Abstract
Background
The majority of phase 3 clinical trials are implemented in multiple sites or centres, which inevitably leads to a correlation between observations from the same site or centre. This correlation must be carefully considered in both the design and the statistical analysis to ensure an accurate interpretation of the results and reduce the risk of biased results. This scoping review aims to provide a detailed statistical method used to analyze data collected from multicentre HIV randomized controlled trials in the African region.
Methods
This review followed the methodological framework proposed by Arksey and O’Malley. We searched four databases (PubMed, EBSCOhost, Scopus, and Web of Science) and retrieved 977 articles, 34 of which were included in the review.
Results
Data charting revealed that the most used statistical methods for analysing HIV endpoints in multicentre randomized controlled trials in Africa were standard survival analysis techniques (24 articles [71%]). Approximately 47% of the articles used stratified analysis methods to account for variations across different sites. Out of 34 articles reviewed, only 6 explicitly considered intra-site correlation in the analysis.
Conclusions
Our scoping review provides insights into the statistical methods used to analyse HIV data in multicentre randomized controlled trials in Africa and highlights the need for standardized reporting of statistical methods.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12874-024-02441-w.
Keywords: Multicentre trials, Randomized control trials, Scoping review, HIV/AIDS trials
Background
Randomized controlled trials (RCTs) are considered the gold standard worldwide for evaluating intervention effectiveness [1, 2]. The two commonly used RCT designs are individual randomized controlled trials and cluster (group) randomized controlled trials. In individual RCTs, individuals are randomly assigned to the intervention, whereas in cluster RCTs, clusters or groups (of individuals) are randomized. In this study, we focused exclusively on individual RCTs.
The first RCT in Africa began in 1987 to test the effectiveness of a microbicide gel in preventing human immunodeficiency virus (HIV) infection among women in Nairobi, Kenya [3, 4]. Since then, the methodology has evolved to include multicentre RCTs. A multicentre RCT is a randomized clinical trial design in which individuals are recruited from multiple distinct sites or centres. For instance, the study conducted by [5] is an example of the use of multicentre RCTs to evaluate the safety and efficacy of lopinavir-ritonavir compared to lamivudine for preventing HIV-1 transmission through breastfeeding in infants. This multicentre RCT involved the collaboration of four different sites in 4 African countries.
Similarly, [6] conducted a multicentre RCT investigating the effectiveness of a latex diaphragm and lubricant gel in preventing heterosexual HIV acquisition among women. This study included participants from three different sites in Zimbabwe and South Africa. Both studies highlight the importance of multicentre RCTs to provide robust evidence for HIV prevention and treatment strategies across diverse geographical settings.
Conducting research across multiple centres offers many advantages over single-centre RCTs, such as larger sample sizes for more generalizable findings and the promotion of networking [7]. Multicentre RCTs are essential for understanding the epidemic's diverse dynamics, influenced by the continent's unique social culture and epidemiological settings. These multicentre RCTs have tested a wide range of interventions, from pharmaceutical drugs to behavioural strategies for adherence.
There are numerous advantages to conducting a multicentre RCT. However, including multiple sites is often associated with some form of clustering; that is, individuals are not independent and may be correlated with individuals in the same cluster [8, 9]. Clustering is common in individual RCTs involving multiple sites. The most common example is the natural clustering of participants within a centre in a multicentre RCT [10]. Clustering in an RCT refers to the fact that individuals within the same centre may be more similar to each other than to individuals in other centres, potentially violating the assumption of independence [11]. Almost all individual RCTs assume that the observed outcomes of participants are independent. However, there is a lack of independence among outcomes when there is clustering (such as clustering by centre in a multicentre RCT). Therefore, the use of standard statistical methods may lead to narrower confidence limits and smaller p-values and potentially invalid results. When conducting an analyses of multicentre RCTs, adjustment for the centre is recommended when there are between-centre differences [12]. Although, adjustment for centre is often more complex and can be problematic, especially with numerous centres compared to the total sample [13]. Most common analysis methods often require a large sample size per centre to obtain robust results [14].
Study rationale
Ninety percent of the individual RCTs were observed to have some clustering in the design [10]. Regardless of the high level of clustering in individual RCTs, most studies have emphasized the need for more awareness regarding the issues of clustering present in analysis [10, 15]. Several statistical methods have been developed to address these challenges, but their application and reporting in the context of multicentre RCTs in Africa are poorly understood. Standard statistical methods are not appropriate for the analysis of complex data [16]. Failure to use appropriate statistical methods can lead to underestimation of standard errors and overestimation of results. To our knowledge, this is the first literature review to address the statistical methods applied to multicentre individual RCTs of HIV in the African region. This scoping review aims to provide a detailed overview of the statistical methods used to analyse HIV data collected from multicentre RCTs in the African region and assess whether these methods consider the complexity of the data. The objectives are: i) to identify the statistical methods used and ii) to review these statistical methods.
Methods
This scoping review followed the methodological framework proposed by Arksey and O’Malley [17], which involves five steps: (1) identifying the research question, (2) identifying relevant studies, (3) study selection, (4) charting the data, and (5) collating, summarizing, and reporting the results [17–19].
Eligibility criteria
The inclusion criteria for this review included any study that included the initial search terms in the title and/or abstract. In addition, studies had to have been published in English with no date restrictions. Primary data analysis was required for inclusion to ensure the relevance and reliability of our findings.
On the other hand, studies were excluded if they met any of the following criteria: multicentre RCT studies conducted outside Africa, cluster RCTs, articles written in a language other than English, studies for which full-text articles could not be obtained, grey literature, clinical case studies, review articles, editorials, or perspectives, opinions, and comments.
Search strategy
A comprehensive search was conducted using the following databases: PubMed, EBSCOhost, Scopus, and Web of Science. An experienced research librarian and the primary author developed the search strategy, which was formulated using the search terms listed in Table 1. The PubMed search strategy was adapted to the other databases. The final search strategy used in all the databases is shown in Additional file 1. The search included studies published up until January 2023.
Table 1.
Search terms
| Concept | Search terms |
|---|---|
| Multicentre Randomized controlled trial | "Multicentre randomized controlled trials" or “Multicentre RCT" or "multicenter randomized trials" or "multicenter RCT" or "multi-country randomized controlled trial" or “multicountry randomized controlled trial" or "multisite randomized trial” or “multi-site randomized trial" |
| HIV | "HIV Prevention" or "HIV Treatment" or "HIV" |
| Africa | “Africa” |
Selection criteria
All the search results were retrieved and imported into EndNote reference management software, and duplicate entries were removed [20]. The articles were then uploaded to the review management software Rayyan for the screening process [21]. The first screening of titles and abstracts was conducted according to the inclusion criteria. Studies that did not meet the inclusion criteria were excluded. The full texts of the remaining articles were then assessed for eligibility by two independent reviewers. Any discrepancies were resolved by discussion between the reviewers. Thirty-eight articles were included for full-text screening.
Charting the data
In the scoping review, data charting refers to the process of data extraction, that is, the process of providing the reader with a clear and concise summary of the results from relevant articles included in the study. The study extraction form was used to capture relevant information from the included studies. The data extraction form was developed using Excel and included the following fields: statistical analysis conducted, the aim of the study, title, sample size, and country of study origin. Two reviewers independently charted the data, discussed the results, and updated the data charting form for each eligible article [22].
Results
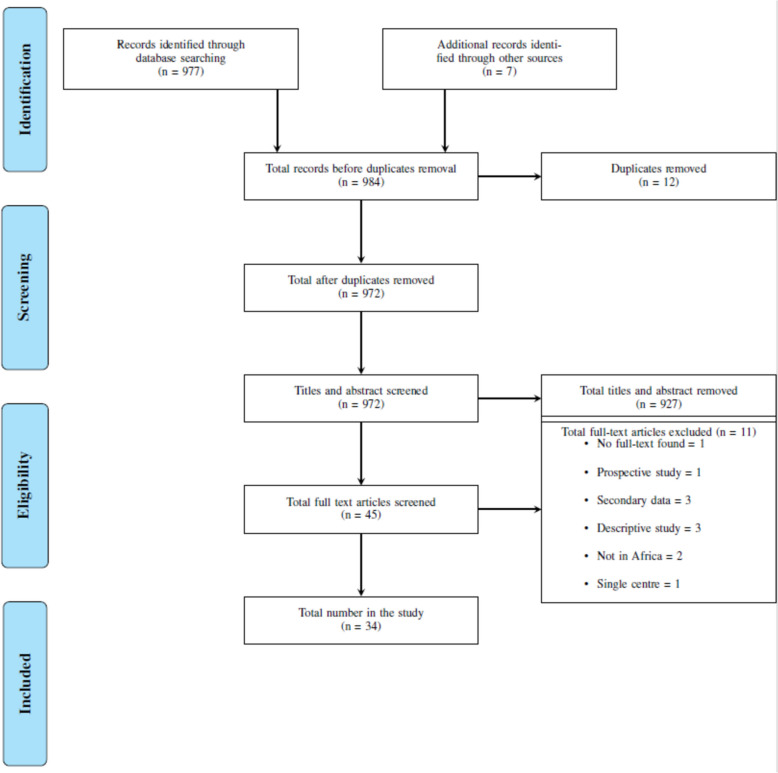
A total of 977 articles were retrieved in the initial search. After removing duplicates, 965 articles remained. Following the title and abstract screening, 38 articles were selected for full-text screening. An additional 7 articles were identified from the reference lists of eligible articles. Ultimately, 34 articles met the inclusion criteria and were included in this scoping review. The review of full-text articles led to 11 articles being excluded. The main reasons for exclusion after full-text review were secondary data analysis (n = 3), descriptive studies (n = 3), studies not conducted in Africa (n = 2), study not available in full text (n = 1), prospective study design (n = 1), and single-centre study (n = 1) (Fig. 1). The details and summaries of each included article are listed in Table 2.
Fig. 1.
Flow diagram describing the selection of relevant studies per PRISMA-ScR
Table 2.
Description of included studies
| Author | Year | Location of data | No. of sites or centres | Sample | Statistical analysis conducted | Clustering by site taken into account |
|---|---|---|---|---|---|---|
| Abrahams, N., et al. [23] | 2010 | South Africa | 2 | 253 | Per-protocol analysis stratified by site, Logistic regression, chi-square | No |
| Baeten,J.M. et al. [24] | 2012 | Uganda and Kenya | 2 | 4758 | Kaplan-Meier analysis, Cox regression, | No |
| Baeten, J. M., et al. [25] | 2016 | Malawi, South Africa, Uganda, and Zimbabwe. | 15 | 2629 | Cox regression stratified according to the site | No |
| Celum, C. et. Al. [26] | 2021 | South Africa and Zimbabwe | 3 | 451 | Logistic and linear regression stratified by site, per-protocol analysis, Kaplan-Meier plot, and complete case analysis | No |
| Chung, M. H., et al. [27] | 2020 | Kenya | 3 | 991 | Sensitivity analysis, Kaplan-Meier curves, the log-rank test, chi-square test, Mann-Whitney U test and logistic regression | No |
| Coovadia, H. M., et al. [28] | 2012 | South Africa, Tanzania, Uganda, and Zimbabwe | 4 | 1527 | Kaplan-Meier method using Greenwood's formula with log-rank test and Pearson χ² test statistic: | No |
| Dabis, F., et al [29] | 1999 | Côte d’Ivoire and Burkina Faso. | 2 | 421 | Student's t-test or Mann-Whitney test, chi-squared test or Fisher's exact test. Kaplan-Meier survival technique and log-rank test. Cox multivariate proportional hazards model. | No |
| de Bruyn, G., et al. [30] | 2011 | Zimbabwe and South Africa | 3 | 2016 | Stratified Cox model, sensitivity analyses | No |
| Delany-Moretlwe, S. et al. [31] | 2018 | South Africa | 9 | 2059 | Kaplan-Meier method, log-rank test stratified by site. Poisson model with study group and site as the main effects. Cox regression adjusted for baseline covariates. Logistic regression generalized estimation equation model, and logistic regression and time-varying Cox regression model stratified by site | Yes |
| Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium [32] | 2019 | Eswatini, Kenya, South Africa, and Zambia. | 12 | 7829 | Cox proportional hazards regression, Kaplan-Meier plots, subgroup analyses | No |
| Halpern, V., et al. [33] | 2008 | Nigeria | 4 | 1644 | Exact log-rank test stratified by site, proportional hazards regression model, and Poisson assumption | No |
| Karim A, et al. [34] | 2011 | South Africa | 2 | 1085 | Poisson distribution, Fisher's exact test, unpaired t-test/Wilcoxon two-sample test and Proportional hazards regression models | No |
| Marrazzo, J. M. et al. [35] | 2015 | South Africa, Uganda, and Zimbabwe. | 15 | 5029 | Cox proportional-hazards models stratified by site (cox regression), Multivariate survival analyses. GEE with a binomial link, exchangeable correlation structure, and robust standard errors | Yes |
| Maskew, M., et al. [36] | 2020 | South Africa | 3 | 601 | Generalized linear models | No |
| Mavedzenge, S. N. et al. [37] | 2010 | Zimbabwe and South Africa | 3 | 4968 | Cox proportional hazard regression | No |
| McCormack, S. et. Al. [38] | 2010 | South Africa, Tanzania, Uganda, and Zambia | 13 | 9385 | Cox proportional hazards regression was stratified by the clinic. Logistic regression. Subgroup analysis: one subgroup analysis was stratified by the research centre, and one was done with post-randomisation | No |
| Mensch, B. S., et al. [39] | 2016 | South Africa, Uganda, and Zimbabwe | 15 | 5029 | Linear and logistic regression models | No |
| Moodley, D., et al. [40] | 2003 | South Africa | 11 | 1317 | Kaplan-Meier analysis, logistic regression, Cox regression, and t-tests and chi-square tests | No |
| Mugo, N. R., et al. [41] | 2014 | Kenya and Uganda | 9 | 1785 | Cox proportional hazards were stratified by study site, GEE logistic regression, linear mixed-effects models, and their interaction as fixed effects and participants as random effects. | Yes |
| Nagot, N., et al. [5] | 2016 | Burkina Faso, South Africa, Uganda, and Zambia. | 4 | 1273 | Turnbull’s extension of the Kaplan-Meier, log-rank test, piecewise model to compare survival curves and Fisher’s exact test | No |
| Nel, A. et. Al. [42] | 2021 | South Africa and Uganda | 6 | 941 | A paired t-test and a mixed model with repeated measures of age, research centre, and centre-by-visit interaction were used. | Yes |
| Nel, A. et. Al. [43] | 2016 | Kenya, Malawi, Tanzania and South Africa | 10 | 280 | Risk ratio | No |
| Nel, A. et. Al. [44] | 2016 | South Africa and Uganda | 7 | 1959 | Two-sided log-rank test stratified according to the research centre and Subgroup analysis | No |
| Odeny, T. A., et al. [45] | 2012 | Kenya | 12 | 1200 | Poisson regression with robust error variance | No |
| Padian, N. S., et al. [6] | 2007 | Zimbabwe and South Africa | 3 | 4948 | subgroup analyses, stratified Cox model, Generalized estimating equation (GEE) logistic regression | Yes |
| Rosen, S., et al. [46] | 2019 | South Africa and Kenya | 6 | 1077 | Linear probability model with robust standard errors and log-linear generalized linear model with robust standard errors | Yes |
| Shapiro, R. L., et al. [47] | 2006 | Botswana | 4 | 709 | Fisher's exact test, Zelen's exact tests, and logistic regression models | No |
| Skoler-Karpoff, S., et al. [48] | 2008 | South Africa | 3 | 6202 | Log-rank test stratified by site, Cox proportional hazards regression, Wilcoxon test | No |
| Taha, T. E., et al. [49] | 2004 | Malawi | 6 | 889 | Exact tests and t-tests, Kaplan-Meier curves, and Logistic regression to adjust comparisons of HIV infection | No |
| The Khesho Bora team [50] | 2011 | Burkina Faso, Kenya and South Africa | 5 | 824 | Student's t-test and χ² test, Kaplan-Meier product-limit estimates, log-rank tests stratified by centre, and logistic regression stratified by centre. | No |
| The Petra study team [51] | 2002 | Uganda, Tanzania and South Africa | 5 | 1457 | Relative risks, analysis stratified by site, Turnbull analysis, Student's t-test, chi-square, and Fisher's exact test. | No |
| Thior, I., et al. [52] | 2006 | Botswana | 4 | 1200 | Fisher exact test (Wilcoxon rank-sum test) for discrete (continuous), Kaplan-Meier estimator, the log-rank test, and Cox proportional hazards modelling. | No |
| Van Damme L., et Al. [53] | 2012 | Kenya, South Africa and Tanzania | 4 | 2021 | Proportional-hazards regression model stratified according to the study site | No |
| Waitt, C. et. Al. [54] | 2019 | South Africa and Uganda | 2 | 60 | Chi-squared test and Wilcoxon rank-sum test, Kaplan-Meier survival curves, and sensitivity analysis | No |
Overview of statistical methods used in the analysis of multicentre HIV RCTs in Africa
The analysis of the 34 articles included in this scoping review revealed a wide range of statistical methods used in the analysis of RCTs focused on HIV research in the African region (Table 3). The sample sizes of these multicentre RCTs varied from less than 100 to nearly 10,000 individuals. Of the total number of articles, six were multicentre RCTs conducted in four countries [5, 25, 28, 32, 38, 43]. South Africa had the highest number of country-specific articles, with 25, followed by Uganda, with 12. The number of sites or centres per RCT ranged from 2 (5 articles) to 15 (3 articles), with most RCTs having 3 sites (7 articles).
Table 3.
Description of the statistical methods of the reviewed articles
| Statistical methods | Frequency (n)b | Number of articles (n) | Percentage of articles (%) | |
|---|---|---|---|---|
| Survival | 24 | 71 | ||
| Log-rank test (unstratified)a | 11 | |||
| Log-rank test (stratified by site)a | 7 | |||
| Cox regression (unstratified) | 7 | |||
| Cox regression (stratified by site) | 9 | |||
| Fixed effects cox regression | 1 | |||
| Regression (not in a survival analysis framework) | 12 | 35 | ||
| Logistic regression (unstratified) | 9 | |||
| Logistic regression (stratified by site) | 1 | |||
| Fixed effects logistics regression | 2 | |||
| Poisson regression | 5 | |||
| Linear regression | 1 | |||
| Basic hypothesis tests and measures of effect | 17 | 50 | ||
| Chi-squared test | 10 | |||
| Fisher test | 6 | |||
| t-test | 7 | |||
| Mann Whitney | 1 | |||
| Wilcoxon rank-sum test | 4 | |||
| Hierarchical models | 6 | 18 | ||
| GEE logistic regression [6, 31, 35, 41] | 4 | |||
| Nonlinear GLM [45] | 1 | |||
| LMM [41, 43] | 2 | |||
aInclude studies that utilized Kaplan-Meier curves
bTotal frequency each statistical method was applied across all included studies (some articles/studies used multiple methods)
The most used statistical approach was survival analysis (24 articles [71%]). Survival analysis techniques, specifically Kaplan-Meier curves and the log-rank test, were used extensively in 18 articles [5, 27–30, 32, 38, 49, 54]. These methods allow evaluation of time-to-event outcomes such as time to HIV infection or disease progression. Kaplan-Meier curves provide a graphical representation of survival probability over time, while the log-rank test compares survival curves between different groups. These methods are essential for evaluating the effectiveness of interventions and estimating survival probabilities in HIV research. Notably, 7 articles used the log-rank test stratified by site, reflecting the frequent use of stratification to account for potential effect heterogeneity in survival analyses. Seventeen articles [29, 33, 35, 38, 41, 48, 52] used Cox proportional hazard regression, a time-to-event analysis method that accounts for censoring. This method allows the estimation of hazard ratios, which measure the relative risk of an event occurring over time. Cox regression is particularly important for assessing the impact of various factors on HIV-related outcomes, such as disease progression or mortality. Among the 17 articles, nine articles specifically used stratified Cox regression, emphasizing the importance of accounting for site-level variability. Additionally, one article used a fixed-effects Cox regression approach. In [35], Cox proportional hazards models stratified according to the site were used to assess the time to HIV-1 seroconversion. Two articles used multivariate survival analyses [35, 52], which involved the simultaneous consideration of multiple predictors when analysing survival outcomes.
A total of 17 articles reported fundamental statistical analyses such as t-tests, chi-square tests, and Fisher’s tests, with 5 articles having sample sizes greater than 1000.
Logistic, linear and poison regression analysis was the third most used type of analysis (12 articles). Logistic regression appeared as a commonly used method in 10 articles [6, 23, 26, 27, 38, 47]. This method allows the examination of associations between predictor variables and binary outcomes, such as the presence or absence of HIV infection. Logistic regression models can provide estimates of odds ratios that quantify the strength of associations between predictors and outcomes. Additionally, fixed-effects logistic regression was used in 2 articles. Fixed-effects models, though less frequently used, provide an alternative approach to control for site-level effects.
In addition, several studies used stratified analysis, a technique to examine heterogeneity between subgroups [24, 38, 50–52]. Notably, about 47% of these studies employed this approach to account for variations across different sites. This approach allows for separate analyses within each study site, allowing researchers to assess whether the treatment effect or association between variables differs across strata. The stratified analysis provides valuable insights into subgroup-specific effects in the context of HIV research in the African region.
While most studies did not perform statistical analyses that accounted for the intra-site correlation, a subset of articles used more advanced techniques. These included generalized estimating equation (GEE) logistic regression, linear mixed-effects models (LMMs), Poisson regression with robust error variance, and log-linear generalized linear model with robust standard errors [6, 31, 35, 41, 42, 46]. GEE logistic regression accounts for correlations within clusters when analysing correlated data, such as repeated measures within individuals or clustering of participants within study sites. Poisson regression with large error variance is helpful for analysing count outcomes such as the number of HIV infections or events. On the other hand, LMMs are statistical models that extend linear regression to handle correlated or clustered data with hierarchical structures. They incorporate fixed effects, representing population-level relationships, and random effects, capturing variability at different levels of the hierarchy. LMMs are particularly useful when dealing with nested data, such as individuals within groups. They provide estimates at multiple levels, making them ideal for modelling complex data structures. Additionally, they can incorporate both fixed and random effects, which further increases their flexibility and usefulness. LMMs are a powerful tool for analysing data with hierarchical structures, especially when traditional linear regression models are inappropriate. These advanced methods allowed for confounding factors and site-specific variation.
Approximately 47% of the studies employed stratified analysis approaches to account for variations across different sites. Out of the 34 studies examined, only 6 explicitly considered intra-site correlation in their analyses. Although the point estimates may remain largely unaffected, neglecting to account for clustering can lead to inaccurate variance, potentially leading to misleading p-values and confidence intervals.
Discussion
The search yielded 984 articles, of which 972 remained after duplicates were removed. After screening the titles, abstracts, and full texts, 34 articles were included in the review. Survival analysis approaches remain the most popular method used. Survival analysis techniques are well-equipped to accommodate clustering through extensions to frailty models [55]. Furthermore, Kaplan-Meier curves can also be computed, taking the site into account via a stratified approach [56]. Only one article utilized a fixed effects Cox regression approach to account for clustering, whereas half of the articles did not consider clustering in their survival analyses.
Although several statistical methods are well developed to accommodate clustering, we found that most studies have ignored the clustering in the data. Depending on the degree of clustering present in the data, failure to account for clustering can substantially affect the accuracy of variance estimates. This may inflate Type I error or produce overly narrow confidence intervals, ultimately leading to erroneous conclusions about statistical significance. Such errors in inference can have significant implications for the validity and generalizability of research findings. If the site or centre is not used as a stratification factor, both adjusted and unadjusted analyses provided unbiased p-values and confidence intervals [13].
Giganti et al. [57] reinforces this assertion by indicating that the method chosen to account for clustering - be it stratified Cox regression, fixed effects or random effects - may have limited impact on hazard ratio estimates under low heterogeneity, with stratified and meta-analyses recommended for their adaptability and ability to deal with non-proportional hazards [58]. Alternatively, Kahan and Morris [12] have shown that clustering cannot be overlooked if there is intraclass correlation (ICC) and treatment correlation within sites, which is often the case in multicentre trials [12]. Ignoring such clustering leads to increased Type I error rates, emphasizing the need to explicitly consider this in trial design and analysis. Similarly, Kahan [13] found that GEE and random-effects models perform better than fixed-effects models in large multicentre studies with binary outcomes, especially when standard errors are not robustly estimated [13].
In the past, the methods used to handle clustered data were not as well developed or widely understood as those used for independent data. As a result, the most straightforward approach has been adopted in many studies that produce clustered data. This has been to ignore the clustering and treat all observations as if they were independent [59]. This trend was evident in this review, where most articles relied on basic statistical methods, such as t-tests, chi-square tests, and unstratified regression models, even though the data were clustered.
The African continent, particularly in the countries of Eastern and Southern Africa within sub-Saharan Africa, bears the heaviest burden of the HIV epidemic. As of 2023, 26 million people are living with HIV in the region, which represents more than two-thirds of the worldwide total and accounts for 50% of all new HIV infections globally. This is noteworthy considering that sub-Saharan Africa comprises only about 11% of the Earth's population. Despite this high prevalence, the region has seen significantly fewer HIV RCTs compared to Europe and North America. As a result, it is crucial to conduct thorough analyses of the limited clinical trials available to ensure that the findings are robust and persuasive [3, 59].
The lack of awareness and understanding of appropriate statistical methods for analyzing complex data in the context of multicentre RCTs could have significant implications for the validity and generalizability of research findings. Incorrect inferences may lead to an investigational product erroneously being concluded as effective or ineffective, leading to incorrect policy decisions. This issue is particularly concerning in the context of HIV research, where decisions derived from these analyses can significantly influence public health interventions.
To mitigate these shortcomings, it is crucial to adopt advanced methods such as GEE, LMMs, or stratified analyses. While these techniques are often underutilized, they are well-suited to address the complexities of correlated or clustered data, as demonstrated in this review and supported by other methodological studies. Currently, there is a lack of formal guidance on the optimal approach for different outcomes, as existing literature generally focuses on each outcome separately under specific conditions. Further research is necessary, including comprehensive simulation studies, to deepen our understanding of the performance of these methods. Thus, it is crucial to identify and review the statistical methods commonly used in this setting to ensure that researchers are using the appropriate tools to analyse the data accurately and make well-informed decisions.
Limitations
A notable limitation of this study is the challenge of identifying relevant multicentre RCTs within the search strategy. Although the aim was to include all relevant studies, some multicentre RCTs did not explicitly specify their multicentre nature in the title or abstract. Therefore, it is possible that relevant studies may have been missed during the initial screening process. However, despite these limitations, this study provides valuable insights into the statistical methods used in HIV research in the African region.
Conclusion
This scoping review revealed that various statistical methods are used to analyse multicentre RCTs that focus on HIV treatment and prevention in the African region. Most of these studies did not account for the study site/centre in the analysis. Neglecting clustering in studies can compromise the validity and generalizability of findings, posing significant challenges for public health decision-making in HIV research. This issue is particularly pressing in the African context, where research outcomes directly inform critical interventions and the allocation of resources. Researchers are encouraged to employ advanced statistical techniques that account for site-level variability to ensure accurate and reliable conclusions.
To enhance the quality of HIV research, it is essential to increase awareness and promote the adoption of these statistical methods in RCTs. By addressing these methodological gaps, future studies can make more meaningful contributions to evidence-based practices and the ongoing fight against HIV in Africa.
Supplementary Information
Acknowledgements
The authors express appreciation to Dr Natasha Langdown for her contribution to developing the search strategy and database searches.
Abbreviations
- AIDS
Acquired immunodeficiency syndrome
- GEE
Generalized estimating equation
- HIV
Human immunodeficiency virus
- LMMs
Linear mixed-effects models
- PRISMA-ScR
Preferred Reporting Items for Systematic reviews and Meta-Analysis flow diagram
- RCT
Randomised controlled trial
- SAMRC
South Africa Medical Research Council
Authors’ contributions
TR and MM conceived the idea for this review. MM conducted the search, full-text assessment, data extraction, analysis, and manuscript writing. NG conducted the full-text assessment, data charting, and editing. TR contributed to interpreting and reviewing selected articles, editing the manuscript, and providing critical insight. SM contributed to the editing and providing critical insight. All authors have read and approved the manuscript.
Funding
As part of the PhD degree, this study was funded by the South African Medical Research Council.
Data availability
The data used during the current study is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125(13):1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zabor EC, Kaizer AM, Hobbs BP. Randomized Controlled Trials. Chest. 2020;158(1s):S79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siegfried N, Clarke M, Volmink J. Randomised controlled trials in Africa of HIV and AIDS: descriptive study and spatial distribution. BMJ (Clinical Res ed). 2005;331(7519):742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, Ruminjo I, Sajabi R, Kimata J, Fleming TR, et al. Efficacy of nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA. 1992;268(4):477–82. [PubMed] [Google Scholar]
- 5.Nagot N, Kankasa C, Tumwine JK, Meda N, Hofmeyr GJ, Vallo R, Mwiya M, Kwagala M, Traore H, Sunday A, et al. Extended pre-exposure prophylaxis with lopinavir-ritonavir versus lamivudine to prevent HIV-1 transmission through breastfeeding up to 50 weeks in infants in Africa (ANRS 12174): a randomised controlled trial. Lancet (London England). 2016;387(10018):566–73. [DOI] [PubMed] [Google Scholar]
- 6.Padian NS, van der Straten A, Ramjee G, Chipato T, de Bruyn G, Blanchard K, Shiboski S, Montgomery ET, Fancher H, Cheng H, et al. Diaphragm and lubricant gel for prevention of HIV acquisition in southern African women: a randomised controlled trial. Lancet (London England). 2007;370(9583):251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng A, Kessler D, Mackinnon R, Chang TP, Nadkarni VM, Hunt EA, Duval-Arnould J, Lin Y, Pusic M, Auerbach M. Conducting multicenter research in healthcare simulation: Lessons learned from the INSPIRE network. Adv Simul. 2017;2(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stuart B, Becque T, Moore M, Little P. Clustering of continuous and binary outcomes at the general practice level in individually randomised studies in primary care - a review of 10 years of primary care trials. BMC Med Res Methodol. 2020;20(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng A, Kessler D, Mackinnon R, Chang TP, Nadkarni VM, Hunt EA, Duval-Arnould J, Lin Y, Pusic M, Auerbach M. Conducting multicenter research in healthcare simulation: Lessons learned from the INSPIRE network. Adv Simul (Lond). 2017;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee KJ, Thompson SG. Clustering by health professional in individually randomised trials. BMJ (Clinical Res ed). 2005;330(7483):142–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oltean H, Gagnier JJ. Use of clustering analysis in randomized controlled trials in orthopaedic surgery. BMC Med Res Methodol. 2015;15(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kahan BC, Morris TP. Analysis of multicentre trials with continuous outcomes: when and how should we account for centre effects? Stat Med. 2013;32(7):1136–49. [DOI] [PubMed] [Google Scholar]
- 13.Kahan BC. Accounting for centre-effects in multicentre trials with a binary outcome – when, why, and how? BMC Med Res Methodol. 2014;14(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahan BC, Harhay MO. Many multicenter trials had few events per center, requiring analysis via random-effects models or GEEs. J Clin Epidemiol. 2015;68(12):1504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahan BC, Morris TP. Assessing potential sources of clustering in individually randomised trials. BMC Med Res Methodol. 2013;13(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gould AL. Multi-centre trial analysis revisited. Stat Med. 1998;17(15–16):1779–97. discussion 1799–1800. [DOI] [PubMed] [Google Scholar]
- 17.Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8(1):19–32. [Google Scholar]
- 18.Peters MDJ, Godfrey C, McInerney P, Khalil H, Larsen P, Marnie C, Pollock D, Tricco AC, Munn Z. Best practice guidance and reporting items for the development of scoping review protocols. JBI Evid Synth. 2022;20(4):953–68. [DOI] [PubMed] [Google Scholar]
- 19.Peters MDJ, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, McInerney P, Godfrey CM, Khalil H. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth. 2020;18(10):2119–26. [DOI] [PubMed] [Google Scholar]
- 20.The EndNote Team. EndNote. In., EndNote 20 edn. Philadelphia, PA: Clarivate; 2013. [Google Scholar]
- 21.Ouzzani Mourad and Hammady HaF. Zbys and Elmagarmid, Ahmed: Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016. [DOI] [PMC free article] [PubMed]
- 22.Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467–73. [DOI] [PubMed] [Google Scholar]
- 23.Abrahams N, Jewkes R, Lombard C, Mathews S, Campbell J, Meel B. Impact of telephonic psycho-social support on adherence to post-exposure prophylaxis (PEP) after rape. AIDS Care. 2010;22(10):1173–81. [DOI] [PubMed] [Google Scholar]
- 24.Baeten JM, Donnell D, Ndase P, Mugo NR, Campbell JD, Wangisi J, Tappero JW, Bukusi EA, Cohen CR, Katabira E, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, Mgodi NM, Matovu Kiweewa F, Nair G, Mhlanga F, et al. Use of a Vaginal Ring Containing Dapivirine for HIV-1 Prevention in Women. N Engl J Med. 2016;375(22):2121–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye BJ, Pathak S, Mgodi N, Bekker LG, Donnell DJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: Results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung MH, McGrath CJ, Beck IA, Levine M, Milne RS, So I, Andersen N, Dross S, Coombs RW, Chohan B, et al. Evaluation of the management of pretreatment HIV drug resistance by oligonucleotide ligation assay: a randomised controlled trial. lancet HIV. 2020;7(2):e104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coovadia HM, Brown ER, Fowler MG, Chipato T, Moodley D, Manji K, Musoke P, Stranix-Chib aL, Chetty V, et al. Efficacy and safety of an extended nevirapine regimen in infant children of breastfeeding mothers with HIV-1 infection for prevention of postnatal HIV-1 transmission (HPTN 046): a randomised, double-blind, placebo-controlled trial. Lancet (London England). 2012;379(9812):221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dabis F, Msellati P, Meda N, Welffens-Ekra C, You B, Manigart O, Leroy V, Simonon A, Cartoux M, Combe P, et al. 6-month efficacy, tolerance, and acceptability of a short regimen of oral zidovudine to reduce vertical transmission of HIV in breastfed children in Côte d'Ivoire and Burkina Faso: a double-blind placebo-controlled multicentre trial. DITRAME Study Group. DIminution de la Transmission Mère-Enfant. Lancet (London England). 1999;353(9155):786–92. [DOI] [PubMed] [Google Scholar]
- 30.de Bruyn G, Shiboski S, van der Straten A, Blanchard K, Chipato T, Ramjee G, Montgomery E, Padian N. The effect of the vaginal diaphragm and lubricant gel on acquisition of HSV-2. Sex Transm Infect. 2011;87(4):301–5. [DOI] [PubMed] [Google Scholar]
- 31.Delany-Moretlwe S, Lombard C, Baron D, Bekker LG, Nkala B, Ahmed K, Sebe M, Brumskine W, Nchabeleng M, Palanee-Philips T, et al. Tenofovir 1% vaginal gel for prevention of HIV-1 infection in women in South Africa (FACTS-001): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Infect Dis. 2018;18(11):1241–50. [DOI] [PubMed] [Google Scholar]
- 32.HIV incidence among. women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: a randomised, multicentre, open-label trial. Lancet (London England). 2019;394(10195):303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halpern V, Ogunsola F, Obunge O, Wang CH, Onyejepu N, Oduyebo O, Taylor D, McNeil L, Mehta N, Umo-Otong J, et al. Effectiveness of cellulose sulfate vaginal gel for the prevention of HIV infection: results of a Phase III trial in Nigeria. PLoS ONE. 2008;3(11):e3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science (New York, NY). 2010;329(5996):1168–74. 10.1126/science.1193748. [DOI] [PMC free article] [PubMed]
- 35.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, Palanee T, Nakabiito C, van der Straten A, Noguchi L, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015;372(6):509–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maskew M, Brennan AT, Fox MP, Vezi L, Venter WDF, Ehrenkranz P, Rosen S. A clinical algorithm for same-day HIV treatment initiation in settings with high TB symptom prevalence in South Africa: The SLATE II individually randomized clinical trial. PLoS Med. 2020;17(8):e1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mavedzenge SN, Pol BV, Cheng H, Montgomery ET, Blanchard K, de Bruyn G, Ramjee G, Straten A. Epidemiological synergy of Trichomonas vaginalis and HIV in Zimbabwean and South African women. Sex Transm Dis. 2010;37(7):460–6. [DOI] [PubMed] [Google Scholar]
- 38.McCormack S, Ramjee G, Kamali A, Rees H, Crook AM, Gafos M, Jentsch U, Pool R, Chisembele M, Kapiga S, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet (London England). 2010;376(9749):1329–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mensch BS, Brown ER, Liu K, Marrazzo J, Chirenje ZM, Gomez K, Piper J, Patterson K, van der Straten A. Reporting of Adherence in the VOICE Trial: Did Disclosure of Product Nonuse Increase at the Termination Visit? AIDS Behav. 2016;20(11):2654–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moodley D, Moodley J, Coovadia H, Gray G, McIntyre J, Hofmyer J, Nikodem C, Hall D, Gigliotti M, Robinson P, et al. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187(5):725–35. [DOI] [PubMed]
- 41.Mugo NR, Hong T, Celum C, Donnell D, Bukusi EA, John-Stewart G, Wangisi J, Were E, Heffron R, Matthews LT, et al. Pregnancy incidence and outcomes among women receiving preexposure prophylaxis for HIV prevention: a randomized clinical trial. JAMA. 2014;312(4):362–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nel A, van Niekerk N, Van Baelen B, Malherbe M, Mans W, Carter A, Steytler J, van der Ryst E, Craig C, Louw C, et al. Safety, adherence, and HIV-1 seroconversion among women using the dapivirine vaginal ring (DREAM): an open-label, extension study. lancet HIV. 2021;8(2):e77–86. [DOI] [PubMed] [Google Scholar]
- 43.Nel A, Bekker LG, Bukusi E, Hellstrӧm E, Kotze P, Louw C, Martinson F, Masenga G, Montgomery E, Ndaba N, et al. Safety, Acceptability and Adherence of Dapivirine Vaginal Ring in a Microbicide Clinical Trial Conducted in Multiple Countries in Sub-Saharan Africa. PLoS ONE. 2016;11(3):e0147743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, Kamali A, Kotze P, Louw C, Mabude Z, et al. Safety and Efficacy of a Dapivirine Vaginal Ring for HIV Prevention in Women. N Engl J Med. 2016;375(22):2133–43. [DOI] [PubMed] [Google Scholar]
- 45.Odeny TA, Bailey RC, Bukusi EA, Simoni JM, Tapia KA, Yuhas K, Holmes KK, McClell RS. Text messaging to improve attendance at post-operative clinic visits after adult male circumcision for HIV prevention: a randomized controlled trial. PLoS ONE. 2012;7(9):e43832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen S, Maskew M, Larson BA, Brennan AT, Tsikhutsu I, Fox MP, Vezi L, Bii M, Venter WDF. Simplified clinical algorithm for identifying patients eligible for same-day HIV treatment initiation (SLATE): Results from an individually randomized trial in South Africa and Kenya. PLoS Med. 2019;16(9):e1002912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapiro RL, Thior I, Gilbert PB, Lockman S, Wester C, Smeaton LM, Stevens L, Heymann SJ, Ndung'u T, Gaseitsiwe S, et al. Maternal single-dose nevirapine versus placebo as part of an antiretroviral strategy to prevent mother-to-child HIV transmission in Botswana. AIDS. 2006;20(9):1281–8. [DOI] [PubMed] [Google Scholar]
- 48.Skoler-Karpoff S, Ramjee G, Ahmed K, Altini L, Plagianos MG, Friedl B, Govender S, De Kock A, Cassim N, et al. Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial. Lancet (London England). 2008;372(9654):1977–87. [DOI] [PubMed] [Google Scholar]
- 49.Taha TE, Kumwenda NI, Hoover DR, Fiscus SA, Kafulafula G, Nkhoma C, Nour S, Chen S, Liomba G, Miotti PG, et al. Nevirapine and zidovudine at birth to reduce perinatal transmission of HIV in an African setting: a randomized controlled trial. JAMA. 2004;292(2):202–9. [DOI] [PubMed] [Google Scholar]
- 50.Kesho Bora Study G, de Vincenzi I. Triple antiretroviral compared with zidovudine and single-dose nevirapine prophylaxis during pregnancy and breastfeeding for prevention of mother-to-child transmission of HIV-1 (Kesho Bora study): a randomised controlled trial. Lancet Infect Dis. 2011;11(3):171–80. [DOI] [PubMed] [Google Scholar]
- 51.Efficacy of three short-. course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet (London England). 2002;359(9313):1178–86. [DOI] [PubMed] [Google Scholar]
- 52.Thior I, Lockman S, Smeaton LM, Shapiro RL, Wester C, Heymann SJ, Gilbert PB, Stevens L, Peter T, Kim S, et al. Breastfeeding plus infant zidovudine prophylaxis for 6 months vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi Study. JAMA. 2006;296(7):794–805. [DOI] [PubMed] [Google Scholar]
- 53.Van Damme L, Corneli A, Ahmed K, Agot K, Lombaard J, Kapiga S, Malahleha M, Owino F, Manongi R, Onyango J, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012;367(5):411–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Waitt C, Orrell C, Walimbwa S, Singh Y, Kintu K, Simmons B, Kaboggoza J, Sihlangu M, Coombs JA, Malaba T, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study). PLoS Med. 2019;16(9):e1002895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ha ID, Sylvester R, Legrand C, Mackenzie G. Frailty modelling for survival data from multi-centre clinical trials. Stat Med. 2011;30(17):2144–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.David GK, Mitchel K. Survival Analysis A Self-Learning Text. New York NY: Springer New York; 2005.
- 57.Giganti MJ, Luz PM, Caro-Vega Y, Cesar C, Padgett D, Koenig S, Echevarria J, McGowan CC, Shepherd BE. A Comparison of Seven Cox Regression-Based Models to Account for Heterogeneity Across Multiple HIV Treatment Cohorts in Latin America and the Caribbean. AIDS Res Hum Retroviruses. 2015;31(5):496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sally G, James AD, Bryce V. A Study of Clustered Data and Approaches to Its Analysis. J Neurosci. 2010;30(32):10601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zani B, Pienaar ED, Oliver J, Siegfried N. Randomized controlled trials of HIV/AIDS prevention and treatment in Africa: results from the Cochrane HIV/AIDS Specialized Register. PLoS ONE. 2011;6(12):e28759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used during the current study is available from the corresponding author on reasonable request.