Abstract
Hypervitaminosis D leads to toxic effects, including hypercalcemia, which can cause severe damage to various organs. Fetuin-A, a glycoprotein with anti-inflammatory properties, may protect tissues from such damage. This study explores the role of Fetuin-A in mitigating hypervitaminosis D-induced damage in renal, hepatic, and cardiac tissues. The objectives of this study were to: (1) Assess the extent of tissue damage from high-dose vitamin D in a murine model by examining the histopathological changes in liver, kidney and heart. (2) Investigate Fetuin-A's protective effect against this damage. Thirty-six albino rats were divided into four groups: (1) control, (2) vitamin D toxicity, (3) Fetuin-A + vitamin D, and (4) Fetuin-A only. Vitamin D was administered subcutaneously at 250 μg/20 g/day for 3 days. Fetuin-A was given at 100 μl/20 g, starting 7 days before vitamin D treatment. Histopathological analysis of liver, kidney, and heart tissues was performed using H&E and Alizarin Red staining and findings were analysed statistically. Vitamin D toxicity caused significant tissue damage, including apoptosis, inflammation, and calcification in the liver, kidneys, and heart. Pre-treatment with Fetuin-A reduced calcification and inflammation, preserving tissue architecture. Fetuin-A-only rats showed no damage or calcification. Fetuin-A provided statistically significant protection against vitamin D-induced damage, reducing oxidative stress and calcification in affected organs. These findings suggest Fetuin-A could be a potential therapeutic agent for hypervitaminosis D.
Keywords: Hypervitaminosis D, Fetuin-A, Tissue damage, Calcification, Vitamin D toxicity
Subject terms: Medical research, Signs and symptoms
Introduction
Hypervitaminosis D and vitamin D toxicity are closely related yet distinct conditions caused by excessive vitamin D intake. Hypervitaminosis D refers to elevated levels of vitamin D metabolites, such as 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)₂D], typically resulting from prolonged supplementation or dietary intake1,2. Vitamin D toxicity, on the other hand, describes the clinical consequences of hypervitaminosis D, primarily hypercalcemia, which leads to symptoms such as nausea, vomiting, renal impairment, and cardiovascular complications3. While hypervitaminosis D may occur without immediate clinical symptoms at serum 25(OH)D levels exceeding 100 ng/mL, toxicity generally arises when levels surpass 150 ng/mL, often due to chronic intake of doses exceeding 50,000 IU/day4,5.
The global prevalence of hypervitaminosis D, though less documented compared to vitamin D deficiency, is increasingly recognized as a growing concern, particularly in populations that frequently self-administer supplements without medical supervision. Contributing factors include the misuse of high-dose supplements, the promotion of megadoses for therapeutic purposes, and errors in supplement manufacturing6–10. This trend was exacerbated during the COVID-19 pandemic, with heightened emphasis on vitamin D supplementation, which increased the risk of toxicity2,9. Certain vulnerable populations, such as pediatric cystic fibrosis patients, show hypervitaminosis D prevalence rates as high as 5%, highlighting the need for monitoring vitamin D levels in high-risk groups11. Additionally, conditions such as mutations in enzymes like CYP24A1 or SLC34A1 and diseases such as pulmonary tuberculosis can predispose individuals to hypervitaminosis D, exacerbating hypercalcemia and increasing the risk of severe complications12,13.
Fetuin-A, a glycoprotein primarily produced in the liver, plays a multifaceted role in maintaining physiological balance, particularly in conditions involving calcium-phosphate dysregulation. It binds calcium and phosphate ions to form soluble calciprotein particles (CPPs), thereby preventing ectopic calcification in soft tissues14,15. Fetuin-A also exhibits anti-inflammatory properties and has been shown to inhibit vascular calcification, making it a key regulator in metabolic and cardiovascular health16–18. Studies have demonstrated that mice deficient in Fetuin-A exhibit increased systemic calcification when exposed to a high-vitamin D diet, underscoring its potential role in mitigating hypercalcemia-induced damage19.
This study investigates the protective effects of Fetuin-A against vitamin D toxicity in a murine model. Specifically, it seeks to address the following questions:
What is the extent of cellular and tissue damage caused by high doses of vitamin D in renal, hepatic, and cardiac tissues?
Does the administration of Fetuin-A mitigate vitamin D-induced damage in these tissues?
What histopathological changes occur in the renal, hepatic, and cardiac tissues of murine models subjected to hypervitaminosis D and Fetuin-A treatment?
Are there differences in the protective efficacy of Fetuin-A across renal, hepatic, and cardiac tissues?
By addressing these questions, this study aims to provide insights into the mechanisms underlying Fetuin-A’s protective effects and its potential as a therapeutic agent for managing hypervitaminosis D. The findings could inform future research and therapeutic strategies aimed at reducing the systemic and tissue-specific complications associated with vitamin D toxicity.
Methodology
Reagents
Vitamin D3 (cholecalciferol) was obtained from Memphis Pharmaceutical and Chemical Industries (Egypt). Each ampoule contained 200,000 IU in 2 mL. Fetuin-A was sourced from Roche Products (UK) as a 10 mg/mL solution in a 1 mL ampoule.
Animals and diets
The study utilized 36 healthy adult male albino rats of the C57BL/6 strain, aged 8–10 weeks and weighing between 20 and 30 g. The animals were housed under standard laboratory conditions, including a controlled temperature of 22 ± 2 °C, relative humidity of 50–60%, and a 12-h light/dark cycle. They had free access to water and food during the experimental period. The diet, supplied by Grain Silos and Flour Mills, consisted of 20% crude protein, 4% crude fat, 3.5% crude fiber, 6% ash, 0.5% salt, 1% calcium, 0.6% phosphorus, 20 IU/g vitamin A, 2.2 IU/g vitamin D, and 70 IU/g vitamin E.
Induction of hypervitaminosis-D and fetuin-A intervention
The animals were randomly assigned to one of four experimental groups (n = 9 per group) using randomization software to ensure unbiased allocation:
Group 1 (Control): Received subcutaneous injections of distilled water.
Group 2 (Vitamin D Toxicity): Administered a toxic dose of 250 μg/20 g/day of vitamin D (500,000 IU/kg/day) subcutaneously for three consecutive days at 0, 24, and 48 h20.
Group 3 (Fetuin-A + Vitamin D): Administered an intraperitoneal dose of Fetuin-A (100 mg/kg) daily for seven days before the first subcutaneous vitamin D (500,000 IU/Kg/day) and continuing the same dose of fetuin A for the duration of vitamin D treatment20,21. The rationale to administer fetuin A before Vitamin D in group 3 was to study its capacity for primary prevention of Vitamin D toxicity and to explore its usability in patients who take large doses of Vitamin D for therapeutic reasons, e.g. Rheumatological diseases22.
Group 4 (Fetuin-A Only):Administered Fetuin-A (100 mg/kg) intraperitoneally for ten days21. This group was included to assess any independent effects of Fetuin-A in the absence of hypervitaminosis D which helps to determine if Fetuin-A has any inherent effects on renal, hepatic, and cardiac tissues. This is important for distinguishing between its general impact and its role in specifically countering the effects of vitamin D toxicity. This group addresses the hypothesis that Fetuin-A, beyond its protective role, does not negatively impact tissue health under normal conditions.
The dosing regimen was based on each rat’s body weight, following established protocols20,21 and all injections were performed at 08:00 each day to minimize circadian variability. Vitamin D was administered subcutaneously to ensure reliable absorption and consistency with previous studies20. Fetuin-A was delivered via intraperitoneal injection according to the methodology outlined by Zhang et al.21. The details of the dose calculation are in appendix-1.
According to the dose conversion between rats and humans23 the daily amount of Vitamin D received by group 2 and 3 were equivalent to 80,800 IU/kg/day in humans which is much higher than recommended daily dose and can cause severe toxicity. The human equivalent dose of Fetuin-A would be 16.22 mg/kg/day.
Quantification of clinical signs
General clinical observations, including appetite, neurological symptoms (e.g., muscle twitching), and physical activity, were recorded daily. Physical activity was objectively assessed using a locomotor activity chamber24, with measurements standardized at 08:00 daily (Fig. 1). Data were expressed as steps per minute, and group averages were calculated for comparison. Data were analysed using statistical methods to compare activity levels and other parameters across the four experimental groups.
Fig. 1.

Quantification of locomotor activity of the mice in Locomotor chamber.
Histopathological analysis
After the treatment period (Day 11), the animals were sacrificed using carbon dioxide inhalation, followed by decapitation to confirm death. Tissue specimens from the liver, kidney, and heart were collected, fixed in 10% neutral-buffered formalin, and subjected to routine histological processing. The samples were trimmed, washed, dehydrated in ascending grades of ethanol, cleared in xylene, and embedded in paraffin. Sections of 4–6 μm thickness were prepared and stained with hematoxylin and eosin (H&E) and Alizarin Red.
Histopathological images were captured using an Olympus BX53 microscope equipped with a DP74 digital imaging system. The analysis was conducted under blinded conditions to ensure objective evaluation.
Quantification of histopathological signs
Histopathological analysis was conducted to evaluate key tissue changes, including the quantification of the portal vein diameter. For portal vein diameter, the most dilated vein within the portal triad was identified and measured using image analysis software. The data were expressed as arithmetic mean, and standard deviation of the mean (± SD) and all findings were statistically analysed to determine significant differences between groups.
Statistical analysis
All data were analyzed using statistical methods to compare parameters across the four experimental groups. The mean and standard deviation were calculated for each group to summarize the data. Group differences were analyzed using a one-way analysis of variance (ANOVA) to assess overall statistical significance. Pairwise comparisons were further examined through post-hoc Tukey’s HSD tests to identify specific differences between groups. Statistical significance was determined at a threshold of p < .001, and all analyses were performed using SPSS software, version 20.
Ethical considerations
This study was approved by the Research Ethics Committee of Dubai Medical College for Girls, and it followed the regulations of ethical treatment of laboratory animals and ARRIVE guidelines (REC AY22-23-F-01). Every effort was made to minimize animal distress and Sample size calculations ensured the use of the minimum number of animals required for statistical validity.
Results
Observed clinical signs
The comparison of clinical signs observed across different experimental groups is summarized in Table 1. No significant clinical signs were observed in any of the groups during the first two days. On the third day, the vitamin D toxicity group (Group 2) exhibited reduced appetite, decreased activity (in all mice), and muscle twitches in approximately 66% of the mice, primarily in the abdominal region. On the fourth day, one mouse from this group was found dead. In the Control group and Fetuin-A groups, both with and without vitamin D, no neurologic signs were recorded, and overall health and appetite appeared normal.
Table 1.
Comparison of clinical signs among experimental groups.
| Clinical Signs | Control | Vitamin D Toxicity (Group 2) | Fetuin-A + Vitamin D (Group 3) | Fetuin-A Only (Group 4) |
|---|---|---|---|---|
| Mortality | None | 1 Mouse (Day 4) | None | None |
| Appetite | Normal | Reduced (Day 3 onward) | Largely Preserved | Normal |
| Neurological Signs (Muscle Twitches) | None | Muscle twitches observed in the abdomen of 66%, reduced activity | None | None |
| Activity Level (Steps per Minute) | 124 | 71 | 103 | 120 |
The physical activity of each group was quantitatively recorded using the locomotor activity chamber (Image 1). The statistical analysis of activity levels across the four groups revealed significant (p < .001) differences into the effects of vitamin D toxicity and the protective role of Fetuin-A (Table 2).
Table 2.
Summary of statistical findings for activity levels and portal vein diameter across experimental groups.
| Parameter | Group 1 (Control) (Mean ± SD) |
Group 2 (Vitamin D Toxicity) (Mean ± SD) |
Group 3 (Fetuin-A + Vitamin D) (Mean ± SD) |
Group 4 (Fetuin-A Only) (Mean ± SD) |
ANOVA p value |
|---|---|---|---|---|---|
| Activity Level | 124 ± 3.2 | 71 ± 8.96 | 103 ± 8.2 | 120 ± 1.94 | < .001 |
| Portal Vein Diameter | 2.00 ± 0.12 | 5.92 ± 0.22 | 2.90 ± 0.23 | 2.02 ± 0.08 | < .001 |
Group 1 (Control), which received distilled water, demonstrated the highest activity levels (mean = 124 steps per minute). In contrast, Group 2 (Vitamin D Toxicity) exhibited the lowest activity levels (mean = 71 steps per minute, p < .001 vs. Group 1), highlighting the debilitating effects of vitamin D toxicity, likely due to hypercalcemia-induced neuromuscular disturbances. Group 3 (Fetuin-A + Vitamin D), pre-treated with Fetuin-A, displayed intermediate activity levels (mean = 103 steps per minute). Although significantly lower than the Control group (p < .001), Group 3 exhibited markedly higher activity than Group 2 (p < .001), suggesting that Fetuin-A mitigates the adverse effects of vitamin D toxicity. These results underscore Fetuin-A's potential as a preventive intervention for hypervitaminosis D, especially in scenarios requiring high-dose vitamin D therapy. Group 4 (Fetuin-A Only), included to assess the independent effects of Fetuin-A, showed activity levels comparable to the Control group (mean = 120 steps per minute, p = .55 vs. Group 1). This indicates that Fetuin-A does not impair normal physiological activity, supporting its safety and potential therapeutic usability.
These findings highlight the severe physiological impact of vitamin D toxicity and the protective role of Fetuin-A. The comparable activity levels between the Control and Fetuin-A Only groups confirm its safety, while the improvements in Group 3 emphasize its therapeutic promise. These results provide a strong foundation for further investigation into Fetuin-A as a protective agent in conditions associated with hypervitaminosis D.
Histopathological findings
Liver
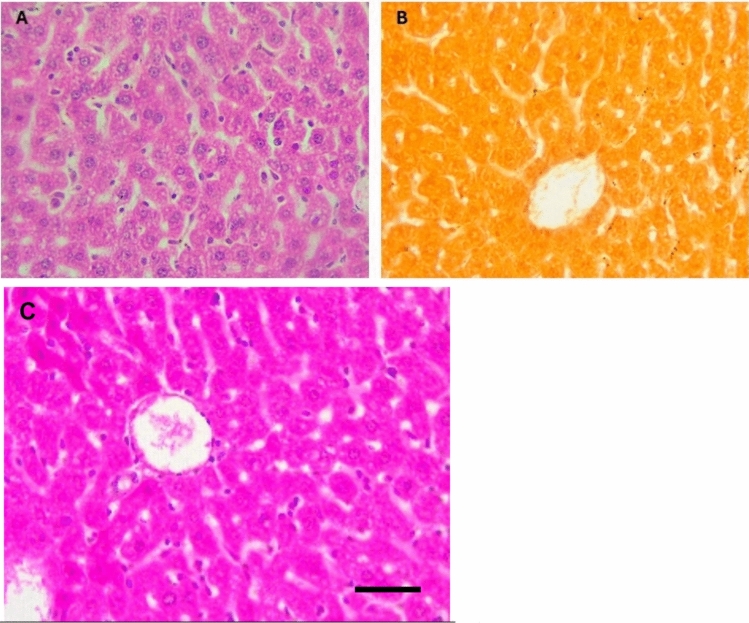
The liver of control mice showed normal histology with a thin capsule, normal portal areas and hepatocytes arranged in plates or cords radiating from the central vein, separated by blood sinusoids lined by thin squamous epithelium (Fig. 2A). However, there was no calcification spots on staining with Alizarin (Fig. 2B). The portal vein caliber appeared normal with maximum diameter noted as 2.2mm (Fig. 2C). In contrast, livers from mice supplemented with a toxic dose of vitamin D exhibited cells showing condensed nuclei and eosinophilic cytoplasm indicative of apoptosis admixed with normal hepatocytes. Kupffer cell hyperplasia, portal vein dilation, inflammatory cells around bile duct, congestion of portal vessels with marked portal vein dilatation (Fig. 3A–C). The maximum diameter of portal vein was noted to be 6. 2mm (Fig. 3E). Calcified deposits were seen with Alizarin Red stain (Fig. 3D). Fetuin-A pre-treated mice exhibited reduced fatty degeneration and preserved hepatocyte structure compared to Group 2. While fatty degeneration was still observed in some hepatocytes, with some cells displaying a signet ring appearance, it was significantly less pronounced in Group 3, and overall tissue integrity appeared more preserved. Occasional cytoplasmic eosinophilia and sparce inflammation was also noted in the hepatocyte demonstrating reversible injury (Fig. 4A). Calcification deposits and portal vein dilatation was less marked in Fetuin-A pre-treated mice compared to Group 2 (Fig. 4B, C). Fetuin A only group showed mild fatty degeneration of hepatocytes (Fig. 5A), no calcification (Fig. 5B) and normal portal vein diameter (Fig. 5C).
Fig. 2.
Histology of negative control mice liver (A) H&E showing normal architecture, (B) Alizarin Red showing no calcification, High Power, (C) Portal Vein diameter (widest area = 2.2mm) (× 40).
Fig. 3.
Histology of mice liver after taking the toxic dose of Vit D (A–C) H&E showing apoptosis, inflammation, central vein dilatation and Kupffer cell hyperplasia, (D) Alizarin Red showing calcification in hepatocytes, (E) Portal Vein diameter (widest area = 6.2mm) High Power (× 40).
Fig. 4.
Histology of mice liver after taking the Fetuin-A followed by toxic dose of Vit D, (A) H&E showing fatty degeneration of hepatocytes, occasional signet ring cells, Occasional cytoplasmic eosinophilia and sparce inflammation was also noted in the hepatocyte demonstrating reversible injury, (B) Alizarin Red showing sparse calcification in hepatocytes, (C) Portal Vein diameter (widest area = 3.2mm) (× 40).
Fig. 5.
Histology of mice liver after taking the Fetuin-A only, (A) H&E showing mild fatty degeneration of hepatocytes, (B) Alizarin Red showing no calcification in hepatocytes, (C) Portal Vein diameter (widest area = 2.1mm) (× 40).
The analysis of portal vein (PV) diameters revealed significant differences among the experimental groups, reflecting the vascular effects of vitamin D toxicity and the protective role of Fetuin-A (Table 2). Group 1exhibited a mean PV diameter of 2.0 ± 0.12 mm, representing the baseline for healthy, untreated rats. In stark contrast, Group 2 (Vitamin D Toxicity) showed a significantly increased PV diameter of 5.92 ± 0.22 mm (p < .001 vs. Group 1). This marked dilation highlights the severe vascular alterations induced by vitamin D toxicity, likely resulting from hypercalcemia-driven endothelial dysfunction, inflammation, and increased vascular permeability. Group 3 (Fetuin-A + Vitamin D), which was pre-treated with Fetuin-A, demonstrated an intermediate PV diameter of 2.9 ± 0.23 mm. While significantly larger than the control group (p < .001), it was markedly reduced compared to Group 2 (p < .001), indicating that Fetuin-A mitigated the vascular effects of vitamin D toxicity. This finding supports Fetuin-A’s potential in preserving vascular integrity by reducing calcium-phosphate imbalance and protecting endothelial function. Group 4 (Fetuin-A only), included to evaluate the independent effects of Fetuin-A, had a mean PV diameter of 2.02 ± 0.08 mm, which was comparable to the control group (p = .99). This suggests that Fetuin-A does not cause any adverse vascular changes under normal physiological conditions, further reinforcing its safety as a therapeutic agent.
These findings collectively demonstrate the detrimental effects of vitamin D toxicity on vascular structure and the protective role of Fetuin-A in mitigating these changes. The absence of significant differences between Group 4 and the Control group confirms that Fetuin-A does not negatively impact vascular health, making it a promising candidate for addressing hypervitaminosis D and related vascular complications.
Kidney
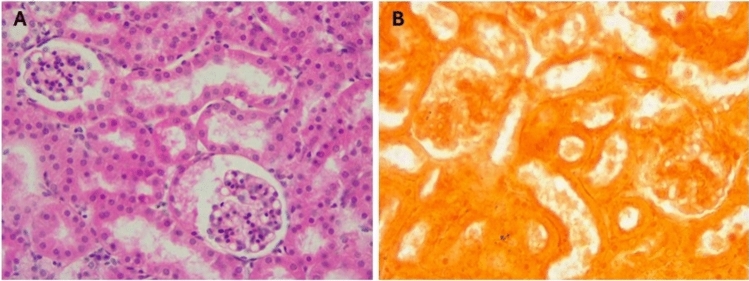
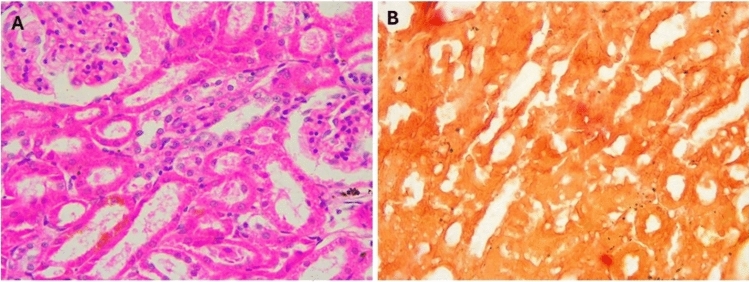
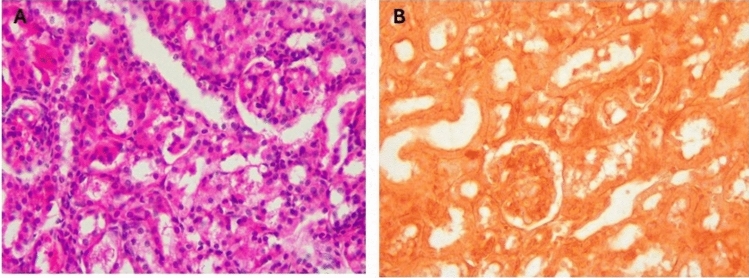
The kidney of control mice (Fig. 6) displayed normal structure with a thin capsule, well-organized renal corpuscles in the outer cortex, and medulla containing parts of different proximal and distal renal tubules (Fig. 6A). No calcification was seen on the renal tissue on staining with Alizarin (Fig. 6B). In mice supplemented with a toxic dose of vitamin D, some renal corpuscles exhibited shrinkage and decreased cellularity of glomerular capillaries with widening of Bowman’s space. Other tubules showed decreased epithelial height, luminal dilation with desquamated cells, hyaline material deposition, focal cellular damage, and inflammatory cell infiltration, alongside apoptosis and necrosis with marked disorganization of renal tubules (Fig. 7A–C). Massive calcification of renal tubules, glomeruli, and renal blood vessels was also observed (Fig. 7D). In mice supplemented with Fetuin-A followed by toxic dose of vitamin D (Fig. 8), the kidneys showed largely normal glomeruli and renal tubules (Fig. 8A) with no detectable calcification by Alizarin Red stains (Fig. 8B). Only fetuin A group showed normal kidney structure (Fig. 9A) and no calcification (Fig. 9B).
Fig. 6.
Histology of negative control mice Kidney, (A) H&E showing normal kidney structure, (B) Alizarin Red showing no calcification, High Power (× 40).
Fig. 7.
Histology of mice kidney after taking the toxic dose of Vit D, (A–C), H&E showing Glomerular shrinkage, wide Bowman’s capsule, inflammatory cell infiltrate, Hyaline casts, damaged tubular epithelium, Apoptosis, necrosis, calcification deposits, (D) Alizarin Red showing massive calcification, High Power (× 40).
Fig. 8.
Histology of mice kidney after taking the Fetuin-A followed by toxic dose of Vit D, (A) H&E showing minimal inflammation, Epithelial degeneration, Hyaline casts, (B) Alizarin Red showing minimal calcification, High Power (× 40).
Fig. 9.
Histology of mice kidney after taking the Fetuin-A only, (A) H&E showing normal kidney structure, (B) Alizarin Red showing no calcification, High Power (× 40).
Heart
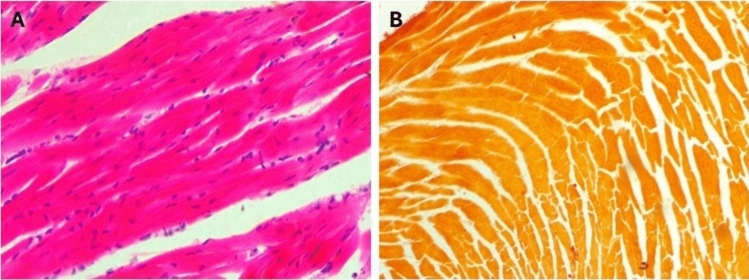
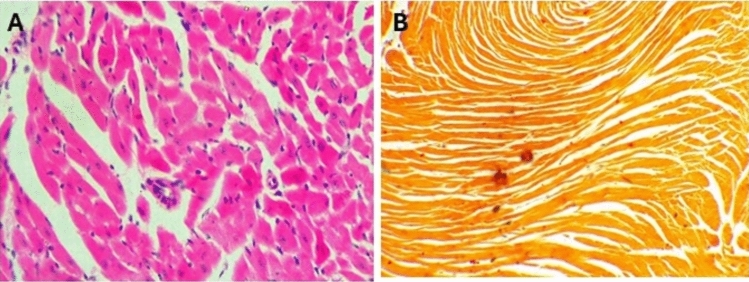
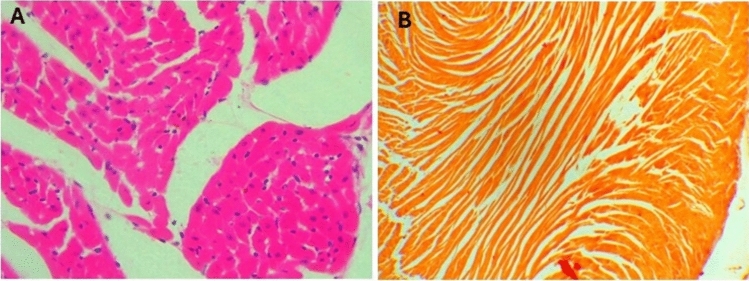
In sections from the hearts of control mice (Fig. 10A), cardiac muscles appeared cylindrical with branched fibers running in various directions, displaying ill-defined striations and oval, vesicular, central nuclei. Scanty loose connective tissue with thin-walled blood capillaries and branches of the coronary artery, lined by simple squamous cells and having a thin muscular wall, were observed among the fibers, no calcification was seen on the renal tissue on staining with Alizarin (Fig. 10B). In mice given a toxic dose of vitamin D, slight changes and congestion were observed in the coronary arteries, with dilated and congested capillaries among the fibers, some showing apoptotic changes, and inflammatory cells, including lymphocytes (Fig. 11A–C). Calcification deposits were detected by Alizarin Red stain (Fig. 11D). In mice supplemented with Fetuin-A as prophylaxis, the cardiac muscles appeared mostly normal, with only minimal inflammatory cell infiltration (Fig. 12A), and very minimal calcification as shown by Alizarin Red stain (Fig. 12cB). Only fetuin A group showed normal cardiac fibers (Fig. 13A) and no calcification (Fig. 13B).
Fig. 10.
Histology of negative control mice heart, (A) H&E showing normal cardiac Fibers, (B) Alizarin Red showing no calcification, Low Power (× 10).
Fig. 11.
Histology of mice heart after taking the toxic dose of Vit D, (A–C), H&E Showing Apoptotic Myocytes, dilated and congested capillaries, inflammatory cell infiltrate, damaged cardiac fibers, (D) Alizarin Red showing calcification, Low Power (× 10).
Fig. 12.
Histology of mice heart after taking the Fetuin-A followed by toxic dose of Vit D, (A) H&E showing minimal inflammatory infiltrate of lymphocytes, (B) Alizarin Red showing minimal calcification, Low Power (× 10).
Fig. 13.
Histology of mice heart after taking the Fetuin-A only, (A), H&E showing normal cardiac Fibers, (B) Alizarin Red showing no calcification, Low Power (× 10).
Discussion
This study highlights the severe physiological and histopathological effects of hypervitaminosis D and the protective potential of Fetuin-A in mitigating these outcomes. Mice exposed to toxic doses of vitamin D exhibited significant reductions in activity levels, extensive tissue calcification, and inflammation in the liver, kidneys, and heart. In contrast, pre-treatment with Fetuin-A significantly alleviated these adverse effects, as evidenced by improved activity levels, reduced calcification, and preserved tissue architecture. These findings underscore the pathological consequences of vitamin D toxicity and emphasize Fetuin-A’s therapeutic promise.
Hypervitaminosis D primarily results from excessive vitamin D intake, leading to hypercalcemia, which disrupts calcium and phosphate homeostasis and causes systemic complications2. The underlying mechanisms involve the activation of the vitamin D receptor (VDR), which forms a heterodimer with the retinoid X receptor (RXR) and binds to vitamin D response elements (VDREs) in DNA, modulating genes that regulate calcium and phosphate metabolism25. High levels of 1,25-dihydroxyvitamin D (1,25(OH)₂D) amplify VDR activation, resulting in increased calcium absorption in the intestines, elevated bone resorption driven by RANKL overexpression, and reduced renal calcium excretion due to decreased transporter expression. These processes overwhelm calcium-regulating mechanisms, such as parathyroid hormone (PTH) and calcium-sensing receptors, leading to persistent hypercalcemia, ectopic calcification, and tissue dysfunction25.
In this study, mice in the Vitamin D Toxicity group exhibited substantial histopathological damage across multiple organ systems, consistent with these mechanisms. The liver showed portal vein dilation (5.92 ± 0.22 mm, p < .001 vs. Control), inflammatory infiltration, Kupffer cell hyperplasia, and calcification, consistent with findings reported by Ali et al.26. Hypercalcemia-induced vascular dysfunction, including endothelial damage, increased permeability, and reduced nitric oxide (NO) production, likely contributed to these changes3,26. Similarly, renal tissues displayed nephrocalcinosis, tubular damage, and widespread calcification, while cardiac tissues exhibited apoptotic fibers, inflammatory infiltration, and calcification deposits. These findings align with prior studies demonstrating the systemic effects of hypervitaminosis D, including nephrocalcinosis, vascular calcification, and cardiac dysfunction27–29.
Pre-treatment with Fetuin-A mitigated these adverse effects, as shown by improved functional and histological outcomes in the Fetuin-A + Vitamin D group. Activity levels, which were significantly reduced in the Vitamin D Toxicity group (71 ± 8.96 steps/min, p < .001 vs. Control), improved to 103 ± 8.2 steps/min (p < .001 vs. Vitamin D Toxicity) with Fetuin-A pre-treatment. Portal vein dilation was markedly reduced in the Fetuin-A + Vitamin D group (2.9 ± 0.23 mm, p < .001 vs. Vitamin D Toxicity), and histological analysis revealed preserved tissue architecture, reduced calcification, and minimal inflammation in the liver, kidneys, and heart. These findings are consistent with Fetuin-A’s molecular mechanisms, including its ability to bind calcium and phosphate ions to form soluble calciprotein particles (CPPs), preventing ectopic calcification in soft tissues15,19.30,31 Fetuin-A also inhibits the osteogenic transition of vascular smooth muscle cells (VSMCs), stabilizes the extracellular matrix, and suppresses pro-inflammatory pathways, reducing systemic inflammation32–35.
Interestingly, the Fetuin-A Only group displayed activity levels (120 ± 1.94 steps/min) and portal vein diameters (2.02 ± 0.08 mm) comparable to the Control group (p > .05), confirming that Fetuin-A does not adversely affect normal physiological or vascular function. However, mild fatty degeneration of hepatocytes, characterized by signet ring-like cells, was observed in both the Fetuin-A + Vitamin D and Fetuin-A Only groups. Similar findings were reported by Dogru et al.36 who concluded that While these changes are reversible, they warrant further investigation into the long-term hepatic effects of Fetuin-A, particularly under conditions of sustained supplementation21,37.
The protective efficacy of Fetuin-A varied across organs. It demonstrated the most pronounced effects in the kidneys and heart, with minimal inflammation and no detectable calcification in these tissues. In contrast, hepatic changes, including mild fatty degeneration, persisted despite Fetuin-A pre-treatment14. These findings emphasize the importance of optimizing dosing strategies and exploring combination therapies to enhance Fetuin-A’s efficacy in the liver.
The broader implications of this study highlight Fetuin-A’s potential as a therapeutic agent for managing hypervitaminosis D. By mitigating calcification, reducing inflammation, and preserving tissue function, Fetuin-A addresses key pathological processes associated with vitamin D toxicity. Combining Fetuin-A with other treatments, such as bisphosphonates, may enhance therapeutic outcomes by simultaneously reducing calcium release from bones and preventing its deposition in soft tissues38. Additionally, novel approaches, such as gene therapy to enhance endogenous production of Fetuin-A, could provide sustainable solutions for patients with chronic hypercalcemia or requiring high doses of vitamin D for therapeutic purposes39,40.
By providing critical insights into the pathological effects of hypervitaminosis D and the protective mechanisms of Fetuin-A, this study highlights a potential therapeutic approach for mitigating the systemic complications of vitamin D toxicity. These findings contribute to a growing body of research aimed at improving the safety and efficacy of vitamin D use in clinical practice.
Study limitations and future directions
One limitation of this study is the lack of quantitative analysis of calcification beyond portal vein diameter. Future research should incorporate advanced imaging techniques and biochemical assessments, such as serum calcium, phosphate, and Fetuin-A levels, to provide a more comprehensive understanding of vitamin D toxicity and Fetuin-A’s protective effects. Investigating hypervitaminosis D’s impact on additional organs, including the gastrointestinal tract, lungs, pancreas, and immune system, could further elucidate its systemic effects 2. Exploring optimal dosing strategies and the long-term safety of Fetuin-A, including its combination with other protective agents like omega-3 fatty acids, could enhance its clinical applicability. Additionally, studies focusing on Fetuin-A’s role in vascular calcification and its interplay with metabolic and inflammatory pathways may provide deeper insights into its therapeutic potential.
Conclusion
This study demonstrated that high doses of vitamin D caused significant cellular and tissue damage in renal, hepatic, and cardiac tissues, including calcification, inflammation, and. The administration of Fetuin-A effectively mitigated these effects, reducing calcification, inflammation, and preserving tissue architecture across all organs examined. Histopathological analysis revealed that the protective effects of Fetuin-A varied between different organs, with the most substantial impact observed in the kidneys and heart, while some hepatic changes, such as fatty degeneration, persisted. These findings highlight the potential of Fetuin-A as a therapeutic agent for managing vitamin D toxicity, though further research is needed to optimize its use and explore combination therapies for enhanced protection, particularly in vulnerable organs like the liver.
With continued research, Fetuin-A may offer a novel approach for managing vitamin D overdose, providing new hope for preventing the harmful effects associated with excessive vitamin D intake and ensuring the safe therapeutic use of vitamin D in clinical practice.
Supplementary Information
Acknowledgements
This work was conducted and funded using the facilities of Dubai Medical College for Girls, Dubai, UAE. We acknowledge Mr. Khan Fayyaz (Lab Technician) and Mr. Omar Hussein (Office boy) for their kind support throughout the work.
Author contributions
M.A., M.H. conceptualised the research.All authors contributed equally for data collection, data analysis, manuscript drafting and revision of final manuscript.
Funding
This study was funded by Dubai Medical College for Girls, Dubai, UAE.
Data availability
The data shall be made available upon request from the corresponding author.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This project was approved by Institutional Review Board in Dubai Medical College for Girls and it was in compliance with the regulations of ethical treatment of laboratory animals. (REC AY22-23-F-01).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-025-85200-1.
References
- 1.Kaur, P., Mishra, S. & Mithal, A. Vitamin d toxicity resulting from overzealous correction of vitamin d deficiency. Clin. Endocrinol.83(3), 327–331. 10.1111/cen.12836 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Marcinowska-Suchowierska, E., Kupisz-Urbańska, M., Łukaszkiewicz, J., Płudowski, P. & Jones, G. Vitamin D toxicity-A clinical perspective. Front. Endocrinol.9, 550. 10.3389/fendo.2018.00550 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silva, C. et al. Vitamin d toxicity from an unusual and unexpected source: a report of 2 cases. Hormone Res. Paediatr.96(3), 332–340. 10.1159/000526755 (2022). [DOI] [PubMed] [Google Scholar]
- 4.Farnaghi, F. et al. Vitamin d toxicity in a pediatric toxicological referral center; A cross-sectional study from iran. BMC Pediatrics10.1186/s12887-020-02240-4 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raizada, N. et al. Changing trend in vitamin d status from 2008 to 2016: an experience from a tertiary care institute in North India. Indian J. Endocrinol. Metab.24(2), 150. 10.4103/ijem.ijem_634_19 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Araki, T. et al. Vitamin D intoxication with severe hypercalcemia due to manufacturing and labeling errors of two dietary supplements made in the United States. J. Clin. Endocrinol. Metab.96(12), 3603–3608 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Galior, K., Grebe, S. & Singh, R. Development of vitamin d toxicity from overcorrection of vitamin d deficiency: a review of case reports. Nutrients10(8), 953. 10.3390/nu10080953 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gérard, A. et al. Case report: severe hypercalcemia following vitamin d intoxication in an infant, the underestimated danger of dietary supplements. Front. Pediatr.10, 816965. 10.3389/fped.2022.816965 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Padalka, V., Kurdil, N., Zinovieva, M. & Petrashenko, H. Vitamin d toxicity syndrome: a toxicologist’s view. One Health Nutr. Probl. Ukraine55(2), 83–93. 10.33273/2663-9726-2021-55-2-83-93 (2021). [Google Scholar]
- 10.Rastogi, S. Hypervitaminosis d due to overdose: A case series. Indian J. Pain38(2), 153–155. 10.4103/ijpn.ijpn_89_23 (2024). [Google Scholar]
- 11.Mangas-Sánchez, C. et al. Vitamin d status in pediatric and young adult cystic fibrosis patients. Are the new recommendations effective?. Nutrients13(12), 4413. 10.3390/nu13124413 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaufmann, M. et al. Differential diagnosis of vitamin d–related hypercalcemia using serum vitamin d metabolite profiling. J. Bone Mineral Res.36(7), 1340–1350. 10.1002/jbmr.4306 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Wada, T., Hashimoto, M. & Saijo, A. Acute hypercalcemia and hypervitaminosis d associated with pulmonary tuberculosis in an elderly patient: A case report and review of the literature. J. Med. Investig.66(34), 351–354. 10.2152/jmi.66.351 (2019). [DOI] [PubMed] [Google Scholar]
- 14.Ashour, W. M. R., Zamzam, M. S. A., Sayed Ali, H. E. E. D. E. & Ebrahim, R. H. Effect of fetuin-A on adenine-induced chronic kidney disease model in male rats. Iran J. Basic Med. Sci.26, 511–516. 10.22038/IJBMS.2023.66346.14584 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brylka, L. & Jahnen-Dechent, W. The role of fetuin-A in physiological and pathological mineralization. Calcif. Tissue Int.93(4), 355–364. 10.1007/s00223-012-9690-6 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Haukeland, J. et al. Fetuin a in nonalcoholic fatty liver disease: in vivo and in vitro studies. Acta Endocrinol.166(3), 503–510. 10.1530/eje-11-0864 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud, A., Mahmoud, H., El-Fadl, A. & Kandeel, M. Serum fetuin-a as a perdictive biomarker of chronic kidney disease. Aswan Univ. Med. J.2(2), 97–111. 10.21608/aumj.2022.147302.1013 (2022). [Google Scholar]
- 18.Naito, C. et al. Facilitatory effects of fetuin-A on atherosclerosis. Atherosclerosis246, 344–351. 10.1016/j.atherosclerosis.2016.01.037 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Hesse, M., Frohlich, L. F., Zeitz, U. & Pischon, N. Fetuin-A—A multifunctional protein at the interface of mineral metabolism, inflammation, and cardiovascular disease. Int. J. Mol. Sci.21(6), 2204. 10.3390/ijms210622044o (2020).32210002 [Google Scholar]
- 20.Kang, Y. H., Jin, J. S., Yi, D. W. & Son, S. M. Bone morphogenetic protein-7 inhibits vascular calcification induced by high vitamin D in mice. Tohoku J. Exp. Med.221(4), 299–307. 10.1620/tjem.221.299 (2010). [DOI] [PubMed] [Google Scholar]
- 21.Zhang, P. et al. Intraperitoneal administration of fetuin-A attenuates D-galactosamine/lipopolysaccharide-induced liver failure in mice. Dig. Dis. Sci.59(8), 1789–1797. 10.1007/s10620-014-3071-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janoušek, J. et al. Vitamin d: sources, physiological role, biokinetics, deficiency, therapeutic use, toxicity, and overview of analytical methods for detection of vitamin d and its metabolites. Crit. Rev. Clin. Lab. Sci.59(8), 517–554. 10.1080/10408363.2022.2070595 (2022). [DOI] [PubMed] [Google Scholar]
- 23.Nair, A. B. & Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm.7(2), 27–31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gancarz, A. M., San George, M. A., Ashrafioun, L. & Richards, J. B. Locomotor activity in a novel environment predicts both responding for a visual stimulus and self-administration of a low dose of methamphetamine in rats. Behav. Proc.86(2), 295–304. 10.1016/j.beproc.2010.12.013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christakos, S., Dhawan, P., Verstuyf, A., Verlinden, L. & Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev.96(1), 365–408. 10.1152/physrev.00014.2015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ali, S. S., Mahassni, S. H. & Alnefaie, R. M. The effects of hypervitaminosis D in rats on histology and weights of some immune system organs and organs prone to calcification. Int. J. Pharmaceut. Phytopharmacol. Res.8(6), 59–71 (2018). [Google Scholar]
- 27.Bouderlique, E. et al. Vitamin D and calcium supplementation accelerate vascular calcification in a model of pseudoxanthoma elasticum. Int. J. Mol. Sci.23(4), 2302. 10.3390/ijms23042302 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chavhan, S. G. et al. Clinicopathological studies on vitamin D(3) toxicity and therapeutic evaluation of aloe vera in rats. Toxicol. Int.18(1), 35–43. 10.4103/0971-6580.75851 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nasri, H. & Mubarak, M. Renal injury due to vitamin D intoxication; a case of dispensing error. J. Renal Injury Prevent.2(2), 85–87. 10.12861/jrip.2013.27 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassan, V. S., Hanifa, M., Navik, U. & Bali, A. Exogenous fetuin-A protects against sepsis-induced myocardial injury by inhibiting oxidative stress and inflammation in mice. Fundam. Clin. Pharmacol.37(3), 607–617. 10.1111/fcp.12870 (2023). [DOI] [PubMed] [Google Scholar]
- 31.Price, P. A., Nguyen, T. M. T. & Williamson, M. K. Biochemical characterization of the serum Fetuin-mineral complex. J. Biol. Chem.278(24), 22153–22160. 10.1074/jbc.M300739200 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Dautova, Y. et al. Fetuin-A and albumin alter cytotoxic effects of calcium phosphate nanoparticles on human vascular smooth muscle cells. Public Library Sci.9(5), e97565–e97565. 10.1371/journal.pone.0097565 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hansen, D., Rasmussen, K., Rasmussen, L. M., Bruunsgaard, H. & Brandi, L. The influence of vitamin d analogs on calcification modulators, n-terminal pro-b-type natriuretic peptide and inflammatory markers in hemodialysis patients: a randomized crossover study. BMC Nephrol.10.1186/1471-2369-15-130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schäfer, C. & Jahnen‐Dechent, W. The biological and cellular role of Fetuin family proteins in biomineralization, 317–328 (2007). 10.1002/9783527619443.ch64
- 35.Westenfeld, R. et al. Fetuin-A protects against atherosclerotic calcification in CKD. J. Am. Soc. Nephrol. JASN20(6), 1264–1274. 10.1681/ASN.2008060572 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dogru, T. et al. The evolving role of fetuin-a in nonalcoholic fatty liver disease: An overview from liver to the heart. Int. J. Mol. Sci.22(12), 6627. 10.3390/ijms22126627 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Etienne, Q. et al. Fetuin-a in activated liver macrophages is a key feature of non-alcoholic steatohepatitis. Metabolites12(7), 625. 10.3390/metabo12070625 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang, S., Wright, J. E. I., Bansal, G., Cho, P. & Uludag, H. Cleavage of disulfide-linked fetuin−bisphosphonate conjugates with three physiological thiols. Biomacromolecules6(5), 2800–2808. 10.1021/bm050273s (2005). [DOI] [PubMed] [Google Scholar]
- 39.Jensen, M. K. et al. Detection of genetic loci associated with plasma fetuin-A: A meta-analysis of genome-wide association studies from the CHARGE Consortium. Hum. Mol. Genet.26(11), 2156–2163. 10.1093/hmg/ddx091.PMID:28379451;PMCID:PMC6075215 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ochieng, J. et al. Impact of Fetuin-A (AHSG) on tumor progression and type 2 diabetes. Int. J. Mol. Sci.19(8), 2211. 10.3390/ijms19082211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data shall be made available upon request from the corresponding author.