Abstract
Sodium aescinate (SA), a natural plant extract with various bioactivities, is widely used to treat oedema and inflammation in clinics. However, adverse events, including liver injury, kidney injury, and phlebitis, have been reported in patients with SA in recent years. In this study, we used BALB/c mice and L02 cells to evaluate the role of ferroptosis in SA-induced liver injury. SA significantly increased AST, ALT, MDA and Fe2+, decreased GSH levels, and induced pathological changes in the liver in vivo. SA also reduced the viability of L02 cells and induced LDH release, intracellular cysteine reduction, GSH depletion, iron accumulation, ROS production, and lipid peroxidation, indicating that SA causes ferroptosis. In addition, SA inhibited transcriptional activity of activating transcription factor 4 (ATF4) and subsequently reduced the expression of the downstream genes xCT (solute carrier family 7a member 11, SLC7A11) and Cystathionine gamma-lyase (CTH) which play vital roles in GSH biosynthesis. Interestingly, the cytotoxic effects of SA were effectively attenuated by ATF4 overexpression, while they were significantly aggravated by ATF4 silencing. These results revealed that SA triggers hepatocyte ferroptosis by inhibiting the activity of ATF4, which causes an oxidative imbalance.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-79723-2.
Keywords: Sodium aescinate, ATF4, GSH, Ferroptosis, Hepatotoxicity
Subject terms: Drug safety, Pharmacology, Toxicology
Sodium aescinate (SA), a triterpene saponin extracted from horse chestnut trees’ seeds and dried fruits, was first marketed in Germany during the 1970s and has anti-inflammatory, anti-edematous, anti-exudation, vasoprotective, and antioxidant effects1–3. Recent studies have shown that SA exerts an antitumour effect on several malignant tumours by inducing cell cycle arrest, oxidative stress, and apoptosis4–6. As an essential bioactive component of Aesculus hippocastanum, the clinic has used SA to treat encephaledema, post-traumatic/surgery oedema, chronic venous insufficiency, and inflammation induced by trauma or fire burns7. However, the clinical application of SA is restricted due to adverse drug reactions. According to case reports, SA exerts severe toxic effects in patients, including phlebitis, kidney injury, liver injury, central nervous system damage, pruritus, and anaphylactic shock8,9. SA can increase aspartate transaminase (AST), alanine aminotransferase (ALT), total bilirubin (T-Bil), and direct bilirubin (D-Bil) levels and cause liver function damage10,11.
Some studies have focused on the mechanism of SA toxicity to provide a reference for its rational clinical use12,13. After exposure to SA, the livers of mice were severely damaged, characterised by elevated ALT and AST levels, inhibition of SOD activity, an increase in the relative liver weight index, and pathological changes in the liver14. However, the mechanism by which SA induces hepatotoxicity has not been fully elucidated. There is evidence that SA toxicity is correlated with glutathione (GSH) depletion, reactive oxygen species (ROS) accumulation, lactate dehydrogenase (LDH) release, and suppression of the cellular intrinsic antioxidant defence system15. Accumulating research found that oxidative stress promotes liver damage16–19. Thus, these previous data suggested that oxidative stress is involved in SA-induced hepatotoxicity.
Recent studies have shown that oxidative stress-mediated ferroptosis may be responsible for various liver diseases, such as hepatitis B virus (HBV) infection, intermittent hypoxia, and drug-induced liver injury20–23. Ferroptosis is a type of iron-dependent cell death. Accumulated ferrous iron promotes the lethal formation of ROS and lipid peroxidation through the Fenton reaction24,25. The GSH-glutathione peroxidase 4 (GPX4) signalling axis is a cellular antioxidant system that defends against ferroptosis26. xCT is a subunit of the cystine/glutamate antiporter that takes up extracellular cystine for intracellular GSH biosynthesis27. CTH provides selenophosphate for synthesising selenocysteine, an essential component of GSH28. GPX4 subsequently consumes GSH to detoxify lipid hydroperoxides and repair lipids, resulting in the suppression of ferroptosis29.
Activating transcription factor 4 (ATF4) is a mediator of oxidative homeostasis and reportedly blunts liver injury and disease development by suppressing ferroptosis in recent research30. ATF4 drives the expression of numerous target genes whose products mitigate oxidative stress, promote amino acid synthesis, and protect cells from damage31. Many studies have reported that ATF4 regulates xCT transcription by binding to amino acid response element (AARE)32,33, and regulates CTH transcription by binding to a cis-regulatory element (CRE)34,35, ultimately enhancing GSH biosynthesis.
The objective of our study was to illustrate the mechanism of liver injury caused by SA. We hypothesized that SA could induce iron overload, oxidative stress and ferroptosis by inhibiting the ATF4 pathway. These findings may provide new information on the molecular mechanism of SA hepatotoxicity and offer a novel hepatoprotective strategy.
Materials and methods
Chemicals and reagents
SA (purity > 99%) was obtained from Solarbio (Beijing, China). Antibodies specific for ATF4 (50 kDa, #CY5873, 1:1000), xCT (55 kDa, #CY7046, 1:1000), CTH (42 kDa, #CY8267, 1:1000) and GPX4 (17 kDa, #CY6959, 1:1000) were obtained from Abways (Shanghai, China). Antibodies for Histone H1.4 (24 kDa, #H7665, 1:1000) and GAPDH (36 kDa, #G9545, 1:1000) were obtained from Sigma (USA). Ferrostatin-1 (Fer-1) and Deferoxamine (DFO) were obtained from MedChem Express (USA).
Animals and treatment
Male BALB/c mice (7–8 weeks, 18–22 g) were bought from Hunan SJA Laboratory Animal Co., Ltd. (China). All of the animal experimental procedures in this study were performed in accordance with the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines and the administration committee of experimental animals of Yichun University (Approval No: IACUC-2023029). Mice were fed adaptively for 7 days before the experiments. After acclimation, 30 mice were randomly divided into 5 groups: vehicle (normal saline), 3.6 mg/kg/d SA, 7.2 mg/kg/d SA, 5 mg/kg/d Fer-1 + 3.6 mg/kg/d SA, 5 mg/kg/d Fer-1groups. After 10 consecutive days of intraperitoneal injection, the mice in each group were weighed and then euthanized with an overdose of 3% pentobarbital sodium (180 mg/kg). Blood and livers were collected. All methods were performed in accordance with the relevant guidelines and regulations.
Serum biochemical assay
AST and ALT in serum were measured according to manufacturer’s instructions to evaluate mice liver function (Leadman, China) as previously reported36.
Hematoxylin and eosin (H&E) staining for histopathological examinations
Paraformaldehyde-fixed and paraffin-embedded livers samples were sectioned. The liver sections were dewaxed, hydrated and stained with H&E. Two pathologists observed the slides to evaluate morphological changes in livers, respectively.
Cell culture and treatment
Human normal hepatocytes (L02 cells) were obtained from the American Type Culture Collection. The cells were cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (HyClone, USA). In subsequent experiments, the cells were treated as follows: solvent (PBS) as a control; SA at different concentrations (40, 50, 60, 70, 80, 90, 100 µmol/L); 1 µmol/L Fer-1 + 70 µmol/L SA; 100 µmol/L DFO + 70 µmol/L SA; 1 µmol/L Fer-1; 100 µmol/L DFO; ATF4 expression plasmid + 70 µmol/L SA; empty vector + 70 µmol/L SA; si-ATF4 + 70 µmol/L SA; si-con + 70 µmol/L SA; si-ATF4 + xCT expression plasmid + 70 µmol/L SA; si-ATF4 + CTH expression plasmid + 70 µmol/L SA; si-ATF4 + xCT expression plasmid + CTH expression plasmid + 70 µmol/L SA.
MTT assay
After treatment, L02 cell viability was measured with a MTT assay. MTT was added and incubated with cells for 4 h. The absorbance of solubilization solution was measured at 570 nm wavelength as previously reported37.
LDH release assay
After treatment, the level of LDH in cell culture medium was measured using a LDH detection kit (Beyotime, China) to assess cytotoxicity.
The measurement of MDA, Cysteine, and GSH
After treatment, supernatant of L02 cells extract were collected. Liver homogenates were centrifuged to collect the supernatant. Malondialdehyde (MDA), cysteine and GSH levels were determined with MDA, cysteine and GSH Detection Kits (Suzhou Grace Biotechnology, China), respectively.
Iron assay
Iron level in cell or tissue extracts was determined by a iron assay kit (Abcam, England) manufacturer. The average optical densities were detected at a 593 nm absorption wavelength and the level of Fe2+ was calculated from the standard curve.
ROS detection assay
After treatment, L02 cells were stained with DCFH-DA probe (Beyotime, China) as previously reported37. The fluorescence intensity of ROS was observed under a fluorescence microscope (Canon, Japan).
Lipid peroxidation assay
After treatment, L02 cells were stained with BODIPY 581/591 C11 dye (Invitrogen, USA). The fluorescence of C11-BODIPY oxidized form (oxC11-BODIPY) was measured using fluorescence microscope (Canon, Japan).
RNA extraction and qRT‑PCR
Total RNAs were isolated from L02 cells using RNAiso Plus (TaKaRa, Japan). cDNA synthesis was conducted using PrimeScript RT Reagent Kit (TaKaRa, Japan). qRT‑PCR reaction was performed using LightCycler 2.0 system (Roche, Germany). Data were calculated using the comparative CT method (2−ΔΔCT). The sequences of primers were listed in Table 1.
Table 1.
Primer sequences for PCR amplification.
| Gene | Species | Type | Sequence |
|---|---|---|---|
| xCT | Human | Forward Primer | 5’-CCTCTATTCGGACCCATTTAGT-3’ |
| Reverse Primer | 5’-CTGGGTTTCTTGTCCCATATAA-3’ | ||
| CTH | Human | Forward Primer | 5’-AGGGCTCTCTTCAACATGCT-3’ |
| Reverse Primer | 5’-AAGCCCACTGAGAGTCGAAT-3’ | ||
| GAPDH | Human | Forward Primer | 5’-GGTCACCAGGGCTGCTTT-3’ |
| Reverse Primer | 5’-CTGTGCCGTTGAACTTGC-3’ |
Western blot analysis
Total protein extracts from L02 cells and liver tissues were prepared in RIPA lysis bufer (Beyotime, China). Nuclear proteins were extracted through a Nuclear Extract Kit (Active Motif, USA). Western blotting was performed as previously described36–38. The blots were cut prior to hybridisation with antibodies. The original blots with three replicates were presented in Supplementary Figure.
Expression plasmid or siRNA transfection
The expression vectors for the dominant positive ATF4 (ATF4-OE), xCT (xCT-OE) and CTH (CTH-OE), the empty vector, the siRNA targeting ATF4 (5’-TCCCTCAGTGCATAAAGGA-3’) and the negative control siRNA (si-con) were synthesized by GenePharma (China). The L02 cells were transfected with the different plasmids or si-RNA using Lipofectamine™ 2000 (Invitrogen, USA).
Luciferase reporter assay
The binding sites of ATF4 in xCT or CTH promoters were predicted by JASPAR database. The xCT promoter reporter pGL3-xCT, CTH promoter reporter pGL3-CTH, and the binding sites mutated plasmids were constructed by GenePharma (China). L02 cells were transfected with the different plasmids using Lipofectamine™ 2000 (Invitrogen, USA). Luciferase and renilla activities were measured by Dual-Luciferase Reporter Assay System (Promega, USA).
Statistical analysis
Data were presented as mean ± SD and analyzed using SPSS v22.0 software (SPSS Inc., USA) by Student’s t-test and one-way ANOVA. p < 0.05 was considered statistically significant.
Results
Hepatotoxicity of SA in mouse livers
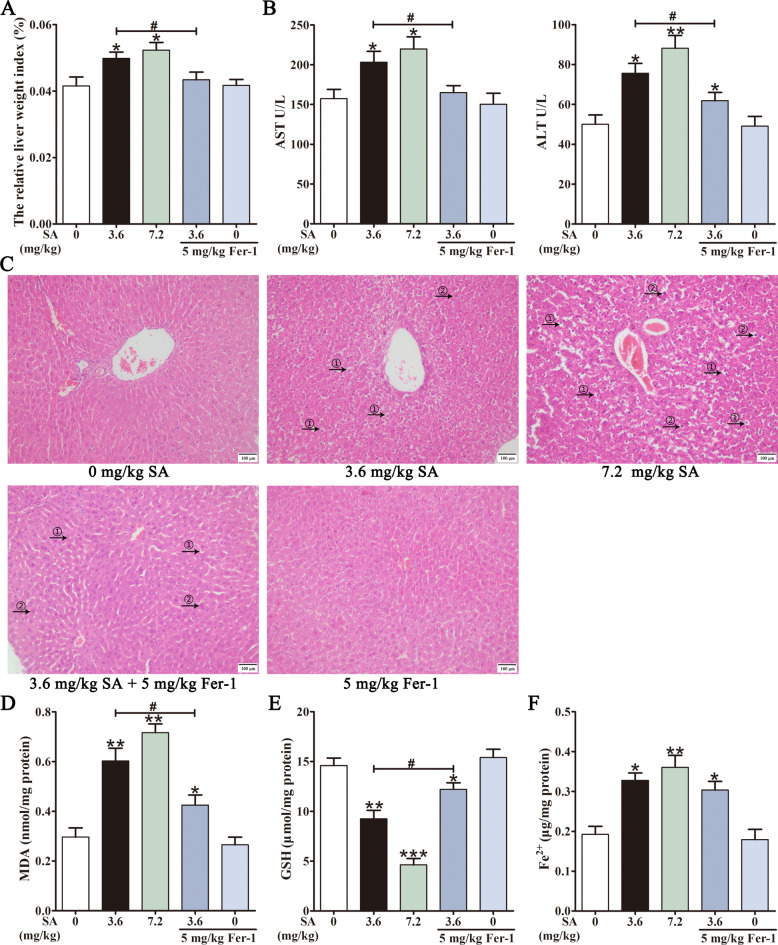
The influence of SA (3.6, 7.2 mg/kg) on the mouse livers was examined after intraperitoneal injection of SA for 10 d. Compared with the control group, SA increased the relative liver weight index (Fig. 1A), elevated ALT and AST levels (Fig. 1B). An overview of histopathological changes in the liver was shown in Fig. 1C. The liver tissues of the control group showed typical lobular architecture, and the hepatic cords were uniformly arranged around the central vein. The liver sections of the SA-treated groups showed significant pathological changes such as a disordered arrangement of hepatocytes, cell oedema, unclear cell boundaries, inflammatory cell infiltration, and hepatocyte regeneration (Fig. 1C). MDA and GSH levels were measured to assess the effects of SA on oxidative stress in the liver. As shown in Fig. 1D-F, SA observably increased MDA, decreased GSH levels, and increased the iron content in the mouse liver. In addition, Fer-1, a ferroptosis inhibitor, markedly decreased SA-elevated liver weight index, AST and ALT levels (Fig. 1A and B). Fer-1 treatment also mitigated SA-induced pathological changes and significantly reduced histological damage (Fig. 1C). Additionally, Fer-1 reversed the changes in MDA and GSH levels under SA conditions (Fig. 1D and E). These results demonstrated that SA causes ferroptosis and liver injury in mice.
Fig. 1.
SA induces ferroptosis in mouse livers. Mice were intraperitoneally (i.p.) injected with vehicle, SA (3.6, 7.2 mg/kg), Fer-1 (5 mg/kg/d) or SA (3.6 mg/kg/d) + Fer-1 (5 mg/kg/d). After 10 d, the relative liver weight index was measured (A). Serum AST and ALT in each group were measured (B). Images of H&E staining (HE) of the liver Sect. (200 × magnification): ① edema of hepatocytes, ② compensatory hypertrophy of liver (C). Hepatic MDA (D), GSH (E) and Fe2+ content (F) were assessed. Values are expressed as the mean ± SD (n = 6). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group.
SA induced cytotoxicity, oxidative stress and ferroptosis in L02 cells
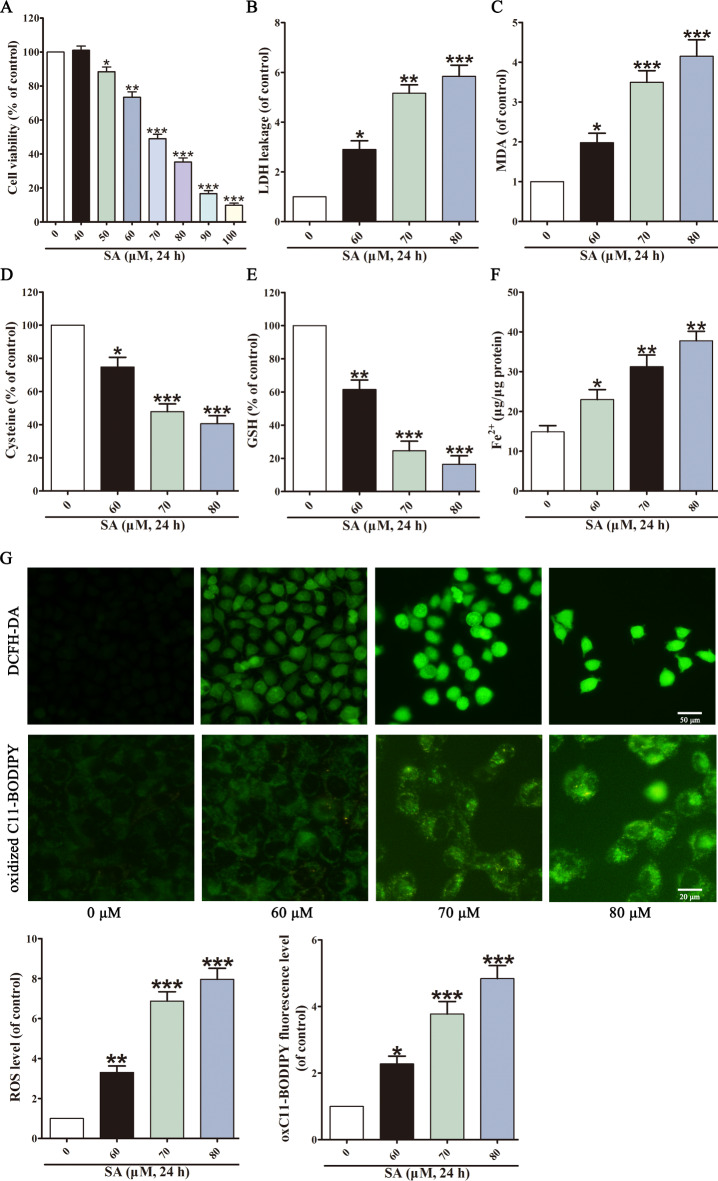
SA-induced hepatotoxicity was investigated in vitro. L02 cells were exposed to various concentrations of SA (0, 40, 50, 60, 70, 80, 90, 100 µM), and the cell viability and cytotoxicity were measured. SA reduced cell viability and released LDH dose-dependent, with an IC50 value of 72.15 ± 3.63 µM (Fig. 2A and B). According to the IC50, 60, 70, and 80 µM SA were selected for subsequent experiments. The results showed that SA markedly increased MDA levels (Fig. 2C), and decreased cysteine (Fig. 2D) and GSH levels (Fig. 2E). In addition, the Fe2+ content was similarly elevated by SA (Fig. 2F). Ferroptosis is characterised by iron-dependent, lipid ROS-related cell death24. DCFH-DA probes were used to measure intracellular ROS levels, and BODIPY 581/591 C11 probes were used to measure lipid peroxidation. Compared with the control group, SA markedly elevated ROS and lipid peroxidation levels (Fig. 2G). These results indicate that SA triggers ferroptosis in liver cells.
Fig. 2.
L02 cells undergo ferroptosis upon SA expose. MTT assay (A) and LDH assay evaluated L02 cell viability after SA stimulation. The levels of MDA (C), cysteine (D), GSH (E) and Fe2+ content (F) in L02 cells were measured. Intracellular ROS was assessed by DCFH-DA probe, and intracellular lipid peroxidation was assessed by BODIPY 581/591 C11 dye (G). Values are expressed as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group.
Ferroptosis inhibitor prevented SA-induced hepatotoxicityin vivo and in vitro
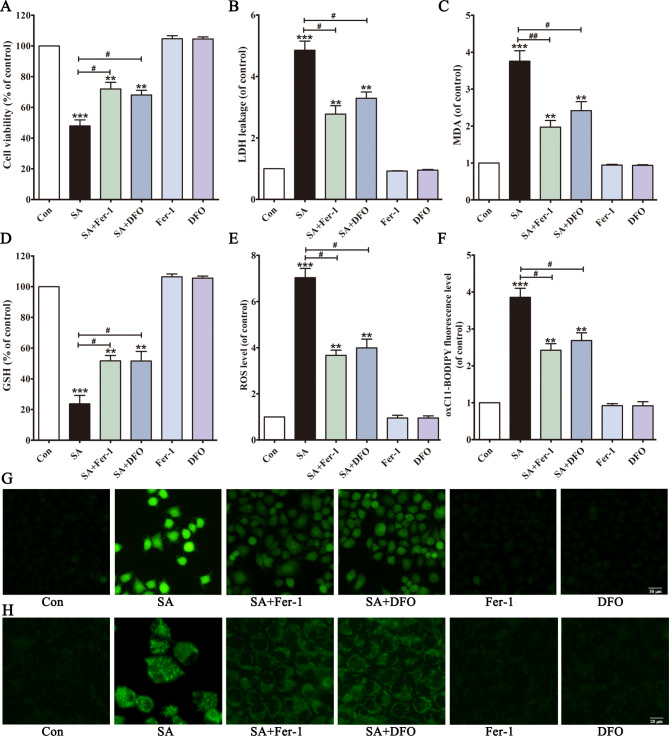
We observed that ferroptosis was involved in SA-induced liver toxicity. The preventive effects of Fer-1 and DFO39 against SA (70 µmol/L) were evaluated. The results showed that Fer-1 and DFO significantly ameliorated SA-induced cell death (Fig. 3A and B). As expected, the cells pretreated with Fer-1 and DFO showed significantly reduced SA-induced MDA accumulation, GSH depletion, ROS generation and lipid peroxidation (Fig. 3C-H). In addition, Fer-1 significantly alleviated SA-induced liver injury in mice (Fig. 1). These results suggested that ferroptosis was an essential contributor to SA-induced hepatotoxicity.
Fig. 3.
Ferroptosis inhibitors alleviate SA-induced cytotoxicity in vitro and in vivo. After pretreated with Fer-1 or DFO, cell viability (A), LDH leakage (B), MDA level (C) and GSH level (D) were detected. Intracellular ROS was assessed by DCFH-DA probe (E, G), and intracellular lipid peroxidation was assessed by BODIPY 581/591 C11 dye (F, H). Values are expressed as the mean ± SD (n = 3). **p < 0.01, ***p < 0.001 vs. control group. #p < 0.05, ##p < 0.01 vs. SA group.
SA induced ferroptosisin vivo and in vitro by regulating ATF4 activity
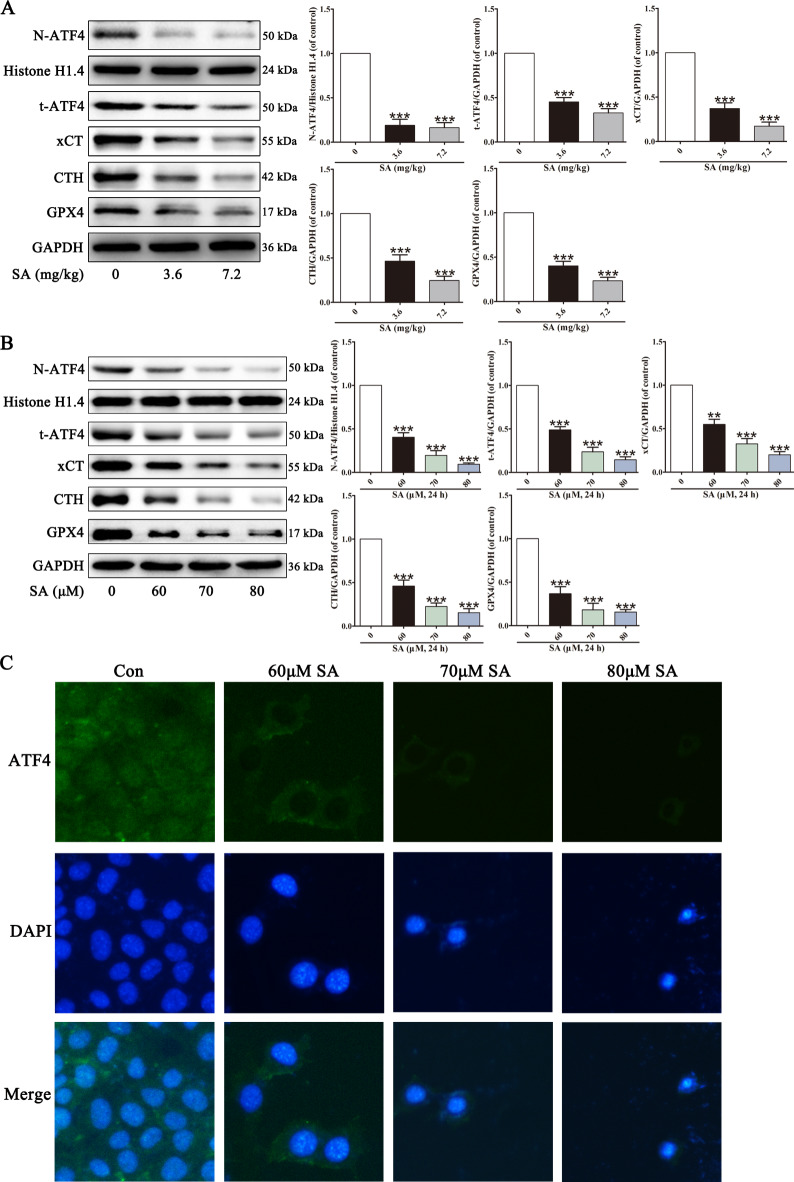
Recent research has shown that ATF4, a critical factor in cell survival and oxidative stress, negatively regulates ferroptosis30,40. As shown in Fig. 4, decreased protein abundance of t-ATF4 and N-ATF4 was observed in SA-treated mice liver and L02 cells, indicating ATF4 inactivation. xCT and CTH are involved in cystine uptake and synthesis, further influencing GSH biosynthesis and GPX4 activity27,41. SA decreased the xCT, CTH, and GPX4 levels. These results suggested that SA-induced ferroptosis is closely related to ATF4 inhibition.
Fig. 4.
SA alters ferroptosis-related proteins in mouse livers and in L02 cells. After different treatments, the abundance of ferroptosis-related proteins N-ATF4, t-ATF4, xCT, CTH and GPX4 in liver tissues (A) and L02 cells (B) were analyzed by western blot. The nuclear translocation of ATF4 in L02 cells was assessed by immunofluorescent staining (C). Values are expressed as the mean ± SD (n = 3). **p < 0.01, ***p < 0.001 vs. control group.
SA suppressed xCT and CTH expression via ATF4-mediated transcription
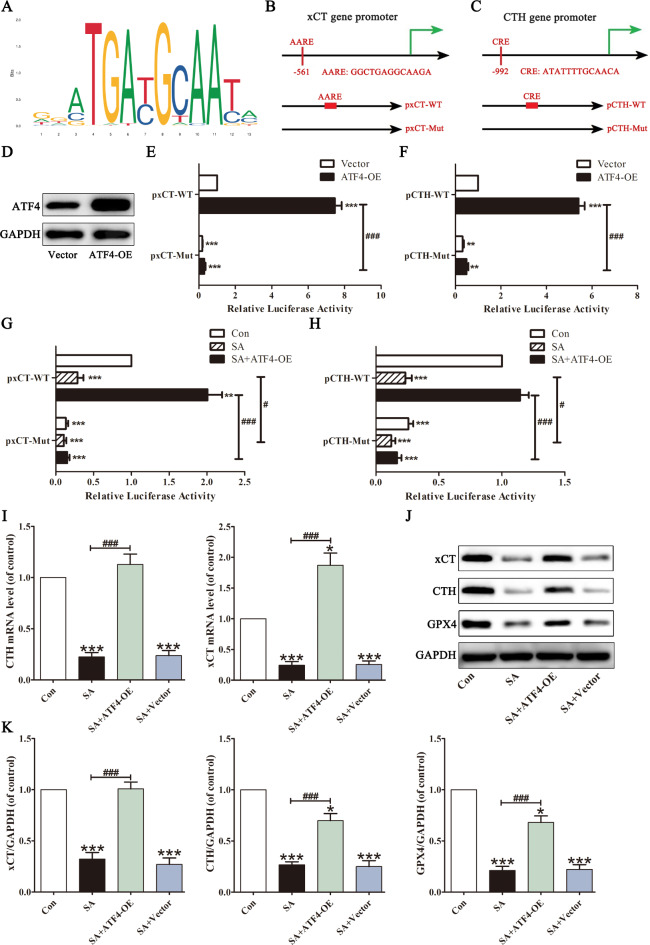
ATF4 binds to various sites in gene promoter regions and regulates various antioxidative genes associated with ferroptosis31. We then attempted to determine whether SA affected xCT and CTH expression in L02 cells via ATF4. ATF4 binding sites were obtained from the JASPAR website (Fig. 5A). After analysing the xCT and CTH promoters, we identified one AARE within 2 kb upstream of the transcription start site of the xCT gene (AARE: GGCTGAGGCAAGA, -561/-573 bp), and one CRE site in the CTH gene promoter (CRE: ATATTTTGCAACA, -992/-1004 bp). Then, site mutations at the vector’s AARE- or CRE-binding site were constructed to determine the significance of the putative AARE or CRE sequence (Fig. 5B and C). We co-transfected the ATF4 plasmid and the xCT or CTH promoters into L02 cells. Western blotting analysis indicated that ATF4 was overexpressed in L02 cells (Fig. 5D). ATF4 overexpression increased the relative luciferase activity of the xCT promoter. Compared with the wild type-vector, the relative luciferase activity was entirely abolished by the AARE mutation (Fig. 5E). Similarly, ATF4 overexpression activated the CTH promoter, and this increased activity was significantly eliminated by CRE mutation (Fig. 5F). These results indicated that the − 561/-573 bp sites of the xCT promoter and the − 992/-1004 bp sites of the CTH promoter play a regulatory role in their transcription.
Fig. 5.
xCT and CTH are regulated by ATF4 in SA-treated L02 cells. A conserved ATF4-binding motif was predicted by JASPAR (A). Schematic images of the potential ATF4 binding sites on the xCT (B) and CTH (C) promoter region were predicted by JASPAR. Solid squares indicate the putative location of the ATF4 binding sites. L02 cells were transiently transfected with ATF4 expression plasmid (ATF4-OE) or the empty vector, and the ATF4 expression levels were measured by Western blot (D). After transfected or not with ATF4 expression plasmid, the predicted AARE site after site-directed mutagenesis in the xCT promoter (E) and predicted CRE site after site-directed mutagenesis in the CTH promoter (F) were analyzed by luciferase activity assays. The effects of SA on ATF4-mediated xCT promoter (G) and CTH promoter (H) activation were determined by luciferase activity assays. The mRNA levels of xCT and CTH were determined (I). xCT, CTH and GPX4 protein levels were determined (J, K). Values are expressed as the mean ± SD (n = 3). *p < 0.05, ***p < 0.001 vs. control group. ###p < 0.001 vs. SA group.
Hence, we investigated the relevance of SA to ATF4 regulation of xCT and CTH. SA significantly decreased ATF4 nuclear translocation from Fig. 4. The transfected cells were then treated with SA (70 µmol/L). As shown in Fig. 5G and H, SA reduced the luciferase activities of the xCT and CTH promoters, whereas ATF4 overexpression increased the SA-decreased binding level of ATF4. Compared to the WT group, the effects of SA and ATF4 overexpression on the recruitment of ATF4 to xCT and CTH promoter were almost abrogated by AARE mutation and CRE mutation, respectively. In addition, the mRNA levels and protein levels of xCT and CTH decreased after SA treatment. ATF4 overexpression improves the ability of ATF4 to activate xCT and CTH expression under SA conditions (Fig. 5I-K). These results suggested that xCT and CTH are ATF4 transcriptional targets and that SA leads to xCT and CTH downregulation by inhibiting ATF4 binding to their promoters.
ATF4 played a pivotal role in SA-induced ferroptosis in L02 cells
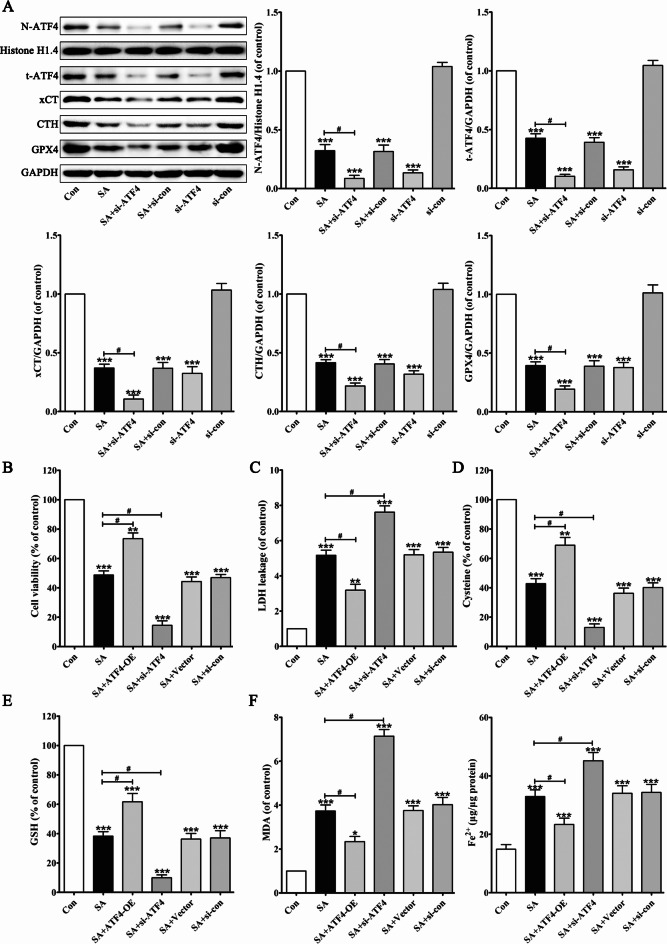
To further validate the effect of ATF4 on SA-induced hepatotoxicity, ATF4 was silenced by small interfering RNA. As shown in Fig. 6A, silencing of ATF4 by siRNA significantly decreased the expression and nuclear translocation of ATF4 and subsequently downregulated the levels of xCT, CTH, and GPX4 in L02 cells. Compared to SA (70 µmol/L) treatment, ATF4 silencing accelerated the inhibitory effects of SA on the ATF4 signalling pathway.
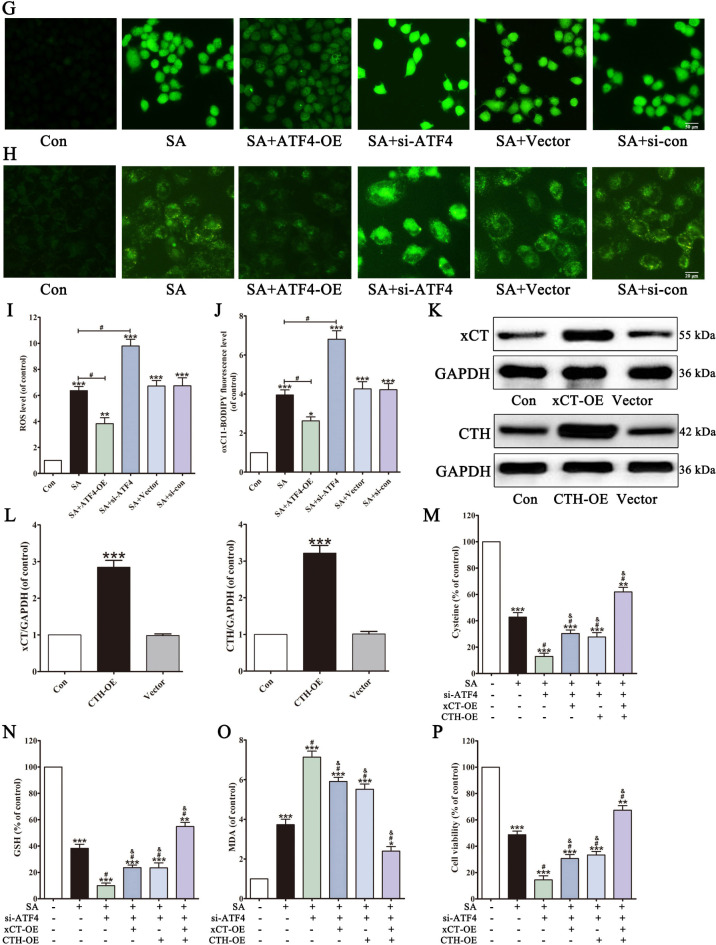
Fig. 6.
ATF4 is involved in SA-induced hepatocyte ferroptosis. After pretreated with ATF4 si-RNA, protein levels of N-ATF4, t-ATF4, xCT, CTH and GPX4 in L02 cells were determined (A). L02 cells were transfected with ATF4 expression plasmid (ATF4-OE) or the empty vector or ATF4 si-RNA or si-con. Cells were then exposed to SA, and cell viability (B), LDH leakage (C), the levels of cysteine (D), GSH (E), MDA and Fe2+ (F), were detected. Intracellular ROS was assessed by DCFH-DA (G, I). Intracellular lipid peroxidation was assessed by BODIPY 581/591 C11 dye (H, J). After transfected with xCT expression plasmid (xCT-OE) or CTH expression plasmid (CTH-OE), the expression levels of xCT or CTH were determined (K, L). L02 cells were transfected with ATF4 si-RNA or not and with xCT expression plasmid (xCT-OE) or CTH expression plasmid (CTH-OE). Cells were then exposed to SA, and the levels of cysteine (M), GSH (N), MDA (O) and cell viability (P) were detected. Values are expressed as the mean ± SD (n = 3). *p < 0.05, **p < 0.01, ***p < 0.001 vs. control group. #p < 0.05 vs. SA group. & p < 0.05 vs. SA + ATF4 si-RNA group.
We examined the effects of ATF4 silencing or ATF4 overexpression on cytotoxicity in SA-treated L02 cells. Notably, ATF4 silencing significantly exacerbated SA-induced cell viability inhibition (Fig. 6B) and LDH release (Fig. 6C). Compared with the SA (70 µmol/L) group, ATF4 silencing accelerated the inhibitory effects of SA on the biosynthesis of cysteine (Fig. 6D) and GSH (Fig. 6E). Additionally, silencing ATF4 increased SA-induced MDA, Fe2+, ROS, and lipid peroxidation synergistically (Fig. 6F-J). However, overexpression of ATF4 reversed SA-induced ferroptosis by improving hepatic antioxidant defense.
In addition, we investigated whether xCT and CTH acted downstream of ATF4 in overcoming SA-induced cytotoxicity and whether them could replace ATF4 activity. After transfecting L02 cells with xCT or CTH overexpression plasmids, Western blot analysis confirmed successful overexpression of both proteins (Fig. 6K and L). Under SA environment, either xCT overexpression or CTH overexpression partially attenuated the accelerated cytotoxicity caused by ATF4 silencing. Only the simultaneous overexpression of xCT and CTH were able to reversed SA-induced ferroptosis in ATF4-deficient L02 cells (Fig. 6M-P). These results suggested that SA induces hepatocyte ferroptosis by inhibiting ATF4 expression.
Discussion
Clinical reports indicated that SA caused liver injury at clinical doses, and adverse reactions were mainly associated with long-term administrative or drug-drug interactions9,11. Therefore, we used L02 cells and BALB/c mice to investigate the mechanisms underlying SA-induced liver damage. SA had a significant cytotoxic effect on L02 cells and caused liver injury in BALB/c mice, accompanied by changes in ferroptosis-related indicators such as increased ROS, lipid peroxidation, and Fe2+ content, and decreased cysteine and GSH level. These results suggested that ferroptosis is involved in SA-induced hepatotoxicity.
Ferroptosis is triggered by excessive iron accumulation and lipid peroxidation and is often associated with excessive ROS, GSH depletion, GPX4 inhibition, and alterations in iron, amino acid, and lipid metabolism genes42,43. The liver plays a crucial role in iron and lipid metabolism and is highly sensitive to oxidative stress24. Ferroptosis is closely associated with various liver disorders, including drug-induced liver injury, liver fibrosis, alcoholic liver injury, nonalcoholic fatty liver disease, hepatic ischemia-reperfusion injury, and liver cancer44,45. Ferrous ion (Fe2+) has high redox potential. Fe2+ generates free radicals from H2O2 through the Fenton reaction46. The increased generation of ROS attacks and oxidises lipids, particularly polyunsaturated fatty acids (PUFAs). ROS reacts with PUFAs and induces lipid peroxidation to form ROS47. MDA is a lipid peroxidation end-product generated by the decomposition of arachidonic acid (AA) and PUFAs48. The accumulation of lipid peroxides in cell membranes destroys membrane integrity and cellular functions, ultimately leading to ferroptosis. GSH is a powerful endogenous antioxidant that scavenges ROS and toxic lipid peroxides to maintain redox homeostasis. GSH depletion is an early hallmark of ferroptosis29. We found that SA decreased cell viability and depleted GSH levels, consistent with previous studies, whereas LDH, MDA, Fe2+, ROS, and lipid peroxidation levels were elevated.
Fer-1 is an effective lipophilic antioxidant49. DFO is an iron-chelating agent with a strong affinity for iron50. Fer-1 and DFO suppress ferroptosis and alleviate liver injury by potentiating GPX4 activity and reducing lipid peroxidation39. L02 cells were pretreated with Fer-1 or DFO to understand the mechanism underlying SA-induced liver injury. As expected, both Fer-1 and DFO significantly reversed the suppression of GSH, reduced lipid peroxidation, improved the integrity of L02 cell membranes, prevented hepatocyte death and improved cell function. In addition, Fer-1 alleviated SA-induced hepatotoxicity in mice. These results support our hypothesis that ferroptosis is involved in SA-induced hepatotoxicity.
ATF4, a key transcription factor, participates in multiple biological processes including proteostasis, cell cycle, energy metabolism, and antioxidant responses and plays a critical role in modulating redox homeostasis and cell survival51. ATF4 can be activated in response to a variety of stresses such as anoxia, starvation, amino acid (cysteine) depletion, endoplasmic reticulum stress, oxidative stress, and exposure to toxic factors52. Recent reports hinted that ATF4 plays a regulatory role in ferroptosis. ATF4 blunts liver damage and suppresses ferroptosis by maintaining GSH production, whereas deletion of ATF4 increases the susceptibility to ferroptosis53,54. In our study, we found that SA downregulated the expression and nuclear translocation of ATF4 both in vivo and in vitro.
ATF4 can positively regulate xCT expression by binding to the promoter region to trigger xCT transcription32. xCT, an amino acid reverse transporter, is an essential gateway for redox homeostasis that transports extracellular cystine into the cytoplasm for cysteine production27. Cysteine is a critical precursor for GSH synthesis, and its uptake rate is considered a limiting factor in the biosynthetic process55. GSH is a substrate of GPX4. GPX4 requires GSH as a cofactor to catalyse the conversion of toxic lipid peroxides into non-toxic alcohols, thereby inhibiting lipid ROS56. Depletion of cysteine and GSH inactivates GPX4, leading to lipid peroxidation, destruction of the cell membrane structure, oxidative damage, and ferroptosis57. Inhibition of xCT blocks cystine uptake, cysteine production, and GSH synthesis, accompanied by GPX4 inactivation and a reduction in cellular antioxidant capacity, ultimately promoting lipid peroxidation-induced ferroptosis58. Our promoter analysis showed that an AARE (amino acid response element) site at -561/-573 bp in the xCT promoter is relative to the transcription start site of the ATF4. SA treatment decreased xCT and cysteine by inhibiting the activity of ATF4, while ATF4 overexpression improved the ability of ATF4 to activate xCT expression.
CTH participates in GSH biosynthesis through the transsulfuration pathway (TSP)59. TSP is the only route for cysteine production60. The first step of TSP is catalysed by cystathionine-synthase, which uses homocysteine to form cystathionine. CTH converts cystathionine to cysteine in the second TSP step61,62. CTH also contributes to selenocysteine (Secys) by providing selenophosphates28,63. Secy can be added to the catalytic centre of GPX4 as the active site. When Secys is deficient, the activity of GPX4 is almost entirely reduced64. Accumulating evidence suggests that CTH can be regulated ATF4 in response to stress31,34. ATF4 drives CTH expression by directly binding to the CRE located in the CTH gene promoter65. Similarly, we found that the − 992/-1004 bp CRE site in the CTH promoter plays a regulatory role in CTH transcription. SA reduced the binding ability of ATF4 to the CRE of the CTH promoter and decreased the expression of CTH. CTH inhibition by SA blocks GSH synthesis and accelerates GPX4 inactivation. These results indicate that ATF4 was responsible for the induction of xCT and CTH in L02 cells and that SA downregulates the expression of xCT and CTH by reducing the transcriptional activity of ATF4, which inhibits the GSH-GPX4 axis and induces hepatocyte ferroptosis.
Consistent with recent studies, we found that ATF4 overexpression counteracted the toxic effects of SA on L02 cells by improving the expression of xCT and CTH. In contrast, knockdown of ATF4 significantly suppressed the xCT and CTH levels, resulted in an imbalance in the antioxidant system and accelerated ferroptosis66,67. It is noteworthy that either xCT overexpression or CTH overexpression in ATF4-deficient cells partially improved cell viability in the presence of SA. This indicated that SA-induced oxidative damage and hepatocyte ferroptosis are closely associated with ATF4, and ATF4 protects against the cytotoxicity of SA by inducing xCT and CTH gene expression. Furthermore, the effects of ATF4 on SA-induced hepatotoxicity will be explored in ATF4-transgenic mouse models to understand the mechanism of SA toxicity better.
Conclusion
In summary, our results clearly demonstrated that SA induced ferroptosis in both L02 cells and mouse livers. SA inhibited the GSH-GPX4 antioxidant system by suppressing ATF4-mediated transcription of genes involved in GSH synthesis in hepatocytes. Disruption of redox homeostasis induced by SA intensified oxidative stress and triggered ferroptosis. ATF4 overexpression alleviated SA-induced hepatotoxicity, whereas ATF4 silencing accelerated cell death. These findings suggested that ATF4 may be a prospective target to protect against SA-induced ferroptosis and liver toxicity.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
Conceptualization and writing-original draft, Chen Xi; supervision, writing-review & editing, Jie Zhou; investigation, Xin Zheng; data curation, Xiaoyi Fu; formal analysis and methodology, Minjuan Xie.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81660593, No. 82460815).
Data availability
Data will be made available from the corresponding author (Dr. Jie Zhou, zj9032@126.com) upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The animal study was reviewed and approved by the administration committee of experimental animals of Yichun University (Approval No: IACUC-2023029).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang, L. et al. Sodium aescinate provides neuroprotection in experimental traumatic brain injury via the Nrf2-ARE pathway. Brain Res. Bull.157, 26–36 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Xu, F. et al. Sodium aescinate inhibits microglia activation through NF-kappaB pathway and exerts neuroprotective effect. Front. Pharmacol.14, 1086429 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang, J. et al. Solid lipid nanoparticles as an effective sodium aescinate delivery system: formulation and anti-inflammatory activity. RSC Adv.12 (11), 6583–6591 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang, X. J. et al. Sodium aescinate and its bioactive components induce degranulation via oxidative stress in RBL-2H3 mast cells. Toxicol. Res. (Camb). 9 (4), 413–424 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li, L. et al. Anti-proliferation and apoptosis-inducing effects of sodium aescinate on retinoblastoma Y79 cells. Int. J. Ophthalmol.13 (10), 1546–1553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, J. et al. FKA-A NPs enhances PTX-A NPs efficacy to suppress ovarian cancer via regulating Skp2/YAP pathway. Fundam Clin. Pharmacol.37 (1), 125–136 (2023). [DOI] [PubMed] [Google Scholar]
- 7.Huang, S. et al. Modification of sodium aescinate into a safer, more stable and effective water-soluble drug by liposome-encapsulation: an in vitro and in vivo study. Drug Deliv. 29 (1), 1132–1141 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan, Y. Analysis of related factors of severe adverse reactions Induced by Sodium Aescinate for Injection (in Chinese). Strait Pharm. J.29 (7), 262–264 (2017). [Google Scholar]
- 9.Ji, H. et al. Adverse event due to a likely interaction between sodium aescinate and ginkgo biloba extract: a case report. J. Clin. Pharm. Ther.42 (2), 237–238 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Deng, Y. Acute hepatorenal damage following intravenous drip of sodium aescinate injection (in Chinese). Adverse Drug React. J.9 (1), 58–59 (2007). [Google Scholar]
- 11.Yu, Y. et al. Analysis and consideration on risk of Sodium Aescinate for Injection (in Chinese). Chin. J. Pharmacovigil.18 (2), 174–177 (2021). [Google Scholar]
- 12.Liang, J. et al. In vivo Cardiotoxicity Induced by Sodium Aescinate in zebrafish larvae. Molecules. 21 (3), 190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, Y. et al. Computational and experimental characterization of isomers of escin-induced renal cytotoxicity by inhibiting heat shock proteins. Eur. J. Pharmacol.908, 174372 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Liu, Y. & Xing, A. Study on hepatorenal toxicity of sodium aesculin in mice (in Chinese). Asia-Pacific Traditional Med.13 (16), 6–7 (2017). [Google Scholar]
- 15.Zhang, X. et al. Oxidative mechanism for toxicity induced by sodium aescinate in HK-2 cells (in Chinese). Chin. J. Pharmacol. Toxicol.24 (6), 471–475 (2010). [Google Scholar]
- 16.Zhao, C. et al. The effect of acute toxicity from tributyltin on Liza Haematocheila liver: Energy metabolic disturbance, oxidative stress, and apoptosis. Aquat. Toxicol.258, 106506 (2023). [DOI] [PubMed] [Google Scholar]
- 17.Banerjee, P. et al. Oxidative stress-Induced Liver damage and remodeling of the liver vasculature. Am. J. Pathol.193 (10), 1400–1414 (2023). [DOI] [PubMed] [Google Scholar]
- 18.Li, Q. et al. Pectolinarigenin ameliorates acetaminophen-induced acute liver injury via attenuating oxidative stress and inflammatory response in Nrf2 and PPARa dependent manners. Phytomedicine. 113, 154726 (2023). [DOI] [PubMed] [Google Scholar]
- 19.Wang, X. et al. Matrine disrupts Nrf2/GPX4 antioxidant system and promotes hepatocyte ferroptosis. Chem. Biol. Interact.384, 110713 (2023). [DOI] [PubMed] [Google Scholar]
- 20.Wang, Y. et al. HBx-Induced HSPA8 stimulates HBV replication and suppresses ferroptosis to support Liver Cancer Progression. Cancer Res.83 (7), 1048–1061 (2023). [DOI] [PubMed] [Google Scholar]
- 21.Chen, L. D. et al. The role of ferroptosis in chronic intermittent hypoxia-induced liver injury in rats. Sleep. Breath.24 (4), 1767–1773 (2020). [DOI] [PubMed] [Google Scholar]
- 22.An, Y. et al. Abietic acid inhibits acetaminophen-induced liver injury by alleviating inflammation and ferroptosis through regulating Nrf2/HO-1 axis. Int. Immunopharmacol.118, 110029 (2023). [DOI] [PubMed] [Google Scholar]
- 23.Cui, J. et al. 4-tert-butylphenol triggers common carp hepatocytes ferroptosis via oxidative stress, iron overload, SLC7A11/GSH/GPX4 axis, and ATF4/HSPA5/GPX4 axis. Ecotoxicol. Environ. Saf.242, 113944 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Cheng, Z. et al. Ferroptosis in non-alcoholic liver disease: molecular mechanisms and therapeutic implications. Front. Nutr.10, 1090338 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu, L. et al. The role of Iron metabolism, lipid metabolism, and Redox Homeostasis in Alzheimer’s Disease: from the perspective of Ferroptosis. Mol. Neurobiol.60 (5), 2832–2850 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Chang, X. & Miao, J. Ferroptosis: mechanism and potential applications in cervical cancer. Front. Mol. Biosci.10, 1164398 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee, J. & Roh, J. L. SLC7A11 as a gateway of metabolic perturbation and ferroptosis vulnerability in Cancer. Antioxid. (Basel)11(12) (2022). [DOI] [PMC free article] [PubMed]
- 28.Ahola, S. et al. OMA1-mediated integrated stress response protects against ferroptosis in mitochondrial cardiomyopathy. Cell. Metab.34 (11), 1875–1891e7 (2022). [DOI] [PubMed] [Google Scholar]
- 29.Tan, M. et al. Glutathione system enhancement for cardiac protection: pharmacological options against oxidative stress and ferroptosis. Cell. Death Dis.14 (2), 131 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He, F. et al. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. (2023). [DOI] [PMC free article] [PubMed]
- 31.Bai, X. et al. Activation of the eIF2alpha/ATF4 axis drives triple-negative breast cancer radioresistance by promoting glutathione biosynthesis. Redox Biol.43, 101993 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen, D. et al. ATF4 promotes angiogenesis and neuronal cell death and confers ferroptosis in a xCT-dependent manner. Oncogene. 36 (40), 5593–5608 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carvajal, P. et al. The integrated stress response is activated in the salivary glands of Sjogren’s syndrome patients. Front. Med. (Lausanne). 10, 1118703 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Statzer, C. et al. ATF-4 and hydrogen sulfide signalling mediate longevity in response to inhibition of translation or mTORC1. Nat. Commun.13 (1), 967 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dickhout, J. G. et al. Integrated stress response modulates cellular redox state via induction of cystathionine gamma-lyase: cross-talk between integrated stress response and thiol metabolism. J. Biol. Chem.287 (10), 7603–7614 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, T. et al. Apigenin attenuates mesoporous silica nanoparticles-Induced Nephrotoxicity by activating FOXO3a. Biol. Trace Elem. Res.200 (6), 2793–2806 (2022). [DOI] [PubMed] [Google Scholar]
- 37.Wang, T. et al. Matrine-induced nephrotoxicity via GSK-3beta/nrf2-mediated mitochondria-dependent apoptosis. Chem. Biol. Interact., 110492 (2023). [DOI] [PubMed]
- 38.Wang, T. et al. Phillyrin ameliorates diabetic nephropathy through the PI3K/Akt/GSK-3beta signalling pathway in streptozotocin-induced diabetic mice. Hum. Exp. Toxicol.40 (12_suppl), S487–S496 (2021). [DOI] [PubMed] [Google Scholar]
- 39.Du, Y. & Guo, Z. Recent progress in ferroptosis: inducers and inhibitors. Cell. Death Discov. 8 (1), 501 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, Y. et al. Ferroptosis Signaling and regulators in atherosclerosis. Front. Cell. Dev. Biol.9, 809457 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Werge, M. P. et al. The role of the Transsulfuration pathway in non-alcoholic fatty liver disease. J. Clin. Med.10(5) (2021). [DOI] [PMC free article] [PubMed]
- 42.Li, L. & Zhu, Z. Pharmacological modulation of ferroptosis as a therapeutic target for liver fibrosis. Front. Pharmacol.13, 1071844 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang, Y. et al. Ferroptosis and its interaction with tumor immune microenvironment in liver cancer. Biochim. Biophys. Acta Rev. Cancer. 1878 (1), 188848 (2023). [DOI] [PubMed] [Google Scholar]
- 44.Xiang, X. et al. The advancements in targets for ferroptosis in liver diseases. Front. Med. (Lausanne). 10, 1084479 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu, L. et al. The emerging role of ferroptosis in various Chronic Liver diseases: opportunity or challenge. J. Inflamm. Res.16, 381–389 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Babaei-Abraki, S., Karamali, F. & Nasr-Esfahani, M. H. Ferroptosis: The functions of Nrf2 in human embryonic stem cells. Cell Signal 106, 110654 (2023). [DOI] [PubMed]
- 47.Mortensen, M. S., Ruiz, J. & Watts, J. L. Polyunsaturated fatty acids drive lipid peroxidation during Ferroptosis. Cells12(5) (2023). [DOI] [PMC free article] [PubMed]
- 48.Rui, T. et al. Ferroptosis-relevant mechanisms and biomarkers for therapeutic interventions in traumatic brain injury. Histol. Histopathol. 35 (10), 1105–1113 (2020). [DOI] [PubMed] [Google Scholar]
- 49.Zhou, Q. et al. Mechanisms and inhibitors of ferroptosis in psoriasis. Front. Mol. Biosci.9, 1019447 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen, Y. et al. Targeting Iron Metabolism and Ferroptosis as Novel Therapeutic approaches in Cardiovascular diseases. Nutrients15(3) (2023). [DOI] [PMC free article] [PubMed]
- 51.Wortel, I. M. N. et al. Surviving stress: modulation of ATF4-Mediated stress responses in normal and malignant cells. Trends Endocrinol. Metab.28 (11), 794–806 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Neill, G. & Masson, G. R. A stay of execution: ATF4 regulation and potential outcomes for the integrated stress response. Front. Mol. Neurosci.16, 1112253 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen, Y. et al. Dihydroartemisinin-induced unfolded protein response feedback attenuates ferroptosis via PERK/ATF4/HSPA5 pathway in glioma cells. J. Exp. Clin. Cancer Res.38 (1), 402 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffiths, A. et al. ATF4-mediated CD36 upregulation contributes to palmitate-induced lipotoxicity in hepatocytes. Am. J. Physiol. Gastrointest. Liver Physiol.324 (5), G341–G353 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paul, B. D. Cysteine metabolism and hydrogen sulfide signaling in Huntington’s disease. Free Radic Biol. Med.186, 93–98 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen, M. et al. Prospects for Anti-tumor mechanism and potential clinical application based on glutathione peroxidase 4 Mediated Ferroptosis. Int. J. Mol. Sci.24(2) (2023). [DOI] [PMC free article] [PubMed]
- 57.Rochette, L. et al. Lipid peroxidation and Iron metabolism: two Corner stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci.24(1) (2022). [DOI] [PMC free article] [PubMed]
- 58.Fan, G. et al. The initiator of neuroexcitotoxicity and ferroptosis in ischemic stroke: glutamate accumulation. Front. Mol. Neurosci.16, 1113081 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thanki, K. K. et al. Deletion of cystathionine-gamma-lyase in bone marrow-derived cells promotes colitis-associated carcinogenesis. Redox Biol.55, 102417 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen, Y. & Betenbaugh, M. J. Reconstruction of reverse transsulfuration pathway enables cysteine biosynthesis and enhances resilience to oxidative stress in Chinese Hamster ovary cells. Metab. Eng.76, 204–214 (2023). [DOI] [PubMed] [Google Scholar]
- 61.Petrosino, M. et al. H(2)S biogenesis by cystathionine beta-synthase: mechanism of inhibition by aminooxyacetic acid and unexpected role of serine. Cell. Mol. Life Sci.79 (8), 438 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cano-Galiano, A. et al. Cystathionine-gamma-lyase drives antioxidant defense in cysteine-restricted IDH1-mutant astrocytomas. Neurooncol Adv.3 (1), vdab057 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Seale, L. A. et al. Relationship between selenoprotein P and selenocysteine lyase: insights into selenium metabolism. Free Radic Biol. Med.127, 182–189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimada, B. K. et al. Metabolism of Selenium, Selenocysteine, and Selenoproteins in Ferroptosis in Solid Tumor Cancers. Biomolecules 12(11) (2022). [DOI] [PMC free article] [PubMed]
- 65.Takahashi, M. et al. Synthesis of reactive Sulfur species in cultured vascular endothelial cells after exposure to TGF-beta(1): induction of Cystathionine gamma-lyase and cystathionine beta-synthase expression mediated by the ALK5-Smad2/3/4 and ALK5-Smad2/3-ATF4 pathways. Int. J. Mol. Sci.22(21) (2021). [DOI] [PMC free article] [PubMed]
- 66.Gao, R. et al. YAP/TAZ and ATF4 drive resistance to Sorafenib in hepatocellular carcinoma by preventing ferroptosis. EMBO Mol. Med.13 (12), e14351 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang, Y. et al. Golgi Stress Response, hydrogen Sulfide Metabolism, and intracellular calcium homeostasis. Antioxid. Redox Signal.32 (9), 583–601 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available from the corresponding author (Dr. Jie Zhou, zj9032@126.com) upon reasonable request.