. The medium was rinsed twice with PBS in order to purge the unattached cells, followed by incubation in DMEM. Inspections on the cell migration was performed at the time period of 0, 6, 12 and 24 h, and photographed for statistical analysis.
Western blot analysis
Lysis buffer was applied to HT22 cells, followed by their lysis on ice. Collected lysates were transferred into EP tubes and centrifuged with the whirring speed at 12,000 rpm for a quarter-hour at the setup of 4 °C. Following centrifugation, supernatant was gathered, followed by the determination of protein concentrations by the application of the BCA method. Amalgamated with SDS-PAGE protein loading buffer, the remaining protein samples were subjected to denaturation by heating for 10 min, followed by packaging. Subsequently, the proteins in each group were subjected to electrophoresis, transferred to membranes, sealed with non-fat milk and subjected to 4 °C overnight incubation with primary antibodies, predominantly rabbit anti-RhoA (1:1000), rabbit anti-ROCK2 (1:3000), rabbit anti-PCNA (1:2000), rabbit anti-Bcl-2(1:800), mouse anti-Bax (1:1000), and mouse anti-β-actin (1:1000). The following day, the membranes were subjected to a further incubation phase with secondary antibodies for 1 h, which were comprised by goat anti-rabbit polyclonal antibody (1:2000) coupled with goat anti-mouse monoclonal antibody (1:10000) conjugated with HRP. Subsequently, membranes have been subjected to ECL chemiluminescence reagent for imaging using an ultra-sensitive chemiluminescence imager, facilitating quantitative analysis of proteins.
Statistical analysis
Statistical one-way ANOVA has been employed to specifically compare amidst multiple groups. Statistical evaluations were executed through the utilization of GraphPad Prism software 7.0. A significance level of P < 0.05 was deemed significant in statistical analysis. Mean ± standard deviation (SD) statistics were used for the showcase of data. A minimum of three trials were performed for each experiment to ensure reliability.
Results
Acquisition of therapeutic targets for VD and PPI network analysis
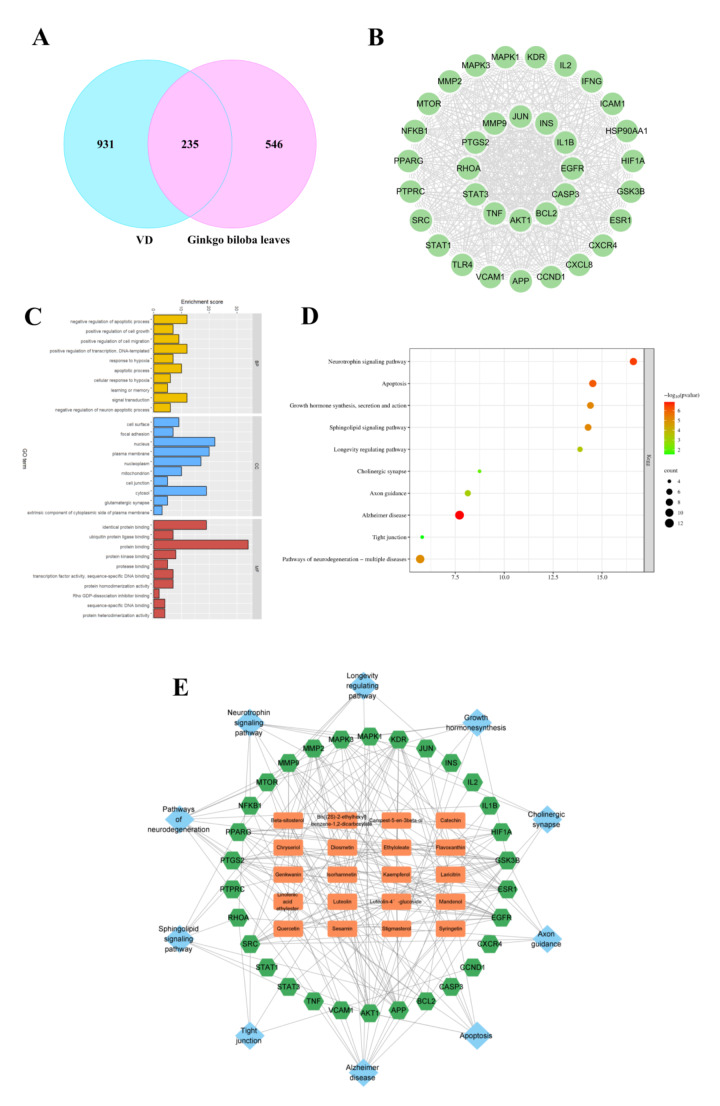
A total of 27 bioactive compounds of Ginkgo biloba leaves were retrieved from the database of TCMSP (Table 1). Subsequently, an assembly of 782 potential targets associated with bioactive compounds were predicted. Further explorations yielded a cumulative count of 1166 targets related to VD. The overlapping zone of predicted targets and anti-VD targets was eventually determined and visually represented, revealing 235 potential targets for therapeutic intervention using Ginkgo biloba leaves against VD (Fig. 1A), including RHOA, BCL2, ROCK2, and PCNA. A PPI network was coherently formed (Fig. S2). By the utilization of Cytoscape, topological relationships were calculated, leading to the identification of 35 core targets with strong interconnections, including RHOA and BCL2 (Fig. 1B).
Table 1.
Twenty-seven bioactive compounds identified from Ginkgo biloba leaves using the TCMSP database
| No. | Molecule Name | OB (%) | DL |
|---|---|---|---|
| 1 | (-)-catechin | 49.68 | 0.24 |
| 2 | (+)-catechin | 54.83 | 0.24 |
| 3 | beta-sitosterol | 36.91 | 0.75 |
| 4 | Bilobalide | 84.42 | 0.36 |
| 5 | bis[(2 S)−2-ethylhexyl] benzene-1,2-dicarboxylate | 43.59 | 0.35 |
| 6 | campest-5-en-3beta-ol | 37.58 | 0.71 |
| 7 | Chryseriol | 35.85 | 0.27 |
| 8 | Diosmetin | 31.14 | 0.27 |
| 9 | Ethyl oleate (NF) | 32.4 | 0.19 |
| 10 | Flavoxanthin | 60.41 | 0.56 |
| 11 | Genkwanin | 37.13 | 0.24 |
| 12 | ginkgolide B | 44.38 | 0.73 |
| 13 | ginkgolide C | 48.33 | 0.73 |
| 14 | ginkgolide J | 44.84 | 0.74 |
| 15 | Ginkgolide M | 49.09 | 0.75 |
| 16 | Isogoycyrol | 40.36 | 0.83 |
| 17 | isorhamnetin | 49.6 | 0.31 |
| 18 | kaempferol | 41.88 | 0.24 |
| 19 | Laricitrin | 35.38 | 0.34 |
| 20 | Linolenic acid ethyl ester | 46.1 | 0.2 |
| 21 | luteolin | 36.16 | 0.25 |
| 22 | Luteolin-4′-glucoside | 41.97 | 0.79 |
| 23 | Mandenol | 42 | 0.19 |
| 24 | quercetin | 46.43 | 0.28 |
| 25 | sesamin | 56.55 | 0.83 |
| 26 | Stigmasterol | 43.83 | 0.76 |
| 27 | Syringetin | 36.82 | 0.37 |
Fig. 1.
Identification of core targets of Ginkgo biloba for the alleviation of VD, and GO and KEGG inspections. A Venn diagram illustrating 235 intersected targets between the bioactive constituents of EGb761 and their relevant VD associated targets. B PPI network interlaced by the relatively pivotal targets. C Bar chart of GO analysis, the bar height represents enrichment score of each section. D Enrichment chart of KEGG analysis. Magnitudes of dots indicate target gene counts of each pathway, and dot colour depicts the related P adjusted values. E The “compound-target-pathway” network
Enrichment analysis of functions and pathways
Analyses through GO and KEGG were conducted revealing significant involvement of the intersected targets such as positive regulation of cell growth, positive regulation of cell migration alongside with negative regulation of neuronal apoptotic process; as well as targeting the cellular nucleus, mitochondrion and cell surface (Fig. 1C). Additionally, notably enriched pathways correlating with network targets were mapped, including growth hormone synthesis, secretion and action and the neurotrophin signalling pathway. (Fig. 1D). Subsequently, a network diagram of “compound-target-pathway” interactions was constructed (Fig. 1E).
Molecular docking
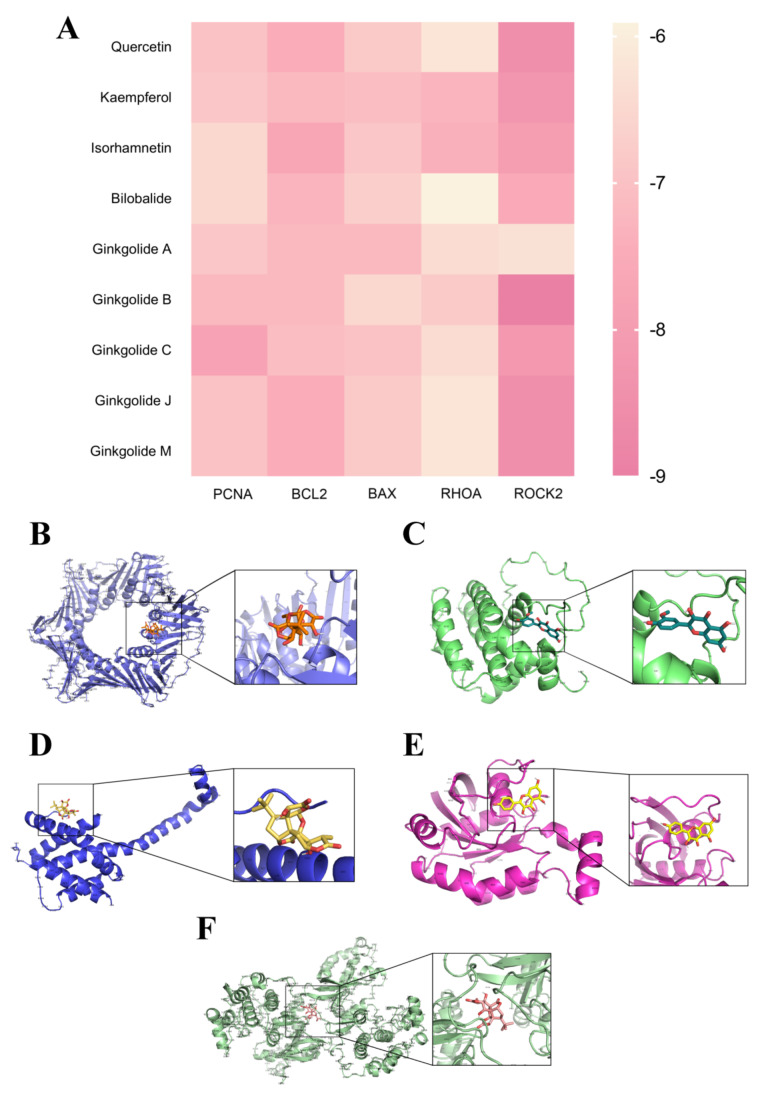
For the purpose of evaluating the binding affinity bridging the active compounds of Ginkgo biloba leaves and the corresponding target proteins, molecular docking trials were implemented, and a heat map was generated to visualise the specific results. Results indicated that the main active ingredients in Ginkgo biloba leaves exhibited strong binding capabilities to core targets of VD and their associated pathway targets, including BAX, BCL2, RHOA, ROCK2, PCNA. Surprisingly, all assessed binding affinity values were below − 5, indicating that the primary components of Ginkgo biloba leaves exhibit significant binding efficiency with the aforementioned targets. Specifically, isorhamnetin demonstrated notable binding affinity with BCL2 (affinity score = −7.7), while quercetin exhibited strong affinity with ROCK2 (affinity score = −8.5) and Ginkgolide B was found to bind effectively with ROCK2 (affinity score = −9) (Fig. 2).
Fig. 2.
Molecular docking results. A Heatmap of molecular docking results (kcal/mol), indicating the magnitude of the binding energy, with lower values signifying heightened estimated binding stability between ligands and targets. B−F Visualization of docking complexes formed by the ligand and receptor protein at the lowest binding energy, presented utilizing PYMOL software: Ginkgolide C and PCNA; Isorhamnetin and BCL2; Ginkgolide A and BAX; Kaempferol and RHOA; Ginkgolide B and ROCK2
EGb761 increased the viability of HT22 cells exposed to OGD/R
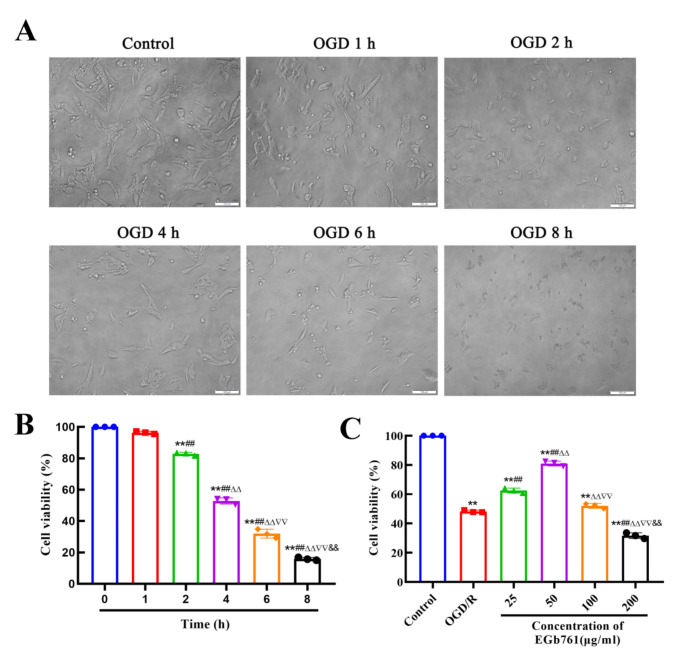
With the intent of simulating the pathological state of cerebral I/R injury, OGD/R was applied to HT22 cells, functioning as a representative in vitro model. By utilizing the CCK-8 assay, the vitality of cells was probed. Following OGD/R, the viability of HT22 cells markedly declined while in contradistinction to the control group. HT22 cells subjected to different durations of OGD were examined under a microscope. For groups with OGD durations of 0, 1, and 2 h, the cell morphology was spindle, the cell membrane was smooth, and the adherent growth was observed. However, following 4 h of OGD, the cells began to shrink and adopt a round shape, accompanied by reduced cytoplasmic volume and a flattened appearance, as the duration of OGD time increased, the cells became progressively rounder, and the number of adherent cells decreased. Results of the CCK-8 experiment demonstrated a gradual decline in cell viability with prolonged OGD duration (Fig. 3A-B). Based on the previous experiment (Yin et al. 2024), an OGD duration resulting in 50% ~ 60% cell viability was deemed optimal, with 4 h identified as the most suitable OGD duration. To explore the impact of assorted EGb761 concentration levels (25, 50, 100, and 200 µg/mL) on HT22 cellular vitality under the stimulated milieu of OGD/R, we utilized the CCK-8 assay. Research outcomes has disclosed a pronounced diminution of cellular vitality within the OGD/R group in opposition to the control group, while the EGb761 group demonstrated a notable elevation in cell viability in comparison with the group subjected to OGD/R. Maximum cellular vitality was notably recorded at an EGb761 concentration level reaching 50 µg/mL, and subsequent cell experiments were conducted at this concentration (Fig. 3C).
Fig. 3.
Microscopic morphology and viability assessment of HT22 cells. A Morphology of HT22 cells at different time of OGD. Bar = 100 μm. B Assessment of HT22 cell viability at different time of OGD conducting CCK-8 assay. (n = 3) **P < 0.01 vs.0 h group, ##P < 0.01 vs.1 h group, ΔΔP < 0.01 vs. 2 h group,∇∇P < 0.01 vs. 4 h group, &&P < 0.01 vs. 6 h group. C Viability of HT22 cell subjected by OGD/R after EGb761 treatment, assessed using CCK-8 asay. (n = 3) **P < 0.01 vs. Control group, ##P < 0.01 vs. OGD/R group, ΔΔP < 0.01 vs. 25 µg/mL group,∇∇P < 0.01 vs. 50 µg/mL group, &&P < 0.01 vs. 100 µg/mL group
EGb761 increased OGD/ R-induced proliferation of HT22 cells
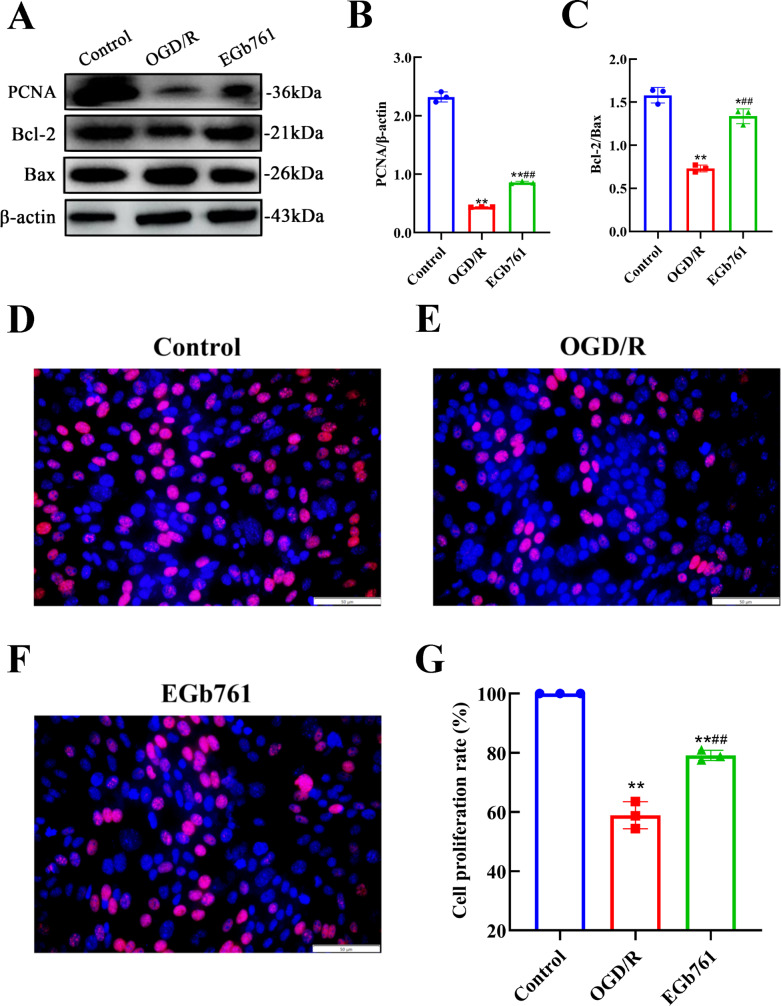
To delve deeper into the specifics about whether EGb761 amplified the proliferation of HT22 cells under OGD/R-induced circumstances, assessment of the expression of PCNA and Bcl-2/Bax was conducted. Results revealed lower expression levels of PCNA and Bcl-2/Bax in OGD/R group while evaluated against the control group, and the expression levels in EGb761 group was higher measured against the group underwent OGD/R solely (Fig. 4A-C), indicating a significant enhancement in post-OGD/R HT22 cell proliferation due to EGb761 treatment. In order to further validate our findings, EdU staining was conducted. Findings revealed that compared to control group, the OGD/R group had a substantially reduced number of cells with proliferative activity, indicating a weakened proliferative capacity of cells after treated with OGD/R. Conversely, the EGb761-treated group exhibited a significantly higher number of proliferated cells in opposition to the OGD/R group (Fig. 4D-G), which highlighted a substantial elevation in the proliferative ability of the cells underwent EGb761treatments.
Fig. 4.
EGb761 amplified HT22 cells’ proliferation provoked by OGD/R. A−C EGb761’s influence on PCNA level and Bcl-2/Bax in OGD/R-intervened HT22 cells. D−G The proliferative influence attributed by EGb761 on OGD/R-induced HT22 cells, assessed by EdU assay. Bar = 50 μm. (n = 3) *P < 0.05, **P < 0.01 vs. Control group, ##P < 0.01 vs. OGD/R group
EGb761 increased OGD/ R-induced migration of HT22 cells
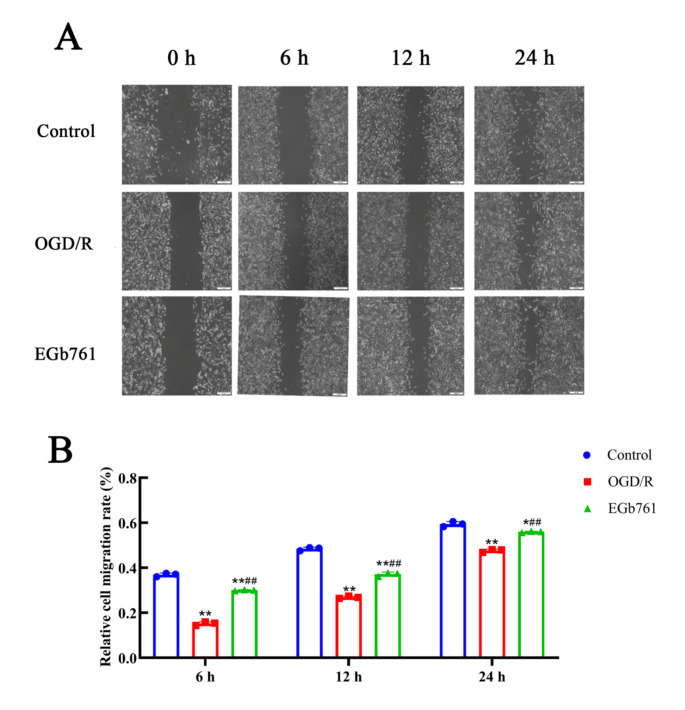
HT22 cells underwent allocations among following groups: control, OGD/R and EGb761 groups to investigate the capability of EGb761 on migration, and wound healing assays were employed, wherein cells from all groups migrated towards the bare zone of the scratch as the experiment progressed (Fig. 5A). In comparison with the control group, the migration rate was notably slower in the group of OGD/R, whereas the EGb761 group demonstrated increased migration velocity surpassing the findings of the OGD/R group (Fig. 5B). These findings suggest that EGb761 may promote the migration of HT22 cells amidst the OGD/R circumstances.
Fig. 5.
Migration effect of EGb761 on OGD/R-induced HT22 cells detected by the wound healing assay. A The migration of post-OGD/R HT22 cells treated with EGb761 observed under the microscope at 6, 12, and 24 h. Bar = 200 μm. B Statistical analysis of migration in OGD/R-induced HT22 cells receiving EGb761 treatment at 6, 12, and 24 h. (n = 3) *P < 0.05, **P < 0.01 vs. Control group, ##P < 0.01 vs. OGD/R group
RhoA-ROCK2 signalling pathway involved in the effects of EGb761 on OGD/R-induced proliferation and migration of HT22 cells
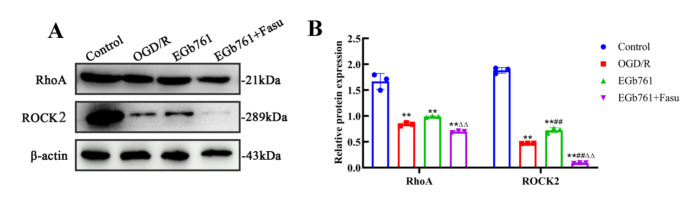
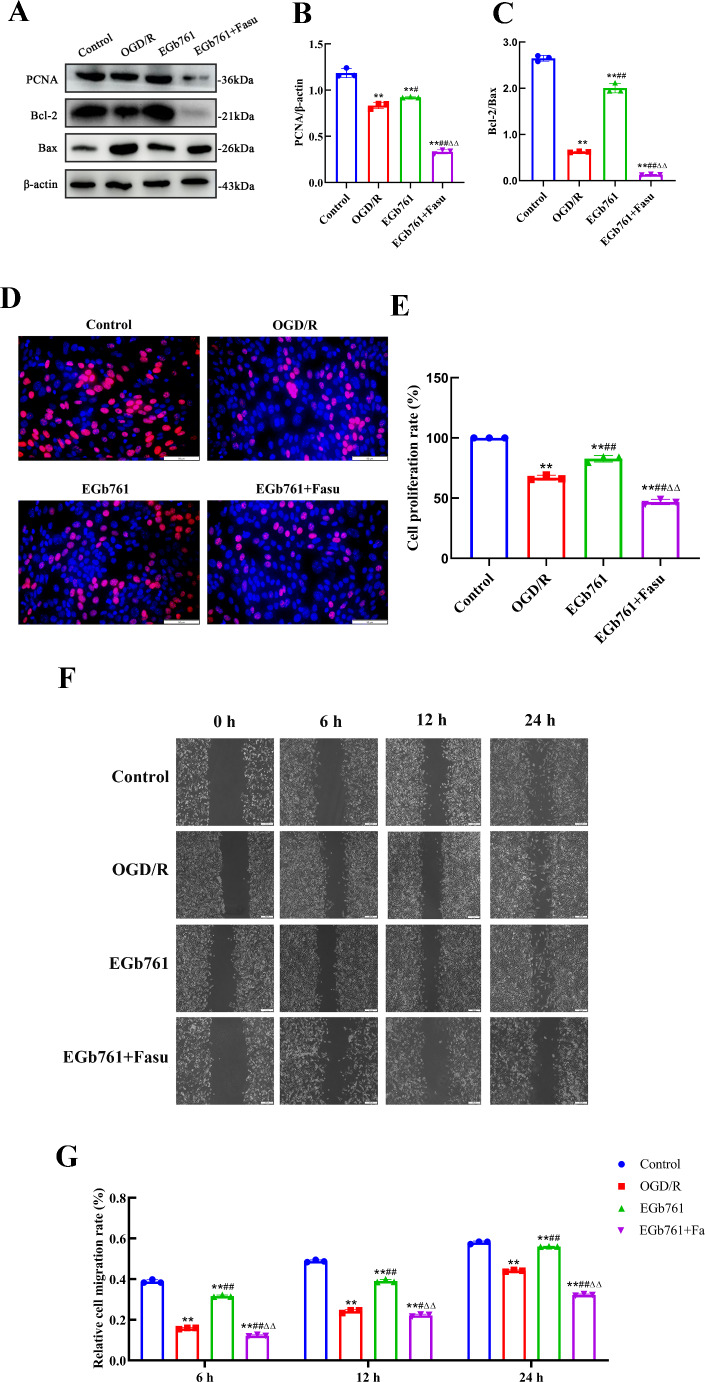
Pursuing an investigation of the involvement of RhoA-ROCK2 signalling pathway in the modulatory effects of EGb761 on cell proliferation and migration prompted by OGD/R, Fasu, the inhibitor of RhoA-ROCK2 signalling pathway, was used. A total of four groups of cells were allocated: control, OGD/R, EGb761 and EGb761 + Fasu groups, and various assays including western blot, wound healing and EdU were employed. Through western blot examination, a reduction in the observed quantities of RhoA and ROCK2 within the group of EGb761 + Fasu has been revealed, while matched against the EGb761 assembly (Fig. 6). Contrasting with the EGb761 group, findings pointed to subdued lower expression of PCNA and Bcl-2/Bax ratio in the EGb761 + Fasu group. (Figs. 7A-C) Similarly, EdU results showed significantly fewer proliferating cells within the assembly of EGb761 + Fasu while juxtaposed against the EGb761 group, indicating diminished proliferation ability. (Fig. 7D-E) The wound healing indicated a reduced migration capability of cells in the group of EGb761 + Fasu as opposed to the EGb761 group at 6, 12, and 24 h (Fig. 7F-G). Overall, it has been conclusively proposed and inferred that the inhibition of RhoA-ROCK2 signalling pathway reduces cell proliferation and migration, and that EGb761 boosts these through the RhoA-ROCK2 signalling pathway.
Fig. 6.
Effect of the inhibitor Fasu on the RhoA-ROCK2 signalling pathway in OGD/R-intervened HT22 cells. A Band articulation of RhoA and ROCK2 levels in the experimental context of HT22 cells in the control, OGD/R, EGb761, and EGb761 + Fasu groups. B Statistical analysis of RhoA and ROCK2 expression.(n = 3) **P < 0.01 vs. Control group, ##P < 0.01 vs. OGD/R group. ΔΔP < 0.01 vs. EGb761 group
Fig. 7.
Involvement of the signalling pathway of RhoA-ROCK2 in the EGb761 mediated proliferative and migratory responses of OGD/R-induced HT22 cells. A−C The expression of PCNA and Bcl-2/Bax in the control, OGD/R, EGb761, and EGb761 + Fasu groups. D−E Cell proliferation of OGD/R-induced HT22 cells in the control, OGD/R, EGb761, and EGb761 + Fasu groups, assessed by EdU assay. Bar = 50 μm. F−G Cell migration of OGD/R-induced HT22 cells in the control, OGD/R, EGb761, and EGb761 + Fasu groups, evaluated by the wound healing assay. Bar = 200 μm. (n = 3) **P < 0.01 vs. Control group, #P < 0.05, ##P < 0.01 vs. OGD/R group. ΔΔP < 0.01 vs. EGb761 group
Discussion
Neuronal proliferation and migration are essential for the formation of neural circuits and are critical for cognitive functions, including memory acquisition and retention. Enhancing neurogenesis, along with promoting neuronal proliferation and migration, emerges as a vital therapeutic approach for alleviating cognitive impairments. In response to neurological insults such as cerebral I/R injury, neurons located within the CA1 region of the hippocampus, one of the most sensitive areas, undergo apoptosis (Wang et al. 2011). Endogenous neural stem cells differentiate into neurons that subsequently proliferate rapidly and migrate towards damaged regions. (Tang et al. 2022) These new neurons integrate into existing neural networks, thereby markedly aiding in the rejuvenation of cognitive functions (Toda et al. 2018). Investigations has discovered that suppression of miR-155 has demonstrated a capacity to offer protective effects by enhancing the survival capacity and migration regarding SH-SY5Y cell that experienced OGD/R conditions, while also mitigating apoptosis and reducing the necrotic zone in middle cerebral artery occlusion (MCAO) mouse (Zhang et al. 2020). Therefore, this study aims to explore strategies for enhancing neurogenesis, as well as promoting neuronal proliferation and migration in the context of cerebral I/R injuries, which are hypothesized to be critical therapeutic approaches for ameliorating cognitive disorders associated with VD.
EGb761 is a standardised extract extensively utilized for the amelioration and deterrence of neurodegenerative dementias. Its multifaceted neuroprotective effects are attributed to its ability to modulate several biological processes, including potent anti-inflammatory and antioxidant activities, the inhibition of apoptosis, and other critical cellular protective mechanisms. Researches had discovered that EGb 761 conspicuously intensified the proliferation of cells within the hippocampal region of 6 months and 22 months TgAPP/PS1 mice (Tchantchou et al. 2007). In addition to its effects on neurogenesis, EGb761 induced prominent reductions within the infarct volume in I/R rats, as in comparison to the vehicle-treated group. This reduction is accompanied by a marked inhibition of neuronal apoptosis, as well as substantial increases in hippocampal cellular proliferation and migration (Sun et al. 2013). Furthermore, our previous experiments on rat models revealed that EGb761 not only delays the progression of cognitive decline but also improves learning and memory capacities (Yin et al. 2024). Despite these promising findings, whether EGb761 can promote neuronal proliferation and migration to exert its neuroprotective effects in VD is yet not clear and remains to be elucidated.
Network pharmacology is a systems biology approach that addresses multiple targets, drug-drug interactions and the overall effect of a drug within an organism. Core targets of Ginkgo biloba for the treatment of VD were initially identified, enabling a deeper understanding of the intricate molecular mechanisms underlying its therapeutic potential. Results of the analysis revealed 235 potential targets, with a PPI network highlighting 35 crucial targets, including RHOA and BCL2. To further explore the biological pathways involved in the treatment of VD, GO enrichment and KEGG pathway analyses were also performed in order to provide valuable insights into the contributions of the identified targets and their interconnected pathways. Molecular docking confirmed strong binding between the main active compounds of Ginkgo biloba and the identified core targets, including BAX, BCL2, RHOA, ROCK2, PCNA. These findings provide compelling evidence that the identified core targets focus on proliferation and migration, and play a pivotal role in the therapeutic mechanisms of EGb761.One of the core targets identified in this study is proliferating cell nuclear antigen (PCNA), a fundamental protein which would be crucial for DNA synthesis and repair processes within proliferating cells, signifies active cell proliferation and serves as a vital role in preserving cellular equilibrium (Witko-Sarsat et al. 2010). In neurological diseases, elevated PCNA expression has been linked to enhanced DNA repair, facilitating the recovery from cerebral I/R injury by promoting cell proliferation, and ultimately improving cognitive function (Wang et al. 2022). Furthermore, studies by Matthew K. Tobin have disclosed an interrelation in the plenitude of DCX+ PCNA+ cells and interactive functionalities among presynaptic SNARE proteins, suggesting that an elevated count of DCX+ PCNA+ cells are associated with improved cognitive performance and mental acuity (Tobin et al. 2019). BAX and BCL2 are also closely linked to cellular proliferation and apoptosis, making their regulation as a potential therapeutic strategy for VD. In addition, RHOA and ROCK2, key components of the RhoA-ROCK2 signaling pathway, regulate cellular growth, differentiation, migration, and development, with its activation promoting cellular proliferation (Ma et al. 2018). This pathway enhances neurite extension in hippocampal neurons, while suppression of RhoA diminishes axon arborization in embryonic mouse neurons (Chan et al. 2007; Ahnert-Hilger et al. 2004). By modulating these core targets and the signalling pathways involved in neuronal proliferation and migration, it remains uncertain whether these targets indeed contribute to the therapeutic effects of EGb761 in VD.
While our findings suggest the potential role of BAX, BCL2, PCNA and RhoA-ROCK2 signalling pathway as therapeutic targets in these processes, their precise involvement remain unclear and warrant further investigation to validate their relevance for drug intervention. Building upon the previous findings, cellular experiments were conducted to further elucidate the neuroprotective mechanisms of EGb761 and its effects on these targets. The experimental results demonstrated that EGb761 significantly increased PCNA expression and upregulated the Bcl-2/Bax ratio, thereby enhancing the proliferation and migration abilities of OGD/R-exposed HT22 cells, confirming the neuroprotective effects of EGb761. Moreover, detailed cellular assays demonstrated that EGb761 treatment led to pronounced upregulation of RhoA and ROCK2 expression levels compared to the untreated model group. Conversely, the group treated with EGb761 in combination with the Fasu exhibited downregulation amidst the levels of RhoA, ROCK2, PCNA, and the Bcl-2/Bax ratio, accompanied by diminished cellular proliferation and migration compared to treatment with EGb761 alone. These findings underscore the vital influence of RhoA-ROCK2 signalling pathway in stimulating the proliferation and migration of neurons, suggesting that EGb761 can effectively promote neuronal proliferation and migration in OGD/R-induced HT22 cells through the RhoA-ROCK2 signalling pathway.
During this study, core targets were identified as pivotal regulators in these processes and are considered potential therapeutic candidates for the treatment of VD. Experimental findings demonstrated that EGb761 effectively enhances neuronal proliferation and migration in HT22 cells subjected to OGD/R, primarily through the activation of the RhoA-ROCK2 signalling pathway, underscoring the significance of this pathway in the neuroprotective effects of EGb761. However, in vitro experiments may not be able to fully replicate the in vivo environment of VD. While cell culture models provide valuable insights into the molecular and cellular effects of EGb761, they lack the complexity of whole organisms, such as interactions between different cell types, the influence of systemic factors like hormones and cytokines, as well as the impact of the blood-brain barrier. To comprehensively elucidate the therapeutic effects and mechanisms of EGb761, our future research will incorporate in vivo studies to simulate the complicated biological environment of living organisms, integrating bioinformatics and metabolomics to explore EGb761’s impact from molecular interactions to systemic outcomes, offering deeper insights into its potential for treating VD. In summary, EGb761 has shown potential in enhancing neuronal proliferation and migration through the pathway of RhoA-ROCK2, with neuroprotective effects evident in cellular models, highlighting the remedial promise of aiming at the signalling pathway of RhoA-ROCK2 in mitigating VD.
Conclusion
This study employed an integrated approach merging network pharmacology alongside with the vitro experimental validation in order to delve into the curative potential of EGb761 for VD. Several key targets including RHOA, ROCK2, and PCNA were initially identified as potential therapeutic targets for VD. Subsequent in vitro experiments demonstrated that EGb761 effectively promotes neuronal proliferation and migration through the pathway of RhoA-ROCK2 amidst OGD/R-exposed HT22 cells, underscoring its therapy effect in brain tissue injury repair in VD and provide further preclinical evidence supporting its clinical application.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Dexiu Wang: Writing– original draft, Conceptualization, Methodology, Investigation, Funding acquisition. Xin Zhao: Methodology, Investigation, Formal analysis. Jinghan Li: Methodology, Investigation, Data curation. Yang Song: Investigation, Formal analysis, Data curation. Weida Chen: Methodology, Investigation, Funding acquisition, Data curation. Xin Cai: Methodology, Investigation, Formal analysis, Resources. Ruofan Liu: Writing– review & editing, Project administration, Conceptualization. Zetao Chen: Writing– review & editing, Project administration, Conceptualization.
Funding
This work was supported by the Shandong Natural Science Foundation (ZR2022MC060), Shandong Traditional Chinese Medicine Science and Technology Project (2021Q071), Shandong Province Medical and Health science and technology project (Health care project) (2023BJ000051), Shandong Medicine Health Science and Technology Development Plan (202102080543), Weifang Science and Technology Development Plan Project (2021YX044).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Consent for publication
All authors agreed with the content and that all gave explicit consent to submit and publish.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ruofan Liu, Email: 71002179@sdutcm.edu.cn.
Zetao Chen, Email: 71000808@sdutcm.edu.cn.
References
- Ahnert-Hilger G, Höltje M, Große G, Pickert G, Mucke C, Nixdorf-Bergweiler B, Boquet P, Hofmann F, Just I (2004) Differential effects of rho GTPases on axonal and dendritic development in hippocampal neurones. J Neurochem 90(1):9–18. 10.1111/j.1471-4159.2004.02475.x [DOI] [PubMed] [Google Scholar]
- Billuart P, Winter CG, Maresh A, Zhao X, Luo L (2001) Regulating axon branch stability: the role of p190 RhoGAP in repressing a retraction signaling pathway. Cell 107(2):195–207. 10.1016/s0092-8674(01)00522-0 [DOI] [PubMed] [Google Scholar]
- Bir SC, Khan MW, Javalkar V, Toledo EG, Kelley RE (2021) Emerging concepts in vascular dementia: a review. J Stroke Cerebrovasc Dis 30(8):105864. 10.1016/j.jstrokecerebrovasdis.2021.105864 [DOI] [PubMed] [Google Scholar]
- Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlova I, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ (2018) Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22(4):589. 10.1016/j.stem.2018.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PC, Xia Q, Fu PP (2007) Ginkgo biloba leave extract: biological, medicinal, and toxicological effects. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25(3):211–244. 10.1080/10590500701569414 [DOI] [PubMed] [Google Scholar]
- Chao JCJ, Chu CC (2004) Effects of Ginkgo biloba extract on cell proliferation and cytotoxicity in human hepatocellular carcinoma cells. World J Gastroenterol 10(1):37. 10.3748/wjg.v10.i1.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFeudis F, Drieu K (2000) Ginkgo biloba extract (EGb 761) and CNS functions basic studies and clinical applications. Curr Drug Targets 1(1):25–58. 10.2174/1389450003349380 [DOI] [PubMed] [Google Scholar]
- DeFeudis FV, Papadopoulos V, Drieu K (2003) Ginkgo biloba extracts and cancer: a research area in its infancy. Fundam Clin Pharmacol 17(4):405–417. 10.1046/j.1472-8206.2003.00156.x [DOI] [PubMed] [Google Scholar]
- Fujita Y, Yamashita T (2014) Axon growth inhibition by RhoA/ROCK in the central nervous system. Front Neurosci 8:338. 10.3389/fnins.2014.00338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A, Lalli G (2010) Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol 2(2):18. 10.1101/cshperspect.a001818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S, Ridley AJ, Lutz S (2015) The function of rho-associated kinases ROCK1 and ROCK2 in the pathogenesis of cardiovascular disease. Front Pharmacol 6:276. 10.3389/fphar.2015.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wen W, Li P, Fu Y, Chen H, Wang F, Dai Y, Xu S (2021) Mitochondrial protection and against glutamate neurotoxicity via Shh/Ptch1 signaling pathway to ameliorate cognitive dysfunction by Kaixin San in multi-infarct dementia rats. Oxid Med Cell Longev 2021:1–15. 10.1155/2021/5590745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu WZ, Chen ZW, Wen JY (2023) The role of RhoA/ROCK pathway in the ischemic stroke-induced neuroinflammation. Biomed Pharmacother 165:115141. 10.1016/j.biopha.2023.115141 [DOI] [PubMed] [Google Scholar]
- Ma W, Xie X, Shao X, Zhang Y, Mao C, Zhan Y, Zhao D, Liu M, Li Q, Lin Y (2018) Tetrahedral DNA nanostructures facilitate neural stem cell migration via activating RHOA/ROCK2 signalling pathway. Cell Prolif 51(6):e12503. 10.1111/cpr.12503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa R, Ibrahim B, Jaleel GA (2016) Neuro-protective effects of Ginkgo biloba leaves extract on cerebral ischemia-reperfusion injury induced experimentally in ovariectomized rats. Int J Mol Sci 8:237–242. 10.1016/j.neuroscience.2023.05.015 [Google Scholar]
- Müller WE, Heiser J, Leuner K (2012) Effects of the standardized Ginkgo biloba extract EGb 761® on neuroplasticity. Int Psychogeriatr 24(S1):S21–S24. 10.1017/S1041610212000592 [DOI] [PubMed] [Google Scholar]
- National Toxicol Program (2013) Toxicology and carcinogenesis studies of Ginkgo biloba extract (CAS 90045-36-6) in F344/N rats and B6C3F1/N mice (gavage studies). Natl Toxicol Program Tech Rep Ser 00(578):1–183 [PubMed]
- Park SY, Kang MJ, Han JS (2018) Interleukin-1 beta promotes neuronal differentiation through the Wnt5a/RhoA/JNK pathway in cortical neural precursor cells. Mol Brain 11(1):39. 10.1186/s13041-018-0383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng CC, Liu JH, Chang CH, Chung JY, Chen KC, Chou KY, Peng RY (2013) Action mechanism of Ginkgo biloba leaves extract intervened by exercise therapy in treatment of benign prostate hyperplasia. Evid Based Complement Alternat Med 2013:1–12. 10.1155/2013/408734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico B, Beggs HE, Schahin-Reed D, Kimes N, Schmidt A, Reichardt LF (2004) Control of axonal branching and synapse formation by focal adhesion kinase. Nat Neurosci 7(10):1059–1069. 10.1038/nn1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi XW, Yu WJ, Yang TT, Liu W, Zhao YZ, Sun YK, Chai LM, Gao YH, Dong B, Zhu LQ (2016) Panax notoginseng saponins provide neuroprotection by regulating NgR1/RhoA/ROCK2 pathway expression, in vitro and in vivo. J Ethnopharmacol 190:301–312. 10.1016/j.jep.2016.06.017 [DOI] [PubMed] [Google Scholar]
- Sun L, Zhuang W, Xu X, Yang J, Teng J, Zhang F (2013) The effect of injection of EGb 761 into the lateral ventricle on hippocampal cell apoptosis and stem cell stimulation in situ of the ischemic/reperfusion rat model. Neurosci Lett 555:123–128. 10.1016/j.neulet.2013.09.015 [DOI] [PubMed] [Google Scholar]
- Tang H, Li Y, Tang W, Zhu J, Parker GC, Zhang JH (2022) Endogenous neural stem cell–induced neurogenesis after ischemic stroke: processes for brain repair and perspectives. Transl Stroke Res 14(3):297–303. 10.1007/s12975-022-01078-5 [DOI] [PubMed] [Google Scholar]
- Tchantchou F, Xu Y, Wu Y, Christen Y, Luo Y (2007) EGb 761 enhances adult hippocampal neurogenesis and phosphorylation of CREB in transgenic mouse model of Alzheimer’s disease. FASEB J 21(10):2400–2408. 10.1096/fj.06-7649com [DOI] [PubMed] [Google Scholar]
- Tian Z, Ji X, Liu J (2022) Neuroinflammation in vascular cognitive impairment and dementia: current evidence, advances, and prospects. Int J Mol Sci 23(11):6224. 10.3390/ijms23116224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin MK, Musaraca K, Disouky A, Shetti A, Bheri A, Honer WG, Kim N, Dawe RJ, Bennett DA, Arfanakis K, Lazarov O (2019) Human hippocampal neurogenesis persists in aged adults and Alzheimer’s disease patients. Cell Stem Cell 24(6):974–982e973. 10.1016/j.stem.2019.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T, Parylak SL, Linker SB, Gage FH (2018) The role of adult hippocampal neurogenesis in brain health and disease. Mol Psychiatry 24(1):67–87. 10.1038/s41380-018-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Simon L, Doval S, Nebreda A, Llinas SJ, Marsh EB, Maestu F (2022) Understanding brain function in vascular cognitive impairment and dementia with EEG and MEG: a systematic review. Neuroimage Clin 35:19. 10.1016/j.nicl.2022.103040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Xia Q, Chu KT, Pan J, Sun LN, Zeng B, Zhu YJ, Wang Q, Wang K, Luo BY (2011) Severe global cerebral ischemia-induced programmed necrosis of hippocampal CA1 neurons in rat is prevented by 3-Methyladenine: a widely used inhibitor of autophagy. J Neuropathol Exp Neurol 70(4):314–322. 10.1097/NEN.0b013e31821352bd [DOI] [PubMed] [Google Scholar]
- Wang W, Yan T, Guo X, Cai H, Liang C, Huang L, Wang Y, Ma P, Qi S (2022) KAP1 phosphorylation promotes the survival of neural stem cells after ischemia/reperfusion by maintaining the stability of PCNA. Stem Cell Res Ther 13(1):290. 10.1186/s13287-022-02962-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witko-Sarsat V, Mocek J, Bouayad D, Tamassia N, Ribeil J-A, Candalh C, Davezac N, Reuter N, Mouthon L, Hermine O, Pederzoli-Ribeil M, Cassatella MA (2010) Proliferating cell nuclear antigen acts as a cytoplasmic platform controlling human neutrophil survival. J Exp Med 207(12):2631–2645. 10.1084/jem.20092241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuerui G, Zhongdong L, Zhaohao W, Erlan S, Jianchang W (2016) Effects of Ginkgo biloba extract on production of ROS in human coronary artery endothelial cells induced by activated platelets. Med J Air Force 32(04):246–251 [Google Scholar]
- Yin Y, Yan C, Zhang R, Wang Y, Song Y, Hu S, Zhao X, Liu R, Guo M, Wang Y, Cai X, Wang D (2024) Ginkgo biloba extract (EGb761) inhibits autophagy and apoptosis in a rat model of vascular dementia via the AMPK-mTOR signalling pathway. J Funct Foods 116:106168. 10.1016/j.jff.2024.106168 [Google Scholar]
- Yoo DY, Nam YY, Kim W, Yoo KY, Park J, Lee CH, Choi JH, Yoon YS, Kim DW, Won MH, Hwang IK (2011) Effects of Ginkgo biloba extract on promotion of neurogenesis in the hippocampal dentate gyrus in C57BL/6 mice. J Vet Med Sci 73(1):71–76. 10.1292/jvms.10-0294 [DOI] [PubMed] [Google Scholar]
- Yoon MS, Cho CH, Lee KS, Han JS (2006) Binding of Cdc42 to phospholipase D1 is important in neurite outgrowth of neural stem cells. Biochem Biophys Res Commun 347(3):594–600. 10.1016/j.bbrc.2006.06.111 [DOI] [PubMed] [Google Scholar]
- Zhang L, Liu C, Huang C, Xu X, Teng J (2020) miR-155 knockdown protects against cerebral ischemia and reperfusion injury by targeting MafB. Biomed Res Int 2020:1–11. 10.1155/2020/6458204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Li KX, Wang XY, Ding YY, Ren ZR, Fang JL, Sun T, Guo Y, Chen ZW, Wen JY (2021) CSE-derived H2S inhibits reactive astrocytes proliferation and promotes neural functional recovery after cerebral ischemia/ reperfusion injury in mice via inhibition of RhoA/ROCK2 pathway. ACS Chem Neurosci 12(14):2580–2590. 10.1021/acschemneuro.0c00674 [DOI] [PubMed] [Google Scholar]
- Zhang GJ, Zheng D, Yu H, Luo XP, Wu W (2022) Ginkgo biloba extract ameliorates scopolamine-induced memory deficits via rescuing synaptic damage. Curr Med Sci 42(3):474–482. 10.1007/s11596-022-2582-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.