Abstract
The layer-stacking mode of a two-dimensional (2D) material plays a dominant role either in its topology or properties, but remains challenging to control. Herein, we developed alkali-metal ion-regulating synthetic control on the stacking structure of a vinylene-linked covalent triazine framework (termed sp2c-CTF) for improving hydrogen peroxide (H2O2) photoproduction. Upon the catalysis of EtONa in Knoevenagel polycondensation, a typical eclipsed stacking mode (sp2c-CTF-4@AA) was built, while a staggered one (sp2c-CTF-4@AB) was constructed using LiOH. The AB stacking might be induced by the Li+ promoted Lewis acid–base interactions with the nitrogen atoms of s-triazine units which would endow the s-triazine units with a charged state and enlarge the total crystal stacking energy. Specifically, the shift in the stacking mode speeds up electron transfer within each layer and along interlayers, thereby improving the photocatalytic activity. sp2c-CTF-4@AB features superior activity over the eclipsed stacking counterpart (sp2c-CTF-4@AA) in sacrificial agent-free H2O2 generation, comparable to the state-of-the-art COF photocatalysts, which has not been demonstrated in this field before. This work demonstrates that regulating the interlayer-stacking mode of COFs can endow them with high photocatalytic activity, further inspiring the development of heterogeneous catalysis.
This work reports an efficient strategy to tailor the stacking mode in 2D vinylene-linked covalent triazine frameworks by regulating the base-catalyst with different alkali-metal ions.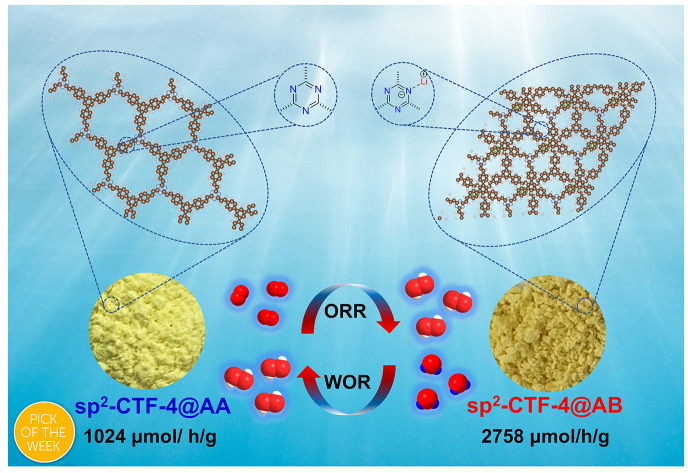
Introduction
Two-dimensional (2D) materials, including graphene, hexagonal BN, MoS2, etc., have been reported and extensively studied in various applications such as optoelectronics, spintronics, catalysts, chemical and biological sensors, supercapacitors, solar cells, and lithium-ion batteries for their distinct properties.1–4 Controlling the stacking mode of 2D materials endows them with unconventional mechanical, photo-electrical, and chemical properties.5–7 For instance, the evolution of monolayer molybdenum disulfide (ML-MoS2) from the 2H phase (AB-stack) to the 1T phase (ABC-stack) gave significantly different conductivity.8,9 2D covalent organic frameworks (COFs) have emerged as a new class of materials with defined molecular stacking, large surface areas and open regular channels, featuring extended molecular sheets interacting through non-covalent interactions like π–π and van der Waals forces.10–13 The pioneer 2D COF was disclosed by Yaghi et al. in 2005, which features an unusual AB stacking mode through boronate/boroxine polycondensation chemistry.14 However, most known 2D COFs are AA stacked, and the known reports are focused on exploring available synthetic methods for 2D COFs, reticulating building blocks, or establishing new covalent linkages and topologies for task-specific functionalities and applications. The topology and properties of 2D COFs were mainly tailored through in-plane molecular structures, but seldom by the vertical stacking modes.15–18 Several limited pioneering efforts including judicious regulation of building blocks, and catalyst-triggered (like CF3SO3H) or solvent-assisted methods have been found to be effective in weakening the attraction of π–π stacking layers and facilitating interlayer sliding.19–21
More recently, numbered vinylene-linked covalent organic frameworks (sp2c-COFs) have been developed via Knoevenagel or aldol condensation routes, and they have exhibited high chemical stability and attractive photocatalytic activity due to their robust and fully π-conjugated backbones.22–24 The photocatalytic transformations enabled by sp2c-COFs, such as photocatalytic H2O2 generation, provide an alternative approach to dismiss the disadvantages (e.g., high energy consumption, safety risks)25,26 of the traditional anthraquinone method. However, very limited structures or topologies of sp2c-COFs have been developed so far, and unveiling the relationship between the bulk structure and photocatalytic activity remains a highly challenging subject. Herein, we report an alkali-metal ion-regulating strategy to control the stacking structure of a vinylene-linked covalent triazine framework (sp2c-CTF-4) during the Knoevenagel polycondensation. An eclipsed AA stacking mode (denoted as sp2c-CTF-4@AA) was achieved by applying sodium ethoxide (EtONa) as the catalyst. A staggered stacking mode (sp2c-CTF-4@AB) was formed upon catalysis of lithium hydroxide (LiOH). Such phenomena were ascribed to the enlarged total crystal stacking energy from the coordination of Li+ ions to the nitrogen atoms of the s-triazine unit during the growth of frameworks. The two stacking-mode COFs showed significantly different physical properties and photocatalytic activities toward the production of H2O2 under visible light irradiation. Because of the much stronger capability in separation and transport of photogenerated charge carriers, the rate of H2O2 generation of sp2c-CTF-4@AB can reach 2758 μmol h−1 g−1, which is unprecedentedly 2.7 fold that of the eclipsed stacking counterpart without adding any sacrificial agent. This finding provides new guidance in the design and synthesis of efficient and stable heterogeneous catalysts for H2O2 production.
Results and discussion
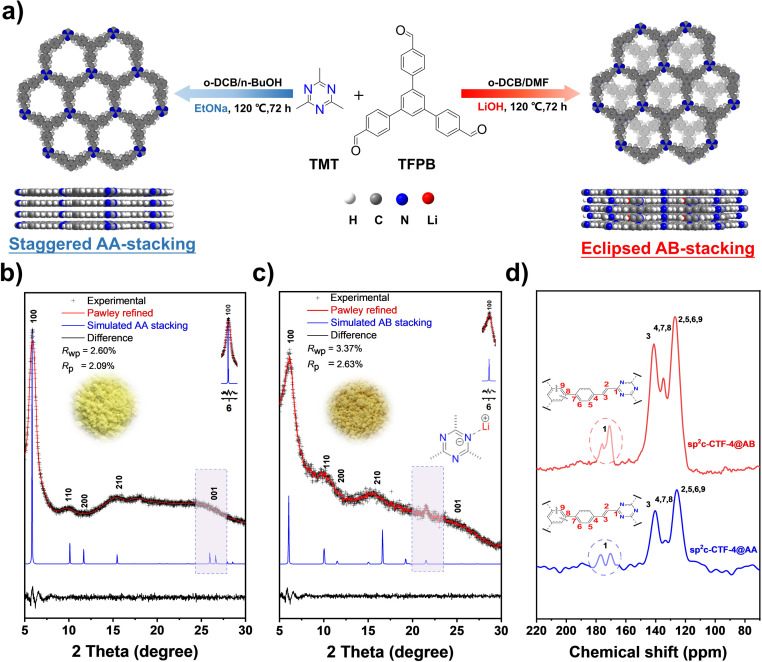
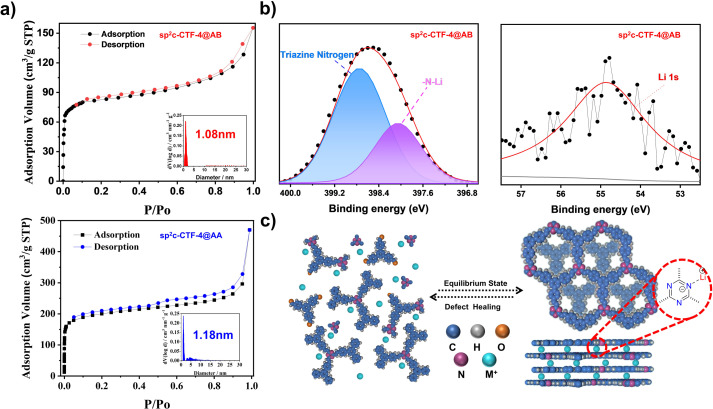
The vinylene (–CH CH–) connected covalent triazine framework (sp2c-CTF-4) was synthesized through the Knoevenagel condensation between TMT and TFPB under selected conditions (Fig. 1a). The sp2c-CTF-4@AA sample with an eclipsed stacking mode was synthesized in a binary solvent of o-dichlorobenzene/n-butanol (3/7 v/v) under the catalysis of sodium ethoxide (EtONa). The staggered-stacking sp2c-CTF-4@AB was prepared under optimized solvothermal conditions of dimethyl formamide/o-dichlorobenzene/methanol (3/1/0.2 v/v/v) in the presence of lithium hydroxide (LiOH) (detailed in the synthesis section of the ESI†). sp2c-CTF-4@AA is obtained as a pale-yellow powder (yield: 87%), whereas the staggered-stacking sp2c-CTF-4@AB is yellow (fluffy, yield: 86%). The crystallinity of the resultant frameworks was revealed by PXRD measurements. sp2c-CTF-4@AA shows clear characteristic peaks at 5.9°, 10.2°, and 11.7°, representing the (100), (110) and (200) facets, respectively, for an eclipsed stacking model (Fig. 1b). The peak at 25.3° corresponds to the layer-to-layer eclipsed stacking distance of 3.43 Å (001). In contrast, the experimental PXRD results for sp2c-CTF-4@AB, including the number, position, and strength of peaks, matched well with a staggered stacking model (PM space group, unit cell parameters: a = b = 17.53, c = 3.1, α = β = 90°, and γ = 120°). The signal observed at 21.5° (Fig. 1c) clearly indicates the formation of Li–N interaction around the triazine units.27,28 The chemical structure, porosity, morphology, and photoelectric properties of two sp2c-CTF-4 samples were characterized and compared. The formation of olefin (–C C–) linkage was proved by Fourier transform infrared (FT-IR) spectra through the emergence of vinylene stretching vibration (1630 cm−1). Both of the two samples show structural similarity without clear distinction on the absorbance intensity location of specific peaks (Fig. S1†).29 Besides, the band at 1628 cm−1 in Raman spectroscopy further proves the formation of C C linkages for the as-synthesized frameworks (Fig. S2†). High-resolution TEM (HR-TEM) images of sp2c-CTF-4 further confirmed the aligned porous structure (Fig. S3†). A solid 13C cross-polarization magic angle spinning nuclear magnetic resonance (CP-MAS NMR) spectrum (Fig. 1d), in which all prominent signals are well consistent with the proposed polymer structure, further confirms the successful polycondensation. Besides, different aggregation structures of 2D COFs can be investigated using 13C CP/MAS solid-state NMR. Compared with sp2c-CTF-4@AA, the peak intensity of the triazine carbon at 176.2 ppm for sp2c-CTF-4@AB is sharply attenuated, indicating that the intimate interactions between the neighboring layers of the sp2c-CTF-4@AB are weakened, and the change in the fine chemical environment further proves the formation of the AB stacking mode.30 N2 sorption isotherm measurements were conducted to probe the porous structure of the obtained frameworks (Fig. 2a). Based on the nonlocal density functional theory (NLDFT) model, pore size distribution (PSD) was evaluated. sp2c-CTF-4@AA delivers a high BET surface area of 752 m2 g−1 with a dominant pore diameter at 1.18 nm. In contrast, a lower BET surface area of 278 m2 g−1 for sp2c-CTF@AB, and a slightly narrow dominant pore width in the PSD curve suggests the presence of meandering channels and certain inaccessible pore volumes for nitrogen probes, probably ascribed to its relatively lower crystallinity and the presence of Li+ within its lattice.
Fig. 1. (a) Schematic diagram for the synthesis of sp2c-CTF-4 and views of the corresponding refined 2D crystal structure; PXRD patterns of (b) sp2c-CTF-4@AA and (c) sp2c-CTF-4@AB; comparison between the experimental (black cross) and Pawley refined (red line) profiles, the simulated patterns for AA and AB stacking mode (blue line); (d) 13C CP/MAS solid-state NMR spectra of sp2c-CTF-4@AA and sp2c-CTF-4@AB.
Fig. 2. (a) (top) Nitrogen adsorption (black) and desorption (red) isotherms of sp2c-CTF-4@AB ((inset) pore size distribution curve of sp2c-CTF-4@AB); (bottom) nitrogen adsorption (black) and desorption (blue) isotherms of sp2c-CTF-4@AA ((inset) pore size distribution of sp2c-CTF-4@AA); (b) XPS spectra of N 1s (left) and Li 1s (right) for sp2c-CTF-4@AB; (c) plausible formation mechanism for sp2c-CTF-4@AB.
A few reports have shown that the phase conversion of staggered modes to eclipsed ones in COFs was triggered by certain synthetic conditions. The synthetic conditions indeed dominate the polymorph formation and crystalline evolution.31,32 Of particular interest is the possible mechanism for the formation of the AB-stacking structure. In our case, N atoms of the triazine unit with a lone pair of electrons appear to be potential Lewis-base sites, coordinating with Lewis-acid sites (metal ions). The possible interaction between the triazine unit and Li+ was probed via in situ1H-NMR spectroscopic measurements on model compounds like 2,4,6-trimethyl-1,3,5-triazine (TMT). After mixing with various concentrations of LiOH·H2O, the 1H NMR signals at 2.60 ppm for hydrogens of the methyl group in TMT transfer to the high field (2.46–2.50 ppm). This indicates that there would be an interaction between Li+ and the triazine unit (Fig. S4†). X-ray photoelectron spectroscopy (XPS) measurements were further conducted to distinguish the chemical state of nitrogen atoms. The N 1s spectra of sp2c-CTF-4@AB are deconvoluted into a single peak located at 398.7 eV, which was attributed to triazine N. An additional peak is found at 398.1 eV for the N 1s signal of the N–Li peak. Accordingly, the deconvoluted peak in the Li 1s spectrum could be assigned to the Li–N coordinate bond (Fig. 2b), further identifying the Li–N interaction.33,34 Notably, inductively coupled plasma-atomic emission spectrometry (ICP-AES) results (residual Li+: 0.03 wt%) indicate that each triazine ring would be coordinated with one Li+ in a cell. The Na+ content of sp2c-CTF-4@AA based on ICP-AES analysis is merely 0.01 wt%. Therefore, the residual Na+ would exert an almost negligible effect on the crystallinity evolution of our frameworks.
To unveil the stability of different stacking modes, first-principles calculations were carried out (Fig. S5 and 6†). Total energy calculations show that the Li coordination strengthens the stability of sp2c-CTF-4@AB by Li–N interaction.35 The corresponding topology of sp2c-CTF-4@AB was modeled with Pawley refinement, exhibiting a staggered stacking model with good R factors (Rp = 3.37%, Rwp = 2.63%). DFT calculations reveal that such stacking mode also exhibits lower energy than those of sp2c-CTF-4@AA with or without Li+ coordination by 95.46 kcal m−1 or 49.58 kcal m−1, respectively, which is also much lower than that of Li-free sp2c-CTF-4@AB by 48.65 kcal m−1 (Table S1†). These results indicate that the formation of AB stacking might be induced by the Lewis acid–base interactions between Li+ and the nitrogen atoms of s-triazine units. The interaction would endow the s-triazine units with a charged state, enlarging the total crystal stacking energy. This means there is a certain amount of electronic repulsion between sp2c-CTF-4 layers, which leads to a staggered stacking mode (Fig. 2c).36 sp2c-CTF-4@AB could maintain the primary skeleton connectivity after being treated in saturated KOH methanol/water (1 : 1) solution (Fig. S7†), as revealed by XPS spectra, manifesting its relative stability.
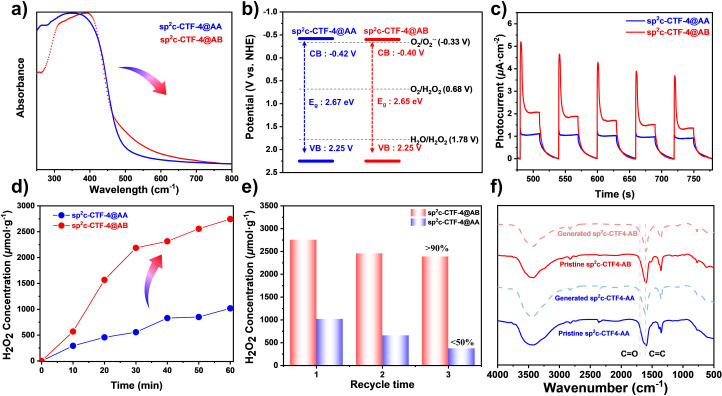
sp2c-CTF-4 samples exhibited broad visible-light absorbance extended to 600 nm in UV-visible diffuse reflectance spectra (DRS), which would be attractive for the efficient utilization of natural sunlight (Fig. 3a).37 From the UV-vis DRS, the corresponding Tauc plot analysis exhibits optical energy band gaps of 2.67 and 2.65 eV for sp2c-CTF-4@AA and sp2c-CTF-4@AB, respectively (Fig. S8 and 9†). Mott–Schottky measurements were conducted to study the electronic structure and relative band positions. The positive slope indicates typical n-type semiconductor characteristics for sp2c-CTF-4@AA and sp2c-CTF-4@AB (Fig. S10 and S11†). Accordingly, the corresponding conduction band (CB) and valence band (VB) were calculated (Fig. 3b). The CB bottoms of both COFs lie above the potential for the O2/H2O2 (+0.68 V vs. NHE), ensuring sufficient reduction potential for H2O2 generation from the ORR. In addition, the oxidation potential of sp2c-CTF-4 also meet the theoretical prerequisite for the WOR to produce H2O2. The transient-photocurrent-response intensity of sp2c-CTF-4@AB is much higher than that of sp2c-CTF-4@AA, as shown in Fig. 3c. In the meantime, according to electrochemical impedance spectroscopy (EIS), the electronic conductivity of sp2c-CTF-4@AB is also superior to that of sp2c-CTF-4@AA (Fig. S12†). Furthermore, the PL intensity of sp2c-CTF-4@AB is slightly quenched in comparison with that of sp2c-CTF-4@AA (Fig. S13†), verifying a more effective suppression of photogenerated carrier recombination. More efficient separation of charge carriers and a higher interfacial charge transfer rate have been demonstrated by sp2c-CTF-4@AB, which is beneficial for photocatalytic processes.38–43 The stacking mode evolution of the as-prepared COFs led to intense differences in their photophysical properties and hence photocatalytic performance. The electron–hole pair distribution of the two frameworks was further compared by theoretical calculations (Fig. S14†). sp2c-CTF-4@AB gave a significantly weaker electron–hole complexing ability than sp2c-CTF-4@AA. We conjecture that the shift in the stacking pattern might allow electrons to transfer either within each layer of COFs or along the interlayers. Thus, all of this further proves that the photophysical properties of sp2c-CTF-4@AB are superior to those of sp2c-CTF-4@AA.
Fig. 3. (a) UV/vis DRS of sp2c-CTF-4@AA and sp2c-CTF-4@AB. (b) Band structures of sp2c-CTF-4@AA and sp2c-CTF-4@AB. (c) Chopped photocurrent density vs. time recorded on sp2c-CTF-4@AA and sp2c-CTF-4@AB at 0.6 V vs. RHE. (d) Time-dependent H2O2 photogeneration using visible light for sp2c-CTF-4@AA and sp2c-CTF-4@AB (5 mg catalyst in 10 mL pure water); (e) recycling H2O2 production on sp2c-CTF-4 (reaction time: 1 h); (f) FTIR spectra of sp2c-CTF-4 before and after the H2O2 photogeneration cycle.
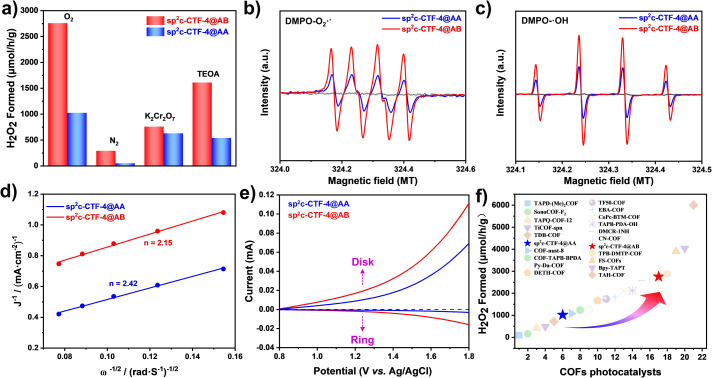
The photocatalytic H2O2 production measurements were carried out using sp2c-CTF-4@AA and sp2c-CTF-4@AB as photocatalysts in pure water and O2 without the addition of any sacrificial reagent under visible light irradiation. sp2c-CTF-4@AB showed an amazing photocatalytic H2O2 production performance with a production rate over 2758 μmol h−1 g−1 (Fig. 3d), whereas sp2c-CTF-4@AA offered a lower H2O2 production rate (1020 μmol h−1 g−1). Compared with the monomer TMT, TFPB and the model product (TST), sp2c-CTF-4 showed significantly superior H2O2 properties, suggesting that the formation of the conjugated structure is more favourable for the generation and transport of photogenerated carriers (Fig. S15 and 16†). sp2c-CTF-4@AB showed 2.7 fold better performance than sp2c-CTF-4@AA, and this trend is consistent with their physical properties. In addition, sp2c-CTF-4@AB produced H2O2 with a much better cycling performance as compared with sp2c-CTF-4@AA (Fig. 3e). The IR spectra show that sp2c-CTF-4 maintains good structural stability after H2O2 cycling performance tests (Fig. 3f). To clarify the exact H2O2 production process, a series of control experiments were carried out. Under high-purity nitrogen conditions (continuous ventilation to prevent the interference of oxygen), the two sp2c-CTF-4 samples still have some ability to produce H2O2 (Fig. 4a). This means that they are likely to have a water oxidation pathway in this process. In addition, potassium dichromate (K2Cr2O7) and triethanolamine (TEOA) are chosen as electron and hole sacrificial agents to study the H2O2 production capacity of the sp2c-CTF-4, and the results show that both electrons and holes play important roles in this process. To probe the reactive oxygen species in the photocatalytic process, electron paramagnetic resonance (EPR) spectra were measured using DMPO as a spin-trapping agent. As illustrated in Fig. 4b and c, under visible light irradiation, typical characteristic peaks of DMPO-O2˙− for sp2c-CTF-4 were observed, indicating the production of O2˙− intermediate species. This result further suggests that this ORR is the 2e two-step oxygen reduction pathway for H2O2 production (O2 → O2˙− → H2O2) in this system. Meanwhile, DMPO-˙OH signals are detected for sp2c-CTF-4 samples. In addition, the typical characteristic peaks for sp2c-CTF-4@AB are significantly stronger than those for sp2c-CTF-4@AA, indicating the superior ability of the former to produce both O2˙− and ˙OH intermediate species. To further elucidate the mechanism of H2O2 generation, more control experiments (Fig. 4d and e) were carried out. The rotating disk electrode tests show that the number of transferred electrons is 2.42 for sp2c-CTF-4@AA and 2.15 for sp2c-CTF-4@AB, respectively. In addition, the rotating ring disk electrode tests show that both materials could oxidize water into H2O2. Therefore, it can be concluded that these catalytic systems produce H2O2 mainly by the two-step single-electron oxygen reduction pathway (O2 → O2˙− → H2O2) supplemented by the water oxidation pathway (Fig. S17†).
Fig. 4. (a) H2O2 production of sp2c-CTF-4@AA and sp2c-CTF-4@AB under different conditions (O2, N2, K2Cr2O7, TEOA); (b) DMPO spin-trapping EPR spectra of sp2c-CTF-4 for measuring O2˙−; (c) DMPO spin-trapping EPR spectra of sp2c-CTF-4 for measuring ˙OH; (d) the Koutecky–Levich plots obtained via RDE measurements in phosphate buffer (pH 7) solution with continuous O2 purging; (e) polarization curves recorded with simultaneous detection of H2O2 at the ring electrode at 1600 rpm; (f) summary of photocatalytic H2O2 evolution rates of sp2c-CTF-4 and other COF-based photocatalysts.
The H2O2 production performance of the sp2c-CTF-4 photocatalyst in the presence of alkali catalysts (LiCl: 0.03% or NaCl: 0.01%) was investigated (Fig. S18†). Neither the addition of LiCl to the sp2c-CTF-4@AA photocatalytic system nor the addition of NaCl to the sp2c-CTF-4@AB photocatalytic system caused any obvious change in the H2O2 production rate. It is demonstrated that the photocatalytic activity is not influenced by free Li+, and the performance is improved only when Li+in situ interacted with the N atoms in the s-triazine units during the polycondensation. Among most known COF photocatalysts for H2O2 production (Fig. 4f and Table S2†),44–62 sp2c-CTF-4@AB is comparable to many known COF photocatalysts, exhibiting 2.7 fold as high performance as that of sp2c-CTF-4@AA. In addition, our sp2c-CTF-4 photocatalysts show prominent stability under various conditions, including treatment in aqueous 35% H2O2, concentrated HCl (12 M), and saturated KOH methanol/water (1 : 1) solution (Fig. S19 and 20†). This stability is superior to that of SNW-4 (ref. 63) or CTF-T1 (ref. 64) (Fig. S21–24†), and is adaptable for versatile functional platforms like catalysis, sensing and separation.
Conclusions
In summary, we have established an efficient strategy to tailor the stacking mode in 2D vinylene-linked covalent triazine frameworks by regulating the base-catalyst with different alkali-metal ions during Knoevenagel polycondensation. A rare sp2c-CTF example with a staggered stacking mode (sp2c-CTF-4@AB) was obtained by utilizing the Lewis acid–base interactions between the Li+ ions of the base catalyst and nitrogen atoms of s-triazine blocks. The evolution on stacking modes of 2D frameworks directly led to the significant difference in photophysical properties. Accordingly, they exhibited efficient but significantly different photocatalytic activities for the production of H2O2. sp2c-CTF-4@AB shows attractive activity with a H2O2 production rate of 2758 μmol h−1 g−1 due to its much stronger capability in separation and transport of photogenerated charge carriers. It is envisaged that either the in-plane molecular structure or vertical aggregation of a 2D vinylene-linked COF might play the key role in the design and exploration of a robust high-performance photocatalyst. This study may shed light on the regulation of crystalline structures for 2D COFs, and pave the way for developing smart framework materials with desirable properties and functionalities.
Data availability
The data supporting this article have been included as part of the ESI.†
Author contributions
Guipeng Yu, Fan Zhang, Chunyue Pan, and Juntao Tang proposed the idea and designed the experiments. Qiujian Xie and Anqi Chen performed the experiments and carried out the structural characterization. Xiaofeng Li helped to synthesize the materials. Shuai Bi helped with the structural characterization analysis. Weijie Zhang helped to analyse the results of photocatalytic experiments. Qiujian Xie and Anqi Chen wrote the manuscript with contributions from all the authors.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 52173212, 52103275, and 22271188), the Science and Technology Innovation Program of Hunan Province (2024JK2074), Hunan Provincial Natural Science Foundation for Distinguished Young Scientists (2022JJ10080), the Key Science and Technology Project of Changsha (KH2301015) and the Open Sharing Fund for the Largescale Instruments and Equipment of Central South University (CSUZC202115). We acknowledge the XRD and NMR measurements by The Modern Analysis and Testing Center of Central South University, and we are grateful to the High-Performance Computing Center of Central South University for partial support of this work.
Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc06451h
Notes and references
- Fu Z. Wang X. Gardner A. M. Wang X. Chong S. Y. Neri G. Cowan A. J. Liu L. Li X. Vogel A. Clowes R. Bilton M. Chen L. Sprick R. S. Cooper A. I. Chem. Sci. 2020;11:543–550. doi: 10.1039/c9sc03800k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae S.-H. Kum H. Kong W. Kim Y. Choi C. Lee B. Lin P. Park Y. Kim J. Nat. Mater. 2019;18:550–560. doi: 10.1038/s41563-019-0335-2. [DOI] [PubMed] [Google Scholar]
- Zhang S. Ma T. Erdemir A. Li Q. Mater. Today. 2019;26:67–86. [Google Scholar]
- Mounet N. Gibertini M. Schwaller P. Campi D. Merkys A. Marrazzo A. Sohier T. Castelli I. E. Cepellotti A. Pizzi G. Marzari N. Nat. Nanotechnol. 2018;13:246–252. doi: 10.1038/s41565-017-0035-5. [DOI] [PubMed] [Google Scholar]
- Yazyev O. V. Chen Y. P. Nat. Nanotechnol. 2014;9:755–767. doi: 10.1038/nnano.2014.166. [DOI] [PubMed] [Google Scholar]
- Xie M. Chen X.-R. Wu K. Lu Z. Wang K. Li N. Wei R.-J. Zhan S.-Z. Ning G.-H. Zou B. Li D. Chem. Sci. 2021;12:4425–4431. doi: 10.1039/d0sc07058k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G. Li X. Huang M. Zhen Z. Zhong Y. Chen Q. Zhao X. He Y. Hu R. Yang T. Zhang R. Li C. Kong J. Xu J.-B. Ruoff R. S. Zhu H. Chem. Soc. Rev. 2017;46:4417–4449. doi: 10.1039/c7cs00256d. [DOI] [PubMed] [Google Scholar]
- Zhu J. Wang Z. Yu H. Li N. Zhang J. Meng J. Liao M. Zhao J. Lu X. Du L. Yang R. Shi D. Jiang Y. Zhang G. J. Am. Chem. Soc. 2017;139:10216–10219. doi: 10.1021/jacs.7b05765. [DOI] [PubMed] [Google Scholar]
- Lin Y. C. Dumcenco D. O. Huang Y. S. Suenaga K. Nat. Nanotechnol. 2014;9:391–396. doi: 10.1038/nnano.2014.64. [DOI] [PubMed] [Google Scholar]
- Smith B. J. Dichtel W. R. J. Am. Chem. Soc. 2014;136:8783–8789. doi: 10.1021/ja5037868. [DOI] [PubMed] [Google Scholar]
- Sen Xu H. Ding S. Y. An W. K. Wu H. Wang W. J. Am. Chem. Soc. 2016;138:11489–11492. doi: 10.1021/jacs.6b07516. [DOI] [PubMed] [Google Scholar]
- Liu C. Jin Y. Yu Z. Gong L. Wang H. Yu B. Zhang W. Jiang J. J. Am. Chem. Soc. 2022;144:12390–12399. doi: 10.1021/jacs.2c03959. [DOI] [PubMed] [Google Scholar]
- Wei P. F. Qi M. Z. Wang Z. P. Ding S. Y. Yu W. Liu Q. Wang L. K. Wang H. Z. An W. K. Wang W. J. Am. Chem. Soc. 2018;140:4623–4631. doi: 10.1021/jacs.8b00571. [DOI] [PubMed] [Google Scholar]
- Ockwig N. W. Co A. P. Keeffe M. O. Matzger A. J. Yaghi O. M. Science. 2005;310:1166–1171. doi: 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- Sasmal H. S. Kumar Mahato A. Majumder P. Banerjee R. J. Am. Chem. Soc. 2022;144:11482–11498. doi: 10.1021/jacs.2c02301. [DOI] [PubMed] [Google Scholar]
- Evans A. M. Parent L. R. Flanders N. C. Bisbey R. P. Vitaku E. Kirschner M. S. Schaller R. D. Chen L. X. Gianneschi N. C. Dichtel W. R. Science. 2018;361:52–57. doi: 10.1126/science.aar7883. [DOI] [PubMed] [Google Scholar]
- Niu J. Li L. Wang Y. Su J. Li J. Wang X. Science. 2018;52:48–52. [Google Scholar]
- Zhang B. Wei M. Mao H. Pei X. Alshmimri S. A. Reimer J. A. Yaghi O. M. J. Am. Chem. Soc. 2018;140:12715–12719. doi: 10.1021/jacs.8b08374. [DOI] [PubMed] [Google Scholar]
- Yang Z. Chen H. Wang S. Guo W. Wang T. Suo X. Jiang D. E. Zhu X. Popovs I. Dai S. J. Am. Chem. Soc. 2020;142:6856–6860. doi: 10.1021/jacs.0c00365. [DOI] [PubMed] [Google Scholar]
- Emmerling S. T. Schuldt R. Bette S. Yao L. Dinnebier R. E. Kästner J. Lotsch B. V. J. Am. Chem. Soc. 2021;143:15711–15722. doi: 10.1021/jacs.1c06518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Zhang Z. Wee V. Usadi A. K. Calabro D. C. Baugh L. S. Wang S. Wang Y. Zhao D. J. Am. Chem. Soc. 2020;142:12995–13002. doi: 10.1021/jacs.0c03691. [DOI] [PubMed] [Google Scholar]
- Jadhav T. Fang Y. Patterson W. Liu C. H. Hamzehpoor E. Perepichka D. F. Angew. Chem. Int. Ed. 2019;58:13753–13757. doi: 10.1002/anie.201906976. [DOI] [PubMed] [Google Scholar]
- Acharjya A. Pachfule P. Roeser J. Schmitt F. J. Thomas A. Angew. Chem. Int. Ed. 2019;58:14865–14870. doi: 10.1002/anie.201905886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin E. Asada M. Xu Q. Dalapati S. Addicoat M. A. Brady M. A. Xu H. Nakamura T. Heine T. Chen Q. Jiang D. Science. 2017;676:673–676. doi: 10.1126/science.aan0202. [DOI] [PubMed] [Google Scholar]
- Chen Z. Yao D. Chu C. Mao S. Chem. Eng. J. 2023;451:138489. [Google Scholar]
- Campos-Martin J. M. Blanco-Brieva G. Fierro J. L. G. Angew. Chem. Int. Ed. 2006;45:6962–6984. doi: 10.1002/anie.200503779. [DOI] [PubMed] [Google Scholar]
- Zhou T. Zhao Y. Choi J. W. Coskun A. Angew. Chem. Int. Ed. 2019;131:16951–16955. [Google Scholar]
- Guo Y. Niu P. Liu Y. Ouyang Y. Li D. Zhai T. Li H. Cui Y. Adv. Mater. 2019;31:1–10. doi: 10.1002/adma.201900342. [DOI] [PubMed] [Google Scholar]
- Wang Z. Yang Y. Zhao Z. Zhang P. Zhang Y. Liu J. Ma S. Cheng P. Chen Y. Zhang Z. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-22288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang C. Zhang Z. Usadi A. K. Calabro D. C. Baugh L. S. Yu K. Wang Y. Zhao D. J. Am. Chem. Soc. 2022;144:3192–3199. doi: 10.1021/jacs.1c12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y. Calabro D. Wooler B. Li Q. Cundy S. Kamakoti P. Colmyer D. Mao K. Ravikovitch P. J. Phys. Chem. C. 2014;118:399–407. [Google Scholar]
- Sick T. Rotter J. M. Reuter S. Kandambeth S. Bach N. N. Döblinger M. Merz J. Clark T. Marder T. B. Bein T. Medina D. D. J. Am. Chem. Soc. 2019;141:12570–12581. doi: 10.1021/jacs.9b02800. [DOI] [PubMed] [Google Scholar]
- Bin Jin C. Shi P. Zhang X. Q. Huang J. Q. Xinxing Tan Cailiao. 2022;37:1–24. [Google Scholar]
- Zheng Y. Xia S. Dong F. Sun H. Pang Y. Yang J. Huang Y. Zheng S. Adv. Funct. Mater. 2021;31:1–10. [Google Scholar]
- Wu X. Han X. Liu Y. Liu Y. Cui Y. J. Am. Chem. Soc. 2018;140:16124–16133. doi: 10.1021/jacs.8b08452. [DOI] [PubMed] [Google Scholar]
- Haase F. Lotsch B. V. Chem. Soc. Rev. 2020;49:8469–8500. doi: 10.1039/d0cs01027h. [DOI] [PubMed] [Google Scholar]
- Higashi M. Domen K. Abe R. J. Am. Chem. Soc. 2013;135:10238–10241. doi: 10.1021/ja404030x. [DOI] [PubMed] [Google Scholar]
- Choi Y. Kim H. Moon G. Jo S. Choi W. ACS Catal. 2016;6:821–828. [Google Scholar]
- Hou Y. Wen Z. Cui S. Guo X. Chen J. Adv. Mater. 2013;25:6291–6297. doi: 10.1002/adma.201303116. [DOI] [PubMed] [Google Scholar]
- Wang X. Park J. Susztak K. Zhang N. R. Li M. Nat. Commun. 2019;10:380. doi: 10.1038/s41467-018-08023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Y. Li J. Wen Z. Cui S. Yuan C. Chen J. Nano Energy. 2014;8:157–164. [Google Scholar]
- Sun T. Song J. Jia J. Li X. Sun X. Nano Energy. 2016;26:83–89. [Google Scholar]
- Li K. Huang Z. Zeng X. Huang B. Gao S. Lu J. ACS Appl. Mater. Interfaces. 2017;9:11577–11586. doi: 10.1021/acsami.6b16191. [DOI] [PubMed] [Google Scholar]
- Wang H. Yang C. Chen F. Zheng G. Han Q. Angew. Chem. Int. Ed. 2022;61:e202202328. doi: 10.1002/anie.202202328. [DOI] [PubMed] [Google Scholar]
- Zhou Z. Sun M. Zhu Y. Li P. Zhang Y. Wang M. Shen Y. Appl. Catal., B. 2023;334:122862. [Google Scholar]
- Zhao W. Yan P. Li B. Bahri M. Liu L. Zhou X. Clowes R. Browning N. D. Wu Y. Ward J. W. Cooper A. I. J. Am. Chem. Soc. 2022;144:9902–9909. doi: 10.1021/jacs.2c02666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T. Wang Z. Zhang W. An S. Wei L. Guo S. Huang Y. Jiang S. Zhu M. Zhang Y.-B. Zhu W.-H. J. Am. Chem. Soc. 2024;146:20107–20115. doi: 10.1021/jacs.4c04244. [DOI] [PubMed] [Google Scholar]
- Li L. Xu L. Hu Z. Yu J. C. Adv. Funct. Mater. 2021;31:2106120. [Google Scholar]
- Yang Y. Kang J. Li Y. Liang J. Liang J. Jiang L. Chen D. He J. Chen Y. Wang J. New J. Chem. 2022;46:21605–21614. [Google Scholar]
- Di X. Lv X. Wang H. Chen F. Wang S. Zheng G. Wang B. Han Q. Chem. Eng. J. 2023;455:140124. [Google Scholar]
- Das P. Chakraborty G. Roeser J. Vogl S. Rabeah J. Thomas A. J. Am. Chem. Soc. 2023;145:2975–2984. doi: 10.1021/jacs.2c11454. [DOI] [PubMed] [Google Scholar]
- Zhang X. Zhang J. Miao J. Wen X. Chen C. Zhou B. Long M. Chem. Eng. J. 2023;466:143085. [Google Scholar]
- Zhi Q. Liu W. Jiang R. Zhan X. Jin Y. Chen X. Yang X. Wang K. Cao W. Qi D. Jiang J. J. Am. Chem. Soc. 2022;144:21328–21336. doi: 10.1021/jacs.2c09482. [DOI] [PubMed] [Google Scholar]
- Sun J. Sekhar Jena H. Krishnaraj C. Singh Rawat K. Abednatanzi S. Chakraborty J. Laemont A. Liu W. Chen H. Liu Y. Leus K. Vrielinck H. Van Speybroeck V. Van Der Voort P. Angew. Chem. Int. Ed. 2023;62:e202216719. doi: 10.1002/anie.202216719. [DOI] [PubMed] [Google Scholar]
- Krishnaraj C. Sekhar Jena H. Bourda L. Laemont A. Pachfule P. Roeser J. Chandran C. V. Borgmans S. Rogge S. M. J. Leus K. Stevens C. V. Martens J. A. Van Speybroeck V. Breynaert E. Thomas A. Van Der Voort P. J. Am. Chem. Soc. 2020;142:20107–20116. doi: 10.1021/jacs.0c09684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y. Han W.-K. Chi W. Mao Y. Jiang Y. Yan X. Gu Z.-G. Appl. Catal., B. 2023;331:122691. [Google Scholar]
- Luo Y. Zhang B. Liu C. Xia D. Ou X. Cai Y. Zhou Y. Jiang J. Han B. Angew. Chem. Int. Ed. 2023;62:e202305355. doi: 10.1002/anie.202305355. [DOI] [PubMed] [Google Scholar]
- Han W.-K. Lu H.-S. Fu J.-X. Liu X. Zhu X. Yan X. Zhang J. Jiang Y. Dong H. Gu Z.-G. Chem. Eng. J. 2022;449:137802. [Google Scholar]
- Pan G. Hou X. Liu Z. Yang C. Long J. Huang G. Bi J. Yu Y. Li L. ACS Catal. 2022;12:14911–14917. [Google Scholar]
- Wu M. Shan Z. Wang J. Liu T. Zhang G. Chem. Eng. J. 2023;454:140121. [Google Scholar]
- Yang T. Chen Y. Wang Y. Peng X. Kong A. ACS Appl. Mater. Interfaces. 2023;15:8066–8075. doi: 10.1021/acsami.2c20506. [DOI] [PubMed] [Google Scholar]
- Zhai L. Xie Z. Cui C.-X. Yang X. Xu Q. Ke X. Liu M. Qu L.-B. Chen X. Mi L. Chem. Mater. 2022;34:5232–5240. [Google Scholar]
- Schwab M. G. Fassbender B. Spiess H. W. Thomas A. Feng X. Müllen K. J. Am. Chem. Soc. 2009;131:7216–7217. doi: 10.1021/ja902116f. [DOI] [PubMed] [Google Scholar]
- Bi J. Fang W. Li L. Wang J. Liang S. He Y. Liu M. Wu L. Macromol. Rapid Commun. 2015;36:1799–1805. doi: 10.1002/marc.201500270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this article have been included as part of the ESI.†