Abstract
Background
About 30% of ischemic strokes do not have a clear cause, which is called cryptogenic stroke (CS). Increasing evidence suggests a potential link between CS and right-to-left shunt (RLS). RLS may lead to CS via paradoxical embolic mechanism. Hence, current study aims to explore the correlation between different RLS indexes and the occurrence of CS and its clinical diagnostic value in CS.
Methods
A total of 117 patients diagnosed with CS from October 2020 to June 2024 were randomly collected, and 93 patients with only headache and dizziness were randomly collected as the control group. All patients underwent agitated saline contrast echocardiography (ASCE) and the semi-quantitative classification, type and duration of RLS were analyzed. Spearman correlation analysis was used to analyze the correlation between RLS grade and type and the occurrence of CS, and the correlation between RLS duration and RLS grade and type. The efficacy of different RLS grades, types and durations in the diagnosis of CS were analyzed by receiver operating characteristic (ROC) curve.
Results
The included population ranged in age from 20–73 years, with 90 males and 120 females. There was no significant difference in basic data (e.g., gender, smoking history, drinking history, and the number of people with hypertension and diabetes) and serum biological indicators [triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL) and low-density lipoprotein (LDL)] between the CS group and the control group (all P>0.05). The proportion of RLS (77.78%) in the CS group was significantly higher than that in the control group (35.48%) (P<0.001). Spearman correlation analysis showed that RLS grade (r=0.569) and type (r=0.346) were significantly correlated with the occurrence of CS (both P<0.001). In addition, RLS duration was significantly correlated with RLS type (r=0.902, P<0.001), but not with RLS size (P>0.05). ROC curve analysis showed that RLS grade had the highest area under the curve (AUC) in CS diagnosis, which was 0.807 [95% confidence interval (CI): 0.748–0.866], the diagnostic sensitivity was 68.4%, and the specificity was 87.1%. In addition, the diagnostic AUC of RLS type and RLS duration in CS were similar, at 0.700 (95% CI: 0.626–0.773) and 0.707 (95% CI: 0.634–0.780), respectively.
Conclusions
RLS grade and RLS type are significantly correlated with the occurrence of CS. As an auxiliary means of CS diagnosis, RLS grade can effectively reduce the misdiagnosis rate of CS, which is of great clinical significance for early detection of CS risk.
Keywords: Cryptogenic stroke (CS), right-to-left shunt (RLS), right heart contrast echocardiography, patent foramen ovale (PFO), receiver operating characteristic curve (ROC curve)
Highlight box.
Key findings
• Right-to-left shunt (RLS) grade and RLS type are significantly correlated with the occurrence of cryptogenic stroke (CS).
• RLS type, RLS grade, and RLS duration time can be used as an auxiliary means for CS diagnosis.
What is known and what is new?
• RLS is significantly associated with the development of CS.
• Both RLS classification and RLS type are significantly associated with the development of CS.
What is the implication, and what should change now?
• In this study, we propose a brand-new aid for CS diagnosis. RLS grade can effectively reduce the misdiagnosis rate of CS, which is of great clinical significance for early detection of CS risk.
Introduction
Background
Stroke seriously endangers human health and has become one of the main causes of death (1). Among them, ischemic stroke accounts for about 80% of deaths (2). The main clinical causes of some cases were attributed to pulmonary vascular disease, cardiac valve embolism syndrome and various small artery vascular diseases. However, about 30% of ischemic strokes do not have a clear cause, which is called cryptogenic stroke (CS) (3,4). In the context of CS, the presence of a microthrombus originating from the right atrium prevents it from fully traversing the cardiopulmonary blood vessels, ultimately leading to its entry into the systemic circulation. Thrombosis formed by lower extremity venous thrombosis and atrial septal aneurysm can cause cerebral basilar artery plexus occlusion through abnormal vascular channels from right-to-left shunt (RLS). This phenomenon is called paradoxical embolism, which is more common in young and middle-aged ischemic stroke (5,6). Furthermore, a classic cardioembolic presentation includes the onset of symptoms after a Valsalva provoking activity (coughing, bending, etc.), suggesting paradoxical embolism facilitated by a transient rise in right atrial pressure and the co-occurrence of cerebral and systemic emboli (7). The common possible causes of CS are vasospasm, paroxysmal atrial fibrillation, venous embolism, patent foramen ovale (PFO), etc. (8).
The foramen ovale is a tunnel-like structure formed by the fetus growing between the primary septum and the secondary septum of the atrium, which is a physiological pathway between the atrial septum (9). With the improvement of the function of the neonatal pulmonary circulation system, the pressure of the left atrium gradually increases, and the primary septum and the secondary septum are closely fitted to the closure (10). If the baby is still not closed after 3 years old, it is called PFO (10). Studies have found that the incidence of PFO in patients with CS is more than 60% (11,12). A large number of studies have shown that PFO may be the most important risk factor for CS, but the mechanism of CS (PFO-RLS) caused by PFO is still controversial (12,13). In recent years, the relationship between CS and RLS has attracted more and more attention (13). Studies have shown that PFO size is an independent predictor of severe RLS (13,14). Multiple ischemic lesions of the brain are more common in CS patients with severe RLS (14). In addition, studies suggest that RLS may lead to CS through abnormal embolization mechanism (6,15). Therefore, the detection of RLS is of great significance for clarifying the etiology of CS.
Rationale and knowledge gap
Recent study has found that in addition to PFO-RLS, lung-related RLS [pulmonary RLS (P-RLS)] is also more common (16). Physiological pulmonary arteriovenous anastomotic branches or anastomotic channels of 20–50 µm are common in healthy people, suggesting that P-RLS not only comes from pulmonary arteriovenous fistula, but may also play a role in the pathogenesis of CS (17). The agitated saline contrast echocardiography (ASCE) has the advantages of high sensitivity, safety and wide application range for RLS (18). ASCE currently commonly uses oscillating sterile saline or sugar water to prepare acoustic contrast agents. The resulting microbubbles are large in volume and cannot pass through the pulmonary circulation, and can be only developed in the right heart. In addition, transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) can evaluate the anatomical structure and shunt of atrial septum. TTE combined with ASCE can be used to analyze the shunt flow, shunt source and duration of RLS. TTE combined with ASCE can more clearly evaluate the shunt flow, shunt source and duration of RLS (19). For patients with poor TTE sound window and in situation in which the source of shunt cannot be determined, TEE combined with ASCE can be performed. TEE combined with ASCE can directly show whether the microbubbles are derived from the foramen ovale or pulmonary vein, and the accuracy of RLS type screening is higher (20). However, TEE combined with ASCE requires esophageal intubation, which is a semi-invasive examination and is not suitable for large-scale RLS screening.
Objective
Therefore, this study explored the correlation between different types and different grades of RLS and CS by combining TTE with ASCE and partially combining TEE with ASCE. At the same time, we introduced the concept of RLS duration, and further explored the application value of RLS type, grade and duration in CS diagnosis. We present this article in accordance with the STROBE reporting checklist (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-288/rc).
Methods
Clinical data
This is a retrospective case-control study. Data of a total of 228 patients were retrospectively collected from The Second Affiliated Hospital of Zhejiang Chinese Medical University, of which four patients with incomplete imaging data, three patients with incomplete blood biochemical examination data, and one patient who did not sign the informed consent form were excluded. Finally, a total of 117 patients with CS (study group) and 93 patients with headache and dizziness (control group) were retrospectively included. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Zhejiang Chinese Medical University (No. 2023-LW-030-01), and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived. Patients with CS were previously confirmed for the presence of aortogenic etiology by computed tomography angiography (CTA) of the aortic arch and cerebral angiography. Bedside electrocardiogram monitoring and TEE were performed to confirm the presence of cardiogenic etiology. Pulmonary angiography was performed to confirm the presence of pulmonary arterial etiology. All enrolled patients underwent ASCE examination and related auxiliary examinations [such as head magnetic resonance imaging (MRI), carotid ultrasound, transcranial Doppler, echocardiography, blood biochemical examination, etc.].
Inclusion criteria: (I) positive brain computed tomography (CT) and/or brain MRI in CS group, and negative brain CT and/or brain MRI in control group; (II) perfect related imaging and blood biochemical examination; (III) informed consent was acquired from all the patients.
Exclusion criteria: (I) migraine patients; (II) history of severe heart and lung diseases, such as ventricular septal defect, severe arrhythmia or severe pneumonia; (III) patients with temporal window closure and limited exploration; cognitive impairment cannot cooperate with ASCE examiners.
ASCE examination
TTE and/or TEE combined with ASCE were performed using Philips IE 33 color Doppler ultrasound diagnostic instrument. TTE (probe S5-1, frequency 1.0–5.0 MHz) was used to measure the size of each atrioventricular cavity, and to observe the absence of echo loss in the atrial septum, atrial fibrillation, left ventricular thrombosis and valvular vegetations. The patient was placed in the left lateral position, and the venous channel was retained in the median vein of the left or right elbow, which was connected with the three-way tube. Two 20 mL syringes were taken. One of the syringes extracted 5 mL normal saline + 4 mL 50% glucose (if the patient had diabetes, it was 8 mL normal saline + 1 mL 50% glucose) + 1 mL air. The two syringes were connected by a three-way switch, and quickly pushed back and forth for 15–20 times to generate a large number of microbubbles.
TTE combined with ASCE was used to observe the number, source and duration of microbubbles in the left atrium after the right heart was fully developed in the resting state and the Valsalva state, respectively. All patients included in this study completed TTE. Of these, 32 patients required further TEE. For TEE (probe S7-2 Omni, frequency 2.2–6.5 MHz), the patient was fasted for 8 hours, and local anesthesia was performed for 5 minutes with tetracaine mucilage. The left lateral position was taken, and the venous channel was placed in the median vein of the right elbow and connected with the three-way tube. The probe was inserted into the middle and lower part of the esophagus, and the probe was rotated 45–110° to clearly show the primary septum and the secondary septum, and to observe whether there was a fissure between the two. Color Doppler flow imaging (CDFI) was used to observe whether there was left-to-right shunt or RLS blood flow signal in the fissure. At the same time, the mode of microbubbles entering the left atrium was observed by combining ASCE, in which microbubbles entered the left atrium from the fissure between the primary septum and the secondary septum as PFO-RLS; the continuous entry of microbubbles from the pulmonary vein into the left atrium is P-RLS. All analyses were performed independently by an examiner and with more than 6 years of experience and a data analyst with more than 10 years of experience. Only examiner and data analyst will be blinded in this study.
RLS semi-quantitative analysis
As mentioned above, the image with the largest number of microbubbles in the left atrium was selected to count the number of microbubbles in the left atrium (21). The degree of RLS was graded according to the number of microbubble signals detected after calm breathing or Valsalva maneuver, and the number of microbubble signals was the largest after calm breathing or Valsalva maneuver.
Level 0: no microbubbles in the left atrium on each frame of image; level 1: 1–10 microbubbles were visible in the left atrium on each frame of image; level 2: 11–30 microbubbles were seen in the left atrium on each frame of image; level 3: more than 30 microbubbles can be seen in the left atrium on each frame of the image (21).
Statistical analysis
SPSS 27.0 statistical software was used to analyze the data. All continuous variables were tested for normal distribution. Variables conforming to a normal distribution were presented as mean ± standard deviation. Continuous variables that did not follow a normal distribution were presented as median (interquartile range). The two groups were compared by independent sample t-test. The count data were expressed by quantity and percentage, and the two groups were compared by χ2 test. Correlation analysis was performed by Spearman. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were obtained for different RLS types, grades and durations. Receiver operating characteristic (ROC) curve analysis were applied to evaluate diagnostic performance of three indexes and obtain the area under the curve (AUC). Two-sided P<0.05 was considered statistically significant.
Results
General data analysis of patients with CS
According to the inclusion and exclusion criteria, a total of 210 patients were included in this study, including 117 CS patients (55.71%) and 93 control patients (44.29%) with only headache and dizziness. The baseline data of CS patients and control group are compared in Table 1. Compared with the control group, there was no significant difference in gender, age, body mass index (BMI), smoking history, drinking history, and the number of people with hypertension and diabetes in CS patients (all P>0.05). Subsequently, we analyzed serum biological indicators. There was no significant difference in TG, TC, HDL and LDL levels between the CS group and the control group. RLS blood flow signals of all patients were examined by ASCE combined with TTE or TEE (Figures 1,2). The number of patients with RLS in the CS group was significantly higher than that in the control group [91 cases (77.78%) vs. 33 cases (35.48%), P<0.001].
Table 1. Comparison of general data between cryptogenic stroke and control group.
| Characteristics | Cryptogenic stroke group (n=117) | Control group (n=93) | P value |
|---|---|---|---|
| Sex (male/female) | 53/64 | 37/56 | 0.42 |
| Age (years) | 46.50±11.21 | 44.65±9.91 | 0.21 |
| BMI (kg/m2) | 24.08±1.13 | 24.31±1.00 | 0.21 |
| Smoking history | 20 (17.09) | 18 (19.35) | 0.67 |
| Drinking history | 11 (9.40) | 4 (4.30) | 0.15 |
| Hypertension | 24 (20.51) | 12 (12.90) | 0.15 |
| Diabetes | 2 (1.71) | 0 (0.00) | 0.21 |
| Triglyceride (mmol/L) | 1.57±0.71 | 1.67±0.67 | 0.31 |
| Total cholesterol (mmol/L) | 4.42±1.21 | 4.28±1.10 | 0.39 |
| HDL (mg/dL) | 1.20±0.21 | 1.24±0.22 | 0.21 |
| LDL (mg/dL) | 2.49±0.77 | 2.53±0.77 | 0.75 |
| RLS | 91 (77.78) | 33 (35.48) | <0.001 |
Measurement data conforming to normal distribution were presented as mean ± standard deviation; count data were presented as number or number (%). BMI, body mass index; HDL, high-density lipoprotein; LDL, low-density lipoprotein; RLS, right-to-left shunt.
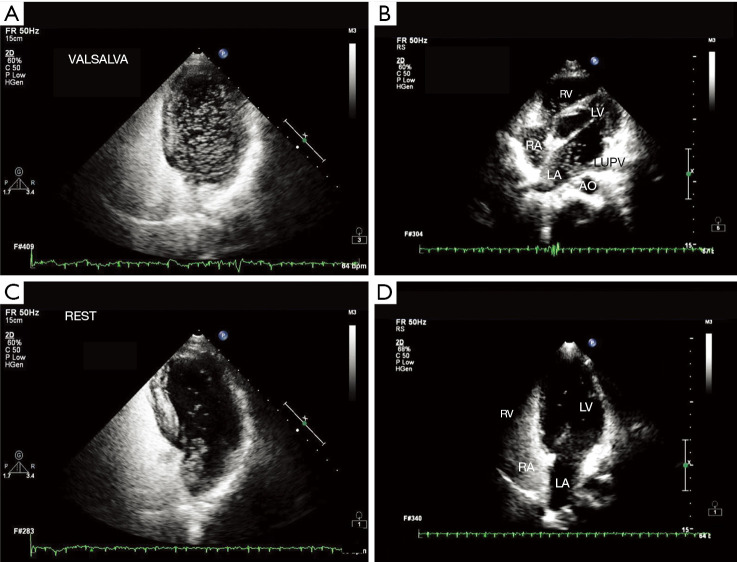
Figure 1.
Representative images of RLS signal diagnosed by TTE combined with ASCE. (A) A large number of RLS signals under Valsalva action; (B) left atrial RLS signal from left superior pulmonary vein; (C) RLS signal from interatrial septum in resting state; (D) a small amount of RLS signals at rest. FR, frequency; C, dynamic range; P, afterglow; HGen, Harmonic General mode; VALSALVA, Valsalva action; bpm, beats per minute; RV, right ventricle; LV, left ventricle; RA, right atrium; LA, left atrium; AO, aorta; LUPV, left upper pulmonary vein; REST, resting state; RLS, right-to-left shunt; TTE, transthoracic echocardiography; ASCE, agitated saline contrast echocardiography.
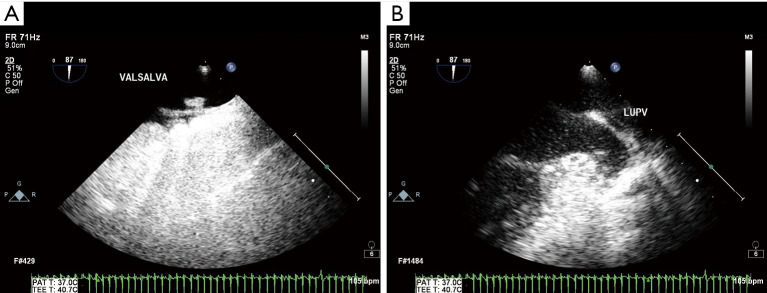
Figure 2.
Representative images of RLS signal diagnosed by TEE combined with ASCE. (A) PFO-RLS signal under Valsalva maneuver; (B) P-RLS signal under Valsalva maneuver. FR, frequency; C, dynamic range; P, afterglow; Gen, General mode; VALSALVA, Valsalva action; PAT, photoacoustic tomography; TEE, transesophageal echocardiography; bpm, beats per minute; LUPV, left upper pulmonary vein; RLS, right-to-left shunt; ASCE, agitated saline contrast echocardiography; PFO-RLS, patent foramen ovale right-to-left shunt; P-RLS, pulmonary right-to-left shunt.
The relationship between CS patients and RLS type and grade
The occurrence of CS may be closely related to RLS type and RLS grade. Spearman correlation analysis was used to analyze the correlation between the occurrence of CS and RLS type and RLS grade. As shown in Table 2, a total of 91 patients in the CS group had RLS. Among them, the number of patients with PFO-RLS type was the largest, with a total of 63 cases. In contrast, the number of patients with PFO-RLS and P-RLS types in the control group was similar. Correlation analysis showed that the occurrence of CS was significantly positively correlated with RLS type (r>0, P<0.001) (Table 2). In terms of RLS grade, the number of patients with RLS grade 3 in the CS group was the highest, with a total of 58 cases. In contrast, patients with RLS grade 3 in the control group were the least, only 2 cases. Correlation analysis showed that the occurrence of CS was significantly positively correlated with RLS grade (r>0, P<0.001) (Table 3).
Table 2. Correlation analysis between the occurrence of CS and RLS type.
| RLS type | CS group | Control group | r | P |
|---|---|---|---|---|
| No RLS | 26 | 60 | 0.346 | <0.001 |
| PFO-RLS | 63 | 18 | ||
| P-RLS | 25 | 15 | ||
| PFO-RLS + P-RLS | 3 | 0 |
CS, cryptogenic stroke; RLS, right-to-left shunt; PFO-RLS, patent foramen ovale right-to-left shunt; P-RLS, pulmonary right-to-left shunt.
Table 3. Correlation analysis between the occurrence of CS and RLS grade.
| RLS semi-quantitative grading | CS group | Control group | r | P |
|---|---|---|---|---|
| 0 | 26 | 60 | 0.569 | <0.001 |
| 1 | 11 | 21 | ||
| 2 | 22 | 10 | ||
| 3 | 58 | 2 |
CS, cryptogenic stroke; RLS, right-to-left shunt.
The relationship between RLS duration and RLS type and grade in patients with CS
Subsequently, we further analyzed the correlation between RLS duration and RLS type and grade. As shown in Table 4, the RLS duration of different RLS types are different. The duration of P-RLS was longer than that of PFO-RLS. Correlation analysis showed that RLS duration was significantly correlated with RLS type (P<0.001, r=0.902). In addition, there was no significant correlation between the duration of RLS and RLS semi-quantitative grading (P>0.05).
Table 4. Correlation analysis of RLS duration with RLS type and grade.
| Contents | RLS duration time (cardiac cycles) | r | P |
|---|---|---|---|
| RLS type | 0.902 | <0.001 | |
| PFO-RLS | 8.0 (7.0, 9.0) | ||
| P-RLS | 19.0 (17.0, 20.0) | ||
| PFO-RLS + P-RLS | 20.0 (18.0, 22.0) | ||
| RLS semi-quantitative grading | −0.154 | 0.09 | |
| 1 | 10.0 (8.0, 20.0) | ||
| 2 | 9.0 (8.0, 17.0) | ||
| 3 | 9.0 (8.0, 10.0) | ||
Continuous variables that did not follow a normal distribution were presented as median (interquartile range). RLS, right-to-left shunt; PFO-RLS, patent foramen ovale right-to-left shunt; P-RLS, pulmonary right-to-left shunt.
The diagnostic value of RLS related indicators in CS
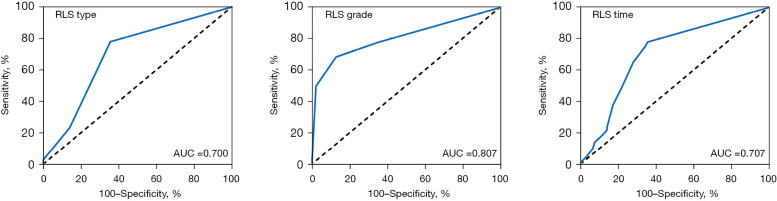
In view of the correlation between RLS type and grade and the occurrence of CS, the preliminary application value of different RLS indicators in the diagnosis of CS was analyzed by ROC curve. As shown in Figure 3 and Table 5, the RLS grade had the highest diagnostic AUC of 0.807 in CS, suggesting that the RLS grade has the best diagnostic value. This result is consistent with the results of correlation analysis. In addition, the diagnostic AUC of RLS type and RLS duration in CS was similar, which may be related to the high correlation between the two (r=0.902). In addition, the sensitivity of RLS grade in the diagnosis of CS was 68.4%, which was slightly lower than that of RLS type and RLS duration, suggesting that the detection rate of RLS grade in the diagnosis of CS was low. However, RLS grade has a high diagnostic specificity (87.1%), suggesting that RLS grade is not prone to misdiagnosis in CS diagnosis. The above results indicate that these three different RLS-related indicators can be used as an auxiliary means for CS diagnosis. Among them, the diagnostic value of RLS grade is the best.
Figure 3.
ROC curve of different RLS diagnostic methods in the diagnosis of cryptogenic stroke. RLS, right-to-left shunt; AUC, area under the curve; ROC, receiver operating characteristic.
Table 5. Comparison of different RLS indexes in the diagnosis of cryptogenic stroke.
| Variables | AUC (95% CI) | Youden index | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | P |
|---|---|---|---|---|---|---|---|
| RLS type | 0.700 (0.626, 0.773) | 0.423 | 77.8 | 64.5 | 77.8 | 63.4 | <0.001 |
| RLS grade | 0.807 (0.748, 0.866) | 0.555 | 68.4 | 87.1 | 68.4 | 87.1 | <0.001 |
| RLS duration time | 0.707 (0.634, 0.780) | 0.423 | 77.8 | 64.5 | 77.8 | 64.5 | <0.001 |
RLS, right-to-left shunt; AUC, area under the curve; CI, confidence interval; PPV, positive predictive value; NPV, negative predictive value.
Discussion
Key finding
RLS grade and RLS type are significantly correlated with the occurrence of CS. RLS type, RLS grade, and RLS duration time can be used as an auxiliary means for CS diagnosis.
Strengths and limitations
Studies have shown that RLS may be one of the risk factors for CS and is associated with the occurrence and development of CS (6,22). In addition, about a quarter of adult patients have RLS (23). RLS can be seen in many diseases, such as cryptogenic ischemic stroke, migraine (especially aura), white matter lesions, Alzheimer’s disease, sleep apnea, etc. Therefore, identifying the risk of CS through different RLS indicators has important clinical significance for the early diagnosis and timely treatment of CS. In this study, RLS grade, type and duration were used as predictors of CS, and the ROC curve of these indicators in CS diagnosis was analyzed. Among these RLS-related indicators, the RLS grade had the highest diagnostic AUC in CS, suggesting that the RLS grade has the best diagnostic value. This result is also consistent with the results of correlation analysis. Moreover, the specificity of RLS grade in the diagnosis of CS was as high as 87.1%, suggesting that RLS grade was not prone to misdiagnosis in the diagnosis of CS. In addition, the diagnostic AUC of RLS type and RLS duration in CS is similar, which may be related to the high correlation between the two. The above results indicate that these three different RLS-related indicators can be used as an auxiliary means for CS diagnosis.
There are still some shortcomings in this study. First of all, this study only involves PFO-RLS and P-RLS, and no further analysis is made for intrinsic RLS and latent RLS. Furthermore, the sample size of this study is relatively small, and the etiology of highly occult CS requires more clinical evaluation. Therefore, larger sample size and multicenter studies are needed to further verify and improve our conclusions in the future. In addition, the sensitivity of RLS grading is too low, only 68.4%.
Comparison with similar researches
At present, there are few studies related to RLS duration, and the correlation between RLS duration and other RLS indicators is still unknown. RLS duration is described by cardiac cycle. In general, P-RLS is not affected by left and right atrial pressure, and the path is relatively smooth, so the left heart microbubbles last longer (24). The duration of left heart microbubbles is generally more than 15 cardiac cycles, and even the regression time is later than that of the right heart (24). PFO-RLS is affected by the pressure of the left and right atrium (24). When the right atrial pressure is greater than the left atrial pressure, the oval foramen will open at a moment, and the left heart will have a short duration of microbubbles (24). The left heart microbubbles generally fade away around 10 cardiac cycles (24). In this study, we found that RLS duration was significantly correlated with RLS type. However, there was no significant correlation between RLS duration and RLS size, which may be related to the small sample size. The above results show that the duration of RLS depends more on the type of RLS, the duration of PFO-RLS is shorter, and the duration of P-RLS is longer.
Explanations of findings
First, we compared the baseline data of patients in the CS group and the control group. There was no significant difference in the proportion of smoking, drinking, hypertension and diabetes between the CS group and the control group, suggesting that traditional cerebrovascular disease risk factors were not significantly different between the CS and general headache populations. In addition, the vascular risk factors of the patients were evaluated. Similarly, we found that there was no significant difference in TG, TC, HDL and LDL levels between CS patients and normal controls. In addition, recent study has shown that female CS patients have different clinical characteristics and poorer early prognosis compared with male CS patients (25). Our study has not found similar results, which may be related to the small sample size we included, and subsequent study still needs to expand the sample size. Subsequently, RLS was detected by ASCE in all patients. We found that the proportion of RLS in CS patients was significantly higher than that in the control group, suggesting that RLS may be a risk factor for CS. Therefore, we further analyzed the correlation between the occurrence of CS and different RLS indicators.
There was a big difference in the overall RLS grade distribution between the CS group and the control group. In this study, due to the low sample size, there was no further subdivision study on whether there was a significant difference in the distribution between the CS group and the control group under the same RLS classification. It can be seen from the existing results that for CS patients, the proportion of RLS grade 3 is the highest, while most of the patients in the control group did not detect RLS. The detection rate of RLS in the control group was about 35%, which was slightly higher than the detection rate of RLS in normal people in previous study (22). It may be related to the symptoms of dizziness and headache in patients (22). In addition, in the control group with RLS, the proportion of patients with RLS grade 1 was the highest. Previous study has also shown that RLS detected in the normal population is mostly small shunts, which also explains why the detection rate of RLS in the population is as high as 30%, but the incidence of CS is far lower than this (23). Some studies have also suggested that PFO-RLS flow should not be the only criterion for measuring CS, because there is no correlation between RLS size and cerebral infarction volume in CS patients (6,26). CS patients with a small amount of shunt have a larger infarct volume. However, this study also found a correlation between PFO-RLS size and the incidence of CS, consistent with our findings (26). The limitation of this study is that it ignores the type of RLS (26). Our results show that the incidence of CS is also significantly correlated with the type of RLS. However, given space limitations and the article’s main objectives, the correlation between RLS type and cerebral infarction volume in CS patients needs to be clarified in subsequent study.
Implications and actions needed
Previous study has shown that CS populations have different clinical characteristics and prognosis in different genders (25). The sample size is subsequently expanded to explore the value of RLS type, RLS grade, or duration of RLS in CS patients of different genders.
Conclusions
In summary, different RLS grades and types are significantly correlated with the occurrence of RLS. The duration of RLS was related to different RLS types, but has no significant correlation with RLS grade. RLS grade, RLS type and RLS duration can be used as auxiliary means for CS diagnosis. The RLS grade has the highest diagnostic efficiency and diagnostic specificity in CS diagnosis. RLS grade as an auxiliary means of CS diagnosis can effectively reduce the misdiagnosis rate of CS, which has important clinical significance for early detection of CS risk.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: None.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by the Ethics Committee of The Second Affiliated Hospital of Zhejiang Chinese Medical University (No. 2023-LW-030-01), and conducted in accordance with the Declaration of Helsinki (as revised in 2013). Individual consent for this retrospective analysis was waived.
Footnotes
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-288/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-288/coif). The authors have no conflicts of interest to declare.
Data Sharing Statement
Available at https://cdt.amegroups.com/article/view/10.21037/cdt-24-288/dss
References
- 1.Vera A, Cecconi A, Ximénez-Carrillo Á, et al. Left Atrial Strain Predicts Stroke Recurrence and Death in Patients With Cryptogenic Stroke. Am J Cardiol 2024;210:51-7. 10.1016/j.amjcard.2023.10.001 [DOI] [PubMed] [Google Scholar]
- 2.Fang J, Wang Z, Miao CY. Angiogenesis after ischemic stroke. Acta Pharmacol Sin 2023;44:1305-21. 10.1038/s41401-023-01061-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poisson SN, Leppert MH, Orjuela KD. Cryptogenic Stroke and PFO Closure: Does Sex Matter? J Am Heart Assoc 2023;12:e031857. 10.1161/JAHA.123.031857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ekker MS, Verhoeven JI, Schellekens MMI, et al. Risk Factors and Causes of Ischemic Stroke in 1322 Young Adults. Stroke 2023;54:439-47. 10.1161/STROKEAHA.122.040524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kwok CS, Alisiddiq Z, Will M, et al. The Modified Risk of Paradoxical Embolism Score Is Associated with Patent Foramen Ovale in Patients with Ischemic Stroke: A Nationwide US Analysis. J Cardiovasc Dev Dis 2024;11:213. 10.3390/jcdd11070213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu YH, Liu H, He JH, et al. Risk factors for the development of cryptogenic stroke and the predictive value of right-to-left shunt in patent foramen ovale. Eur Rev Med Pharmacol Sci 2024;28:1027-35. [DOI] [PubMed] [Google Scholar]
- 7.Pujadas Capmany R, Arboix A, Casañas-Muñoz R, et al. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol 2004;95:129-34. 10.1016/j.ijcard.2003.02.007 [DOI] [PubMed] [Google Scholar]
- 8.Lucà F, Pino PG, Parrini I, et al. Patent Foramen Ovale and Cryptogenic Stroke: Integrated Management. J Clin Med 2023;12:1952. 10.3390/jcm12051952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teshome MK, Najib K, Nwagbara CC, et al. Patent Foramen Ovale: A Comprehensive Review. Curr Probl Cardiol 2020;45:100392. 10.1016/j.cpcardiol.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 10.Collado FMS, Poulin MF, Murphy JJ, et al. Patent Foramen Ovale Closure for Stroke Prevention and Other Disorders. J Am Heart Assoc 2018;7:e007146. 10.1161/JAHA.117.007146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riaz IB, Dhoble A, Mizyed A, et al. Transcatheter patent foramen ovale closure versus medical therapy for cryptogenic stroke: a meta-analysis of randomized clinical trials. BMC Cardiovasc Disord 2013;13:116. 10.1186/1471-2261-13-116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diener HC, Wachter R, Wong A, et al. Monitoring for atrial fibrillation prior to patent foramen ovale closure after cryptogenic stroke. Int J Stroke 2023;18:400-7. 10.1177/17474930221124412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanemaru K, Ueno Y, Kikuno M, et al. High-risk patent foramen ovale and elderly in cryptogenic stroke. J Stroke Cerebrovasc Dis 2023;32:107344. 10.1016/j.jstrokecerebrovasdis.2023.107344 [DOI] [PubMed] [Google Scholar]
- 14.Ji MH, Seoung YH. Right-to-Left Shunt Evaluation in Cardiac Patent Foramen Ovale Using Bubble Contrast Transcranial Color-Coded Doppler: A Cryptogenic Stroke Case. Healthcare (Basel) 2023;11:2655. 10.3390/healthcare11192655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crispino SP, Segreti A, La Porta Y, et al. A sudden right-to-left shunt: the importance of evaluating patent foramen ovale during exercise. Monaldi Arch Chest Dis 2023;94. doi: . 10.4081/monaldi.2023.2443 [DOI] [PubMed] [Google Scholar]
- 16.Reimann L, Mayer L, Schneider SR, et al. Change in Right-to-Left Shunt Fraction in Patients with Chronic Thromboembolic Pulmonary Hypertension after Pulmonary Endarterectomy. J Cardiovasc Dev Dis 2023;10:442. 10.3390/jcdd10110442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrero P, Constantine A, Chessa M, et al. Pulmonary arterial hypertension related to congenital heart disease with a left-to-right shunt: phenotypic spectrum and approach to management. Front Cardiovasc Med 2024;11:1360555. 10.3389/fcvm.2024.1360555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau VI, Mah GD, Wang X, et al. Intrapulmonary and Intracardiac Shunts in Adult COVID-19 Versus Non-COVID Acute Respiratory Distress Syndrome ICU Patients Using Echocardiography and Contrast Bubble Studies (COVID-Shunt Study): A Prospective, Observational Cohort Study. Crit Care Med 2023;51:1023-32. 10.1097/CCM.0000000000005848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo L, Xie Z, Wu Q, et al. Transesophageal echocardiography guidance for percutaneous closure of PFO and a new method to improve the diagnosis and safety during the procedures. Front Cardiovasc Med 2024;11:1428380. 10.3389/fcvm.2024.1428380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen A, Zhu J, Zhu L, et al. Neglected intrapulmonary arteriovenous anastomoses: A comparative study of pulmonary right-to-left shunts in patients with patent foramen ovale. Front Cardiovasc Med 2023;10:1111818. 10.3389/fcvm.2023.1111818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Ji S, Li H, et al. Association of patent foramen ovale with epilepsy: A hospital-based case-control study. Epilepsia Open 2023;8:1075-83. 10.1002/epi4.12787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tian L, Zhang M, Nie H, et al. Contrast-enhanced transcranial doppler versus contrast transthoracic echocardiography for right-to-left shunt diagnosis. J Clin Monit Comput 2023;37:1145-51. 10.1007/s10877-023-00979-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimura T, Okada T, Obata N, et al. Right-to-left shunt due to iatrogenic atrial septal defect manifested by aorto-caval fistula: a case report. JA Clin Rep 2024;10:50. 10.1186/s40981-024-00735-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng C, Luo T, Luo Y, et al. Contrast-enhanced transthoracic echocardiography applied in evaluation of pulmonary right-to-left shunt: A preliminary study. Comput Med Imaging Graph 2018;68:55-60. 10.1016/j.compmedimag.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 25.Inogés M, Arboix A, García-Eroles L, et al. Gender Predicts Differences in Acute Ischemic Cardioembolic Stroke Profile: Emphasis on Woman-Specific Clinical Data and Early Outcome-The Experience of Sagrat Cor Hospital of Barcelona Stroke Registry. Medicina (Kaunas) 2024;60:101. 10.3390/medicina60010101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin P, Jiao P, Feng J, et al. The predictive value of abnormal electrocardiogram for patent foramen ovale: A retrospective study. Clin Cardiol 2023;46:1504-10. 10.1002/clc.24133 [DOI] [PMC free article] [PubMed] [Google Scholar]