Abstract
Anopheles stephensi is a major vector of malaria in Asia and the Arabian Peninsula, and its recent invasion into Africa poses a major threat to malaria control and elimination efforts on the continent. The mosquito is well adapted to urban environments, and its presence in Africa could potentially lead to an increase in malaria transmission in cities. Most of the knowledge about An stephensi ecology in Africa has been generated from studies conducted during the rainy season, when vectors are most abundant. Here, we provide evidence from the peak of the dry season in the city of Jigjiga in Ethiopia, and report An stephensi immature stages infesting predominantly in water reservoirs made to support construction operations (ie, in construction sites or associated with brick-manufacturing businesses). Political and economic changes in Ethiopia (particularly the Somali Region) have fuelled an unprecedented construction boom since 2018 that, in our opinion, has been instrumental in the establishment, persistence, and propagation of An stephensi via the year-round availability of perennial larval habitats associated with construction. We argue that larval source management during the dry season might provide a unique opportunity for focused control of An stephensi in Jigjiga and similar areas.
Introduction
Most African countries have been remarkably successful in reducing malaria burden since the year 2000, thanks to the scaling up of vector control tools (insecticide-treated nets and indoor residual spraying) and effective preventive and treatment drugs.1 Increasing evidence suggests that rapid urbanisation of Africa’s human population (driven primarily by rural to urban migration) is also contributing to a reduction in malaria burden.2–5 Lower habitat suitability for Anopheles spp breeding and improvements in housing within African cities reduce human–mosquito contact and can lead to lower Plasmodium spp inoculation rates compared with rural settings.2–5 Environmental management in the form of housing improvement has gained research interest due to its sustained effect on Anopheles spp mosquitoes and its positive effect on livelihoods.6,7 WHO calls this approach “building the vector out”, and it involves the adoption of practices that range from improved housing structures to retrofitting eave tubes and other approaches to limit mosquito entry indoors.8 This approach is also seen as a novel aspect of malaria control in urban settings, given that most human population growth over the next century will be accounted for by the growing number of city dwellers.9
As most sub-Saharan countries continue their push towards malaria elimination with large-scale delivery of long-lasting insecticide nets and indoor residual spraying, increased insecticide resistance in Anopheles spp is becoming a serious challenge.10 Currently, resistance to insecticides is reinforcing the need to consider novel approaches, such as attractive targeted sugar baits, spatial repellents, and housing improvement in the form of eave tubes and screening.11,12 A new threat with the potential to derail public health gains in the reduction of malaria burden in Africa is the invasion and establishment of Anopheles stephensi, a malaria vector native to Asia and commonly found in cities throughout India, Iran, Pakistan, and the Arabian Peninsula.13,14
Since it was first detected in Africa in Djibouti in 2012,15 An stephensi has spread to Ethiopia,16 Somalia,17 Sudan,18 Kenya, Nigeria, and Ghana.19 Niche modelling predicts suitable environmental conditions for An stephensi establishment throughout tropical African cities, putting an additional 126 million people potentially at risk of malaria.20 While in its native range An stephensi has different forms, with the type form mainly found in urban environments and the mysorensis form being mostly rural,21 reports from Africa have predominantly described the type form in urban areas.22,23 Given An stephensi’s dependency on artificial containers as primary larval breeding habitats, rainfall alone was a poor predictor of An stephensi-driven malaria transmission.24 In Djibouti, a 2000-times exponential increase in the number of malaria cases has been observed since the detection of An stephensi.14,19 The contribution of An stephensi to increases in malaria transmission outside of Djibouti has started to be investigated, especially in light of the malaria outbreak (2018–2022). From 2018 to 2020 in Ethiopia, Plasmodium vivax was detected in wild-caught An stephensi from the cities of Dire Dawa (infection rate 0·5%) and Kebridehar (0·3%),23 and P vivax (2·8%) and P falciparum (1·4%) infection was reported in An stephensi from Awash, in 2021.25 Furthermore, experimental membrane feeding experiments showed that field-caught An stephensi from Ethiopia became considerably more infectious with local P vivax and P falciparum than Anopheles arabiensis (the primary malaria vector in Ethiopia), indicating that An stephensi is a highly competent vector for African Plasmodium.25
Given the entomological and epidemiological evidence gathered so far, WHO launched a new initiative to stop the further spread of An stephensi in Ethiopia that is based on a 5-pronged approach: (1) increasing collaboration, (2) strengthening surveillance, (3) improving information exchange, (4) developing guidance, and (5) prioritising research.19 To execute an effective plan for An stephensi elimination, key sources of information about its biology and bionomics in its new habitats are needed, and vector control tools that are better suited for urban settings will need to be investigated.
Several studies (most from Ethiopia, cross-sectional, and conducted during the rainy season) have characterised An stephensi habitats and bionomics, with many knowledge gaps remaining.22,23,25,26 The evidence gathered so far shows that in the rainy season, An stephensi larvae are found in a wide array of small and large artificial containers, ranging from large water cisterns to car tyres and buckets.22,23,25 In addition to Plasmodium infection, such studies have characterised up to 48% human biting (14 of 29 mosquitoes) in Awash,25 but low human biting (<1% human biting) in Dire Dawa and Kebridehar (where a high frequency of domestic animal feeding was also observed).23 Such discrepancies might have originated, in part, due to the opportunistic collection of adult mosquitoes in or near animal shelters. Indeed, the finding of P vivax and P falciparum infected mosquitoes can only be explained by human biting. Furthermore, given its egg-laying behaviour (eggs that resist desiccation27 and are laid in small containers), the fact that it bites humans not only at night when they are sleeping and that it is found in urban and peri-urban areas, An stephensi has more similarities with Aedes aegypti mosquitoes (which vector viruses such as dengue, chikungunya, and Zika) than with other Anophelines.28 One of the many factors that remains to be studied is how An stephensi persists in Ethiopia and other African countries that have a prolonged dry season, as this period might offer unique opportunities for surveillance and control.
Here, we report the habitat use of An stephensi during the dry season in eastern Ethiopia. Although increased focus on characterising larval habitats in rainy periods can provide information of niche breadth for the species, our goal of focusing on the dry season was to explore possible windows for control in periods where the population size might be smallest.
Dry season An stephensi collections in Jigjiga, Ethiopia
During March 6 to March 14, 2023, mosquito surveys were conducted in Jigjiga city (capital of Somali Region with a population of approximately 800 000) during the dry season. An stephensi was first detected in Jigjiga in 201822 and has persisted in the city since then despite a harsh dry season (the rainless period of the year lasts for around 3 months). Molecular analysis of cytochrome oxidase subunit I (COI) and cytochrome B genes shows Jigjiga as one of the locations with highest diversity, suggesting it was likely an early introduction point of An stephensi into Ethiopia.29 Jigjiga is of relevance because of its large population size, rapid urbanisation, and connection to other malaria-endemic regions and the port of Berbera in Somaliland.
We used methods developed for standard larval and pupal sampling of container-breeding mosquitoes8 that included collecting all the larvae and pupae in small water-holding containers and using dippers and large fish nets to sample large water-holding habitats. Dipping and nets were used to exhaustively collect immature mosquitoes from the sides and centre of each habitat. All the larvae and pupae were reared to adulthood at Jigjiga University Entomology Laboratory. The emerged Anopheles spp adults were identified to species level with standard keys30 and molecular means.16 From a total of 60 potential larval sites with water that were sampled across the city, we identified a major habitat consistently positive for An stephensi larvae and pupae during the dry season: man-made pits related with construction operations (figure 1A). We term such habitats construction pits, as they were primarily built for the storage of water in construction sites or in small-scale brick-manufacturing businesses (figure 1B). An stephensi positivity in construction pits was 62·5% (presence-absence), which was significantly higher (by an order of magnitude) than the 5·9% positivity in water cisterns made of cement (Fisher’s exact test; χ2=0·0008; p<0·01) and the 0% positivity in 200 L plastic drums (χ2=0·0001; p<0·01; figure 1B). All abandoned tyres sampled did not contain any water. Of note, from all the sites that we found positive for An stephensi larvae and pupae, 63·6% of them also had Culiseta longiareolata and Aedes hirsutus larvae in them, whereas only 18·2% and 9·1% sites positive for An stephensi were cohabited by C longiareolata or Ae hirsutus only, respectively (figure 1C). In construction pits, the frequency of positivity of the three species did not differ significantly (χ2=0·740, p>0·05 for An stephensi vs C longiareolata; χ2=1, p>0·05 for An stephensi vs Ae hirsutus; and χ2=0·740, p>0·05 for C longiareolata vs Ae hirsutus). Although the drivers that enabled cohabitation of multiple species remain to be studied, we found that all positive containers for all species were open and exposed to direct sunlight, and also had a presence of algae, but not turbid water.
Figure 1: Results from field collections in Jigjiga during the dry season of 2023.
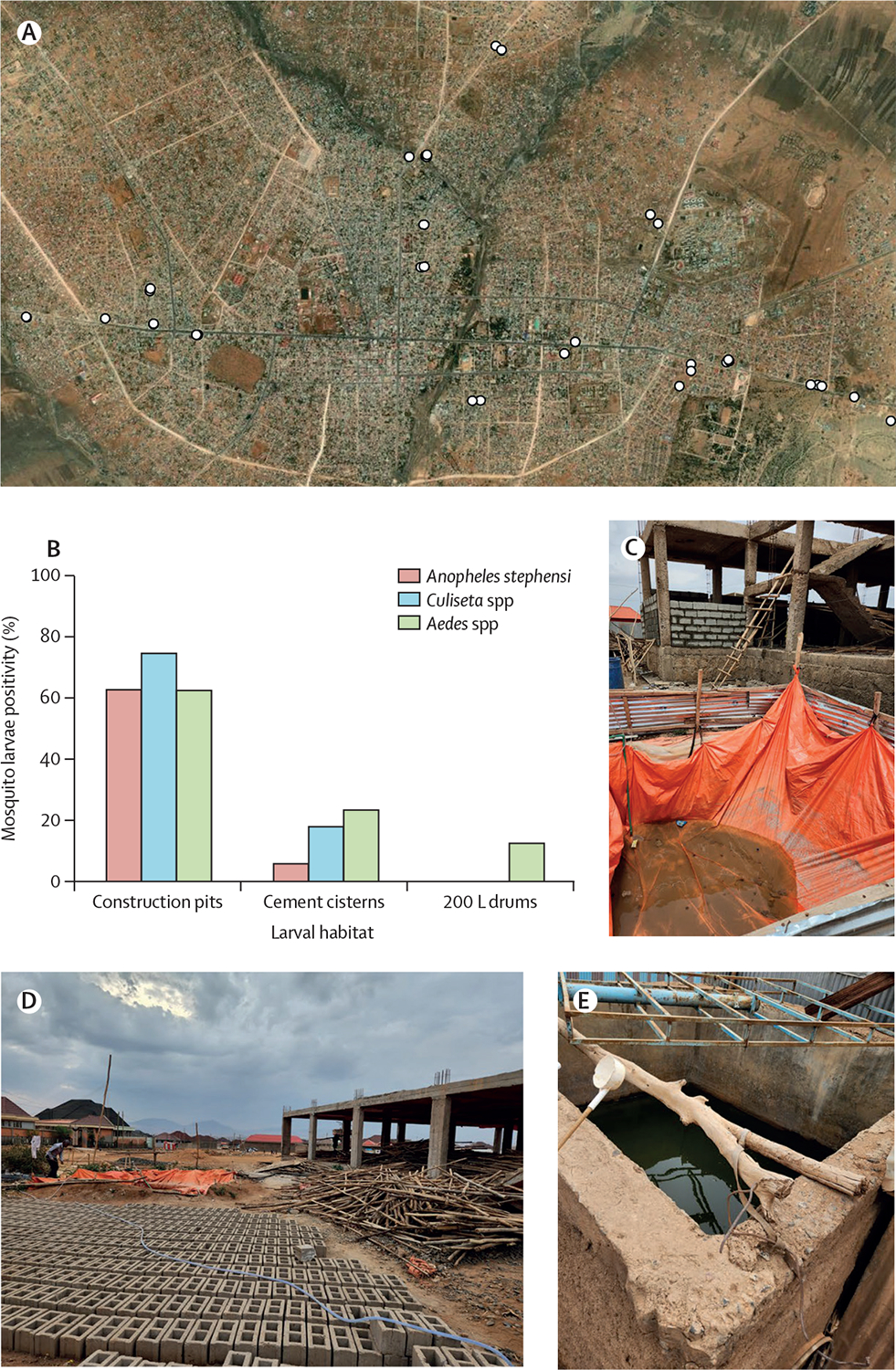
(A) Distribution of Anopheles stephensi positive larval habitats in the city of Jigjiga, Somali Region, Ethiopia. Map scale 1:39840. (B) Positivity of An stephensi immature stages stratified by habitat type and by species or genus of mosquito found. Examples of sampled habitats, such as (C) construction pits associated with house construction, (D) a construction pit associated with brick manufacturing, and (E) a cement cistern. Image (A) sourced from ESRI World Imagery, MAXAR, and the GIS User Community.31
A subset of 20 adults emerging from the pupae collected in construction pits and visually identified with standard keys was molecularly confirmed to be An stephensi using an allele specific PCR and the sequencing of ITS2 and COI loci.16 While ITS2 haplotypes were all identical for the An stephensi samples, three COI haplotypes were detected: Hap 1 (7 of 14 adult An stephensi), Hap 2 (6 of 14), and Hap 3 (1 of 14; using published haplotypes designations),29 mostly consistent with previous studies. Notably, the presence of the COI Hap 1 (common to south Asia and detected in northern Ethiopia and Djibouti) supports the notion of Jigjiga’s connectivity with regions outside of the continent with long-established An stephensi populations and as a likely entry point for An stephensi into the southern part of the country.
We incorporated the GPS coordinates from the construction pits sampled in March 2023 into Google Earth to visualise their location within Jigjiga (figure 1A). After mapping the construction pits to Google Earth, it was evident that their unique spectral signature (size, colour contrast, and presence of water) allowed the visual identification of other pits not sampled by our team (figure 2A, B). In Google Earth, we then used a satellite image taken in November, 2022 (4 months previous to sampling and the closest point in time to our collections) to digitise all construction pits visible in a swath of Jigjiga centred on the road connecting the city with Somaliland and ranging from a dense urban area (the city’s downtown) at the city’s edge (figure 2C). Within this urban to rural area of transition, we identified a total of 101 construction pits. In ArcMap 10.8 (ESRI, Redlands, CA), we implemented a kernel density estimation of pits per hectare within the swath and found that pits were concentrated to the centre of the swath (figure 2D). This area of Jigjiga was experiencing rapid construction and development at the time.
Figure 2: Construction pits can be mapped using high-resolution satellite imagery.
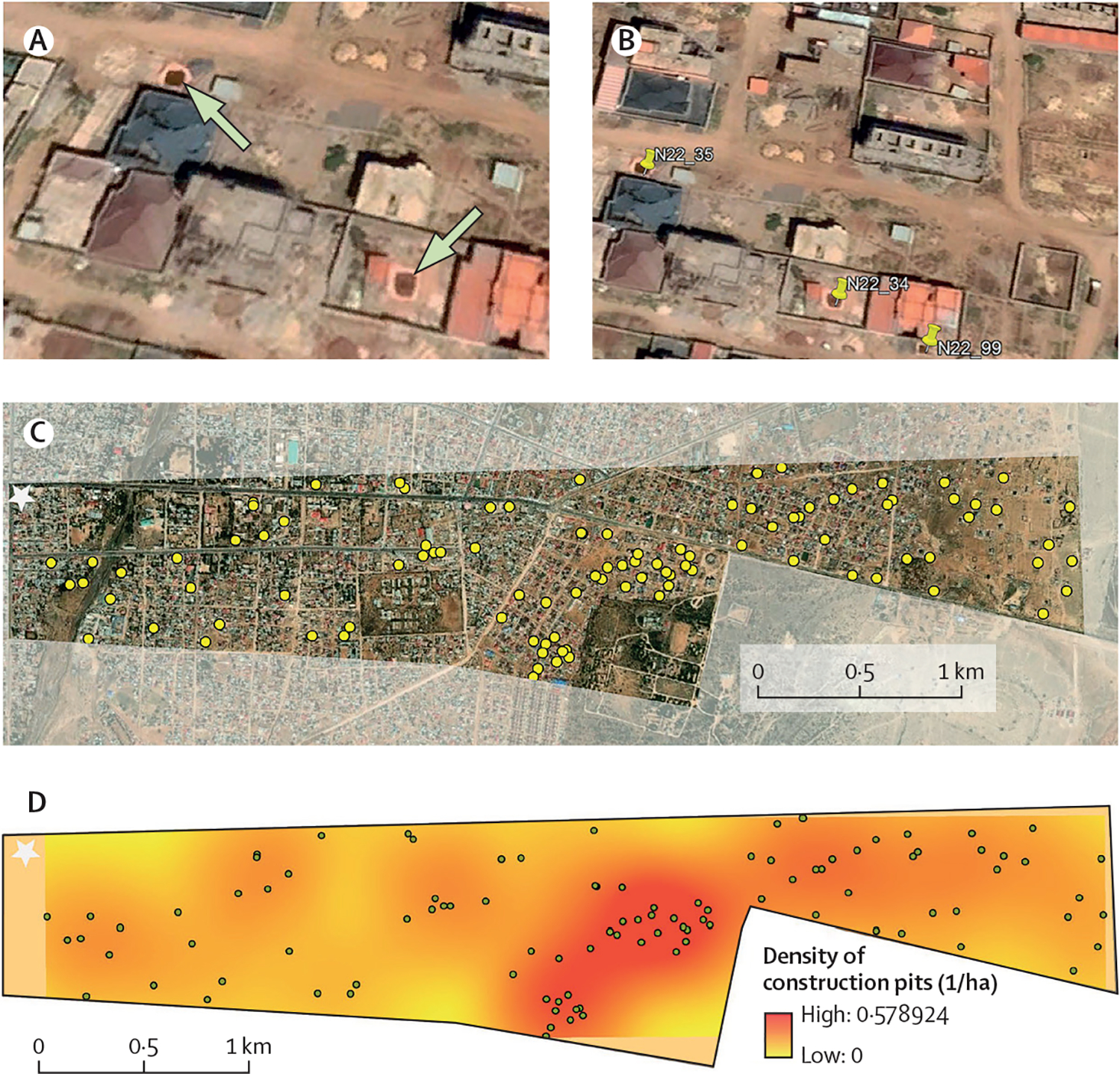
(A) Construction pits identified as positive for Anopheles stephensi larvae during March, 2023 in Jigjiga, Ethiopia (indicated by the arrows). (B) Use of Google Earth Pro to digitise all visible construction pits (a total of three pits are identified with a pin). (C) Distribution of the 101 construction pits visually identified in November, 2022, 4 months before our sampling, within a rural-urban swath measuring 4·3 km2 and centred on the highway connecting Jigjiga with Somaliland (one of the busiest corridors in the region). (D) Kernel density estimate of the density of construction pits per hectare (coloured surface) and location of all identified pits (dots) using a bandwidth of 500 m and a pixel size of 10 m. Stars in (C) and (D) indicate the location of Jigjiga’s downtown. Images (A–C) were sourced from ESRI World Imagery, MAXAR, and the GIS User Community.31
We acknowledge several limitations to our findings. We might have missed habitat types that, while common and infested by An stephensi in the rainy season,22,23 were not found holding water in the dry season. Increasing the number of sampled locations might have provided an opportunity to detect such infested habitats. Although we provide information on the infestation of construction pits, we were unable to estimate the productivity of such habitats. For container-breeding mosquitoes (ie, Ae aegypti) pupal surveys are used to quantify productivity.32 Unfortunately, An stephensi sampling has not relied on such measures and is restricted to the counting of larvae collected from dipping, which has challenges when comparing habitats of different sizes. As more evidence from studies similar to this emerge, formal guidelines for immature and adult An stephensi sampling (as the ones developed for Ae aegypti)33 will provide an opportunity to better quantify productivity and the effect of vector control interventions.
Political and economic development and An stephensi invasion in Jigjiga
We suggest that an unprecedented urban development boom in Jigjiga has been crucial in favouring An stephensi establishment and rapid spread. Since its first rare detection in Jigjiga from 2018, An stephensi has been consistently and more commonly found in 2020 and now in 2023. Jigjiga increased its built-up area from 4·2% in 1985 to 5·2% in 2005 and then to 24·0% in 2015, primarily driven by a change in status from zonal capital to regional capital, which opened political and economic opportunities, leading to high rural to urban migration.34 Since 2018, when transformative political reform of Ethiopia was enacted by the Ethiopian Government, Jigjiga has seen an even larger population and urban footprint increase. The recent declaration by the national Government of Ethiopia that 19·0% of commodity imports for the country should enter via Berbera Port in Somaliland and be transported through Jigjiga to the rest of the country,35 led to an increased interest in investment and even higher migration into the city.35 Jigjiga’s population grew from 125 876 inhabitants in 2007 to more than 700 000 in 2020.
Since the 2018 political reform in Ethiopia, different political groups began to accept Jigjiga’s new regional status as a safe regional hub, opening the window of opportunity to increased investment and business development.35 Diaspora Somalis started to make investments, purchase land, and construct homes leading to a construction boom and increases in the price of land.35 New hotels, restaurants, and businesses are being built in preparation for the increased trade (and truck traffic) with Berbera Port and from Somaliland.35 As the city continues its unprecedented expansion, it is also increasingly facing crucial water shortages (particularly during the dry season); the mean water accessibility of Jigjiga in 2016 was only 19·0%.36 In response to these water shortages, communities build cement cisterns to store water for domestic use.36 Similarly, for building construction or brick-manufacturing purposes, people in the town are accustomed to construct temporary construction pits lined with plastic sheets (figure 1). During the dry season, water for construction pits is generally purchased and delivered in truck cisterns, which source the water from underground wells located outside the city. We can see evidence of the unprecedented construction boom in Jigjiga using historical satellite imagery (figure 3). From the images, the striking expansion of construction pits in 2018 and the construction further along the periphery of the city can be seen. The sector went from 62–84 pits between 2016–18 to 232 in 2020 and 192 in 2021, showcasing the rapid urban expansion of Jigjiga during that time (figure 3).
Figure 3: Historical sequence of the distribution of construction pits in an urban-rural swath of Jigjiga, Somali Region, Ethiopia.
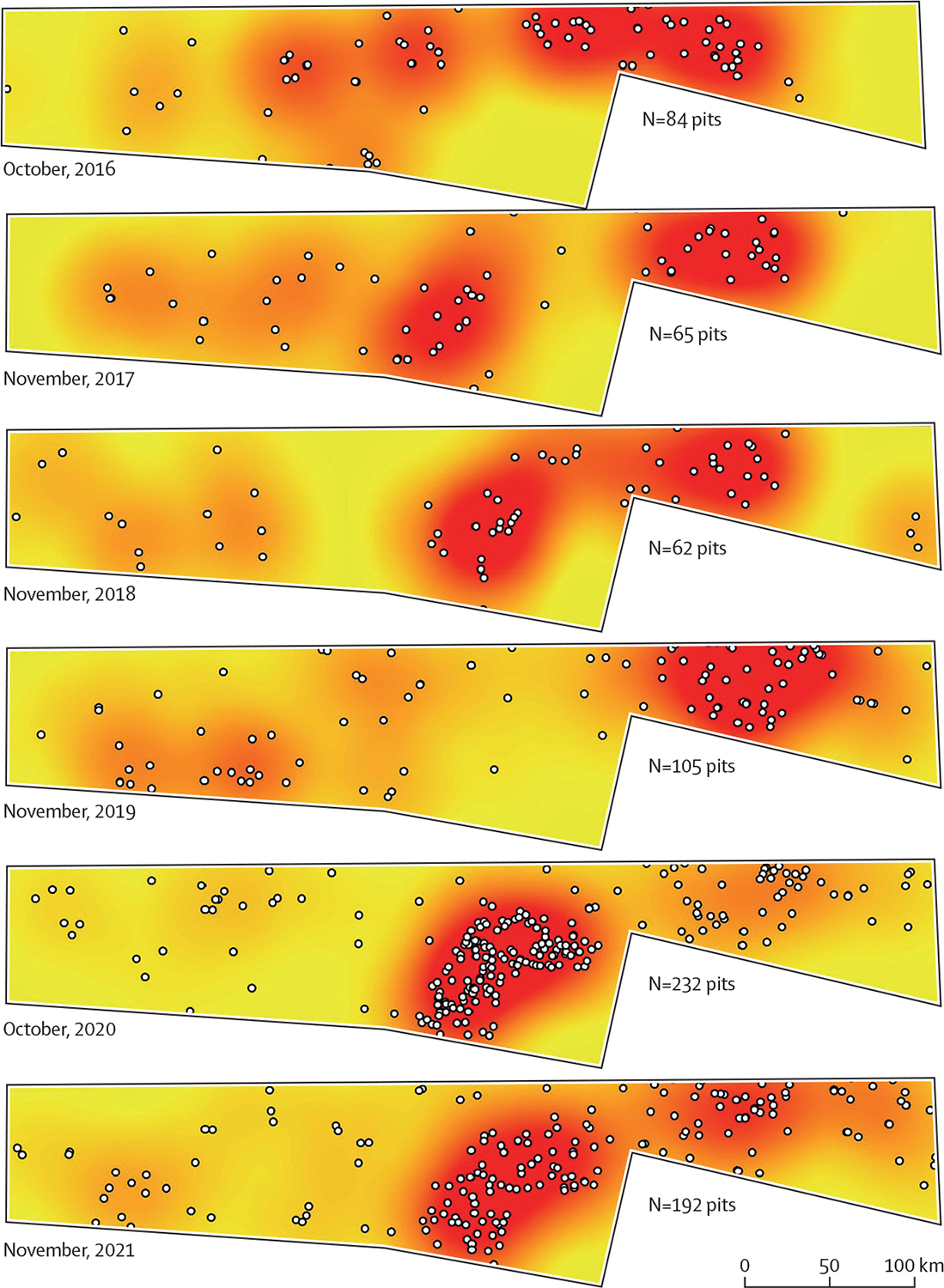
The white dots represent digitised construction pits, observed with high-resolution satellite imagery historically archived in Google Earth. The surface was generated using kernel density function in ArcMap 10.8. For each year we used October–November, as they were the months that had most complete information.
Building An stephensi into Africa
In sub-Saharan Africa, residents of modern rural houses experience half the risk of malaria infection compared with those living in traditional rural houses.37 Such findings have led to a package of interventions intended to build out malaria that focuses on practices, such as house screening in windows, doors and eaves, and sensitive building designs that lower the suitability of the indoor environment for Anopheles spp mosquitoes.8,38 Improved housing and the increased number of construction sites in rural and peri-urban areas might be perceived as a sign of positive development with the potential to reduce malaria transmission. We argue that with An stephensi, construction sites might become a pivotal environment for species establishment and propagation within sub-Saharan cities.
As new evidence emerges, it is apparent that the presence of An stephensi in construction areas might not be a unique feature of Jigjiga City. Balkew and colleagues22 described construction pits infested by An stephensi larvae during the rainy season across multiple cities of Ethiopia in the Afar, Dire Dawa, and Amhara regions. In Sudan, construction pits made of cement were also identified as productive An stephensi larval habitats in Port Sudan and Tokar City.39 In India, it is widely recognised that many An stephensi breeding sites are built into the finished structures of offices, homes, and factories in urban areas.40 Less widely recognised but also as important as An stephensi breeding sites are the transient structures created during and as part of the construction process.41 Therefore, by capitalising on the temporary water reservoirs predominant in construction sites, An stephensi might be exploiting unique environmental conditions that could be favouring its establishment and spread in Africa. This association between urban development and An stephensi resembles the finding of cutaneous leishmaniasis outbreaks associated with urban growth and, more specifically, construction sites in Israeli settlements.42,43
Opportunities for An stephensi containment: larval source management of construction pits
The finding of discrete and easily identifiable An stephensi larval habitats in Jigjiga could provide a unique opportunity for immediate larval source management (LSM) and targeted control during the dry season, particularly with larviciding or biological control. A similar concept of dry season LSM has been proposed for An gambiae in semi-arid Kenya as an approach to maximise the effectiveness of larval control.44 An extensive list of larvicides prequalified by WHO for vector control is available.45 While temephos and Bacillus thuringiensis have shown important larviciding effects on An Stephensi from Ethiopia,46 they require frequent reapplication, which can be challenging given the number of construction pits that need to be treated. Long-lasting larvicide formulations, that could be potential candidates for control in large water volumes are Spinosad 7·48% DT (Clarke Mosquito Control Products) and SumiLarv 2 MR (Sumitomo Chemical Company). Spinosad DT is a tablet for direct application used at a dose of 0·5 mg per L active ingredient (1 tablet per 200 L of water) for control of container-breeding mosquitoes with a minimum expected duration of optimum efficacy of 4–6 weeks under field conditions.47 SumiLarv 2 MR is a 2 g plastic disc containing 2% (20 g active ingredient per kg ± 25% w/w) pyriproxyfen used at the dose of one disk in a water container with a volume of 40 L.48 Long-lasting methoprene briquettes are commonly used for Culex pipiens control in catch basins in the USA and, if prequalified, would provide an additional long-lasting tool since there is a 6-month extended release formulation (Altosid 150-Day Briquets, Zoecon).49
Given the water source and use, long duration of construction pits, and constant availability of water, a biological control option that can be considered is the use of larvivorous fish.50 Fish that feed on mosquito larvae have been widely used around the world in attempts to control malaria, other mosquito-borne diseases, and mosquito nuisance biting,50 and could be used in this case as a textbook example.51 Locally native larvivorous fish exist near Jigjiga.50 Furthermore, LSM in Jigjiga could include both larviciding and larvivorous fish if larvicides with low toxicity (Spinosad, metoprene, or pyriproxyfen) are chosen. More importantly, our finding of high cohabitation between An stephensi with Culiseta spp and Aedes spp mosquitoes provides an opportunity for integrated LSM across vectors, which can lead to important co-benefits and a higher justification for the implementation of such programmes within Jigjiga and other cities. The emphasis for malaria is typically on the protection of people inside their home by deployment of insecticide-treated bed nets and indoor residual spraying. In the case of An stephensi in Jigjiga, vectors could be controlled outside the house by conducting LSM during the dry season (the period when mosquito populations are lower and primary larval sites are easier to identify) to reduce the risk of vector establishment and further transmission of malaria. More broadly, incorporating LSM within a comprehensive integrated vector management package52 that includes adult control and targets other cohabiting species, such as Aedes aegypti, might provide an opportunity to tackle both the invasion of An stephensi and the growing threat of arbovirus transmission in African cities.
Conclusions
The spread of An stephensi in Africa might be facilitated in some Ethiopian cities by high urban migration and immigration and an unprecedented construction boom, which is generating novel larval habitats that the vector exploits during the dry season. We highlight that the spread of An stephensi is a planetary health problem that requires holistic consideration of the environmental, social, and political changes ongoing in rapidly growing African cities.
Acknowledgments
We thank the Jigjiga residents for allowing us to conduct this research. Emory Global Health Institute Rapid Response Grant funding was provided by the Emory Global Health Institute to conduct this research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Emory Global Health Institute or the official policy or position of the Centers for Disease Control and Prevention.
Footnotes
Declaration of interests
We declare no competing interests.
Data sharing
Files used for generating figure 2 and figure 3 are shared as supplementary Google Earth kml files. Please contact the corresponding author Gonzalo M Vazquez-Prokopec for data used in the study.
References
- 1.Bhatt S, Weiss DJ, Cameron E, et al. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature 2015; 526: 207–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasili S, Odemba N, Ngere FG, Kamanza JB, Muema AM, Kutima HL. Entomological assessment of the potential for malaria transmission in Kibera slum of Nairobi, Kenya. J Vector Borne Dis 2009; 46: 273–79. [PubMed] [Google Scholar]
- 3.Killeen GF, Govella NJ, Mlacha YP, Chaki PP. Suppression of malaria vector densities and human infection prevalence associated with scale-up of mosquito-proofed housing in Dar es Salaam, Tanzania: re-analysis of an observational series of parasitological and entomological surveys. Lancet Planet Health 2019; 3: e132–43. [DOI] [PubMed] [Google Scholar]
- 4.Trape JF, Zoulani A. Malaria and urbanization in central Africa: the example of Brazzaville. Part III: relationships between urbanization and the intensity of malaria transmission. Trans R Soc Trop Med Hyg 1987; 81 (suppl 2): 19–25. [DOI] [PubMed] [Google Scholar]
- 5.Musiime AK, Krezanoski PJ, Smith DL, et al. House design and risk of malaria, acute respiratory infection and gastrointestinal illness in Uganda: a cohort study. PLoS Glob Public Health 2022; 2: e0000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirby MJ, Ameh D, Bottomley C, et al. Effect of two different house screening interventions on exposure to malaria vectors and on anaemia in children in The Gambia: a randomised controlled trial. Lancet 2009; 374: 998–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsay SW, Jawara M, Paine K, Pinder M, Walraven GE, Emerson PM. Changes in house design reduce exposure to malaria mosquitoes. Trop Med Int Health 2003; 8: 512–17. [DOI] [PubMed] [Google Scholar]
- 8.WHO. Keeping the vector out: housing improvements for vector control and sustainable development. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 9.UN Department of Economic and Social Affairs PD. World urbanization prospects: the 2018 revision (ST/ESA/SER.A/420). New York, USA: United Nations, 2019. [Google Scholar]
- 10.Killeen GF, Ranson H. Insecticide-resistant malaria vectors must be tackled. Lancet 2018; 391: 1551–52. [DOI] [PubMed] [Google Scholar]
- 11.Cotter C, Sturrock HJ, Hsiang MS, et al. The changing epidemiology of malaria elimination: new strategies for new challenges. Lancet 2013; 382: 900–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anaele BI, Varshney K, Ugwu FSO, Frasso R. The efficacy of insecticide-treated window screens and eaves against Anopheles mosquitoes: a scoping review. Malar J 2021; 20: 388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samarasekera U. A missed opportunity? Anopheles stephensi in Africa. Lancet 2022; 400: 1914–15. [DOI] [PubMed] [Google Scholar]
- 14.Vogel G Invasive mosquito adds to Africa’s malaria toll. Science 2022; 378: 582–83. [DOI] [PubMed] [Google Scholar]
- 15.Faulde MK, Rueda LM, Khaireh BA. First record of the Asian malaria vector Anopheles stephensi and its possible role in the resurgence of malaria in Djibouti, Horn of Africa. Acta Trop 2014; 139: 39–43. [DOI] [PubMed] [Google Scholar]
- 16.Carter TE, Yared S, Gebresilassie A, et al. First detection of Anopheles stephensi Liston, 1901 (Diptera: Culicidae) in Ethiopia using molecular and morphological approaches. Acta Trop 2018; 188: 180–86. [DOI] [PubMed] [Google Scholar]
- 17.Ali S, Samake JN, Spear J, Carter TE. Morphological identification and genetic characterization of Anopheles stephensi in Somaliland. Parasit Vectors 2022; 15: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ahmed A, Khogali R, Elnour MB, Nakao R, Salim B. Emergence of the invasive malaria vector Anopheles stephensi in Khartoum State, Central Sudan. Parasit Vectors 2021; 14: 511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO. WHO initiative to stop the spread of Anopheles stephensi in Africa. Geneva, Switzerland: World Health Organization, 2022. [Google Scholar]
- 20.Sinka ME, Pironon S, Massey NC, et al. A new malaria vector in Africa: predicting the expansion range of Anopheles stephensi and identifying the urban populations at risk. Proc Natl Acad Sci USA 2020; 117: 24900–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alam MT, Bora H, Das MK, Sharma YD. The type and mysorensis forms of the Anopheles stephensi (Diptera: Culicidae) in India exhibit identical ribosomal DNA ITS2 and domain-3 sequences. Parasitol Res 2008; 103: 75–80. [DOI] [PubMed] [Google Scholar]
- 22.Balkew M, Mumba P, Dengela D, et al. Geographical distribution of Anopheles stephensi in eastern Ethiopia. Parasit Vectors 2020; 13: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balkew M, Mumba P, Yohannes G, et al. An update on the distribution, bionomics, and insecticide susceptibility of Anopheles stephensi in Ethiopia, 2018–2020. Malar J 2021; 20: 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whittaker C, Hamlet A, Sherrard-Smith E, et al. Seasonal dynamics of Anopheles stephensi and its implications for mosquito detection and emergent malaria control in the Horn of Africa. Proc Natl Acad Sci USA 2023; 120: e2216142120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tadesse FG, Ashine T, Teka H, et al. Anopheles stephensi mosquitoes as vectors of Plasmodium vivax and falciparum, Horn of Africa, 2019. Emerg Infect Dis 2021; 27: 603–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yared S, Gebressielasie A, Damodaran L, et al. Insecticide resistance in Anopheles stephensi in Somali Region, eastern Ethiopia. Malar J 2020; 19: 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chalam BS. The resistance of Anopheles eggs to desiccation. Indian J Med Res 1927; 14: 863–66. [Google Scholar]
- 28.Allan R, Budge S, Sauskojus H. What sounds like Aedes, acts like Aedes, but is not Aedes? Lessons from dengue virus control for the management of invasive Anopheles. Lancet Glob Health 2023; 11: e165–69. [DOI] [PubMed] [Google Scholar]
- 29.Carter TE, Yared S, Getachew D, et al. Genetic diversity of Anopheles stephensi in Ethiopia provides insight into patterns of spread. Parasit Vectors 2021; 14: 602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Coetzee M. Key to the females of Afrotropical Anopheles mosquitoes (Diptera: Culicidae). Malar J 2020; 19: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Esri. World imagery. 2023. https://www.arcgis.com/home/item.html?id=10df2279f9684e4a9f6a7f08febac2a9 (accessed Oct 11, 2023).
- 32.WHO. Operational guide for assessing the productivity of Aedes aegypti breeding sites. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 33.WHO. Operational guide for assessing the productivity of Aedes aegypti breeding sites. Geneva, Switzerland: World Health Organization, 2011. [Google Scholar]
- 34.Barow I, Megenta M, Megento T. Spatiotemporal analysis of urban expansion using GIS and remote sensing in Jigjiga town of Ethiopia. Appl Geomat 2019; 11: 121–27. [Google Scholar]
- 35.Thompson DK, Mohamoud K, Mahamed JY. Geopolitical boundaries and urban borderlands in an Ethiopian frontier city. Urban Geogr 2023; 44: 301–25. [Google Scholar]
- 36.Dereje AC, Tesfaye G, Bezatu M. Water supply accessibility and associated factors among households of Jigjiga town, eastern Ethiopia. Landscape Architecture and Regional Planning 2020; 5: 1–11. [Google Scholar]
- 37.Tusting LS, Ippolito MM, Willey BA, et al. The evidence for improving housing to reduce malaria: a systematic review and meta-analysis. Malar J 2015; 14: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindsay SW, Davies M, Alabaster G, et al. Recommendations for building out mosquito-transmitted diseases in sub-Saharan Africa: the DELIVER mnemonic. Philos Trans R Soc Lond B Biol Sci 2021; 376: 20190814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahmed A, Pignatelli P, Elaagip A, Abdel Hamid MM, Alrahman OF, Weetman D. Invasive malaria vector Anopheles stephensi mosquitoes in Sudan, 2016–2018. Emerg Infect Dis 2021; 27: 2952–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas S, Ravishankaran S, Justin JA, et al. Overhead tank is the potential breeding habitat of Anopheles stephensi in an urban transmission setting of Chennai, India. Malar J 2016; 15: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kolsky P. Engineers and urban malaria: part of the solution, or part of the problem? Environ Urban 1999; 11: 159–64. [Google Scholar]
- 42.Gandacu D, Glazer Y, Anis E, et al. Resurgence of cutaneous leishmaniasis in Israel, 2001–2012. Emerg Infect Dis 2014; 20: 1605–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singer SR, Abramson N, Shoob H, Zaken O, Zentner G, Stein-Zamir C. Ecoepidemiology of cutaneous leishmaniasis outbreak, Israel. Emerg Infect Dis 2008; 14: 1424–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mala AO, Irungu LW, Shililu JI, et al. Dry season ecology of Anopheles gambiae complex mosquitoes at larval habitats in two traditionally semi-arid villages in Baringo, Kenya. Parasit Vectors 2011; 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.WHO. Prequalified vector control products. 2023. https://extranet.who.int/pqweb/vector-control-products/prequalified-product-list (accessed Oct 11, 2023).
- 46.Teshome A, Erko B, Golassa L, et al. Laboratory-based efficacy evaluation of Bacillus thuringiensis var. israelensis and temephos larvicides against larvae of Anopheles stephensi in Ethiopia. Malar J 2023; 22: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.WHO. WHOPES. Report of the eleventh WHOPES working group meeting: review of Spinosad 7.48% DT, Netprotect, Duranet, Dawaplus, Icon Maxx. Geneva, Switzerland: World Health Organization, 2008. [Google Scholar]
- 48.WHO. Report of the twentieth WHOPES working group meeting: review of Interceptor G2LN, DawaPlus 3.0 LN, DawaPlus 4.0 LN, SumiLarv 2 MR, Chlorfenapyr 240 SC. Geneva, Switzerland: World Health Organization, 2017. [Google Scholar]
- 49.Knepper RG, Leclair AD, Strickler JD, Walker ED. Evaluation of methoprene (Altosid XR) sustained-release briquets for control of culex mosquitoes in urban catch basins. J Am Mosq Control Assoc 1992; 8: 228–30. [PubMed] [Google Scholar]
- 50.WHO. Use of fish for mosquito control. Geneva, Switzerland: World Health Organization, 2003. [Google Scholar]
- 51.Rozendaal J. Vector control: methods for use by individuals and communities. Geneva, Switzerland: World Health Organization, 1997. [Google Scholar]
- 52.Mnzava A, Monroe AC, Okumu F. Anopheles stephensi in Africa requires a more integrated response. Malar J 2022; 21: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Files used for generating figure 2 and figure 3 are shared as supplementary Google Earth kml files. Please contact the corresponding author Gonzalo M Vazquez-Prokopec for data used in the study.


