Abstract
Weight loss, a key indicator of malnutrition in amyotrophic lateral sclerosis (ALS) patients, negatively impacts patient prognosis. However, effective nutritional interventions have not been adequately established. Research in ALS model mice has shown that L-arginine can prolong survival; however, no human intervention studies have been conducted. We conducted a single-center, single-arm, prospective, open-label, and comparative trial to assess the safety and tolerability of L-arginine hydrochloride in ALS patients. ALS patients were administered 15 g/day L-arginine hydrochloride for 90 days. The primary outcome of safety was evaluated on days 45 and 90. The secondary outcome of efficacy was evaluated by measuring nutritional status, ALS Functional Rating Scale (ALSFRS) scores, and the occurrence of events such as the initiation of tracheostomy positive pressure ventilation (TPPV) and death. The study included 20 patients (40% female; mean age, 62.0 ± 6.9 years; median disease duration, 1.9 years). Six participants (30%) experienced treatment-emergent adverse events (TEAEs), including elevated creatine kinase levels, liver function test abnormalities, glucose tolerance issues, hyperammonemia, anorexia, dysgeusia, and vasculitis. No serious TEAEs were associated with L-arginine hydrochloride. Over the course of three months, the average changes in body weight, body mass index, and the ALSFRS score were − 0.37 kg, -1.1 kg/m2, and − 1.7 points, respectively. There were no events requiring TPPV initiation or deaths. This study demonstrated that the oral administration of L-arginine hydrochloride over three months was well tolerated by ALS patients, with no serious TEAEs or deaths attributed to the study drug.
Trial Registration number: Japan Registry of Clinical Trials (jRCTs061230001), first registered 11/04/2023.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-84944-6.
Keywords: Amyotrophic lateral sclerosis, L-arginine hydrochloride, Nutrition, Body weight, Body mass index
Subject terms: Health care, Neurology
Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, fatal neurodegenerative disorder for which there is no effective cure. Moreover, malnutrition significantly impacts the prognosis and overall outcomes of this disease and worsens quality of life. Hence, thorough assessment and appropriate management of nutritional status are critical components of ALS care. Despite extensive research, effective nutritional interventions have yet to be established. Most trials have focused predominantly on high-calorie, high-fat diets. Studies in ALS mouse models have reported that the administration of a high-calorie, high-fat diet leads to weight gain and disease progression inhibition1, contributing to prolonged survival2. In clinical studies investigating high-calorie diets for ALS patients, Wang et al. reported that six months of high-calorie nutritional supplementation following PEG placement improved nutritional status and potentially extended survival3. In contrast, Ludolph et al. reported no significant survival benefit in ALS patients receiving a high-calorie, high-fat diet (equivalent to an additional 45 g of fat and 405 kcal per day) compared with a placebo4, highlighting inconsistent findings. A systematic review and meta-analysis on the efficacy, safety, and tolerability of high-calorie diets in ALS patients confirmed that such supplementation is generally safe and well tolerated5. However, no evidence was found to support its efficacy in improving weight or functional disability. These findings indicate that high-calorie, high-fat diets may not exert disease-modifying effects on ALS.
In ALS, one of the mechanisms contributing to motor neuron death is believed to be excessive activation of glutamate receptors due to abnormalities in amino acid metabolism. Riluzole, a drug that inhibits glutamate release, exemplifies the critical link between amino acid metabolism and ALS pathophysiology. It is postulated that L-arginine confers neuroprotection to motor neurons against glutamate toxicity and is a potential therapeutic target for ALS. Additionally, L-arginine is thought to increase skeletal muscle glucose metabolism and sustain muscle energy production, which may protect against ALS progression. In vitro studies using mature C2C12 myotubes have demonstrated that L-arginine can protect against skeletal muscle cell atrophy through the activation of the mechanistic target of rapamycin complex 1 and increased synthesis of skeletal muscle proteins4. Observations indicate that serum L-arginine levels are typically low in ALS patients5, which is correlated with the malnutrition often observed in this population. Preclinical trials involving presymptomatic mtSOD1(G93A) mice, an animal model of ALS, have shown that administering L-arginine delays the onset of motor symptoms and prolongs survival6. Moreover, nitric oxide synthase (NOS), which is implicated in motor neuron degeneration in ALS models, was found to be downregulated following L-arginine supplementation in G93A ALS mice, underscoring its neuroprotective effects7.
Despite these promising preclinical findings, clinical studies investigating L-arginine supplementation in ALS patients are lacking. To address this gap, we conducted a prospective clinical trial to evaluate the safety and efficacy of oral L-arginine supplementation in ALS patients.
Results
Background characteristics
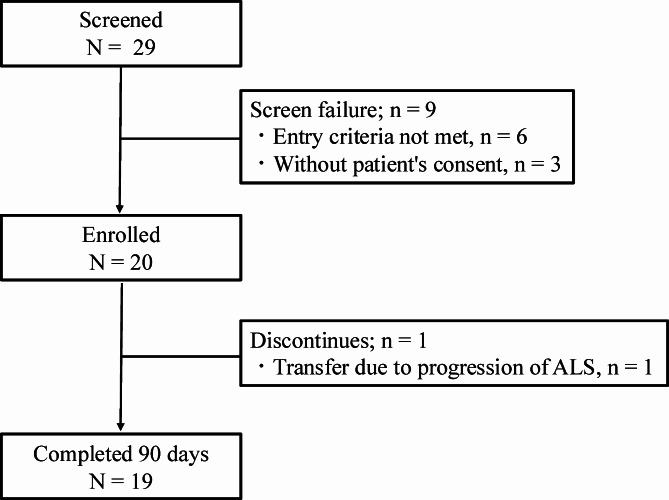
From May 2023 to October 2023, a total of 29 patients were screened for this study, 20 of whom were enrolled and received oral L-arginine hydrochloride. Six patients did not meet the inclusion criteria, and three did not provide consent. All 20 patients completed 45 days of intervention, and 19 completed 90 days, reflecting a discontinuation rate of 5% (Fig. 1). One participant discontinued the study on day 75 because of the progression of ALS-related symptoms. The clinical and demographic data are presented in Table 1. The majority of patients (75%) were concurrently using riluzole, and most had not yet undergone PEG or received NPPV. Before propensity score matching, the L-arginine hydrochloride group was younger than the nonadministration group was, but after matching, there were no significant differences in baseline characteristics between the two groups (Supplementary Table S1).
Fig. 1.
Patient selection flow diagram. Amyotrophic lateral sclerosis
Table 1.
Patient background information.
| N = 20 | |
|---|---|
| Age, years | 62.0 ± 6.9 |
| Sex (female), n (%) | 8 (40.0) |
| Disease duration, years | 1.9 (0.4, 4.8) |
| Initial symptom, n (%) | |
| Bulbar onset | 5 (25.0) |
| Limb onset | 14 (70.0) |
| Respiratory failure | 1 (5.0) |
| ALSFRS-R score | 38.4 ± 6.5 |
| Body weight, kg | 59.7 ± 12.5 |
| Body mass index, kg/m2 | 22.3 ± 3.4 |
| Soft lean mass, kg | 40.1 ± 10.0 |
| Body fat mass, kg | 17.0 ± 5.0 |
| MNA score | 23.1 ± 4.5 |
| mRMR, kcal | 1294 ± 221 |
| TEE, kcal | 1934 ± 396 |
| Plasma amino acids (arginine), nmol/ml | 73.2 ± 17.4 |
| Urine amino acids (arginine), µmol/g・cre | 20.0 ± 15.3 |
| Concomitant use of riluzole, n (%) | 15 (75.0) |
| Concomitant use of edaravone, n (%) | 6 (30.0) |
| PEG, n (%) | 1 (5.0) |
| NPPV, n (%) | 1 (5.0) |
ALSFRS-R, revised ALS functional rating scale; MNA, mini nutritional assessment; mRMR, measured resting metabolic rate; TEE, total energy expenditure; PEG, percutaneous endoscopic gastrostomy; NPPV, noninvasive positive pressure ventilation.
The data are expressed as the mean ± standard deviation or median (minimum, maximum) for continuous variables and as frequencies and percentages for discrete variables.
Safety assessments
During the study period, 4 patients (25%) experienced TEAEs within the first 45 days, and 6 patients (31.6%) experienced TEAEs within 90 days (Table 2). By day 45, the reported TEAEs included elevated creatinine kinase (CK) levels in 3 patients (CK levels of 900, 350, and 648 U/L, respectively) and anorexia in 1 patient. By the end of the 90-day intervention, the reported TEAEs were elevated CK levels in 2 patients (384 and 584 U/L); liver function test abnormalities, including elevated aspartate aminotransferase (AST) and γ-glutamyl transpeptidase (γ-GTP) levels, in 1 patient (AST level of 123 U/L, γ-GTP level of 545 U/L); glucose tolerance abnormalities in 1 patient (fasting blood glucose level of 173 mg/dL and hemoglobin A1c level of 6.6%); hyperammonemia in 1 patient (ammonia level of 70 µg/dL); dysgeusia in 1 patient; and vasculitis in 1 patient. Among the TEAEs, elevated serum CK levels, liver function test abnormalities, glucose tolerance abnormalities, hyperammonemia, and vasculitis were determined by the investigators to be unrelated to the study drug. The investigator considered anorexia and dysgeusia to be related to the study drug. No AEs were severe, and there were no instances of study discontinuation or death due to AEs. With respect to clinical events, there was one instance where NPPV was initiated during the study. However, there were no cases of pneumonia, gastrostomy tube placement, TPPV initiation, or death. For the administration of L-arginine hydrochloride, the median number of missed doses was 1 (range: 0–43), with an adherence rate of 99.7%.
Table 2.
TEAEs, events, and compliance rates at 90 days or until discontinuation.
| TEAE | N = 20 |
|---|---|
| Any TEAEs, n (%) | 6 (30) |
| Elevated creatinine kinase levels, n (%) | 3 (15) |
| Liver function test abnormalities, n (%) | 1 (5) |
| Glucose tolerance abnormalities, n (%) | 1 (5) |
| Hyperammonemia, n (%) | 1 (5) |
| Anorexia, n (%) | 1 (5) |
| Dysgeusia, n (%) | 1 (5) |
| Vasculitis, n (%) | 1 (5) |
| Event | |
| Pneumonia, n (%) | 0 |
| PEG, n (%) | 0 |
| NPPV, n (%) | 1 (5) |
| TPPV, n (%) | 0 |
| Death, n (%) | 0 |
| Medication intake | |
| Number of missed medication doses | 1 (0, 43) |
| Adherence rate (%) | 99.7 (76.1, 100) |
TEAE, treatment-emergent adverse event; PEG, percutaneous endoscopic gastrostomy; NPPV, noninvasive positive pressure ventilation; TPPV, tracheostomy positive pressure ventilation. Data are expressed as the mean ± standard deviation or median (minimum, maximum) for continuous variables and as frequencies and percentages for discrete variables.
Effect of L-arginine on clinical outcomes
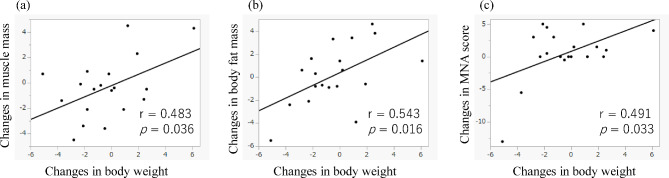
From the initiation of L-arginine hydrochloride administration to the end of the study, the average change in body weight was − 0.37 kg, and the average change in BMI was − 0.11 kg/m². There were 12 patients (63.2%) whose body weight decreased. The average change in muscle mass was − 0.38 kg, whereas the average change in body fat mass was + 0.17 kg. After propensity score matching, no significant differences were observed between the two groups in terms of the monthly weight change rate (arginine hydrochloride group: -0.10 kg/month; nonarginine group: -0.12 kg/month; p = 0.968). The MNA total score increased by 0.5 points on average. The ALSFRS-R score decreased by an average of -1.7 points. Changes in muscle mass, fat mass, and the MNA score from baseline to 3 months were significantly correlated with changes in body weight (muscle mass, p = 0.036; body fat mass, p = 0.016; MNA score, p = 0.033) (Fig. 2). No significant differences were found between the weight increase and weight loss groups regarding baseline data, changes in the ALSFRS-R score, body composition, or nutritional assessment (Supplementary Table S2). Although no significant differences in baseline plasma or urinary arginine levels were detected between the groups, the proportions of individuals with high plasma and urinary arginine levels at 90 days after the start of the intervention and the urinary arginine ratio (day 90/baseline) were significantly greater in the weight increase group than in the weight loss group (p = 0.020, p = 0.020, and p = 0.019, respectively). Additionally, the urinary arginine ratio (day 45/baseline) was significantly greater in the weight increase group (p = 0.048). There were no significant differences in the frequency of events or TEAEs between the groups.
Fig. 2.
(a–c) Scatter plot of the changes in body weight and nutritional status, including muscle mass, body fat mass, and the MNA score, from baseline to 3 months. Linear correlations were observed between the changes in body weight and each measure of nutritional status.
Discussion
In this study, we investigated the safety of L-arginine hydrochloride administered orally over a 90-day period in patients with ALS and explored its effects on nutritional status. L-arginine hydrochloride was generally well tolerated, as evidenced by a low rate of study discontinuation and high medication adherence, with no treatment-related serious AEs or deaths reported. Changes in muscle mass, fat mass, and the MNA score were significantly correlated with changes in body weight, and patients without weight loss presented increased L-arginine serum and urine levels.
With respect to the use of oral L-arginine formulations that combine L-arginine and L-arginine hydrochloride, AEs such as liver function abnormalities, nausea, vomiting, and diarrhea have been reported8. Additionally, arginine hydrochloride loading has been linked to renal dysfunction and glucose tolerance abnormalities. In this study, the reported TEAEs included elevated CK levels, liver function test abnormalities, glucose tolerance abnormalities, hyperammonemia, taste alterations, appetite loss, and vasculitis. Elevated CK levels are associated with the progression of ALS, whereas liver function test abnormalities and glucose tolerance abnormalities are related to comorbid conditions (alcoholic liver injury and type 2 diabetes, respectively). Given that L-arginine is used to treat hyperammonemia in patients with urea cycle disorders9, the occurrence of hyperammonemia in this study was considered unrelated to L-arginine hydrochloride administration. In previous safety studies in healthy individuals, L-arginine hydrochloride was administered at dosages of 0, 15, and 30 g/day, with safety confirmed to be up to 30 g/day10. However, considering the substantial load at 30 g/day, a dosage of 15 g/day was selected for this study. No serious AEs or deaths occurred during the study. One patient discontinued treatment due to ALS progression, and the study confirmed good tolerability given the high medication adherence rate.
Weight loss, which is recognized as a marker of malnutrition in individuals with ALS, has been identified as a poor prognostic factor and is utilized for the longitudinal assessment of nutrition. In Japan, a prediagnosis rate of BMI reduction of 2–2.5 kg/m2/year has been reported as an indicator of poor prognosis11,12. Additionally, a decrease in BMI of 1.7 kg/m2/year or more from diagnosis to tracheostomy has been associated with poorer functional prognosis post-TPPV13. In ALS patients, weight loss is often driven by a combination of factors such as skeletal muscle atrophy, decreased fat mass, and increased respiratory muscle energy demands14, suggesting that BMI maintenance could improve life expectancy. In this study, three months of L-arginine administration in ALS patients who did not require TPPV resulted in a modest decrease in BMI of 0.11 kg/m2 and a decrease in body weight of 0.37 kg. Ngo ST et al. reported that Caucasian ALS patients experienced monthly changes, with a decrease in BMI of -0.13 kg/m2 and a weight loss of -0.28 kg15. To address potential racial disparities in ALS, we compared a cohort that was administered L-arginine hydrochloride with a Japanese ALS patient cohort from an epidemiological study that investigated the natural history of ALS. After propensity score matching, no significant differences were observed between the two groups in terms of the monthly weight change rate, indicating that differences in the patient population may influence BMI change rates and must be considered. However, the observed BMI reduction rate in this study was lower than the rates reported as prognostic indicators of poor outcomes in Japanese ALS patients11–13. Furthermore, in the group that gained weight, the serum and urine L-arginine ratios were significantly greater than those in the group that lost weight, suggesting a potential role of L-arginine in weight maintenance. L-arginine is suggested to improve skeletal muscle glucose metabolism4 and protect motor neurons against glutamate toxicity through nitric oxide (NO) synthesis7. Latif S et al. reported that L-arginine supplementation restored neural NOS expression in ALS disease model cell lines and provided neuroprotective effects against the oxidative stress-inducing agent H2O216. Arginine methylation is implicated in the pathology of ALS. Asymmetric demethylation has been shown to regulate muscle function, and proteomics analysis of skeletal muscle biopsies from ALS patients revealed site-specific changes in asymmetric dimethyl arginine (ADMA)17. Protein arginine methyltransferase 1, which is responsible for ADMA production, has been reported to regulate the cytotoxicity of ALS-associated FUS mutants18,19. Furthermore, Ikenaka et al. reported that the ratio of asymmetric dimethylarginine (ADMA) to arginine is a predictor of outcomes in ALS20 and demonstrated a significant correlation between neurofilament and serum ADMA levels21, suggesting the potential use of ADMA as a biomarker for ALS. Recent studies have also used L-arginine in animal models of spinocerebellar ataxia and spinal muscular atrophy, in which polyglutamine aggregate formation was inhibited and motor symptoms were improved22. Additionally, arginine has been shown to inhibit abnormal aggregation of the RNA-binding protein fused in sarcoma, which is implicated in ALS pathogenesis23, making it a promising target for ALS treatment. In this study, the ALSFRS-R score decreased by an average of 1.7 points over three months of L-arginine administration. Importantly, there were no deaths or new initiations of TPPV during the trial period.
This study has several limitations. First, this was a single-site, single-group intervention trial that presents the first investigation into the safety of L-arginine hydrochloride in patients with ALS. As an exploratory trial aimed at examining the potential benefits of L-arginine hydrochloride for improving nutritional status and outcomes in ALS patients, no control group was established, and the study design involved a pre- and postintervention comparison within the same group. In addition, we did not evaluate exploratory efficacy biomarkers such as the neurofilament light chain or target engagement biomarkers indicating the efficacy of L-arginine hydrochloride. Future studies should consider employing randomized controlled trials, including a nonintervention group, to facilitate intergroup comparisons. Second, the limited number of participants represents a challenge. This study focused primarily on the safety of L-arginine hydrochloride; therefore, the sample size was determined on the basis of the incidence rate of AEs related to L-arginine hydrochloride reported in clinical trials regarding its approval and postmarketing surveillance, as well as accounting for potential dropouts. Third, this study did not evaluate dietary energy or protein intake in ALS patients during the trial period. Therefore, it is unclear whether the observed weight maintenance was due to L-arginine hydrochloride supplementation itself or an increase in the patients’ energy intake. Fourth, the study excluded patients who used TPPV and thus did not include those with advanced ALS. ALS is characterized by weight loss due to hypermetabolism in the early stages, but after the initiation of TPPV, metabolic changes often lead to weight gain24, necessitating different nutritional interventions. A comprehensive examination and analysis of the diverse clinical courses of ALS patients requires the inclusion of a greater number of patients. Therefore, the recruitment of a greater number of patients is necessary for future research.
In conclusion, this study demonstrated that oral L-arginine hydrochloride administered over the course of 90 days was well tolerated, with no serious TEAEs or deaths related to the intervention. In ALS patients who received L-arginine hydrochloride, the maintenance of body weight—an indicator of nutritional status—was observed throughout the intervention period. This maintenance was suggested to correlate with the concentrations of L-arginine hydrochloride in the blood and urine. Clinically, randomized controlled trials comparing the treatment to placebo, as well as biological validation in basic experiments, are needed in the future.
Methods
Study design and protocol
This study employed a single-arm, open-label comparative design to evaluate the safety and tolerability of L-arginine hydrochloride supplementation (before and after treatment). The study was conducted at Hiroshima University Hospital and targeted ALS patients aged 18 years or older who met the Gold Coast criteria25 and were capable of being observed for a minimum of three months. The exclusion criteria included patients with contraindications for arginine formulations, such as arginase deficiency or lysinuric protein intolerance; patients who were already on arginine formulations; patients who were using ventilators; and patients who were scheduled for surgery at the time of study registration.
The primary endpoints were the safety and tolerability of oral L-arginine hydrochloride. Safety assessments included adverse events (AEs), such as any events occurring during the clinical trial period, total treatment-emergent adverse events (TEAEs) during the L-arginine hydrochloride administration period, serious TEAEs, TEAEs leading to death, TEAEs leading to the discontinuation of L-arginine hydrochloride, and TEAEs related to L-arginine hydrochloride (side effects). Patients were evaluated at 45 days (± 14 days) and 90 days (± 14 days) following the initiation of the treatment. The safety analysis population included all enrolled patients who received at least one dose of L-arginine hydrochloride. Tolerability was determined on the basis of the incidence of AEs and the proportion of patients who discontinued the study drug owing to TEAEs.
As a secondary endpoint, efficacy was assessed by evaluating changes in nutritional status and outcomes in ALS patients. Nutritional status was assessed at baseline and after three months of treatment by measuring changes in body weight, body mass index (BMI), body fat mass, soft lean mass, and the Mini Nutritional Assessment (MNA) score26, an 18-item nutritional assessment tool designed for elderly individuals. The resting metabolic rate (RMR) was calculated via the Harris–Benedict equation 7,27 and represents the energy expenditure of the body at rest, i.e., the basal metabolic rate. The total energy expenditure (TEE) was derived from the Shimizu formula28, which indicates total daily caloric consumption. Multifrequency bioelectrical impedance analysis using an InBody S10 (InBody Japan, Tokyo, Japan) was performed to assess the fat mass and lean soft mass. Lean body mass was calculated by subtracting the fat mass from the total body weight. We measured arginine in plasma and urine before the intake of L-arginine hydrochloride and estimated its concentration by liquid chromatography/mass spectrometry29. Outcomes were evaluated via the revised ALS Functional Rating Scale (ALSFRS-R)27, and ALS-related events, including percutaneous endoscopic gastrostomy (PEG), pneumonia, noninvasive positive pressure ventilation (NPPV), tracheostomy positive pressure ventilation (TPPV), and death, were monitored.
Ethics statement
This study received ethical approval from the Certified Review Board at Hiroshima University, Hiroshima, Japan (approval ID: CRB2022-0013-01) and conformed to directives from the federal government in accordance with the ethical principles outlined in the 1964 Declaration of Helsinki. This trial is registered in the jRCT database (trial registration number: jRCTs061230001, first registered 11/04/2023). The recruitment period from this study was from April 11, 2023, to November 30, 2023. Comprehensive written informed consent was obtained from all participants prior to their inclusion in the study.
L-arginine intervention
Patients received oral L-arginine hydrochloride (Ajinomoto Co., Inc., Tokyo, Japan) at a dose of 15 g per day for 90 days. Two packets were dissolved in water and administered orally following breakfast and dinner. Patients who were capable of oral intake consumed oral L-arginine hydrochloride directly, whereas those receiving enteral nutrition received it through the enteral feeding route. Adherence to the treatment regimen was monitored via a medication diary.
Data acquisition
Clinical evaluation and diagnosis were conducted by three neurologists (HN, MN, and MT). The recorded data included age, sex, weight, BMI, muscle mass, body fat mass, date of symptom onset and current neurological symptoms, disease duration, weekly alcohol consumption, smoking habits, ALSFRS-R scores, MNA scores, current medications, and blood and urine test results. Physical measurements, physical examinations, and nutritional assessments, along with blood and urine tests, were performed at baseline, 45 days after treatment (± 14 days), 90 days after treatment (at completion) or at the time of discontinuation (± 14 days).
Sample size
On the basis of the frequency of AEs observed in clinical trials and postmarketing surveillance of arginine formulations (5 out of 40 cases and 18 out of 222 cases, respectively), the incidence rate of AEs associated with L-arginine hydrochloride was estimated to be 8.7%. To calculate the number of patients needed where the probability of observing no events, given the assumed rate of 8.7%, was less than 20%, a minimum of 18 patients was needed. Considering an anticipated dropout rate of approximately 10%, the target sample size for this study was 20 patients.
Statistical analysis
Categorical variables are presented as numbers and percentages, whereas continuous variables are presented as the means with standard deviations (SDs) or medians (minimum, maximum). The safety of L-arginine hydrochloride was evaluated using descriptive statistics, which included the number and incidence of AEs during the administration period, the detailed breakdown of each AE, and the severity of the AEs. To assess the efficacy of L-arginine hydrochloride, we compared the nutritional status and outcomes of each patient before the intervention with those recorded 90 days after the start of the intervention or at the time of discontinuation. Furthermore, we conducted statistical comparisons between groups in which body weight was maintained or increased postintervention and those in which body weight decreased. Appropriate statistical tests, such as the χ2 test, Mann–Whitney U test, or unpaired t test, were used to evaluate differences between groups. Pearson correlation analysis was performed to examine the correlation between changes in body weight from baseline to 3 months and changes in muscle mass, fat mass, and the MNA score. We compared a cohort of patients administered L-arginine hydrochloride with a control cohort based on data from an epidemiological study in which we observed the natural history of ALS since 2014 via propensity score matching according to the presence or absence of weight loss. This cohort study received ethical approval from the Certified Review Board at Hiroshima University, Hiroshima, Japan (approval ID: E2014-0942-03). For the control group, we excluded patients treated with L-arginine hydrochloride or TPPV and included those whose BMI was measured at the time of registration and within one year. To reduce the effects of confounding factors, we carried out greedy propensity score matching at a 1:1 ratio to divide the patients into those with L-arginine hydrochloride and those without L-arginine hydrochloride. The variable factors for this procedure were age, sex, and BMI at baseline. For the logit of the propensity score, we used caliper widths equal to 0.2 standard deviations. A p value less than 0.05 was considered to indicate statistical significance. All analyses were conducted using JMP 17.0 software (SAS Institute, Inc., Cary, NC).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Clinical Research Center in Hiroshima at the Hiroshima University Hospital for their assistance.
Author contributions
HN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Validation, Writing—original draft. MN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Validation, Writing—original draft. MT: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing—original draft. YH: Data curation, Writing—original draft. TT: Conceptualization, Methodology. TW: Conceptualization, Methodology. KI: Data curation. KT: Data curation. YY: Conceptualization, Supervision, Writing—review & editing. HM: Conceptualization, Supervision, Validation, Writing—review & editing.
Funding
HN received funding from Grants-in-Aid for Scientific Research (23K16642) and the ALS Foundation, Japan ALS Association. The other author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Data availability
The datasets used and/or analyzed during the current study are available from the first author upon reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dupuis, L. et al. Evidence for defective energy homeostasis in amyotrophic lateral sclerosis: Benefit of a high-energy diet in a transgenic mouse model. Proc. Natl. Acad. Sci. U S A. 101, 11159–11164. 10.1073/pnas.0402026101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coughlan, K. S., Halang, L., Woods, I. & Prehn, J. H. A high-fat jelly diet restores bioenergetic balance and extends lifespan in the presence of motor dysfunction and lumbar spinal cord motor neuron loss in TDP-43A315T mutant C57BL6/J mice. Dis. Model. Mech.9, 1029–1037. 10.1242/dmm.024786 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludolph, A. C. et al. Effect of high-caloric nutrition on survival in amyotrophic lateral sclerosis. Ann. Neurol.87, 206–216. 10.1002/ana.25661 (2020). [DOI] [PubMed] [Google Scholar]
- 4.Ham, D. J., Caldow, M. K., Lynch, G. S. & Koopman, R. Arginine protects muscle cells from wasting in vitro in an mTORC1-dependent and NO-independent manner. Amino Acids. 46, 2643–2652. 10.1007/s00726-014-1815-y (2014). [DOI] [PubMed] [Google Scholar]
- 5.Iłzecka, J. et al. Plasma amino acids concentration in amyotrophic lateral sclerosis patients. Amino Acids. 25, 69–73. 10.1007/s00726-002-0352-2 (2003). [DOI] [PubMed] [Google Scholar]
- 6.Lee, J., Ryu, H. & Kowall, N. W. Motor neuronal protection by L-arginine prolongs survival of mutant SOD1 (G93A) ALS mice. Biochem. Biophys. Res. Commun.384, 524–529. 10.1016/j.bbrc.2009.05.015 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee, J., Ryu, H. & Kowall, N. W. Differential regulation of neuronal and inducible nitric oxide synthase (NOS) in the spinal cord of mutant SOD1 (G93A) ALS mice. Biochem. Biophys. Res. Commun.387, 202–206. 10.1016/j.bbrc.2009.07.007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda, I. et al. Clinical evaluation of arginine preparations (ARG-U granule and ARG-U injection) against hyperammonemia due to congenital urea cycle enzyme abnormality (excluding arginase abnormality) and congenital amino acid transfer abnormality in 17 centers nationwide. Japanese Pharmacol. Ther.25, 585–598 (1997). (in Japanese). [Google Scholar]
- 9.Häberle, J. et al. Suggested guidelines for the diagnosis and management of urea cycle disorders. Orphanet J. Rare Dis.7, 32. 10.1186/1750-1172-7-32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McNeal, C. Safety of dietary supplementation with arginine in adult humans. Amino Acids. 50, 1215–1229. 10.1007/s00726-018-2594-7 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Shimizu, T. et al. Reduction rate of body mass index predicts prognosis for survival in amyotrophic lateral sclerosis: A multicenter study in Japan. Amyotroph. Lateral Scler.13, 363–366. 10.3109/17482968.2012.678366 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Shimizu, T. et al. Prognostic significance of body weight variation after diagnosis in ALS: A single-centre prospective cohort study. J. Neurol.266, 1412–1420. 10.1007/s00415-019-09276-2 (2019). [DOI] [PubMed] [Google Scholar]
- 13.Nakayama, Y. et al. Body weight variation predicts disease progression after invasive ventilation in amyotrophic lateral sclerosis. Sci. Rep.9, 12262. 10.1038/s41598-019-48831-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bouteloup, C. et al. Hypermetabolism in ALS patients: An early and persistent phenomenon. J. Neurol.256, 1236–1242. 10.1007/s00415-009-5100-z (2009). [DOI] [PubMed] [Google Scholar]
- 15.Ngo, S. T. et al. Loss of appetite is associated with a loss of weight and fat mass in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 497–505 10.1080/21678421.2019.1621346 (2019). [DOI] [PubMed] [Google Scholar]
- 16.Latif, S. & Kang, Y. S. Differences of transport activity of arginine and regulation on neuronal nitric oxide synthase and oxidative stress in amyotrophic lateral sclerosis model cell lines. Cell10, 3554 (2021). 10.3390/cells10123554 [DOI] [PMC free article] [PubMed]
- 17.Wong, J. P. H. et al. Characterization of the skeletal muscle arginine methylome in health and disease reveals remodeling in amyotrophic lateral sclerosis. FASEB J.38, e23647. 10.1096/fj.202400045R (2024). [DOI] [PubMed] [Google Scholar]
- 18.Tradewell, M. L. et al. Arginine methylation by PRMT1 regulates nuclear-cytoplasmic localization and toxicity of FUS/TLS harbouring ALS-linked mutations. Hum. Mol. Genet.21, 136–149. 10.1093/hmg/ddr448 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Scaramuzzino, C. et al. Protein arginine methyltransferase 1 and 8 interact with FUS to modify its sub-cellular distribution and toxicity in vitro and in vivo. PLoS One. 8, e61576. 10.1371/journal.pone.0061576 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ikenaka, K. et al. Increase of arginine dimethylation correlates with the progression and prognosis of ALS. Neurology92, e1868–e1877. 10.1212/WNL.0000000000007311 (2019). [DOI] [PubMed] [Google Scholar]
- 21.Ikenaka, K. et al. Serum asymmetric dimethylarginine level correlates with the progression and prognosis of amyotrophic lateral sclerosis. Eur. J. Neurol.29, 1410–1416. 10.1111/ene.15254 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minakawa, E. N. et al. Arginine is a disease modifier for polyQ disease models that stabilizes polyQ protein conformation. Brain143, 1811–1825. 10.1093/brain/awaa115 (2020). [DOI] [PubMed] [Google Scholar]
- 23.Li, S. et al. Mechanism underlying liquid-to-solid phase transition in fused in sarcoma liquid droplets. Phys. Chem. Chem. Phys.24, 19346–19353. 10.1039/d2cp02171d (2022). [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama, Y., Abe, K., Tokunaga, J. & Numayama, T. Metabolic syndrome in advanced amyotrophic lateral sclerosis patients with tracheostomy–invasive ventilation. Neurol. Clin. Neurosci.7, 174–179. 10.1111/ncn3.12289 (2019). [Google Scholar]
- 25.Shefner, J. M. et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol.131, 1975–1978. 10.1016/j.clinph.2020.04.005 (2020). [DOI] [PubMed] [Google Scholar]
- 26.Vellas, B. et al. Overview of the MNA–Its history and challenges. J. Nutr. Health Aging. 10, 456–463 (2006). [PubMed] [Google Scholar]
- 27.Cedarbaum, J. The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J. Neurol. Sci.169, 13–21. 10.1016/s0022-510x(99)00210-5 (1999). [DOI] [PubMed] [Google Scholar]
- 28.Shimizu, T. et al. The measurement and estimation of total energy expenditure in Japanese patients with ALS: A doubly labelled water method study. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 37–45. 10.1080/21678421.2016.1245756 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Shimbo, K. et al. Automated precolumn derivatization system for analyzing physiological amino acids by liquid chromatography/mass spectrometry. Biomed. Chromatogr.24, 683–691. 10.1002/bmc.1346 (2010). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the first author upon reasonable request.