Abstract
Background
Biomass allocation reflects functional tradeoffs among plant organs and thus represents life history strategies. However, little is known about the patterns and drivers of biomass allocation between reproductive and vegetative organs along large environmental gradients. Here, we examined how environmental gradients affect biomass and the allocation between reproductive and vegetative organs. We also tested whether the allocation patterns conform optimal or allometric partitioning theory.
Methods
We collected 22 Artemisia species along a large environmental gradient in China and measured reproductive (infructescences including seeds) and vegetative (leaves, stems and roots) mass for each plant. We then used standardized major axes regressions to quantify the relationships between reproductive and vegetative organs and linear mixed-effect models to examine the effect of environmental gradients (climate and soil) on biomass allocation patterns.
Results
We found significant negative correlations between total biomass of Artemisia and the first principal component of climate, an axis that was negatively correlated with temperature and precipitation. Overall, there were significant isometric relationships between reproductive and vegetative mass. In addition, the ratio of reproductive to vegetative mass increased with the second principal component of climate (representing climate variability), but decreased with the second principal component of soil (representing bulk density and available water capacity). These patterns were consistent at the individual and interspecific levels, but were mixed at the intraspecific level.
Conclusions
Our findings of the plastic responses of biomass allocation to environmental gradients support the optimal partitioning theory (OPT). The isometric relationships between reproductive and vegetative organs indicate that plant growth and reproduction are intricately linked. Furthermore, the plasticity of biomass ratios of reproductive to vegetative organs to climate variability and soil physical properties suggests that the flexible allocation between growth and reproduction is crucial for successful adaptation to diverse habitats.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-06030-3.
Keywords: Allometric partitioning theory, Artemisia, Biomass allocation pattern, Environmental gradient, Optimal partitioning theory, Reproductive mass, Vegetative mass
Introduction
Biomass allocation, the process by which plants allocate finite biomass to different organs, determines plant performance along environmental gradients [1–4]. In specific, proper biomass allocations guarantee the functions of different plant organs: leaves perform photosynthesis to fix carbon, stems and branches act as transport corridors and mechanical support, roots take up nutrients and water and anchor the plant, and seeds/fruits execute sexual reproduction [5–7]. Therefore, the patterns of biomass allocation reflect functional tradeoffs among plant organs and hence life history strategies [8, 9]. At the ecosystem level, patterns of plant biomass allocation are critical for the functioning of terrestrial ecosystems, because they impact biomass distribution, carbon cycling, and vegetation responses to environmental changes [10–12]. To date, most studies on biomass allocation patterns have focused on vegetative organs such as leaves, shoots and roots [13].
However, an essential aspect of plant life history strategy is reproductive allocation, which refers to the proportion of the total resources devoted to reproductive structures [5, 14] and represents the cost of reproduction [15, 16]. Allocation between vegetative and reproductive organs influences seed quality and yield, which in turn determines plant fitness [17]. Variation in reproductive allocation typically indicates distinct plant strategies imposed by natural selection [14]. At a broad scale, environmental gradients (e.g., soil and climate) directly affect plant growth and reproduction, as well as biomass allocation between the two processes. For instance, in suitable environments, larger plants with higher growth rate allocate more resources to reproductive processes; however, in unsuitable environments, plants reduce their allocation to reproduction to cope with environmental constraints [18, 19]. In addition, the availability of essential resources such as light, water and nutrients influences biomass allocation among plant organs [20]. Yet it is still uncertain how biomass allocation between growth and reproduction shifts across large environmental gradients.
There are two prevailing theories on the patterns of biomass allocation among plant organs: The optimal allocation theory (OPT; also called the balanced growth theory) [11, 21, 22] and the allometric allocation theory (APT) [19, 23, 24]. The OPT states that plants allocate more biomass toward the organ with the greatest capacity to absorb limiting resources to maximize their performance [11, 21, 22]. According to the OPT, the ratio of reproductive biomass to total biomass does not change significantly with plant size [11, 21, 25]. Instead, plants can adjust their resource allocation to favor growth or reproduction depending on the prevailing conditions, thereby maximizing their fitness [9]. In contrast, the APT posits a uniform allocation pattern for all plants, subject to allometric constraints that limit biomass partitioning to different organs [10, 26]. Based on the APT, biomass allocation depends solely on plant size rather than on environmental conditions [26, 27]. To date, most studies testing the two theories of reproductive allocation have focused on the flowering stage, during which time reproductive mass typically increases as vegetative mass decreases [11, 17, 23, 24, 26]. However, the studies at the flowering stage cannot provide us with a complete understanding of life history strategies, as plant fitness is more directly linked to seeds produced at a later stage.
Although many studies have investigated the allocation patterns of reproductive and vegetative organs separately [1, 7, 27–32], few studies have examined the allocation patterns between them simultaneously. Among them, some studies supported APT [24, 33, 34], while others supported OPT [9, 21, 35]. Hence, it is still unclear whether biomass allocation patterns conform APT or OPT. Furthermore, biomass allocation patterns between reproductive and vegetative organs have seldom been investigated along large environmental gradients or among closely related species.
Here, we sampled 22 Artemisia species to explore how biomass allocation between reproductive and vegetative organs varies along a broad environmental gradient in China. We chose this genus because: (1) It is a specis-rich genus, comprising 350–500 species; (2) It is widely distributed in the natural habitats across Asia, Europe, and North America; (3) Most species in this genus have similar life forms (perennial herbs), with a few annuals, semi-shrubs and small shrubs. These features make it an ideal genus to study allocation patterns along large environmental gradients [36–38]. We proposed three hypotheses. First, we hypothesize that plant size (total biomass) should vary along environmental gradients, because the environment provides plants with resources for growth and reproduction (Fig. 1). With respect to climate, temperature can alter physiological processes and consequently determine plant growth [39, 40]. In addition, plants in regions with high precipitation tend to grow larger than those in arid regions, while extreme precipitation and seasonal changes have negative effects on plant growth [41]. For soil conditions, nutrient availability strongly influences plant growth, as reported in natural Pinus tabuliformis forests [42]. Further, soil texture and structure are also important determinants of plant size, as demonstrated in rhizomatous wetland plants [43].
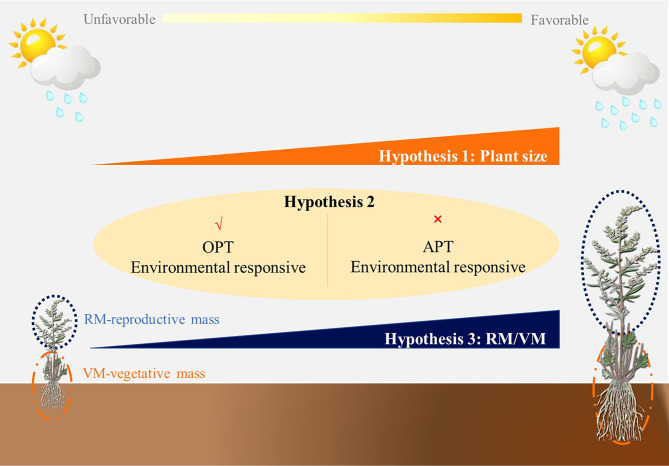
Fig. 1.
Conceptual diagram illustrating the three hypotheses tested in this study. Hypothesisis 1 is that plant size (total biomass) should vary along environmental gradients. Hypothesisis 2 is that biomass allocation between reproductive and vegetative organs conforms optimal partition theory (OPT) rather than allometric partition theory (APT). Hypothesisis 3 is that the allocation between reproductive and vegetative organs responds plastically to the environmental gradient
Second, we hypothesize that biomass allocation between reproductive and vegetative organs conforms OPT (Fig. 1). Plants allocate resources differently to their reproductive and vegetative organs [44, 45], which results in OPT [25]. Some studies have already reported evidence to support OPT at small scales. For example, light exposure influences allocation patterns between growth and reproduction of Plantago species to optimize resource use [46, 47]. Perennial plants adjust their resource allocation to optimize growth and reproductive output, thereby maximizing fitness under varying conditions [9]. In addition, the optimal allocation of resources between growth and reproduction influences size of the plant at maturity [48]. Therefore, plants could adopt optimal partitioning strategies to balance growth and reproduction and thus maximize fitness.
Finally, we hypothesize that the allocation between reproductive and vegetative organs responds plastically to the environmental gradient (Fig. 1). Increased plant growth in favorable environments has been reported to result in larger plants that can allocate more resources to reproduction [5, 19, 49]. Some large-scale studies also show apparent differences in the allocation between reproductive and vegetative biomass. For instance, environmental factors influence the allocation of reproductive and vegetative biomass of Leymus chinensis in northeastern China, with plants allocating more biomass to vegetative growth under nutrient-rich conditions, but more to reproductive structures under optimal climatic conditions to maximize reproductive success [50, 51]. In addition, soil temperature drives elevational patterns of reproductive allocation in the Gaoligong Mountains of China, with plants allocating more resources to reproductive structures at higher elevations to maximize reproductive success [52]. In grasslands, plants adjust their biomass allocation to optimize growth and reproduction along temperature and precipitation gradients [53].
To test the three hypotheses, we first quantified how the environmental gradient affects plant size of Artemisia; then we determined whether biomass allocation between reproductive and vegetative organs conforms OPT or APT; finally, we assessed how biomass allocation changes along the environmental gradient. In addition, we identified these patterns at the individual, interspecific and intraspecific levels to determine whether the biomass allocation strategy was consistent across the three taxonomic levels.
Materials and methods
Plant sampling
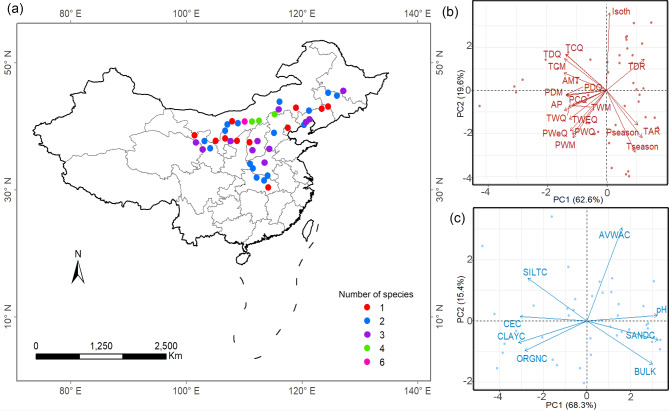
We collected Artemisia species from 42 sites along a large environmental gradient in China in November, 2023 (Fig. 2A), when the seeds on most plants were fully matured. We focused on natural vegetation and established sites in areas with minimal human disturbance, avoiding those impacted by agriculture, urbanization, or significant habitat modification. Sites were located along a large environmental gradient in climate and soil to capture diverse conditions that influence Artemisia species. At each site, we recorded longitude, latitude and altitude, and four plots of 1 m × 1 m with distance > 5 m were established randomly. Then, all species of Artemisia were identified, and four reproductive individuals of each species were randomly sampled and put into separate paper bags. In total, we collected 376 individuals of 22 species with fully matured seeds from all sites (Table 1). All species were formally identified by Associate Professor Ruiru Gao (The School of Life Sciences, Shanxi Normal University). The voucher specimens are preserved in the Herbarium of the School of Life Sciences, Shanxi Normal University.
Fig. 2.
The location and environmental gradients of 42 sampling sites across China. (A) The location of sampling sites. (B) The first two PC axes of 19 climate variables. (C) The first two PC axes of 8 soil variables
Table 1.
List of species sampled, growth form, and number of sites of the occurrence of each species investigated in this study
| No. | Latin name | Growth from | Number of sites |
|---|---|---|---|
| 1 | Artemisia anethifolia | Annual or biennial | 1 |
| 2 | Artemisia annua | Annual | 6 |
| 3 | Artemisia capillaris | Perennial | 10 |
| 4 | Artemisia caruifolia | Annual or biennial | 1 |
| 5 | Artemisia demissa | Annual or biennial | 1 |
| 6 | Artemisia dubia | Subshrub | 2 |
| 7 | Artemisia edgeworthii | Annual or biennial | 3 |
| 8 | Artemisia freyniana | Subshrub | 1 |
| 9 | Artemisia frigida | Perennial | 3 |
| 10 | Artemisia giraldii | Subshrub | 3 |
| 11 | Artemisia incisa | Perennial | 1 |
| 12 | Artemisia indica | Perennial | 2 |
| 13 | Artemisia japonica | Perennial | 1 |
| 14 | Artemisia klementze | Subshrub | 1 |
| 15 | Artemisia lavandulaefolia | Perennial | 12 |
| 16 | Artemisia mongolica | Perennial | 2 |
| 17 | Artemisia ordosica | Shrubs | 7 |
| 18 | Artemisia sacrorum | Subshrub | 5 |
| 19 | Artemisia scoparia | Perennial | 14 |
| 20 | Artemisia sieversiana | Annual or biennial | 11 |
| 21 | Artemisia sphaerocephala | Shrub | 2 |
| 22 | Artemisia tangutica | Perennial | 1 |
Organ biomass determination
In the laboratory, we separated each individual into four modules, including one reproductive organ (infructescences including seeds) and three vegetative organs (leaves, stems and roots). The separated organs (infructescence, leaf, stem and root) were oven-dried at 65 ºC for 48 h to constant weight and weighed to determine their dry mass. Reproductive mass (RM) was the weight of the infructescences of each individual, while vegetative mass (VM) was the sum of the mass of stems, leaves and roots.
Environmental variables
According to the geographical coordinates of the sampling sites, we extracted nineteen bioclimatic variables (eleven temperature and eight precipitation variables; Table 2) from the World Climate Information Database [54]. Our sampling sites covered a wide climate gradient, with annual mean temperature (AMT, ranging from 0.29 °C to 17.1 °C) and annual precipitation (AP, from 146 mm to 1260 mm). Additionally, eight soil variables from the global SoilGrids250m database [55], including bulk density (BULK, ranging from 1044.8 to 1578.2 kg/m³) and cation exchange capacity (CEC, ranging from 2.4 to 32.56 cmolc/kg).
Table 2.
Principle components analysis of climate and soil variables across all sites
| Variables | Description | PC1 | PC2 |
|---|---|---|---|
| Climate | |||
| AMT | Annual mean temperature | -0.265 | 0.109 |
| TDR | Mean diurnal range (Mean of monthly (max temp - min temp)) | 0.236 | 0.196 |
| Isoth | Isothermality | 0.017 | 0.485 |
| Tseason | Temperature seasonality (standard deviation ×100) | 0.179 | -0.386 |
| TWM | Max temperature of warmest month | -0.215 | -0.093 |
| TCM | Min temperature of coldest month | -0.262 | 0.197 |
| TAR | Temperature annual range | 0.222 | -0.293 |
| TWEQ | Mean temperature of wettest quarter | -0.231 | -0.180 |
| TDQ | Mean temperature of driest quarter | -0.255 | 0.225 |
| TWQ | Mean temperature of warmest quarter | -0.236 | -0.100 |
| TCQ | Mean temperature of coldest quarter | -0.254 | 0.227 |
| AP | Annual precipitation | -0.265 | -0.125 |
| PWM | Precipitation of wettest month | -0.209 | -0.294 |
| PDM | Precipitation of driest month | -0.253 | -0.034 |
| Pseason | Precipitation seasonality (coefficient of variation) | 0.195 | -0.214 |
| PWeQ | Precipitation of wettest quarter | -0.225 | -0.252 |
| PDQ | Precipitation of driest quarter | -0.252 | -0.027 |
| PWQ | Precipitation of warmest quarter | -0.213 | -0.273 |
| PCQ | Precipitation of coldest quarter | -0.252 | -0.027 |
| Soil | |||
| BULK | Bulk density of the fine earth fraction | 0.366 | -0.363 |
| CEC | Cation exchange capacity of the soil | -0.374 | 0.038 |
| CLAYC | Proportion of clay particles (< 0.002 mm) in the fine earth fraction | -0.383 | -0.182 |
| ORGNC | Organic carbon content | -0.347 | -0.253 |
| pH | Soil pH | 0.392 | 0.047 |
| SANDC | Proportion of sand particles (> 0.05 mm) in the fine earth fraction | 0.398 | -0.160 |
| SILTC | Proportion of silt particles (≥ 0.002 mm and ≤ 0.05 mm) in the fine earth fraction | -0.330 | 0.358 |
| AVWAC | Available water capacity (%) | 0.196 | 0.783 |
The first two PC axes were shown for the 19 climate variables and 8 soil variables
Statistical analysis
All analyses were conducted using R version 4.3.1 (https://www.r-project.org/). First, a principal component analysis (PCA) was carried out by applying the prcomp function in the stats package to determine the gradients in the climate and soil data separately. Before PCAs, the climate and soil data were centered and scaled.
To test the first hypothesis, we used linear mixed-effect models (LMMs) to determine how plant size (total biomass) varied with environmental gradients using the lme4 package [56]. Plant size was log10-transformed to enhance residual normality. We analyzed the data on three levels. On the individual level, we used pooled samples to determine overall patterns, with environmental gradients and species nested in sites as fixed and random effects, respectively. On the interspecific level, we used species means at each site to determine interspecific patterns, with environmental gradients and sites as fixed and random effects, respectively. On the intraspecific level, we used 10 species occurring in at least three sites to determine intraspecific patterns.
To test the second hypothesis, standardized major axes (SMA) regressions were performed to quantify the allometric power functions between RM and VM. The sma function in the smart package [57] was used. SMA regression is a standard allometric technique for calculating scaling exponents (α) and allometric constants (β) [10]. We also analyzed the data on three levels. First, we used the data of all individuals to determine overall patterns. Second, we used the means of the 22 species at each site to determine interspecific patterns. Third, we used species occurring in at least three sites to test intraspecific patterns. We also analyzed the allometric relationships between RM and each of the three vegetative organs (leaf, stem and root) separately.
To test the third hypothesis, we performed additional LMMs to examine the effects of climate and soil gradients on biomass allocation (the ratio of RM to VM). LMMs were also performed on three levels. First, we used the data from all individuals to determine overall patterns. Second, we calculated species means at each site, which were used to determine interspecific patterns. Third, at the intraspecific level, we used species occuring in at least three sites to determine intraspecific patterns. To determine whether the relationship was consistent across the three vegetative organs, we further carried out the analyses on the ratios of RM to stem, leaf and root mass separately.
Results
The effect of environmental gradients on plant size
PCAs of nineteen climate and eight soil variables revealed that the first two axes explained most of the variation in the climate (82.2%) and soil (83.7%) variables (Fig. 2B and C). Climate PC1 was a temperature and precipitation axis that was negatively correlated with AMT, AP and TCM, while climate PC2 was a climate variability axis that was positively correlated with Isoth but negatively correlated with Tseason and Pseason (Fig. 2B; Table 2). For soil gradients, PC1 was a chemical axis that was positively correlated with pH and SANDC but negatively with CEC, while PC2 was a physical axis that was correlated negatively with BULK but positively to AVWAC (Fig. 2C; Table 2).
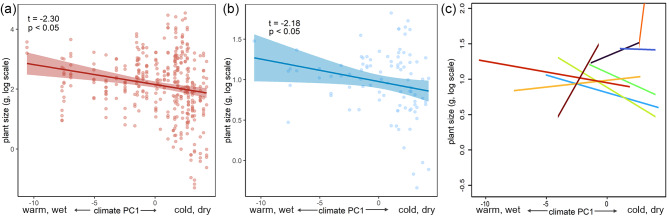
LMMs showed that plant size decreased significantly with climate PC1 at the individual level (Fig. 3A). A similar trend was observed at the interspecific level (Fig. 3B), while the relationships were mixed at the intraspecific level (Fig. 3C; Table 3). In contrast, climate PC2, soil PC1 and soil PC2 had no significant effect on plant size at the individual, interspecific or intraspecific level.
Fig. 3.
Relationships between plant size of Artemisia species and environmental gradients. (A) The individual level. (B) The interspecific level. (C) The intraspecific level, where different colour lines represent all relationships of the 10 different species. Species names are provided in Table 3. t and p values are from linear mixed models
Table 3.
Linear mixed models for analyses of the relationships between plant size of Artemisia species occuring in at least three sites and environmental gradients
| Species name | Climate | Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t-value | p | Estimate | SE | t-value | p | ||
| A.sacrorum | Intercept | 1.31 | 0.14 | 9.13 | 0.01 | 1.54 | 0.20 | 7.68 | 0.02 |
| PC1 | 0.07 | 0.06 | 1.16 | 0.37 | 0.01 | 0.10 | 0.13 | 0.91 | |
| PC2 | -0.04 | 0.09 | 0.46 | 0.69 | -0.21 | 0.16 | -1.36 | 0.31 | |
| A.ordosica | Intercept | 1.43 | 0.45 | 3.20 | 0.03 | 1.13 | 0.49 | 2.29 | 0.08 |
| PC1 | -0.01 | 0.16 | -0.04 | 0.97 | 0.15 | 0.18 | 0.86 | 0.44 | |
| PC2 | 0.05 | 0.11 | 0.41 | 0.70 | 0.17 | 0.43 | 0.39 | 0.72 | |
| A.scoparia | Intercept | 0.80 | 0.06 | 12.28 | < 0.001 | 0.81 | 0.07 | 12.14 | < 0.001 |
| PC1 | -0.05 | 0.02 | -2.03 | 0.06 | -0.03 | 0.03 | -0.87 | 0.40 | |
| PC2 | -0.06 | 0.03 | -2.26 | 0.04 | -0.10 | 0.05 | -1.93 | 0.07 | |
| A.edgeworthii | Intercept | -2.62 | 6.48 | -0.40 | 0.70 | 1.07 | 0.79 | 1.35 | 0.21 |
| PC1 | -3.21 | 8.63 | -0.37 | 0.72 | -0.17 | 0.30 | -0.57 | 0.58 | |
| PC2 | 3.35 | 8.24 | 0.41 | 0.69 | -0.39 | 0.39 | -1.00 | 0.34 | |
| A.sieversiana | Intercept | 1.08 | 0.09 | 11.88 | < 0.001 | 1.05 | 0.06 | 17.01 | < 0.001 |
| PC1 | -0.08 | 0.03 | -2.55 | 0.03 | -0.11 | 0.04 | -2.60 | 0.03 | |
| PC2 | -0.05 | 0.03 | -1.36 | 0.21 | -0.15 | 0.05 | -2.85 | 0.02 | |
| A.capillaris | Intercept | 0.87 | 0.08 | 10.95 | < 0.001 | 0.91 | 0.08 | 10.84 | < 0.001 |
| PC1 | -0.10 | 0.03 | -3.19 | 0.02 | -0.21 | 0.08 | -2.76 | 0.03 | |
| PC2 | -0.08 | 0.05 | -1.38 | 0.21 | -0.10 | 0.11 | -0.94 | 0.38 | |
| A.annua | Intercept | 0.97 | 0.18 | 5.46 | 0.01 | 0.87 | 0.18 | 4.89 | 0.02 |
| PC1 | 0.02 | 0.04 | 0.48 | 0.66 | 0.07 | 0.09 | 0.81 | 0.48 | |
| PC2 | 0.24 | 0.63 | 0.39 | 0.72 | -0.26 | 0.37 | -0.71 | 0.53 | |
| A.frigida | Intercept | -2.08 | 0.61 | -3.38 | 0.01 | 0.16 | 0.38 | 0.41 | 0.69 |
| PC1 | 1.35 | 0.31 | 4.28 | < 0.001 | -0.02 | 0.28 | -0.07 | 0.95 | |
| PC2 | 2.18 | 0.41 | 5.26 | 0.00 | 1.46 | 0.27 | 5.46 | < 0.001 | |
| A.lavandulaefoliada | Intercept | 0.94 | 0.09 | 9.97 | < 0.001 | 0.91 | 0.13 | 7.25 | < 0.001 |
| PC1 | -0.03 | 0.02 | -1.57 | 0.15 | -0.06 | 0.04 | -1.41 | 0.19 | |
| PC2 | -0.06 | 0.04 | -1.70 | 0.12 | -0.02 | 0.05 | -0.39 | 0.71 | |
| A.giraldii | Intercept | 1.69 | 0.39 | 4.37 | 0.00 | 1.64 | 1.28 | 1.28 | 0.23 |
| PC1 | 0.30 | 0.15 | 2.06 | 0.07 | 1.40 | 2.41 | 0.58 | 0.58 | |
| PC2 | 0.30 | 0.16 | 1.85 | 0.10 | 1.67 | 2.82 | 0.59 | 0.57 | |
The bold values mean the effects of environmental gradients are significant
The relationships between RM and VM
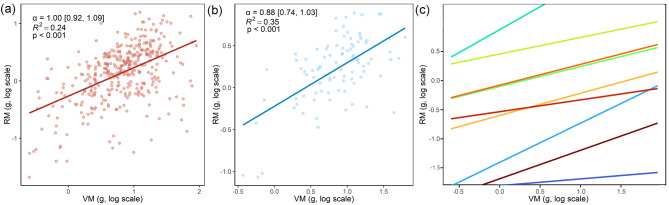
SMA analyses revealed significant isometric relationships between RM and VM at both individual and interspecific levels (Fig. 4A and B). Additionally, most relationships at the intraspecific level were similar to those at the individual level (Fig. 4C, Table S1).
Fig. 4.
Standardized major axis (SMA) regressions between reproductive mass (RM) and vegetative mass (VM) of Artemisia. (A) The individual level. (B) The interspecific level. (C) The intraspecific level, where different color lines represent all relationships of the 10 different species. Species names are provided in Table S1. α is the observed allometric exponent, and numbers in square brackets are the lower and upper 95% confidence intervals. All data are log10-transformed before analysis
For the three vegetative organs, the relationship between RM and leaf mass was not significant at both individual and interspecific levels (Figs S1A and B). However, the relationship between RM and stem mass was isometric (Figs. S1D and E), but that between RM and root mass was allometric (Figs S1G and H). At the intraspecific level, these relationships differed among species (Figs S1C, F and I, Table S1).
The patterns of biomass allocation between reproductive and vegetative organs along environmental gradients
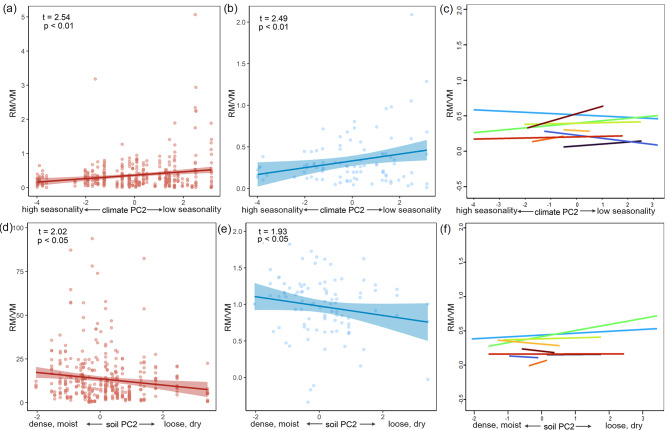
LMMs showed that climate PC1 (individual level: t = 1.07, p = 0.28; interspecific level: t = 0.87, p = 0.38) and soil PC1 (individual level: t = 1.44, p = 0.15; interspecific level: t = 1.28, p = 0.20) did not significantly affect the ratio of RM to VM, but climate PC2 and soil PC2 did (Fig. 5). In specific, the ratio of RM to VM increased with climate PC2 (Fig. 5A and B), but decreased with soil PC2 (Fig. 5D and E). These trends were consistent at both interspecific and individual levels (Fig. 5). At the intraspecific level, however, the relationships were mixed (Fig. 5C and F; Table 4).
Fig. 5.
Relationships between the ratio of reproductive mass to vegetation mass (RM/VM) of Artemisia and environmental gradients. (A, D) The individual level. (B, E) The interspecific level. (C, F) The intraspecific level, where different colored lines represent all relationships for the 10 species analyzed. Species names are provided in Table 4. t and p values are from linear mixed models
Table 4.
Linear mixed models for analyses of the relationships between the ratio of reproductive to vegetation mass (RM/VM) of Artemisia species occuring in at least three sites and environmental gradients
| Species name | Climate | Soil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Estimate | SE | t-value | p | Estimate | SE | t-value | P | ||
| A.sacrorum | Intercept | 0.07 | 0.05 | 1.45 | 0.28 | 0.16 | 0.11 | 1.53 | 0.27 |
| PC1 | 0.03 | 0.02 | 1.34 | 0.31 | 0.00 | 0.05 | 0.08 | 0.94 | |
| PC2 | 0.04 | 0.03 | 1.39 | 0.30 | -0.03 | 0.08 | -0.31 | 0.79 | |
| A.ordosica | Intercept | 0.23 | 0.09 | 2.66 | 0.06 | 0.12 | 0.12 | 0.98 | 0.38 |
| PC1 | -0.05 | 0.03 | -1.49 | 0.21 | -0.03 | 0.04 | -0.67 | 0.54 | |
| PC2 | -0.04 | 0.02 | -1.81 | 0.14 | -0.10 | 0.10 | -0.95 | 0.40 | |
| A.scoparia | Intercept | 0.52 | 0.04 | 4.13 | < 0.001 | 0.45 | 0.05 | 9.34 | < 0.001 |
| PC1 | -0.02 | 0.01 | -1.32 | 0.21 | 0.03 | 0.02 | 1.30 | 0.21 | |
| PC2 | 0.07 | 0.02 | 4.52 | < 0.001 | 0.07 | 0.04 | 1.92 | 0.07 | |
| A.edgeworthii | Intercept | 2.91 | 28.02 | 0.10 | 0.92 | 4.22 | 5.06 | 0.83 | 0.43 |
| PC1 | -4.81 | 37.31 | -0.13 | 0.90 | -1.09 | 1.93 | -0.56 | 0.59 | |
| PC2 | 2.93 | 35.63 | 0.08 | 0.94 | -0.63 | 2.47 | -0.26 | 0.80 | |
| A.sieversiana | Intercept | 0.40 | 0.14 | 2.88 | 0.02 | 0.42 | 0.07 | 5.68 | < 0.001 |
| PC1 | 0.03 | 0.05 | 0.74 | 0.48 | 0.09 | 0.05 | 1.79 | 0.08 | |
| PC2 | 0.01 | 0.05 | 0.24 | 0.82 | 0.06 | 0.06 | 0.94 | 0.35 | |
| A.capillaris | Intercept | 0.40 | 0.04 | 9.64 | < 0.001 | 0.39 | 0.04 | 9.57 | < 0.001 |
| PC1 | 0.01 | 0.02 | 0.46 | 0.66 | 0.01 | 0.04 | 0.36 | 0.73 | |
| PC2 | 0.04 | 0.03 | 1.45 | 0.19 | 0.07 | 0.05 | 1.38 | 0.21 | |
| A.annua | Intercept | 0.29 | 0.09 | 3.12 | 0.05 | 0.32 | 0.10 | 3.18 | 0.05 |
| PC1 | -0.02 | 0.02 | -0.93 | 0.42 | -0.04 | 0.05 | -0.87 | 0.45 | |
| PC2 | 0.02 | 0.33 | 0.07 | 0.95 | 0.04 | 0.21 | 0.17 | 0.87 | |
| A.frigida | Intercept | 0.24 | 0.38 | 0.64 | 1.00 | 0.05 | 0.22 | 0.25 | 0.81 |
| PC1 | 0.06 | 0.19 | 0.33 | 1.00 | 0.15 | 0.16 | 0.95 | 0.36 | |
| PC2 | 0.20 | 0.26 | 0.77 | 1.00 | 0.25 | 0.15 | 1.60 | 0.14 | |
| A.lavandulaefoliada | Intercept | 0.20 | 0.03 | 6.01 | < 0.001 | 0.17 | 0.04 | 3.85 | < 0.001 |
| PC1 | 0.01 | 0.01 | 1.14 | 0.28 | 0.00 | 0.02 | 0.04 | 0.97 | |
| PC2 | 0.01 | 0.01 | 1.17 | 0.27 | 0.01 | 0.02 | 0.60 | 0.56 | |
| A.giraldii | Intercept | 0.53 | 0.19 | 2.86 | 0.02 | 0.21 | 1.12 | 0.19 | 0.85 |
| PC1 | 0.11 | 0.07 | 1.50 | 0.17 | -0.06 | 2.10 | -0.03 | 0.98 | |
| PC2 | 0.19 | 0.08 | 2.38 | 0.04 | 0.18 | 2.45 | 0.07 | 0.94 | |
The bold values mean the effects of environmental gradients are significant
For the three vegetative organs, the ratio of RM to stem and root mass increased significantly, but that of RM to leaf mass decreased, with climate PC2 at both individual and interspecific levels (Fig. S2, Table S2). Although soil PC2 had no significant effect on the ratio of RM to leaf or root mass at all three levels, it had a significant positive effect on the ratio of RM to stem mass at both individual and interspecific levels (Fig. S3, Table S3). At the intraspecific level, the relationships were mixed; while some species had positive relationships, others were negatively correlated with climate PC2 and soil PC2 (Figs S2 and S3, Tables S2 and S3).
Discussion
Temperature and precipitation strongly affect plant size of Artemisia
In support of our first hypothesis, we found that plant size was negatively correlated with climate PC1 (Fig. 3), an axis that was negatively correlated with temperature and precipitation (Fig. 2B; Table 2). These results suggest that high temperature and precipitation favor plant growth and biomass accumulation of Artemisia species. Three main mechanisms could explain such positive effects. (1) Favorable climatic conditions enhance plant metabolic processes during growth [20, 58, 59]. For instance, an adequate precipitation is essential to maintain plant productivity, whereas water deficit can impair metabolic processes such as photosynthesis and respiration, resulting in reduced plant growth [60, 61]. For Artemisia species, a previous study also highlighted the significant role of precipitation in determining biomass accumulation across northern China [62]. (2) Climatic conditions impact plant growth by determining how plants acquire resources. In particular, an increase in temperature enhances the availability of water and nutrients in the soil, causing plants to grow and develop faster [63]. (3) Climate affects plant growth by shifting the timing of life cycle events (phenology; e.g., seedling emergence, flowering and fruiting) and subsequently the timing for biomass accumulation [61].
Surprisingly, we found that soil conditions did not significantly affect plant size of Artemisia species. A possible reason could be that the gradients in soil chemical and physical properties in our study are not large enough to influence plant size. The weak effect of soil on plant growth has also been reported in several previous studies. For instance, a meta-analysis demonstrates that soil properties have a weaker effect on plant growth compared to elevated atmospheric CO₂ levels [64]. Similarly, compared to seasonal climatic conditions, soil properties, also have a minor effect in determining net ecosystem production in C3 grasslands, highlighting the dominant role of climate in determining plant size [65].
The consistent trends at the interspecific level suggest the generalizability of the effect of climate across species along the environmental gradient (Fig. 3B). These results align with the report that temperature and precipitation significantly influence plant biomass in Leymus chinensis along a large-scale gradient in northeastern China [51]. This consistency between our study and others underscores the strong influence of climate on plant growth across different species. However, at the intraspecific level, we observed mixed relationships between plant size and environmental gradients (Fig. 3C; Table 3). This suggests that while climatic gradients may consistently influence plant size across species, within-species variability may be influenced by additional factors such as genetic diversity or microenvironmental conditions [66].
Biomass allocation between reproductive and vegetative organs conforms OPT
In line with our second hypothesis, we found significant isometric relationships between reproductive and vegetative mass at both individual and interspecific levels (Fig. 4A and B), supporting the prediction of OPT. In addition, the consistent interspecific patterns suggest that different species follow a similar biomass allocation strategy, possibly due to evolutionary pressures that favor consistent reproductive investment across broad environmental conditions. The isometric relationships suggest an underlying strategy of balanced biomass allocation between reproduction and growth to optimize fitness. Our findings are consistent with the isometric allocation pattern, with a slope close to 1, in cereal-legume intercropping systems [67] and the consistent reproductive allocation of Gentiana species across elevation gradients on the Yunnan-Guizhou Plateau, China [68]. However, other studies have found an allometric relationship between plant reproductive and vegetative mass [19, 24, 69–71]. These studies focused on different species, life-forms, environmental contexts, nutrient additions or land-use changes, which may result in the different results from our study. Furthermore, Artemisia species, being well-adapted to diverse and sometimes harsh environments, may have evolved a strategy to maintain an isometric resource allocation to balance growth and reproductive success under variable conditions.
Furthermore, our study on Artemisia species revealed distinct patterns of reproductive allocation in relation to different vegetative organs, with the relationships between reproductive mass and root mass being allometric, but that between reproductive mass and stem mass being isometric (Fig. S1). The different allocation patterns could be attributed to the different roles among plant organs. Leaves and roots are directly involved in photosynthesis and nutrient uptake, which are critical for supporting reproduction. These findings are consistent with the allometric models of seed plant reproduction, which posited that plants allocate resources to maximize reproductive success while maintaining essential vegetative functions [72]. In addition, our findings align with the dynamic optimization theory, which suggests that plants dynamically adjust their growth and resource allocation to balance immediate growth with reproductive success [8]. The isometric relationship between reproductive mass and stem mass (Fig. S1D and E) indicates that reproductive output also increases proportionately with increasing stem growth to maintain structural integrity and efficient nutrient transport. Conversely, the allometric relationship between reproductive mass and root mass (Fig. S1G and H) indicates that Artemisia species prioritize reproductive investment over root growth during reproduction. Similar results have been reported in Tibetan alpine grasslands, where reproductive mass allocation varies allometrically with root mass in response to environmental conditions, reflecting an adaptive response to maximize reproductive output while maintaining sufficient root function [69].
It is important to distinguish between the intra- and interspecific biomass allocation patterns, as they can differ significantly [24]. In our study, the allocation patterns within species (i.e., the intraspecific level) are largely consistent with the general patterns observed at the individual level and the overall trends seen across different species (i.e., the interspecific level), whereas the relationships differed among species at the intraspecific level (Figs S1C, F and I, Table S1), highlighting the potentially diverse intraspecific adaptations to environmental gradients. Similarly, in cereal-legume intercropping systems, both interspecific and intraspecific factors play crucial roles in determining biomass allocation patterns [67]. Also, life forms (e.g., annual, perennial and semi-shrub) have different reproductive allocations at the intraspecific and interspecific levels. For instance, an experiment with fifteen species showed that long-lived iteroparous species typically exhibit very low reproductive allocation, whereas species with shorter lifespans exhibit relatively high reproductive allocation [73]. For two Plantago species, reproductive allocation of P. major decreases with vegetative mass, but there is no consistent relationship between reproduction allocation for P. asiatica [46, 47].
Biomass allocation between reproductive and vegetative organs is plastic to environmental gradients
In accordance with our third hypothesis, we found that biomass allocation between reproductive and vegetative organs was plastic in response to environmental gradients (Fig. 5). Our findings align with those of previous studies reporting significant effects of climatic factors and soil water availability on plant reproductive strategies [17, 74]. In North American, biomass allocation to reproduction of sunflowers varied with climate and soil variables [75]. With regard to climate, we found a significant increase in the ratio of reproductive to vegetative mass with climate variability (Fig. 5A and B). Because climate variability axis (PC2) was positvely associated with isothermality but negatively with Tseason and Pseason (Fig. 2), our results suggest that plants invest more in reproduction under lower climate variability (i.e., more stable climatic conditions). However, an earlier study on Artemisia reported that climate has a weak effect on biomass allocation among vegetative organs [66]. The key difference between the present study and that study is that they focused on biomass allocation in different ontogenetic stages of Artemisia. Therefore, our results suggest that allocation patterns differ fundamenally between growth and reproduction stages.
For the three vegetative organs, our analysis revealed significant increases in the ratio of reproductive mass to stem and root mass with climate variability, while the ratio to leaf mass decreased (Fig. S2). Our findings partially support the general trend that plants often decrease allocation to reproductive structures, but increase that to roots, under climatic stresses [76]. The disparate responses among the organs may be indicative of organ-specific allocation mechanisms. For Artemisia, a robust stem structure may be essential for maintaining the health and function of reproductive organs under stressful conditions. The promotion of reproduction by the stem has also been documented in other Asteraceae herbs, in which the increased stem allocation is associated with enhanced reproductive success across varying soil depths and water availability [77]. Conversely, we found the biomass allocation to leaves exhibited a contrasting pattern, indicating a trade-off where resources are redirected from leaves to reproductive and supportive structures including stems, roots and fruits. Furthermore, the reduction in leaf allocation of Artemisia could minimize water loss and enhance resource use efficiency under stressful conditions. Similarly, Vallisneria spinulosa has been observed to reduce leaf mass in favor of reproductive success when resources are limited [74].
With regard to the soil, we found a significant change in the biomass ratio of reproductive to vegetative organs with physical properties (Fig. 5D and E). This points to a potentially unique role of soil conditions in influencing structural growth components such as stems, which may be of particular importance for supporting reproductive structures. Soil physical properties, including texture and water availability, are critical factors influencing nutrient uptake, plant health, structure and reproductive success [34]. In a semi-arid grassland, an increase in soil water and nutrient availability causes plants to invest less in belowground biomass, with a corresponding increase in allocation to reproduction [63]. Under high nutrient conditions, Plantago lanceolata maximizes fitness by increasing root biomass to enhance nutrient uptake and allocating more resources to reproductive organs [35]. Similarly, soil water availability significantly affects reproductive strategies in Asteraceae herbs by influencing biomass allocation, reproductive phenology and seed production [77].
At the intraspecific level, the relationships were mixed. While some species had positive relationships with climate variability and soil physical properties, others had negative relationships (Fig. 5C and F; Table 4), suggesting that species-specific adaptive strategies significantly influence how biomass allocation responds to environmental gradients. Reproductive allocation is known to vary significantly among species with different life forms and even among populations of a species growing in different environmental conditions [15, 18, 19, 78]. For example, semelparous annual species allocate a greater proportion of their resources to reproduction than iteroparous perennials [78]. Similarly, distinct biomass allocation patterns have been observed in different sunflower species growing in diverse soil pH, organic matter content, cation exchange capacity across North America [75]. Together, our findings of diverse intraspecific responses indicate that genetic diversity and micro-environmental conditions may play an important role in shaping species-specific responses. However, further research is needed to explore these factors in a broader range of species groups and environmental gradients.
Conclusions
Our study has demonstrated that climate has a more pronounced effect on plant size than soil conditions along the environmental gradient for pooled species. However, this does not rule out the important role of soil on plant growth of some species, because our study involved different species that may evolve according to environmental conditions. In addition, the allocation between reproductive and vegetative organs is isometric, which does not support the OPT, at both the individual and interspecific levels. This suggests a proportional allocation between reproductive and vegetative growth that transcends species-specific variation. Furthermore, our findings show the plasticity in biomass allocation between reproductive and vegetative organs along environmental gradients, aligning with the prediction of the OPT. Such plasticity is merged among individuals, across species and even within species, indicating a consistent adaptation in plant growth and reproduction across different taxonomic levels. These findings highlight the necessity of considering climatic and soil factors in understanding plant ecological strategies in growth and reproduction.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
X.Y. and Z.H. conceived the ideas and designed methodology; T.T., X.Y., R.G., J.L., W.T., G.L. and X.Y. collected the data; T.T. and X.Y. analyzed the data; T.T., X.Y. and Z.H. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Funding
This work was supported by the National Natural Science Foundation of China [grant numbers 31770514 and 32071524].
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Xuejun Yang, Email: xjyang_jx@ibcas.ac.cn.
Zhenying Huang, Email: zhenying@ibcas.ac.cn.
References
- 1.Poorter H, Nagel O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol. 2000;27(6):595–607. [Google Scholar]
- 2.Crosby SC, Ivens-Duran M, Bertness MD, Davey E, Deegan LA, Leslie HM. Flowering and biomass allocation in U.S. Atlantic coast Spartina alterniflora. Am J Bot. 2015;102(5):669–76. [DOI] [PubMed] [Google Scholar]
- 3.Crosby SC, Sax DF, Palmer ME, Booth HS, Deegan LA, Bertness MD, et al. Salt marsh persistence is threatened by predicted sea-level rise. Estuar Coast Shelf Sci. 2016;181:93–9. [Google Scholar]
- 4.Husáková I, Weiner J, Münzbergová Z. Species traits and shoot-root biomass allocation in 20 dry-grassland species. J Plant Ecol. 2018;11(2):273–85. [Google Scholar]
- 5.Bazzaz FA, Chiariello NR, Coley PD, Pitelka LF. Allocating resources to reproduction and defense. Bioscience. 1987;37:58–67. [Google Scholar]
- 6.Kleyer M, Minden V. Why functional ecology should consider all plant organs: an allocation-based perspective. Basic Appl Ecol. 2015;16:1–9. [Google Scholar]
- 7.Poorter H, Jagodzinski AM, Ruiz-Peinado R, Kuyah S, Luo Y, Oleksyn J, et al. How does biomass distribution change with size and differ among species? An analysis for 1200 plant species from five continents. New Phytol. 2015;208(3):736–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwasa Y. Dynamic optimization of plant growth. Evol Ecol Res. 2000;2:434–55. [Google Scholar]
- 9.Mironchenko A, Kozlowski J. Optimal allocation patterns and optimal seed mass of a perennial plant. J Theor Biol. 2014;354:12–24. [DOI] [PubMed] [Google Scholar]
- 10.Niklas KJ, Enquist BJ. Canonical rules for plant organ biomass partitioning and annual allocation. Am J Bot. 2002;89(5):812–9. [DOI] [PubMed] [Google Scholar]
- 11.McCarthy MC, Enquist BJ. Consistency between an allometric approach and optimal partitioning theory in global patterns of plant biomass allocation. Funct Ecol. 2007;21:713. [Google Scholar]
- 12.Yan B, Ji Z, Fan B, Wang X, He G, Shi L, et al. Plants adapted to nutrient limitation allocate less biomass into stems in an arid hot grassland. New Phytol. 2016;211:1232–40. [DOI] [PubMed] [Google Scholar]
- 13.Kumordzi BB, Gundale MJ, Nilsson MC, Wardle DA. Shifts in aboveground biomass allocation patterns of dominant shrub species across a strong environmental gradient. PLoS ONE. 2016;11(6):e0157136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bazzaz FA, Ackerly DD, Reekie EG. Reproductive allocation in plants. In: Fenner M, editor. Seeds: the Ecology of Regeneration in Plant communities. 2nd ed. CABI Publishing; 2005. pp. 1–29.
- 15.Wilson AM, Thompson K. A comparative study of reproductive allocation in 40 British grasses. Funct Ecol. 1989;3:297–302. [Google Scholar]
- 16.Wenk EH, Abramowicz K, Westoby M, Falster DS. Investment in reproduction for 14 iteroparous perennials is large and associated with other life-history and functional traits. J Ecol. 2017;106:1338–48. [Google Scholar]
- 17.Tang L, Zhou QS, Gao Y, Li P. Biomass allocation in response to salinity and competition in native and invasive species. Ecosphere. 2022;13:e4027. [Google Scholar]
- 18.Bazzaz FA, Grace J. Plant Resource Allocation. New York: Academic; 1997. [Google Scholar]
- 19.Weiner J. Allocation, plasticity and allometry in plants. Perspect Plant Ecol Evol Syst. 2004;6(4):207–15. [Google Scholar]
- 20.Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytol. 2012;193:30–50. [DOI] [PubMed] [Google Scholar]
- 21.Shipley B, Meziane D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct Ecol. 2002;16(3):326–31. [Google Scholar]
- 22.Lugli LF. Digging deeper? Biomass allocation patterns in trees and lianas in tropical seasonal forests. New Phytol. 2020;226:639–40. [DOI] [PubMed] [Google Scholar]
- 23.Müller I, Schmid B, Weiner J. The effect of nutrient availability on biomass allocation patterns in 27 species of herbaceous plants. Perspect Plant Ecol Evol Syst. 2000;3(2):115–27. [Google Scholar]
- 24.Weiner J, Campbell LG, Pino J, Echarte L. The allometry of reproduction within plant populations. J Ecol. 2009;97:1220–33. [Google Scholar]
- 25.Gedroc JJ, McConnaughay KDM, Coleman JS. Plasticity in root/shoot partitioning: optimal, ontogenetic, or both? Funct Ecol. 1996;10:44–50. [Google Scholar]
- 26.Enquist BJ, Niklas KJ. Global allocation rules for patterns of biomass partitioning in seed plants. Science. 2002;295(5559):1517–20. [DOI] [PubMed] [Google Scholar]
- 27.Fang T, Rao M, Chen Q, Liu S, Lai J, Chen T, et al. Different biomass allocation strategies of geophytes and non-geophytes along an altitude gradient. Ecol Indic. 2023;146:110828. [Google Scholar]
- 28.Yin Q, Tian T, Han X, Xu J, Chai Y, Mo J, et al. The relationships between biomass allocation and plant functional traits. Ecol Indic. 2019;102:302–8. [Google Scholar]
- 29.Boonman CCF, Langevelde FV, Oliveras L, Couedon J, Luijken N, Martini D, et al. On the importance of root traits in seedlings of tropical tree species. New Phytol. 2020;227(1):156–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jin Y, Liu C, Qian SS, Luo Y, Zhou R, Tang J, et al. Large-scale patterns of understory biomass and its allocation across China’s forests. Sci Total Environ. 2022;804:150169. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Wang R, Gao J. Precipitation and soil nutrients determine the spatial variability of grassland productivity at large scales in China. Front Plant Sci. 2022;13:996313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carvalho RB, Pizo MA. Seed removal, seed dispersers, and the allocation of tissues in Myrtaceae seeds. Biotropica. 2023;55:719–28. [Google Scholar]
- 33.Wang T, Zhou D, Wang P, Zhang H. Size-dependent reproductive effort in Amaranthus retroflexus: the influence of planting density and sowing date. Can J Bot. 2006;84:485–92. [Google Scholar]
- 34.Zhang J, Zhao Y, Wang Y. The trade-off between growth and reproduction in an alpine herbaceous plant along an elevation gradient. Pak J Bot. 2019;51(3):533–9. [Google Scholar]
- 35.Janeček Š, Patáčová E, Klimešová J. Effects of fertilization and competition on plant biomass allocation and internal resources: does Plantago lanceolata follow the rules of economic theory? Folia Geobot. 2013;49:49–64. [Google Scholar]
- 36.Vallès J, Garcia S, Hidalgo O, Martín J, Pellicer J, Sanz M, et al. Biology, genome evolution, biotechnological issues and research including applied perspectives in Artemisia (Asteraceae). Advances in Botanical Research. Academic; 2011. pp. 349–419.
- 37.Yang X, Huang Z, Zhang K, Cornelissen JHC. C:N:P stoichiometry of Artemisia species and close relatives across northern China: unravelling effects of climate, soil, and taxonomy. J Ecol. 2015a;103(4):1020–31. [Google Scholar]
- 38.Yang X, Huang Z, Zhang K, Cornelissen JHC. Geographic pattern and effects of climate and taxonomy on nonstructural carbohydrates of Artemisia species and their close relatives across northern China. Biogeochemistry. 2015b;125(3):337–48. [Google Scholar]
- 39.Khodorova NV, Boitel-Conti M. The role of temperature in the growth and flowering of geophytes. Plants. 2013;2:699–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hatfield JL, Prueger JH. Temperature extremes: Effect on plant growth and development. Weather Clim Extremes. 2015;10:4–10. [Google Scholar]
- 41.Zeppel MJB, Wilks JV, Lewis JD. Impacts of extreme precipitation and seasonal changes in precipitation on plants. Biogeosciences. 2014;11(11):3083–93. [Google Scholar]
- 42.Gao J, Wang J, Li Y. Effects of soil nutrients on plant nutrient traits in natural Pinus tabuliformis forests. Plants. 2023;12(4):735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang L, Dong BC, Xue W, Peng YK, Zhang MX, Yu FH. Soil particle heterogeneity affects the growth of a rhizomatous wetland plant. PLoS ONE. 2013;8(7):e76252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poorter H, Sack L. Pitfalls and possibilities in the analysis of biomass allocation patterns in plants. Front Plant Sci. 2012;3:259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheplick GP. Life-history variation in a native perennial grass (Tridens flavus): reproductive allocation, biomass partitioning, and allometry. Plant Ecol. 2020;221(2):103–15. [Google Scholar]
- 46.Reekie EG. An explanation for size-dependent reproductive allocation in Plantago major. Can J Bot. 1998;76:43–50. [Google Scholar]
- 47.Kobayashi T, Okamoto K, Hori Y. Variations in size structure, growth, and reproduction in Japanese plantain (Plantago asiatica L.) between exposed and shaded populations. Plant Spec Biol. 2001;16:13–28. [Google Scholar]
- 48.Kozlowski J. Optimal allocation of resources to growth and reproduction: implications for age and size at maturity. Trends Ecol Evol. 1992;7(1):9–15. [DOI] [PubMed] [Google Scholar]
- 49.Samson DA, Werk KS. Size-dependent effects in the analysis of reproductive effort in plants. Am Nat. 1986;127(5):667–80. [Google Scholar]
- 50.Wang R, Qiong G, Quansheng C. Effects of climatic change on biomass and biomass allocation in Leymus chinensis (Poaceae) along the North-East China Transect (NECT). J Arid Environ. 2003;54(4):653–65. [Google Scholar]
- 51.Zheng Y, Xue J, Lv Y, Zhang C, Wang R. Plant mass variations of Leymus chinensis (Poaceae) and their relationships with environmental factors on a large-scale gradient, northeastern China. Ecol Evol. 2024;14:e11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen K, Liu Q, Chen ZH, Li ZL. Soil temperature drives elevational patterns of reproductive allometry in a biodiversity hotspot. Plant Ecol. 2020;221(10):979–88. [Google Scholar]
- 53.Skarpaas O, Meineri E, Bargmann T, Pötsch C, Töpper J, Vandvik V. Biomass partitioning in grassland plants along independent gradients in temperature and precipitation. Perspect Plant Ecol Evol Syst. 2016;19:1–11. [Google Scholar]
- 54.Fick SE, Hijmans RJ. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int J Climatol. 2017;37(12):4302–15. [Google Scholar]
- 55.Hengl T, Mendes de Jesus J, Heuvelink GBM, Ruiperez Gonzalez M, Kilibarda M, Blagotić A, et al. SoilGrids250m: global gridded soil information based on machine learning. PLoS ONE. 2017;12(2):e0169748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 57.Warton DI, Duursma RA, Falster DS, Taskinen S. Smatr smatr 3 – an R package for estimation and inference about allometric lines. Methods Ecol Evol. 2012;3(2):257–59. [Google Scholar]
- 58.Chaves MM, Pereira JS, Maroco J, Rodrigues ML, Ricardo CPP, Osório ML, et al. How plants cope with water stress in the field: photosynthesis and growth. Ann Bot. 2002;89:907–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu XG, Long SP, Ort DR. Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol. 2010;61:235–61. [DOI] [PubMed] [Google Scholar]
- 60.Chaves MM, Maroco JP, Pereira JS. Understanding plant responses to drought – from genes to the whole plant. Funct Plant Biol. 2003;30:239–64. [DOI] [PubMed] [Google Scholar]
- 61.Morecroft MD, Paterson JS. Effects of temperature and precipitation changes on plant communities. In: Morison JIL, Morecroft MD, editors. Plant Growth and Climate Change. Academic; 2006. pp. 96–122.
- 62.Yang X, Huang Z, Venable DL, Wang L, Zhang K, Baskin JM, et al. Linking performance trait stability with species distribution: the case of Artemisia and its close relatives in northern China. J Veg Sci. 2016;27:123–32. [Google Scholar]
- 63.Mueller KE, LeCain DR, McCormack ML, Pendall E, Carlson M, Blumenthal DM, et al. Root responses to elevated CO2, warming, and irrigation in a semi-arid grassland: integrating biomass, length, and lifespan in a 5-year field experiment. J Ecol. 2018;106(6):2176–89. [Google Scholar]
- 64.Poorter H, Navas ML. Plant growth and competition at elevated CO2: on winners, losers, and functional groups. New Phytol. 2003;157:175–98. [DOI] [PubMed] [Google Scholar]
- 65.Peichl M, Sonnentag O, Wohlfahrt G, Varlagin A, Merbold L, Nilsson M, et al. Convergence of potential net ecosystem production among contrasting C3 grasslands. Ecol Lett. 2013;16(4):502–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu R, Yang X, Gao R, Hou X, Huo L, Huang Z, et al. Allometry rather than abiotic drivers explains biomass allocation among leaves, stems, and roots of Artemisia across a large environmental gradient in China. J Ecol. 2021;109(2):1026–40. [Google Scholar]
- 67.Gaudio N, Violle C, Gendre X, Dumora D, Jeuffroy M-H, Pellerin S, et al. Interspecific interactions regulate plant reproductive allometry in cereal–legume intercropping systems. J Appl Ecol. 2021;58(11):2579–89. [Google Scholar]
- 68.Zhang J, Wang YZ, Gao HK, Zuo ZT, Yang SB, Cai CT. Different strategies in biomass allocation across elevation in two Gentiana plants on the Yunnan-Guizhou Plateau, China. J Mt Sci. 2020;17(11):2750–7. [Google Scholar]
- 69.Niu K, Choler P, Zhao B, Du G. The allometry of reproductive biomass in response to land use in tibetan alpine grasslands. Funct Ecol. 2009;23:274–83. [Google Scholar]
- 70.Guo H, Weiner J, Mazer SJ, Zhao Z, Du G, Li B. Reproductive allometry in Pedicularis species changes with elevation. J Ecol. 2012;100:452–8. [Google Scholar]
- 71.Tian D, Pan Q, Simmons M, Chaolu H, Du B, Bai Y, et al. Hierarchical reproductive allocation and allometry within a perennial bunchgrass after 11 years of nutrient addition. PLoS ONE. 2012;7(9):e42833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niklas KJ, Enquist BJ. An allometric model for seed plant reproduction. Evol Ecol Res. 2003;5:79–88. [Google Scholar]
- 73.Wenk EH, Falster DS. Quantifying and understanding reproductive allocation schedules in plants. Ecol Evol. 2015;5(23):5521–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li L, Ding M, Lan Z, Zhao Y, Chen J. Light availability and patterns of allocation to reproductive and vegetative biomass in the sexes of the dioecious macrophyte Vallisneria Spinulosa. Front Plant Sci. 2019;10:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mason CM, Goolsby EW, Davis KE, Bullock DV, Donovan LA. Importance of whole-plant biomass allocation and reproductive timing to habitat differentiation across the north American sunflowers. Ann Bot. 2017;119:1131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tian D. Drought effect on plant biomass allocation: a meta-analysis. Ecol Evol. 2017;7(24):11002–10. Han W, Tang Z, Fang J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li S, Liu J, Li J, Deng Y, Chen J, Wang J, et al. Reproductive strategies involving biomass allocation, reproductive phenology, and seed production in two Asteraceae herbs growing in karst soil varying in depth and water availability. Plant Ecol. 2021;222:737–47. [Google Scholar]
- 78.Karlsson PS, Méndez M. The resource economy of plant reproduction. In: Reekie EG, Bazzaz FA, editors. Reproductive allocation in plants. San Diego: Elsevier Academic; 2005. pp. 1–49. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.