Abstract
Aims: Previous evidence suggests that serum lung cancer biomarkers are associated with inflammatory conditions; however, their relationship with peripheral arterial stiffness remains unclear. Therefore, the present study investigated the relationship between serum lung cancer biomarkers and peripheral arterial stiffness in middle-aged Chinese adults.
Methods: In total, 3878 middle-aged Chinese adults were enrolled in this study. Increased peripheral arterial stiffness was assessed using the brachial-ankle pulse wave velocity and ankle-brachial index. Univariate and multivariate logistic regression analyses were used to determine the independent effects of serum lung cancer biomarkers on the risk of increased peripheral arterial stiffness. A receiver operating characteristic curve analysis was used to assess the diagnostic ability of serum lung cancer biomarkers in distinguishing increased peripheral arterial stiffness.
Results: Serum levels of carcinoembryonic antigen (CEA), neuron-specific enolase (NSE), cytokeratin-19 fragment 21-1, and pro-gastrin-releasing peptide were higher in subjects with increased peripheral arterial stiffness than in those without (P<0.05). After adjusting for other risk factors, serum CEA and NSE levels were found to be independently associated with increased peripheral arterial stiffness. The corresponding adjusted odds ratios (ORs) for increased peripheral arterial stiffness in CEA level quartiles were 1.00, 1.57, 2.15, and 6.13. The ORs for increased peripheral arterial stiffness in the quartiles of NSE levels were 1.00, 4.92, 6.65, and 8.01.
Conclusions: Increased serum CEA and NSE levels are closely linked to increased peripheral arterial stiffness, and high serum CEA and NSE levels are potential risk markers for peripheral arterial stiffness in middle-aged Chinese adults.
Keywords: Carcinoembryonic antigen, Neuron-specific enolase, Peripheral arterial stiffness, Middle-aged Chinese adults
Yun Li and Jian-Wei Gu contributed equally to this work.
Introduction
Peripheral arterial stiffness is a primary risk factor for cardiovascular disease and is associated with atherosclerosis. It can predict the risk of future fatal and nonfatal cardiovascular disease events (e.g. heart failure and myocardial infarction) based on the results of individual studies 1 - 4) . The brachial–ankle pulse wave velocity (baPWV) is a composite measure of central and peripheral arterial stiffness, which is considered the gold standard for assessing arterial stiffness. It has been widely adopted in routine health checkups 5) . The pathology of peripheral arterial stiffness is multifactorial and includes a reduced elastin-to-collagen ratio, increased crosslinking of elastin, vascular smooth muscle cell stiffness, and vascular endothelial dysfunction 6) . Chronic inflammatory conditions are a risk factor for peripheral arterial stiffness, which has been well established by numerous studies 7 - 9) .
Carcinoembryonic antigen (CEA), squamous cell carcinoma-associated antigen (SCCA), cytokeratin-19 fragment 21-1 (CYFRA 21-1), neuron-specific enolase (NSE), and pro-gastrin-releasing peptide (proGRP) are widely used in clinical management to select subjects with a high risk of lung cancer because of their high specificity and sensitivity for the presence and type of lung cancer 10) . Furthermore, numerous studies have reported that these tumor biomarkers are associated with various inflammatory conditions within normal or near-normal ranges. For instance, serum CEA is closely associated with oxidative stress and tissue injury 11) . CYFRA 21-1 is positively correlated with interstitial lung diseases 12) , and serum NSE is positively correlated with the degree of inflammation in patients with Crohn’s disease 13) . Nevertheless, the relationships between these tumor biomarkers and peripheral arterial stiffness have not been fully studied. Middle-aged people who face great social, economic, and work pressure are at a high risk of lung cancer and peripheral arterial stiffness.
Aims
The present study investigated whether or not the aforementioned lung cancer biomarkers are associated with increased peripheral arterial stiffness independently of traditional risk factors in middle-aged Chinese adults via a cross-sectional large-sample study.
Methods
Study Populations
The study participants were individuals between 45 and 65 years old who visited the Health Management Center for a routine health checkup on a voluntary basis between March 2022 and December 2023 (n=9883). Subjects with available data on both levels of biomarkers of lung cancer (including CEA, SCCA, CYFRA 21-1, NSE, and proGRP) for the screening of lung cancer and baPWV for the detection of peripheral arterial stiffness as components of the health checkup list were voluntarily selected for the analysis (n=4780). From among these participants, we excluded those who had severe vascular disease and lower-extremity vascular stent implantation (n=189), severe hepatic and renal insufficiency (n=234), amputation (n=3), or hemodialysis or kidney transplantation (n=121). Subjects who were diagnosed with malignant tumors, had abnormal findings for a high risk of lung cancer on chest radiography or computed tomography (CT) (n=156, before or during this health checkup), or refused to participate in the study (n=199) were also excluded. A total of 3878 subjects were thus ultimately enrolled in this study.
All procedures performed in the study were in accordance with the ethical standards of the institutional research committee and the 1964 Declaration of Helsinki and its later amendments. This study was approved by the Ethics Committee of the Affiliated Hospital of North Sichuan Medical College, and written informed consent was obtained from all participants included in the study.
Clinical Characteristics
All subjects underwent a routine physical examination and completed detailed questionnaires to establish a clinical database, which included the age, sex, body mass index (BMI), medical history of hypertension and diabetes mellitus, and smoking status. The BMI was calculated as weight (kg) / height (cm)2. Blood pressure was measured after the subjects had rested for at least 5 min. Smoking status was defined as positive if the participant had ever smoked or was a current smoker.
Measurement of Laboratory Indicators
Fasting venous blood samples were collected and immediately analyzed at the clinical laboratory of the Affiliated Hospital of North Sichuan Medical College to determine the serum levels of fasting blood glucose (FBG), total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), creatinine, uric acid, and high-sensitivity C-reactive protein (hs-CRP), white blood cell (WBC) count, neutrophil count, lymphocyte count, and monocyte count. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the number of neutrophils by the number of lymphocytes, while the lymphocyte-to-monocyte ratio (LMR) was calculated by dividing the number of lymphocytes by the number of monocytes. The levels of lung cancer biomarkers were measured by a chemiluminescence immunoassay using a Cobase 602 analyzer (Roche, Germany).
Measurement of the baPWV
The baPWV was measured using an automatic waveform analyzer (BP-203RPE III; Colin, Komaki, Japan), as reported in a previous study 14) . The baPWV value was calculated as distance/time (cm/s). The time delay between the arrival of the pulse wave at the brachium and ankle on each side was measured automatically by gating the pulse wave to the peak of the R wave on an electrocardiogram. The distance between the brachium and ankle on each side was estimated from the body height. After bilateral determination of the baPWV, the average of the right and left baPWV values was used to assess arterial stiffness 15) . The ankle-brachial pressure index (ABI) was also recorded, which was measured concurrently with the baPWV. Increased peripheral arterial stiffness was defined as a baPWV ≥ 1400 cm/s or ABI ≤ 0.9 or >1.4 16) .
Statistical Analyses
Data are expressed as mean±standard deviation for continuous variables with normal distributions, median (interquartile range) for continuous variables with skewed distributions, and frequencies (proportions) for categorical variables. An independent Student’s t-test, chi-squared test, and Mann–Whitney U-test were conducted to assess statistically significant differences between the subjects with and without increased peripheral arterial stiffness. Univariate and multivariate logistic regression analyses were performed to investigate the independent risk factors for increased peripheral arterial stiffness. Collinearity statistics were used to assess collinearity of the parameters used in the multivariate models, and values >10 for the VIF and <0.1 for the tolerance were considered to require action.
The clinical and laboratory indicators of study subjects according to quartiles of CEA or NSE were further compared using a one-way analysis of variance with a post hoc analysis by Bonferroni for normally distributed data and Mann-Whitney U test for parameters with skewed distribution. A receiver operating characteristic curve analysis was used to assess the diagnostic ability of the lung cancer biomarkers to distinguish increased peripheral arterial stiffness. The optimal cutoff value was defined as the value corresponding to the maximum sum of the sensitivity and specificity.
Statistical analyses were performed using the standard statistical software package SPSS Statistics for Windows version 22.0 (International Business Machines Corporation). All reported P-values were two-sided for consistency, and a value of P<0.05 was considered statistically significant.
Results
Clinical Characteristics of Study Subjects
The eligible subjects had a mean age of 52±5 years old, 36.7% were female, no subjects were diagnosed with cancer during these health checkups, and all lung cancer biomarkers were within the normal or near normal range in the present study. The clinical data, medical histories, and levels of metabolic and inflammatory indicators are summarized in Table 1 . In addition, the age, proportion of males, BMI, blood pressure, rate of a medical history of hypertension and diabetes mellitus, proportion of smokers, and serum levels of metabolic and inflammatory indicators (except TC and LDL-C) were all higher in subjects with increased peripheral arterial stiffness than in those without increased peripheral arterial stiffness (all P<0.05). Similarly, serum levels of the lung cancer biomarkers CEA, NSE, CYFRA 21-1, and proGRP were higher in subjects with increased peripheral arterial stiffness than in those without increased peripheral arterial stiffness (all P<0.01). However, the serum levels of SCCA were not significantly different between the groups (P>0.05).
Table 1. Comparison of clinical and laboratory indicators of study subjects with and without increased peripheral arterial stiffness.
| Variable | Subjects without increased peripheral arterial stiffness (n= 1970) | Subjects with increased peripheral arterial stiffness (n= 1908) | P |
|---|---|---|---|
| Demographic data | |||
| Age, (years) | 51.05±4.42 | 53.65±5.07 | <0.01 |
| Male, n (%) | 1073 (54.5) | 1383 (72.5) | <0.01 |
| BMI (kg/m2) | 24.49±2.91 | 25.52±2.97 | <0.01 |
| SBP (mmHg) | 116.37±11.22 | 134.99±15.65 | <0.01 |
| DBP(mmHg) | 71.75±8.72 | 84.36±10.39 | <0.01 |
| Medical history | |||
| Hypertension, n (%) | 83 (4.2) | 797 (41.8) | <0.01 |
| Smoker, n (%) | 46 (2.3) | 455 (23.8) | <0.01 |
| Diabetes, n (%) | 92 (4.6) | 275 (14.4) | <0.01 |
| Blood chemistry | |||
| FBG (mmol/L) | 5.07±1.17 | 5.72±1.83 | <0.01 |
| TC (mmol/L) | 4.82±0.58 | 4.81±0.58 | 0.50 |
| TG (mmol/L) | 1.12±0.33 | 1.21±0.33 | <0.01 |
| LDL-C (mmol/L) | 2.72±0.40 | 2.74±0.39 | 0.31 |
| HDL-C(mmol/L) | 1.24±0.19 | 1.20±0.19 | <0.01 |
| Creatinine (umol/L) | 69.62±14.33 | 73.01±13.87 | <0.01 |
| Uric acid (umol/L) | 310.1±64.5 | 327.3±58.7 | <0.01 |
| Serum levels of inflammatory factors | |||
| WBC (109/L) | 5.68±1.23 | 6.07±1.32 | <0.01 |
| NLR, (%) | 1.98±0.76 | 2.01±0.78 | <0.01 |
| LMR, (%) | 6.09±2.30 | 5.87±1.94 | <0.01 |
| hs-CRP (mg/L) | 0.43 (017, 0.98) | 0.66 (0.28, 1.47) | <0.01 |
| Serum levels of biomarkers of lung cancer | |||
| CEA, ug/l | 0.79±0.54 | 1.57±0.94 | <0.01 |
| NSE, ug/L | 12.29±3.08 | 15.16±3.23 | <0.01 |
| CYFRA21-1, ng/ml | 2.11±0.66 | 2.24±0.49 | <0.01 |
| SCCA, ng/ml | 1.16±0.47 | 1.16±0.37 | 0.55 |
| proGRP, pg/ml | 40.45±10.57 | 43.36±8.34 | <0.01 |
Abbreviations: BMI, body mass index; CEA, carcinoembryonic antigen; DBP, diastolic blood pressure; CYFRA 21-1, cytokeratin-19 fragment 21-1; FBG, fast blood glucose; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high sensitivity C protein; LDL-C, low-density lipoprotein cholesterol; LMR, lymphocyte-to- monocyte ratio; NLR, neutrophil-to lymphocyte ratio; NSE, neuron-specific enolase; proGRP , pro-gastrin- releasing peptide; SBP, systolic blood pressure; SCCA, squamous cell carcinoma-associated antigen; TC, total cholesterol; TG, triglyceride; WBC, white blood cell.
Potential Risk Factors of Increased Peripheral Arterial Stiffness
The potential risk factors for increased peripheral arterial stiffness are shown in Table 2 . A univariate logistic regression analysis revealed that the age, proportion of males, BMI, blood pressure, rate of a medical history of hypertension and diabetes mellitus, proportion of smokers, and serum levels of metabolic indicators (FBG, TG, HDL-C, creatinine, and uric acid) and inflammatory indicators (WBC count, NLR, LMR, and hs-CRP levels) were all risk factors for increased peripheral arterial stiffness (all P<0.05). Similarly, serum levels of the lung cancer biomarkers CEA, NSE, CYFRA 21-1, and proGRP were risk factors for increased peripheral arterial stiffness (all P<0.05). However, serum SCCA levels were not associated with increased peripheral arterial stiffness (P>0.05).
Table 2. Evaluation of potential risk factors of increased peripheral arterial stiffness.
| Variable | Univariate analysis | multivariate analysis | ||
|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | P | ||
| Age, (years) | 1.12 (1.11-1.34) | <0.01 | 1.13 (1.08-1.18) | <0.01 |
| Male, n (%) | 2.20 (1.93-2.52) | <0.01 | 1.07 (0.50-2.29) | 0.87 |
| BMI (kg/m2) | 1.13 (1.10-1.15) | <0.01 | 0.89 (0.82-0.97) | 0.01 |
| SBP (mmHg) | 1.12 (1.11-1.12) | <0.01 | 1.06 (1.03-1.09) | <0.01 |
| DBP (mmHg) | 1.15 (1.14-1.16) | <0.01 | 1.08 (1.04-1.12) | <0.01 |
| Hypertension, n (%) | 16.31 (12.86-20.69) | <0.01 | 0.81 (0.25-2.66) | 0.73 |
| Smoker, n (%) | 13.09 (9.60-17.87) | <0.01 | 2.95 (0.67-13.03) | 0.15 |
| Diabetes, n (%) | 3.55 (2.78-4.54) | <0.01 | 3.07 (0.28-34.07) | 0.36 |
| FBG (mmol/L) | 1.35 (1.28-1.42)) | <0.01 | 0.85 (0.55-1.32) | 0.48 |
| TC (mmol/L) | 0.96 (0.84-1.09) | 0.50 | NA | |
| TG (mmol/L) | 2.29 (1.80-2.92) | <0.01 | 1.30 (0.67-2.52) | 0.43 |
| LDL-C (mmol/L) | 1.11 (0.91-1.35) | 0.32 | NA | |
| HDL-C (mmol/L) | 0.33 (0.22-0.49) | <0.01 | 0.69 (0.21-2.36) | 0.56 |
| Creatinine (umol/L) | 1.02 (1.01-1.02) | <0.01 | 1.00 (0.98-1.02) | 0.99 |
| Uric acid (umol/L) | 1.00 (1.00-1.01) | <0.01 | 0.99 (0.99-1.00) | 0.01 |
| WBC (109/L) | 1.28 (1.21-1.34) | <0.01 | 1.00 (0.82-1.22) | 0.99 |
| NLR, (%) | 1.15 (1.06-1.25) | <0.01 | 1.12 (0.79-1.58) | 0.54 |
| LMR, (%) | 0.95 (0.92-0.98) | <0.01 | 1.00 (0.87-1.14) | 0.98 |
| hs-CRP (mg/L) | 1.24 (1.16-1.32) | <0.01 | 1.33 (0.88-1.99) | 0.17 |
| CEA, ug/l | 4.17 (3.73-4.66) | <0.01 | 2.38 (1.72-3.30) | <0.01 |
| NSE, ug/L | 1.40 (1.36-1.44) | <0.01 | 1.26 (1.17-1.35) | <0.01 |
| CYFRA21-1, ng/ml | 1.44 (1.291.61) | <0.01 | 1.06 (0.65-1.72) | 0.82 |
| SCCA, ng/ml | 1.02 (0.88-1.19) | 0.89 | NA | |
| proGRP, pg/ml | 1.03 (1.03-1.04) | <0.01 | 0.99 (0.96-1.02) | 0.56 |
Abbreviations: Similar to Table 1
The VIF between parameters of the multivariate model were all below 10, tolerances between parameters of the multivariate model were all above 0.1 (shown in Supplementary Table 3 ), and all parameters showed no significant collinearity and were thus entered into the multivariate logistic regression analysis. After adjusting for all of the aforementioned risk factors using a multivariate logistic regression analysis, the age, systolic and diastolic blood pressure, uric acid, and serum levels of CEA and NSE were found to be independent risk factors for increased peripheral arterial stiffness (all P<0.05).
Supplementary Table 3. Assessing collinearity of the parameters used in the multivariate models.
| Variable | Tolerance | VIF |
|---|---|---|
| Age, (years) | 0.88 | 1.13 |
| Male, n (%) | 0.29 | 3.51 |
| BMI (kg/m2) | 0.78 | 1.26 |
| SBP (mmHg) | 0.20 | 5.09 |
| DBP(mmHg) | 0.25 | 3.98 |
| Hypertension, n (%) | 0.26 | 3.83 |
| Smoker, n (%) | 0.36 | 2.77 |
| Diabetes, n (%) | 0.85 | 6.18 |
| FBG (mmol/L) | 0.12 | 5.38 |
| TG (mmol/L) | 0.88 | 1.13 |
| HDL-C (mmol/L) | 0.80 | 1.24 |
| Creatinine (umol/L) | 0.33 | 2.96 |
| Uric acid (umol/L) | 0.60 | 1.67 |
| WBC (109/L) | 0.79 | 1.30 |
| NLR, (%) | 0.58 | 1.71 |
| LMR, (%) | 0.60 | 1.68 |
| Hs-CRP (mg/L) | 0.88 | 1.14 |
| CEA, ug/l | 0.73 | 1.36 |
| NSE, ug/L | 0.89 | 1.13 |
| CYFRA21-1, ng/ml | 0.39 | 2.54 |
| proGRP, pg/ml | 0.40 | 2.51 |
Abbreviations: Similar to supplementary Table 1, CEA, carcinoembryonic antigen; CYFRA 21-1, cytokeratin-19 fragment 21-1; NSE, neuron- specific enolase; proGRP, pro-gastrin-releasing peptide.
Odds Ratios (ORs) for Peripheral Arterial Stiffness According to Quartiles of Serum CEA and NSE Levels
The detailed influences of serum levels of CEA and NSE on increased peripheral arterial stiffness were further investigated by dividing the subjects into four groups (Q1–Q4) according to the quartiles of serum levels of CEA and NSE (shown in Table 3 ). The median circulating level of CEA was 1.08 (0.49–1.60) µg/L, and that of NSE was 13.95 (10.98–16.08) µg/L. The clinical and laboratory indicators of the study subjects according to quartiles of CEA or NSE are shown in Supplementary Tables 1 and 2 . The age, proportion of males, BMI, blood pressure, rate of a medical history of hypertension and diabetes mellitus, proportion of smokers, and serum levels of metabolic and inflammatory indicators (except TC and LDL-C) all differed significantly among CEA and NSE quartiles (all P<0.05).
Table 3. Odds ratios for increased peripheral arterial stiffness according to quartiles of serum CEA and NSE levels.
| CEA (ug/L) | NSE (ug/L) | |||||
|---|---|---|---|---|---|---|
| n | Means values | OR (95%CI) | n | Means values | OR (95%CI) | |
| Q1 | 961 | 0.23±0.12 | 1 | 955 | 9.56±1.25 | 1 |
| Q2 | 974 | 0.79±0.18 | 1.57 (0.93-2.65) | 963 | 12.47±0.88 | 4.92 (2.70-8.96) |
| Q3 | 958 | 1.31±0.15 | 2.15 (1.26-3.67) | 982 | 15.02±0.61 | 6.65 (3.59-12.30) |
| Q4 | 985 | 2.34±0.67 | 6.13 (3.28-11.44) | 978 | 17.78±2.91 | 8.01 (4.32-14.85) |
| P for trend | <0.01 | <0.01 | ||||
Adjusted for age, male gender, BMI, blood pressure, medical history of hypertension and diabetes mellitus, smoking status, and serum levels of metabolic indicators (FBG, TG, HDL-C, creatinine, and uric acid) and inflammatory indicators (WBC counts, NLR, MLR, and hs-CRP levels). Abbreviations: Similar to Table 1.
Supplementary Table 1. Comparison of clinical and laboratory indicators of study subjects according to quartile of carcinoembryonic antigen.
| Variable |
1st Quartile (n = 961) |
2nd Quartile (n = 974) |
3rd Quartile (n = 958) |
4th Quartil (n = 985) |
P |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age,(years) | 51.19±4.47 | 51.54±4.71 | 52.66±4.82§* | 53.88±5.20†#& | <0.01 |
| Male, n (%) | 485 (50.5) | 578 (59.3) | 638 (66.6) | 755 (76.6) | <0.01 |
| BMI (kg/m2) | 24.78±3.09 | 24.80±2.79 | 25.02±3.00 | 25.37±3.01†# | <0.01 |
| SBP (mmHg) | 118.92±12.63 | 120.84±13.24¶ | 126.12±15.88§* | 136.05±17.83†#& | <0.01 |
| DBP(mmHg) | 73.68±9.57 | 74.80±10.14 | 78.51±10.96§* | 84.70±11.70†#& | <0.01 |
| Medical history | |||||
| Hypertension, n (%) | 84 (8.7) | 114 (11.7) | 230 (24.0)§* | 452 (45.9)§#& | <0.01 |
| Smoker, n (%) | 53 (5.5) | 75 (7.7) | 123 (12.8) | 250 (25.4)§#& | <0.01 |
| Diabetes, n (%) | 42 (4.4) | 77 (7.9) | 81 (8.4) | 167 (17.0)§#& | <0.01 |
| Blood chemistry | |||||
| FBG (mmol/L) | 5.11±1.17 | 5.26±1.45 | 5.35±1.52§ | 5.84±1.94†#& | <0.01 |
| TC (mmol/L) | 4.80±0.58 | 4.81±0.59 | 4.84±0.57 | 4.81±0.58 | 0.66 |
| TG (mmol/L) | 1.15±0.34 | 1.13±0.32 | 1.16±0.35 | 1.20±0.32†# | <0.01 |
| LDL-c (mmol/L) | 2.72±0.40 | 2.75±0.40 | 2.74±0.40 | 2.71±0.38 | 0.43 |
| HDL-c (mmol/L) | 1.23±0.19 | 1.23±0.20 | 1.22±0.19 | 1.19±0.19†#& | <0.01 |
| Creatinine (umol/L) | 68.10±14.30 | 70.77±13.77¶ | 72.28±14.45§ | 73.88±13.68†# | <0.01 |
| Uric acid (umol/L) | 308.6±65.5 | 314.0±62.5 | 320.4±60.8§ | 329.9±58.9†#& | <0.01 |
| Serum levels of inflammatory factors | |||||
| WBC (109/L) | 5.63±1.19 | 5.79±1.23¶ | 5.83±1.29§ | 6.24±1.35†#& | <0.01 |
| NLR, (%) | 2.00±0.79 | 2.00±0.75 | 2.00±0.77 | 2.09±0.79 | 0.02 |
| LMR, (%) | 6.10±2.64 | 6.03±1.92 | 5.97±2.03 | 5.83±1.84† | 0.04 |
| hs-CRP (mg/L) | 0.40 (0.15-1.12) | 0.47 (0.20-1.07) | 0.51 (0.23-1.18) | 0.68 (0.30-1.41) †#& | <0.01 |
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; FBG, fast blood glucose; HDL-C, high-density lipoprotein cholesterol; hs-CRP, high sensitivity C protein; LDL-C, low-density lipoprotein cholesterol; LMR, lymphocyte-to- monocyte ratio; NLR, neutrophil-to lymphocyte ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; WBC, white blood cell.
¶ P<0.05 between 1st quartile and 2nd quartile
§ P<0.05 between 1st quartile and 3rd quartile
† P<0.05 between 1st quartile and 4th quartile
* P<0.05 between 2nd quartile and 3rd quartile
# P<0.05 between 2nd quartile and 4th quartile
& P<0.05 between 3rd quartile and 4th quartile
Supplementary Table 2. Comparison of clinical and laboratory indicators of study subjects according to quartile of neuron-specific enolase.
| Variable |
1st Quartile (n=955) |
2nd Quartile (n=963) |
3rd Quartile (n=982) |
4th Quartil (n=978) |
P |
|---|---|---|---|---|---|
| Demographic data | |||||
| Age, (years) | 51.17±4.47 | 51.97±4.92¶ | 52.77±4.88§* | 53.46±5.14†#& | <0.01 |
| Male, n (%) | 531 (54.9) | 589 (60.5) | 654 (67.3)§ | 676 (69.8)†# | <0.01 |
| BMI (kg/m2) | 24.48±2.94 | 24.74±2.85 | 25.31±3.02§* | 25.45±3.03†# | <0.01 |
| SBP (mmHg) | 117.57±11.56 | 122.81±15.18¶ | 129.27±15.41§* | 132.98±18.58†#& | <0.01 |
| DBP (mmHg) | 72.62±9.17 | 76.31±10.72¶ | 80.82±10.75§* | 82.47±12.26†#& | <0.01 |
| Medical history | |||||
| Hypertension, n (%) | 55 (5.7) | 156 (16.0) | 287 (29.2)§* | 382 (39.5)†#& | <0.01 |
| Smoker, n (%) | 35 (3.6) | 81 (8.3) | 180 (18.5)§* | 205 (21.2)†# | <0.01 |
| Diabetes, n (%) | 53 (5.5) | 66 (6.8) | 127 (13.1)§* | 121 (12.5)†# | <0.01 |
| Blood chemistry | |||||
| FBG (mmol/L) | 5.12±1.28 | 5.24±1.38 | 5.62±1.77§* | 5.60±1.75§* | <0.01 |
| TC (mmol/L) | 4.80±0.59 | 4.81±0.57 | 4.83±0.57 | 4.82±0.58 | 0.78 |
| TG (mmol/L) | 1.13±0.34 | 1.14±0.32 | 1.18±0.33§ | 1.18±0.33† | 0.01 |
| LDL-c (mmol/L) | 2.72±0.41 | 2.72±0.39 | 2.74±0.39 | 2.74±0.39 | 0.77 |
| HDL-c (mmol/L) | 1.25±0.19 | 1.23±0.19 | 1.21±0.19§ | 1.20±0.19† | <0.01 |
| Creatinine (umol/L) | 69.75±14.35 | 70.70±14.61 | 71.90±13.77§ | 72.99±13.93†# | <0.01 |
| Uric acid (umol/L) | 310.4±64.9 | 314.0±63.1 | 325.3±60.7§* | 324.2±59.7†# | <0.01 |
| Serum levels of inflammatory factors | |||||
| WBC (109/L) | 5.68±1.23 | 5.84±1.28¶ | 5.92±1.27§ | 6.06±1.33†# | <0.01 |
| NLR, (%) | 1.95±0.77 | 2.07±0.80¶ | 2.04±0.76 | 2.03±0.77 | <0.01 |
| LMR, (%) | 6.17±2.67 | 5.88±1.96¶ | 5.89±1.80§ | 5.97±1.98 | 0.01 |
| hs-CRP (mg/L) | 0.42 (0.17-1.02) | 0.52 (0.19-1.24) | 0.54 (0.25-1.24) | 0.62 (0.25-1.28)† | 0.04 |
Abbreviations: Similar to supplementary Table 1.
¶ P<0.05 between 1st quartile and 2nd quartile
§ P<0.05 between 1st quartile and 3rd quartile
† P<0.05 between 1st quartile and 4th quartile
* P<0.05 between 2nd quartile and 3rd quartile
# P<0.05 between 2nd quartile and 4th quartile
& P<0.05 between 3rd quartile and 4th quartile
When the lowest quartile of serum levels of CEA was used as a reference, a multivariate logistic regression analysis showed that the ORs (95% confidence intervals [CIs]) for increased peripheral arterial stiffness were 1.00 (Q1), 1.57 (0.93-2.65; Q2), 2.15 (1.26-3.67; Q3), and 6.13 (3.28-11.44; Q4) after adjusting for the age, proportion of males, BMI, blood pressure, rate of a medical history of hypertension and diabetes mellitus, proportion of smokers, and serum levels of metabolic indicators (FBG, TG, HDL-C, creatinine, and uric acid) and inflammatory indicators (WBC counts, NLR, LMR, and hs-CRP levels) (P<0.01; Table 3 ). When the lowest quartile of serum NSE levels was used as a reference, the ORs (95% CIs) for increased peripheral arterial stiffness were 1.00 (Q1), 4.92 (2.70-8.96; Q2), 6.65 (3.59-12.30; Q3), and 8.01 (4.32–14.85; Q4) after adjusting for all of the aforementioned risk factors (P<0.01; Table 3 ).
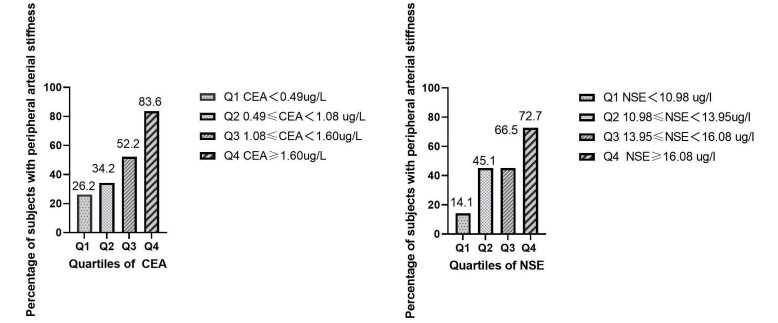
In addition, the percentage of subjects with increased peripheral arterial stiffness increased gradually in the CEA and NSE quartiles (CEA: 26.2% in Q1 group, 34.2% in Q2 group, 52.2% in Q3 group, and 83.6% in Q4 group; NSE: 14.1% in Q1 group, 45.1% in Q2 group, 66.5% in Q3 group, and 72.7% in Q4 group) ( Fig.1 ) .
Fig.1. The percentage of subjects with increased peripheral arterial stiffness according to the quartiles of the serum CEA and NSE levels.
Abbreviations: CEA, carcinoembryonic antigen; NSE, neuron-specific enolase.
Receiver Operating Characteristic Curves for the Diagnostic Value of CEA and NSE for Increased Peripheral Arterial Stiffness
Receiver operating characteristic curves were generated to determine the accuracy of CEA and NSE levels in the diagnosis of increased peripheral arterial stiffness. As shown in Fig.2 , the AUC of CEA and NSE in the present study was 0.76 (95% CI 0.74–0.77), 0.76 (95% CI 0.75–0.78), respectively. A cutoff value of 1.22 µg/L for CEA provided a sensitivity of 62.6% and a specificity of 77.0%, whereas the cutoff value of 14.32 µg/L for NSE had a sensitivity of 66.0% and a specificity of 74.3%. In the present study, 41.3% (n=1601) of subjects had a CEA level of >1.22 µg/L, and 40.6% (n=1574) had an NSE level of >14.32 µg/L. The combination of a CEA level of >1.22 µg/L and an NSE level of >14.32 µg/L had a sensitivity of 45.5% and a specificity of 93.4%, whereas the presence of either a CEA level of >1.22 µg/L or an NSE level of >14.32 µg/L had a sensitivity of 80.9% and a specificity of 58.7%.
Fig.2. Receiver operating characteristic curves for the diagnostic value of CEA and NSE for increased peripheral arterial stiffness.

Abbreviations: Similar to Figure 1
Discussion
The present study demonstrated that serum CEA and NSE levels were correlated with increased peripheral arterial stiffness, as measured by the baPWV, in middle-aged Chinese adults. The association persisted when adjusted for the age, proportion of males, BMI, blood pressure, rate of a medical history of hypertension and diabetes mellitus, proportion of smokers, serum levels of metabolic indicators (FBG, TG, HDL-C, creatinine, and uric acid), and inflammatory indicators (WBC count, NLR, LMR, and hs-CRP levels). This indicated that the effects of CEA and NSE on increased peripheral arterial stiffness were independent of the traditional risk factors.
Peripheral arterial stiffness occurs as a consequence of aging 17) and is affected by multiple factors, including high blood pressure, obesity, hypertension, diabetes mellitus, metabolic syndrome, and smoking 6) . Chronic inflammation is a well-established risk factor of arteriosclerosis. It has been reported that an increase in the WBC count is correlated with damage to vascular endothelial cells and mediates the occurrence and development of arteriosclerosis, which is positively correlated with the baPWV 18) . Many other novel inflammatory markers, such as the NLR, platelet-to-lymphocyte ratio, LMR, and CRP, have all been shown to accelerate the pathogenesis of peripheral arterial stiffness and are positively correlated with the baPWV 19) . With reference to the above-mentioned studies, the present study included the age, sex, BMI, blood pressure, medical history of hypertension and diabetes mellitus, smoking status, and serum levels of metabolic indicators (FBG, TC, TG, HDL-C, LDL-C, creatinine, and uric acid) and inflammatory indicators (WBC count, NLR, LMR, and hs-CRP levels). All of the above parameters, except TC and LDL-C, were related to increased peripheral arterial stiffness in a univariate logistic analysis. After adjusting for all of the aforementioned risk factors by a multivariate logistic regression analysis, serum levels of CEA and NSE remained independent risk factors for increased peripheral arterial stiffness.
CEA is the most widely used serological marker of malignant tumors, and CEA levels may also exhibit a modest increase in some nonmalignant conditions, such as aging and smoking 20) . It have been confirmed that CEA levels are correlated with peripheral arterial stiffness in Asian people. In a cross-sectional study involving 2909 Koreans, CEA levels correlated with the baPWV. The higher the CEA concentration, the greater the correlation coefficient with the baPWV 21) . Another cross-sectional study involving 4181 adult Japanese males found that serum CEA levels were closely correlated with carotid atherosclerotic plaque. After quartile grouping of serum CEA levels, the OR for carotid atherosclerotic plaque formation gradually increased with increasing CEA levels 22) . In the present study, 3878 middle-aged Chinese adults from southeast China were enrolled and divided into four groups according to quartiles of serum CEA levels. The OR for increased peripheral arterial stiffness gradually increased with CEA levels, and a cutoff value of 1.22 µg/L had a sensitivity of 62.6% and a specificity of 77.0% in distinguishing increased peripheral arterial stiffness. The underlying mechanism may be related to chronic inflammatory response. Studies have confirmed that CEA can stimulate monocytes and macrophages to release proinflammatory factors, activate endothelial cells, increase the expression of adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1) in endothelial cells, and promote the migration of endothelial cells 23 , 24) , which are associated with the early pathogenesis of arterial stiffness.
NSE is known for its specific presence in neuroendocrine cells and is closely associated with diseases such as disorders of nervous system development, injuries, and tumors 25) . In recent years, many studies have confirmed that NSE acts as a proinflammatory factor and is correlated with adverse outcomes in cerebrovascular disease 26 , 27) . In the present study, when 3878 middle-aged Chinese adults were divided into four groups according to the quartiles of serum NSE levels, the OR for increased peripheral arterial stiffness gradually increased with an increase in NSE levels. The optimal cutoff value of 14.32 µg/L provided a sensitivity and specificity of 66.0% and 74.3%, respectively. A possible underlying mechanism of NSE in arterial stiffness may be related to the degradation of the extracellular matrix and promotion of inflammation induced by NSE, which further leads to damage to blood vessels in the stromal microenvironment and an imbalance in vascular homeostasis. First, NSE degrades the extracellular matrix by inhibiting the activity of matrix metalloproteinases 28) , which destroy blood vessels in the stromal microenvironment. Second, the C-terminal region of NSE can promote the production of proinflammatory cytokines (such as tumor necrosis factor-α, interleukin-1β, interferon-γ, transforming growth factor-β, and monocyte chemoattractant protein-1) and disrupt vascular homeostasis by activating phosphoinositide-3-kinase and mitogen-activated protein kinase pathways 29) .
Limitations
Nevertheless, some limitations of the present study must be considered. First, most of the study subjects were from southeast China, and the sensitivity and specificity of CEA and NSE in predicting peripheral arterial stiffness need to be improved. Follow-up experiments can be performed in multicenter studies to increase the sample size and improve the sensitivity and specificity of the optimal cutoff value. Second, because this was a cross-sectional study, no causative relationship between high serum CEA or NSE levels and peripheral arterial stiffness was established. Therefore, the effects of CEA and NSE on the occurrence and development of peripheral arteriosclerosis need to be further studied by extending the observation time in future studies. Third, CEA and NSE levels are abnormally elevated in patients with tumors, and their predictive value for arterial stiffness in these patients has not been reported. Because tumor patients were excluded from this study, further research is needed to investigate whether or not CEA and NSE can be used to predict peripheral arterial stiffness in tumor patients by increasing the number of subgroups of tumor patients. Fourth, subjects were not required to divulge a family history of cancer unless they wished to undergo a health checkup, and further research is needed to investigate whether lung cancer biomarkers and the baPWV differed in subjects with a family history of cancer compared to subjects without a family history of cancer.
Conclusion
In summary, our results demonstrated that increased serum CEA and NSE levels were associated with increased peripheral arterial stiffness in middle-aged Chinese adults, independent of traditional risk factors. Patients with a CEA >1.22 µg/L or NSE >14.32 µg/L may undergo further examinations, such as baPWV measurements, to evaluate arteriosclerosis.
Acknowledgement
This study was supported by the Sichuan Medical Association, Sichuan Province, China (no.2019HR42), and the Sports and Health Innovation Research Center of Zigong City, Sichuan Province, China (no.YDJKZ22-03).
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1).Stone K, Fryer S, Faulkner J, Meyer ML, Heffernan K, Kucharska-Newton A, Zieff G, Paterson C, Matsushita K, Hughes TM, Tanaka H and Stoner L: Associations of lower-limb atherosclerosis and arteriosclerosis with cardiovascular risk factors and disease in older adults: The Atherosclerosis Risk in Communities (ARIC) study. Atherosclerosis, 2022; 340: 53-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).Sumin AN, Shcheglova AV, Ivanov SV and Barbarash OL: Long-Term Prognosis after Coronary Artery Bypass Grafting: The Impact of Arterial Stiffness and Multifocal Atherosclerosis. Journal of clinical medicine, 2022; 11: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Liu H, Liu J, Zhao H and Wang H: Association of brain white matter lesions with arterial stiffness assessed by cardio-ankle vascular index. The Beijing Vascular Disease Evaluation STudy (BEST). Brain imaging and behavior, 2021; 15: 1025-1032 [DOI] [PubMed] [Google Scholar]
- 4).Tuttolomondo A, Casuccio A, Della Corte V, Maida C, Pecoraro R, Di Raimondo D, Vassallo V, Simonetta I, Arnao V and Pinto A: Endothelial function and arterial stiffness indexes in subjects with acute ischemic stroke: Relationship with TOAST subtype. Atherosclerosis, 2017; 256: 94-99 [DOI] [PubMed] [Google Scholar]
- 5).Tomiyama H and Shiina K: State of the Art Review: Brachial-Ankle PWV. J Atheroscler Thromb, 2020; 27: 621-636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6).Lacolley P, Regnault V and Laurent S: Mechanisms of Arterial Stiffening: From Mechanotransduction to Epigenetics. Arteriosclerosis, thrombosis, and vascular biology, 2020; 40: 1055-1062 [DOI] [PubMed] [Google Scholar]
- 7).Aminuddin A, Lazim M, Hamid AA, Hui CK, Mohd Yunus MH, Kumar J and Ugusman A: The Association between Inflammation and Pulse Wave Velocity in Dyslipidemia: An Evidence-Based Review. Mediators of inflammation, 2020; 2020: 4732987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Dregan A: Arterial stiffness association with chronic inflammatory disorders in the UK Biobank study. Heart (British Cardiac Society), 2018; 104: 1257-1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Peyster E, Chen J, Feldman HI, Go AS, Gupta J, Mitra N, Pan Q, Porter A, Rahman M, Raj D, Reilly M, Wing MR, Yang W and Townsend RR: Inflammation and Arterial Stiffness in Chronic Kidney Disease: Findings From the CRIC Study. American journal of hypertension, 2017; 30: 400-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Molina R, Marrades RM, Augé JM, Escudero JM, Viñolas N, Reguart N, Ramirez J, Filella X, Molins L and Agustí A: Assessment of a Combined Panel of Six Serum Tumor Markers for Lung Cancer. American journal of respiratory and critical care medicine, 2016; 193: 427-437 [DOI] [PubMed] [Google Scholar]
- 11).Dery KJ, Wong Z, Wei M and Kupiec-Weglinski JW: Mechanistic Insights into Alternative Gene Splicing in Oxidative Stress and Tissue Injury. Antioxidants & redox signaling, 2023; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12).Gan YZ, Zhang LH, Ma L, Sun F, Li YH, An Y, Li ZG and Ye H: Risk factors of interstitial lung diseases in clinically amyopathic dermatomyositis. Chinese medical journal, 2020; 133: 644-649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Yang RX, Song WJ, Wu ZQ, Goyal H and Xu HG: Association of Serum Neuron-Specific Enolase and C-Reactive Protein With Disease Location and Endoscopic Inflammation Degree in Patients With Crohn’s Disease. Frontiers in medicine, 2021; 8: 663920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Yang YF, Li Y, Liu JH, Wang XM, Wu BH, He CS and Gu JW: Relation of Helicobacter pylori infection to peripheral arterial stiffness and 10-year cardiovascular risk in subjects with diabetes mellitus. Diabetes& vascular disease research, 2020; 17: 1479164120953626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Stone K, Veerasingam D, Meyer ML, Heffernan KS, Higgins S, Maria Bruno R, Bueno CA, Döerr M, Schmidt-Trucksäss A, Terentes-Printzios D, Voicehovska J, Climie RE, Park C, Pucci G, Bahls M and Stoner L: Reimagining the Value of Brachial-Ankle Pulse Wave Velocity as a Biomarker of Cardiovascular Disease Risk-A Call to Action on Behalf of VascAgeNet. Hypertension (Dallas, Tex : 1979), 2023; 80: 1980-1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).2018 Practice Guidelines for the management of arterial hypertension of the European Society of Hypertension and the European Society of Cardiology: ESH/ESC Task Force for the Management of Arterial Hypertension: Erratum. Journal of hypertension, 2019; 37: 456 [DOI] [PubMed] [Google Scholar]
- 17).Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF and O’Rourke MF: Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation, 1983; 68: 50-58 [DOI] [PubMed] [Google Scholar]
- 18).Liu Y, Lai X, Guo W, Ma L, Li W, Fang Q, Yang H, Cai Y, Liu M, Zhang X and Yang L: Total White Blood Cell Count Mediated the Association Between Increased Arterial Stiffness and Risk of Type 2 Diabetes Mellitus in Chinese Adults. Arteriosclerosis, thrombosis, and vascular biology, 2020; 40: 1009-1015 [DOI] [PubMed] [Google Scholar]
- 19).Ning P, Yang F, Kang J, Yang J, Zhang J, Tang Y, Ou Y, Wan H and Cao H: Predictive value of novel inflammatory markers platelet-to-lymphocyte ratio, neutrophil-to-lymphocyte ratio, and monocyte-to-lymphocyte ratio in arterial stiffness in patients with diabetes: A propensity score-matched analysis. Frontiers in endocrinology, 2022; 13: 1039700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Ruibal Morell A: CEA serum levels in non-neoplastic disease. The International journal of biological markers, 1992; 7: 160-166 [DOI] [PubMed] [Google Scholar]
- 21).Bae U, Shim JY, Lee HR and Shin JY: Serum carcinoembryonic antigen level is associated with arterial stiffness in healthy Korean adult. Clinica chimica acta; international journal of clinical chemistry, 2013; 415: 286-289 [DOI] [PubMed] [Google Scholar]
- 22).Ishizaka N, Ishizaka Y, Toda E, Koike K, Yamakado M and Nagai R: Are serum carcinoembryonic antigen levels associated with carotid atherosclerosis in Japanese men? Arteriosclerosis, thrombosis, and vascular biology, 2008; 28: 160-165 [DOI] [PubMed] [Google Scholar]
- 23). Aarons CB, Bajenova O, Andrews C, Heydrick S, Bushell KN, Reed KL, Thomas P, Becker JM and Stucchi AF: Carcinoembryonic antigen-stimulated THP-1 macrophages activate endothelial cells and increase cell-cell adhesion of colorectal cancer cells. Clinical& experimental metastasis, 2007; 24: 201-209 [DOI] [PubMed] [Google Scholar]
- 24).Libby P: The changing landscape of atherosclerosis. Nature, 2021; 592: 524-533 [DOI] [PubMed] [Google Scholar]
- 25).Mjønes P, Sagatun L, Nordrum IS and Waldum HL: Neuron-Specific Enolase as an Immunohistochemical Marker Is Better Than Its Reputation. The journal of histochemistry and cytochemistry : official journal of the Histochemistry Society, 2017; 65: 687-703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26).Stammet P, Collignon O, Hassager C, Wise MP, Hovdenes J, Åneman A, Horn J, Devaux Y, Erlinge D, Kjaergaard J, Gasche Y, Wanscher M, Cronberg T, Friberg H, Wetterslev J, Pellis T, Kuiper M, Gilson G and Nielsen N: Neuron-Specific Enolase as a Predictor of Death or Poor Neurological Outcome After Out-of-Hospital Cardiac Arrest and Targeted Temperature Management at 33℃ and 36℃. J Am Coll Cardiol, 2015; 65: 2104-2114 [DOI] [PubMed] [Google Scholar]
- 27).Wang K, Hong T, Liu W, Xu C, Yin C, Liu H, Wei X, Wu SN, Li W and Rong L: Development and validation of a machine learning-based prognostic risk stratification model for acute ischemic stroke. Scientific reports, 2023; 13: 13782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28).Haque A, Polcyn R, Matzelle D and Banik NL: New Insights into the Role of Neuron-Specific Enolase in Neuro-Inflammation, Neurodegeneration, and Neuroprotection. Brain sciences, 2018; 8: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).Hafner A, Obermajer N and Kos J: γ-Enolase C-terminal peptide promotes cell survival and neurite outgrowth by activation of the PI3K/Akt and MAPK/ERK signalling pathways. The Biochemical journal, 2012; 443: 439-450 [DOI] [PubMed] [Google Scholar]