Abstract
Therapeutic human papillomavirus (HPV) DNA vaccine is an attractive option to control existed HPV infection and related lesions. The two early viral oncoproteins, E6 and E7, are continuously expressed in most HPV-related pre- and cancerous cells, and are ideal targets for therapeutic vaccines. We have previously developed an HPV 16 DNA vaccine encoding a modified E7/HSP70 (mE7/HSP70) fusion protein, which demonstrated significant antitumor effects in murine models. In this study, we employed multifaceted approach to enhance the potency of the HPV16 DNA vaccine. Strategies including inserting CpG oligodeoxynucleotide (CpG ODNs) into the vaccine vector backbone, selecting cytokine gene adjuvants, combining plasmids encoding mE6/HSP70 and mE7/HSP70, and utilizing electroporation for vaccination. Our findings revealed that mice immunized with CpG-modified vaccines, coupled with an IL-28B gene adjuvant exhibited heightened antigen-specific CD8+ T cell responses. Additionally, the combination of mE6/HSP70 and mE7/HSP70 plasmids synergistically enhanced the specific CD8+ T cell response. Furthermore, vaccination with CpG-modified mE7/HSP70 and mE6/HSP70 plasmids, alongside the Interleukin-28B (IL-28B) gene adjuvant, generated substantial preventive and therapeutic antitumor effects against HPV E6- and E7-expressing tumors in C57BL/6 mice. These results suggested that integrating these multiple strategies into an HPV DNA vaccine holds promise for effectively controlling HPV infection and related diseases.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12985-024-02604-7.
Keywords: CpG oligodeoxynucleotide, IL-28B gene adjuvant, E7, E6, HSP70, HPV DNA vaccine
Introduction
Carcinogenic human papillomaviruses (HPVs) are associated with anogenital and oropharyngeal malignancies [1]. Among these, HPV16 is the predominant type causing invasive cervical cancer globally. Although the advent of prophylactic HPV vaccines, such as Gardasil and Cervarix, have proven effective in preventing HPV infections, they offer no remedy for pre-existing HPV infections and associated lesions [2]. Presently, the management of high-grade precancerous conditions and early-stage cancers associated with HPV relies heavily on surgical intervention, which is fraught with complications. In cases of advanced cancers, conventional treatments like surgery, radiotherapy, and chemotherapy yield disappointing results [3]. Consequently, the development of therapeutic HPV vaccines emerge as a promising strategy to curtail the progression of cervical and related diseases.
The viral oncoproteins HPV E6 and E7 are constitutively expressed in HPV-infected, pre-malignant and malignant cells, and are ideal tumor specific antigens [4]. Recently several HPV therapeutic DNA vaccines targeting E6 and E7 delivered by electroporation could induce complete regression of high-grade cervical lesions and viral clearance in cervical intraepithelial neoplasia 2 and 3 (CIN2/3) patients [5–7]. These clinical trials indicated that the magnitude of systemic polyfunctional CD8+ T cell response is the main contributing factor for histopathological regression and cleared HPV infection [5, 7]. However, although the HPV E6/E7 DNA vaccines have shown positive therapeutic effects in clinical trials for precancerous lesions, its clinical efficacy in cancer patients is still not significant. For example, one study of HPV DNA vaccine VGX-3100 with IL-12 adjuvant was conducted in HPV-associated head and neck cancer which also induced antigen-specific CD8+ T cell response and increased CD8+ T cell infiltration in tumors, but the clinical regression of tumors was not shown [8]. Another clinical trial of GX-188E therapeutic DNA vaccine plus pembrolizumab was conducted in patients with recurrent or advanced cervical cancer showed preliminary antitumor activity, while only 4 of 26 patients (15%) had a complete response [9]. These findings highlight the imperative need to enhance the potency of CD8+ T cell immune responses induced by DNA vaccines, a key focus in the ongoing quest for effective HPV therapeutic vaccines.
In our previous studies, we discovered that a HPV DNA vaccine encoding a fusion of modified E7 and a human heat shock protein 70 (mE7/HSP70) successfully elicited E7-specific CD8+ T cell responses [10]. Building upon this foundation, we embarked on a multifaceted strategy to amplify the potency of the CD8+ T cell response generated by the HPV16 DNA vaccine. Specifically, we constructed plasmids expressing mE7/HSP70 and mE6/HSP70, integrated CpG ODN motifs into the plasmid backbone to enhance immunogenicity, selected cytokine genes as adjuvants, and administered the vaccines via electroporation in mice. Subsequent evaluations focused on assessing the magnitude of antigen-specific cytotoxic T cell responses and the anti-tumor effect in vivo, aiming to determine if this comprehensive strategy could indeed potentiate the effectiveness of HPV DNA vaccine.
Materials and methods
CpG-modified DNA plasmids construction
The optimized CpG ODN sequence (5’-TCGTCGTTTTGTCGTTTTGTCGTT-3’) was synthesized by Sangon Biotech (Shanghai, China). To investigate the “built-in” adjuvant activity of CpG motifs within the DNA plasmid pVAX1EI backbone, a construct featuring 20 tandem repeats of the CpG sequence was synthesized and inserted into the pVAX1EI vector via the PmaC I restriction site. This manipulation, carried out by Sangon Biotech, resulted in the CpG-modified immunization vector pmVAX1EI.
The mE7/HSP70 fusion antigen gene was previously engineered by our group, distinguished by its enhanced T-cell epitope presentation and the elimination of transforming activity. This advanced antigen was derived from the wild-type E7 gene through a meticulous process involving gene shuffling, site-directed mutagenesis and codon optimization [10, 11]. To prepare for further experimentation, the mE7/HSP70 fusion gene was amplified by polymerase chain reaction (PCR) using pVR1012-mE7 /HSP70 (conserved by our group) as the template. The PCR amplification was carried out with the following primers: forward 5’-CGGGATCCGCCACCATGGATCTGCTCAT-3’ and reverse 5’-CCGCTCGACTAATCTACCTCCTCAATGGTGGG-3’. Following successful amplification, the PCR product was cloned into BamH I/Xho I restriction enzyme-digested vectors pmVAX1EI and pVAX1EI, thereby generating the recombinant plasmids: pmVAX1EI-mE7/HSP70 and pVAX1EI-mE7/HSP70.
The wild-type E6 protein was strategically dissected at amino acid position 70 to disrupt sequences essential for transformation. Subsequently, two segments of HLA-I epitopes, specifically E6aa29-38 [12, 13] and E6aa49-67 [12] were integrated into these cleaved regions. The artificial gene was then codon-optimized for the human system and synthesized by Sangon Biotech, designated as mE6. A key feature of the mE6 design was its augmented presentation of T-cell epitopes, coupled with the abrogation of its transforming capabilities. To create the mE6/HSP70 fusion gene, the mE7 component within our established plasmid pVR1012-mE7/HSP70 was substituted with mE6, this novel fusion gene was then cloned into the BamH I/Xho I-digested vectors pmVA1XEI and pVAX1EI, yielding the recombinant constructs pmVAX1EI-mE6/HSP70 and pVAX1EI-mE6/HSP70.
The human IL-28B and IL-15 genes were subjected to codon optimization and synthesized by Sangon Biotech, these optimized genes were subsequently ligated into BamH I/Xba I restriction sites of both pmVAX1EI and pVAX1EI vectors. This process yielded for recombinant plasmids: pmVAX1EI-IL-28B, pmVAX1EI-IL-15, pVAX1EI-IL-28B and pVAX1EI-IL-15.
All DNA constructs were validated through restriction enzyme digestion and subsequent DNA sequencing.
SDS-PAGE and western blotting
293T cells were respectively transfected with pmVAX1EI, pVAX1EI, pmVAX1EI-mE6/HSP70, pmVAX1EI-mE7/HSP70, pmVAX1EI-IL28B, pmVAX1EI-IL15, pVAX1EI-mE6/HSP70, pVAX1EI-mE7/HSP70, pVAX1EI-IL28B, or pVAX1EI-IL15 plasmids. 48 h post transfection, cell lysates were prepared. 50 µg of protein samples were mixed with the loading buffer containing 2% SDS and 5% β-mercaptoethanol, then denatured at 95 °C for 8 min. These denatured proteins were loaded onto 10% SDS-PAGE gels for separating and subsequently transferred onto polyvinylidene difluoride membranes (PALL Life Sciences). The membranes were blocked with 0.05% Tween-80/PBS containing 5% non-fat milk at room temperature for 1–2 h and incubated with anti-HPV16 E6 polyclonal antibody (1:3000), anti-HPV16 E7 polyclonal antibody (1:3000), anti-IL-28B polyclonal antibody (1:1000, Abcam, Cat#ab181411), or anti-IL15 polyclonal antibody (1:1000, Abcam, Cat#ab109082) at 4 °C overnight. Finally, horseradish peroxidase-conjugated secondary antibody (1:5000, CWBio, Cat#CW0102S) was added and incubated at room temperature for 1–2 h. The protein bands were visualized using chemiluminescence with the EasySee Western Bolt Kit (TransGen Biotech, Cat#DW101-02). The anti-HPV16 E6 polyclonal antibody and anti-HPV16 E7 polyclonal antibody were prepared in our laboratory.
Animals
Six- to eight-week-old female C57BL/6 mice were purchased from SPF (Beijing) Biotechnology Co.,Ltd. (License No. SCXK 2019-0004, Beijing, China). All animals were housed under pathogen-free conditions at the animal facility of the Institute of Basic Medical Sciences (IBMS), CAMS. All experimental procedures adhered strictly to the guidelines set forth by the Institutional Animal Care and Use Committee (IACUC) of IBMS, CAMS, with all protocols having been thoroughly reviewed and approved by IACUC (Approval Number: HBNU20231222116). At the end of study, all mice were humanely sacrificed by CO2 euthanasia.
Immunization of mice
DNA plasmids intended for injection were prepared using Plasmid Maxprep Kits from Vigorous Biotechnology (Beijing China, Cat#N001), resuspended in endotoxin-free TE buffer at a final concentration of 1 µg/µl. The plasmids were stored at -20˚C until they were used for injection. The quadriceps muscles of C57BL/6 mice were injected 2 times, 10 days apart followed by electroporation using ECM830 electroporation system (BTX, America). Each plasmid was used as 15 µg in 15 µl buffer. To consider the effect of DNA dose in the experiment, the empty vector was used to supplement the inoculation component in each immunization group, thus the total plasmid dose was consistent in each group. For example, when comparing mE7/HSP70 and mE7/HSP70 + IL-28B, the mE7/HSP70 group were immunized with 15 µg pVAX1EI-mE7/HSP70 plasmid and 15 µg pVAX1EI empty plasmid, while the mE7/HSP70 + IL-28B group were immunized with 15 µg pVAX1EI-mE7/HSP70 plasmid and 15 µg pVAX1EI-IL-28B plasmid.
Splenocyte purification
Mice were sacrificed 1 week after the final immunization. The spleens were isolated and placed onto a 200-mesh screen, then gently ground with a tissue grinding rod until no visible red lumps. The screen was rinsed with 15 mL of DMEM, the rinsing solution was collected in a 15 mL centrifuge tube. Following this, it was centrifuged at 1200 rpm for 5 min, after which the supernatant was discarded. The resuspended cells were treated with ACK lysing buffer (Solarbio, Cat#R1010) for 5–10 min, washed twice in IMDM, resuspended in IMDM medium containing 10% fetal bovine serum, and counted using a hemocytometer for subsequent ELISPOT assays.
IFN-γ ELISPOT
Interferon-γ (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assay was employed to assess the HPV16 E6 and E7 specific CD8+ T cell response using peptides that correspond to immunodominant epitopes for CD8+ T cells. This approach ensured that the assay readouts were specifically aligned to focus solely on CD8+ T cell responses. The ELISPOTs were conducted in accordance with the manufacturer’s protocols (BD biosciences, Cat#551083), using 96-well plates. Briefly, each well was coated with 5 µg/ml rat anti-mouse IFN-γ antibody in 100 µl of phosphate-buffered saline (PBS) overnight at 4˚C. Subsequently, the wells were washed and blocked with IMDM culture medium containing 10% fetal bovine serum for 2 h at room temperature. Splenocytes from each vaccinated mouse were then added to the wells along with 50 IU/ml of IL-2, 10 µg/ml of E7-specific Major Histocompatibility Complex (MHC) class I immunodominant epitope (H-2Db, amino acids 49–57, RAHYNIVTF [14]) and/or 10 µg/ml E6-specific MHC class I immunodominant epitope (H-2Db, amino acids 50–57, YDFAFRDL [15]) for 40 h at 37˚C as part of a cultured ELISPOT. Following incubation, the wells were washed and incubated with 2.5 µg/ml biotinylated IFN-γ antibody in 100 µl PBS for 2 h at room temperature. For detection, HRP-streptavidin and AEC substrate were used. The spots were counted using the ImmunoSpot Analyzer (CTL Corporation, USA, Model: Immun spot 4.0).
Real time PCR
Real time PCR was employed to assess the expression of granzyme B in spleen cells from immunized mice. In Brief, total RNA was extracted from splenocytes that were restimulated with E7-(H-2Db, amino acids 49–57, RAHYNIVTF) and E6-specific MHC class I cytotoxic T Lymphocyte (CTL) epitope (H-2Db, amino acids 50–57, YDFAFRDL) for 20 hours at 37˚C. 1 µg RNA was reverse transcribed into cDNA using SuperRT cDNA Synthesis Kit (Beijing Comwin Biotech Co., Ltd, Cat#CW0741M). The resulting cDNA was quantified utilizing the UltraSYBR Mixture(Low ROX)(Beijing Comwin Biotech Co., Ltd, Cat#CW2601M), employing granzyme B sense primer 5’-CCTCCAGGACAAAGGCAG-3’ and antisense primer 5’-CAGTCAGCACAAAGTCCTCTC-3’. GAPDH served as reference gene, its sense primer is 5’-GCACAGTCAAGGCCGAGAAT-3’, while its antisense primer is 5’-GCCTTCTCCATGGTGGTGAA-3’. The quantitative real-time PCR was conducted under the following conditions: initial denaturation at 95˚C for 15 min followed by 40 cycles of denaturation at 95˚C for 15 s and annealing/extension at 60˚C for 1 min. The data were normalized relative to GAPDH using the following formula: relative mRNA expression = 2-△△Ct, Ct represents the cycle threshold.
In vivo tumor protection experiments
C57BL/6 mice (six per group) were vaccinated via intramuscular injection by electroporation with 15 µg of plasmid, delivered twice at an interval of 10 days using electroporation, as previously described. One week following the last vaccination, each mouse was subcutaneously challenged with 7.5 × 104 TC-1 cells on the right flank. Tumor growth was monitored biweekly using digital calipers. The tumor volume was calculated using the formula: volume=(length ×width2)/2. Additionally, the percentage of tumor-free mice was also recorded.
In vivo tumor treatment experiments
Each mouse was subcutaneously challenged with 7.5 × 104 TC-1 cells on the right flank. 3 days following this challenge, mice were immunized with the plasmids as previously described (six per group). Then the tumor growth was subsequently monitored according to the method of “in vivo tumor protection experiments”.
Statistical analysis
Statistical analysis was conducted using SPSS 13.0 software. The differences between immunization groups were assessed via ANOVA. Comparisons with a P value less than 0.05 were considered statistically significant.
Results
Generation of a plasmid vector containing optimized CpG ODNs
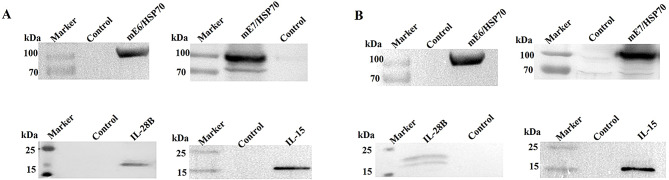
CpG oligodeoxynucleotides (CpG ODNs) serve as agonists of TLR9 and enhance adaptive immune response by stimulating human B cells and plasmacytoid dendritic cells [16]. Numerous studies have demonstrated that CpG elements can improve vaccine immunogenicity for the prevention and treatment of cancers, allergies, and infectious diseases [17]. The number and type of unmethylated CpG motifs within a plasmid backbone are crucial for optimizing immune responses [18]. In our study, we selected CpG motifs that exhibited strong stimulatory activity on immune cells [17]. We then inserted 20 tandem copies of these motifs into the backbone of pVAX1EI to create the CpG-modified vector pmVAX1EI (Fig. 1). The following recombinant plasmids were successfully constructed: pmVAX1EI-mE7/HSP70, pVAX1EI-mE7/HSP70, pmVAX1EI-mE6/HSP70, pVAX1EI-mE6/HSP70, pmVAX1EI-IL-28B, pmVAX1EI-IL-15, pVAX1EI-IL-28B, and pVAX1EI-IL-15. Partial schematic maps of these plasmids are available in the supplementary materials. To verify whether these constructs expressed the corresponding proteins, we tested them in vitro via transfection of 293T cells with 5 µg of each plasmid. The cells were subsequently lysed, and the lysates were used for Western blots to assess protein expression. The blots for mE6/HSP70, mE7/HSP70, IL-28B, and IL-15 demonstrated that all constructs were well expressed in vitro (Fig. 2).
Fig. 1.
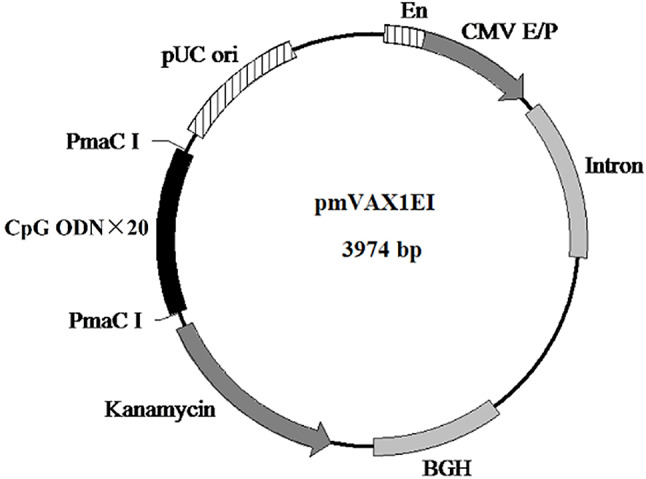
Map for CpG-modified vector. The optimized CpG ODN sequence (5’-TCGTCGTTTTGTCGTTTTGTCGTT-3’) was synthesized by Sangon (Shanghai, China). 20 tandem copies of this sequence were inserted into the backbone of pVAX1EI via the PamC I restriction site, resulting in the CpG-modified immunization vector pmVAX1EI
Fig. 2.
Western blot was performed to assess the expression of mE6/HSP70, mE7/HSP70, IL-28B and IL-15 in lysates from 293T cells transfected with either pVAX1EI (A) or pmVAX1EI (B) vectors, 48 h post-transfection. Control samples were prepared from cells transfected with empty pVAX1EI or pmVAX1EI vectors
IL-28B gene adjuvant could significantly enhance the CD8+ T cell response induced by mE7/HSP70
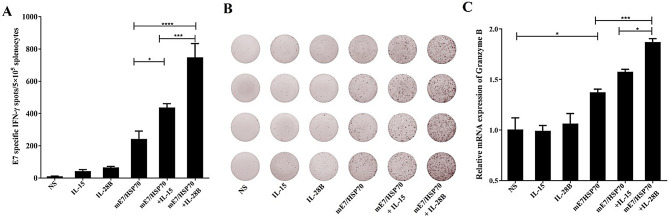
Our previous research has confirmed that the mE7/HSP70 DNA vaccine can effectively induce cellular immune responses [10]. In this study, we explored the potential of IL-28B and IL-15 plasmids as adjuvants for HPV DNA vaccines, aiming to enhance the cellular immune response triggered by the mE7/HSP70 DNA vaccine. Female C57BL/6 mice were randomly divided into 6 groups of 4 mice each. The experimental groups received injections of the mE7/HSP70 plasmid combined with either the IL-28B or IL-15 plasmid, according to the protocols described in the Materials and Methods section. Control groups were injected with IL-28B alone, IL-15 alone, or saline. 7 days after the final vaccination, the splenocytes were isolated and stimulated with 10 µg/ml E749 − 57 peptide for 40 h. The results from the IFN-γ ELISPOT assay revealed that inclusion of IL-28B or IL-15 significantly boosted E7-specific IFN-γ release (748 or 438 spots per 5 × 105 splenocytes, respectively) (Fig. 3A). Notably, the use of the IL-28B adjuvant resulted in a markedly higher IFN-γ release compared to the IL-15 adjuvant.
Fig. 3.
Effects of cytokine gene adjuvants on the quantity of IFN-γ-specific CD8+ T cells and mRNA levels of granzyme B in isolated splenocytes. (A) The quantity of IFN-γ-secreting E7-specific CD8+ T cells was assessed using ELISPOT. The spot counts were presented as the mean ± SE for 4 mouse samples per vaccinated group, normalized to 5 × 105 splenocytes. (B) Representative ELISPOT images from each vaccinated group were shown. (C) The mRNA levels of specific granzyme B were analyzed via real-time PCR and normalized relative to GAPDH. *:P<0.05, ***:P<0.001, ****:P<0.0001
After confirming that IL-28B and IL-15 could increase the release of IFN-γ, we next investigated their effects on granzyme B expression. The mRNA levels of specific granzyme B were analyzed by real-time PCR. Consistently, the inclusion of IL-28B adjuvant resulted in significantly higher mRNA level of granzyme B compared to IL-15 adjuvant (Fig. 3C). Therefore, IL-28B plasmid adjuvant appears to be more effective in enhancing the mE7/HSP70 DNA vaccine response than IL-15 plasmid. Consequently, IL-28 plasmid was selected as the adjuvant for subsequent experiments.
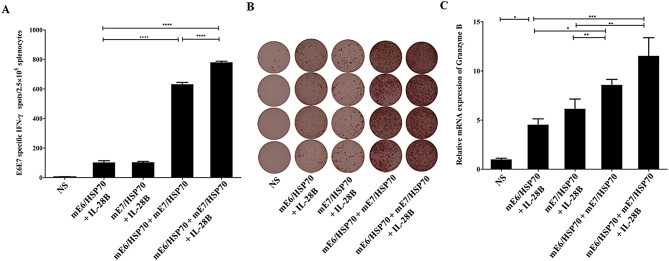
CpG ODN incorporated into the vaccine vector could significantly enhance the CD8+ T cell response induced by mE7/HSP70 vaccine
To explore the intrinsic adjuvant activity of CpG ODN integrated into the DNA plasmid backbone, we constructed both CpG ODN-modified or unmodified plasmids encoding mE7/HSP70 (pmVAX1EI-mE7/HSP70, pVAX1EI-mE7/HSP70) and IL-28B (pmVAX1EI-IL-28B, pVAX1EI-IL-28B). Female C57BL/6 mice were randomly divided into 3 groups of 4 mice each. The plasmids were administered as detailed in the Materials and Methods section, 7 days post the final vaccination, the splenocytes were isolated and stimulated with 10 µg/ml E749 − 57 peptide for 40 h. IFN-γ ELISPOT assay results revealed that the CpG-modified plasmids induced significantly higher E7-specific IFN-γ release compared to the unmodified group (Fig. 4A). Additionally, real-time PCR analysis for granzyme B expression demonstrated that the CpG-modified plasmids elicited significantly higher level than unmodified group (Fig. 4C). Consequently, embedding CpG ODN within the plasmid backbone as an intramolecular adjuvant significantly enhanced specific CD8+ T cell responses. Therefore, the CpG-modified vector pmVAX1EI was utilized in subsequent experiments.
Fig. 4.
Effects of CpG ODN modified plasmids on the quantity of IFN-γ -specific CD8+ T cells and mRNA levels of granzyme B in isolated splenocytes. (A) The quantity of IFN-γ-secreting E7-specific CD8+ T cells was assessed using ELISPOT. The spot counts were presented as the mean ± SE for 4 mouse samples per vaccinated group, normalized to 5 × 105 splenocytes. (B) Representative ELISPOT images from each vaccinated group are shown. (C) The mRNA levels of specific granzyme B were analyzed via real-time PCR and normalized relative to GAPDH. *:P<0.05, **:P<0.01, ***:P<0.001, ****:P<0.0001
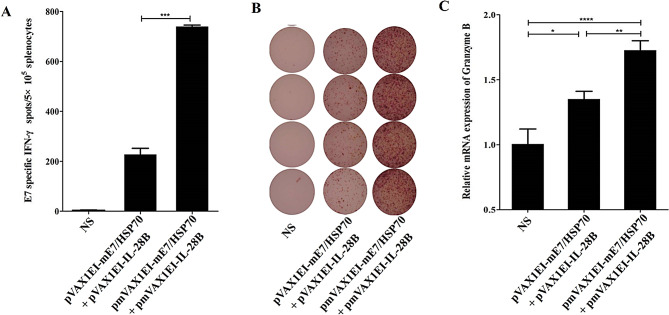
The CpG-modified plasmids encoding mE7/HSP70, when combined with those encoding mE6/HSP70, exhibited a synergistic effect in enhancing the specific CD8+ T cell response
Currently, HPV16 therapeutic vaccines focusing on E7 or E6 oncoproteins have been reported, but there are relatively few DNA vaccines targeting both E6 and E7 oncoproteins. In this study, we investigated the combined effects of the mE7/HSP70 and mE6/HSP70 in conjunction with IL-28B gene adjuvant.
Female C57BL/6 mice were randomly assigned to 5 groups, each consisting of 4 mice. The groups were as follows: mE7/HSP70 combined with IL-28B, mE6/HSP70 combined with IL-28B, mE7/HSP70 combined with mE6/HSP70 in the presence or absence of IL-28B, and saline. The plasmids were administered as detailed in the Materials and Methods Sect. 7 days after the final vaccination, the splenocytes were isolated and stimulated with 10 µg/ml E749 − 57 and 10 µg/ml E650 − 57 peptides for 40 h. The results of the IFN-γ ELISPOT assay demonstrated that vaccination with a combination of mE7/HSP70 and mE6/HSP70 elicited a significantly higher number of specific IFN-γ-secreting CD8+T cells compared to vaccination with either mE7/HSP70 or mE6/HSP70 alone (780 vs. 103 vs. 102 spots per 2.5 × 105 splenocytes) (Fig. 5A). Notably, the number of specific IFN-γ-secreting CD8+ T cells was found to be 3.8 times greater than the cumulative total observed for mE6/HSP70 and mE7/HSP70 administered individually. The results of real-time PCR for the detection of granzyme B indicated that the combination of mE7/HSP70 and mE6/HSP70 resulted in significantly higher mRNA levels compared to either mE7/HSP70 or mE6/HSP70 administered alone (Fig. 5C). furthermore, the findings demonstrated that the inclusion of IL-28B adjuvant could markedly enhance the CD8+ T cell response induced by the combination of mE7/HSP70 and mE6/HSP70.
Fig. 5.
Effects of CpG-modified plasmids combining mE7/HSP70 and mE6/HSP70 on the quantity of IFN-γ-specific CD8+ T cells and mRNA levels of granzyme B in isolated splenocytes. (A) The quantity of IFN-γ-secreting E7-specific CD8+ T cells was assessed using ELISPOT. The spot counts were presented as the mean ± SE for 4 mouse samples per vaccinated group, normalized to 5 × 105 splenocytes. (B) Representative ELISPOT images from each vaccinated group are shown. (C) The mRNA levels of specific granzyme B were analyzed via real-time PCR and normalized relative to GAPDH. *:P<0.05, **:P<0.01, ***:P<0.001, ****:P<0.0001
Based on the results obtained, it is evident that vaccination with the CpG-modified plasmids mE7/HSP70 in conjunction with mE6/HSP70, along with the IL-28B gene adjuvant, elicited the most potent cellular immune response.
Vaccination with CpG-modified plasmids mE7/HSP70 in combination with mE6/HSP70, along with the IL-28B gene adjuvant, has been shown to effectively inhibit the growth of TC-1 transplanted tumors
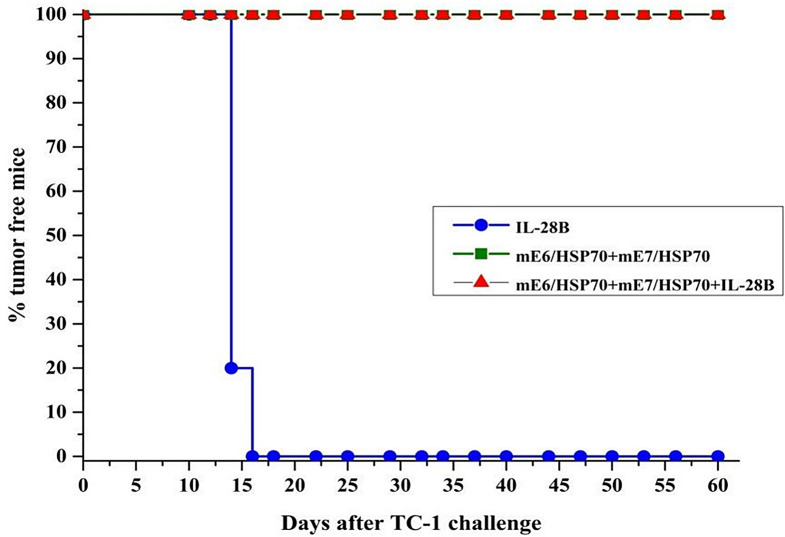
Based on the promising results from our cellular immune response assays, which indicated that CpG-modified plasmids mE7/HSP70 in combination with mE6/HSP70, and further enhanced by the IL-28B gene adjuvant, had the potential to induce robust cellular immunity, we proceeded to evaluate their efficacy in preventing tumor growth in vivo. As illustrated in Fig. 6, by day 60, all mice immunized with a combination of mE6/HSP70 and mE7/HSP70, regardless of the presence or absence of the IL-28B gene adjuvant, remained tumor-free. In contrast, mice immunized solely with the IL-28B gene adjuvant began to develop tumors 16 days after TC-1 injection, with an average tumor size of approximately 387 ± 18 mm2 by day 60. These mice were subsequently sacrificed. The results suggested that vaccination with mE6/HSP70 combined with mE7/HSP70, either alone or synergistically with the IL-28B gene adjuvant, generates potent antitumor effects capable of completely preventing tumor growth in C57BL/6 mice following prophylactic challenge.
Fig. 6.
The analysis of prophylactic immune activity in vivo. C57BL/6 mice were immunized with 15 µg of plasmid via electroporation, administered twice at a 10-day interval. 7 days following the final vaccination, each mouse was challenged subcutaneously with 7.5 × 104 TC-1 tumor cells, and the growth of tumors was monitored for a period of 60 days
Vaccination with CpG-modified plasmids mE7/HSP70 in combination with mE6/HSP70, along with the IL-28B gene adjuvant, has demonstrated significant efficacy in suppressing the progression of established TC-1 transplanted tumors
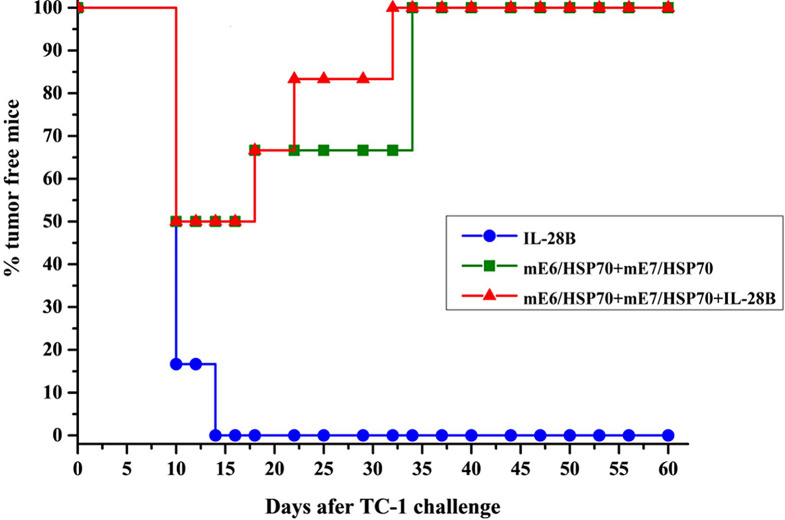
We also evaluated the efficacy of CpG-modified plasmids mE7/HSP70 in combination with mE6/HSP70, along with the IL-28B gene adjuvant, in eradicating established tumors. The therapeutic effects were illustrated in Fig. 7. on day 14 following TC-1 challenge, a tumor-free rate of 50% was observed in mice receiving either mE6/HSP70 combined with mE7/HSP70, regardless of the presence or absence of the IL-28B gene adjuvant. By day 32, the presence of the IL-28B gene adjuvant enhanced the efficacy, resulting in a 100% tumor-free rate among mice inoculated with mE6/HSP70 and mE7/HSP70, compared to a 67% tumor-free rate without the adjuvant. In contrast, all mice receiving only the IL-28B plasmid developed tumors by day 60, with an average tumor size measuring approximately 433 ± 27 mm2. All mice survived until day 60 when they were euthanized using CO2 asphyxiation. These results indicated that the combination of mE6/HSP70 and mE7/HSP70, particularly in the presence of IL-28B gene adjuvant, exhibited the strongest immune clearance activity against the transplanted tumor.
Fig. 7.
The analysis of therapeutic immune activity in vivo. Each C57BL/6 mouse was subcutaneously inoculated with 7.5 × 104 TC-1 tumor cells. Three days post-inoculation, the mice were immunized with 15 µg of plasmid via electroporation, administered twice at a 10-day interval. Subsequently, tumor growth was monitored for a period of 60 days
Discussion
In the past, DNA vaccines have demonstrated efficacy in animal models. However, they have not elicited sufficient immunogenicity in human clinical trials [19]. Therefore, it is crucial to modulate and enhance the effectiveness of DNA vaccines. Currently, several strategies have been explored to improve their immunogenicity, including the use of adjuvants such as cytokines [20, 21], CpG motifs or CpG oligonucleotides [22–25], liposomes [26, 27], and lipopolysaccharide [28, 29]. Additionally, modifications like altering codon bias [30, 31] and employing electroporation techniques [32–34], along with heterologous prime/boost immunization [32, 35, 36], have shown promise in boosting the immune response.
In the current study, we investigated the efficacy of a combination of multiple strategies on HPV DNA vaccine. Our findings revealed that the integrating CpG motifs into the plasmid vector backbone, combining mE7/HSP70 and mE6/HSP70 plasmids, incorporating an IL-28B gene adjuvant, and administering the vaccine via electroporation significantly enhanced specific CD8+ T cell responses and eradicated the established tumor graft in C57BL/6 mice. Notably, We did not detect a specific CD4+ T cell response or humoral immune response, this is consistent with previous studies from our group and others which demonstrated that HPV DNA vaccines fused with HSP70 predominantly enhance specific CD8+ T cell responses, without substantial improvement in specific CD4+ T cell response or antibody production [10, 37–39]. This phenomenon may be attributed to HSP70’s primary function in delivering antigenic peptides to MHC-I molecules and its cross-presentation activity [40, 41]. Furthermore, clinical researches have indicated that CD8+ T cells are crucial for the antitumor effects of therapeutic vaccines, while specific CD4+ T cell responses and antibody responses are not the most critical factors [5, 41–44]. Therefore, our focus was on evaluating the specific CD8+ T cell response.
In this study, we engineered the HPV16 E6 wild-type protein gene by inserting two HLA-A2 restriction epitopes between amino acids 70 and 71 in the zinc finger binding region. Similarly, the E7 wild-type protein gene was also modified through a combination of strategies that involved mutating the Rb binding site, cleaving and rearranging the gene, as well as adding one HLA-A2 restriction epitope at both the N-terminus and C-terminus. These modifications introduced additional human CD8+ T cell epitopes into the E6 and E7 genes, laying the groundwork for eliciting a robust CD8+ T cell response and paving the way for future clinical applications. The transforming functions of HPV E6 and E7 proteins depend on their native spatial structure. The E7 protein requires dimerization and must be localized in the nucleus. The CR2 homology region of the E7 protein includes a binding site for the tumor suppressor protein Rb, facilitating the release of the E2F factor, which is crucial for its transforming activity [45]. Studies have shown that mutating the Rb binding site or the zinc finger motif (CXXC) of the E7 protein can knock out its transforming activity [46]. There are few reports on the key sites of the transforming activity of the E6 protein, but E6 also contains two zinc finger structures. Introducing point mutations into the zinc finger motif can disrupt its structure, potentially preventing the E6 protein from degrading p53 via E6-AP-mediated pathways, thereby knocking out its transforming activity. Additionally, cutting the E6 and E7 genes into sub-fragments and rearranging them to disrupt their native protein structures can also knock out the transforming activities when used alone or in combination with other strategies [38, 47–50]. In our study, we employed a multifaceted approach to modify E6/E7 by introducing point mutations (at the Rb binding site and the zinc finger motif), gene shuffling and CTL epitope peptide insertion. We assessed the effect of mE6/HSP70 on degradation of cellular p53 proteins was detected. As shown in Figure S2, after transfection of wild-type HPV16 E6 expression plasmid into 293T cells, intracellular degradation of p53 was observed. However, no degradation of p53 was observed in 293T cells transfected with the mE6/HSP70 vaccine plasmid, indicating that the antigen design of the vaccine can effectively eliminate the transforming activity of E6. This may be due to the fact that the modified E6 not only lost the key sites and structures related to transforming activity but also lost the spatial structure of the native protein.
Notably, our findings revealed that the combined immunization with plasmids expressing E6/HSP70 or E7/HSP70 antigens induced significantly higher levels of specific CD8+ T cell responses compared to individual immunization (Fig. 5). We speculated that the synergistic enhancement observed with combined immunization may stem from multiple mechanisms. Firstly, the combined immunization utilizing two plasmids likely results in enhanced expression of fusion proteins, all containing HSP70. Consequently, the total expression of HSP70 may be elevated, potentially amplifying the adjuvant effect. Secondly, the presence of more fusion antigens could enhance the immune response through various factors, such as recruiting a greater number of immune cells, providing additional helper T cell epitopes, and elevating local cytokine levels. Future studies will aim to elucidate the underlying mechanisms of this synergistic effect.
To further enhance the immunogenicity of our HPV DNA vaccine, we incorporated cytokine gene adjuvants. Previous studies have demonstrated the adjuvant activity of various cytokines, including IL-12, IL-15, IL-28B, IFN-γ and Granulocyte-macrophage colony-stimulating factor (GM-CSF). However, the efficacy of a particular cytokine as an adjuvant can be unpredictable when used in different vaccines or administered in varying ways. IL-15 gene adjuvant has been shown to significantly boost specific CD8+T cell response in HIV DNA vaccine and Trypanosoma cruzi DNA vaccine [51–53]. Although IL-28B gene adjuvant has been reported to augment the function of CD8+ T cells and protective immune responses in HIV DNA vaccine [54–56], HSV DNA vaccines [57] and Flu vaccines [58], it had no effect on the immune activity or protective immunity of a tuberculosis fusion protein vaccine [59]. Similarly, GM-CSF gene adjuvant significantly enhanced the immunogenicity of SARS-CoV-2 DNA vaccines [60], anti-Lewis lung carcinoma DNA vaccines [61] and HPV16 E6/E7 DNA vaccines [62], but it inhibited antibody level and protective effects induced by Japanese encephalitis virus DNA vaccine [63]. For the first time, we employed the IL-28B plasmid as an adjuvant for our HPV DNA vaccine in comparison with IL-15 plasmid due to our research group’s findings that IL-15 enhance CD8+ T cell response. Our analysis of this comparison suggested that IL-28B may be more effective than IL-15 in enhancing CD8+ T cell response induced by HPV DNA vaccine.
The previous studies have demonstrated that the immunogenicity of plasmid DNA can be modulated and enhanced by the presence of CpG motifs or ODN in the backbone. In humans, four classes of synthetic CpG ODN have been identified, differing in structure and immune activity: A-, B-, C- and P-types [64]. One study suggested that insertion of C-type motifs in anthrax DNA vaccines enhance higher cell-mediated and protective immune responses compared to A- and B-type motifs [18]. Another study showed that mice vaccinated with C-type CpG-modified vaccines exhibited enhanced Th1-biasd T-cell response [65]. Morever, several clinical studies indicated that B-type CpG such as CpG7909 or CpG1018, used as adjuvants in vaccines targeting infectious diseases and cancers, could enhance Th1-skewed immune response of these vaccines [66, 67]. These findings suggested that CpG motifs might be appropriate for use as “bulit-in” adjuvants in DNA vaccine. Furthermore, a study comparing the efficacy of vaccines modified with 5, 20 and 40 copies of CpG motifs showed that adding more copies of CpG motifs produced stronger adjuvant effects [18]. In our study, we synthesized one CpG motifs, including B type, which exhibited good stimulating activity on human immune cells [16], then inserted 20 copies of CpG motif into the plasmid backbone. Our data showed that CpG-modified HPV DNA vaccine induced significantly higher CD8+ T cell responses than non-modified HPV DNA vaccine.
Electroporation (EP) was employed in our experiment as the delivery method, which has been previously proved to enhance the efficacy of DNA vaccine efficacy in various pre-clinical [54] and clinical trials [5, 7]. EP has also been shown to improve the immunogenicity of low-dose of DNA plasmid. For instance, previous studies in mice had utilized 2 µg [68] or 10 µg [54, 69] of plasmid to demonstrate that electroporation-mediated intramuscular plasmid delivery could induce significant cellular immunity. In our study, we initially used 15 µg of plasmid to explore the induced cellular immunity, with plans to test other doses in subsequent experiments.
TC-1 cells, commonly used as in vivo tumor model for evaluating HPV16 DNA vaccine efficacy, were derived from epithelial cells of C57BL/6 mice co-transformed with HPV16 E6, E7 and c-Ha-ras oncogenes [70]. According to previous studies [50, 71, 72], the commonly accepted dose of TC-1 cells was used in this study, and the results demonstrated that both mE6/HSP70 and mE7/HSP70 bivalent vaccines, with or without IL-28B plasmid, induced complete protection against TC-1 challenges (Figs. 5 and 6). Notably, the vaccine combined with IL-28B plasmid showed faster tumor clearance (though not significantly) in the therapeutic experiments (Fig. 6). These findings suggested that the protective activity of the mE6/HSP70 and mE7/HSP70 bivalent vaccines is potent and may have reached the detection limit of the TC-1 challenge assay using the commonly employed TC-1 dose. Therefore, it is necessary to optimize the dose of TC-1 challenge in future studies, to clarify the role of IL-28B in enhancing in vivo protective activity.
In general, our findings might offer one efficient strategy for optimizing DNA vaccines by incorporating CpG ODNs into plasmid vector, combining mE6/HSP70 and mE7/HSP70 plasmids, and using IL-28B as a gene adjuvant, with delivery via electroporation.
Deficiencies and prospects
Our study is the first to demonstrate that IL-28B gene adjuvant can enhance the CD8+ T cell immune response induced by HPV16 DNA vaccine. Previous research has indicated that IL-28B gene adjuvant also significantly down-regulated the number of Treg cells in the spleen of immunized mice [54, 59]. Therefore, further analysis is needed to investigate the effect of IL-28B on Treg cells in HPV16 DNA vaccine.
In this study, we observed no obvious difference in the anti-tumor activity of HPV16 DNA vaccine with or without IL-28B in vivo. The possible reason for this might be the low tumor burden in mice. Future study should consider increasing the number of TC-1 tumor cells inoculated or prolonging the interval between tumor cell inoculation and the first immunization to better assess the impact of IL-28B on anti-tumor activity.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Author contributions
Y.Z. did the experiments and wrote the main manuscript text; T.Z. prepared Figs. 1, 2, 3, 4, 5 and 6. ZR.W. did the data analysis;X.X. revised and edited manuscripts. All authors reviewed the manuscript.
Funding
This work was supported by CAMS Innovation Fund for Medical Sciences (Grant No. 2021-I2M-1-043) and the basic scientific research business of Hebei North University (JYT2022005).
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Ethical approval
This study was approved by the Ethical Committee of Hebei North University (Approval No. HBNU20231222116).
Consent for publication
All authors approved the publication of this manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.de Sanjosé S, Brotons M, Pavón MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. PMID:28964706. [DOI] [PubMed] [Google Scholar]
- 2.Cheng MA, Farmer E, Huang C, Lin J, Hung CF, Wu TC. Therapeutic DNA vaccines for human Papillomavirus and associated diseases. Hum Gene Ther. 2018;29(9):971–96. PMID: 29316817. PMCID: PMC6152857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orbegoso C, Murali K, Banerjee S. The current status of immunotherapy for cervical cancer. Rep Pract Oncol Radiother. 2018;23(6):580–588. PMID: 30534022. PMCID: PMC6277269. [DOI] [PMC free article] [PubMed]
- 4.Lee SJ, Yang A, Wu TC. Immunotherapy for human papillomavirus-associated disease and cervical cancer: review of clinical and translational research. J Gynecol Oncol. 2016;27(5):e51. PMID: 27329199. PMCID: PMC4944018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim TJ, Jin HT, Hur SY, Yang HG. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. PMID: 25354725. PMCID: PMC4220493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi YJ, Hur SY, Kim TJ, A Phase II, Prospective. Randomized, Multicenter, Open-Label Study of GX-188E, an HPV DNA Vaccine, in Patients with Cervical Intraepithelial Neoplasia 3. Clin Cancer Res. 2020;26(7):1616–23. PMID:31727676. [DOI] [PubMed] [Google Scholar]
- 7.Trimble CL, Morrow MP, Kraynyak KA. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasis 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015;386(10008):2078–2088. PMID: 26386540. PMCID: PMC4888059. [DOI] [PMC free article] [PubMed]
- 8.Aggarwal C, Cohen RB, Morrow MP, Kraynyak KA, Sylvester AJ, Knoblock DM, et al. Immunotherapy targeting HPV16/18 generates potent immune responses in HPV-associated head and neck cancer. Clin Cancer Res. 2019;25(1):110–24. PMID: 30242022. PMCID: PMC6320307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn JW, Hur SY, Woo JW, Kim YM, Lim MC, Park SY, et al. Pembrolizumab plus GX-188E therapeutic DNA vaccine in patients with HPV-16-positive or HPV-18-positive advanced cervical cancer: interim results of a single-arm, phase 2 trial. Lancet Oncol. 2020;21(12):1653–60. PMID: 33271094. [DOI] [PubMed]
- 10.Zong JB, PENG QL, WANG QY, et al. Human HSP70 and modified HPV16 E7 fusion DNA vaccine induces enhanced specific CD8+ T cell responses and anti-tumor effects. Oncol Rep. 2009;22(4):953–61. PMID:19724878. [DOI] [PubMed] [Google Scholar]
- 11.Wang QY, Xu YF, Fan DS et al. Linkage of modified human papillomavirus type 16 E7 to CD40 ligand enhances specific CD8+ T-lymphocyte induction and anti-tumor activity of DNA vaccine. Zhong guo Yi Xue Ke Xue Yuan Xue Bao. 2007;29(5):584 – 91. (Article in Chinese). PMID: 18051710. [PubMed]
- 12.de Oliveira LM, Morale MG, Chaves AA, et al. Design, Immune Responses and Anti-Tumor Potential of an HPV16 E6E7 Multi-Epitope Vaccine. PLoS ONE. 2015;10(9):e0138686. 2015.PMID: 26390407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas KJ, Smith KL, Youde SJ, et al. HPV16 E6 29-38-specific T cells kill cervical carcinoma cells despite partial evasion of T-cell effector function. Int J Cancer. 2008;122(12):2791–9. PMID: 18366058. [DOI] [PubMed] [Google Scholar]
- 14.Feltkamp MC, Smits HL, Vierboom MP, Minnaar RP, de Jongh BM, Drijfhout JW, ter Schegget J, Melief CM, Kast MW. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–9. PMID: 7690326. [DOI] [PubMed] [Google Scholar]
- 15.Lipford GB, Bauer S, Wagner H, Heeg K. Peptide engineering allows cytotoxic T-cell vaccination against human papilloma virus tumor antigen, E6. Immunology. 1995;84(2):298–303. PMID: 7751006. [PMC free article] [PubMed] [Google Scholar]
- 16.Gao F, Zheng M, Fan J. A trimeric spike-based COVID-19 vaccine candidate induces broad neutralization against SARS-CoV-2 variants. Hum Vaccin Immunother. 2023;19(1):2186110. PMID: 36882925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kayraklioglu N, Horuluoglu B, Klinman DM. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol Biol. 2021;2197:51–85. PMID: 32827132. [DOI] [PubMed] [Google Scholar]
- 18.Yu YZ, Ma Y, Xu WH, et al. Combinations of various CpG motifs cloned into plasmid backbone modulate and enhance protective immunity of viral replicon DNA anthrax vaccines. Med Microbiol Immunol. 2015;204:481–91. PMID:25265876. [DOI] [PubMed] [Google Scholar]
- 19.Saade F, Petrovsky N. Technologies for enhanced efficacy of DNA vaccines. Expert Rev Vaccines. 2012;11(2):189–209. PMID: 22309668. PMCID: PMC3293989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grasse M, Meryk A, Miggitsch C, Grubeck-Loebenstein B. GM-CSF improves the immune response to the diphtheria-component in a multivalent vaccine. Vaccine. 2018;36:4672–80. PMID: 29961602. PMCID: PMC7116485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suschak JJ, Shoemaker KBCJ, et al. The Genetic Adjuvants Interleukin-12 and Granulocyte-Macrophage Colony Stimulating Factor Enhance the Immunogenicity of an Ebola Virus Deoxyribonucleic Acid Vaccine in Mice. J Infect Dis. 2018;218(suppl5):S519–S. 527.PMID:30053157. [DOI] [PubMed] [Google Scholar]
- 22.Klinman DM, Klaschik S, Sato T, Tross D. CpG oligonucleotides as adjuvants for vaccines targeting infectious disease. Adv Drug Deliv Rev. 2009;61(3):248–55. PMID:19272313. [DOI] [PubMed] [Google Scholar]
- 23.Bode C, Zhao G, Steinhagen F, Kinjo T, Klinman DM. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011;10(4):499–511. PMID: 21506647. PMCID: PMC3108434. [DOI] [PMC free article] [PubMed]
- 24.Yu ZY, Li N, Ma Y, et al. Three types of human CpG motifs differentially modulate and augment immunogenicity of nonviral and viral replicon DNA vaccines as built-in adjuvants. Eur J Immunol. 2013;43(1):228–39. PMID: 23037552. [DOI] [PubMed] [Google Scholar]
- 25.Shirota H, Klinman DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014;13(2):299–312. PMID: 24308579. PMCID: PMC6335645. [DOI] [PMC free article] [PubMed]
- 26.Didierlaurent AM, Laupèze B, Pasquale AD, et al. Adjuvant system AS01:helping to overcome the challenges of modern vaccines. Expert Rev Vaccines. 2017;16(1):55–63. PMID:27448771. [DOI] [PubMed] [Google Scholar]
- 27.Christensen D, et al. Cationic liposomes as vaccine adjuvants. Expert Rev Vaccines. 2011;10(4):513–21. PMID:21506648. [DOI] [PubMed] [Google Scholar]
- 28.Hawkins WG, Trcka J, Segal N, et al. The role of lipopolysaccharide in T-cell responses following DNA vaccination. Vaccine. 2003;21(13–14):1548–53. PMID:12615452. [DOI] [PubMed] [Google Scholar]
- 29.Kianmehr Z, Soleimanjahi H, Ardestani SK, Fotouhi F, Abdoli A. Influence of Brucella abortus lipopolysaccharide as an adjuvant on the immunogenicity of HPV-16 L1VLP vaccine in mice. Med Microbiol Immunol. 2015;204(2):205–13. PMID: 25187406. [DOI] [PubMed] [Google Scholar]
- 30.Li K, Gao L, Gao H, et al. Codon optimization and woodchuck hepatitis virus posttranscriptional regulatory element enhance the immune responses of DNA vaccines against infectious bursal disease virus in chickens. Virus Res. 2013;175(2):120–7. PMID:23631937. [DOI] [PubMed] [Google Scholar]
- 31.Duan F, Chen J, Yao H, et al. Enhanced therapeutic efficacy of Listeria-based cancer vaccine with codon-optimized HPV16 E7. Hum Vaccin Immunother. 2021;17(6):1568–77. PMID:33449866. PMCID: PMC8115762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li L, Petrovsky N. Molecular mechanisms for enhanced DNA vaccine immunogenicity. Expert Rev Vaccine. 2016;15(3):313–29. PMID:26707950. PMCID: PMC4955855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lambricht L, Vanvarenberg K, Beuckelaer AD, et al. Coadministration of a plasmid encoding HIV-1 Gag Enhances the efficacy of cancer DNA vaccines. Mol Ther. 2016;24(9):1686–96. PMID:27434590. PMCID: PMC5223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen H, Zheng XY, Wang R, et al. Immunization with electroporation enhances the protective effect of a DNA vaccine candidate expressing prME antigen dengue virus serotype 2 infection. Clin Immunol. 2016;171:41–9. PMID:27578400. [DOI] [PubMed] [Google Scholar]
- 35.Li LL, Wang HR, Zhou ZY, et al. One-prime multi-boost strategy immunization with recombinant DNA, adenovirus, and MVA vector. Antiviral Res. 2016;128:20–7. PMID:26821205. [DOI] [PubMed] [Google Scholar]
- 36.Peng SW, Qiu J, Yang A, et al. Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus-associated disease. Cell Biosci. 2016;6:16. PMID:26918115. PMCID: PMC4766698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y, Subjeck J, Yang G, et al. Generation of anti-tumor immunity using mammalian heat shock protein 70 DNA vaccines for cancer immunotherapy. Vaccine. 2006;24(25):5360–70. PMID:16714072. [DOI] [PubMed] [Google Scholar]
- 38.Zong JB, Wang CH, Wang QY, et al. HSP70 and modified HPV16 E7 fusion gene without the addition of a signal peptide gene sequence as a candidate therapeutic tumor vaccine. Oncol Rep. 2013;30(6):3020–6. PMID:24065282. [DOI] [PubMed] [Google Scholar]
- 39.Zhang X, Yu C, Zhao J, et al. Vaccination with a DNA vaccine based on human PSCA and HSP70 adjuvant enhances the antigen-specific CD8+ T cell response and inhibits the PSCA+ tumors growth in mice. J Gene Med. 2007;9(8):715–26. PMID:17595048. [DOI] [PubMed] [Google Scholar]
- 40.Murshid A, Gong J, Calderwood SK. The role of heat shock protein in antigen cross presentation. Front Immunol. 2012;3:63. PMID:22566944. PMCID: PMC3342350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzhova IV, Margulis BA. HSP70-based anti-cancer immunotherapy. Hum Vaccin Immunother. 2016;12(10):2529–35. PMID:27294301. PMCID: PMC5084976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragonnaud E, Andersson AC, Mariya S, Pedersen AG, Burk RD, Folgori A, et al. Therapeutic vaccine against primate papillomavirus infections of the cervix. J Immunother. 2017;40(2):51–61. PMID: 28166180. [DOI] [PubMed]
- 43.Stanley MA, Sterling JC, et al. Host responses to infection with human papillomavirus. Curr Probl Dermatol. 2014;45:58–74. PMID:24643178. [DOI] [PubMed] [Google Scholar]
- 44.de Sanjose S, Brotons M, Pavon MA. The natural history of human papillomavirus infection. Best Pract Res Clin Obstet Gynaecol. 2018;47:2–13. PMID:28964706. [DOI] [PubMed] [Google Scholar]
- 45.Klingelhutz AJ, Roman A. Cellular transformation by human papillomaviruses: Lessons learned by comparing high- and low-risk viruses. Virology. 2012;424(2):77–98. PMID:22284986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi W, Bu P, Liu J, et al. Human Papillomavirus Type 16 E7 DNA Vaccine: Mutation in the Open Reading Frame of E7 Enhances Specific Cytotoxic T-Lymphocyte Induction and Antitumor Activity. J Virol. 1999;73(9):7877–81. PMID: 10438884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Osen W, Peiler T, Öhlschläger P, et al. A DNA vaccine based on a shuffled E7 oncogene of the human papillomavirus type 16 (HPV 16) induces E7-specific cytotoxic T cells but lacks transforming activity. Vaccine. 2001;19(30):4276–86. PMID:11457555. [DOI] [PubMed] [Google Scholar]
- 48.Ohlschläger P, Pes M, Osen W, Dürst M, Schneider A, Gissmann L, et al. An improved rearranged human Papillomavirus type 16 E7 DNA vaccine candidate (HPV-16 E7SH) induces an E7 wildtype-specific T cell response. Vaccine. 2006;24(15):2880–93. PMID: 16472545. [DOI] [PubMed]
- 49.Almajhdi FN, Senger T, Amer HM, et al. Design of a highly effective therapeutic HPV16 E6/E7-specific DNA vaccine: Optimization by different ways of sequence rearrangements (shuffling). PLoS ONE. 2014;9(11):e113461. PMID:25422946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brinkman JA, Xu X, Kast WM. The efficacy of a DNA vaccine containing inserted and replicated regions of the E7 gene for treatment of HPV-16 induced tumors. Vaccine. 2007;25(17):3437–44. PMID: 17241713. PMCID: PMC1885421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kutzler MA, Robinson TM, Chattergoon MA, et al. Coimmunization with an optimized IL-15 plasmid results in enhanced function and longevity of CD8+T cells that function and longevity of CD8+ T cells that are partially independent of CD4+ T cell help. J Immunol. 2005;175(1):112–23. PMID:15972637. [DOI] [PubMed] [Google Scholar]
- 52.Li W, Li S, Hu Y, Tang B, et al. Efficient augmentation of a long-lasting immune responses in HIV-1 gag DNA vaccination by IL-15 plasmid boosting. Vaccine. 2008;26(26):3282–90. PMID:18472194. [DOI] [PubMed] [Google Scholar]
- 53.Eickhoff CS, Vasconcelos JR, Sullivan NL, et al. Co-administration of a plasmid DNA encoding IL-15 improves long-term protection of a genetic vaccine against Trypanosoma cruzi. PLoS Negl Trop Dis. 2011;5(3):e983. PMID:21408124. PMCID: PMC3050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrow MP, Pankhong P, Laddy DJ, et al. Cooparative ability of IL-12 and IL-28B to regulate Treg populations and enhance adaptive cellular immunity. Blood. 2009;113(23):5868–77. PMID:19304955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Morrow MP, Yan J, Pankhong P, et al. Unique Th1/Th2 phenotypes induced during priming and memory phases by use of interleukine-12(IL-12) or IL-28B vaccine adjuvants in rhesus macaques. Clin Vaccine Immunol. 2010;17(10):1493–9. PMID:20685940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morrow MP, Yan J, Pankhong P, et al. IL-28B/IFN-λ3 drives granzyme B loading and significantly increases CTL killing activity in Macaques. Mol Ther. 2010;18(9):1714–23. PMID:20571540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, Wang ZY, Xu YQ, et al. Optimized DNA vaccine enhanced by adjuvant IL-28B induces protective immune responses against herpes simplex virus type 2 in mice. Viral Immunol. 2017;30(8):601–14. PMID:28650722. [DOI] [PubMed] [Google Scholar]
- 58.Sabbaghi A, Zargar M, Zolfaghari MR, et al. Protective cellular and mucosal immune responses following nasal administration of a whole gamma-irradiated influenza A (subtype H1N1) vaccine adjuvanted with interleukin-28B in a mouse model. Arch Virol. 2021;166(2):545–57. PMID: 33409549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo Y, Ma X, Liu X, et al. IL-28B down-regulates regulatory T cells but does not improve the protective immunity following tuberculosis subunit vaccine immunization. Int Immunol. 2016;28(2):77–85. PMID:26521300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liu C, Xue RY, Li GC et al. pGM-CSF as an adjuvant in DNA vaccination against SARS-CoV-2. Int J Biol Macromol. 2024;264(Pt 2):130660. PMID: 38460634. [DOI] [PubMed]
- 61.Ruan JZ, Duan Y, Li FG, et al. Enhanced synergistic anti-Lewis lung carcinoma effect of a DNA vaccine harboring a MUC1-VEGFR2 fusion gene used with GM-CSF as an adjuvant. Clin Exp Pharmacol Physiol. 2017;44(1):71–8. PMID:27562635. [DOI] [PubMed] [Google Scholar]
- 62.Ding Z, Zhu H, Mo L, et al. FLT3L and granulocyte macrophage colony-stimulating factor enhance the anti-tumor and immune effects of an HPV16 E6/E7 vaccine. Aging. 2019;11(24):11893–904. PMID: 31881013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen H, Gao N, Fan DY, et al. Suppressive effects on the immune response and protective immunity to a JEV DNA vaccine by co-administration of a GM-CSF-expressing plasmid in mice. PLoS ONE. 2012;7(4):e34602. PMID:22493704. PMCID: PMC3321030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shirota H, Tross D, Klinman DM, et al. CpG oligonucleotides as cancer vaccine adjuvants. Vaccine. 2015;3:390–407. PMID:26343193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma Y, Jiao YY, Yu YZ et al. A Built-In CpG Adjuvant in RSV F Protein DNA Vaccine Drives a Th1 Polarized and Enhanced Protective Immune Response. Viruses.2018;10(1):38–50. PMID:29342954. PMCID: PMC5795451. [DOI] [PMC free article] [PubMed]
- 66.Pant S, Wainberg ZA, Weekes CD, et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat Med. 2024;30(2):531–42. PMID: 38195752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hsieh SM, Liu WD, Huang YS, Lin YJ, Hsieh EF, Lian WC, et al. Safety and immunogenicity of a recombinant stabilized prefusion SARS-CoV-2 spike protein vaccine (MVC-COV1901) adjuvanted with CpG 1018 and aluminum hydroxide in healthy adults: a phase 1, dose-escalation study. EClinicalMedicine. 2021;38:100989. PMID: 34222848. PMCID: PMC8233066. [DOI] [PMC free article] [PubMed]
- 68.Peng S, Tomson TT, Trimble C, et al. A combination of DNA vaccines targeting human papillomavirus type 16 E6 and E7 generates potent antitumor effects. Gene Ther. 2006;13(3):257–65. PMID: 16177818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liang Y, Cui L, Xiao L et al. Immunotherapeutic Effects of Different Doses of Mycobacterium tuberculosis ag85a/b DNA Vaccine Delivered by Electroporation. Front Immunol. 2022;13:876579. PMID: 35603155. [DOI] [PMC free article] [PubMed]
- 70.Lin KY, Guarnier FG, Staveley-O’Carroll KF, Levitsky HI, August JT, Pardoll DM, Wu TC. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56(1):21–6. PMID: 8548765. [PubMed] [Google Scholar]
- 71.Qian X, Lu Y, Liu Q, Chen K, Zhao Q, Song J. Prophylactic, therapeutic and anti-metastatic effects of an HPV-16 mE6∆/mE7/TBhsp70∆ fusion protein vaccine in an animal model. Immunol Lett. 2006;102(2):191–201. PMID:16242781. [DOI] [PubMed] [Google Scholar]
- 72.Yan J, Reichenbach DK, Corbitt N, Hokey DA, Ramanathan MP, McKinney KA, Weiner DB, Sewell D. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine [Internet]. 2009;27(3):431–40. PMID: 19022315. PMCID: PMC4477831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.