ABSTRACT
Background
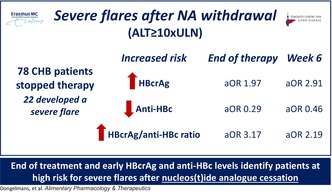
Severe flares (ALT ≥ 10×ULN) are a well‐recognised adverse outcome after nucleos(t)ide analogue (NA) cessation and may lead to liver failure. Thus, identification of patients at risk for these flares is of major importance.
Methods
Data were used from two prospective studies on NA cessation conducted in the Netherlands and Canada. Patients were eligible based on EASL criteria. HBcrAg and anti‐HBc levels were measured at end of treatment (EOT) and week 6 (FUW6). Logistic regression was used to study the association with severe flares.
Results
Seventy‐eight patients were analysed with a mean age of 49 years, 16 (21%) Caucasian and a majority (65%) were treated with Tenofovir. Overall, 22 patients (28%) developed a severe flare, and 29 (37%) patients were retreated. At EOT, higher HBcrAg levels (aOR: 1.97, p = 0.05; ≥ 4log: 47% severe flare vs. < 3log: 19%, p = 0.036), lower anti‐HBc (aOR: 0.29, p = 0.036; < 2log: 50% vs. ≥ 3log: 11%, p = 0.029) and higher HBcrAg/anti‐HBc‐ratio (aOR: 3.17, p = 0.015; ≥ 2: 58% vs. < 1.5: 14%, p < 0.001) were associated with an increased risk of severe flares, adjusted for HBsAg. At FUW6, higher HBcrAg (aOR: 2.91, p = 0.035; ≥ 5log: 83%, < 3log: 4%, p < 0.001), lower anti‐HBc (aOR: 0.46, p = 0.29; < 2log: 50% vs. ≥ 3log: 0%, p = 0.003) and higher HBcrAg/anti‐HBc‐ratio (aOR: 2.19, p = 0.048; ≥ 1.75: 52% vs. < 1.75: 8%, p < 0.001) were associated with an increased risk of severe flares, adjusted for HBV DNA and ALT.
Conclusion
Higher HBcrAg, lower anti‐HBc and higher HBcrAg/anti‐HBc ratio at EOT and during the first weeks of post‐treatment follow‐up are associated with an increased risk of hepatic flares after NA withdrawal and could therefore potentially be used to select patients eligible for therapy cessation and to identify patients requiring retreatment.
Trial Registration: This study was a post hoc and follow‐up study of two previously registered clinical trials (NCT01911156 & NTR7001). No new patients were prospectively included
Keywords: anti‐HBc, discontinuation, flares, HBcrAg, HBV
Higher HBcrAg and lower anti‐HBc levels at EOT and early follow‐up seem to be associated with an increased risk of severe flares (ALT ≥ 10×ULN) after nucleos(t)ide analogue withdrawal and could potentially be used to identify high‐risk patients in whom therapy withdrawal should be discouraged and/or need retreatment.
1. Introduction
Cessation of nucleos(t)ide analogue's (NAs) is a novel strategy to increase the probability of functional cure in a selected group of chronic hepatitis B (CHB) patients, especially those with lower HBsAg‐levels [1, 2, 3, 4]. However, stopping therapy is not without risks since withdrawal could result in viral rebound potentially leading to severe hepatic flares and liver failure [5, 6, 7]. The risk of hepatic flares and the probability of achieving functional cure represent a delicate balance between host‐ and virus‐related factors [8, 9]; a response of the immune system is required for viral clearance, whereas failure to clear the virus or hyper‐activation of the immune response could lead to severe hepatic flares. Thus, identification of biomarkers that reflect this interplay is needed.
Two candidate biomarkers are hepatitis B core‐related antigen (HBcrAg) and anti‐hepatitis B core antibodies (anti‐HBc). HBcrAg is a combination of hepatitis B core antigen (HBcAg), hepatitis B envelop antigen (HBeAg) and 22 kDa precore protein (p22Cr), and serum HBcrAg correlates strongly with intrahepatic replicational activity, even in patients who are virally suppressed with NA [10, 11, 12, 13]. Lower HBcrAg levels at end of treatment (EOT) have previously been linked to superior virological outcomes after NA withdrawal, such as lower risks of clinical relapses and higher rates of HBsAg loss [14, 15]. However, data regarding off‐treatment HBcrAg kinetics and the risk of subsequent flares is lacking.
Where HBcrAg reflects viral activity, anti‐HBc may be reflective of certain aspects of the host anti‐viral immune response. Anti‐HBc is produced by HBV‐specific B‐cells as a response to Hepatitis B Core antigen and therefore reflects immune activity, with higher levels previously linked to superior immune responses [16, 17, 18, 19]. Based on these prior observations, we hypothesised that serum levels of HBcrAg and anti‐HBc, and their ratio could be used to predict the risk of severe flares after NA withdrawal.
Therefore, the aim of this study was to investigate the association between EOT and (early) off‐treatment HBcrAg and anti‐HBc levels and (severe) hepatic flares after treatment withdrawal.
2. Methods
2.1. Study Cohort
The study included patients with CHB who withdrew NA therapy during two previously published prospective studies conducted in Canada (NCT01911156) [20] and the Netherlands (NTR7001) [21]. The follow‐up time after NA cessation was 72 or 96 weeks, respectively. More comprehensive details regarding the study designs have been published before [20, 21]. Patients were eligible if they were HBeAg negative at NA cessation and achieved long‐term viral suppression. Exclusion criteria were a co‐infection (i.e., HCV/HDV/HIV), history of advanced fibrosis or hepatocellular carcinoma (HCC) prior to NA cessation. All patients gave written informed consent for re‐use of their data and samples when included in the original studies. Both studies were approved by the research ethical board of each participating center and performed in concordance with Good Clinical Practice guidelines and the Declaration of Helsinki in 1964 as modified by the 59th World Medical Association General Assembly, Seoul, South Korea, in October 2008.
2.2. Primary Outcome
The primary outcome was the association between EOT and early off‐treatment HBcrAg‐ and anti‐HBc levels and the occurrence of severe ALT‐flares (ALT ≥ 10×ULN) during subsequent follow‐up.
2.3. Laboratory Assays
Quantitative HBcrAg was determined using the Lumipulse G HBcrAg assay (Fujirebio Europe, Ghent, Belgium) on the LUMIPULSE G1200 analyser (Fujirebio Inc., Tokyo, Japan), having a lower limit of detection (LOD) and quantification (LOQ) of 2 and 3 IU/mL log, respectively. Anti‐HBc was quantified using ChemiLuminescent Enzyme Immunoassay (CLEIA) kits (Fujirebio Inc., Tokyo, Japan), with a LOQ of 0.1 log IU/mL. More details about the qualitative/quantitative HBsAg, HBeAg and HBV DNA assays can be found elsewhere [20, 21].
2.4. Statistical Analyses
HBcrAg and anti‐HBc were analysed separately after log10 transformation and as a ratio (HBcrAg/anti‐HBc ratio). Continuous variables were presented as mean ± standard deviation or median [P25–P75], dependent on normal distribution. For statistical comparison, Student's t‐test, Wilcoxon rank‐sum‐ or signed‐rank test (continuous data) or Chi‐square test and Fisher's exact test (categorical data) were used, where appropriate. For the association between EOT levels with subsequent severe flares, a logistic regression model was constructed with the occurrence of severe flares (ALT ≥ 10×ULN) during the study period (76/96 weeks) as a binary outcome. Subsequently, the association between off‐treatment HBcrAg and anti‐HBc kinetics was studied using the ‘geom_smooth’ function from ‘ggplot2’ package, including the ‘loess’ model for fitting a trend line and standard error (se) confidence interval.
In addition, the area under the receiver operating characteristic curve (AUROC) was calculated for the different biomarkers by using “pROC” package, including the occurrence of severe flares during the study period as a binary outcome.
Finally, a sub‐analysis was performed using HBcrAg and anti‐HBc data obtained at follow‐up week 6–8 (FUW6), excluding patients who developed any flare (ALT ≥ 5×ULN) or were retreated before/at this visit. To avoid overfitting, logistic regression models were constructed with only the variables of interest (HBcrAg and anti‐HBc), HBV DNA‐ and/or ALT levels.
All statistical tests were performed in SPSS statistical software [22] and R version 4.2.2 [23]. All tests were two‐sided, and a p‐value < 0.05 was considered to be statistically significant.
3. Results
In total, 78 patients discontinued NA therapy. The mean age at EOT was 49 (±9.7) years, 64% were male and 21% were Caucasian, Table 1. Mean HBsAg, HBcrAg and anti‐HBc levels at EOT were 2.88 (±0.75), 3.36 (±0.83) and 2.38 (±0.50) log10 IU/mL, respectively. Thirty‐two (41%) patients had HBcrAg < 3 log10 IU/mL at EOT. During follow‐up, 22 (28%) patients developed a severe flare, with a median time of 12 [9–19] weeks after NA cessation. Time until a severe flare was shorter in patients who discontinued TDF/TAF therapy vs. ETV (11 [9–13] vs. 27 [24–34] weeks, p = 0.003). No patients developed hepatic decompensation or HBsAg loss afterwards. Further characteristics of these flares and outcomes have been described in the respective papers [20, 21].
TABLE 1.
Patient characteristics of included patients and comparison between patients with and without a severe flare (≥ 10×ULN).
| Total number of patients (N = 78) | No severe flare (≥ 10×ULN) (N = 56) | Severe flare (≥ 10×ULN) (N = 22) | p b | |
|---|---|---|---|---|
| Patient characteristics | ||||
| Sex, male (n, %) | 50 (64) | 33 (59) | 17 (77) | 0.129 |
| Age, years (mean ± SD) | 49 (±9.7) | 50 (±10.1) | 47 (±8.5) | 0.257 |
| Ethnicity, Asian/Caucasian/Other (n, %) | 54 (69)/16 (21)/8 (10) | 36 (64)/13 (23)/7 (13) | 18 (82)/3 (14)/1 (5) | 0.304 |
| Type of therapy, ETV/TDF/TAF (n, %) | 24 (31)/51 (65)/3 (4) | 20 (36)/34 (61)/2 (4) | 4 (18)/17 (77)/1 (5) | 0.320 |
| Duration of NA therapy, years (mean ± SD) | 8.4 (4.1) | 8.1 (3.9) | 9.3 (4.5) | 0.260 |
| HBeAg positive at SOT (n, %) | 21 (27) | 11 (20) | 10 (46) | 0.021 |
| Liver stiffness, kPa (mean ± SD) | 4.8 (±1.1) | 4.8 (±1.1) | 4.8 (±1.1) | 0.738 |
| Serology at NA cessation a | ||||
| HBsAg, log 10 IU/mL (mean ± SD) | 2.88 (±0.75) | 2.83 (±0.81) | 3.01 (±0.57) | 0.273 |
| HBsAg categorised (n, %) | 0.529 | |||
| > 1000 | 38 (49) | 26 (46) | 12 (57) | |
| 100–1000 | 30 (39) | 23 (41) | 7 (33) | |
| < 100 | 9 (12) | 7 (13) | 2 (10) | |
| HBcrAg, log 10 IU/mL (mean ± SD) | 3.36 (±0.83) | 3.23 (±0.80) | 3.68 (±0.83) | 0.040 |
| HBcrAg, < 3 log 10 IU/mL (n, %) | 32 (41) | 26 (47) | 6 (29) | 0.140 |
| Anti‐HBc, log 10 IU/mL (mean ± SD) | 2.38 (±0.50) | 2.46 (±0.47) | 2.16 (±0.54) | 0.034 |
| HBcrAg/antiHBc‐ratio (mean ± SD) | 1.51 (±0.62) | 1.39 (±0.58) | 1.84 (±0.63) | 0.010 |
| ALT × ULN (mean ± SD) | 0.62 (±0.24) | 0.59 (±0.24) | 0.70 (±0.22) | 0.061 |
Abbreviations: Anti‐HBc, hepatitis B core antibody; ETV, entecavir; HBcrAg, hepatitis B core‐related antigen; HBsAg, hepatitis B surface antigen; IQR, interquartile range; NA, nucleos(t)ide analogue; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; ULN, upper limit of normal.
Due to lack of sample residue, HBcrAg and anti‐HBc could not be determined at end of therapy in 2 and 4 patients, respectively. HBsAg at EOT was not available from 1 patient.
Compared using the independent t‐test (normal distribution, presented as mean (SD)), Wilcoxon rank sum test (if not normally distributed, presented as median [IQR]) and Chi‐square test (categorical).
3.1. End of Treatment Factors Associated With Severe Flares
Patients who developed severe flares after NA withdrawal, compared to those without, had significantly higher HBcrAg levels at EOT (3.68 (±0.83) vs. 3.23 (±0.80), p = 0.040), whereas anti‐HBc levels were lower (2.16 (±0.54) vs. 2.46 (±0.47), p = 0.034). This was reflected in a significantly higher HBcrAg/anti‐HBc ratio (1.84 (±0.63) vs. 1.39 (±0.58), p = 0.010). No statistical differences in type of therapy (TDF/TAF: 82% vs. 64%, p = 0.131) or duration of NA therapy (9.3 vs. 8.1 years, p = 0.260) were found between patients with or without a severe flare. When comparing ETV with TDF/TAF‐treated patients, mean HBcrAg (3.44 (±0.93) vs. 3.32 (±0.79), p = 0.605), anti‐HBc (2.34 (±0.54) vs. 2.40 (±0.49), p = 0.652) and HBcrAg/anti‐HBc ratio (1.60 (±0.77) vs. 1.48 (±0.54), p = 0.500) were comparable at EOT.
In both univariable‐ and multivariable logistic regression models adjusting for HBsAg‐levels at EOT, higher HBcrAg levels (aOR 1.97; 95%CI 1.00–3.90, p = 0.050), lower anti‐HBc levels (aOR 0.29; 95%CI 0.09–0.92, p = 0.036) and higher HBcrAg/anti‐HBc ratio (aOR: 3.17; 95%CI 1.25–8.07, p = 0.015) at EOT were associated with an increased flare risk (Table 2).
TABLE 2.
Uni‐ and multivariate logistic regression models for HBcrAg‐ and anti‐HBc at end of therapy and severe flares (≥ 10×ULN).
| At end of therapy | Univariate models | Multivariate models a | ||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p | aOR | 95% CI | p | |
| Model 1 (continuous) | ||||||
| HBcrAg (log10) | 1.946 | 1.036–3.654 | 0.038 | 1.974 | 0.999–3.901 | 0.050 |
| Anti‐HBc (log10) | 0.291 | 0.098–0.867 | 0.027 | 0.294 | 0.094–0.921 | 0.036 |
| Model 2 (categorical) | ||||||
| HBcrAg (log10) ≥ 4.00 | 3.375 | 1.120–10.169 | 0.031 | 3.032 | 0.953–9.645 | 0.060 |
| Anti‐HBc(log10) ≥ 2.00 | 0.277 | 0.082–0.932 | 0.038 | 0.321 | 0.090–1.141 | 0.079 |
| Model 3 (continuous) | ||||||
| HBcrAg/anti‐HBc ratio | 3.167 | 1.259–7.964 | 0.014 | 3.174 | 1.248–8.068 | 0.015 |
| Model 4 (categorical) | ||||||
| HBcrAg/anti‐HBc ratio ≥ 1.75 | 5.378 | 1.760–16.431 | 0.003 | 5.846 | 1.852–18.454 | 0.003 |
Abbreviations: Anti‐HBc, anti‐hepatitis B core; aOR, adjusted odds ratio; HBcrAg, hepatitis B core related antigen; HBsAg, hepatitis B surface antigen.
Adjusted for HBsAg (log10) levels at end of therapy.
The highest risk of severe flares was observed in patients with HBcrAg > 4log (47% vs. 21%, p = 0.026), in patients with anti‐HBc < 2log (50% vs. 22%, p = 0.032) and in those with a HBcrAg/anti‐HBc ratio ≥ 1.75 (52% vs. 17%, p = 0.002), Figure 1. These findings were consistent in both univariable‐ and multivariable regression models, adjusted for HBsAg levels at EOT (Table 2).
FIGURE 1.
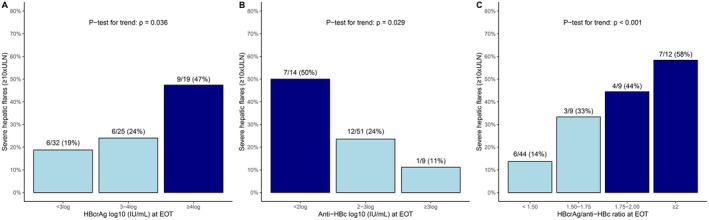
Rates of severe flares (≥ 10×ULN) during 72–96 weeks follow up based on HBcrAg and anti‐HBc levels at end of therapy. Dark blue bars = sub‐group with the highest risk of severe flares, light blue bars = sub‐group with lower risk of severe flares.
The highest discriminatory performance was obtained with a combination of HBcrAg and anti‐HBc (as a ratio), with an AUROC of 0.73, which was higher than for HBcrAg alone (AUROC: 0.68), anti‐HBc alone (AUROC: 0.64) or HBsAg (AUROC: 0.56; Table S1).
3.2. Off‐Treatment Biomarker Kinetics in Patients With and Without Severe Flares
As shown in Figure 2, HBV DNA increases were seen in both patients with and without flares, although peak HBV DNA levels were higher in those with severe flares (mean peak HBV DNA: 6.83 (±0.95) vs. 4.36 (±1.57), p < 0.001). Conversely, HBcrAg remained relatively stable after withdrawal in those without severe flares, whereas an increase was seen in those who developed a severe flare (mean peak HBcrAg levels: 6.28 (±1.44) vs. 4.29 (±1.49), p < 0.001). No statistically significant differences were seen between both groups with regards to mean peak anti‐HBc (3.52 (±0.26) vs. 3.43 (±0.29), p = 0.23) or lowest anti‐HBc levels (2.12 (±0.49) vs. 2.32 (±0.47), p = 0.11). Compared to patients who stopped ETV, patients who withdrew TDF/TAF therapy had significantly higher HBcrAg (3.90 (±1.23) vs. 3.37 (±0.83), p = 0.042), HBV DNA (4.12 (±1.44) vs. 1.40 (±0.56), p < 0.001), anti‐HBc (2.84 (±0.58) vs. 2.31 (±0.62), p = 0.002) and ALT‐levels (1.02 [0.55–1.88] vs. 0.58 [0.47–0.78] × ULN, p = 0.004) at 6 weeks after NA withdrawal.
FIGURE 2.
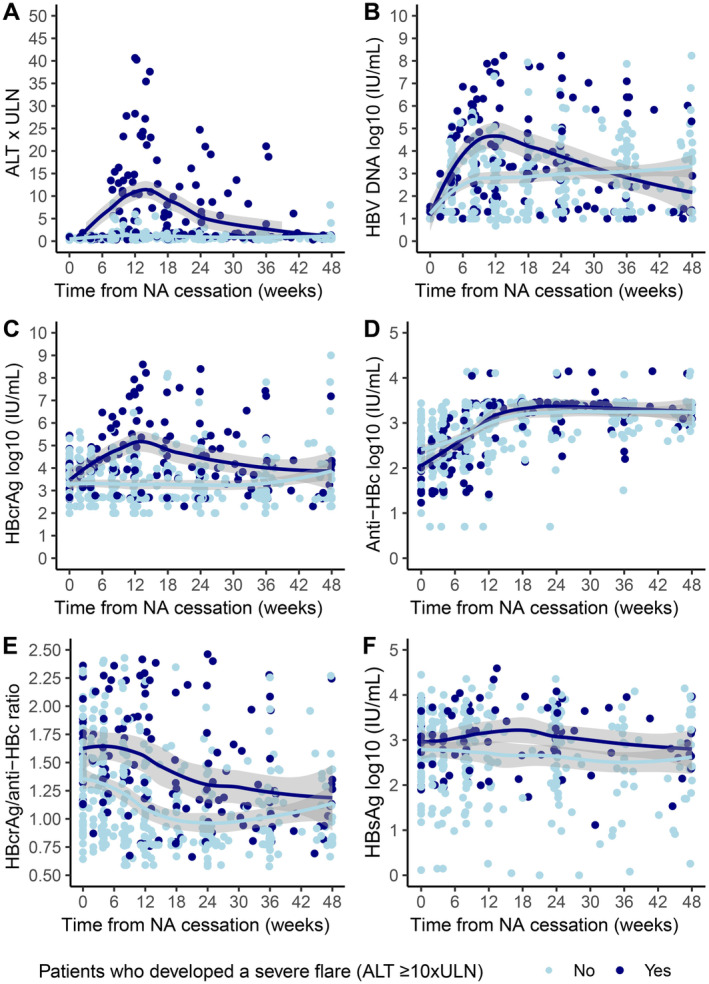
Kinetics after NA cessation using a trend line including standard error for (A) ALT × ULN, (B) log10 HBV DNA, (C) log10 HBcrAg, (D) log10 anti‐HBc, (E) HBcrAg/anti‐HBc ratio, (F) log10 HBsAg. Dark blue line = patients who developed a severe flare (≥ 10×ULN), light blue line = patients who didn't develop a severe flare (≥ 10×ULN).
3.3. Association Between HBcrAg and Anti‐HBc Levels at Off‐treatment Week 6 With Subsequent Severe Flares
This sub‐analysis was performed in 63 patients after exclusion of 15 patients (11 had developed a flare before this time, 1 patient was retreated and 3 patients had no sample available for measuring both markers). Patients who developed a severe flare after FUW6 had higher mean HBcrAg levels (4.71 (±1.10) vs. 3.43 (±0.93) log IU/mL, p < 0.001), lower anti‐HBc levels (2.23 (±0.48) vs. 2.69 (±0.60), log IU/mL, p = 0.006) and a higher HBcrAg/anti‐HBc ratio (2.24 (±0.79) vs. 1.43 (±0.89), p = 0.003) at FUW6.
In both univariable‐ and multivariable logistic regression models, adjusted for ALT‐ and HBV DNA levels at FUW6, again higher HBcrAg levels (aOR 2.91; 95%CI 1.08–7.88, p = 0.035), lower anti‐HBc (aOR 0.46; 95%CI 0.11–1.92, p = 0.29) and higher HBcrAg/anti‐HBc ratio (aOR 2.19; 95%CI 1.01–4.78, p = 0.048) were associated with a higher risk of severe flares (Table 1, 3).
TABLE 3.
Uni‐ and multivariate logistic regression models for HBcrAg‐ and anti‐HBc at week 6 and severe flares (≥ 10×ULN).
| At week 6 | Univariate models | Multivariate models a | ||||
|---|---|---|---|---|---|---|
| aOR | 95% CI | p | aOR | 95% CI | p | |
| Model 1 (continuous) | ||||||
| HBcrAg (log10) | 3.421 | 1.633–7.170 | 0.001 | 2.912 | 1.076–7.884 | 0.035 |
| Anti‐HBc (log10) | 0.253 | 0.081–0.794 | 0.018 | 0.458 | 0.109–1.915 | 0.285 |
| Model 2 (categorical) | ||||||
| HBcrAg (log10) ≥ 5.00 | 25.556 | 2.659–245.570 | 0.005 | 16.502 | 1.362–199.90 | 0.028 |
| Anti‐HBc (log10) ≥ 2.00 | 0.205 | 0.049–0.856 | 0.030 | 0.152 | 0.027–0.857 | 0.033 |
| Model 4 (continuous) | ||||||
| HBcrAg/anti‐HBc ratio | 2.663 | 1.164–6.093 | 0.020 | 2.194 | 1.008–4.777 | 0.048 |
| Model 5 (categorical) | ||||||
| HBcrAg/anti‐HBc ratio ≥ 1.75 | 13.567 | 3.166–58.139 | < 0.001 | 10.815 | 2.340–49.998 | 0.002 |
Abbreviations: aOR, adjusted odds ratio; HBcrAg, hepatitis B core‐related antigen; anti‐HBc, anti‐hepatitis B core.
Adjusted for ALT × ULN (log10) and HBV DNA (log10) levels at week 6.
The highest rate of severe flares was observed in those with HBcrAg ≥ 5log (83% vs. 16%, p = 0.002), in patients with anti‐HBc < 2log (50% vs. 17%, p = 0.021) and in those with a HBcrAg/anti‐HBc ratio ≥ 1.75 (52% vs. 8%, p < 0.001), Figure 3. These findings were consistent in both univariable‐ and multivariable regression models, adjusted for ALT‐ and HBV DNA levels at the same visit (Table 3).
FIGURE 3.
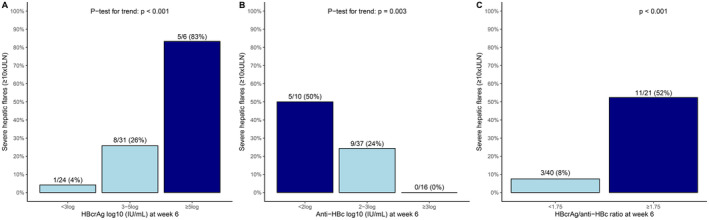
Rates of severe flares (≥ 10×ULN) during 72–96 weeks of follow‐up based on HBcrAg and anti‐HBc levels at week 6. Dark blue bars = sub‐group with the highest risk of severe flares, light blue bars = sub‐group with lower risk of severe flares.
The highest discriminatory performance was obtained with a combination of HBcrAg and anti‐HBc; the AUC was 0.82 which was higher than for HBcrAg alone (AUC: 0.80), anti‐HBc alone (AUC: 0.73), HBV DNA (AUC: 0.67) or HBsAg (AUC: 0.61), Table S1.
4. Discussion
In this combined analysis of two prospective studies, including 78 CHB patients who withdrew NA therapy, we show that higher HBcrAg, lower anti‐HBc and a higher HBcrAg/anti‐HBc ratio at EOT are associated with a higher risk of severe off‐treatment flares. Furthermore, higher HBcrAg, lower anti‐HBc and a higher HBcrAg/anti‐HBc ratio at FUW6 were also highly predictive of subsequent flares, independent from HBV DNA. HBcrAg and anti‐HBc levels, and their ratio, can therefore potentially be used to guide follow‐up and the decision to restart antiviral therapy in patients who discontinue NA therapy.
Withdrawal of NA therapy has recently emerged as a novel strategy to increase functional cure rates. Unfortunately, these potential benefits are counterbalanced by a significant risk of severe viral rebound and subsequent hepatic flares, which may precipitate liver failure and death [5, 6, 7]. In the current cohort, severe flares were observed in 28% of patients, which is rather high compared to previously described [24]. A possible explanation could be the restrictive retreatment criteria that were applied in both studies. This emphasises that risk stratification is essential for optimal patient selection for finite therapy and to identify patients who require urgent retreatment.
Although previous studies [15, 25, 26, 27, 28, 29] have suggested that EOT HBcrAg and anti‐HBc could be predictive of virological outcomes after NA withdrawal, the current report describes the first study on the use of combining HBcrAg and anti‐HBc levels for assessing the risk of severe flares after NA cessation. This was enabled by the prospective design with restrictive retreatment criteria, which resulted in high rates of severe flares. In our cohort, patients with higher HBcrAg and lower anti‐HBc levels were at increased risk of severe flares after therapy withdrawal. Prohibitively high flare rates were observed for patients with HBcrAg levels ≥ 4log (47%), anti‐HBc levels < 2log (50%) and high HBcrAg/anti‐HBc ratio (≥ 1.75: 52%) at EOT. Conversely, patients with low HBcrAg and/or high anti‐HBc were at reduced risk of flares, and these patients may therefore be the best candidates for NA withdrawal. Findings were consistent in multivariable analysis, underscoring that both HBcrAg and anti‐HBc, and their combination, provide important new information over existing biomarkers including HBsAg.
Since even patients with the most favourable EOT profile remain at risk for flares, careful off‐treatment monitoring remains essential. Recent studies have shown that HBV DNA and HBsAg levels at off‐treatment week 24 are associated with excellent long‐term outcomes [30]. However, since many severe flares occur early after NA cessation (median time of 12 weeks in the current cohort), biomarkers obtained sooner after therapy withdrawal (preferable during the first weeks after withdrawal of treatment) are needed for early risk stratification [24]. Since HBsAg levels remain relatively stable (early) after therapy withdrawal, even in patients developing severe flares, the use of HBsAg as a predictor during the early off‐treatment phase is limited. Thus, alternative biomarkers are therefore needed to guide off‐treatment follow‐up and management. A recent study showed that lower HBcrAg levels after therapy withdrawal might be associated with a lower risk of viral relapse [31]. The current study now shows for the first time that HBcrAg and anti‐HBc levels obtained at 6 weeks post‐treatment are predictive of subsequent flares. In our cohort, both high HBcrAg and low anti‐HBc and a higher ratio were associated with a higher subsequent risk of severe flares, independent of HBV DNA and ALT. For instance, severe flares were observed in nearly all patients with HBcrAg ≥ 5 log at week 6, whereas flare risk was negligible in patients with HBcrAg < 3log (4%), anti‐HBc ≥ 3log (0%) and/or HBcrAg/anti‐HBc ratio < 1.75 (8%). Also, it is important to note that these associations remained significant even after adjusting for HBV DNA levels at the same visit. Recently, it has been recommended to restart treatment when HBV DNA levels exceed 100.000 IU/mL [24]. Four of the 10 patients in our cohort who had HBV DNA levels > 5log and available HBcrAg levels at the week 6 visit developed a severe flare afterwards, of whom all had HBcrAg > 5log, resulting in a PPV of 80%, NPV of 100%, specificity of 83% and sensitivity of 100%. These findings suggest that HBcrAg and anti‐HBc levels at week 6 could potentially be used as an additional tool to stratify flare risk, and to identify patients with such a high risk of flares that increased monitoring and consideration of restarting treatment is required. It remains, however, important to highlight that severe flares can still occur in patients with the most favourable characteristics, underscoring that careful monitoring remains essential in all patients who discontinue treatment.
Furthermore, the combination of HBcrAg and anti‐HBc yielded superior accuracy over either marker alone. This might be due to the fact that these biomarkers combine two aspects that determine the outcome of NA withdrawal: viral replication and the host immune response. Our study, therefore, introduces new clinical applications for these markers. Interestingly, thresholds predictive of a low risk of flares are well above the lower limits of quantification for these markers, overcoming a limitation typically observed in studies aiming to predict HBsAg loss [32, 33].
Our study also sheds some new light on the previous observation that withdrawal of TDF therapy is a risk factor for hepatic flares [24, 34]. Flares after TDF cessation occur earlier and are more severe compared to flares occurring after ETV withdrawal [24, 35, 36]. Despite similar HBcrAg and anti‐HBc levels at EOT, (early) HBcrAg‐ and anti‐HBc kinetics seemed to differ between patients who withdrew TDF/TAF vs. ETV, with both higher HBcrAg and higher anti‐HBc levels observed at 6–8 weeks after withdrawal of tenofovir therapy.
The current study has several strengths, including the prospective designs of the included cohorts with detailed off‐treatment follow‐up data available, and the multiethnic population enrolled. The main limitation of our study is the limited sample size and thus validation of our findings in a larger cohort is needed. Furthermore, the cohort included both pretreatment HBeAg‐positive and pretreatment HBeAg‐negative patients. Since pretreatment HBeAg‐positive patients may have higher HBcrAg levels, we also performed stratified analyses and found consistent results, although statistical significance could not be obtained due to lack of statistical power (Figure S1).
In conclusion, higher HBcrAg, lower anti‐HBc and a higher HBcrAg/anti‐HBc ratio at end of therapy and at FUW6 seem to be associated with a higher risk of severe flares after therapy withdrawal. If confirmed, these markers could potentially be used to identify patients with such a high risk of flares that therapy withdrawal should be discouraged and/or early retreatment advised. Nevertheless, a risk of flares remains even in patients with favourable characteristics, thus necessitating careful follow‐up in all patients who discontinue treatment.
Author Contributions
Edo J. Dongelmans: writing – original draft, methodology, visualization, writing – review and editing, conceptualization, investigation, formal analysis, data curation. Jordan J. Feld: data curation, writing – review and editing. André Boonstra: writing – review and editing, data curation. Sylvia M. Brakenhoff: writing – review and editing, data curation. David Wong: writing – review and editing, data curation. Colina Yim: writing – review and editing, data curation. Mark Claassen: writing – review and editing, data curation. Pieter Honkoop: writing – review and editing, data curation. Bettina E. Hansen: writing – review and editing, data curation. Robert A. de Man: data curation, writing – review and editing. Scott Fung: data curation, writing – review and editing. Thomas Berg: data curation, writing – review and editing. Florian van Bömmel: data curation, visualization. Harry L. A. Janssen: data curation, writing – review and editing, conceptualization, funding acquisition, investigation, methodology, supervision. Milan J. Sonneveld: data curation, supervision, methodology, writing – review and editing, conceptualization, investigation, funding acquisition.
Ethics Statement
This study was approved by the research ethical board of each participating centre.
Conflicts of Interest
J.F. receives research grants from Abbvie, Gilead, Janssen, Enanta, Eiger and is a consultant for Abbvie, Gilead, Finch, Arbutus and GlaxoSmithKline. A.B. has been in consulting or in advisory boards for Gilead Sciences and Bristol‐Myers Squibb and has received research grants from Roche, Gilead Sciences, Fujirebio and Janssen. B.E.H. has received grants from Intercept, CymaBay, Albireo, Mirum, Calliditas and Gliad and is a consultant for Intercept, CymaBay, Albireo, Mirum, Genfit, Calliditas, Eiger, and ChemomAb. H.L.A.J. received grants from: Gilead Sciences, GlaxoSmithKline, Janssen, Roche, Vir Biotechnology Inc. and is consultant for: Aligos, Gilead Sciences, GlaxoSmithKline, Janssen, Roche, Vir Biotechnology Inc., Precision Biosciences and Griffols. M.J.S. receives speakers' fees and research support from Roche, Bristol Myers Squibb, Gilead Sciences and Fujirebio. All other authors disclose no conflicts.
Supporting information
Data S1.
Acknowledgements
The authors thank all the participating centers, investigators, and research staff for their time and effort. This study was sponsored by the Foundation for Liver and Gastrointestinal Research. Materials for HBcrAg and anti‐HBc testing were provided free of charge by Fujirebio. Both the Foundation for Liver and Gastrointestinal Research and Fujirebio had no influence on the study design, data collection and analyses, writing of the manuscript and the decision to submit for publication.
Handling Editor: Grace L‐H Wong
Funding: This work was supported by Stichting voor Lever‐en Maag‐Darm Onderzoek.
Data Availability Statement
The data that support these findings are not publicly available, since they are subject to (inter)national data protection laws to ensure data privacy of the study participants. The data can therefore not be shared.
References
- 1. Terrault N. A., Lok A. S. F., McMahon B. J., et al., “Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance,” Hepatology 67, no. 4 (2018): 1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. European Association for the Study of the Liver and Electronic address eee, European Association for the Study of the L , “EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection,” Journal of Hepatology 67, no. 2 (2017): 370–398. [DOI] [PubMed] [Google Scholar]
- 3. Kao J. H., Jeng W. J., Ning Q., et al., “APASL Guidance on Stopping Nucleos(t)ide Analogues in Chronic Hepatitis B Patients,” Hepatology International 15, no. 4 (2021): 833–851. [DOI] [PubMed] [Google Scholar]
- 4. Hirode G., Choi H. S. J., Chen C. H., et al., “Off‐Therapy Response After Nucleos(t)ide Analogue Withdrawal in Patients With Chronic Hepatitis B: An International, Multicenter, Multiethnic Cohort (RETRACT‐B Study),” Gastroenterology 162, no. 3 (2022): 757–771.e754. [DOI] [PubMed] [Google Scholar]
- 5. Hirode A. S. G., Hansen B. E., Chen C. H., et al., “Incidence of Hepatic Decompensation After Nucleos(t)ide Analogue Withdrawal: Results From a Large, International, Multi‐Ethnic Cohort of Patients With Chronic Hepatitis B (RETRACT‐B Study),” American Journal of Gastroenterology 118, no. 9 (2023): 1601–1608. [DOI] [PubMed] [Google Scholar]
- 6. Agarwal K., Lok J., Carey I., et al., “A Case of HBV‐Induced Liver Failure in the REEF‐2 Phase II Trial: Implications for Finite Treatment Strategies in HBV ‘cure’,” Journal of Hepatology 77, no. 1 (2022): 245–248. [DOI] [PubMed] [Google Scholar]
- 7. Hsu Y. C., Lin Y. H., Lee T. Y., et al., “Severe Hepatitis B Flares With Hepatic Decompensation After Withdrawal of Nucleos(t)ide Analogues: A Population‐Based Cohort Study,” Alimentary Pharmacology & Therapeutics 58, no. 4 (2023): 463–473. [DOI] [PubMed] [Google Scholar]
- 8. García‐López M., Lens S., Pallett L. J., et al., “Viral and Immune Factors Associated With Successful Treatment Withdrawal in HBeAg‐Negative Chronic Hepatitis B Patients,” Journal of Hepatology 74, no. 5 (2021): 1064–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang M. L. and Liaw Y. F., “Hepatitis B Flare in Hepatitis B e Antigen‐Negative Patients: A Complicated Cascade of Innate and Adaptive Immune Responses,” International Journal of Molecular Sciences 23, no. 3 (2022): 1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mak L. Y., Wong D. K., Cheung K. S., Seto W. K., Lai C. L., and Yuen M. F., “Review Article: Hepatitis B Core‐Related Antigen (HBcrAg): An Emerging Marker for Chronic Hepatitis B Virus Infection,” Alimentary Pharmacology & Therapeutics 47, no. 1 (2018): 43–54. [DOI] [PubMed] [Google Scholar]
- 11. Inoue T., Kusumoto S., Iio E., et al., “Clinical Efficacy of a Novel, High‐Sensitivity HBcrAg Assay in the Management of Chronic Hepatitis B and HBV Reactivation,” Journal of Hepatology 75, no. 2 (2021): 302–310. [DOI] [PubMed] [Google Scholar]
- 12. Sandmann L., Bremer B., Ohlendorf V., et al., “Kinetics and Value of Hepatitis B Core‐Related Antigen in Patients With Chronic Hepatitis B Virus Infection During Antiviral Treatment,” Viruses 16, no. 2 (2024): 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Suzuki F., Miyakoshi H., Kobayashi M., and Kumada H., “Correlation Between Serum Hepatitis B Virus Core‐Related Antigen and Intrahepatic Covalently Closed Circular DNA in Chronic Hepatitis B Patients,” Journal of Medical Virology 81, no. 1 (2009): 27–33. [DOI] [PubMed] [Google Scholar]
- 14. Hsu Y. C., Nguyen M. H., Mo L. R., et al., “Combining Hepatitis B Core‐Related and Surface Antigens at End of Nucleos(t)ide Analogue Treatment to Predict Off‐Therapy Relapse Risk,” Alimentary Pharmacology & Therapeutics 49, no. 1 (2019): 107–115. [DOI] [PubMed] [Google Scholar]
- 15. Sonneveld M. J., Park J. Y., Kaewdech A., et al., “Prediction of Sustained Response After Nucleo(s)tide Analogue Cessation Using HBsAg and HBcrAg Levels: A Multicenter Study (CREATE),” Clinical Gastroenterology and Hepatology 20, no. 4 (2022): e784–e793. [DOI] [PubMed] [Google Scholar]
- 16. Luo M., Zhou B., Hou J., and Jiang D., “Biomarkers for Predicting Nucleos(t)ide Analogs Discontinuation and Hepatitis B Virus Recurrence After Drug Withdrawal in Chronic Hepatitis B Patients,” Hepatology Research 52, no. 4 (2022): 337–351. [DOI] [PubMed] [Google Scholar]
- 17. Yuan Q., Song L. W., Liu C. J., et al., “Quantitative Hepatitis B Core Antibody Level May Help Predict Treatment Response in Chronic Hepatitis B Patients,” Gut 62, no. 1 (2013): 182–184. [DOI] [PubMed] [Google Scholar]
- 18. Osmani Z. and Boonstra A., “Recent Insights Into the Role of B Cells in Chronic Hepatitis B and C Infections,” Pathogens 12, no. 6 (2023): 815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vanwolleghem T., Groothuismink Z. M. A., Kreefft K., Hung M., Novikov N., and Boonstra A., “Hepatitis B Core‐Specific Memory B Cell Responses Associate With Clinical Parameters in Patients With Chronic HBV,” Journal of Hepatology 73, no. 1 (2020): 52–61. [DOI] [PubMed] [Google Scholar]
- 20. Liem K. S., Fung S., Wong D. K., et al., “Limited Sustained Response After Stopping Nucleos(t)ide Analogues in Patients With Chronic Hepatitis B: Results From a Randomised Controlled Trial (Toronto STOP Study),” Gut 68, no. 12 (2019): 2206–2213. [DOI] [PubMed] [Google Scholar]
- 21. Brakenhoff S. M., Claassen M., Honkoop P., et al., “Sustained Response and HBsAg Loss After Nucleo(s)tide Analogue Discontinuation in Chronic Hepatitis B Patients: The Prospective SNAP Study,” Clinics and Research in Hepatology and Gastroenterology 48, no. 2 (2023): 102257. [DOI] [PubMed] [Google Scholar]
- 22. IBM Corp , IBM SPSS Statistics for Windows, Version 28 (Armonk, NY: IBM Corp, 2021), https://hadoop.apache.org. [Google Scholar]
- 23. R Core Team , R: A Language and Environment for Statistical Computing (Vienna: R Foundation for Statistical Computing, 2022). [Google Scholar]
- 24. Dongelmans E. J., Hirode G., Hansen B. E., et al., “Predictors of Hepatic Flares After Nucleos(t)ide Analogue Cessation ‐ Results of a Global Cohort Study (RETRACT‐B Study),” Journal of Hepatology (2024), 10.1016/j.jhep.2024.08.015 . [DOI] [Google Scholar]
- 25. Chi H., Li Z., Hansen B. E., et al., “Serum Level of Antibodies Against Hepatitis B Core Protein Is Associated With Clinical Relapse After Discontinuation of Nucleos(t)ide Analogue Therapy,” Clinical Gastroenterology and Hepatology 17, no. 1 (2019): 182–191.e181. [DOI] [PubMed] [Google Scholar]
- 26. Ohlendorf V., Wübbolding M., Gineste P., et al., “Low Anti‐HBc Levels Are Associated With Lower Risk of Virological Relapse After Nucleos(t)ide Analogue Cessation in HBe Antigen‐Negative Patients,” Liver International 42, no. 12 (2022): 2674–2682. [DOI] [PubMed] [Google Scholar]
- 27. Brakenhoff S. M., de Knegt R. J., van Campenhout M. J. H., et al., “End‐of‐Treatment HBsAg, HBcrAg and HBV RNA Predict the Risk of Off‐Treatment ALT Flares in Chronic Hepatitis B Patients,” Journal of Microbiology, Immunology, and Infection 56, no. 1 (2022): 31–39. [DOI] [PubMed] [Google Scholar]
- 28. Zeng G., Koffas A., Mak L. Y., Gill U. S., and Kennedy P. T. F., “Utility of Novel Viral and Immune Markers in Predicting HBV Treatment Endpoints: A Systematic Review of Treatment Discontinuation Studies,” JHEP Reports 5, no. 6 (2023): 100720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaroenlapnopparat A., Chayanupatkul M., and Tangkijvanich P., “Novel Viral Markers and the Prediction of Off‐Treatment Relapse in Chronic Hepatitis B Patients: A Systematic Review,” Journal of Gastroenterology and Hepatology 36, no. 9 (2021): 2349–2362. [DOI] [PubMed] [Google Scholar]
- 30. Sonneveld M. J., Chiu S. M., Park J. Y., et al., “HBV DNA and HBsAg Levels at 24 Weeks Off‐Treatment Predict Clinical Relapse and HBsAg Loss in HBeAg‐Negative Patients Who Discontinued Antiviral Therapy,” Gastroenterology 166, no. 1 (2024): 168–177.e168. [DOI] [PubMed] [Google Scholar]
- 31. Tsai Y. N., Wu J. L., Tseng C. H., et al., “Hepatitis B Core‐Related Antigen Dynamics and Risk of Subsequent Clinical Relapses After Nucleos(t)ide Analog Cessation,” Clinical and Molecular Hepatology 30, no. 1 (2024): 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adraneda C., Tan Y. C., Yeo E. J., Kew G. S., Khakpoor A., and Lim S. G., “A Critique and Systematic Review of the Clinical Utility of Hepatitis B Core‐Related Antigen,” Journal of Hepatology 78, no. 4 (2023): 731–741. [DOI] [PubMed] [Google Scholar]
- 33. Sonneveld M. J., Chiu S. M., Park J. Y., et al., “Probability of HBsAg Loss After Nucleo(s)tide Analogue Withdrawal Depends on HBV Genotype and Viral Antigen Levels,” Journal of Hepatology 76, no. 5 (2022): 1042–1050. [DOI] [PubMed] [Google Scholar]
- 34. Huang Y. J., Li T. C., Chen C. H., et al., “Hepatitis Flares or Hepatic Decompensation After Discontinuation of Tenofovir Disoproxil Fumarate and Entecavir in Non‐Cirrhotic Hepatitis B e Antigen‐Negative Patients,” Journal of Clinical Medicine 12, no. 24 (2023): 7565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu Y. C., Jeng W. J., Peng C. W., Chien R. N., and Liaw Y. F., “The Role of Off‐Therapy Viral Kinetics in the Timing and Severity of Flares in Hepatitis B e Antigen‐Negative Patients,” Clinical Gastroenterology and Hepatology 21, no. 6 (2023): 1533–1541.e1511. [DOI] [PubMed] [Google Scholar]
- 36. Hall S. A. L., Burns G. S., Anagnostou D., et al., “Stopping Nucleot(s)ide Analogues in Non‐cirrhotic HBeAg‐Negative Chronic Hepatitis B Patients: HBsAg Loss at 96 Weeks Is Associated With Low Baseline HBsAg Levels,” Alimentary Pharmacology & Therapeutics 56, no. 2 (2022): 310–320. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support these findings are not publicly available, since they are subject to (inter)national data protection laws to ensure data privacy of the study participants. The data can therefore not be shared.


