ABSTRACT
Background
We sought to comprehensively describe drug-related components associated with acute kidney injury (AKI) in patients with chronic kidney disease (CKD), describing the incidence of drug-related AKI, the proportion of preventable AKI, identified the various drugs potentially associated with it, explored the risk factors, and assessed the 1-year incidences of the recurrence of drug-related AKI, kidney failure, and death.
Methods
CKD-REIN is a French national prospective cohort of 3033 nephrology outpatients with a confirmed diagnosis of CKD (eGFR <60 ml/min/1.73 m²). AKIs and adverse drug reactions (ADRs) were prospectively identified from hospital reports, medical records, and patient interviews. Expert nephrologists used the KDIGO criteria to adjudicate all stages of AKI, and expert pharmacologists used validated tools to adjudicate ADRs (including drug-related AKIs).
Results
Over a median [interquartile range] period of 4.9 [3.4–5.1] years, 832 cases of AKI were reported in 639 (21%) of the 3033 study participants. The drug-related component associated with AKI accounted for 236 cases, and 28% were judged to be preventable or potentially preventable. The three most frequently implicated drug classes were diuretics, renin-angiotensin system inhibitors, and contrast agents. A history of cardiovascular events, diabetes, lower levels of hemoglobin and eGFR, poor medication adherence, and ≥5 drugs taken daily were associated with a greater risk of drug-related AKI. Full recovery was not attained in 64 (27%) of the 236 cases of drug-related AKI. The 1-year cumulative incidences of recurrence of drug-related AKI, kidney replacement therapy, and death were 7%, 15%, and 11%, respectively, after the first drug-related AKI.
Conclusions
Drug-related AKI is prevalent among patients with CKD. Even though a substantial proportion of these events were classified as stage 1, our findings point to a poor prognosis.
Keywords: acute kidney injury, adverse drug reaction, chronic kidney disease, drugs, pharmacoepidemiology
KEY LEARNING POINTS.
What was known:
Acute kidney injury (AKI) and chronic kidney disease (CKD) are intricately interconnected and one probably exacerbates the other.
Drugs are a common cause of AKIs in both hospital and community settings.
This study adds:
In a French national prospective cohort of 3033 nephrology outpatients with a confirmed diagnosis of CKD, we found that the drug-related component associated with AKI accounted for over a quarter of the acute-on-chronic kidney disease in the cohort, and 28% of these events were judged to be preventable or potentially preventable.
At least two concomitant medications were implicated in more than half of the AKIs with a drug-related component, RASis and diuretics, two diuretics, and diuretics and antibiotics were the three most frequent associations.
Potential impact:
Within 1 year of the episode, having undergone a first drug-related AKI, 7% of patients experienced a recurrence of drug-related AKI, 16% of the cases required KRT, and 12% died.
INTRODUCTION
Acute kidney injury (AKI) is a worldwide public health issue associated with increased morbidity and mortality rates and healthcare expenditure [1, 2]. Each episode of AKI is associated with a significant higher risk of death and adverse long-term outcomes, including cardiovascular complications, chronic kidney disease (CKD), and kidney failure [3–6].
It is increasingly acknowledged that AKI and CKD are intricately interconnected and that one probably exacerbates the other. Underlying CKD is now considered to be a risk factor for AKI [6, 7]. Diverse risk factors have been identified for acute-on-chronic kidney disease [7, 8], with certain factors being inherently non-modifiable, while others belong to the modifiable category, notably including drug-induced AKI, warranting focused investigation.
Indeed, drugs are a common cause of AKIs in both hospital and community settings. The injuries can be attributed to a variety of mechanisms, including tubular injury, interstitial nephritis, intratubular crystal precipitation, parenchymal damage from vascular/glomerular injuries, and hemodynamic effects that reduce the GFR. Furthermore, the AKI-related accumulation of a parent drug or its metabolites usually excreted by the kidneys may be harmful. The avoidance of medications with renal toxicity is a crucial means of preventing AKI or reducing its duration [9]. It is essential to thoroughly assess exposure to drugs known to be linked to kidney impairment, detect potential drug interactions, and identify individuals at risk of drug-related AKI (especially those with underlying CKD) [10]. However, there is a lack of quantitative data regarding the contribution of drugs to the occurrence of AKI in CKD patients, and detailed information on specific drugs, including drug combinations, as well as the preventability of these events in this specific population, is limited.
Indeed, patients with CKD have a significant comorbidity burden, which often leads to polypharmacy and complex drug prescriptions [11, 12]. We have shown that CKD patients are at a high risk of developing adverse drug reactions (ADRs) [13]. Prospective data regarding the factors influencing and the prognosis of drug-induced AKI in individuals with CKD are currently unavailable.
The primary objective of the present study was to thoroughly examine the contribution of drugs to the occurrence of acute-on-chronic kidney disease in a CKD cohort. We documented the incidence of drug-related AKI, the proportion of preventable AKIs, identified drugs potentially associated with drug-related AKI, explored the risk factors, and assessed the 1-year outcomes.
MATERIALS AND METHODS
The results of this cohort study are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [14].
Study design and participants
CKD-REIN is a prospective cohort study conducted in 40 nationally representative outpatient nephrology centers in France. Eligible adult patients had a confirmed diagnosis of moderate or advanced CKD with an estimated glomerular filtration rate (eGFR) <60 ml/min per 1.73 m²; they were not on dialysis and had not undergone kidney transplantation. Between July 2013 and March 2016, the CKD-REIN investigators enrolled 3033 patients, all of whom gave their written, informed consent. Details of the study protocol and study flow chart have been published previously [15, 16]. The study was approved by the institutional review board at the French National Institute of Health and Medical Research (INSERM; reference: IRB00003888) and was registered at ClinicalTrials.gov (NCT03381950).
Study data
Data were collected at baseline and then annually by trained clinical research associates (CRAs) from patient interviews and medical records. The study data included baseline sociodemographic characteristics and any history of diabetes, cirrhosis, cardiovascular disease or AKI, as defined in the Table S1. Medication adherence was assessed with the validated, questionnaire-based Girerd score (Table S1) [17]. Health literacy was evaluated from the answer to a question in the patient interview ‘How often do you need to seek assistance from someone when you read prescriptions, instructions, brochures, or other written documents from your doctor or pharmacist?’. Low health literacy was defined as an answer of ‘rarely’, ‘often’ or ‘always’. Participants underwent blood and urine tests, including measurements of serum creatinine, hemoglobin, and albumin, and urinary albumin-to-creatinine ratio (Table S1). We used the 2009 creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation to estimate the baseline GFR [18]. Patients were asked to bring all their drug prescriptions for the preceding 3 months (for the enrollment visit) or all the year's prescriptions (for each annual follow-up visit). Accordingly, drug prescriptions were continuously recorded from 3 months preceding study inclusion to the end of the follow-up period, including the drugs prescribed at the time of ADRs (if any). We used the Anatomical Therapeutic and Chemical (ATC) thesaurus [19] to code medications, and we recorded the drug start and stop dates (with reasons for stopping, if recorded). Longitudinal data (appointments with the nephrologist, hospital admissions, laboratory tests—whether prescribed annually as per the study protocol or routinely by the nephrologist or by any other physician—and medications) were recorded at 1-year intervals.
Identification and staging of AKI events
During the study follow-up, all AKI episodes in outpatients or inpatients were identified. Several sources were used to identify AKI episodes: (i) the study's CRAs were trained to identify any mention of AKI in hospital reports, and (ii) a study physician reviewed all the hospital discharge reports for any mention of elevated serum creatinine levels, regardless of whether or not AKI was specified. Additional data on the AKI adjudication process in the CKD-REIN cohort have been published elsewhere [7]. The AKI classification was based on the 2012 KDIGO-AKI criteria, using the baseline and peak serum creatinine levels as follows: Stage 1: 1.5–1.9 × baseline serum creatinine or ≥0.3 mg/dl increase in serum creatinine; Stage 2: 2.0–2.9 × baseline serum creatinine; Stage 3: 3 × baseline serum creatinine or ≥4.0 mg/dl increase, or dialysis initiation [1]. For AKIs that occurred in hospital, the timeframe for diagnosis and staging was based on an increase in serum creatinine of 50% within a week or 0.3 mg/dl within 48 h, as recommended in the guidelines [1]. For AKIs considered as community-acquired AKI, we assumed that the baseline serum creatinine was the mean of all values measured the year before each episode; according to the literature, this is the best approximation [20]. Urine output was not considered to capture AKI events. The baseline serum creatinine corresponded to the average of the values measured in the year preceding the AKI. All patients who developed AKI had at least one serum creatinine measurement in the previous year. For those with only one measurement in the year preceding the event, that value was used as the baseline. Hospital-acquired AKI referred to AKI that occurs during a hospital stay, whereas community-acquired AKI referred to events that did not involve a hospital or were reported as a cause of hospital admission.
Identification and adjudication of ADRs
According to the World Health Organization (WHO), an ADR is defined as ‘an appreciably harmful or unpleasant reaction, resulting from an intervention related to the use of a medicinal product, which predicts hazard from future administration and warrants prevention or specific treatment, or alteration of the dosage regimen, or withdrawal of the product’ [21]. An ADR is considered to be serious when the outcome is death, a life-threatening situation, hospital admission, disability, permanent damage, or another important medical event (i.e. the ADR was considered to be serious but did not require hospitalization, only required a short stay in the emergency room, or occurred during a hospital stay but did not prolong it) [22].
Over a 5-year follow-up period, we collected essential data on ADRs via a study-specific electronic case report form. We used several sources to identify potential ADRs: (i) medical records (examined by CRAs) (ii) patient interviews with CRAs, (iii) hospital reports, and (iv) AKI adjudication. Thus, the event identifier could be the patient, a medical record, or both. All drugs prescribed to the patient at the time of an ADR were recorded. Two expert pharmacists (S.L. and S.M.L.) contributed to adjudication of the identified ADRs, although each ADR was reviewed by a single expert. The expert evaluated the potential causal relationship with the patient's drugs, coded the event according to the Medical Dictionary for Regulatory Activities (MedDRA) and rated the seriousness of the ADR (nonserious or serious), the drug(s) thought to be responsible for the ADR, the dose level, and the immediate drug management action (discontinuation, dose adjustment, or no change). When the ADR was considered to be serious, a committee of four expert pharmacologists from the Amiens Pharmacovigilance center (V.G.-C., J.M., S.L. and S.M.L.) applied Bégaud's method for the imputability assessment of ADRs [23, 24]. This algorithmic method attributes an intrinsic score on the basis of chronological and semiological criteria. The cause and effect relationship is assessed independently for each drug taken by the patient before the occurrence of the event and is not influenced by the extent of imputability to other drugs. This method allows us to identify the drug most responsible for ADRs with the highest degree of imputation (i.e. that with the highest intrinsic score). A first drug-related AKI was defined as the first occurrence of an AKI potentially associated with a drug during the CKD-REIN study follow-up.
Assessment of the preventability of serious ADRs
Drug-related AKIs that met the criteria for a serious ADR were assessed for preventability (‘preventable’, ‘potentially preventable’, ‘not assessable’, or ‘not preventable’) on a seven-item scale by the pharmacovigilance committee [25]. This preventability scale is based on several criteria: compliance with prescription guidelines, the presence or absence of risk factors for ADRs at the time of the prescription, adjustment of the prescription to the patient's condition and environment, and the absolute need to take the implicated drug (Table S2). When there was doubt concerning the prescriber's or patient's compliance with treatment guidelines and/or the patient's real need for the prescription, the expert committee rated the preventability as ‘not assessable’. When preventable or potentially preventable, the preventability criteria were assessed: the appropriateness of the prescription, a medication error by the patient, and self-medication (i.e. self-administration of a medication in the absence of a current prescription and/or without consulting a healthcare professional).
Recovery and one-year outcomes after a first drug-related AKI during study follow-up
We evaluated the rate of kidney function recovery in the year following AKI. Full renal recovery was defined as a decrease in the serum creatinine level to below 1.10 × baseline serum creatinine [26].
We also assessed the cumulative incidence rates of recurrent drug-related AKI, KRT, and death within 12 months of the first drug-related AKI. KRT events were defined as initiation of maintenance dialysis or pre-emptive kidney transplantation, identified from medical records, patient interviews, or by linkage with the national REIN (Renal Epidemiology and Information Network) registry [27]. Deaths were identified from medical records or the French national death registry [27].
Statistical analyses
Baseline characteristics were described for the participants as whole and according to the number of prescription drugs taken daily. The data were expressed as the mean [standard deviation (SD)], median [interquartile range (IQR)], or the number (percentage). The crude incidence rates [95% confidence interval (CI)] per 100 person-years for all AKI episodes and by AKI subtype (hospital or community acquired) were estimated for the study population as a whole.
We used cause-specific Cox proportional hazard models to investigate the patients’ baseline clinical characteristics associated with the risk of drug-related AKI. Data were censored at the date of death, the date of KRT initiation or the date of last follow-up, whichever came first (i.e. the first of these competing events). Variables were preselected via a literature review and were analyzed in a crude model that included age, sex, health literacy, educational level, history of cardiovascular disease, diabetes, AKI, or cirrhosis, baseline eGFR, baseline hemoglobin, baseline serum albumin, number of prescription drugs taken daily, and medication adherence. The proportional hazards assumption was checked by testing the Schoenfeld residuals.
Long-term outcomes were analyzed by estimating the cumulative incidence [95%CI] of the competing risks of recurrence of drug-related AKI, kidney failure, and death after the first drug-related AKI. The cumulative incidence [95%CI] of these competing risks was further investigated after a first hospital-acquired drug-related AKI and after a first community-acquired drug-related AKI.
To handle missing data, we performed multiple imputation by chained equations (20 iterations and 25 datasets) and included all the patient variables used in the adjusted models [28]. The data patterns suggested that the ‘data missing at random’ assumption was plausible. Out of the patients, 26% had at least one missing data item. The proportions of missing data by variable are listed in Table 1. Fitted Cox models were generated for each dataset, and pooled regression coefficients were obtained using Rubin's rules. All statistical analyses were performed with R software [29].
Table 1:
Baseline characteristics of the study participants.
| Number of drugs taken daily | |||||
|---|---|---|---|---|---|
| Total (n = 3033) | <5 (n = 595) | 5–10 (n = 1685) | >10 (n = 753) | Imputed data (n = 3033) | |
| Age at baseline (years) | 69 [60–76] | 61 [48–70] | 69 [62–77] | 71 [66–77] | |
| Men | 65% | 63% | 67% | 63% | |
| Health literacy | 81% | 87% | 82% | 75% | |
| Educational level (years) | 1% | ||||
| < 9 | 15% | 8% | 15% | 21% | |
| 9–12 | 49% | 40% | 51% | 53% | |
| ≥ 12 | 36% | 52% | 35% | 26% | |
| Smoking | 0.8% | ||||
| Never-smokers | 41% | 50% | 40% | 38% | |
| Current smokers | 12% | 15% | 11% | 10% | |
| Former smokers | 47% | 35% | 49% | 52% | |
| Hypertension | 96% | 90% | 97% | 99% | 0.2% |
| Diabetes | 43% | 11% | 43% | 70% | 0.2% |
| Dyslipidemia | 73% | 43% | 76% | 91% | 0.5% |
| History of AKI | 23% | 16% | 22% | 32% | 8% |
| Cardiovascular disease | 53% | 21% | 54% | 76% | 1% |
| Coronary artery disease | 25% | 5% | 22% | 45% | 2% |
| Heart failure | 13% | 3% | 12% | 24% | 0.3% |
| Cerebrovascular disease | 12% | 4% | 13% | 17% | 2% |
| History of stroke or TIA | 10% | 3% | 11% | 14% | 2% |
| Peripheral artery disease | 17% | 4% | 16% | 28% | 2% |
| Body mass index (kg/m2) | 28.7 (5.86) | 26.0 (4.63) | 28.5 (5.43) | 31.3 (6.51) | 2% |
| Systolic blood pressure (mmHg) | 142 (20) | 137 (17) | 142 (20) | 144 (20) | 0.6% |
| Diastolic blood pressure (mmHg) | 78 (12) | 81 (11) | 78 (12) | 76 (12) | 0.7% |
| eGFR at baseline (ml/min/1.73 m2) | 34.1 (13.1) | 38.2 (13.4) | 33.9 (13.2) | 31.2 (11.8) | |
| uACR (mg/g) | 109 [21.8–509] | 54.1 [14.3–293] | 112 [22.3–508] | 182 [31.4–680] | 12% |
| Normal or slightly elevated (A1) | 30% | 38% | 30% | 24% | |
| Moderately elevated (A2) | 35% | 37% | 35% | 34% | |
| Severely elevated (A3) | 35% | 25% | 35% | 42% | |
| Serum albumin (g/l) | 40.1 (4.32) | 41.2 (3.84) | 40.2 (4.32) | 39.3 (4.52) | 16% |
| Hemoglobin (g/dl) | 13.0 (1.65) | 13.5 (1.59) | 13.1 (1.63) | 12.5 (1.60) | 0.7% |
| Diuretic | 55% | 20% | 56% | 79% | 0.3% |
| Renin-angiotensin system inhibitor | 76% | 67% | 77% | 80% | 0.3% |
| Beta-blocker | 42% | 14% | 43% | 63% | 0.3% |
| Calcium channel inhibitor | 48% | 24% | 49% | 64% | 0.3% |
| Proton pump inhibitor | 33% | 7% | 29% | 61% | 0.3% |
| Antiplatelet therapy | 41% | 8% | 43% | 63% | 0.3% |
| Anticoagulant | 15% | 4% | 13% | 26% | 0.3% |
| Nonsteroidal anti-inflammatory drug | 2% | 1% | 2% | 3% | 0.3% |
| Allopurinol | 26% | 12% | 26% | 38% | 0.3% |
| Colchicine | 2% | 1% | 2% | 3% | 0.3% |
| Poor adherence to medication | 62% | 47% | 61% | 76% | 1% |
TIA: transient ischemic attack; uACR: urinary albumin-to-creatinine ratio.
RESULTS
Baseline characteristics
Two thirds of the study participants were male (Table 1). At baseline, the median age was 69 years [IQR, 60–76] and patients were taking a median of eight medications [IQR, 5–10] per day. Most patients were taking at least five medications at baseline (80%). The more medications patients were taking at baseline, the more comorbidities they had (hypertension, diabetes, dyslipidemia, AKI history, a history of cardiovascular disease), and the lower the eGFR, serum albumin, and hemoglobin levels.
Characteristics of the AKIs
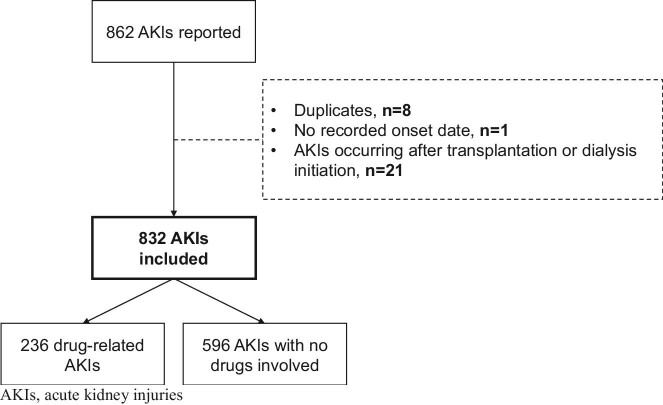
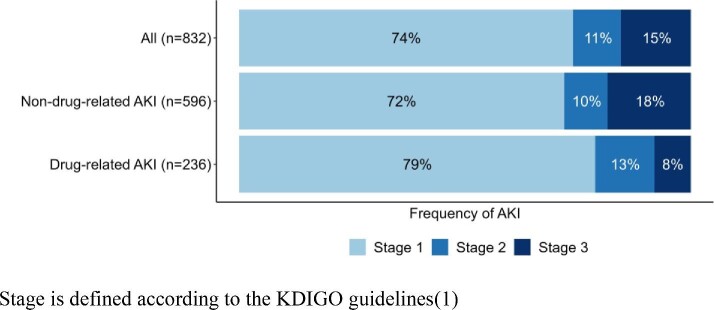
Over a median follow-up period of 4.9 years [IQR, 3.4–5.1], 832 AKIs were reported in 639 (21%) of the 3033 participants (Fig. 1); 236 AKIs in 208 participants were classified as drug-related (Table 2). The incidence rates [95%CI] for AKI and drug-related AKI were 7.1 [6.2; 8.1] and 2.0 [1.7; 2.4], respectively. Most (n = 615; 74%) of all the AKIs were classified as stage 1 (of which 173 corresponded to a 50% rise in creatinine from the baseline), and 15% were categorized as stage 3. The proportion of stage 3 events was greater for non-drug-related AKI than for drug-related AKI (Fig. 2).
Figure 1:
AKI flowchart.
Table 2:
Incidence rates of AKI.
| Total n = 3033 |
|
|---|---|
| AKI | |
| Person-years | 11 737 |
| Number of events | 832 |
| Incidence rate per 100 person-years [95%CI] | 7.1 [6.2; 8.1] |
| Drug-related AKI | |
| Person-years | 11 737 |
| Number of events | 236 |
| Incidence rate per 100 person-years [95%CI] | 2.0 [1.7; 2.4] |
| Drug-related community-acquired AKI | |
| Person-years | 11 737 |
| Number of events | 126 |
| Incidence rate per 100 person-years [95%CI] | 1.1 [0.9; 1.3] |
| Drug-related hospital-acquired AKI | |
| Person-years | 11 737 |
| Number of events | 110 |
| Incidence rate per 100 person-years [95%CI] | 0.9 [0.7; 1.2] |
CI: confidence interval.
Figure 2:
Stage of AKI, according to the medication component.
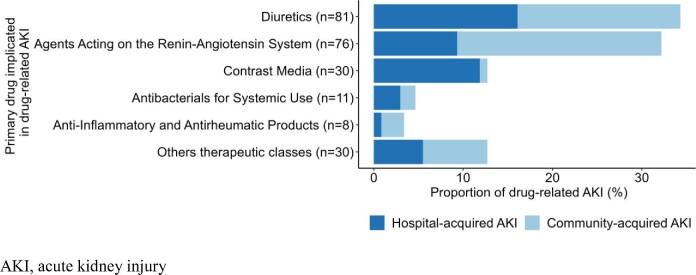
Of the drug-related AKIs, 126 were classified as community acquired [incidence rate (IQR): 1.1 (0.9; 1.3)], and 110 were categorized as hospital-acquired [incidence rate 0.9 (0.7; 1.2)]. The number of medications associated with the occurrence of AKI was higher in community-acquired cases than in hospital-acquired cases. The proportion of drug-related AKIs involving three drugs was 26% for community-acquired cases and 15% for hospital-acquired cases. The proportion of drug-related AKIs involving a single drug was over 50% for hospital-acquired cases. The drugs most frequently implicated in drug-related AKI cases were diuretics (34%), renin-angiotensin system inhibitors (RASis) (32%), and contrast agents (13%) (Fig. 3). However, the drug class distribution depended on whether the AKI was hospital or community acquired. While RASis, diuretics and anti-inflammatory drugs were the classes most commonly implicated in community-acquired AKI (43%, 34%, and 5% respectively), diuretics, contrast agents and RASis were most commonly implicated in hospital-acquired cases (35%, 25%, and 20% respectively) (Table S3).
Figure 3:
Medications primarily implicated in drug-related acute kidney injuries, by setting of occurrence.
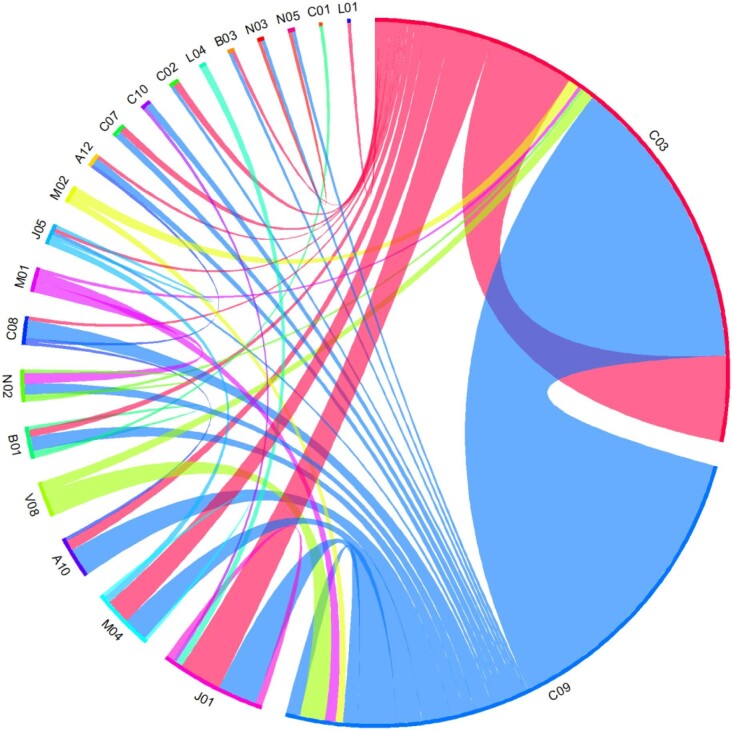
Among the cases of drug-related AKI with at least two drugs implicated, the three most frequent associations were RASis and diuretics, two diuretics, and diuretics and antibiotics (Fig. 4). Most of these drug interactions were additive or synergistic pharmacodynamic interactions.
Figure 4:
Diagram of drug associations implicated in drug-related AKI. A10, drugs used in diabetes; A12, mineral supplements; B01, antithrombotic agents; B03, antianemic preparations; C01, cardiac therapy; C02, antihypertensives; C03, diuretics; C07, beta blocking agents; C08, calcium channel blockers; C09, agents acting on the renin-angiotensin system; C10, lipid modifying agents; J01, antibacterials for systemic use; J05, antivirals for systemic use; L01, antineoplastic agents; L04, immunosuppressants; M01, anti-inflammatory and antirheumatic products; M02, topical products for joint and muscular pain; M04, antigout preparations; N02, analgesics; N03, antiepileptics; N05, psycholeptics; V08, contrast media. Note: Associations of therapeutic classes are shown for drug-related acute kidney injuries for which at least two drugs were implicated (n = 132). The wider the link between two therapeutic classes, the more frequently the association is found to be involved in drug-induced AKI. The combination of two drugs from the same therapeutic class may have contributed to drug-related AKI (e.g. diuretics, C03). RASis and diuretics, two diuretics, and diuretics and antibiotics were the three most frequent associations.
In most cases, the drug primarily implicated in the AKI (i.e. with the highest degree of imputation) was discontinued (83%); this happened more frequently in community-acquired AKIs (88%) than in hospital-acquired AKIs (76%). Dose adjustment was more frequent when the drug-related AKI occurred in hospital (in 17% of cases, compared with 9% for community-acquired AKIs).
Of the 236 drug-related AKIs, 168 (71%) were considered serious ADRs (according to the WHO definition), 28% were preventable or potentially preventable, and 48% were not preventable. The most frequent preventability criteria were prescriptions that did not comply with the summary of product characteristics, such as contraindications (n = 18) and an inappropriately high-dose level (n = 10) relative to the patient's level of kidney function (Table S4). Community-acquired drug-related AKIs were more often judged to be preventable or potentially preventable (35%) than hospital-acquired drug-related AKIs (17%). Most serious drug-related AKIs were mentioned solely in medical documents (95%): only eight cases of drug-related AKI were also reported by patients during the annual interview, and these were all community-acquired cases.
Risk factors for drug-related AKI
After adjusting for other associated variables, the risk of a first drug-related AKI significantly increased with the participant's baseline number of prescribed drugs, a history of cardiovascular disease, a history of diabetes, and poor treatment adherence (Table 3). Lower levels of eGFR and hemoglobin level (but not serum albumin) were associated with an increased risk of first drug-related AKI. Men were at a higher risk of first drug-related AKI than women. After adjustment, there was no significant associations with age, a history of AKI, cirrhosis, and education level. Literacy was significantly associated with a lower risk of first drug-related AKI in the univariable model but not after adjustment. An exploratory analysis of factors linked to preventable drug-related AKI identified sex, decrease in eGFR, and urinary albumin-to-creatinine ratio as statistically significant associations (Table S5).
Table 3:
Risk factors for a first drug-related AKI (n events = 208).
| Crude model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| HR | [95% CI] | P value | HR | [95% CI] | P value | |
| Age (per year) | 1.02 | [1.00; 1.04] | .02 | 1.01 | [0.99; 1.02] | .49 |
| Men (ref. women) | 1.76 | [1.14; 2.73] | .01 | 1.83 | [1.15; 2.92] | .01 |
| Literacy | 0.82 | [0.52; 1;30] | .40 | 0.92 | [0.58; 1.46] | .72 |
| Level of education | ||||||
| <9 | Ref | |||||
| 9–11 | 0.79 | [0.46; 1.34] | .37 | 0.85 | [0.49; 1.46] | .55 |
| ≥12 | 0.89 | [0.51; 1.53] | .66 | 1.19 | [0.67; 2.11] | .54 |
| History of cardiovascular disease | 2.04 | [1.37; 3.04] | <.001 | 1.29 | [0.84; 1.99] | .24 |
| History of diabetes | 1.91 | [1.31; 2.77] | <.001 | 1.23 | [0.82; 1.85] | .31 |
| History of AKI | 1.31 | [0.85; 2.00] | .22 | 1.11 | [0.72; 1.70] | .64 |
| eGFR (per 5 ml/min/1.73 m2 decrease) | 1.14 | [1.05; 1.24] | .001 | 1.08 | [0.99; 1.15] | .08 |
| Urinary albumin-to-creatinine ratio | ||||||
| Normal or slightly elevated (A1) | Ref | Ref | ||||
| Moderately elevated (A2) | 1.34 | [0.81; 2.22] | .25 | 1.14 | [0.69; 1.90] | .61 |
| Severely elevated (A3) | 1.88 | [1.14; 3.08] | .01 | 1.38 | [0.81; 2.34] | .23 |
| Hemoglobin (per g/dl decrease) | 1.19 | [1.06; 1.34] | .004 | 1.12 | [1.01; 1.23] | .04 |
| Number of daily drugs | ||||||
| <5 | Ref | Ref | ||||
| 5–9 | 3.29 | [1.48; 7.32] | .004 | 2.34 | [1.02; 5.39] | .046 |
| ≥10 | 6.06 | [2.74; 13.39] | <.001 | 3.36 | [1.39; 8.12] | .008 |
| Poor medication compliance (ref. good) | 1.74 | [1.14; 2.65] | .01 | 1.37 | [0.89; 2.10] | .15 |
Recovery from an AKI
Of the 832 AKI episodes (and regardless of their relationship to a drug), 563 (68%) led to a full renal recovery within the following year. Full recovery was not attained in 64 (27%) of the 236 cases of drug-related AKI and 205 (35%) of the 596 cases of non-drug-related AKI.
One-year outcomes after a first drug-related AKI
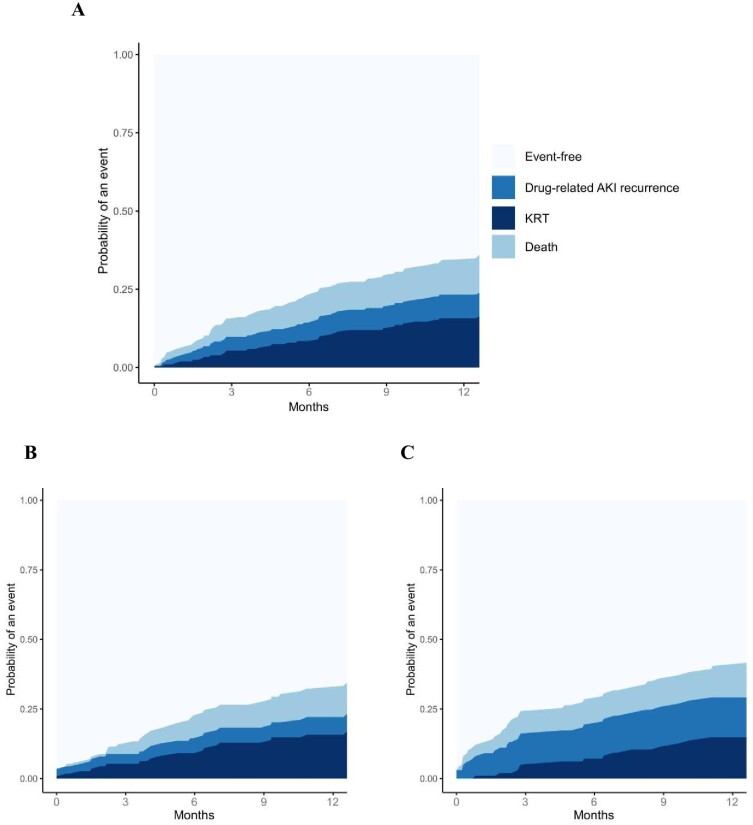
One year after the first episode of drug-related AKI during study follow-up, 7% [95%CI, 4; 11] of patients had experienced a recurrence of drug-related AKI, 15% [95%CI, 10; 20] required KRT, and 11% [95%CI, 6; 15] had died (Fig. 5a).
Figure 5:
Cumulative incidence rates of various events after a first drug-related AKI, by setting of occurrence. (a) Cumulative incidence rates of various events after a first drug-related AKI. (b) After a first community-acquired drug-related AKI. (c) After a first hospital-acquired drug-related AKI.
The incidence rates depended slightly on the setting of the first drug-related AKI. After a community-acquired drug-related AKI, respectively, 6% [95%CI, 2; 10], 15% [95%CI, 8; 21], and 10% [95%CI, 4; 15] of the patients experienced a recurrence of drug-related AKI, required KRT, or died (Fig. 5b). By contrast, after a first hospital-acquired drug-related AKI, respectively, 14% [95%CI, 7; 21], 14% [95%CI, 7; 21], and 11% [95%CI, 5; 17] of the patients experienced a recurrence of drug-related AKI, required KRT, or died (Fig. 5c).
DISCUSSION
On the basis of data from a French cohort of 3033 CKD patients followed up by a nephrologist, we found that the drug-related component associated with AKI accounted for 236 of the 832 cases of acute-on chronic AKI in the cohort (of which 47% were hospital-acquired AKI and 53% were community acquired AKI), and 28% of the 168 serious AKIs were judged to be preventable or potentially preventable. The three most frequently implicated drug classes were diuretics (35%), contrast agents (25%), and RASis (20%), for hospital-acquired drug-related AKIs and RASis (43%), diuretics (34%), and anti-inflammatory drugs (5%) for community-acquired drug-related AKIs. At least two concomitant medications were implicated in more than half of the AKIs with a drug-related component, RASis and diuretics, two diuretics, and diuretics and antibiotics were the three most frequent associations. Furthermore, we found that certain patient-related characteristics increased the risk of developing a first drug-related AKI; these included a history of cardiovascular disease, diabetes, poor medication compliance, ≥5 drugs taken daily, and lower baseline eGFR and hemoglobin level. Within 1 year of the episode, patients having undergone a first drug-related AKI experienced in 7% a recurrence of drug-related AKI, required KRT in 16% of the cases, when 12% died.
The incidence of drug-related AKI and implicated medication classes
Over a median follow-up of 4.9 years, the incidence rates [95%CI] for AKI and drug-related AKI were 7.1 [6.2; 8.1] and 2.0 [1.7; 2.4], respectively. To the best of our knowledge, this information on drug-related AKI has not been previously reported for a CKD population.
Our analyses revealed that the medication with the highest intrinsic imputability depended on the setting of occurrence. For hospital-acquired AKIs, diuretics were most frequently implicated. For community-acquired AKIs, RASi were most frequently implicated. Most drugs we identified as being associated with AKI are well known for their propensity to induce functional AKI [10, 30]. However, these were clinically important medications, e.g. diuretics in cases of heart failure decompensation, and contrast agents for life-saving diagnostic tests or coronary interventions. Indeed, we found that most hospital-acquired AKIs were not preventable, due to the clinical context and the essential nature of the prescription.
We also found that two or more medications were implicated in more than half of the drug-related AKIs, and we thoroughly described the associations. In a disproportionality analysis of the French national pharmacovigilance database (within the general population), Pierson-Marchandise et al. demonstrated that at least two suspect medications were involved in the occurrence of >60% of AKIs [31]. These data are important for prescribers to alert them about the potential pharmacodynamic interactions posing a risk of AKI in CKD patients.
It is noteworthy that 90% of the community-acquired AKIs led to the temporary discontinuation of the drug treatment. Failure to reinitiate certain treatments after an AKI might constitute a missed opportunity for the patient, e.g. RASis and their nephroprotective effects [32]. Furthermore, the fact that self-medication with NSAIDs accounted for 26% of the preventable, drug-induced AKI highlights the importance of patient education for the avoidance of this inappropriate practice.
Factors associated with drug-related AKI
We found that a poor compliance and high number of medications were associated with the risk of drug-related AKI in CKD patients. Chang et al. found that patients exposed to polypharmacy might have an increased risk of AKI [33]. The risk of developing AKI increases with the number of prescribed drugs. Indeed, a meta-analysis has demonstrated that each additional medication with potential renal toxicity increases the risk of developing AKI by 53% [34]. Therefore, our results extended these literature findings to a population of patients with CKD.
Furthermore, polypharmacy might reduce compliance, particularly by complicating the management of patients' underlying conditions. This complexity could manifest itself as poor control of concurrent illnesses and could potentially lead to conditions such as hypertensive crises and heart failure decompensation: both of which are risk factors for AKI [35]. Hence, polypharmacy may be linked to the development of AKI directly by exerting the effects of medications or indirectly by altering patient behavior (e.g. compliance). When exploring factors associated with preventable AKI, eGFR emerged as a significant contributor. These findings are consistent with our identification of preventability causes, namely contraindicated or excessively high-dose prescriptions. This emphasizes the need for regular reassessment of the benefit-risk balance of prescriptions, particularly as eGFR declines, to reduce the incidence of preventable drug-related AKI.
Besides, we observed associations between various comorbidities (such as diabetes and a history of cardiovascular disease) and the risk of drug-related AKI. These associated factors have already been identified in cases of AKI, although the medication component was notably absent from the published studies [36–38]. We also enhanced the role of lower eGFR as a risk factor for acute-on-chronic kidney disease. We recently evidenced a linear relationship between a decrease in the eGFR and an increase in the risk of drug-related AKI [13]. This identification of risk factors paves the way for prediction studies to develop a risk stratification tool, enabling personalized medicine approaches.
Outcomes after drug-related AKI
We found that 12 months after the drug-induced AKI, 16% of the patients had developed KRT and 12% had died. This result is consistent with other reports on AKI [7, 39, 40]. Even though 79% of the drug-induced AKI were classified as KDIGO stage 1 and most (73%) patients with drug-related AKI reached full renal recovery in the following year, the 1-year kidney outcomes and survival are poor. However, the current study was not designed to assess optimal care following drug-induced AKI. Therefore, we were unable to identify the most effective strategy to prevent AKI recurrence or renal progression. Moreover, we found that 28% of the observed drug-related AKI cases were preventable or potentially preventable, and the proportion was higher for community-acquired AKIs. This finding suggests that preventive measures should be enhanced. The discontinuation of medications (as a means of preventing AKI) during episodes of acute illness (e.g. following sick day guidance) or during medical procedures has also been suggested and studied [1]. Patient education appears to be crucial because only eight drug-related AKIs were reported by the patients; the level of awareness of AKIs is low. This point has already been reported [41]. Among the strategies considered for proactive identification of AKI, one involves the use of electronic alerts (e-alerts) based on automated algorithms that detect changes in serum creatinine levels through laboratory data. While this approach is feasible in a hospital setting and can facilitate early detection of AKI (which is typically more severe than in the community setting), implementing such a system in outpatient settings may be challenging [42].
Our study's main strengths were the large sample size and the representative study population (i.e. patients with a confirmed diagnosis of CKD and nationally representative of this population under nephrology follow-up). Furthermore, the study featured a long (5-year) follow-up period. Last, the meticulous, extensive data collection on patient characteristics and medications, together with a careful expertise of AKIs and ADRs, enhanced the quality and relevance of our results.
However, the study had some limitations. Evaluation of drug causality in the context of ADRs is complicated. This is particularly true for cases of drug-induced AKI, as the latter is multifactorial. In the present study, our standardized, validated pharmacovigilance approach based on the individual analysis of cases through a detailed review of patients' medical records, hospitalization reports, and laboratory results. However, in these events, drugs were often just one contributing factor in a multifactorial process, making it difficult to establish a clear causal relationship. Given that the cohort included solely patients with CKD under nephrology follow-up, it might not be possible to extrapolate our results to other populations. However, the situation could be potentially even more serious within a population that does not benefit from this specialized monitoring. Even though we actively sought to identify AKIs, the incidence might have been underestimated because certain events might not have been captured (particularly within community settings). While the KDIGO criteria are widely accepted, they may not capture all cases of AKI, particularly in patients with mild or transient increases in creatinine that might be clinically relevant. Last, the study's observational design precluded any causal interpretation, especially with regard to the association between AKI and KRT or death, and does not provide insights into the effectiveness of interventions to prevent drug-related AKI.
In conclusion, drug-related AKI is prevalent among patients with CKD. Even though a substantial proportion of these events were classified as stage 1, our findings point to a poor prognosis.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the CKD-REIN study coordination staff for their efforts in setting up the cohort: Elodie Speyer, Céline Lange, Reine Ketchemin, Oriane Lambert, Madonna Salib, Marie Metzger, and all the clinical research associates.
We would also like to express our gratitude to Dr Victor Fages and Dr Xavier Vincent, both of whom assessed AKI.
Contributor Information
Solène M Laville, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France; MP3CV Laboratory, Jules Verne University of Picardie, Amiens, France.
Janice Vendar, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France.
Ziad A Massy, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France; Department of Nephrology, Ambroise Paré University Hospital, APHP, Boulogne-Billancourt/Paris, France.
Valérie Gras-Champel, Pharmacovigilance Center, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France.
Julien Moragny, Pharmacovigilance Center, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France.
Luc Frimat, Nephrology Department, Centre Hospitalier Régional Universitaire de Nancy, Vandoeuvre-lès-Nancy, France; Lorraine University, APEMAC, Vandoeuvre-lès-Nancy, France.
Maurice Laville, Université de Lyon, CarMeN INSERM 1060, Lyon, France.
Christian Jacquelinet, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France; Biomedicine Agency, Saint Denis, France.
Roberto Pecoits-Filho, Arbor Research Collaborative for Health, Ann Arbor, MI, USA.
Natalia Alencar De Pinho, Centre for Research in Epidemiology and Population Health (CESP), INSERM UMRS 1018, Université Paris-Saclay, Université Versailles Saint Quentin, Villejuif, France.
Aghilès Hamroun, Department of Public Health – Epidemiology, Lille University, Lille Regional University Medical Center, Lille, France; UMR1167 RID-AGE, INSERM, Institut Pasteur de Lille, Lille University, Lille, France.
Sophie Liabeuf, Pharmacoepidemiology Unit, Department of Clinical Pharmacology, Amiens-Picardie University Medical Center, Amiens, France; MP3CV Laboratory, Jules Verne University of Picardie, Amiens, France.
the Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) Study Group:
Natalia Alencar de Pinho, Christian Combe, Denis Fouque, Luc Frimat, Aghilès Hamroun, Christian Jacquelinet, Oriane Lambert, Céline Lange, Maurice Laville, Sophie Liabeuf, Ziad A Massy, Marie Metzger, Christophe Pascal, Roberto Pecoits-Filho, Bénédicte Stengel, T Hannedouche, B Moulin, A Klein, C Combe, J P Bourdenx, A Keller, C Delclaux, B Vendrely, B Deroure, A Lacraz, T Lobbedez, I Landru, Z Massy, P Lang, X Belenfant, E Thervet, P Urena, M Delahousse, C Vela, M Essig, D Clément, H Sekhri, M Smati, M Jamali, B Hacq, V Panescu, M Bellou, Luc Frimat, N Kamar, C Noël, F Glowacki, N Maisonneuve, R Azar, M Hoffmann, M Hourmant, A Testa, D Besnier, G Choukroun, G Lambrey, S Burtey, G Lebrun, E Magnant, M Laville, D Fouque, L Juillard, C Chazot, P Zaoui, and F Kuentz
CKD-REIN STUDY GROUP
Steering committee and coordinators: The CKD-REIN Study Group steering committee and coordinators include: Natalia Alencar de Pinho, Christian Combe, Denis Fouque, Luc Frimat, Aghilès Hamroun, Christian Jacquelinet, Oriane Lambert, Céline Lange, Maurice Laville, Sophie Liabeuf, Ziad A. Massy, Marie Metzger, Christophe Pascal, Roberto Pecoits-Filho, Bénédicte Stengel.
Investigators: Alsace : Prs T. Hannedouche et B. Moulin (CHU, Strasbourg), Dr A. Klein (CH Colmar) Aquitaine : Pr C. Combe (CHU, Bordeaux), Dr J.P. Bourdenx (Clinique St Augustin, Bordeaux), Dr A. Keller, Dr C. Delclaux (CH, Libourne), Dr B. Vendrely (Clinique St Martin, Pessac), Dr B. Deroure (Clinique Delay, Bayonne), Dr A. Lacraz (CH, Bayonne) Basse Normandie : Dr T. Lobbedez (CHU, Caen), Dr I. Landru (CH, Lisieux) Ile de France : Pr Z. Massy (CHU, Boulogne—Billancourt), Pr P. Lang (CHU, Créteil), Dr X. Belenfant (CH, Montreuil), Pr E. Thervet (CHU, Paris), Dr P. Urena (Clinique du Landy, St Ouen), Dr M. Delahousse (Hôpital Foch, Suresnes) Languedoc—Roussillon : Dr C. Vela (CH, Perpignan) Limousin : Pr M. Essig, Dr D. Clément (CHU, Limoges) Lorraine : Dr H. Sekhri, Dr M. Smati (CH, Epinal) Dr M. Jamali, Dr B. Hacq (Clinique Louis Pasteur, Essey-les-Nancy), Dr V. Panescu, Dr M. Bellou (Polyclinique de Gentilly, Nancy), Pr Luc Frimat (CHU, Vandœuvre-les-Nancy) Midi-Pyrénées : Pr N Kamar (CHU, Toulouse) Nord-Pas-de-Calais : Prs C. Noël et F. Glowacki (CHU, Lille), Dr N. Maisonneuve (CH, Valenciennes), Dr R. Azar (CH, Dunkerque), Dr M. Hoffmann (Hôpital privé La Louvière, Lille) Pays-de-la Loire : Pr M. Hourmant (CHU, Nantes), Dr A. Testa (Centre de dialyse, Rezé), Dr D. Besnier (CH, St Nazaire) Picardie : Pr G. Choukroun (CHU, Amiens), Dr G. Lambrey (CH, Beauvais) Provence-Alpes—Côte d'Azur : Pr S. Burtey (CHU, Marseille), Dr G. Lebrun (CH, Aix-en-Provence), Dr E. Magnant (Polyclinique du Parc Rambot, Aix-en-Provence) Rhône-Alpes : Pr M. Laville, Pr D. Fouque (CHU, Lyon-Sud) et L. Juillard (CHU Edouard Herriot, Lyon), Dr C. Chazot (Centre de rein artificiel Tassin Charcot, Ste Foy-les-Lyon), Pr P. Zaoui (CHU, Grenoble), Dr F. Kuentz (Centre de santé rénale, Grenoble).
AUTHORS’ CONTRIBUTIONS
S.M.L. and S.L. designed the present project. A.H. evaluated AKIs. S.M.L., S.L., V.G-C., and J.M. evaluated ADRs. S.M.L. and J.V. analyzed the data. S.M.L., S.L., J.V., A.H., V.G-C., J.M., Z.A.M., L.F., M.L., and N.A.P helped to interpret the results. Each author contributed important intellectual content during manuscript drafting or revision and agrees to be personally accountable for the individual's own contributions and to ensure that questions pertaining to the accuracy or integrity of any portion of the work—even one in which the author was not directly involved—are appropriately investigated and resolved, including with documentation in the literature if appropriate.
FUNDING
CKD-REIN is funded by the French Agence Nationale de la Recherche through the 2010 ‘Cohortes-Investissements d'Avenir’ program (ANR-IA-COH-2012/3731) and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public-private partnership with Fresenius Medical Care and GlaxoSmithKline (GSK) since 2012, Boehringer Ingelheim France since 2022, Vifor France from 2018 to 2023, Sanofi-Genzyme from 2012 to 2015, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017, Amgen from 2012 to 2020, Lilly France from 2013 to 2018, Otsuka Pharmaceutical from 2015 to 2020, and AstraZeneca from 2018 to 2021. INSERM Transfer set up and has managed this partnership since 2011.
A specific project was funded by the National Agency for the Safety of Medicines and Health Products (ANSM) through the EPI-PHARE group of scientific interest. It should be noted that the authors of this article were solely responsible for interpreting the data; ANSM was not involved.
The funding sources had no roles in study design, conduct, reporting, or the decision to submit for publication.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available upon reasonable request by contacting the corresponding author. The data are not publicly available, due to privacy or ethical restrictions.
CONFLICT OF INTEREST STATEMENT
S.M.L., S.L., J.V., V.G.C., and J.M. have nothing to declare. Z.A.M. reports having received grants for CKD-REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp & Dohme-Chibret, Sanofi- Genzyme, Lilly, Otsuka, AstraZeneca, Vifor and the French government, as well as fees and grants to charities from AstraZeneca, Boehringer Ingelheim, and GlaxoSmithKline. N.A.P. declare financial support from pharmaceutical companies integrating the public-private partnership of the CKD-REIN cohort: Fresenius Medical Care, GlaxoSmithKline (GSK), Vifor France, and Boeringher Ingelheim; all grants are made to Paris Saclay University.
REFERENCES
- 1. Kidney Disease: Improving Global, Outcomes (KDIGO) Acute Kidney Injury work group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2012;2:1–138. [Google Scholar]
- 2. Susantitaphong P, Cruz DN, Cerda J et al. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 2013;8:1482–93. 10.2215/CJN.00710113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoste EAJ, Kellum JA, Selby NM et al. Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 2018;14:607–25. 10.1038/s41581-018-0052-0 [DOI] [PubMed] [Google Scholar]
- 4. Odutayo A, Wong CX, Farkouh M et al. AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 2017;28:377. 10.1681/ASN.2016010105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schulman IH, Chan K, Der JS et al. Readmission and mortality after hospitalization with acute kidney injury. Am J Kidney Dis 2023;82:63–74.e1. 10.1053/j.ajkd.2022.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heung M, Steffick DE, Zivin K et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of Veterans Health Administration data. Am J Kidney Dis 2016;67:742–52. 10.1053/j.ajkd.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hamroun A, Frimat L, Laville M et al. New insights into acute-on-chronic kidney disease in nephrology patients: the CKD-REIN study. Nephrol Dial Transplant 2022;37:1700–9. 10.1093/ndt/gfab249 [DOI] [PubMed] [Google Scholar]
- 8. Leblanc M, Kellum JA, Gibney RTN et al. Risk factors for acute renal failure: inherent and modifiable risks. Curr Opin Crit Care 2005;11:533. 10.1097/01.ccx.0000183666.54717.3d [DOI] [PubMed] [Google Scholar]
- 9. Goldstein SL, Kirkendall E, Nguyen H et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 2013;132:e756–e67. 10.1542/peds.2013-0794 [DOI] [PubMed] [Google Scholar]
- 10. Perazella MA, Rosner MH. Drug-induced acute kidney injury. Clin J Am Soc Nephrol 2022;17:1220. 10.2215/CJN.11290821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schmidt IM, Hübner S, Nadal J et al. Patterns of medication use and the burden of polypharmacy in patients with chronic kidney disease: the German Chronic Kidney Disease study. Clin Kidney J 2019;12:663–72. 10.1093/ckj/sfz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Laville SM, Metzger M, Stengel B et al. Evaluation of the adequacy of drug prescriptions in patients with chronic kidney disease: results from the CKD-REIN cohort: adequacy of drug prescriptions in CKD patients. Br J Clin Pharmacol 2018;84:2811–23. 10.1111/bcp.13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laville SM, Gras-Champel V, Hamroun A et al. Kidney function decline and serious adverse drug reactions in patients with CKD. Am J Kidney Dis 2024;83:601–14. 10.1053/j.ajkd.2023.09.012 [DOI] [PubMed] [Google Scholar]
- 14. von Elm E, Altman DG, Egger M et al. The strengthening the reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet North Am Ed 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [Google Scholar]
- 15. Stengel B, Combe C, Jacquelinet C et al. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant 2014;29:1500–7. 10.1093/ndt/gft388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stengel B, Metzger M, Combe C et al. Risk profile, quality of life and care of patients with moderate and advanced CKD: the French CKD-REIN Cohort Study. Nephrol Dial Transplant 2019;34:277–86. 10.1093/ndt/gfy058 [DOI] [PubMed] [Google Scholar]
- 17. Girerd X, Radauceanu A, Achard JM et al. [Evaluation of patient compliance among hypertensive patients treated by specialists]. Arch Mal Coeur Vaiss 2001;94:839–42. [PubMed] [Google Scholar]
- 18. Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World Health Organization Collaborating Centre for Drug Statistics Methodology, Oslo . Guidelines for ATC Classification and DDD Assignment 2016, 20th Edition. 2016. https://atcddd.fhi.no/filearchive/publications/2024_guidelines__final_web.pdf [Google Scholar]
- 20. Siew ED, Ikizler TA, Matheny ME et al. Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 2012;7:712. 10.2215/CJN.10821011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet North Am Ed 2000;356:1255–9. 10.1016/S0140-6736(00)02799-9 [DOI] [Google Scholar]
- 22. World Health Organization, Quality Assurance and Safety of Medicines Team . Safety of medicines: a guide to detecting and reporting adverse drug reactions: why health professionals need to take action. 2002.
- 23. Bégaud B, Evreux JC, Jouglard J et al. Imputation of the unexpected or toxic effects of drugs. Actualization of the method used in France. Therapie 1985;40:111–8. [PubMed] [Google Scholar]
- 24. Miremont-Salamé G, Théophile H, Haramburu F et al. Imputabilité en pharmacovigilance : de la méthode française originelle aux méthodes réactualisées. Thérapie 2016;71:171–8. [Google Scholar]
- 25. Olivier P, Caron J, Haramburu F et al. Validation d'une échelle de mesure: exemple de l’échelle française d’évitabilité des effets indésirables médicamenteux. Therapies 2005;60:39–45. 10.2515/therapie:2005005 [DOI] [Google Scholar]
- 26. Jones J, Holmen J, De Graauw J et al. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis 2012;60:402–8. 10.1053/j.ajkd.2012.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Couchoud C, Stengel B, Landais P et al. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant 2006;21:411–8. 10.1093/ndt/gfi198 [DOI] [PubMed] [Google Scholar]
- 28. van BS, mice G-OK. Multivariate imputation by chained equations in R. J Stat Softw 2011;45:1–67. [Google Scholar]
- 29. R Core Team . R: a language and environment for statistical computing. 2021; Accessed 31 March 2021. Available from: https://www.R-project.org/
- 30. Morcos R, Kucharik M, Bansal P et al. Contrast-induced acute kidney injury: review and practical update. Clin Med Insight Cardiol 2019;13:1179546819878680. 10.1177/1179546819878680 [DOI] [Google Scholar]
- 31. Pierson-Marchandise M, Gras V, Moragny J et al. The drugs that mostly frequently induce acute kidney injury: a case –noncase study of a pharmacovigilance database. Br J Clin Pharmacol 2017;83:1341–9. 10.1111/bcp.13216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bhandari S, Mehta S, Khwaja A et al. Renin–Angiotensin system inhibition in advanced chronic kidney disease. N Engl J Med 2022;387:2021–32. 10.1056/NEJMoa2210639 [DOI] [PubMed] [Google Scholar]
- 33. Chang Y-P, Huang S-K, Tao P et al. A population-based study on the association between acute renal failure (ARF) and the duration of polypharmacy. BMC Nephrol 2012;13:96. 10.1186/1471-2369-13-96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cartin-Ceba R, Kashiouris M, Plataki M et al. Risk factors for development of acute kidney injury in critically ill patients: a systematic review and meta-analysis of observational studies. Crit Care Res Practice 2012;2012:e691013. 10.1155/2012/691013 [DOI] [Google Scholar]
- 35. Knafl GJ, Riegel B. What puts heart failure patients at risk for poor medication adherence? Patient Pref Adherence 2014;8:1007–18. [Google Scholar]
- 36. Lombardi Y, Ridel C, Touzot M. Anaemia and acute kidney injury: the tip of the iceberg? Clin Kidney J 2021;14:471–3. 10.1093/ckj/sfaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kaur A, Sharma GS, Kumbala DR. Acute kidney injury in diabetic patients: a narrative review. Medicine (Baltimore) 2023;102:e33888. 10.1097/MD.0000000000033888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holmes J, Rainer T, Geen J et al. Acute kidney injury in the era of the AKI E-Alert. Clin J Am Soc Nephrol 2016;11:2123. 10.2215/CJN.05170516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lafrance J-P, Djurdjev O, Levin A. Incidence and outcomes of acute kidney injury in a referred chronic kidney disease cohort. Nephrol Dial Transplant 2010;25:2203–9. 10.1093/ndt/gfq011 [DOI] [PubMed] [Google Scholar]
- 40. See EJ, Jayasinghe K, Glassford N et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int 2019;95:160–72. 10.1016/j.kint.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 41. Siew ED, Parr SK, Wild MG et al. Kidney disease awareness and knowledge among survivors of acute kidney injury. Am J Nephrol 2019;49:449–59. 10.1159/000499862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ivica J, Sanmugalingham G, Selvaratnam R. Alerting to acute kidney injury—Challenges, benefits, and strategies. Pract Lab Med 2022;30:e00270. 10.1016/j.plabm.2022.e00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon reasonable request by contacting the corresponding author. The data are not publicly available, due to privacy or ethical restrictions.