Abstract
Objective
The incidence of suicide is high among adolescents and young adults, especially those suffering from psychiatric diseases. Because of the reported association between depression and suicidality, exploring suicide risk factors in depressed patients is crucial for the identification of those at high risk and preventing suicide. In recent decades, electroencephalography parameters have been considered for identifying biomarkers of suicide ideation and attempts in depressed patients. This study aimed to review the available literature on resting-state EEG for suicidality in depressed patients.
Method
A systematic search was performed in five electronic databases, including APA PsycINFO, Embase, Medline (via PubMed), Scopus, and Web of Science. Papers with full text available in English in which resting-state EEG was evaluated in depressed patients with suicide ideation or suicide attempts compared to a control group of healthy subjects or non-suicidal depressed patients were included. The risk of bias was assessed by using the Newcastle–Ottawa scale.
Results
A total of 4665 references were retrieved from five electronic databases from which eleven studies were included in this systematic review. A meta-analysis was not performed due to the substantial heterogeneity of the studies. Five of the eleven reviewed papers were classified as high-quality, and six had moderate quality.
Conclusions
According to the included studies in this review, the EEG signals of depressed patients with suicide ideation or suicide attempts may be different from patients with low risk of suicidality or healthy subjects. Connectivity measures sound more promising parameters than the power spectral analysis and EEG asymmetry.
Protocol registration
The protocol of this review was registered in PROSPERO (No. CRD42024502056).
Supplementary Information
The online version contains supplementary material available at 10.1186/s12888-024-06464-x.
Keywords: Electroencephalography, Suicide ideation, Suicide attempt, MDD, Major depressive disorder
Introduction
Suicide is defined as an intentional, lethal self-harm that may or may not be associated with a conscious attempt to die. It is considered a global challenge for public health [1, 2]. Suicide is one of the leading causes of death all over the world, especially among adolescents and young adults [3]. Suicidality is often viewed as a spectrum that can range from feelings of wanting to die and being tired of life to having thoughts of suicide, planning and actual attempts [4]. To differentiate behaviors encompassed in suicidality, explanations provided by the US Centers for Disease Control and Prevention (CDC) are useful. Based on the definitions provided by the US CDC, the suicidal ideation (SI) term refers to thinking about, considering, or planning suicide, and suicide attempt (SA) is a term that describes a non-fatal, potentially damaging, self-directed behavior with an intention to die even if it does not result in death [5].
According to the literature, the incidence of suicides and suicide attempts is remarkably higher in patients with psychiatric diseases than in the general population [6]. Longitudinal studies recruiting people suffering from mood disorders indicate that suicidal acts are usually occurred during major depressive episodes [7]. The overall lifetime prevalence of suicide attempts and suicidal ideation among patients with MDD is estimated to be approximately 31% and 38%, respectively. Further, suicide attempts are observed to occur five times more frequently in individuals with depression compared to the general population [8].
On the other hand, there is an increasing trend in the prevalence of depression among the general population [9] especially adolescents [10]. Based on a recent systematic review, about 34% of adolescents globally are at risk of experiencing clinical depression [10].
Because of the reported association between depression and suicidal ideation and suicide attempts [11–13], exploration of suicide risk factors in depressed patients sounds crucial for the identification of those at high risk and preventing suicide [14].
Psychological evaluation is often based on questionnaires, and the subjectivity of the patient’s answers to questionnaires makes it hard to diagnose psychopathologies precisely [15]. Insufficient sensitivity and specificity of self-reported scales may result in underrating or overrating suicidal ideation that may become problematic by inadequate follow-up of those at high risk or unnecessary hospitalization and consequently increased cost of healthcare, respectively [16].
As an association has been observed between biological phenomena and psychiatric illness, intensive efforts have been made to identify specific and sensitive biomarkers for psychopathology as add-ons or alternatives to the current subjective clinical parameters [17]. In this line, many studies have adopted a neurophysiological approach for identifying brain-based markers of suicidal ideation and suicide attempts in healthy subjects and psychiatric patients using electroencephalography (EEG) [18–21].
EEG is a non-invasive method of recording the ongoing electrical activity of the brain through scalp electrodes [22]. As a brain imaging method with excellent temporal resolution and improved spatial resolution acquired through advances in signal processing and visualization, EEG is becoming a valuable tool for developing biomarkers in psychiatry. Further, EEG is less expensive and more widely available than other brain imaging methods such as functional magnetic resonance imaging (fMRI), which makes it beneficial for collecting data in large samples required for analyzing possible contributing factors to the development of psychopathology [17].
Resting-state activity in major depressive disorder shows topographical and spatiotemporal alterations, including changes in functional connectivity (FC) [23] as well as changes in spectral power of different frequency bands [24]. These changes have been associated with depressive symptoms [25] and suicidal ideation in patients with depression [20]. Further, studies have shown that the resting-state band powers have the potential to predict response to antidepressants in patients with depression [26–28]. These findings suggest that different resting-state EEG parameters may be informative about the underlying neurobiological mechanisms of various symptoms, including suicide in depressed patients [24].
Accordingly, this systematic review aimed to synthesize the relevant literature on the relationship between suicidality and resting-state EEG parameters in depression.
Methods
Materials and methods
This review adheres to the guidelines set forth by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (S1 Table) [29]. Additionally, the protocol of this review has been registered in the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42024502056.
Information sources
A systematic search was performed in five electronic databases, including APA PsycINFO, Embase, Medline (via PubMed), Scopus, and Web of Science. Furthermore, a manual check was conducted on the references of all included studies to identify any additional eligible studies.
Search strategy
A set of keywords, determined by the study's inclusion criteria, was utilized to search for relevant studies from inception to February 2024. In order to capture all relevant studies, no restriction was imposed on publication dates.
The search details in PubMed were as follows:
("electroencephalog*"[Title/Abstract] OR "electroencephalog*"[MeSH Terms] OR "Resting state electroencephalography"[Title/Abstract] OR "Resting-state EEG"[Title/Abstract] OR "rest EEG"[Title/Abstract] OR "QEEG"[Title/Abstract] OR "quantitative EEG"[Title/Abstract] OR "brain changes"[Title/Abstract] OR "brain adaptations"[Title/Abstract] OR "brain activity"[Title/Abstract] OR "brain function"[Title/Abstract] OR "cortex activation"[Title/Abstract] OR "EEG power"[Title/Abstract] OR "EEG spectral power"[Title/Abstract] OR "coherence"[Title/Abstract] OR "EEG coherence"[Title/Abstract] OR "connectivity"[Title/Abstract] OR "functional connectivity"[Title/Abstract] OR "brain networks"[Title/Abstract] OR "delta power"[Title/Abstract] OR "theta power"[Title/Abstract] OR "alpha power"[Title/Abstract] OR "beta power"[Title/Abstract] OR "gamma power"[Title/Abstract] OR "asymmetry"[Title/Abstract] OR "EEG asymmetry"[Title/Abstract] OR "electroencephalographic asymmetry"[Title/Abstract] OR "delta asymmetry"[Title/Abstract] OR "theta asymmetry"[Title/Abstract] OR "alpha asymmetry"[Title/Abstract] OR "beta asymmetry"[Title/Abstract] OR "gamma asymmetry"[Title/Abstract] OR "frontal asymmetry"[Title/Abstract] OR "frontal alpha asymmetry"[Title/Abstract] OR "FAA"[Title/Abstract] OR "posterior asymmetry"[Title/Abstract] OR "lateral asymmetry"[Title/Abstract]) AND ("suicid*"[Title/Abstract] OR "suicid*"[MeSH Terms] OR "suicidality"[Title/Abstract] OR "suicidal thoughts"[Title/Abstract] OR "suicidal behavior"[Title/Abstract] OR "suicidal attempt"[Title/Abstract] OR "suicide attempt"[Title/Abstract] OR "suicide planning"[Title/Abstract] OR "suicidal ideation"[Title/Abstract] OR "suicide risk"[Title/Abstract] OR "ideators"[Title/Abstract] OR "self-harm"[Title/Abstract] OR "self-injury"[Title/Abstract]) AND ("depress*"[Title/Abstract] OR "depress*"[MeSH Terms] OR "depressive disorder"[Title/Abstract] OR "major depressive disorder"[Title/Abstract] OR "MDD"[Title/Abstract]).
The basic search strategy was appropriately modified to optimize the approach for different databases (S1 File). Data management was conducted using the reference management software EndNote V.21 (Clarivate Analytics).
Inclusion and exclusion criteria
The retrieved results were exported into the EndNote software for screening. First, duplicate articles were automatically removed, and then two reviewers (FSH and FA) screened the titles and abstracts of the remaining records independently to identify eligible papers based on the inclusion–exclusion criteria. If the abstract lacked sufficient data for inclusion, the full text was considered. Any disagreements between the reviewers upon including an article were adjudicated by a third reviewer (FV) until consensus was reached.
Studies were included in the final list for review based on the following criteria:
Inclusion criteria:
Peer-reviewed journal articles with available full-text written in English.
Participants diagnosed with depression or major depressive disorder (MDD) based on a valid scale/questionnaire for diagnosis of depression such as the Diagnostic and Statistical Manual of Mental Disorders (DSM IV or later versions), the International Classification of Diseases (ICD-9 or ICD-10), and so on.
Papers in which resting-state EEG data was assessed in a group of patients with depressive disorders with suicidal ideation, suicidal behaviors, and suicide attempts compared to a control group of healthy subjects or non-suicidal depressed patients.
Prospective cohort, case-control, or cross-sectional studies. Baseline data from randomized clinical trials were included only if suicidal ideation, suicidal behaviors, and suicide attempts were measured and compared at baseline.
Exclusion criteria:
Non- English written papers.
Studies in which participants had mood disorders other than depression.
Papers in which dynamic EEG signals were recorded in responses to various tasks or cues, including acoustic sounds, oral presentation, or resting-state EEG, were recorded after any intervention or in stress conditions.
Non-controlled studies.
Case report, letter to the editor, editorials, dissertation, book chapter, personal opinions or commentary, reviews.
Data extraction
Two reviewers (FSH and FA) independently extracted data from the included studies for descriptive analyses. Any disagreements between the reviewers were adjudicated by a third reviewer (FV) until consensus was reached. The following information was extracted for each study: first author’s name, publication year, participants’ characteristics (sex, age, state of health), the number of participants, control/comparison group, outcome measures, depression and suicidality diagnosis criteria, EEG recording setting, and findings.
Evaluating the risk of bias
The Newcastle–Ottawa scale recommended by the Cochrane Non-Randomized Studies Methods Working Group for assessing the observational studies was used for the risk of bias assessment in the included studies. This scale was developed by Wells et al. [30] for the assessment of the quality of nonrandomized studies using a “star system”. Observational studies (case–control and cohort studies) are judged in terms of selection, comparability, and exposure or outcome. There are four items for selection, three items for outcome, and one item for comparability subscale. Each item in the selection and outcome subscales can get a maximum of one star, while the only item in the compatibility subscale can get a maximum of two stars, adding up to a maximum of 9 stars for each study [30]. The quality of a study is judged based on the total score as follows: scores ≤ 3 = low quality, 4 ≤ scores ≤ 6 = moderate quality, and scores ≥ 7 = high quality [31].
The quality assessment was done independently by the same two authors. Any disagreements between the reviewers were adjudicated by a third reviewer (FV) until consensus was reached.
Summary measures and data synthesis
This review focuses on the narrative interpretation of the results because a meta-analysis was inappropriate due to substantial methodological heterogeneity among the studies. For instance, although four studies evaluated EEG asymmetry, at most two studies used similar methods (electrode pair and frequency bands) and participants (MDD patients and controls). Also, there was substantial variation in selected electrodes or brain regions, frequency sub-bands, and patients’ sub-groups and controls in seven studies that reported EEG spectral power.
Results
A total of 4665 references were retrieved from five electronic databases. After removing duplicates, 2779 records remained. Screening titles and abstracts of the remaining articles resulted in the exclusion of 2719 records. The remaining 60 records were sought for retrieval, from which 35 full texts were retrieved and assessed for eligibility. Screening full-text resulted in the exclusion of 24 articles because of the following reasons: non-English language [32, 33], no report of suicidal thoughts and behaviors [34–41], not recording resting-state EEG [42–44], having no control group [21, 45–52], and being a commentary or book chapter [53, 54]. Finally, 11 studies [55–65] were included in this systematic review. Figure 1 shows the process of study selection.
Fig. 1.
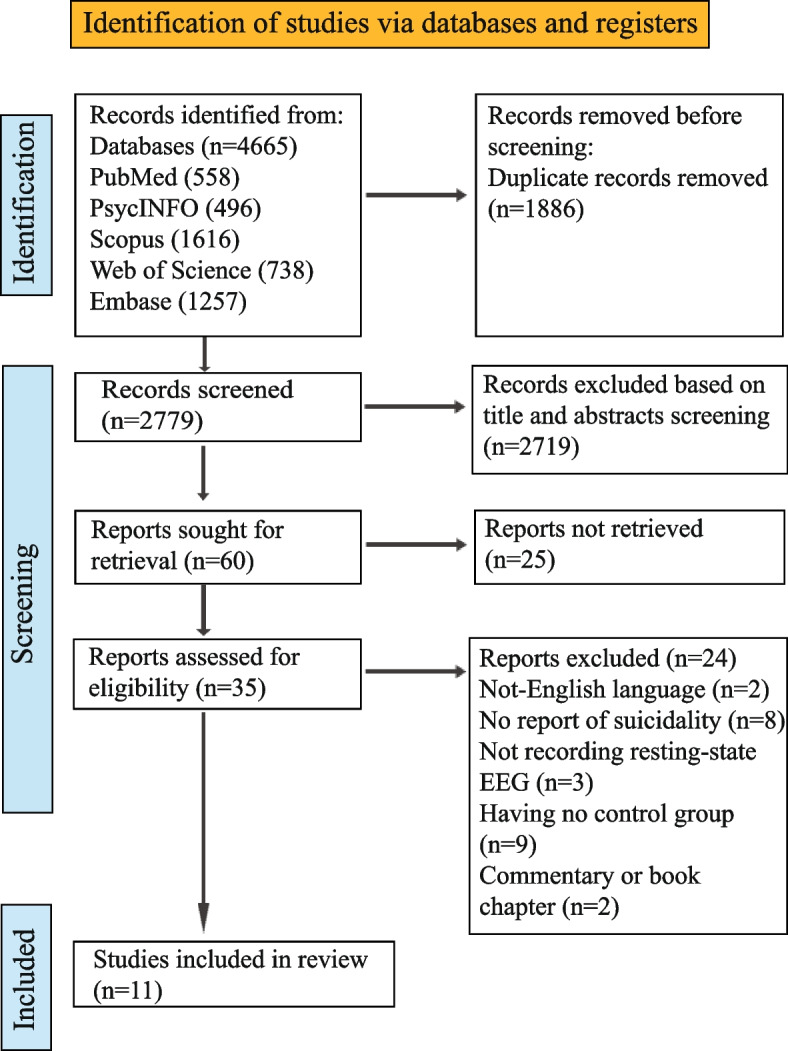
The PRISMA 2020 flow diagram for the search strategy and study selection
Characteristics of the included studies
Eleven studies were included in this systematic review. Characteristics of the included studies are summarized in Table 1.
Table 1.
Characteristics of the included studies
| Author (year) | Participants | Measure of suicidality | Measure of depression | EEG data acquisition | EEG measures | Findings | ||
|---|---|---|---|---|---|---|---|---|
| State of health (number) | Age (years) Mean (range) | Gender Female/Male | ||||||
| Graae et al. (1996) [55] | SA (MDD:9, NMDD:7) HS (22) | Mean = 14 (12–17) | All female |
Harkavy, Asnis Suicide Scale; Pierce Suicide Intent Scale |
Diagnostic Interview Schedule for Children, (DISC-C) and parents (DISC-P) Version 2.3 |
3 min RS-EEG (EO and EC); 11 EEG scalp electrodes; Ref: nose; bandpass = 0.01–30 Hz, 1.28-s epochs (62–227 epochs) | Brain spectral power at delta, theta, alpha, beta-1, beta-2 frequency bands; alpha asymmetry | No difference between groups in overall power of any frequency band; greater alpha power over the right hemisphere for healthy controls |
| Arikan et al. (2019) [56] |
MDD patients (NS:218, SI: 211, SA:74) / HS (30) |
NS = 38.42 SI = 35.06 SA = 31.36 HS = 43.9 (15–85) |
NS = 107/111 SI = 109/102 SA = 45/29 HS = 15/15 |
Clinical interview and Beck Scale for Suicidal Ideation |
Diagnostic and Statistical Manual of Mental Disorders (DSM) 4 or 5 |
7 min RS-EEG (EC); 19 EEG scalp electrodes; Ref: linked mastoid electrodes |
Absolute power for delta (1–4 Hz), theta (4–7 Hz), alpha (8–12 Hz), alpha1 (8–10 Hz), alpha2 (10–12 Hz), beta (12–25 Hz), beta1 (12–15 Hz), beta2 (15–18 Hz), beta3 (18–25 Hz), high beta (25–30 Hz), gamma (30–50 Hz), gamma1 (30–35 Hz), gamma2 (35–40 Hz), high gamma (40–50 Hz) |
High-gamma absolute power differed between groups at 8 electrode sites (F4, Fz, C4, Cz, O2, F8, T5 and T6), showing higher power in SA group compared to others, especially the NS group |
| Benschop et al. (2019) [57] |
MDD patients (SA:19, SI:36, NS:23) |
SA:37.26 SI:45 NS:44.13 |
All female |
Mini-International Neuropsychiatric Interview (MINI) |
MINI; DSM-IV |
2 min RS-EEG (EC); 26 EEG scalp electrodes; Ref: average; Frequency decomposition through complex Morlet wavelet convolution; Power extraction using the fast-Fourier transform |
Spatial-Frequency Cluster Analysis at (delta to gamma frequency bands; 2–100 Hz); at frontal lobe, temporal lobe, and limbic lobe | Less resting state beta and gamma power in the frontal regions of the brain for the patients with suicide attempts and suicidal ideations compared to the low-risk controls |
| Roh et al. (2020) [58] |
MDD patients (SI: 44, NS:23) / HS (60) |
SI:37.48 NS:39.3 HS: 34.83 |
SI: 19/4 NS: 39/5 HS: 50/10 |
DSM-IV | 3 min RS-EEG (EO); 62 EEG scalp electrodes; Ref: between Cz and CPz; 2-s epochs (30 epochs); sampling rate: 1000 Hz |
Frontal alpha asymmetry (FAA) at pre-frontal (Fp1 − Fp2), mid-frontal (F3 − F4), and lateral-frontal (F7 − F8) areas; delta (1 − 4 Hz), theta (4 − 8 Hz), alpha (8 − 12 Hz), beta (12 − 30 Hz), and gamma (30 − 50 Hz) bands |
A significantly higher FAA at pre-frontal area in MDD patients with SI compared to HSs; a significant main effect of SI on FAA at lateral frontal area | |
| Iznak et al. (2021) [59] |
Depressive patients (NSSI + SA:24, NSSI: 21) |
NSSI + SA:18.5 NSSI: 17.4 (16–25) |
All female | The 10th revision of the international statistical classification of diseases and related health problems (ICD-10) |
RS-EEG (EC); 16 EEG scalp electrodes, Ref: A1 and A2; Sampling rate: 200 Hz |
EEG asymmetry,EEG coherence, and absolute power at delta (2–4 Hz); theta-1 (4–6 Hz); theta-2 (6–8 Hz); alpha-1 (8–9 Hz); alpha-2 (9–11 Hz); alpha-3 (11–13 Hz); beta-1 (13–20 Hz); and beta-2 (20–30 Hz) bands | More distribution of mid-alpha frequency with higher power in the right hemisphere and higher EEG coherence at theta and alpha sub-bands in SA group | |
| Krepel et al. (2021) [60] | MDD patients (SI:188, NS: 214) | SI: 37.2 (18–65)/ NS: 37.7 (19–85) | All female | MINI-plus | MINI-plus | 2 min RS-EEG (EO); 26 EEG scalp electrodes; LORETA analyses |
Absolute power at SMR (12 -15 Hz), beta (14.5-30 Hz), beta I (14.5-20 Hz), beta II (20-25 Hz), beta III (25-30 Hz), and gamma I (31-49 Hz) bands |
No significant difference between patients with suicidal ideation and low-risk individuals at any frequency band |
| Amico et al. (2023) [61] | MDD patients (15) / HS (12) | Patients (37)/ HS (37.5) | Patients: 7 /8 HS: 7/ 5 | Reasons for Living Inventory | ICD-10 | 10 min RS-EEG (EO); 14 EEG scalp electrodes, Ref: CMS and DRL; ICA for artifact rejection; spectral power density calculation (Welch’s method) |
Spectral power and alpha asymmetry for F3, F4 channels at delta (1.5–3.5 Hz), theta (4–7.5 Hz), alpha (8–13 Hz), and beta (13–29 Hz) bands |
No significant differences between patients and HS for power spectral density or FAA |
| Bankwitz et al. (2023) [62] |
MDD patients (70) / HS (70) |
Patients (31.3) /HS (31.8) |
Patients: 40 /30 HS: 40 / 30 |
Beck scale for suicide ideation | ICD-10; MINI |
15 min RS-EEG (EC); 64 EEG scalp electrodes; Ref: CPz; sampling rate: 500 Hz; 4-s epochs; ICA for artifact rejection; eLORETA based functional connectivity mapping |
Lagged source-based measures of whole-brain connectivity within the standard alpha frequency range | Increased lagged nonlinear functional connectivity within the alpha frequency range in MDD patients compared to HS |
| Jiang et la. (2023) [63] |
MDD patients (NS: 47 SI: 40 SA:42) / HS (60) |
NS = 25.62 SI = 26.23 SA = 22.36 HS = 25.33 |
NS: 31/16; SI:18/22; SA:31/16; HS: 33/27 |
MINI-part C | DSM-IV |
5 min RS-EEG (EC); 68 EEG scalp electrodes; Ref: FCz; Samplimg rate: 1000 Hz; ICA for artifact rejection; 2-s epochs |
Absolute power at delta (1–4 Hz), theta (4–7 Hz), alpha 1 (8–10 Hz), alpha 2 (10–12 Hz), beta 1 (12–15 Hz), beta 2 (15–18 Hz), beta 3 (18–25 Hz), high beta (25–30 Hz), gamma (30–50 Hz), gamma 1 (30–35 Hz), gamma 2 (35–40 Hz), gamma 3 (40–48 Hz), gamma 4 (52–70 Hz) and gamma 5 (70–100 Hz) bands | Lower delta, beta, and gamma 1 power on the right hemisphere in SA group compared to SI group, higher powers in delta, theta, alpha 2 and beta 2 on the right frontal-central sites of SI group compared to NS group; significant differences between patients and HS at beta and gamma bands |
| Ozger et al. (2023) [64] |
MDD patients (SI: 9, SB:17) / HS (28) |
SI = 15.4 SB = 15.7 HS = 15.5 (12–18) |
SI:7/2 SB:13/4 HS:16/12 |
Columbia Suicide Severity Rating Scale | MINI-KID |
3 min RS-EEG (EC); 64 EEG scalp electrodes; sampling rate: 1000 Hz; 10-s epochs; automatic artifact rejection using TBT toolbox in MATLAB |
EEG coherence at the delta (0.5–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), and beta (12–20 Hz) frequency bands |
No significant difference between the HS and SI groups; higher delta, alpha, and beta coherence over the right prefrontofrontal electrode pair in the SB group compared with the SI group; higher alpha coherence over the right centroparietal electrode pair in the HS group compared to the SB group |
| He et al. (2024) [65] |
MDD patients (SI: 47, NS:26) / HS (26) |
SI = 14.83 NS = 15.31 HS = 15.35 |
SI:29/18 NS:17/9 HS:13/13 |
Columbia Suicidal Ideation Severity Scale |
DSM-5; ICD-10, MINI-KID; Children and Adolescents Questionnaire-9 (PHQ-9); |
7 min RS-EEG (EC); 64 EEG scalp electrodes; sampling: 500 Hz; ICA for artifacts rejection: bandpass filter 1–40 Hz |
EEG microstate dynamics including duration, occurrence, coverage, and transition probability | A significant reduction in the occurrence and coverage of microstate B within the SI group compared with the NS group; a notable increase in the occurrence and coverage of microstate A in the SI group compared to the HS group; changes in transition probabilities between different microstates in the SI and NS groups |
EEG electroencephalography, SA suicide attempt, MDD major depressive disorder, HS healthy subject, RS-EEG resting-state EEG, EO eyes open, EC eyes closed, NS non-suicidal, SI suicidal ideation, NSSI non-suicidal self-injuries, SB suicidal behavior, LORETA Low Resolution Brain Electromagnetic Tomography, ICA Independent Component Analysis
Studies are categorized based on outcome measures into three groups as follows: EEG asymmetry, EEG spectral power, and connectivity.
EEG asymmetry
Participants
Four studies considered EEG asymmetry as a primary outcome measure [55, 58, 59, 61]. Two studies recruited female adolescents aged between 12 and 25 years old [55, 59], while in the other studies, patients were selected from both genders with a mean age of 37 to 38.67 years old [58, 61]. Two studies recruited MDD patients with suicidal ideation (SI) [58, 61], while participants in two other studies were people with a suicide attempt (SA) [55, 59]. Three studies included healthy subjects (HSs) as controls [55, 58, 61], while one study served MDD patients with non-suicidal self-injuries as controls [59]. One study used the Diagnostic Interview Schedule for Children (DISC-C) and parents (DISC-P) for psychiatric assessment [55]. Exclusion of psychiatric conditions other than depression was based on the International Classification of Diseases and Related Health Problems (ICD) in two studies [59, 61], and one used the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM) [58].
EEG signal acquisition and processing
Resting-state EEG was collected for at least 3 min in all studies. One study collected EEG in both open and closed eye conditions [55]; the eyes open condition was considered in two studies [58, 61], and EEG was recorded with closed eyes in one study [59].
Three studies focused on the alpha frequency band [55, 58, 61], while one considered EEG asymmetry in the delta to beta frequency bands [59]. One study measured asymmetry in all brain regions [59], one focused on frontal and parietal regions [55], and two others selected the frontal brain area as their region of interest [58, 61].
EEG spectral power
Participants
EEG spectral power was reported in seven studies [55–57, 59–61, 63]. Four studies recruited only females [55, 57, 59, 60], while the subjects in three studies were of both genders [56, 61, 63]. Participants in two studies were adolescents with a mean age of 14 [55] and 18.5 [59] years old; however, the mean age of subjects in five studies was between 31 and 45 years old [56, 57, 60, 61, 63].
In two studies, only persons with suicide attempts were compared to HSs [55] or non-suicidal MDD patients [59]. Patients with suicidal ideation were compared to HSs in one study [61] and to low-risk patients in another study [60]. Patients with suicidal ideation and suicide attempts were compared to non-suicidal patients and HSs in two studies [56, 63], while one study recruited patients with suicidal ideation and suicide attempts compared to non-suicidals [57].
Psychiatric assessments were based on different tools, including ICD [59, 61], DSM-IV or V [56, 57, 63], Mini-International Neuropsychiatric Interview (MINI) [60], Diagnostic Interview Schedule for Children DISC-C and DISC-p (Parent version) [55]. One study used two scales, including DSM-IV and MINI, for clinical assessment of patients [57].
EEG signal acquisition and processing
Resting-state EEG recording time ranged between 2 and 10 min in six studies [55–57, 60, 61, 63], while in one study the duration of EEG records was not reported [59]. Signals acquired in closed eyes condition were analyzed in four studies [56, 57, 59, 63]; in two studies, power extraction was applied on eyes open data [60, 61]; and both eyes closed and eyes open conditions were considered in one study [55].
EEG power was calculated for all frequency bands, from delta to gamma, in one study [63]; three studies considered delta to beta band powers [55, 59, 61]; alpha to gamma frequency bands were considered in two studies [57, 60]; and one study focused on high gamma band power [56]. Three studies computed EEG power at different individual electrodes [56, 59, 63]; in two studies, average power over the frontal region was considered [57, 60]; one study focused on anterior and posterior brain regions [55]; and in one study, the power was computed for only F3 and F4 electrodes [61].
Connectivity
Participants
Four studies addressed brain connectivity in suicidality [59, 62, 64, 65]. Patients and healthy subjects of both genders participated in three studies; however, two studies recruited 12–18-year-old adolescents with an average age of 15.5 years old [64, 65], but the subjects in the other study were 31.5 years old on average [62]. One study recruited female adolescents aged 16–25 years old [59]. One study compared patients with suicide attempts to HSs [62]; in one, MDD patients with suicidal ideation and suicidal behaviors were compared to HSs [64]; and in one, patients with suicidal ideation were compared to non-suicidal patients and HSs [65]. However, in one study, patients with suicide attempts were compared to non-suicidal patients [59]. Psychiatric assessment for depression was based on the ICD-10 and MINI [62], MINI [64], DSM-V and IC-10 [65] and ICD-10 [59].
EEG signal acquisition and processing
All studies recorded resting-state EEG with closed eyes lasting for 3 min [64], 7 min [65], and 15 min [62]. While one study did not provide information regarding duration of signal [59]. These studies adopted different approaches for connectivity, including whole-brain functional connectivity in a specified frequency band (alpha frequency range) [62], EEG coherence at delta to beta frequency bands for specified EEG channels [59, 64], and microstate dynamics to uncover brain network alteration [65].
Quality assessment in the included studies
A summary of the quality assessment of the included papers is represented in Table 2. Five out of eleven reviewed papers were classified as high-quality [55, 59, 60, 62, 63] and six were deemed to be of moderate quality [56–58, 61, 64, 65]. Regarding the selection domain, patients were clearly defined in all studies while two studies lacked a clear definition of controls [61, 65]. The selection of control was not well documented in five studies [56, 61, 62, 64, 65], and the representativeness of patients was confirmed only in one study [60]. Regarding the comparability domain, more than half of the included studies matched groups for gender [55, 57, 59, 60, 62, 63] while age was matched between groups just in four studies [55, 62, 63, 65]. The outcome measures were collected using the same methods for case and control groups in all studies, while only three studies provided information regarding non-response rate activity [59, 61, 62].
Table 2.
Quality assessment of the included studies
| Selection | Comparability | Outcome measurement | Total score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient definition | Representat-iveness of patients | Selection of controls | Definition of controls | Age | Gender/ education | Ascertainment of outcome | Same method of ascertainment for case and control | Non-response rate | ||
| Graae et al. (1996) [55] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Arikan et al. (2019) [56] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 4 |
| Benschop et al. (2019) [57] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 6 |
| Roh et al. (2020) [58] | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 5 |
| Iznak et al. (2021) [59] | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 7 |
| Krepel et al. (2021) [60] | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 7 |
| Amico et al. (2023) [61] | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Bankwitz et al. (2023) [62] | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| Jiang et la. (2023) [63] | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| Ozger et al. (2023) [64] | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 4 |
| He et al. (2024) [65] | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 4 |
Discussion
The current study provides a comprehensive review of studies that used EEG to find out possible biomarkers for suicidal thoughts and behaviors in depression. Eleven studies were included. We organized the studies into three groups based on the outcome measures as follows: EEG asymmetry, EEG spectral power, and connectivity. Due to substantial methodological heterogeneity in terms of selected electrodes and frequency bands and patients’ states in terms of suicidal ideation and suicide attempts, a meta-analysis was inappropriate. Therefore, this review focuses on narrative interpretation of results.
EEG asymmetry and suicidal thoughts and behaviors in depression
The alteration of resting-state EEG asymmetry as a biomarker of major depressive disorder has been investigated for several decades, and most previous studies have focused on frontal alpha asymmetry (FAA) [66]. Although some found an asymmetry resulting from unequal alpha activity between hemispheres [67–69], others did not find clear correlations between EEG asymmetry and psychiatric tests [70]. The observed link between negative emotionality and right EEG asymmetry has brought up the question if there is any connection between adverse thoughts and experiences, like suicidal thoughts and suicidal behaviors and EEG asymmetry.
In this regard, the possible association of resting-state EEG asymmetry and suicidal ideation and suicide attempt has been addressed in healthy subjects and those with mental disorders [46, 55]. In this review, four studies, two with high quality [55, 59] and two with moderate quality [58, 61], considered EEG asymmetry as a primary outcome measure. As a primary effort to examine the role of EEG asymmetry in suicidal thoughts and behaviors, Graae et al. (1996) compared the resting-state EEG in a group of female adolescent with suicide attempts to that of healthy adolescents. Overall, persons with suicide attempts showed a non-significant asymmetry in favor of greater alpha power over the left hemisphere compared to normal controls who had a significantly higher alpha power over the right hemisphere, especially at posterior electrode sites. Grouping persons with suicide attempts based on the diagnosis of depression showed that the alpha asymmetry at posterior regions of the left hemisphere was only significant in persons with suicide attempts without a depressive disorder, which was correlated with suicide intent in this subgroup of persons with suicide attempts [55].
In another study, Iznak et al. (2021) evaluated resting-state EEG in depressive female adolescents with suicide attempts (SA) and those with non-suicidal behaviors. Comparing EEG asymmetry at different frequency sub-bands showed significant differences in alpha sub-bands between groups. Increased alpha power was found over the right hemisphere in those with SA, showing a significant inter-hemispheric difference in the occipital area across all sub-bands but in the parietal area for the alpha-2 sub-band. However, in patients with non-suicidal self-injuries, the power of alpha-2 and alpha-3 sub-bands was significantly higher on left occipital, parietal, and temporal leads [59].
Comparing FAA in MDD patients with or without suicidal ideation (SI) with that of healthy subjects has suggested a moderating role for SI on FAA in patients with MDD. In line with previous studies, Rho et al. (2020) observed higher alpha power in the left frontal lobe of patients with depression compared with healthy subjects. However, subgrouping depressed patients based on the presence of SI showed significantly higher asymmetry in the prefrontal region in those with SI compared with patients without SI and HSs. Despite higher left asymmetry in the lateral-frontal area in MDD patients compared to HSs, the difference was significant only for patients without SI. No significant difference was observed between groups at mid-frontal area (F3-F4) [58].
Findings by Amico et al. (2023) also showed no significant difference in FAA at F4-F3 channels between a group of depressed patients with SI and healthy subjects [61], a finding that is in line with the reports of Roh et al. (2020) [58].
In short, the available literature does not provide sufficient evidence for supporting the prognostic value of FAA in SI and SA in depressed patients. Discrepancies observed in the included studies may be in part due to the substantial methodological heterogeneity in these studies. At most two studies have compared patients with SA and/or those with SI with patients without suicidal thoughts and behaviors or healthy controls. Differences at selected frequency bands or brain regions might have resulted in various results. Another source of discrepancy might be the comparison group; although both studies recruited female adolescents, suicide attempters were compared with healthy subjects in one study [55] and with low-risk depressed patients in the other one [59]. Another issue that needs consideration is the assessment of suicidal thoughts and behaviors. Graae et al. (1996) assessed suicidal thoughts and behaviors using the Harkavy, Asnis Suicide Scale, and the Pierce Suicide Intent Scale [55], while Iznak et al. (2021) did not provide any information about suicidal thoughts and behavior measures in their study [59]. Regarding the studies considering suicidal ideation [58, 61], besides substantial methodological differences in terms of EEG recording and signal processing between the two studies, differences in sample size and female-to-male ratio are other possible factors influencing the results.
However, the findings suggest that suicidal ideation and suicide attempts modulate the brain electrical activity of depressed patients so that the inter-hemispheric asymmetry of patients with suicide attempts differs from that of depressed patients with non-suicidal behaviors. Based on the available literature, further studies considering different frequency bands and sub-bands and different brain regions not restricted to the frontal area are required to delineate the possible role of EEG power spectral asymmetry in suicidal thoughts and behaviors in depressed patients.
EEG power and suicidal thoughts and behaviors in depression
Resting-state frequency power is another EEG parameter studied as a potential biomarker of suicidal behavior in depressed patients [56, 57]. Seven studies included in this review, four with high quality [55, 59, 60, 63] and three with moderate quality [56, 57, 61], assessed EEG power as a main outcome measure.
Graae et al. (1996) considered overall EEG power at the delta to beta frequency bands in a small group of female adolescent suicide attempters and a sex and age-matched healthy group. Comparing overall power at different frequency bands yielded no significant difference at any band between groups [55].
Benschop et al. (2019) investigated the resting-state EEG of 78 female MDD patients with or without suicide thoughts or attempts. Comparing EEG power at frequencies ranging from 2–100 Hz revealed hypoactivity of beta and low gamma in both patients with suicidal ideation and suicide attempts, especially in frontal brain regions, compared to low-risk patients. Further, beta and low gamma activity were lower over the temporal region in patients with suicide attempts compared to those with suicidal ideation. However, patients with suicidal ideation were characterized by increased alpha, high beta, and low gamma activity over the posterior regions compared with non-suicidal patients. The activity at lower frequency bands was not significantly different between the three groups [57].
Arikan et al. (2019) investigated gamma band power as a potential biomarker for suicidal thoughts and behaviors in a large group of patients (both males and females) with depression. Dividing patients into non-suicidal, suicidal ideation, and suicide attempt groups revealed a significantly higher power of the high-gamma band at different brain regions over the right hemisphere in patients with suicide attempt compared to the other groups, especially the non-suicidal MDD group. However, significant differences between suicide attempt and suicidal ideation groups were limited to lateral-frontal, mid-frontal, and mid-central channels [56].
The other study by Krepel et al. (2021) [60] on 402 female MDD patients that aimed to replicate the study by Benschop et al. (2019) found no significant difference between those with SI and low-risk individuals for any frequency band from alpha to gamma range [57]. However, the authors reported a trend more in line with the findings by Arikan et al. (2019) who reported higher gamma power in patients with suicidal ideation compared to non-suicidal individuals [56].
Evaluating QEEG in a small sample of depressive female adolescents and young adults with non-suicidal behavior and those with suicide attempts by Iznak et al. (2021) has shown the wider distribution of the mid-frequency alpha band in centro-parieto-ocipital regions with higher spectral power, particularly over the right hemispheres of patients with suicide attempts. Considering alpha sub-bands, the power of the high-alpha was higher than the low-alpha band in patients with suicide attempts, while in patients with non-suicidal behaviors, the power of the low-alpha band was higher [59].
In a recent study by Amico et al. (2023) power spectral density was computed for delta, theta, alpha, and beta bands at F3 and F4 channels in a group of depressive patients with suicidal ideation and healthy controls. No difference was found between the patients and healthy subjects in any frequency band at either channel. The correlation analysis also did not show any association between spectral powers and suicidal ideation in the patients [61].
Jiang et al. (2023) also addressed EEG power at different frequency sub-bands in depressed patients with and without suicidal thoughts and behaviors compared to healthy subjects. The authors reported an increase of power in different frequencies, including delta, theta, and beta bands, from non-suicidality to suicidal ideation in depressed patients, which showed a significant decrease after an acute suicide attempt. Changes in the power of beta sub-bands in patients with different levels of suicidality and the negative correlation of decreased beta 1 power with suicidality in MDD patients with suicide attempts suggested the beta-frequency characteristics as possible identifiers of suicide risk and facilitators of transition from suicidal ideation to suicide attempt [63].
Collectively, the available evidence suggests alpha, beta, and gamma powers as potential contributors to suicidality in depressed patients. Benschop et al. (2019) found decreased beta and gamma activity in MDD patients with suicide attempts compared to other patients, especially those with a low risk of suicidality [57], while Arikan et al. (2019) reported higher power of the high-gamma band in patients with suicide attempts at certain brain regions [56]. However, the study by Jiang et al. (2023) found a significant decrease in low beta power after a suicide attempt compared to non-attempters, especially those with suicidal ideation [63]. Although delta to beta power were not significantly different between patients with suicide attempts and healthy subjects in the study by Graae et al. (1996) [55], Iznak et al. (2021) observed a wider distribution of the mid-frequency alpha band in some brain regions with higher spectral power in patients with suicide attempts compared to non-suicidal patients [59]. Despite these variations, the majority of these studies show differences between patients with suicide attempts and other groups of MDD patients and healthy subjects in beta and gamma frequencies. These discrepancies may arise from differences in signal processing. The reported studies have selected different regions of interest. In two studies power has been calculated for every single electrode [56, 59], while in two other studies brain regions composed of different electrodes have been defined [57, 63], and one study calculated averaged power over all electrodes [55]. Variations in defined frequency bands reported in these studies may be another source of discrepancy. Arikan et al. (2019) [56] and Jiang et la. (2023) [63] defined several sub-bands for beta and gamma frequencies, while Benschop et al. (2019) investigated EEG power upon a continuous frequency spectrum ranging from 2–100 Hz [57]. Further, two studies reported power in the delta to beta frequency range with different sub-bands and took the gamma band into account [55, 59]. The number of patients, ranging from 16 to 530, in different studies may also have a role in various results. In addition, age-related changes in brain oscillations must also be considered. EEG studies have shown a linear increase in alpha power during maturation followed by a decrease with physiological aging [71]. Further, evidence shows an increasing trend in beta power but a slightly decreasing trend in delta and theta power with age [72]. Recruiting participants ranging from 18–85 years old in large-sample studies without age-match comparison might have influenced their results. Combining these factors may somehow justify different results in various studies. Altogether, higher frequencies, especially beta and gamma frequencies, sound to be worth investigating in future studies on suicide attempts in MDD patients.
The role of power spectral analysis in suicidal ideation has been considered in five studies. Like patients with suicide attempts, the patients with suicidal ideation also differed in the beta and gamma power from the non-suicidal patients and/or healthy subjects in two studies [56, 57]. However, the study by Krepel et al. (2021) failed to find any significant difference at the gamma frequency band between patients with suicidal ideation and low-risk patients [60].
Some studies have evaluated resting-state EEG-based power spectral density in depressive patients with suicidal ideation and suicide attempts compared to non-suicidal patients and healthy subjects. The results of these studies recruiting both genders at different ages suggest that suicidality, especially in the form of a suicide attempt, may be associated with changes in spectral power in fast frequencies, particularly the gamma band. However, further research considering the same population with age and gender match comparisons is required to support previous findings.
EEG-based brain connectivity and suicidal thoughts and behaviors in depression
The possible contribution of brain connectivity to suicidal thoughts and behaviors in depressed patients was considered in four studies, two with high quality [59, 62] and two with moderate quality [64, 65].
Two studies reported EEG coherence, which measures synchrony of neural oscillations across different brain regions, as a measure of brain connectivity [59, 64]. Iznak et al. (2021) found higher coherence in theta and alpha frequency bands in female adolescents with suicide attempts compared to those with non-suicidal behavior, especially in fronto-centro-temporal regions [59]. These findings were somehow supported by reports of Ozger et al. (2023) who found increased delta, alpha, and beta intra-hemispheric coherence at the frontal brain region of patients with suicide attempts compared to those with suicidal ideation. However, compared to healthy subjects, patients with suicide attempts had significantly lower theta to beta coherence over the right temporoparietal region [64].
Further, evaluating EEG-based functional connectivity within the alpha frequency range by Bankwitz et al. (2023) has shown a significant increase in nonlinear functional connectivity mostly in right-hemispheric posterior brain regions in MDD patients with suicide attempt compared to matched healthy subjects [62]. A finding that might be suggestive of impaired down-regulation of brain arousal in MDD patients.
Possible alteration in brain network connectivity in MDD patients with suicidal ideation was assessed using microstate analysis in the study by He et al. [65]. The results showed increased occurrence in a microstate associated with neural activity in the key brain regions involved in auditory processing in MDD patients with suicidal ideation compared to healthy controls, suggesting possible abnormal auditory processing in this population. However, non-suicidal patients showed increased occurrence of a microstate associated with brain regions involved in visual processes contributing to self-reflection and autobiographical memory compared to those with suicidal ideation and healthy controls [65].
Collectively, the association of EEG-based functional connectivity with suicidal thoughts and behaviors in depressed patients has been considered in a few studies using different measures of connectivity. Although one study showed decreased alpha coherence over the right temporoparietal region in suicide attempters compared to the healthy subjects [64], alpha frequency range connectivity analysis showed increased connectivity in the same brain regions [62]. The method of connectivity measures might have resulted in this discrepancy. There is accumulating evidence in the literature indicating the role of the right temporoparietal junction in social cognition [73, 74]. On the other hand, depressed patients with suicide attempts have shown impairment in some aspects of social cognition compared to healthy subjects [75]. Accordingly, decreased EEG coherence over the right temporoparietal brain region in patients with suicide attempts might be due to impaired social cognition in these patients. Suicidal thoughts and behaviors in these people may be due to their insufficient capacity to regulate perceived social stress [76].
EEG coherence analyses have shown increased coherence at different frequency bands in patients with suicide attempts compared to patients with suicidal ideation or non-suicidal behaviors. In this regard, Chen et al. (2021) also found increased functional connectivity over the right prefrontal cortex in patients with a suicide attempt compared to patients with non-suicidal behaviors in resting-state fMRI [77]. Since the frontal cortex consists of brain structures contributing to regulation of behavioral impulsivity and response inhibition, alteration of neural circuitry in this brain area may be associated with a higher vulnerability to suicidal behavior [78]. Further, increased occurrence of a microstate contributing to auditory processing in patients with suicidal ideation is somehow in line with increased functional connectivity mostly in posterior brain regions in MDD patients with suicide attempts, findings that suggest brain connectivity as a possible biomarker of suicidality in depressed patients. However, further high-quality studies are required to support this idea.
Strength and limitation
We comprehensively searched five databases to explore the possible links between resting-state EEG parameters and suicidal thoughts and behaviors in depressed patients. This study is the first systematic review in this field. However, due to the heterogeneity of the included studies regarding participants and outcome measures, a meta-analysis was not performed; instead, we focused on the comprehensive interpretation of the included studies. We included studies that recruited both depressed patients without suicidality and healthy subjects as control groups. Comparing patients with suicidal ideation and those with suicide attempts against each control group separately resulted in at most two studies that had identical case and control groups, as well as the same outcome measures, which made a meta-analysis and sub-group analysis impossible. Another limitation was the inconsistent definition of frequency bands across different studies. Although spectral power was reported as a primary outcome measure, some studies subdivided frequency bands into multiple sub-bands, complicating the interpretation of results. In addition, the available studies have predominantly focused on linear analyses, including amplitude and frequency analyses, while the human brain functions as a non-linear system. Therefore, we highly recommend incorporating non-linear analyses, such as entropy measures that quantify the degree of randomness in the EEG signal, in future research. Furthermore, we included only papers published in English; however, relevant research may exist in other languages, and excluding these studies could lead to a biased and incomplete understanding of the evidence.
Implication for the future
According to our results, using some EEG parameters like power analysis in fast frequency bands and brain connectivity analyses sounds promising in the study of suicidal thoughts and behaviors in depressed patients. However, because of the small number of studies and high clinical and methodological heterogeneity in the included studies, future high-quality studies with large sample sizes are required to provide more information in this field. Considering both linear and non-linear analyses in future research shed light on the applicability of EEG parameters as possible biomarkers of suicidality in depressed patients.
Conclusion
This review did not find definitive evidence linking EEG parameters to suicidal thoughts and behaviors in depressed patients, because of the limited number of studies with clinical and methodological variability. However, the findings from the included studies indicate that EEG signals in individuals with suicidal ideation or suicide attempts may differ from those of patients at low risk for suicidality or healthy controls. The existing literature suggests that connectivity measures may serve as more promising indicators than spectral power and EEG asymmetry. However, future research with more standardized clinical and methodological approaches is essential to further elucidate the potential of EEG analysis in assessing suicidal thoughts and behaviors among depressed patients.
Supplementary Information
Acknowledgements
None.
Clinical trial number
Not applicable.
Abbreviations
- SI
Suicidal Ideation
- SA
Suicide Attempts
- EEG
Electroencephalography
- fMRI
Functional Magnetic Resonance Imaging
- FC
Functional Connectivity
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
Prospective Register of Systematic Reviews
- MDD
Major Depressive Disorder
- DSM
Diagnostic and Statistical Manual of Mental Disorders
- ICD
International Classification of Diseases
- HS
Healthy Subject
- DISC-C
Diagnostic Interview Schedule for Children
- DISC-P
Diagnostic Interview Schedule for parents
- MINI
Mini-International Neuropsychiatric Interview
- FAA
Frontal Alpha Asymmetry
Authors’ contributions
F.Sh. contributed to conceptualization, data curation, project administration, methodology, supervision, writing – original draft and writing – review & editing. F. A. contributed to methodology, writing – review & editing and F.V.contributed to methodology, writing – review & editing.
Funding
This study received no financial support.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weinberg I. The prisoners of despair: right hemisphere deficiency and suicide. Neurosci Biobehav Rev. 2000;24(8):799–815. [DOI] [PubMed] [Google Scholar]
- 2.Turecki G, Brent DA, Gunnell D, O’Connor RC, Oquendo MA, Pirkis J, Stanley BH. Suicide and suicide risk. Nat Rev Dis Primers. 2019;5(1):74. [DOI] [PubMed] [Google Scholar]
- 3.Arensman E, Scott V, De Leo D, Pirkis J. Suicide and suicide prevention from a global perspective. Crisis. 2020;41(Suppl 1):S3–7. [DOI] [PubMed] [Google Scholar]
- 4.Carrasco-Barrios MT, Huertas P, Martín P, Martín C, Castillejos MC, Petkari E, Moreno-Küstner B. Determinants of suicidality in the European general population: a systematic review and meta-analysis. Int J Environ Res Public Health. 2020;17(11):4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klonsky ED, May AM, Saffer BY. Suicide, suicide attempts, and suicidal ideation. Annu Rev Clin Psychol. 2016;12(1):307–30. [DOI] [PubMed] [Google Scholar]
- 6.Bachmann S. Epidemiology of suicide and the psychiatric perspective. Int J Environ Res Public Health. 2018;15(7):1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isometsä E. Suicidal behaviour in mood disorders—who, when, and why? Canadian J Psychiatry. 2014;59(3):120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riera-Serra P, Navarra-Ventura G, Castro A, Gili M, Salazar-Cedillo A, Ricci-Cabello I, et al. Clinical predictors of suicidal ideation, suicide attempts and suicide death in depressive disorder: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2024;274(7):1543–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moreno-Agostino D, Wu Y-T, Daskalopoulou C, Hasan MT, Huisman M, Prina M. Global trends in the prevalence and incidence of depression: a systematic review and meta-analysis. J Affect Disord. 2021;281:235–43. [DOI] [PubMed] [Google Scholar]
- 10.Shorey S, Ng ED, Wong CH. Global prevalence of depression and elevated depressive symptoms among adolescents: A systematic review and meta-analysis. Br J Clin Psychol. 2022;61(2):287–305. [DOI] [PubMed] [Google Scholar]
- 11.Chang H-J, Lin H-C, Lee H-C, Lin C-C, Pfeiffer S. Risk of mortality among depressed younger patients: a five-year follow-up study. J Affect Disord. 2009;113(3):255–62. [DOI] [PubMed] [Google Scholar]
- 12.Lesage AD, Boyer R, Grunberg F, Vanier C, Morissette R, Ménard-Buteau C, Loyer M. Suicide and mental disorders: a case-control study of young men. Am J Psychiatry. 1994;151(7):1063–8. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Fung S, Tsang A, Liu Z, Huang Y-Q, He Y, et al. Lifetime prevalence of suicide ideation, plan, and attempt in metropolitan China. Acta Psychiatr Scand. 2007;116(6):429–37. [DOI] [PubMed] [Google Scholar]
- 14.Rihmer Z. Suicide risk in mood disorders. Curr Opin Psychiatry. 2007;20(1):17–22. [DOI] [PubMed] [Google Scholar]
- 15.de AguiarNeto FS, Rosa JLG. Depression biomarkers using non-invasive EEG: A review. Neurosci Biobehav Rev. 2019;105:83–93. [DOI] [PubMed] [Google Scholar]
- 16.Runeson B, Odeberg J, Pettersson A, Edbom T, JildevikAdamsson I, Waern M. Instruments for the assessment of suicide risk: A systematic review evaluating the certainty of the evidence. PLoS ONE. 2017;12(7):e0180292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLoughlin G, Makeig S, Tsuang MT. In search of biomarkers in psychiatry: EEG-based measures of brain function. Am J Med Genet B Neuropsychiatr Genet. 2014;165(2):111–21. [DOI] [PubMed] [Google Scholar]
- 18.Shim S. Electrophysiological changes between patients with suicidal ideation and suicide attempts: An event-related potential study. Eur Psychiatry. 2023;66:S499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon SH, Shim SH, Kim JS. Electrophysiological changes between patients with suicidal ideation and suicide attempts: an event-related potential study. Front Psychiatry. 2022;13:900724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SM, Jang KI, Chae JH. Electroencephalographic correlates of suicidal ideation in the theta band. Clin EEG Neurosci. 2017;48(5):316–21. [DOI] [PubMed] [Google Scholar]
- 21.Park Y, Jung W, Kim S, Jeon H, Lee SH. Frontal alpha asymmetry correlates with suicidal behavior in major depressive disorder. Clin Psychopharmacol Neurosci. 2019;17(3):377–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Light GA, Williams LE, Minow F, Sprock J, Rissling A, Sharp R, et al. Electroencephalography (EEG) and event-related potentials (ERPs) with human participants. Curr Protoc Neurosci. 2010;52(1):6.25. 1-6.. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Northoff G. How do resting state changes in depression translate into psychopathological symptoms? From ‘Spatiotemporal correspondence’to ‘Spatiotemporal Psychopathology.’ Curr Opin Psychiatry. 2016;29(1):18–24. [DOI] [PubMed] [Google Scholar]
- 24.Koshiyama D, Kirihara K, Usui K, Tada M, Fujioka M, Morita S, et al. Resting-state EEG beta band power predicts quality of life outcomes in patients with depressive disorders: A longitudinal investigation. J Affect Disord. 2020;265:416–22. [DOI] [PubMed] [Google Scholar]
- 25.Merkl A, Neumann W-J, Huebl J, Aust S, Horn A, Krauss JK, et al. Modulation of beta-band activity in the subgenual anterior cingulate cortex during emotional empathy in treatment-resistant depression. Cereb Cortex. 2016;26(6):2626–38. [DOI] [PubMed] [Google Scholar]
- 26.Iosifescu DV, Greenwald S, Devlin P, Mischoulon D, Denninger JW, Alpert JE, Fava M. Frontal EEG predictors of treatment outcome in major depressive disorder. Eur Neuropsychopharmacol. 2009;19(11):772–7. [DOI] [PubMed] [Google Scholar]
- 27.Baskaran A, Farzan F, Milev R, Brenner CA, Alturi S, McAndrews MP, et al. The comparative effectiveness of electroencephalographic indices in predicting response to escitalopram therapy in depression: a pilot study. J Affect Disord. 2018;227:542–9. [DOI] [PubMed] [Google Scholar]
- 28.Arikan MK, Metin B, Tarhan N. EEG gamma synchronization is associated with response to paroxetine treatment. J Affect Disord. 2018;235:114–6. [DOI] [PubMed] [Google Scholar]
- 29.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. [DOI] [PubMed] [Google Scholar]
- 30.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000.
- 31.Pourahmadi M, Negahban H, Koes BW, Fernández-de-Las-Peñas C, EbrahimiTakamjani I, Bahramian M. The effect of dual-task conditions on postural control in adults with low back pain: a systematic review and meta-analysis. J Orthop Surg Res. 2023;18(1):555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voitsekh VF, Melnikova TS, Lapin IA. Clinical-neurophysiological aspects of suicidal behavior. Zhurnal Nevrologii i Psihiatrii imeni SS Korsakova. 2009;109(10):14–20. [PubMed] [Google Scholar]
- 33.Iznak AF, Klyushnik TP, Zozulya SA, Iznak EV, Oleichik IV. Clinical-neurobiological correlations in young depressive patients with a history of suicidal attempts. Zh Nevrol Psikhiatr Im S S Korsakova. 2022;122(11):105–9. [DOI] [PubMed] [Google Scholar]
- 34.Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J Psychiatr Res. 2016;78:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishitha P, Reji M, Kishore Raja PC. EEG based depression level monitoring using signal processing technique. Indian J Public Health Res Dev. 2017;8(4):1346–51. [Google Scholar]
- 36.Ulke C, Wittekind DA, Spada J, Franik K, Jawinski P, Hensch T, Hegerl U. Brain arousal regulation in SSRI-medicated patients with major depression. J Psychiatr Res. 2019;108:34–9. [DOI] [PubMed] [Google Scholar]
- 37.Chen MH, Su TP. Effects of adjunctive ketamine ntravenous infusion in taiwanese patients with treatment-resistant depression: Antidepression, antisuicidality, BDNF Val66Met, and brain imaging. Ketamine: From Abused Drug to Rapid-Acting Antidepressant; 2020. p. 175–89. [Google Scholar]
- 38.Chen F, Zhao L, Li B, Yang L. Depression evaluation based on prefrontal EEG signals in resting state using fuzzy measure entropy. Physiolog Measurement. 2020;41(9):095007. [DOI] [PubMed] [Google Scholar]
- 39.Jang KI, Kim S, Chae JH, Lee C. Machine learning-based classification using electroencephalographic multi-paradigms between drug-naïve patients with depression and healthy controls. J Affect Disord. 2023;338:270–7. [DOI] [PubMed] [Google Scholar]
- 40.Khadidos AO, Alyoubi KH, Mahato S, Khadidos AO, Mohanty SN. Machine learning and electroencephalogram signal based diagnosis of depression. Neurosci Lett. 2023;809:137313. [DOI] [PubMed] [Google Scholar]
- 41.Zhang J, Xu B, Yin H. Depression screening using hybrid neural network. Multimedia Tools Appl. 2023;82(17):26955–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stange J, Jenkins L, Pocius S, Kreutzer K, Bessette K, DelDonno S, et al. Using resting state intrinsic network connectivity to identify suicide risk in mood disorders. Neuropsychopharmacology. 2018;43:S241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anijärv TE, Can AT, Gallay CC, Forsyth GA, Dutton M, Mitchell JS, et al. Spectral changes of EEG following a 6-week low-dose oral ketamine treatment in adults with major depressive disorder and chronic suicidality. Int J Neuropsychopharmacol. 2023;26(4):259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang HG, Meng QH, Jin LC, Hou HR. AMGCN-L: an adaptive multi-time-window graph convolutional network with long-short-term memory for depression detection. J Neural Eng. 2023;20(5):056038. [DOI] [PubMed] [Google Scholar]
- 45.Meerwijk EL, Weiss SJ. Does suicidal desire moderate the association between frontal delta power and psychological pain? Peerj. 2016;4:e1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thompson C, Ong ELC. The association between suicidal behavior, attentional control, and frontal asymmetry. Front Psychiatry. 2018;9:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Can AT, Schwenn PE, Isbel B, Beaudequin D, Bouças AP, Dutton M, et al. Electrophysiological phenotypes of suicidality predict prolonged response to oral ketamine treatment. Progress Neuro Psychopharmacol Biol Psychiatry. 2023;123:110701. [DOI] [PubMed] [Google Scholar]
- 48.Cáceda R, Mirmina J, Kim DJ, Rafiaa M, Carbajal JM, Akram F, et al. Low global frontal brain activity is associated with non-planned or impulsive suicide attempts. A preliminary study. J Affective Disord. 2023;326:44–8. [DOI] [PubMed] [Google Scholar]
- 49.Park YM. resting state electroencephalographic gamma activity is associated with circadian preference in patients with depression. Chronobiol Med. 2023;5(2):82–6. [Google Scholar]
- 50.Iosifescu DV, Greenwald S, Devlin P, Perlis RH, Denninger JW, Alpert JE, Fava M. Pretreatment frontal EEG and changes in suicidal ideation during SSRI treatment in major depressive disorder. Acta Psychiatr Scand. 2008;117(4):271–6. [DOI] [PubMed] [Google Scholar]
- 51.Hunter AM, Leuchter AF, Cook IA, Abrams M. Brain functional changes (QEEG cordance) and worsening suicidal ideation and mood symptoms during antidepressant treatment. Acta Psychiatr Scand. 2010;122(6):461–9. [DOI] [PubMed] [Google Scholar]
- 52.de la Salle S, Phillips JL, Blier P, Knott V. Electrophysiological correlates and predictors of the antidepressant response to repeated ketamine infusions in treatment-resistant depression. Prog Neuro Psychopharmacol Biolog Psychiatry. 2022;115:110507. [DOI] [PubMed] [Google Scholar]
- 53.Bress JN, Kiosses DN. Investigating the neurocognitive correlates of suicidal risk in middle-aged and older adults with major depression. Int Psychogeriatr. 2023;35(8):395–7. [DOI] [PubMed] [Google Scholar]
- 54.Ganiga GR, Subramani K, Sharma DK, Sengan S, Anbalagan K, Seenivasan P. Depressive Disorder Prediction Using Machine Learning-Based Electroencephalographic Signal. EAI/Springer Innovations in Communication and Computing. Part F6322023. p. 181–95.
- 55.Graae F, Tenke C, Bruder G, Rotheram MJ, Piacentini J, CastroBlanco D, et al. Abnormality of EEG alpha asymmetry in female adolescent suicide attempters. Biol Psychiat. 1996;40(8):706–13. [DOI] [PubMed] [Google Scholar]
- 56.Arikan MK, Gunver MG, Tarhan N, Metin B. High-Gamma: A biological marker for suicide attempt in patients with depression. J Affect Disord. 2019;254:1–6. [DOI] [PubMed] [Google Scholar]
- 57.Benschop L, Baeken C, Vanderhasselt MA, Van de Steen F, Van Heeringen K, Arns M. Electroencephalogram resting state frequency power characteristics of suicidal behavior in female patients with major depressive disorder. J Clin Psychiatry. 2019;80(6):5459. [DOI] [PubMed] [Google Scholar]
- 58.Roh S-C, Kim JS, Kim S, Kim Y, Lee S-H. Frontal alpha asymmetry moderated by suicidal ideation in patients with major depressive disorder: a comparison with healthy individuals. Clin Psychopharmacol Neurosci. 2020;18(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iznak AF, Iznak EV, Damyanovich EV, Oleichik IV. Differences of EEG frequency and spatial parameters in depressive female adolescents with suicidal attempts and non-suicidal self-injuries. Clin EEG Neurosci. 2021;52(6):406–13. [DOI] [PubMed] [Google Scholar]
- 60.Krepel N, Benschop L, Baeken C, Sack AT, Arns M. An EEG signature of suicidal behavior in female patients with major depressive disorder? A non-replication. Biolog Psychol. 2021;161:108058. [DOI] [PubMed] [Google Scholar]
- 61.Amico F, De Canditiis D, Castiglione F, Pascarella A, Venerelli N, Fagan JV, et al. A resting state EEG study on depressed persons with suicidal ideation. IBRO Neurosci Rep. 2023;14:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bankwitz A, Ruesch A, Adank A, Hormann C, de Araujo TV, Schoretsanitis G, et al. EEG source functional connectivity in patients after a recent suicide attempt. Clin Neurophysiol. 2023;154:60–9. [DOI] [PubMed] [Google Scholar]
- 63.Jiang C, Huang Z, Zhou Z, Chen L, Zhou H. Decreased beta 1 (12–15 Hertz) power modulates the transfer of suicidal ideation to suicide in major depressive disorder. Acta Neuropsychiatrica. 2023. [DOI] [PubMed]
- 64.Ozger C, Chumachenko S, McVoy M, Croarkin PE, Camsari DD. Evidence for altered electroencephalography coherence in depressed adolescents with suicidal ideation and behaviors. J Child Adolesc Psychopharmacol. 2023;33(7):287–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He XQ, Hu JH, Peng XY, Zhao L, Zhou DD, Ma LL, et al. EEG microstate analysis reveals large-scale brain network alterations in depressed adolescents with suicidal ideation. J Affect Disord. 2024;346:57–63. [DOI] [PubMed] [Google Scholar]
- 66.Umemoto A, Panier LY, Cole SL, Kayser J, Pizzagalli DA, Auerbach RP. Resting posterior alpha power and adolescent major depressive disorder. J Psychiatr Res. 2021;141:233–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen JJ, Urry HL, Hitt SK, Coan JA. The stability of resting frontal electroencephalographic asymmetry in depression. Psychophysiology. 2004;41(2):269–80. [DOI] [PubMed] [Google Scholar]
- 68.Davidson RJ. Anterior cerebral asymmetry and the nature of emotion. Brain Cogn. 1992;20(1):125–51. [DOI] [PubMed] [Google Scholar]
- 69.Gollan JK, Hoxha D, Chihade D, Pflieger ME, Rosebrock L, Cacioppo J. Frontal alpha EEG asymmetry before and after behavioral activation treatment for depression. Biol Psychol. 2014;99:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gold C, Fachner J, Erkkilä J. Validity and reliability of electroencephalographic frontal alpha asymmetry and frontal midline theta as biomarkers for depression. Scand J Psychol. 2013;54(2):118–26. [DOI] [PubMed] [Google Scholar]
- 71.Kang J-H, Bae J-H, Jeon Y-J. Age-related characteristics of resting-state electroencephalographic signals and the corresponding analytic approaches: a review. Bioengineering. 2024;11(5):418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hashemi A, Pino LJ, Moffat G, Mathewson KJ, Aimone C, Bennett PJ, et al. Characterizing population EEG dynamics throughout adulthood. ENeuro. 2016;3(6):ENEURO.0275-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13(6):580–93. [DOI] [PubMed] [Google Scholar]
- 74.Santiesteban I, Banissy MJ, Catmur C, Bird G. Enhancing social ability by stimulating right temporoparietal junction. Curr Biol. 2012;22(23):2274–7. [DOI] [PubMed] [Google Scholar]
- 75.Szanto K, Dombrovski AY, Sahakian BJ, Mulsant BH, Houck PR, Reynolds CF III, Clark L. Social emotion recognition, social functioning, and attempted suicide in late-life depression. Am J Geriatr Psychiatry. 2012;20(3):257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dickhoff J, Opmeer EM, Heering HD, Bruggeman R, van Amelsvoort T, Bartels-Velthuis AA, et al. Relationship between social cognition, general cognition, and risk for suicide in individuals with a psychotic disorder. Schizophr Res. 2021;231:227–36. [DOI] [PubMed] [Google Scholar]
- 77.Chen Z, Xia M, Zhao Y, Kuang W, Jia Z, Gong Q. Characteristics of intrinsic brain functional connectivity alterations in major depressive disorder patients with suicide behavior. J Magn Reson Imaging. 2021;54(6):1867–75. [DOI] [PubMed] [Google Scholar]
- 78.Zhang H, Chen Z, Jia Z, Gong Q. Dysfunction of neural circuitry in depressive patients with suicidal behaviors: a review of structural and functional neuroimaging studies. Prog Neuropsychopharmacol Biol Psychiatry. 2014;53:61–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No datasets were generated or analysed during the current study.


