Abstract
The advent of immunotherapy represents a significant breakthrough in cancer treatment, with immune checkpoint inhibitors (ICIs) targeting PD-1 and CTLA-4 demonstrating remarkable therapeutic efficacy. However, patient responses to immunotherapy vary significantly, with immunosuppression within the tumor microenvironment (TME) being a critical factor influencing this variability. Immunosuppression plays a pivotal role in regulating cancer progression, metastasis, and reducing the success rates of immunotherapy. Myeloid-derived suppressor cells (MDSCs), due to their potent immunosuppressive capabilities, emerged as major negative regulators within the TME, facilitating tumor immune evasion by modulating various immune cells. In addition to their immunosuppressive functions, MDSCs also promote tumor growth and metastasis through non-immunological mechanisms, such as angiogenesis and the formation of pre-metastatic niches. Consequently, MDSCs in the TME are key regulators of cancer immune responses and potential therapeutic targets in cancer treatment. This review describes the origins and phenotypes of MDSCs, their biological roles in tumor progression, and regulatory mechanisms, with a focus on current therapeutic approaches targeting tumor-associated MDSCs. Furthermore, the synergistic effects of targeting MDSCs in combination with immunotherapy are explored, aiming to provide new insights and directions for cancer therapy.
Keywords: TME, MDSCs, Immunotherapy, Immunosuppression, Therapeutic targets
The definition and phenotype of MDSCs
Definition
Myeloid-derived suppressor cells (MDSCs) are a population of cells that predominantly accumulate in specific pathological conditions, such as cancer and chronic inflammation [1]. MDSCs consist of heterogeneous immature myeloid cells (IMCs) at various stages of transcriptional activity and differentiation [2]. Under homeostatic conditions, hematopoiesis in the bone marrow is a structured process that maintains a stable supply of host myeloid cells. Hematopoietic stem cells in the bone marrow typically develop into IMCs, which further differentiate into mature macrophages, dendritic cells (DCs), and granulocytes, including neutrophils, basophils, and eosinophils. Pathological conditions, such as infection or tissue damage, can trigger emergency myelopoiesis to eliminate potential threats to the host [3]. During these conditions, myeloid cells are rapidly activated for a relatively short duration, leading to intense phagocytic activity, respiratory bursts, and cytokine release [4]. This transient myelopoiesis concludes with the resolution of the stimulus, thereby maintaining the balance of myeloid cells. In contrast, pathological conditions, such as chronic inflammation, cancer, and autoimmune diseases, can lead to abnormal and sustained myelopoiesis. In these scenarios, persistent inflammatory signals cause IMCs to deviate from normal differentiation and become pathologically activated. Compared to physiologically differentiated myeloid cells, these IMCs exhibit distinct characteristics, including an immature phenotype and morphology, relatively weak phagocytic activity, and immunosuppressive functions. Functionally, MDSCs can inhibit T cell proliferation, as demonstrated by in vitro assays where co-culture of MDSCs with T cells results in reduced T cell division [5, 6]. These IMCs are now collectively referred to as MDSCs [7].
Since the early 1970s, sporadic studies have reported on immunosuppressive myeloid cells, primarily in the context of tumor progression [8, 9]. From the 1980s to the early 1990s, research confirmed the immunosuppressive functions of various myeloid cell types in tumor immunity. However, the characteristics and biological significance of these IMCs cells remained largely unclear [10]. By the late 1990s, CD11b + Gr-1 + was recognized as the phenotype of immunosuppressive myeloid cells in mice. Although, these IMCs cells shared phenotypic similarities with monocytes and neutrophils, they differed functionally [11]. Due to the absence of conventional surface markers for T cells, B cells, natural killer (NK) cells, and macrophages, these IMCs cells were also described as NS or “null” cells [12]. Subsequently, the accumulation of a large number of strongly immunosuppressive cells in the spleen and tumors became readily reproducible in most mouse tumor models. It was not until 2007 that the term “MDSCs” was proposed to unify the various descriptions of these immunosuppressive myeloid cells [13], based on their origin and immunosuppressive functions. Following this, research on MDSCs surged, with nearly 2,500 related articles published within a decade. MDSCs are involved in multiple pathological conditions and immunological processes. MDSCs have been implicated in cancer, autoimmune diseases, chronic inflammation, and infections. Additionally, MDSCs play a significant role in various aspects of immune regulation [14, 15]. In some cancer patients and tumor-bearing mice, the frequency of MDSCs correlates closely with disease progression and clinical staging. MDSCs hold significant clinical relevance in cancer and have become a critical component of tumor immunology.
Phenotype
Unlike monocytes, macrophages, and dendritic cells (DCs), MDSCs lack distinct and identifiable surface markers [16]. Based on their density, morphology, and phenotype, MDSCs in both humans and mice are primarily categorized into two major subsets: monocytic MDSCs (M-MDSCs) and granulocytic/polymorphonuclear MDSCs (G-MDSCs/PMN-MDSCs) [17]. The former resembles monocytes in both phenotype and morphology, while the latter is similar to neutrophils. Consequently, phenotypic criteria alone are insufficient for distinguishing MDSCs from other cell types. In most cancer types, PMN-MDSCs constitute over 80% of the total MDSC population, whereas M-MDSCs are direct promoters of tumor metastasis [18]. Additionally, a small population of bone marrow progenitor cells exhibiting MDSC characteristics, referred to as “early-stage MDSCs” (E-MDSCs), has been identified in humans (but not in mice). This group of cells, characterized by strong immunosuppressive properties, primarily consists of bone marrow progenitor and precursor cells, making up less than 5% of the total MDSC population [19].
Movahedi et al. [20] were the first to isolate and purify M-MDSCs and PMN-MDSCs using the Ly6G marker. Subsequent research has refined the characterization of MDSCs, revealing distinct features in both mice and humans, and demonstrating the significant heterogeneity of these MDSC cells. In mice, MDSCs are primarily found in the bone marrow, peripheral blood, spleen, liver, lungs, and tumors across various organs, characterized by the co-expression of CD11b and Gr-1. These cells are further subdivided into two subtypes: PMN-MDSCs, defined as CD11b+Ly6G+Ly6Clo, and M-MDSCs, defined as CD11b+Ly6G−Ly6Chi, which resemble neutrophils and monocytes in morphology and phenotype, respectively [21]. In humans, MDSCs are primarily detected in the blood and tumors in various organs. M-MDSCs are characterized as CD11b+CD14+HLA-DR−/loCD15−, while PMN-MDSCs are identified as CD11b+CD14−CD15+orCD11b+CD14−CD66b+ [2]. Currently, the phenotypic identification of MDSCs using flow cytometry is relatively well established. Additionally, M-MDSCs also express the myeloid marker CD33. However, MDSCs expressing Lin−HLA-DR−/loCD33+ are predominantly immature myeloid progenitor cells, referred to as E-MDSCs [19]. Using multicolor immunofluorescence staining and fluorescence-activated cell sorting (FACS), MDSCs can be further classified into six phenotypes: MDSC1 (CD14+IL-4Rα+), MDSC2 (CD15+IL-4Rα+), MDSC3 (Lin-HLA−DR−CD33+), MDSC4 (CD14+HLA-DRlo/−), MDSC5 (CD11b+CD14−CD15+), and MDSC6 (CD15+FSCloSSChi) [22].
In the tumor microenvironment (TME), more specific characteristics of MDSCs have been identified. Lectin-like oxidized low-density lipoprotein receptor 1 (LOX1), a 50 kDa transmembrane glycoprotein, has been established as a specific marker for PMN-MDSCs [23]. Another notable marker is fatty acid transport protein 2 (FATP2) [24]. PD-L1, primarily expressed in M-MDSCs, plays a significant role in mediating immunoregulation [2]. Furthermore, single-cell transcriptomic analyses have identified CD84 + as a surface marker for detecting MDSCs in breast cancer [25]. Wu et al. have also provided a comprehensive review of surface markers for MDSCs in both humans and mice, serving as a valuable reference for their identification [26, 27]. These highly specific molecules can act as markers for MDSCs, making them promising targets for cancer immunotherapy.
It is important to note that the current definition and classification methods for MDSCs are primarily based on their immunosuppressive functions and surface markers. However, MDSCs exhibit significant heterogeneity and plasticity. The classification of MDSCs lacks a unified standard, as various research teams employ different methodologies. A more comprehensive definition and classification of MDSCs should emphasize the distinctions in their immunosuppressive mechanisms, including the stimulating factors and the pathways or cells they affect [28].
Functions of MDSCs
Immunosuppressive effects
Mechanistic studies and theories of MDSC immunosuppression
The immune system, which is essential for tumor control and elimination, comprises lymphocytes, NK cells, antigen-presenting cells (APCs), and B cells. In hosts with tumors, MDSCs play a critical role in promoting tumor immune escape by inhibiting the immune cells responsible for tumor destruction, with their primary functional characteristic being immunosuppression [21].
In the TME, MDSCs exert immunosuppressive functions and promote tumor growth by inducing various factors, including interleukin (IL)-6, interferon (IFN)-γ, IL-1β, granulocyte-macrophage colony-stimulating factor (GM-CSF), tumor necrosis factor (TNF)-α, and vascular endothelial growth factor (VEGF) [29, 30]. In studies of prostatic cancer (PCa), IL-23 produced by MDSCs enhances the survival and proliferation of androgen-deprived cells in castration-resistant prostate cancer (CRPC) by activating the androgen receptor pathway [31]. Tumor-derived PGE2 promotes the accumulation of p50 NF-κB in M-MDSCs, resulting in a phenotype characterized by elevated levels of Nos2 and reduced levels of TNF-α, which diminishes the effectiveness of immunotherapy. PGE2 antagonists can reprogram M-MDSCs to exhibit a phenotype with low Nos2 and high TNF-α levels, thereby restoring the efficacy of immunotherapy (IFNγ) and inhibiting tumor progression [32]. Furthermore, inhibiting the prostaglandin E2 (PGE2)/P50/NO axis can disrupt MDSC-mediated immunosuppression and enhance anti-tumor immunotherapy. MDSCs regulate amino acid metabolism by secreting various factors that affect T cell activity. For example, arginase-1 (ARG1) secreted by MDSCs depletes the TME of L-arginine, leading to the downregulation of TCR-ζ [33]. Increased ARG1 activity in MDSCs breaks down arginine, resulting in reduced levels of L-arginine, which inhibits T cell proliferation and function [6]. Additionally, adenosine plays a role in MDSC-mediated T cell suppression [34]. Inducible nitric oxide synthase (iNOS) in MDSCs produces NO, which inhibits the JAK3/STAT5 signaling pathway, thereby reducing the expression of MHC II molecules and inducing T cell apoptosis [35]. MDSCs also impede T cell activation by decreasing CD62L expression on immature T cells [36]. Furthermore, indoleamine 2,3-dioxygenase (IDO) in MDSCs can induce T cell autophagy, cell cycle arrest, and apoptosis by depleting tryptophan [37].
Associations of the immunosuppressive effects of MDSCs
n nearly all patients with solid tumors, MDSCs are significant promoters of immunosuppression, facilitating tumor progression [25]. In patients with metastatic breast cancer, G-MDSCs co-transplanted with breast cancer cells lead to the exclusion of myeloid immune cells and promote tumor growth [38]. The levels of MDSCs are associated with clinical outcomes and treatment responses in lung cancer patients [39]. In patients with pancreatic cancer, the number of MDSCs in systemic circulation correlates with the stage of the disease [40]. In head and neck squamous cell carcinoma (HNSCC), there is an increased infiltration of immunosuppressive CXCR1/2 + CD15 + PMN-MDSCs and CD14 + M-MDSCs [41].
MDSCs also inhibit the activity of other tumor-killing immune cells. TGF-β secreted by MDSCs impairs NK cell activation by reducing the expression of NKG2D [42]. In patients with hepatocellular carcinoma, M-MDSCs suppress NK cell function through their interaction with the NKp30 receptor [43]. In tumor-bearing mice, PMN-MDSCs block DC antigen cross-presentation [44]. In a mouse model of lung cancer, MDSCs inhibit B cell differentiation and function [45]. In a mouse model of breast cancer, MDSCs upregulate PD-L1 expression on B cells, converting them into regulatory B cells [46]. The expression of PD-L1 on MDSCs in human tumors is of significant importance. Studies have shown that in various human cancers, including breast cancer and hepatocellular carcinoma (HCC), the level of PD-L1 expression on the surface of MDSCs correlates with tumor progression and the extent of immunosuppression [47, 48]. In breast cancer patients, MDSCs within the tumor microenvironment can enhance PD-L1 expression, inhibit immune cell activity, and facilitate the immune evasion of tumor cells [47]. Furthermore, in human HCC tissues, the presence of PD-L1-positive MDSCs is associated with poor patient prognosis. These cells may inhibit T cell immune responses by binding to PD-1 on the surface of T cells, thereby promoting tumor cell growth and metastasis [48]. In cervical cancer, MDSCs interact with B10 cells via the BAFF/BAFF-R signaling pathway, further contributing to immune suppression [49].
MDSCs stimulate other immunosuppressive cells. In a mouse tumor model, tumor-infiltrating M-MDSCs produce CCR5 to recruit Tregs [50]. MDSCs can induce Treg proliferation through cell-cell interactions or by secreting IL-10 and TGF-β [51]. ARG1, IDO, and CD40 expression in MDSCs are involved in Treg generation [52]. MDSCs promote macrophage polarization to the M2 phenotype by producing IL-10 and reducing macrophage MHC II expression, which diminishes antigen-presenting capability and induces immunosuppression [53, 54]. Overall, MDSCs form an inhibitory network with other immunosuppressive cells, thereby weakening the immune response against tumor cells.
Non-immunosuppressive functions
In addition to their immunosuppressive functions, MDSCs contribute to tumor progression through various non-immunosuppressive mechanisms, including the promotion of tumor angiogenesis, enhancement of cancer stem cell properties, facilitation of epithelial-mesenchymal transition (EMT), and establishment of pre-metastatic niches [55–57]. MDSCs not only utilize the abundant Vascular Endothelial Growth Factors (VEGFs) present in the TME, but also produce VEGFs themselves, creating a positive feedback loop that further promotes tumor angiogenesis [58]. Notably, studies have demonstrated that MDSCs can directly induce angiogenesis and tumor invasion by secreting matrix metalloproteinase-9 (MMP9) and differentiating into endothelial-like cells (ECs) [59]. Additionally, MDSCs also promote angiogenesis by releasing exosomes containing pro-angiogenic factors [60]. For instance, exosomes derived from Granulocytic MDSCs (G-MDSCs) that contain S100A9 have been shown to promote colorectal cancer (CRC) progression in a hypoxia-inducible factor-1α (HIF-1α)-dependent manner [61]. Subsequent research revealed that MDSC-derived exosomal S100A9 facilitates the progression of castration-resistant prostate cancer (CRPC) through the circMID1/miR-506-3p/MID1 axis [62]. In ovarian cancer, MDSCs have been shown to upregulate miR-101 expression in cancer cells while suppressing the expression of C-terminal binding protein-2 (CtBP2), resulting in increased cancer cell stemness and metastatic potential [63]. Importantly, clinical samples from breast cancer patients have demonstrated a correlation between MDSC levels and the presence of cancer stem-like cells (CSCs) [64]. Moreover, in a CRC mouse model, elevated levels of CXCL1 in the pre-metastatic liver were found to recruit CXCR2 + MDSCs, thereby forming pre-metastatic niches and promoting liver metastasis [65]. Recent studies have also linked the frequency of CCR4 + Monocytic MDSCs (M-MDSCs) with increased EMT in patients with pancreatic ductal adenocarcinoma (PDAC) [66]. Ginsenoside Rg3 has exhibited significant anticancer effects by modulating MDSCs to inhibit CSCs and EMT in breast cancer [67].
It is important to note that PMN-MDSCs and M-MDSCs not only differ in phenotype and morphology, but also possess unique functional characteristics, despite some overlapping functions [68]. Within the tumor TME, M-MDSCs are generally more suppressive than PMN-MDSCs [69]. Recent studies have reported that different subsets of MDSCs infiltrate primary tumors and distant organs with distinct temporal dynamics, regulating the spatiotemporal plasticity of tumors [70]. In mouse tumor models, tumor-infiltrating M-MDSCs promote the EMT/CSC phenotype, facilitating the metastasis of tumor cells from primary sites [71]. Conversely, lung-infiltrating PMN-MDSCs promote the growth of metastatic tumors by restoring the EMT/CSC phenotype and enhancing tumor cell proliferation [70]. Therefore, the spatiotemporal dynamics of MDSC infiltration may have clinical significance in tumor progression. In conclusion, the specific functions of MDSCs depend on their phenotype, tumor type and stage, and the affected organ or site.
Accumulation and differentiation of MDSCs
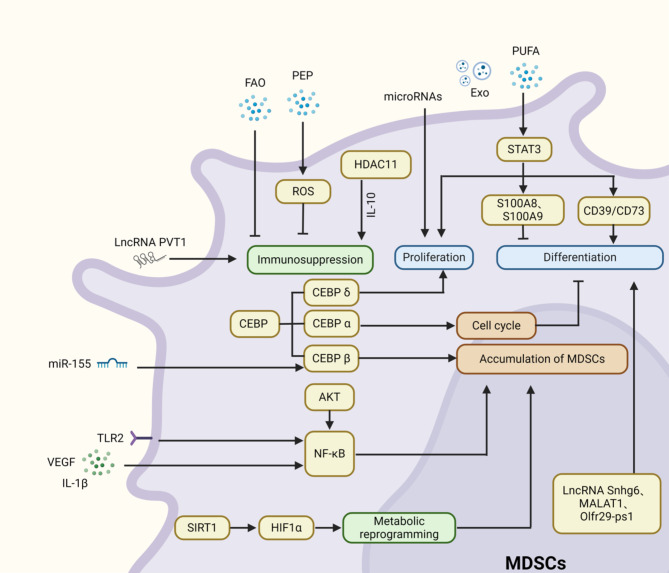
The accumulation and differentiation of MDSCs is an exceptionally complex process. Condamine et al. [72] proposed a two-signal model to elucidate this process, indicating that MDSC accumulation necessitates two distinct types of signals, although some overlap exists. This model is divided into two phases: the first phase involves the expansion of immature myeloid cells (IMCs) and the inhibition of their terminal differentiation; the second phase entails the pathological activation of these IMCs, transforming them into MDSCs. The first phase is primarily driven by tumor-derived growth factors and signaling pathways, including STAT3, IRF8, CCAAT enhancer binding proteins (C/EBP)-β, NOTCH, adenosine receptor A2b, and NOD-like receptor family pyrin domain containing 3 (NLRP3). Retinoblastoma protein 1 (Rb1) plays a role in the differentiation of certain M-MDSCs into PMN-MDSCs. While Rb1hi M-MDSCs predominantly differentiate into macrophages and dendritic cells (DCs), the majority of Rb1low M-MDSCs transition into PMN-MDSCs [73]. The second phase is mainly driven by tumor-derived factors, including pro-inflammatory molecules such as IFN-γ, IL-1β, IL-13, and Toll-like receptor (TLR) ligands [74–76]. These molecules activate various signaling pathways, including STATs [77], NF-κB [78], and HIF-1α [79], all of which are associated with the suppressive activity of MDSCs. Continuous recruitment of MDSCs can also be facilitated by interactions between chemokines and their receptors, particularly the interaction of C-C chemokine receptor type 5 (CCR5) with its ligands, which promotes tumor progression [30].
Related signaling pathways
Myeloid-derived suppressor cells (MDSCs) accumulate and differentiate through tightly regulated by diverse signaling pathways (Fig. 1). Here, we highlight several key pathways, including STATs, C/EBP, and NF-κB. Numerous studies have investigated how STATs signaling pathways govern MDSCs. Targeting STATs pathways can selectively eliminate MDSCs, thereby alleviating tumor-induced immune suppression in neuroblastoma [77]. Among these pathways, STAT3 plays a crucial role in regulating tumor cell proliferation, survival, and invasion, and is also involved in antitumor immune responses. Additionally, several downstream pathways of STAT3 are implicated in regulating MDSCs proliferation and function. Notably, S100A8 and S100A9 are two common molecules regulated by STAT3. The activation of STAT3 enhances the expression of S100A8 and S100A9, which inhibits the differentiation of MDSCs into DCs and promotes their accumulation [80, 81]. However, it is important to note that myeloid-derived suppressor cells (MDSCs) and dendritic cells (DCs) originate from different progenitor cells within the myeloid lineage. Under normal physiological conditions, several limiting factors hinder the differentiation of MDSCs into DCs, making direct differentiation challenging. It is likely that only under specific experimental conditions or pathological states can the differentiation of MDSCs into DCs be influenced, resulting in observable changes in their behavior. Notably, STAT3 can also indirectly regulate the accumulation and differentiation of MDSCs by modulating the expression of relevant factors [82]. In addition to STAT3, other members of the STAT family, such as STAT1 and STAT6, also significantly affect the activity and function of MDSCs. STAT6 promotes expansion of MDSCs and inhibits cytotoxic CD8 + T-cell responses, contributing to intestinal tumor development in adenomatous polyposis coli (APC) mouse models [83]. Although STAT1 is generally considered a tumor suppressor, it exhibits a dual role in MDSCs. In head and neck squamous cell carcinoma (HNSCC), STAT1 serves as a crucial mediator of antitumor responses by inhibiting MDSC accumulation and promoting T-cell-mediated immune responses [84]. Conversely, another study found that STAT1 stimulates MDSCs-mediated immune suppression, thereby facilitating breast cancer progression. Inhibiting STAT1 activity presents a promising therapeutic approach for advanced breast cancer [85].
Fig. 1.
Various pathways play crucial roles in regulating the accumulation and differentiation of MDSCs
C/EBP is a downstream signaling pathway of STAT3; STAT3 can partially increase C/EBP-β, promoting the proliferation of MDSCs [86]. C/EBP-β facilitates the differentiation of myeloid progenitor cells into functional MDSCs, and the loss of miR-155 in tumor cells can enhance C/EBP-β-mediated MDSC infiltration, thereby promoting tumor growth [87]. The deletion of the C/EBP-δ gene in mice significantly inhibits MDSC expansion in response to tumor progression, which aligns with its role in promoting tumor angiogenesis and growth [88].
Additionally, NF-κB plays a significant role in regulating the recruitment and function of MDSCs [89]. Periplocin inhibits MDSC accumulation and suppresses hepatocellular carcinoma (HCC) progression by blocking the AKT/NF-κB pathway [78]. Beyond MDSCs accumulation, the NF-κB pathway can also promote MDSCs activation by IL-1β [90]. Therefore, various signaling pathways collectively regulate the development and differentiation of MDSCs.
Epigenetic modifications
Epigenetic modifications, which alter gene expression without changing the DNA sequence, encompass mechanisms such as DNA methylation, RNA-mediated processes, and histone modifications. These modifications play a crucial role in cellular expansion, differentiation, accumulation, and function [91]. In the context of MDSCs, epigenetic modifications can reshape their characteristics, thereby altering the tumor microenvironment (TME) to counteract tumor growth and metastasis [92]. Increasing evidence indicates that non-coding RNAs (ncRNAs), including microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), are involved in tumor progression and regulate the differentiation and development of MDSCs [93]. miRNAs target cellular signaling pathways to either promote or inhibit MDSCs functions. Conversely, MDSCs can transfer biological information through exosomes containing miRNAs [94]. Specific miRNAs associated with MDSCs, such as miR-146a, miR-155, miR-100, miR-125a, miR-125b, miR-99b, miR-146b, and let-7e, are linked to MDSCs proliferation and contribute to resistance to immunotherapy in melanoma patients [95]. LncRNAs modulate MDSC functions by mediating gene transcription. Recent studies have shown that lncRNA Snhg6 regulates MDSCs differentiation by modulating the ubiquitination of EZH2 [96]. Furthermore, lncRNAs have been identified as regulatory factors for MDSCs in lung cancer [97]. The knockout of lncRNA PVT1 significantly suppresses the immunosuppressive functions of G-MDSCs both in vitro and in vivo, with lncRNA PVT1 expression in G-MDSCs being upregulated under hypoxic conditions, a hallmark of TME [98]. Thus, modulating ncRNA functions in immune cells may unveil new strategies for restoring immune balance without significant toxic side effects.
Histone modifications also influence the differentiation of MDSCs [99]. Histone acetylation regulates gene transcription via histone deacetylases (HDACs) and histone acetyltransferases (HATs) [100]. MDSCs in HDAC11-deficient mice exhibit enhanced suppressive functions [101]. Moreover, the absence of HDAC11 reduces IL-10 secretion, indicating that HDAC11 plays a role in regulating MDSC formation and their suppressive phenotype [102]. Elevated levels of immature myeloid-derived suppressor cells (I-MDSCs) and PMN-MDSCs are observed in colorectal cancer (CRC) tissues. In CRC patients, HDAC-related genes are upregulated in tumor-infiltrating I-MDSCs, while HAT-related genes are downregulated. Conversely, HDAC-related genes are downregulated in tumor-infiltrating PMN-MDSCs [92]. A deeper understanding of the mechanisms by which epigenetic modifications regulate MDSC accumulation and differentiation could provide valuable insights for developing novel therapeutic approaches.
Metabolic reprogramming
During tumor development, excessive metabolic activity promotes tumor cell proliferation and migration, while enhancing immune suppression and altering the TME [103]. As a major heterogeneous population of immune-suppressive cells, MDSCs regulate their immunosuppressive functions not only through the aforementioned mechanisms but also by altering the surrounding metabolic environment [104]. Metabolic reprogramming is a key factor in the functional changes of MDSCs [105]. SIRT1 has been found to coordinate HIF-1α-dependent glycolysis, thereby modulating MDSC function and fate [79]. Fatty acid metabolism is also a significant component of MDSCs development and function. Polyunsaturated fatty acids (PUFAs) promote MDSCs accumulation and enhance their immunosuppressive functions by activating the JAK/STAT3 pathway [106]. Recent studies further propose that FATP2 regulates lipid accumulation, ROS, and immunosuppressive functions in MDSCs in tumor-bearing mice [107]. MDSCs also promote increased fatty acid uptake and activate FAO [108]. Consequently, immunometabolic therapy is emerging as a significant breakthrough in MDSC research, providing new strategies for antitumor treatment.
mTOR, a serine/threonine protein kinase, serves as a central regulator of metabolic reprogramming, reshaping the tumor microenvironment (TME) and influencing the development and treatment of various cancers [109]. Numerous studies have shown that mTOR directly regulates MDSC function by modulating glycolysis. For instance, research by Deng et al. found that tumor-infiltrating M-MDSCs have a higher glycolytic rate than MDSCs in other tissues. Treatment with rapamycin (RAPA), a specific mTOR inhibitor, reduced both the abundance and glycolytic rate of M-MDSCs, thereby inhibiting tumor growth [110]. Wu et al. reported that RAPA suppresses the immunosuppressive function of MDSCs through the iNOS pathway [111]. Recent studies have revealed that RAPA treatment causes significant changes in the genomic landscape of MDSCs, preferentially upregulating genes responsible for the uptake or signaling of lipids and lipoproteins [112]. In tumor-bearing mice, the mTORC1/2 inhibitor AZD2014 reduced MDSC accumulation and delayed tumor growth and recurrence [113]. Additionally, the glycolytic pathway in MDSCs is regulated by HIF-1α, which can be inhibited by the activation of AMP-activated protein kinase (AMPK). AMPK activation also suppresses immune-related pathways involved in MDSC expansion and activation. Studies have shown that the pharmacological activation of AMPK by metformin inhibits the accumulation and immunosuppressive capacity of MDSCs in tumor-bearing mice [114]. Recent research has reported that metformin mediates the suppression of MDSCs and M2 macrophages in the colorectal cancer microenvironment through AMPK activation and subsequent mTOR inhibition, thereby exerting anti-tumor effects [115].
The role of growth factors and signaling molecules
VEGF
VEGF is a potent angiogenic factor that not only stimulates neovascularization in tumor tissues but also plays a role in MDSC biology. It can inhibit DC development and induce MDSC generation [116]. In tumor-bearing mouse models, administration of anti-VEGF monoclonal antibodies (mAb) has been shown to significantly inhibit tumor growth and reduce MDSC numbers in both tumor tissues and peripheral blood [117]. Bevacizumab, the first FDA-approved anti-VEGF mAb, has demonstrated the ability to inhibit tumor angiogenesis and metastatic progression in advanced-stage patients [118]. However, the precise mechanisms by which anti-VEGF mAbs inhibit MDSC expansion remain to be fully elucidated, and the effects may vary depending on factors such as dosage, timing, and treatment intervals.
GM-CSF and G-CSF
These cytokines are well-known promoters of myelopoiesis and hematopoietic progenitor cell mobilization. In the context of cancer, they have been implicated in MDSC accumulation and immunosuppression. For example, anti-VEGF therapy can induce tumor hypoxia and GM-CSF expression, leading to MDSC recruitment and tumor immune suppression [119]. Glutamine deprivation has also been shown to enhance the expression of G-CSF and GM-CSF, promoting MDSC generation and mobilization [120]. In clinical studies, recombinant human GM-CSF administration increased M-MDSC levels in the peripheral blood of recurrent prostate cancer patients [121]. Additionally, G-CSF is a positive regulator of Bv8, which is involved in MDSC mobilization from the bone marrow and tumor angiogenesis [122].
M-CSF
M-CSF is another growth factor that can influence MDSC function and differentiation. It has been shown to play a role in the polarization of macrophages, and since MDSCs share some characteristics with macrophages, M-CSF may also impact MDSC behavior within the tumor microenvironment [123]. However, further research is needed to fully understand its specific role in MDSC biology and its potential as a therapeutic target.
TLR signaling in MDSC regulation
Toll-like receptors (TLRs) are a family of pattern recognition receptors that play a crucial role in the innate immune response. In the context of MDSCs, TLR signaling has complex effects. TLR ligands can act as inducers of MDSCs, and the MyD88 signaling pathway has been shown to be involved in mediating MDSC induction and promoting tumor growth [124]. However, the role of TLR signaling in MDSC differentiation and function is not straightforward. Some studies suggest that TLR activation can reduce MDSC immunosuppressive activity by promoting their differentiation into tumoricidal macrophages. For example, TLR3/7/8/9 agonists have been shown to reduce MDSCs frequencies and enhance anti-tumor efficacy. The anti-cancer effects of the TLR9 agonist CpG ODN partly result from its ability to induce MDSCs differentiation [125]. In contrast, TLR1/2 agonists can lead to the maturation of M-MDSCs into immunosuppressive M2 macrophages. The differential effects of TLR agonists on MDSCs may depend on the specific TLR activated and the context of the tumor microenvironment [126].
Other factors and pathways
Chemokines and Chemokine receptors
Chemokines are key mediators of MDSC recruitment to the tumor site, among which CCL2 is of particular significance [127, 128]. CCL2 is found to be overexpressed in a variety of cancers. This overexpression is closely associated with the recruitment of MDSCs and the progression of tumors [129, 130]. Notably, the CCL2/CCR2 signaling pathway has been clearly demonstrated to have a critical function in mouse models of melanoma and hepatocellular carcinoma [131, 132]. Inhibition of this pathway can reduce MDSC recruitment and enhance anti-tumor immunity. Additionally, the CXCL/CXCR1/2 axis is also involved in MDSC trafficking, and its activation can support immune evasion and tumor progression by promoting the recruitment of neutrophils and PMN-MDSCs [133].
IL-6
In experimental cerebral malaria, IL-6 promotes the expansion of MDSCs in a STAT3-dependent manner, induces the expression of inflammatory markers in MDSCs with an M1-like phenotype, affects the immune balance, regulates the percentage of Th-17 cells, and is closely related to the disease process [134]. IL-6 secreted by cancer-associated fibroblasts (CAFs) synergizes with exosomal miR-21 to activate signal transducer and activator of STAT3 in an autocrine manner, thereby promoting the differentiation of monocytes into M-MDSCs. Functionally, CAF-induced M-MDSCs can promote the drug resistance of tumor cells during cisplatin treatment. Moreover, inhibiting the IL-6 receptor and miR-21 can reverse the generation of - MDSCs mediated by CAFs, and inhibiting the STAT3 signal can abolish the induction effect of CAFs on M-MDSCs [135].
IL-1β and the NLRP3 inflammasome
IL-1β has been shown to play a novel role in the accumulation of G-MDSCs by activating Erk1/2 [136]. The NLRP3 inflammasome, which promotes IL-1β maturation and secretion, has been implicated in MDSC expansion. Inhibition of the NLRP3/IL-1β signaling axis can enhance anti-tumor immunity by reducing MDSC-mediated T cell suppression [137].
S100A8/A9
These pro-inflammatory cytokines enhance the accumulation and immunosuppressive activity of MDSCs in the TME. Knockdown of S100A8 in tumor cells has been shown to inhibit tumor growth and MDSC recruitment in vivo [138]. Tasquinimod, a small-molecule inhibitor of S100A9, has shown potential in preclinical studies but has had limited success in clinical trials [139].
In summary, the accumulation and differentiation of MDSCs are regulated by a multitude of factors and signaling pathways. Understanding these complex interactions is essential for developing effective therapeutic strategies that target MDSCs and enhance anti-tumor immune responses.
Therapeutic approaches targeting Tumor-MDSCs
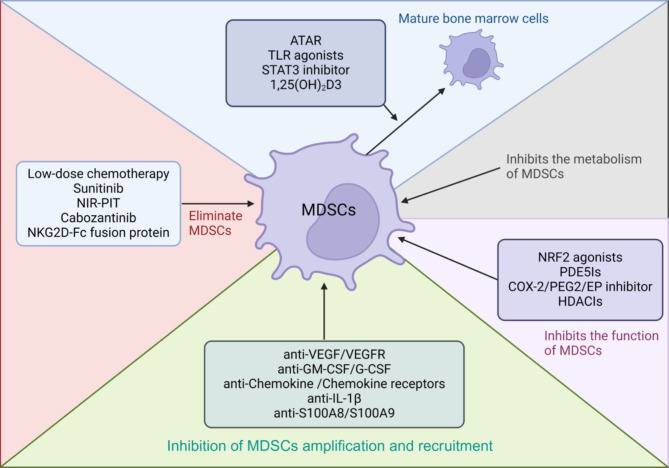
Pathologically activated myeloid-derived suppressor cells (MDSCs) play a critical role in immune suppression in cancer. MDSCs alter the nutrient environment within the tumor microenvironment (TME) and interact with immune cells to suppress anti-tumor activities, resulting in poor cancer treatment outcomes. Consequently, extensive research has focused on developing therapeutic strategies to target MDSCs. These strategies can be broadly categorized into five approaches: (1) promoting the differentiation of MDSCs into mature myeloid cells; (2) inhibiting the proliferation and recruitment of MDSCs; (3) suppressing MDSC function; (4) eliminating MDSCs; and (5) targeting MDSCs metabolism (Fig. 2). In this review, we discuss these approaches and highlight recent research developments in this evolving field.
Fig. 2.
Therapeutic Approaches Targeting Tumor-MDSCs. The developing therapeutic strategies can be broadly categorized into five approaches: (1) promoting the differentiation of MDSCs into mature myeloid cells; (2) inhibiting MDSCs proliferation and recruitment; (3) suppressing MDSC function; (4) eliminating MDSCs; and (5) targeting MDSCs metabolism
Promoting MDSCs differentiation into mature myeloid cells
all-trans retinoic acid (ATRA)
All-trans retinoic acid (ATRA) is an agonist of retinoic acid receptors that inhibits retinoic acid signaling and promotes the differentiation of MDSCs into mature myeloid cells, including granulocytes, macrophages, and dendritic cells (DCs) [140]. This process enhances the host’s anti-tumor immune response by neutralizing reactive oxygen species (ROS) [141]. ATRA is one of the most well-established targeted therapies for acute promyelocytic leukemia [142]. Early studies indicated that high doses of ATRA (> 150 ng/mL) could eliminate MDSC-mediated immune suppression in metastatic renal cell carcinoma by promoting MDSC differentiation into antigen-presenting cell (APC) precursors, thereby enhancing T-cell-induced cytotoxicity against tumor cells [143]. Recent research has demonstrated that ATRA reduces the number of M-MDSCs, G-MDSCs, and tumor-associated macrophages (TAMs) in the spleen. Additionally, ATRA significantly decreases the expression of tumor-infiltrating G-MDSCs and pro-tumor immune suppression molecules (ARG1, iNOS, IDO, S100A8, and S100A9) [140]. ATRA has also been used to enhance the efficacy of conventional chemotherapeutic agents and immunotherapies. Clinical trial data for patients with small-cell lung cancer (SCLC) revealed that ATRA, when combined with vaccination, significantly reduced peripheral blood MDSCs and enhanced the immune response to vaccination in SCLC patients [144]. In a randomized phase II clinical trial, the addition of ATRA to standard ipilimumab treatment in stage IV metastatic melanoma patients significantly reduced circulating MDSC frequencies compared to ipilimumab alone [145]. Furthermore, a recent clinical trial assessing the safety and efficacy of ATRA combined with pembrolizumab in stage IV metastatic melanoma demonstrated that this combination effectively reduced circulating MDSC frequencies, exhibited good tolerability, and had a high response rate, with 50% of patients achieving complete remission and a one-year overall survival rate of 80%. This combination represents a promising first-line treatment strategy for advanced melanoma [146]. Although ATRA as a monotherapy shows limited efficacy in solid tumors, it may enhance immune responses and prolong patient survival by inducing MDSC differentiation.
TLR agonists
Toll-like receptors (TLRs) are type I transmembrane proteins, with ten TLRs identified in humans (TLR1-10) and twelve in mice (TLR1-9, TLR11-13). Previous studies have suggested that TLR ligands serve as inducers of MDSCs and have highlighted the critical role of myeloid differentiation primary response 88 (MyD88) in mediating MDSC induction and promoting tumor growth, a process that can be suppressed by blocking MyD88-mediated signaling [124]. Other studies have reported that MDSC differentiation is influenced by TLR signaling pathways. For example, He et al. demonstrated that TLR4 signaling, through the induction of S100A8 and S100A9 expression, can inhibit MDSC differentiation into DCs, promote their functional maturation, and enhance their migration [147]. Tumor cell-derived extracellular vesicles (EVs) bind to TLR4 on the surface of myeloid cells through the heat shock protein 86 (HSP86) expressed on these cells, activating the NF-κB signaling pathway. This, in turn, upregulates the expression of programmed cell death ligand 1 (PD-L1) on myeloid cells such as murine immature myeloid cells (IMCs) and human CD14 + monocytes, inducing the transformation of normal myeloid cells into immunosuppressive MDSC. Moreover, inhibiting the TLR4 signal can block this process, indicating that TLR4 is a crucial regulator in the generation and immunosuppression of MDSC [148]. Furthermore, TLR4 binds to soluble HSP90α, activating the NF-κB signaling pathway, which induces monocytes to upregulate PD-L1 expression, thereby enabling them to acquire the ability to inhibit T cell proliferation. Simultaneously, this process endows monocytes with anti-apoptotic capabilities and downregulates the expression of HLA-DR, promoting the transformation of monocytes into immunosuppressive MDSC, thus playing a crucial role in the positive regulation of MDSC [149]. However, some research groups have contested these findings, reporting that TLR3 activation can reduce the immunosuppressive activity of MDSCs. TLR3 activation may decrease MDSC populations and promote their differentiation into tumoricidal macrophages [150]. Recent studies further indicate that TLR1/TLR2 signaling activation reduces MDSC-mediated inhibition of T-cell proliferation, thereby suppressing tumor growth. This effect is manifested by a reduction in MDSCs numbers, MDSC differentiation into APCs, and decreased production of suppressive mediators [151]. Agonists of TLR3/7/8/9, by activating their respective TLR pathways, reduce the frequency of MDSCs and enhance anti-tumor efficacy. For instance, it has been revealed that the anti-cancer effects of the TLR9 agonist CpG ODN partly result from its ability to induce MDSCs differentiation [125]. Wang et al. found that the suppressive activity of M-MDSCs isolated from cancer patients could be reversed by treatment with TLR7/8 agonists, which induced differentiation of human M-MDSCs into tumor-killing M1 macrophages.
TLR7/8 agonists can function as monotherapies or in combination with other immunotherapies to enhance anti-tumor effects by inducing non-suppressive MDSC states. In an ongoing phase Ib trial (NCT02124850) involving 14 patients with head and neck squamous cell carcinoma (HNSCC), preoperative treatment with the TLR8 agonist motolimod in combination with cetuximab resulted in a reduction of MDSCs in tumor tissues and an increase in M1 monocyte infiltration [152]. Another recent study reported that the combination of oxaliplatin and the TLR agonist R848 reversed MDSC functional polarization to M1 macrophages, thereby enhancing the anti-tumor effects of oxaliplatin. This study not only uncovered a new immunological mechanism underlying oxaliplatin resistance but also demonstrated the substantial potential of TLR7/8 agonists as novel immune adjuvants in the chemotherapy of oxaliplatin-resistant colorectal cancer [153]. Currently, Bacillus Calmette-Guerin (BCG) (TLR2/TLR4 agonist), mono phosphoryl lipid A (MPL) (TLR4 agonist), and imiquimod (Imiq) (TLR7 agonist) have been approved by the U.S. Food and Drug Administration (FDA) for clinical use in cancer patients [154]. BCG and PD-L1 blockade can synergistically inhibit bladder cancer growth and reduce MDSC frequencies. This suggests that the combination of TLR agonists and PD-1/PD-L1 inhibitors may synergistically disrupt the immunotolerant microenvironment.
STAT3 inhibitors
Constitutive phosphorylation of STAT3 is a critical molecular event that regulates the expansion and immunosuppressive functions of MDSCs in tumors [155]. STAT3 also inhibits the differentiation of immature myeloid cells (IMCs) into mature dendritic cells (DCs) and macrophages. Therefore, targeting STAT3 represents an attractive therapeutic strategy for reducing MDSCs in cancer treatment. Early studies have reported that docetaxel reduces STAT3 phosphorylation in breast cancer mouse models, thereby promoting the differentiation of MDSCs into M1 macrophages with anti-tumor activity [156]. Additionally, oral administration of curcumin B, a selective inhibitor of the JAK2/STAT3 pathway, for seven consecutive days decreased the levels of peripheral blood IMCs and increased the levels of mature myeloid cells in patients with advanced lung cancer [157]. Subsequent trials have shown that systemic administration of AZD9150, an antisense oligonucleotide inhibitor of STAT3, reduced levels of PMN-MDSCs in patients with diffuse large B-cell lymphoma [158]. More recent research has demonstrated the feasibility and rationality of combining STAT3 inhibition with immunotherapy in cancer treatment. For instance, a study found that inhibiting STAT3 significantly enhanced Bax-dependent apoptosis of MDSC in a mouse model of liver metastases, further augmenting the anti-tumor effects of chimeric antigen receptor T cell (CAR-T) therapy [159]. Therefore, targeting the STAT3 signaling pathway represents a promising strategy for cancer immunotherapy.
Other potential therapies
Similar to ATRA, vitamin D3 can induce the differentiation of Myeloid-Derived Suppressor Cells (MDSCs) and enhance antitumor immune responses. 1,25-Dihydroxyvitamin D3 (1,25(OH)2D3), the active metabolite of vitamin D3, has been identified in early studies as a potent natural regulator of both innate and adaptive immunity. In patients with head and neck squamous cell carcinoma (HNSCC) who received preoperative treatment with 1,25(OH)2D3, there was a reduction in the frequency of immunosuppressive CD34 + progenitor cells, along with a significant increase in mature dendritic cells (DCs) within tumor tissues [160]. Another study demonstrated that HNSCC patients treated with 1,25(OH)2D3 exhibited higher levels of intratumoral CD4 + and CD8 + T cells compared to untreated patients, and those who received 1,25(OH)2D3 experienced a longer time to tumor recurrence than those who did not receive preoperative treatment [161]. However, the role of vitamin D3 in the differentiation of bone marrow cells remains largely confined to early-stage research. Therefore, targeting MDSCs with vitamin D3 for clinical cancer therapy warrants further investigation.
In recent years, compounds such as dioscin, ganoderma lucidum polysaccharide (GLP), sanguinarine, and β-glucan have been reported to promote the differentiation of MDSCs and reduce associated immunosuppression in preclinical tumor models. For instance, dioscin has been shown to inhibit colitis-associated colorectal cancer (CRC) tumorigenesis by modulating macrophage polarization and MDSC differentiation [162]. In a Lewis lung carcinoma (LLC) mouse model, GLP, isolated and purified from the fruiting bodies of Ganoderma lucidum, suppressed lung cancer progression by inducing MDSC differentiation and inhibiting their accumulation [163]. Subsequent studies found that sanguinarine alleviated lung cancer-induced immunosuppression by promoting the differentiation of MDSC into macrophages and DCs through the NF-κB pathway [164]. Additionally, treatment with particulate β-glucan for two weeks reduced the levels of polymorphonuclear (PMN)-MDSCs in the peripheral blood of patients with non-small cell lung cancer (NSCLC) [165]. However, further in-depth studies are necessary to confirm the therapeutic potential of these compounds in cancer patients.
Inhibition of MDSC expansion and recruitment
Anti-VEGF/VEGFR
The effects of widely used anti-VEGF/VEGFR therapies on MDSCs have been confirmed in cancer patients. Bevacizumab-based therapy significantly reduced the proportion of PMN-MDSCs in the peripheral blood of patients with NSCLC [118]. In a Phase 0/I dose-escalation clinical trial (NCT02669173), low-dose capecitabine combined with bevacizumab significantly decreased circulating MDSC levels and increased the infiltration of cytotoxic immune cells into the TME in patients with recurrent glioblastoma multiforme (GBM) [166]. However, in another study, bevacizumab monotherapy did not reduce MDSC accumulation in the peripheral blood of patients with renal cell carcinoma (RCC) [167]. These discrepancies may be attributed to variations in dosage, timing, or treatment intervals. Although the use of anti-VEGF mAbs has been validated and assessed in numerous clinical cancer trials, the therapeutic effects cannot be solely be ascribed to the reduction of MDSCs, considering the multifaceted roles of VEGF.
Anti-GM-CSF/G-CSF
Granulocyte Colony-Stimulating Factor (G-CSF) and Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) are well-established promoters of myelopoiesis and the mobilization of hematopoietic progenitor cells. Numerous preclinical studies have demonstrated that GM-CSF and G-CSF facilitate the accumulation of MDSC and suppress antitumor immune responses [119]. Targeting GM-CSF may help overcome resistance to anti-VEGF therapy in ovarian cancer [117]. In summary, while blocking CSF signaling presents a promising strategy for inhibiting MDSC expansion and recruitment in cancer patients, further validation is required.
Anti-chemokine/chemokine receptor
Recent studies have shown that *Clostridium* species can reduce MDSC recruitment to the tumor microenvironment by modulating the PI3K/CCL2/CCR2 axis, thereby increasing CD8 + T cell infiltration in cholangiocarcinoma [168]. Furthermore, in preclinical mouse tumor models, the combination of CCL2/CCR2 blockade with radiotherapy, immunotherapy, and targeted therapy has shown synergistic and enhanced antitumor effects, while reducing tumor-associated MDSCs and increasing tumor-infiltrating lymphocytes. In studies of PD-1 blockade-resistant glioblastoma, the CCR2 antagonist CCX872, when used alone, improved median survival, and in combination with anti-PD-1 therapy, it further enhanced both median and overall survival. CCX872 significantly reduced tumor-associated MDSCs in this context [169]. Additionally, CCL2/CCR2-targeted therapeutic strategies have shown efficacy in cancer treatment. In a Phase I dose-escalation study, primary breast cancer patients safely received the CCL2 inhibitor propagermanium (PG), which is anticipated to possess antitumor metastatic potential [170]. Another Phase Ib trial demonstrated that PF-04136309, a small-molecule CCR2 inhibitor, when combined with chemotherapy, inhibited the migration of inflammatory monocytes from the bone marrow, thereby reducing tumor-associated macrophages (TAMs) and improving tumor control rates in patients with pancreatic ductal adenocarcinoma (PDAC). This indicates that CCR2-targeted therapy is both safe and well-tolerated [171].
However, the inability of anti-CCL2 antibodies and CCR2 inhibitors to effectively block the CCL2/CCR2 axis over prolonged periods has resulted in suboptimal clinical trial outcomes. Additionally, the infiltration of MDSC into tumor sites is regulated by multiple chemokines, which limits the efficacy of therapeutic interventions that target a single chemokine. For example, in tumor-bearing hosts, the activation of the CXCL/CXCR1/2 axis plays a critical role in facilitating immune evasion and tumor progression by promoting the recruitment of neutrophils and PMN-MDSCs. Research has demonstrated that CXCL1 induces PMN-MDSC accumulation, leading to CD8 + T cell exhaustion in gastric cancer [172]. Furthermore, conventional anticancer therapies such as chemotherapy and radiotherapy can lead to treatment resistance. Combining these therapies with blockade of the CXCL/CXCR1/2 axis has shown synergistic effects in enhancing antitumor activity in preclinical tumor models. SX-682, a CXCR1/2 inhibitor, effectively blocks the recruitment of tumor MDSC and enhances T cell activation, thereby making pancreatic ductal adenocarcinoma (PDAC) more responsive to immunotherapy [133].
Anti-IL-1β therapy
Several studies have observed a correlation between IL-1β and the expansion of MDSC. IL-1β promotes MDSC accumulation and inhibits the activation of cytotoxic T cells, thereby contributing to the development of an immunosuppressive microenvironment. Notably, the combination of anti-IL-1β antibodies with androgen deprivation therapy and immune checkpoint inhibitor anti-PD-1 antibodies has shown enhanced anticancer effects in patients with castrated prostate cancer (PCa) [173]. Another study found that IL-1β expression in K-ras-mutant lung adenocarcinoma patients was positively correlated with PMN-MDSC infiltration, indicating that targeting IL-1β could be a viable immunopreventive and therapeutic approach for K-ras-mutant lung adenocarcinoma [174]. Furthermore, the inhibition of NLRP3, when combined with anti-PD-1 therapy, significantly improved the antitumor efficacy of monotherapy by inhibiting MDSC-mediated T cell suppression and tumor progression. This study suggests that NLRP3 activation in melanoma cells is a pro-tumorigenic mechanism that induces MDSC expansion and immune evasion. Therefore, blocking IL-1β in conjunction with chemotherapy or other cancer treatments may yield in synergistic effects.
Anti-S100A8/A9 therapy
In NK/T cell lymphoma (NKTCL), the percentage of MDSCs in peripheral blood was positively correlated with S100A9 levels [175]. S100A9 functions as an immunosuppressor in NKTCL by promoting MDSC accumulation and upregulating PD-L1, suggesting that S100A9 could be a potential target for enhancing the efficacy of immunotherapy in NKTCL [176]. Studies have shown that tasquinimod treatment significantly reduced bone marrow-mediated immunosuppression in myeloma-bearing mice, increased the population of CD11b + cells, and significantly decreased myeloma cell proliferation and colony formation in vitro [139]. However, another clinical trial evaluating the efficacy and tolerability of tasquinimod in patients with advanced solid tumors failed to demonstrate clinical activity in heavily pretreated patients with advanced HCC, RCC, ovarian cancer, and gastric cancer [177]. Therefore, further investigation into the effectiveness of S100A8/A9-targeted tumor strategies is necessary.
Inhibition of MDSC function
Activators of the nuclear factor erythroid 2-related factor 2 (NRF2) pathway
MDSCs primarily express active ROS, ARG1, NOS, and peroxynitrite to exert their immunosuppressive functions [178–180]. Therefore, appropriately targeting these factors as potential therapeutic targets could eliminate the immunosuppressive function of MDSCs. MDSCs release ROS molecules as part of a primary mechanism for inhibiting T cell responses. Despite the toxic effects of ROS on most cells, MDSCs survive and release elevated levels of ROS [107]. NRF2 is a master transcription factor that is ubiquitously present and regulates multiple genes to alleviate oxidative stress [181]. The loss of NRF2 in tumor-bearing mice may lead to abnormal accumulation of ROS in MDSCs, increasing the susceptibility to cancer metastasis. Selective activation of NRF2 can reduce intracellular ROS production, inhibit MDSC immunosuppression, prevent tumor metastasis, and induce tumor regression [182]. Early studies reported that the synthetic triterpenoid CDDO-Me reduces ROS production by activating NRF2, completely eliminating the immunosuppressive effects of MDSCs. CDDO-Me treatment in tumor-bearing mice inhibited the suppressive activity of splenic MDSCs, resulting in reduced tumor growth [183]. Furthermore, analysis of samples from pancreatic cancer patients treated with CDDO-Me in combination with gemcitabine showed no effect on MDSC levels in peripheral blood but significantly improved immune responses in cancer patients [183]. Another synthetic triterpenoid, omaveloxolone (RTA 408), has been reported to effectively activate NRF2 and subsequently exert antioxidant functions. A Phase I clinical trial (NCT02029729) was conducted to determine the appropriate dose for Phase II studies, characterize pharmacokinetics and pharmacodynamics, and evaluate the antitumor activity of omaveloxolone in patients with stage IV relapsed/refractory melanoma or NSCLC. This study supports further exploration of the therapeutic role of omaveloxolone in cancer [184]. Therefore, NRF2 represents a potential target for cancer therapy and warrants further research.
Phosphodiesterase type 5 inhibitors (PDE5Is)
Increasing evidence suggests that PDE5 inhibitors enhance endogenous antitumor immunity by inhibiting MDSC function [185]. Early studies demonstrated that PDE5 inhibition in MDSCs enhances antitumor immunity in mouse models. Furthermore, tadalafil treatment in end-stage relapsed/refractory multiple myeloma patients was found to reduce MDSC function, resulting in significant and lasting anti-myeloma immunity and clinical responses [186]. Subsequent research further evaluated whether tadalafil could reverse tumor-induced immunosuppression and promote tumor immunity in patients with HNSCC. The results showed that tadalafil beneficially modulated the tumor microenvironment in HNSCC patients by dose-dependently reducing MDSCs and Tregs while increasing tumor-specific CD8 + T cells [186]. A Phase II clinical trial demonstrated that tadalafil enhances immune function in patients with HNSCC by inhibiting MDSC function [187]. Additionally, in an open-label, dose-escalation trial indicated that tadalafil treatment is safe and well-tolerated in patients with metastatic melanoma. Clinically stable patients exhibited significant infiltration of CD8 + T cells and a reduction in MDSCs within metastatic lesions [188]. Another study found that sildenafil decreased the function of surgery-derived G-MDSCs by downregulating the expression of ARG1, IL-4Ra, and ROS, thereby enhancing NK cell antitumor cytotoxicity and reducing the risk of postoperative disease recurrence [189]. By counteracting the immunosuppressive effects of surgery-derived MDSCs, sildenafil can be combined with perioperative influenza vaccination targeting NK cells to mitigate postoperative metastasis. Importantly, sildenafil has the potential to reverse MDSC suppression in patients undergoing cancer surgery [189]. A recent interim analysis of a Phase I clinical trial (NCT02544880) is currently underway to evaluate the safety and immunological effects of combining tadalafil with an antitumor vaccine in patients with recurrent HNSCC [190]. Further clinical studies are necessary to validate the immunotherapeutic role of PDE5 inhibitors in cancer treatment.
COX-2/PGE2/EP Axis inhibitors
COX-2 is a critical inflammatory factor that is often overexpressed in various malignancies. Its elevated expression is associated with a poor prognosis [191]. PGE2, a major metabolic product generated by COX-2 from arachidonic acid, can bind to four G protein-coupled EP receptors (EP1-EP4) [192]. Substantial evidence suggests that the COX-2/PGE2/EP axis plays a significant role in tumor progression [193]. More importantly, numerous preclinical studies have investigated the effects of inhibiting the COX-2/PGE2/EP axis on the development of MDSC in cancer. For instance, dietary treatment with the COX-2 inhibitor celecoxib reduces PGE2 levels both in vitro and in vivo, leading to a decrease in the accumulation of all MDSC subtypes, both locally and systemically, while also lowering ROS and NO levels in tumor-bearing mice. This study indicates that celecoxib enhances immunotherapy by reducing MDSC numbers and inhibiting their function [194]. Additionally, combined treatment with the CD40 mAb agonist and celecoxib reduces ARG1 expression in MDSCs and increases the survival rate of glioma-bearing mice compared to monotherapy [195]. Current therapies targeting COX-2, including NSAIDs and COX-2 inhibitors, are associated with severe side effects due to the overall inhibition of prostaglandins. This suggests that targeting downstream molecules in the PGE2 pathway may be a viable strategy. Among these, EP4 antagonists have shown efficacy in inhibiting tumor metastasis in preclinical models. For example, YY001, an EP4 receptor antagonist, inhibits PCa growth by modulating the TME, leading to significant tumor regression, long-term survival, and sustained immune memory [196]. Furthermore, EP4 knockdown significantly inhibits lung metastasis of oral cancer cells in murine tumor models [197]. A phase I clinical trial (NCT02540291) involving patients with advanced solid tumors showed that oral administration of the EP4 inhibitor E7046 significantly enhances CD3 + and CD8 + T cell infiltration within tumors, though the MDSC levels in these patients were not reported [198]. Additionally, YY001 promotes T lymphocyte proliferation and anti-cancer activity while counteracting the immunosuppressive effects of MDSCs [196]. In vitro studies show that PGE2 induces ARG1 expression via EP4 and inhibits effector T cell activity, thereby contributing to MDSC-mediated immunosuppression [199]. Another study found that treatment with NSAID aspirin or an EP antagonist significantly protects CRC mice from tumor formation, reduces MDSC aggregation, and decreases COX-2/Arg-1 expression [200]. Therefore, EP4 antagonists represent a promising strategy for cancer therapy, warranting further investigation into their anti-tumor effects when used in combination with chemotherapy or immunotherapy.
Histone deacetylase inhibitors (HDACIs)
HDACIs are anti-tumor drugs currently under development for clinical use. HDACIs enhance the acetylation of histones or non-histone proteins, promoting gene transcription through epigenetic regulation [201]. More importantly, these drugs exhibit cytotoxic or cytostatic properties, directly inhibiting tumor cells. HDACIs play a crucial role in modulating host immunosuppressive cells, particularly MDSCs [202]. Many isoform-specific HDACIs are currently available on the market and are undergoing clinical trials as anti-tumor agents. Entinostat (ENT), a specific HDACI that targets class I HDAC enzymes (HDAC1, 2, and 3), has been observed to increase the total number of MDSCs in tumors when used alone in lung cell carcinoma and RCC models. However, only a slight increase in MDSC numbers was noted when ENT was combined with anti-PD-1 therapy. Additionally, a reduction in immunosuppressive function was observed, suggesting that ENT can lower the levels of ARG1, iNOS, and COX-2, thereby diminishing the immunosuppressive effects of MDSCs. This study indicates that ENT enhances PD-1-targeted anti-tumor effects by inhibiting MDSC function and breaking the immunosuppressive TME [203]. Valproic acid (VPA), another class I-selective HDACI, has been shown to reduce the proportion of PMN-MDSCs and inhibit the immunosuppressive function of MDSCs in a dose-dependent manner. It can also decrease ARG1 levels by inhibiting IL-4Rα expression, thereby weakening the immunosuppressive effects of MDSCs [204]. Combined treatment with VPA and an anti-PD-L1 antibody effectively inhibits the immunosuppressive function of MDSCs. Although HDACIs have demonstrated efficacy as anti-tumor agents in clinical studies, their overall success has been limited. Additionally, these inhibitors may lead to side effects, including thrombocytopenia, nausea, vomiting, anorexia, and fatigue. Therefore, further research is necessary to explore the regulation of MDSCs by HDACIs is needed to enhance their therapeutic effectiveness against tumors.
Elimination of MDSCs
Low-dose chemotherapy
Low-dose chemotherapy agents, such as 5-fluorouracil (5-FU), paclitaxel, cisplatin, and gemcitabine, have been shown to effectively eliminate MDSCs in tumor-bearing mice and enhance anti-tumor immune responses. Early studies demonstrated that low-dose chemotherapy drugs, particularly paclitaxel, can initiate anti-tumor responses by neutralizing MDSCs in melanoma mouse models. A preliminary study further investigated the potential therapeutic effect of low-dose paclitaxel in melanoma patients resistant to immune checkpoint inhibitors (ICIs), revealing that it reduced both the frequency and immunosuppressive phenotype of MDSCs in peripheral blood and skin metastases [205]. Recent research has found that low-dose 5-FU mediates MDSC depletion, enhancing T-cell infiltration and anti-tumor responses in ICI-resistant lung cancer [206]. Gemcitabine is another chemotherapy agent that eliminates MDSCs; however, its clinical application is often hindered by resistance and low in vivo delivery efficiency. A study developed gemcitabine nanoparticles that effectively depleted MDSCs and enhanced T-cell immune responses, thereby improving anti-tumor efficacy [207]. Compared to gemcitabine, 5-FU is more effective and selective in reducing the number of MDSCs in the spleen and tumor sites of tumor-bearing mice. However, 5-FU treatment activates the NLRP3 inflammasome in MDSCs, leading to increased IL-1β production and limiting its therapeutic efficacy. Notably, these chemotherapeutic agents significantly reduce the number of MDSCs in the spleen without substantially affecting DCs, T cells, NK cells, macrophages, or B cells. The underlying mechanism may involve these chemotherapeutic agents acting as nucleoside analogs, which extend and block DNA synthesis during the cell cycle, ultimately inducing cell death. However, the selective killing mechanism of MDSCs requires further elucidation. Additionally, the effects of chemotherapeutic agents on MDSCs may vary under different conditions, influenced by multiple factors, including chemotherapy dosage, administration regimen, tumor type and stage, and MDSC location. Therefore, certain chemotherapeutic agents, at specific doses and durations, can elicit a positive anti-tumor immune response by targeting MDSCs.
Sunitinib
Sunitinib (Sutent) is a multi-targeted tyrosine kinase inhibitor (TKI) that possesses properties to inhibit angiogenesis and regulate immune dysfunction. It has been approved as a first-line treatment for patients with metastatic RCC. Sunitinib can reduce the percentage of eMDSCs and increase NK cells without affecting the phenotype or effector function of CD4 + or CD8 + T cells [208]. Furthermore, numerous studies have demonstrated the synergistic anti-tumor effects of sunitinib when combined with various immunotherapies in tumor-bearing mice [209]. In a mouse tumor model, treatment with tazemetostat alone had limited inhibitory effects on HNSCC progression. However, the combination of tazemetostat and sunitinib reduced the number of MDSCs and Tregs, promoted intratumoral T-cell infiltration, inhibited T-cell exhaustion, increased intratumoral PD-L1 expression, and improved the response rate to anti-PD-1 therapy. This combination effectively reverses HNSCC-specific resistance to immunotherapy, making it a promising strategy for overcoming ICI treatment resistance [210]. Additionally, the combination of CAR-T therapy and sunitinib showed synergistic effects in a mouse model of RCC lung metastasis, potentially due to sunitinib’s ability to reduce MDSC frequency in the TME [211].
Other potential therapies
Near-infrared photoimmunotherapy (NIR-PIT) is a novel developed treatment that selectively targets and destroys cancer cells while sparing adjacent normal cells [212]. Ly6G-targeted NIR-PIT has been shown to effectively eliminate intratumoral PMN-MDSCs [213]. The combination of NIR-PIT targeting both cancer cells and PMN-MDSCs produces a synergistic effect, significantly enhancing host anti-tumor immunity. Research indicates that the selective local depletion of PMN-MDSCs by NIR-PIT may represent a promising new strategy for cancer immunotherapy [213]. Furthermore, cabozantinib has been reported to enhance the efficacy of anti-HER2 mAb immunotherapy in primary breast cancer by eliminating MDSCs [214]. The NKG2D-Fc fusion protein can also bolster anti-tumor immunity by targeting and eliminating immunosuppressive MDSCs and Tregs within the TME [215]. Consequently, targeting MDSCs in the TME may serve as an effective strategy to disrupt the tumor immune microenvironment and enhance the effectiveness of anti-tumor therapies.
Inhibition of MDSC Metabolism
Fatty acid metabolism
In several mouse tumor models, tumor-infiltrating MDSCs (T-MDSCs) have been shown to enhance their immunosuppressive functions by increasing the uptake of free fatty acids (FFAs) and activating fatty acid oxidation (FAO) [216]. Therefore, selectively targeting the fatty acid metabolism of MDSCs can mitigate associated immunosuppression, thereby enhancing anti-tumor immune responses. Carnitine palmitoyl transferase 1 (CPT1), the first rate-limiting enzyme in the FAO cycle, can be inhibited by etomoxir. The combination of etomoxir with low-dose chemotherapy completely abolishes the immunosuppressive function of T-MDSCs and induces significant anti-tumor effects. Additionally, FATP2, a long-chain fatty acid transporter, is overexpressed in PMN-MDSCs in both mice and humans, but is not expressed in M-MDSCs. FATP2 is regulated by GM-CSF and STAT5, mediating the uptake of arachidonic acid and subsequently promoting PGE2 synthesis in MDSCs. PGE2 production is inhibited when FATP2 is blocked, and the compound Lipofermata can selectively inhibit FATP2 [24]. Liver X receptors (LXRs) are a family of nuclear hormone receptor transcription factors involved in lipid homeostasis in mammals. Treatment with LXR agonists leads to MDSC apoptosis and a reduction in tumor volume, primarily due to the transcriptional activation of the target gene apolipoprotein E (ApoE). ApoE binds to its receptor, which is expressed on MDSCs, inducing MDSC depletion and ultimately inhibiting tumor growth [217]. These studies suggest that tumor-derived MDSCs are compelled to reprogram their lipid metabolism due to the substantial accumulation of lipids and the activation of associated signaling pathways. Although inhibiting lipid metabolism can effectively restrict tumor proliferation, the molecular mechanisms by which MDSCs augment their immunosuppressive function through increased FFA uptake and FAO activation necessitate further investigation.
Amino acid metabolism
Amino acid metabolism is a critical component of the altered processes observed in cancer cells and is closely linked to the development and progression of various cancers. The activation of MDSC drives the high expression of amino acid metabolic enzymes, which are key contributors to the immunosuppressive characteristics of MDSCs [68]. Among these amino acids, glutamine is the most abundant free amino acid in the body. Utilizing small molecule inhibitors of glutamine metabolism can not only inhibit tumor growth but also significantly suppress the production and recruitment of MDSCs. DRP-104, a novel prodrug of the broad-spectrum glutamine antagonist DON, has shown significant anti-tumor activity as a monotherapy, and its combination with immune checkpoint blockade therapy (ICB) can further enhance anti-tumor efficacy. DRP-104 broadly remodels the TME by inducing widespread tumor metabolic effects and enhancing the infiltration and function of various immune cells [218]. Additionally, tryptophan catabolism plays a crucial role in immune regulation. IDO catalyzes the first and rate-limiting step of tryptophan catabolism, producing N-formyl-L-kynurenine, which can be further metabolized into intermediates involved in immune responses. IDO is not only highly expressed in various human cancers and positively correlated with tumor stage and metastatic status, but it is also highly expressed in tumor-infiltrating fibroblasts, endothelial cells, and MDSCs. Recent studies have found that combining STING agonists with the IDO inhibitor 1-MT significantly inhibits tumor growth, promotes the recruitment of CD8 + T cells and DCs, and reduces MDSC infiltration [219]. It is noteworthy that IDO1 inhibitors represent promising candidates for cancer treatment, with some already entering clinical evaluation. Among these, epacadostat (INCB024360), navoximod (GDC-0919, NLG-919), PF-06840003, and BMS-986,205 have been validated to exert anti-tumor immune responses in solid malignancies [220]. However, the recent failure of the widely known ECHO-301 trial (a large phase 3 trial of the IDO inhibitor epacadostat combined with the anti-PD-1 antibody pembrolizumab in advanced melanoma) highlights the inherent challenges of targeting these pathways [221].
Adenosine metabolism
Another molecule widely recognized for its crucial role in tumorigenesis is extracellular adenosine (eADO), which accumulates at significantly high concentrations under hypoxic conditions. As a modulator, eADO undermines anti-tumor responses. More importantly, eADO stimulates the development of MDSCs and Tregs, exerting immunosuppressive effects [222]. Studies have reported that a series of extracellular enzymes, including CD38, produce immunosuppressive adenosine in the bone marrow niche, inhibiting T-cell proliferation and MDSCs. Therefore, anti-CD38 antibodies targeting myeloma cells have the potential to restore T-cell responses against myeloma cells [223]. Additionally, eADO exerts both activating and inhibitory effects on various immune cells by binding to G protein-coupled adenosine receptors, including A1R, A2AR, A2BR, and A3R, thereby activating downstream signaling pathways [224]. Particularly, A2AR and A2BR can influence a wide range of immune cells, such as NK cells, T cells, macrophages, and MDSCs [225]. Numerous studies have shown that eADO-induced A2AR inhibits the function and proliferation of T cells and NK cells, while A2AR stimulation promotes the expression of FOXP3 in Tregs, leading to cancer immune evasion and ultimately poor clinical outcomes [226]. Therefore, reducing eADO-related signaling pathways may represent a feasible therapeutic strategy.
Interactions among different pathways
-
Synergy between transcriptional regulation and epigenetic modification
The activation of the STAT3 pathway may affect the expression or activity of molecules related to epigenetic modification, thereby regulating the status of ncRNAs and histone modifications. For example, STAT3 activation may up-regulate the expression of certain miRNAs, and these miRNAs may in turn target and regulate the expression of histone-modifying enzymes, thus affecting the differentiation and function of MDSCs. Conversely, changes in epigenetic modifications may also affect the activity of transcription factors such as STAT3, forming a feedback regulatory loop. For instance, changes in the histone acetylation status may affect the DNA-binding ability of STAT3 and regulate its transcriptional activity, thereby influencing the proliferation and function of MDSCs.
-
Association between metabolic reprogramming and other pathways
Metabolic reprogramming is closely related to the transcriptional regulatory pathway. The activation of the mTOR pathway not only regulates the metabolism of MDSCs but also may affect the activity of transcription factors such as STAT3, promoting the proliferation and immunosuppressive function of MDSCs. Meanwhile, metabolites such as ATP and NADPH may also act as signaling molecules to regulate the activity of epigenetic-modifying enzymes and affect the epigenetic status of MDSCs. For example, ATP produced by high glycolysis may provide energy for HATs, promoting histone acetylation and then regulating the expression of MDSC-related genes. In addition, lipid mediators produced by fatty acid metabolism may affect intracellular signaling pathways, such as activating transcription factors like NF-κB, regulating the immunosuppressive function of MDSCs, and may also affect the expression of ncRNAs, forming a complex regulatory network.
-
Integration of pathways related to immunosuppressive function
All the above pathways ultimately converge on regulating the immunosuppressive function of MDSCs. The transcriptional regulatory pathway regulates the differentiation and expansion of MDSCs, providing a cellular basis for the immunosuppressive function; epigenetic modification affects the expression of immunosuppressive molecules in MDSCs by regulating gene expression; and metabolic reprogramming provides the energy and material basis for the immunosuppressive function. For example, the activation of the STAT3 pathway promotes the proliferation and differentiation of MDSCs and simultaneously up-regulates the expression of immunosuppressive molecules such as ARG1 and iNOS; histone modification affects the accessibility of genes encoding these immunosuppressive molecules; and metabolic reprogramming provides the energy and substrates for the synthesis and secretion of immunosuppressive molecules. These pathways cooperate with each other to jointly maintain the immunosuppressive role of MDSCs in the tumor microenvironment and promote tumor immune evasion.
Through the above systematic elaboration of the signaling pathways and molecular mechanisms related to MDSCs, it can be seen that they form a complex and orderly regulatory network, jointly regulating the function of MDSCs in the tumor microenvironment, providing an important theoretical basis for further understanding the mechanism of tumor immune evasion and developing targeted MDSC treatment strategies.
Targeting MDSCs in combination with tumor immunotherapy
In recent years, immunotherapy has revolutionized cancer treatment, with several therapies receiving FDA approval. Among them, immune checkpoint blockade (ICB) and CAR-T cell therapy have demonstrated significant advantages in tumor immunotherapy. ICBs target immune checkpoint molecules, primarily PD-1, PD-L1, and CTLA-4, to restore antitumor immune functions. Although certain checkpoint proteins, such as PD-L1, are expressed on both tumors and MDSCs, and a subset of cancer patients exhibit long-term clinical benefits following ICB treatment, most advanced solid tumors eventually develop resistance to immunotherapy. Chemokines and cytokines within the tumor microenvironment (TME) induce MDSCs, which pose a major barrier to ICB efficacy. A strong correlation has been observed between MDSC levels in patients and the effectiveness of anti-PD-1 or anti-CTLA-4 treatments [227]. Thus, targeting MDSCs represents a potential breakthrough in ICB therapy. Furthermore, numerous studies suggest that due to the complex nature of the TME, monotherapy alone is often inadequate for treating various cancers, and combination therapies may enhance the efficacy of single-agent immunotherapies by addressing their limitations.
The combination of ICB treatment with MDSC depletion has been explored in several preclinical studies. Early research demonstrated that combining epigenetic modulators, such as entinostat (ENT) and 5-azacitidine, with immune checkpoint inhibitors targeting PD-1 and CTLA-4, improved treatment outcomes in a triple-negative breast cancer (TNBC) model, curing over 80% of tumor-bearing mice [228]. In HNSCC, the efficacy of anti-CTLA-4 treatment was limited due to the accumulation and recruitment of MDSC. However, depleting G-MDSCs in combination with CTLA-4 inhibition resulted in superior antitumor effects [229]. In TNBC, aberrant SMAD3 activation promotes metastasis through MDSC recruitment. SMAD3 has been identified as a non-histone substrate of lysine acetyltransferase 6 A (KAT6A). Targeting KAT6A in conjunction with anti-PD-L1 therapy reduced MDSC recruitment, significantly decreased the metastatic potential of TNBC, and improved overall survival in xenograft models [230]. Additionally, gene modification may enable CAR-T cells to act as dual-targeting agents against both tumor cells and MDSCs. One study developed CAR-T cells containing IL-15 that specifically target the IL-15Rα receptor expressed on MDSCs in both human and glioblastoma (GBM) models. These modified CAR-T cells established a dual-targeting system that reduced the frequencies of both MDSC and tumor cells, ultimately improving survival in two GBM models [231]. Another study designed CAR-T cells to deliver RN7SL1, an endogenous RNA that activates RIG-I/MDA5 signaling. RN7SL1 promoted the expansion of CAR-T cells and facilitated effector memory differentiation while limiting MDSC development, reducing TGF-β levels in bone marrow cells, and fostering DC subsets with co-stimulatory characteristics. This enabled CAR-T cells to enhance both autologous and endogenous immune functions [232]. Therefore, combining ICB/CAR-T therapies with MDSC-targeting strategies may provide further evidence of their clinical efficacy.
Moreover, tumor vaccines utilize tumors as sources of antigen to induce sequential immune modulation, thereby generating systemic and durable antitumor immunity. A significant challenge for therapeutic vaccines in cancer treatment is maintaining cytotoxic T cell responses while overcoming inhibitory signals from MDSCs within the TME. Understanding the mechanisms of vaccine resistance and developing combination therapies that enhance antitumor immunity and promote durable responses are essential. Although demonstrating the clinical efficacy of therapeutic vaccines as monotherapies for advanced cancers is challenging, combining them with MDSC-targeting treatments to reduce intratumoral immune suppression and enhance vaccine efficacy presents a promising strategy. For example, a prophylactic vaccine (ES-exo/GM-CSF) utilizing exosomes derived from genetically engineered mouse embryonic stem cells that produce GM-CSF successfully protected mice from the growth of implantable mouse lung cancer and offered protection against metastatic lung cancer by reducing the frequency of Tregs and MDSCs [233]. Similarly, a vaccine composed of the tumor antigen survivin peptide (SVX vaccine) combined with the antiangiogenic drug sunitinib amplified the vaccine-mediated immune response in a CRC mouse model [234]. The combination of IFN-α and decitabine combined with a chemokine fusion vaccine, which effectively targets antigens to immature DCs, demonstrated increased tumor infiltration of DCs and NK cells, along with reduced MDSC levels. This combination significantly improved vaccine efficacy [235]. The emergence of various solid tumor vaccines has promoted the development of new therapeutic combinations targeting MDSCs, which play a crucial role in regulating the the tumor immune microenvironment and systemic antitumor responses. However, the underlying mechanisms by which combination therapies modify tumor immune cell infiltration and elicit protective immune responses warrant further investigation.
Conclusion and outlook
MDSCs are crucial in tumorigenesis and development. Cancer-related conditions can promote their generation. They enhance tumor progression via complex mechanisms such as immunosuppression, angiogenesis promotion, and metastasis facilitation, and are also a significant factor in resistance to immunotherapy. In cancer patients, circulating MDSCs have been regarded as negative prognostic biomarkers for predicting disease conditions, possessing value in clinical prognostic assessment. However, the research on MDSCs faces many challenges.
On the one hand, plasticity and heterogeneity are two core challenges in current research. In particular, the lack of specific markers for MDSCs in mice and humans makes the problem even more complicated. MDSCs are generally defined as immature bone marrow cells. During carcinogenesis, the combination of markers expressed by MDSCs reflects the diversity of the bone marrow lineage, and this diversity is affected by cancer type and specific TME. Therefore, the accurate definition of phenotypic markers that include functional changes are crucial for evaluating the role of MDSCs in tumor progression and immune escape. The newly developed high-throughput single-cell multi-omics technology brings hope for solving this problem. By applying this technology to analyze the phenotypic and functional composition of MDSC populations, it will help reveal their diversity and determine effective markers.
On the other hand, there is still a lack of research on specific types of MDSCs. For example, only a few studies involve T-MDSCs, mainly due to the considerable challenge of separating MDSCs that are closely attached to tumor cells. At present, most studies focus on the total MDSC population. However, in order to establish more accurate means of eliminating MDSCs, it is necessary to deeply study the specific phenotypes and characteristics of MDSCs in different types of tumors. Although the phenotypes and inhibitory mechanisms between tumor MDSC subsets seem similar on the surface, it is very necessary to identify and distinguish their differences in detail to formulate accurate individualized treatment plans targeting MDSCs. In addition, there is phenotypic similarity between MDSCs and normal bone marrow cells, which makes selective targeting of MDSCs extremely challenging. Therefore, when designing clinical trials targeting MDSCs in cancer patients, various factors such as tumor location, stage, and tumor pathology type need to be considered comprehensively.
Since the generation, amplification, recruitment, and immunosuppression of MDSCs involve extremely complex mechanisms, a single method cannot effectively regulate or eliminate MDSCs to induce a strong antitumor effect. Combining MDSC-targeted therapy with other immunotherapies is currently a more ideal strategy. This combined strategy has been proven to effectively reduce tumor burden and metastasis, thereby improving overall survival.
In conclusion, although there are still more unknowns than known answers in the field of MDSCs research at present, as our understanding of the characteristics and clinical value of MDSCs continues to deepen, more selective targeted MDSC therapies are expected to emerge in the future, opening up new approaches and strategies for antitumor treatment.
Author contributions
S.H. and C.Q. drafted the manuscript. L.Z. revised and finalized the manuscript.
Funding
This study was supported by the grants from National Natural Science Foundation of China (32270954), Key Project of Jiangsu S &T Plan (BE2023693), Changzhou Science and Technology Bureau (CZ20230017, CE20246003), Changzhou Health Commission (2022CZLJ011), Youth Project of Wuxi Municipal Health Commission (Q202357), Beijing Life Oasis Cancer Research Foundation (BH004506) and Nantong University Special Scientific Research Fund for Clinical Medicine(2023JQ015).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Law AMK, Valdes-Mora F, Gallego-Ortega D. Myeloid-derived suppressor cells as a therapeutic target for Cancer. Cells. 2020;9:561. [DOI] [PMC free article] [PubMed]
- 2.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. 2017;5:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiba Y, Mizoguchi I, Hasegawa H, Ohashi M, Orii N, Nagai T, Sugahara M, Miyamoto Y, Xu M, Owaki T, Yoshimoto T. Regulation of myelopoiesis by proinflammatory cytokines in infectious diseases. Cell Mol Life Sci. 2018;75:1363–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultze JL, Mass E, Schlitzer A. Emerging principles in Myelopoiesis at Homeostasis and during infection and inflammation. Immunity. 2019;50:288–301. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Liu M, Wu H, Tang W, Yang W, Chan TTH, Zhang L, Chen S, Xiong Z, Liang J, et al. PPP1R15A-expressing monocytic MDSCs promote immunosuppressive liver microenvironment in fibrosis-associated hepatocellular carcinoma. JHEP Rep. 2024;6:101087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan J, Feng L, Sun G, Chen P, Zhou Y, Xia M, Li H, Li Y. Interplay between mTOR and STAT5 signaling modulates the balance between regulatory and effective T cells. Immunobiology. 2015;220:510–7. [DOI] [PubMed] [Google Scholar]
- 7.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. 2018;19:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robinson WA. Granulocytosis in neoplasia. Ann N Y Acad Sci. 1974;230:212–8. [DOI] [PubMed] [Google Scholar]
- 10.Brooks-Kaiser JC, Bourque LA, Hoskin DW. Heterogeneity of splenic natural suppressor cells induced in mice by treatment with cyclophosphamide. Immunopharmacology. 1993;25:117–29. [DOI] [PubMed] [Google Scholar]
- 11.Bronte V, Chappell DB, Apolloni E, Cabrelle A, Wang M, Hwu P, Restifo NP. Unopposed production of granulocyte-macrophage colony-stimulating factor by tumors inhibits CD8 + T cell responses by dysregulating antigen-presenting cell maturation. J Immunol. 1999;162:5728–37. [PMC free article] [PubMed] [Google Scholar]
- 12.Oseroff A, Okada S, Strober S. Natural suppressor (NS) cells found in the spleen of neonatal mice and adult mice given total lymphoid irradiation (TLI) express the null surface phenotype. J Immunol. 1984;132:101–10. [PubMed] [Google Scholar]
- 13.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma J, Xu H, Wang S. Immunosuppressive Role of Myeloid-Derived Suppressor Cells and Therapeutic Targeting in Lung Cancer. J Immunol Res. 2018;2018:6319649. [DOI] [PMC free article] [PubMed]
- 15.Blazar BR, MacDonald KPA, Hill GR. Immune regulatory cell infusion for graft-versus-host disease prevention and therapy. Blood. 2018;131:2651–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20:676–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu SY, Chiang CS. Distinct role of CD11b(+)Ly6G(-)Ly6C(-) myeloid-derived cells on the progression of the primary Tumor and Therapy-Associated Recurrent Brain Tumor. Cells. 2019;9:51. [DOI] [PMC free article] [PubMed]
- 18.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol. 2017;14:399–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A, Ostrand-Rosenberg S, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. [DOI] [PubMed] [Google Scholar]
- 21.Damuzzo V, Pinton L, Desantis G, Solito S, Marigo I, Bronte V, Mandruzzato S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytometry B Clin Cytom. 2015;88:77–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18:1254–61. [DOI] [PubMed] [Google Scholar]
- 23.Kattoor AJ, Kanuri SH, Mehta JL. Role of Ox-LDL and LOX-1 in Atherogenesis. Curr Med Chem. 2019;26:1693–700. [DOI] [PubMed] [Google Scholar]
- 24.Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, To TKJ, Schug Z, Basu S, Wang F, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alshetaiwi H, Pervolarakis N, McIntyre LL, Ma D, Nguyen Q, Rath JA, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Sci Immunol. 2020;5:eaay6017. [DOI] [PMC free article] [PubMed]
- 26.Wu Y, Yi M, Niu M, Mei Q, Wu K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022;21:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hegde S, Leader AM, Merad M. MDSC: markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldmann O, Nwofor OV, Chen Q, Medina E: Mechanisms underlying immunosuppression by regulatory cells. Front Immunol. 2024;15:1328193. [DOI] [PMC free article] [PubMed]
- 29.Groth C, Hu X, Weber R, Fleming V, Altevogt P, Utikal J, Umansky V. Immunosuppression mediated by myeloid-derived suppressor cells (MDSCs) during tumour progression. Br J Cancer. 2019;120:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber R, Riester Z, Hüser L, Sticht C, Siebenmorgen A, Groth C, et al. IL-6 regulates CCR5 expression and immunosuppressive capacity of MDSC in murine melanoma. J Immunother Cancer. 2020;8:e000949. [DOI] [PMC free article] [PubMed]
- 31.Calcinotto A, Spataro C, Zagato E, Di Mitri D, Gil V, Crespo M, De Bernardis G, Losa M, Mirenda M, Pasquini E, et al. IL-23 secreted by myeloid cells drives castration-resistant prostate cancer. Nature. 2018;559:363–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Porta C, Consonni FM, Morlacchi S, Sangaletti S, Bleve A, Totaro MG, Larghi P, Rimoldi M, Tripodo C, Strauss L, et al. Tumor-derived prostaglandin E2 promotes p50 NF-κB-Dependent differentiation of monocytic MDSCs. Cancer Res. 2020;80:2874–88. [DOI] [PubMed] [Google Scholar]
- 33.Heuvers ME, Muskens F, Bezemer K, Lambers M, Dingemans AC, Groen HJM, Smit EF, Hoogsteden HC, Hegmans J, Aerts J. Arginase-1 mRNA expression correlates with myeloid-derived suppressor cell levels in peripheral blood of NSCLC patients. Lung Cancer. 2013;81:468–74. [DOI] [PubMed] [Google Scholar]
- 34.Ryzhov S, Novitskiy SV, Goldstein AE, Biktasova A, Blackburn MR, Biaggioni I, Dikov MM, Feoktistov I. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b + Gr1 + cells. J Immunol. 2011;187:6120–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Y, Shen XF, Cao K, Ding J, Kang X, Guan WX, Ding YT, Liu BR, Du JF. Dexamethasone-Induced myeloid-derived suppressor cells prolong Allo Cardiac Graft Survival through iNOS- and glucocorticoid receptor-dependent mechanism. Front Immunol. 2018;9:282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian L, Liu Y, Wang S, Gong W, Jia X, Liu L, Ye F, Ding J, Xu Y, Fu Y, Tian F. NKG2D ligand RAE1ε induces generation and enhances the inhibitor function of myeloid-derived suppressor cells in mice. J Cell Mol Med. 2017;21:2046–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–97. [DOI] [PubMed] [Google Scholar]
- 38.Mehmeti-Ajradini M, Bergenfelz C, Larsson AM, Carlsson R, Riesbeck K, Ahl J, et al. Human G-MDSCs are neutrophils at distinct maturation stages promoting tumor growth in breast cancer. Life Sci Alliance. 2020;3:e202000893. [DOI] [PMC free article] [PubMed]
- 39.Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, Maurer D, Ottensmeier C, van der Burg SH, Welters MJ, Walter S. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. 2016;65:161–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pergamo M, Miller G. Myeloid-derived suppressor cells and their role in pancreatic cancer. Cancer Gene Ther. 2017;24:100–5. [DOI] [PubMed] [Google Scholar]
- 41.Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, Horn LA, Palena C, Schlom J, Maeda DY, et al. Inhibition of MDSC trafficking with SX-682, a CXCR1/2 inhibitor, enhances NK-Cell Immunotherapy in Head and Neck Cancer models. Clin Cancer Res. 2020;26:1420–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lazarova M, Steinle A. Impairment of NKG2D-Mediated tumor immunity by TGF-β. Front Immunol. 2019;10:2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H, Lehner F, Manns MP, Greten TF, Korangy F. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology. 2009;50:799–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ugolini A, Tyurin VA, Tyurina YY, Tcyganov EN, Donthireddy L, Kagan VE, et al. Polymorphonuclear myeloid-derived suppressor cells limit antigen cross-presentation by dendritic cells in cancer. JCI Insight. 2020;5:e138581. [DOI] [PMC free article] [PubMed]
- 45.Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, Ponnazhagan S, Hsu HC, Deshane JS. Myeloid-derived suppressor cells impair B cell responses in Lung Cancer through IL-7 and STAT5. J Immunol. 2018;201:278–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shen M, Wang J, Yu W, Zhang C, Liu M, Wang K, Yang L, Wei F, Wang SE, Sun Q, Ren X. A novel MDSC-induced PD-1(-)PD-L1(+) B-cell subset in breast tumor microenvironment possesses immuno-suppressive properties. Oncoimmunology. 2018;7:e1413520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu W, Li B, Liu D, Zhao B, Sun G, Ding J. Obesity correlates with the immunosuppressive ILC2s-MDSCs axis in advanced breast cancer. Immun Inflamm Dis. 2024;12:e1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.He Q, Liu M, Huang W, Chen X, Zhang B, Zhang T, Wang Y, Liu D, Xie M, Ji X, et al. IL-1β-Induced Elevation of Solute Carrier Family 7 Member 11 promotes Hepatocellular Carcinoma Metastasis through Up-regulating programmed death Ligand 1 and colony-stimulating factor 1. Hepatology. 2021;74:3174–93. [DOI] [PubMed] [Google Scholar]
- 49.Jianyi D, Haili G, Bo Y, Meiqin Y, Baoyou H, Haoran H, Fang L, Qingliang Z, Lingfei H. Myeloid-derived suppressor cells cross-talk with B10 cells by BAFF/BAFF-R pathway to promote immunosuppression in cervical cancer. Cancer Immunol Immunother. 2023;72:73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, Cerwenka A. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–11. [DOI] [PubMed] [Google Scholar]
- 51.Siret C, Collignon A, Silvy F, Robert S, Cheyrol T, André P, Rigot V, Iovanna J, van de Pavert S, Lombardo D, et al. Deciphering the Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells in pancreatic ductal adenocarcinoma. Front Immunol. 2019;10:3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Haist M, Stege H, Grabbe S, Bros M. The functional crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the immunosuppressive Tumor Microenvironment. Cancers (Basel). 2021;13:210. [DOI] [PMC free article] [PubMed]
- 53.Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, Khezri Z, Majidpoor J, Abouzaripour M, Habibi M, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120:2756–65. [DOI] [PubMed] [Google Scholar]
- 54.Zhang X, Meng T, Cui S, Liu D, Pang Q, Wang P. Roles of ubiquitination in the crosstalk between tumors and the tumor microenvironment (review). Int J Oncol. 2022;61:84. [DOI] [PMC free article] [PubMed]
- 55.Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233:3024–36. [DOI] [PubMed] [Google Scholar]
- 56.Younos IH, Dafferner AJ, Gulen D, Britton HC, Talmadge JE. Tumor regulation of myeloid-derived suppressor cell proliferation and trafficking. Int Immunopharmacol. 2012;13:245–56. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y, Ding Y, Guo N, Wang S. MDSCs: key criminals of Tumor pre-metastatic niche formation. Front Immunol. 2019;10:172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kujawski M, Kortylewski M, Lee H, Herrmann A, Kay H, Yu H. Stat3 mediates myeloid cell-dependent tumor angiogenesis in mice. J Clin Invest. 2008;118:3367–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. [DOI] [PubMed] [Google Scholar]
- 60.Deng Z, Rong Y, Teng Y, Zhuang X, Samykutty A, Mu J, Zhang L, Cao P, Yan J, Miller D, Zhang HG. Exosomes miR-126a released from MDSC induced by DOX treatment promotes lung metastasis. Oncogene. 2017;36:639–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, Xu H, Wang S. Granulocytic myeloid-derived suppressor cells promote the stemness of Colorectal Cancer cells through Exosomal S100A9. Adv Sci (Weinh). 2019;6:1901278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao F, Xu Q, Tang Z, Zhang N, Huang Y, Li Z, Dai Y, Yu Q, Zhu J. Exosomes derived from myeloid-derived suppressor cells facilitate castration-resistant prostate cancer progression via S100A9/circMID1/miR-506-3p/MID1. J Transl Med. 2022;20:346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, Vatan L, Szeliga W, Mao Y, Thomas DG, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, et al. Myeloid-derived suppressor cells endow stem-like qualities to breast Cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling. Cancer Res. 2016;76:3156–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 is critical for Premetastatic Niche Formation and metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sharma V, Sachdeva N, Gupta V, Nada R, Jacob J, Sahni D, Aggarwal A. CCR4(+) monocytic myeloid-derived suppressor cells are associated with the increased epithelial-mesenchymal transition in pancreatic adenocarcinoma patients. Immunobiology. 2022;227:152210. [DOI] [PubMed] [Google Scholar]
- 67.Song JH, Eum DY, Park SY, Jin YH, Shim JW, Park SJ, Kim MY, Park SJ, Heo K, Choi YJ. Inhibitory effect of ginsenoside Rg3 on cancer stemness and mesenchymal transition in breast cancer via regulation of myeloid-derived suppressor cells. PLoS ONE. 2020;15:e0240533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmann SHL, Reck DI, Maurer A, Fehrenbacher B, Sceneay JE, Poxleitner M, Öz HH, Ehrlichmann W, Reischl G, Fuchs K, et al. Visualization and quantification of in vivo homing kinetics of myeloid-derived suppressor cells in primary and metastatic cancer. Theranostics. 2019;9:5869–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, et al. Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade. Nat Commun. 2017;8:14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang Z, Yao Z, Zhang Z, Cui L, Zhang L, Qiu G, Song X, Song S. Local radiotherapy for murine breast cancer increases risk of metastasis by promoting the recruitment of M-MDSCs in lung. Cancer Cell Int. 2023;23:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Youn JI, Kumar V, Collazo M, Nefedova Y, Condamine T, Cheng P, Villagra A, Antonia S, McCaffrey JC, Fishman M, et al. Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer. Nat Immunol. 2013;14:211–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, Umansky V. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci U S A. 2011;108:17111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao N, Zhu W, Wang J, Liu W, Kang L, Yu R, Liu B. Group 2 innate lymphoid cells promote TNBC lung metastasis via the IL-13-MDSC axis in a murine tumor model. Int Immunopharmacol. 2021;99:107924. [DOI] [PubMed] [Google Scholar]
- 76.Moon TJ, Ta HM, Bhalotia A, Paulsen KE, Hutchinson DW, Arkema GM, et al. Nanoparticles targeting immune checkpoint protein VISTA induce potent antitumor immunity. J Immunother Cancer. 2024;12:e008977. [DOI] [PMC free article] [PubMed]
- 77.Xu W, Li S, Li M, Yang X, Xie S, Lin L, Li G, Zhou H. Targeted elimination of myeloid-derived suppressor cells via regulation of the STAT pathway alleviates tumor immunosuppression in neuroblastoma. Immunol Lett. 2021;240:31–40. [DOI] [PubMed] [Google Scholar]
- 78.Lin JP, Huang MH, Sun ZT, Chen L, Lei YH, Huang YQ, Qi M, Fan SR, Chen SG, Chung CW, et al. Periplocin inhibits hepatocellular carcinoma progression and reduces the recruitment of MDSCs through AKT/NF-κB pathway. Life Sci. 2023;324:121715. [DOI] [PubMed] [Google Scholar]
- 79.Liu G, Bi Y, Shen B, Yang H, Zhang Y, Wang X, Liu H, Lu Y, Liao J, Chen X, Chu Y. SIRT1 limits the function and fate of myeloid-derived suppressor cells in tumors by orchestrating HIF-1α-dependent glycolysis. Cancer Res. 2014;74:727–37. [DOI] [PubMed] [Google Scholar]
- 80.Dai J, Kumbhare A, Youssef D, McCall CE, El Gazzar M. Intracellular S100A9 promotes myeloid-derived suppressor cells during late Sepsis. Front Immunol. 2017;8:1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sido JM, Yang X, Nagarkatti PS, Nagarkatti M. ∆9-Tetrahydrocannabinol-mediated epigenetic modifications elicit myeloid-derived suppressor cell activation via STAT3/S100A8. J Leukoc Biol. 2015;97:677–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ahn R, Sabourin V, Bolt AM, Hébert S, Totten S, De Jay N, Festa MC, Young YK, Im YK, Pawson T, et al. The Shc1 adaptor simultaneously balances Stat1 and Stat3 activity to promote breast cancer immune suppression. Nat Commun. 2017;8:14638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jayakumar A, Bothwell ALM. Stat6 promotes intestinal tumorigenesis in a mouse model of adenomatous polyposis by expansion of MDSCs and inhibition of cytotoxic CD8 response. Neoplasia. 2017;19:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryan N, Anderson K, Volpedo G, Hamza O, Varikuti S, Satoskar AR, Oghumu S. STAT1 inhibits T-cell exhaustion and myeloid derived suppressor cell accumulation to promote antitumor immune responses in head and neck squamous cell carcinoma. Int J Cancer. 2020;146:1717–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang M. Novel function of STAT1 in breast cancer. Oncoimmunology. 2013;2:e25125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang H, Nguyen-Jackson H, Panopoulos AD, Li HS, Murray PJ, Watowich SS. STAT3 controls myeloid progenitor growth during emergency granulopoiesis. Blood. 2010;116:2462–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kim S, Song JH, Kim S, Qu P, Martin BK, Sehareen WS, Haines DC, Lin PC, Sharan SK, Chang S. Loss of oncogenic miR-155 in tumor cells promotes tumor growth by enhancing C/EBP-β-mediated MDSC infiltration. Oncotarget. 2016;7:11094–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Min Y, Li J, Qu P, Lin PC. C/EBP-δ positively regulates MDSC expansion and endothelial VEGFR2 expression in tumor development. Oncotarget. 2017;8:50582–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu Y, Wong CC, Ding Y, Gao M, Wen J, Lau HC, Cheung AH, Huang D, Huang H, Yu J. Peptostreptococcus anaerobius mediates anti-PD1 therapy resistance and exacerbates colorectal cancer via myeloid-derived suppressor cells in mice. Nat Microbiol. 2024;9:1467–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ramzan F, Vickers MH, Mithen RF. Epigenetics, microRNA and metabolic syndrome: a Comprehensive Review. Int J Mol Sci. 2021;22:5047. [DOI] [PMC free article] [PubMed]
- 92.Sasidharan Nair V, Saleh R, Toor SM, Taha RZ, Ahmed AA, Kurer MA, Murshed K, Alajez NM, Abu Nada M, Elkord E. Transcriptomic profiling disclosed the role of DNA methylation and histone modifications in tumor-infiltrating myeloid-derived suppressor cell subsets in colorectal cancer. Clin Epigenetics. 2020;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Huo S, Liu L, Li Q, Wang J. Modulation of myeloid-derived suppressor cells by long non-coding RNAs in the Tumor Microenvironment and Tumorigenesis. Discov Med. 2022;33:143–51. [PubMed] [Google Scholar]
- 94.Liang L, Xu X, Li J, Yang C. Interaction between microRNAs and myeloid-derived suppressor cells in Tumor Microenvironment. Front Immunol. 2022;13:883683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huber V, Vallacchi V, Fleming V, Hu X, Cova A, Dugo M, Shahaj E, Sulsenti R, Vergani E, Filipazzi P, et al. Tumor-derived microRNAs induce myeloid suppressor cells and predict immunotherapy resistance in melanoma. J Clin Invest. 2018;128:5505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lu W, Cao F, Feng L, Song G, Chang Y, Chu Y, Chen Z, Shen B, Xu H, Wang S, Ma J. LncRNA Snhg6 regulates the differentiation of MDSCs by regulating the ubiquitination of EZH2. J Hematol Oncol. 2021;14:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liu Y, Han Y, Zhang Y, Lv T, Peng X, Huang J. LncRNAs has been identified as regulators of myeloid-derived suppressor cells in lung cancer. Front Immunol. 2023;14:1067520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng Y, Tian X, Wang T, Xia X, Cao F, Tian J, Xu P, Ma J, Xu H, Wang S. Long noncoding RNA Pvt1 regulates the immunosuppression activity of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Mol Cancer. 2019;18:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tian X, Wang T, Shen H, Wang S. Tumor microenvironment, histone modifications, and myeloid-derived suppressor cells. Cytokine Growth Factor Rev. 2023;74:108–21. [DOI] [PubMed] [Google Scholar]
- 100.Zhang C, Li HX, Man Y, Jiang ZH, Yin P, Yu K. Exploring the role of histone deacetylase inhibitors in cancer development and therapeutic potential. J Physiol Pharmacol. 2024;75:117–22. [DOI] [PubMed] [Google Scholar]
- 101.Chen J, Cheng F, Sahakian E, Powers J, Wang Z, Tao J, Seto E, Pinilla-Ibarz J, Sotomayor EM. HDAC11 regulates expression of C/EBPβ and immunosuppressive molecules in myeloid-derived suppressor cells. J Leukoc Biol. 2021;109:891–900. [DOI] [PubMed] [Google Scholar]
- 102.Bi L, Ren Y, Feng M, Meng P, Wang Q, Chen W, Jiao Q, Wang Y, Du L, Zhou F, et al. HDAC11 regulates Glycolysis through the LKB1/AMPK Signaling Pathway to maintain Hepatocellular Carcinoma Stemness. Cancer Res. 2021;81:2015–28. [DOI] [PubMed] [Google Scholar]
- 103.Liu J, Shen H, Gu W, Zheng H, Wang Y, Ma G, Du J. Prediction of prognosis, immunogenicity and efficacy of immunotherapy based on glutamine metabolism in lung adenocarcinoma. Front Immunol. 2022;13:960738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang R, Li B, Huang B, Li Y, Liu Q, Lyu Z, Chen R, Qian Q, Liang X, Pu X, et al. Gut microbiota-derived butyrate induces epigenetic and metabolic reprogramming in myeloid-derived suppressor cells to alleviate primary biliary cholangitis. Gastroenterology. 2024;167:733–e749733. [DOI] [PubMed] [Google Scholar]
- 105.Porta C, Sica A, Riboldi E. Tumor-associated myeloid cells: new understandings on their metabolic regulation and their influence in cancer immunotherapy. Febs j. 2018;285:717–33. [DOI] [PubMed] [Google Scholar]
- 106.den Brok MH, Raaijmakers TK, Collado-Camps E, Adema GJ. Lipid droplets as Immune modulators in myeloid cells. Trends Immunol. 2018;39:380–92. [DOI] [PubMed] [Google Scholar]
- 107.Adeshakin AO, Liu W, Adeshakin FO, Afolabi LO, Zhang M, Zhang G, Wang L, Li Z, Lin L, Cao Q, et al. Regulation of ROS in myeloid-derived suppressor cells through targeting fatty acid transport protein 2 enhanced anti-PD-L1 tumor immunotherapy. Cell Immunol. 2021;362:104286. [DOI] [PubMed] [Google Scholar]
- 108.Yan D, Yang Q, Shi M, Zhong L, Wu C, Meng T, Yin H, Zhou J. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur J Immunol. 2013;43:2943–55. [DOI] [PubMed] [Google Scholar]
- 109.Navarro C, Ortega Á, Santeliz R, Garrido B, Chacín M, Galban N, Vera I, De Sanctis JB. Bermúdez V: Metabolic Reprogramming in Cancer Cells: Emerging Molecular Mechanisms and Novel Therapeutic Approaches. Pharmaceutics 2022, 14. [DOI] [PMC free article] [PubMed]
- 110.Deng Y, Yang J, Luo F, Qian J, Liu R, Zhang D, Yu H, Chu Y. mTOR-mediated glycolysis contributes to the enhanced suppressive function of murine tumor-infiltrating monocytic myeloid-derived suppressor cells. Cancer Immunol Immunother. 2018;67:1355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wu T, Zhao Y, Wang H, Li Y, Shao L, Wang R, Lu J, Yang Z, Wang J, Zhao Y. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci Rep. 2016;6:20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Scheurer J, Reisser T, Leithäuser F, Messmann JJ, Holzmann K, Debatin KM, Strauss G. Rapamycin-based graft-versus-host disease prophylaxis increases the immunosuppressivity of myeloid-derived suppressor cells without affecting T cells and anti-tumor cytotoxicity. Clin Exp Immunol. 2020;202:407–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pi R, Yang Y, Hu X, Li H, Shi H, Liu Y, Wang X, Tong A, Lu T, Wei Y, et al. Dual mTORC1/2 inhibitor AZD2014 diminishes myeloid-derived suppressor cells accumulation in ovarian cancer and delays tumor growth. Cancer Lett. 2021;523:72–81. [DOI] [PubMed] [Google Scholar]
- 114.Xu P, Yin K, Tang X, Tian J, Zhang Y, Ma J, Xu H, Xu Q, Wang S. Metformin inhibits the function of granulocytic myeloid-derived suppressor cells in tumor-bearing mice. Biomed Pharmacother. 2019;120:109458. [DOI] [PubMed] [Google Scholar]
- 115.Kang J, Lee D, Lee KJ, Yoon JE, Kwon JH, Seo Y, et al. Tumor-suppressive effect of Metformin via the regulation of M2 macrophages and myeloid-derived suppressor cells in the Tumor Microenvironment of Colorectal Cancer. Cancers (Basel). 2022;14:2881. [DOI] [PMC free article] [PubMed]
- 116.Hack SP, Zhu AX, Wang Y. Augmenting anticancer immunity through combined targeting of angiogenic and PD-1/PD-L1 pathways: challenges and opportunities. Front Immunol. 2020;11:598877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Horikawa N, Abiko K, Matsumura N, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Taki M, Ukita M, Hosoe Y, et al. Anti-VEGF therapy resistance in ovarian cancer is caused by GM-CSF-induced myeloid-derived suppressor cell recruitment. Br J Cancer. 2020;122:778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Koinis F, Vetsika EK, Aggouraki D, Skalidaki E, Koutoulaki A, Gkioulmpasani M, Georgoulias V, Kotsakis A. Effect of First-Line treatment on myeloid-derived suppressor cells’ subpopulations in the Peripheral blood of patients with Non-small Cell Lung Cancer. J Thorac Oncol. 2016;11:1263–72. [DOI] [PubMed] [Google Scholar]
- 119.Wang L, Hu D, Xie B, Xie L. Blockade of Myd88 signaling by a novel MyD88 inhibitor prevents colitis-associated colorectal cancer development by impairing myeloid-derived suppressor cells. Invest New Drugs. 2022;40:506–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sun HW, Wu WC, Chen HT, Xu YT, Yang YY, Chen J, Yu XJ, Wang Z, Shuang ZY, Zheng L. Glutamine deprivation promotes the generation and mobilization of MDSCs by enhancing expression of G-CSF and GM-CSF. Front Immunol. 2020;11:616367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Triozzi PL, Achberger S, Aldrich W, Elson P, Garcia J, Dreicer R. Differential immunologic and microRNA effects of 2 dosing regimens of recombinant human granulocyte/macrophage colony stimulating factor. J Immunother. 2012;35:587–94. [DOI] [PubMed] [Google Scholar]
- 122.Negri L, Ferrara N. The prokineticins: neuromodulators and mediators of inflammation and myeloid cell-dependent angiogenesis. Physiol Rev. 2018;98:1055–82. [DOI] [PubMed] [Google Scholar]
- 123.Wang H, Ji J, Zhuang Y, Zhou X, Zhao Y, Zhang X. PMA induces the differentiation of monocytes into immunosuppressive MDSCs. Clin Exp Immunol. 2021;206:216–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hong EH, Chang SY, Lee BR, Kim YS, Lee JM, Kang CY, Kweon MN, Ko HJ. Blockade of Myd88 signaling induces antitumor effects by skewing the immunosuppressive function of myeloid-derived suppressor cells. Int J Cancer. 2013;132:2839–48. [DOI] [PubMed] [Google Scholar]
- 125.Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang J, Shirota Y, Bayik D, Shirota H, Tross D, Gulley JL, Wood LV, Berzofsky JA, Klinman DM. Effect of TLR agonists on the differentiation and function of human monocytic myeloid-derived suppressor cells. J Immunol. 2015;194:4215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Le H, Wang Y, Zhou J, Li D, Gong Z, Zhu F, Wang J, Tian C, Cai W, Wu J. Git2 deficiency promotes MDSCs recruitment in intestine via NF-κB-CXCL1/CXCL12 pathway and ameliorates necrotizing enterocolitis. Mucosal Immunol. 2024;17:1060–71. [DOI] [PubMed] [Google Scholar]
- 128.Jiménez-Cortegana C, Gutiérrez-García C, Sánchez-Jiménez F, Vilariño-García T, Flores-Campos R, Pérez-Pérez A, et al. Impact of obesity–associated myeloid–derived suppressor cells on cancer risk and progression (review). Int J Oncol. 2024;65:79. [DOI] [PMC free article] [PubMed]
- 129.Qian Y, Dong J, Zhang W, Xue X, Xiong Z, Zeng W, Wang Q, Fan Z, Zuo Z, Huang Z, Jiang Y. Deguelin inhibits the glioblastoma progression through suppressing CCL2/NFκB signaling pathway. Neuropharmacology. 2024;259:110109. [DOI] [PubMed] [Google Scholar]
- 130.Yoshimura T, Li C, Wang Y, Matsukawa A. The chemokine monocyte chemoattractant protein-1/CCL2 is a promoter of breast cancer metastasis. Cell Mol Immunol. 2023;20:714–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Steinberg SM, Shabaneh TB, Zhang P, Martyanov V, Li Z, Malik BT, Wood TA, Boni A, Molodtsov A, Angeles CV, et al. Myeloid cells that impair immunotherapy are restored in Melanomas with Acquired Resistance to BRAF inhibitors. Cancer Res. 2017;77:1599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie M, Lin Z, Ji X, Luo X, Zhang Z, Sun M, Chen X, Zhang B, Liang H, Liu D, et al. FGF19/FGFR4-mediated elevation of ETV4 facilitates hepatocellular carcinoma metastasis by upregulating PD-L1 and CCL2. J Hepatol. 2023;79:109–25. [DOI] [PubMed] [Google Scholar]
- 133.Liu X, Tang R, Xu J, Tan Z, Liang C, Meng Q, Lei Y, Hua J, Zhang Y, Liu J, et al. CRIP1 fosters MDSC trafficking and resets tumour microenvironment via facilitating NF-κB/p65 nuclear translocation in pancreatic ductal adenocarcinoma. Gut. 2023;72:2329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mukherjee S, Ghosh S, Sengupta A, Sarkar S, Keswani T, Chatterjee R, Bhattacharyya A. IL-6 dependent expansion of inflammatory MDSCs (CD11b + Gr-1+) promote Th-17 mediated immune response during experimental cerebral malaria. Cytokine. 2022;155:155910. [DOI] [PubMed] [Google Scholar]
- 135.Zhao Q, Huang L, Qin G, Qiao Y, Ren F, Shen C, Wang S, Liu S, Lian J, Wang D, et al. Cancer-associated fibroblasts induce monocytic myeloid-derived suppressor cell generation via IL-6/exosomal miR-21-activated STAT3 signaling to promote cisplatin resistance in esophageal squamous cell carcinoma. Cancer Lett. 2021;518:35–48. [DOI] [PubMed] [Google Scholar]
- 136.Shi H, Qin Y, Tian Y, Wang J, Wang Y, Wang Z, Lv J. Interleukin-1beta triggers the expansion of circulating granulocytic myeloid-derived suppressor cell subset dependent on Erk1/2 activation. Immunobiology. 2022;227:152165. [DOI] [PubMed] [Google Scholar]
- 137.Tengesdal IW, Menon DR, Osborne DG, Neff CP, Powers NE, Gamboni F, et al. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118:e2000915118. [DOI] [PMC free article] [PubMed]
- 138.Nguyen NT, Mitsuhashi A, Ogino H, Kozai H, Yoneda H, Afroj T, Sato S, Nokihara H, Shinohara T, Nishioka Y. S-1 eliminates MDSCs and enhances the efficacy of PD-1 blockade via regulation of tumor-derived Bv8 and S100A8 in thoracic tumor. Cancer Sci. 2023;114:384–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fan R, Satilmis H, Vandewalle N, Verheye E, Vlummens P, Maes A, et al. Tasquinimod suppresses tumor cell growth and bone resorption by targeting immunosuppressive myeloid cells and inhibiting c-MYC expression in multiple myeloma. J Immunother Cancer. 2023;11:e005319. [DOI] [PMC free article] [PubMed]
- 140.Li X, Luo X, Chen S, Chen J, Deng X, Zhong J, Wu H, Huang X, Wang C. All-trans-retinoic acid inhibits hepatocellular carcinoma progression by targeting myeloid-derived suppressor cells and inhibiting angiogenesis. Int Immunopharmacol. 2023;121:110413. [DOI] [PubMed] [Google Scholar]
- 141.Hamilton MJ, Bosiljcic M, Lepard NE, Halvorsen EC, Ho VW, Banáth JP, Krystal G, Bennewith KL. Macrophages are more potent immune suppressors ex vivo than immature myeloid-derived suppressor cells induced by metastatic murine mammary carcinomas. J Immunol. 2014;192:512–22. [DOI] [PubMed] [Google Scholar]
- 142.Bi G, Liang J, Bian Y, Shan G, Besskaya V, Wang Q, Zhan C. The immunomodulatory role of all-trans retinoic acid in tumor microenvironment. Clin Exp Med. 2023;23:591–606. [DOI] [PubMed] [Google Scholar]
- 143.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Tobin RP, Jordan KR, Robinson WA, Davis D, Borges VF, Gonzalez R, Lewis KD, McCarter MD. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int Immunopharmacol. 2018;63:282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tobin RP, Cogswell DT, Cates VM, Davis DM, Borgers JSW, Van Gulick RJ, Katsnelson E, Couts KL, Jordan KR, Gao D, et al. Targeting MDSC differentiation using ATRA: a phase I/II clinical trial combining Pembrolizumab and All-Trans Retinoic Acid for metastatic melanoma. Clin Cancer Res. 2023;29:1209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.He XY, Gong FY, Chen Y, Zhou Z, Gong Z, Gao XM. Calreticulin fragment 39–272 promotes B16 melanoma malignancy through myeloid-derived suppressor cells in vivo. Front Immunol. 2017;8:1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fleming V, Hu X, Weller C, Weber R, Groth C, Riester Z, Hüser L, Sun Q, Nagibin V, Kirschning C, et al. Melanoma Extracellular vesicles generate immunosuppressive myeloid cells by upregulating PD-L1 via TLR4 signaling. Cancer Res. 2019;79:4715–28. [DOI] [PubMed] [Google Scholar]
- 149.Arkhypov I, Özbay Kurt FG, Bitsch R, Novak D, Petrova V, Lasser S, et al. HSP90α induces immunosuppressive myeloid cells in melanoma via TLR4 signaling. J Immunother Cancer. 2022;10:e005551. [DOI] [PMC free article] [PubMed]
- 150.Di S, Zhou M, Pan Z, Sun R, Chen M, Jiang H, Shi B, Luo H, Li Z. Combined adjuvant of poly I:C improves Antitumor effects of CAR-T cells. Front Oncol. 2019;9:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Deng Y, Yang J, Qian J, Liu R, Huang E, Wang Y, Luo F, Chu Y. TLR1/TLR2 signaling blocks the suppression of monocytic myeloid-derived suppressor cell by promoting its differentiation into M1-type macrophage. Mol Immunol. 2019;112:266–73. [DOI] [PubMed] [Google Scholar]
- 152.Shayan G, Kansy BA, Gibson SP, Srivastava RM, Bryan JK, Bauman JE, Ohr J, Kim S, Duvvuri U, Clump DA, et al. Phase ib study of Immune Biomarker Modulation with Neoadjuvant Cetuximab and TLR8 stimulation in Head and Neck Cancer to overcome suppressive myeloid signals. Clin Cancer Res. 2018;24:62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Z, Xie Y, Xiong Y, Liu S, Qiu C, Zhu Z, Mao H, Yu M, Wang X. TLR 7/8 agonist reverses oxaliplatin resistance in colorectal cancer via directing the myeloid-derived suppressor cells to tumoricidal M1-macrophages. Cancer Lett. 2020;469:173–85. [DOI] [PubMed] [Google Scholar]
- 154.Urban-Wojciuk Z, Khan MM, Oyler BL, Fåhraeus R, Marek-Trzonkowska N, Nita-Lazar A, Hupp TR, Goodlett DR. The role of TLRs in anti-cancer immunity and tumor rejection. Front Immunol. 2019;10:2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Trovato R, Fiore A, Sartori S, Canè S, Giugno R, Cascione L, Paiella S, Salvia R, De Sanctis F, Poffe O, et al. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer. 2019;7:255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012;27:495–503. [DOI] [PubMed] [Google Scholar]
- 158.Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, Fowler N, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. 2018;6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Guha P, Gardell J, Darpolor J, Cunetta M, Lima M, Miller G, Espat NJ, Junghans RP, Katz SC. STAT3 inhibition induces bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene. 2019;38:533–48. [DOI] [PubMed] [Google Scholar]
- 160.Kulbersh JS, Day TA, Gillespie MB, Young MR. 1alpha,25-Dihydroxyvitamin D(3) to skew intratumoral levels of immune inhibitory CD34(+) progenitor cells into dendritic cells. Otolaryngol Head Neck Surg. 2009;140:235–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Walsh JE, Clark AM, Day TA, Gillespie MB, Young MR. Use of alpha,25-dihydroxyvitamin D3 treatment to stimulate immune infiltration into head and neck squamous cell carcinoma. Hum Immunol. 2010;71:659–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Xun J, Zhou S, Lv Z, Wang B, Luo H, Zhang L, Yang L, Zhang A, Wu X, Wang Z, et al. Dioscin modulates macrophages polarization and MDSCs differentiation to inhibit tumorigenesis of colitis-associated colorectal cancer. Int Immunopharmacol. 2023;117:109839. [DOI] [PubMed] [Google Scholar]
- 163.Wang Y, Fan X, Wu X. Ganoderma Lucidum polysaccharide (GLP) enhances antitumor immune response by regulating differentiation and inhibition of MDSCs via a CARD9-NF-κB-IDO pathway. Biosci Rep. 2020;40:BSR20201170. [DOI] [PMC free article] [PubMed]
- 164.Li B, Luo Y, Zhou Y, Wu J, Fang Z, Li Y. Role of sanguinarine in regulating immunosuppression in a Lewis lung cancer mouse model. Int Immunopharmacol. 2022;110:108964. [DOI] [PubMed] [Google Scholar]
- 165.Albeituni SH, Ding C, Liu M, Hu X, Luo F, Kloecker G, Bousamra M 2nd, Zhang HG, Yan J. Yeast-derived particulate β-Glucan treatment subverts the suppression of myeloid-derived suppressor cells (MDSC) by inducing Polymorphonuclear MDSC apoptosis and monocytic MDSC differentiation to APC in Cancer. J Immunol. 2016;196:2167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019;4:e130748. [DOI] [PMC free article] [PubMed]
- 167.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ma WJ, Li ZH, Wu ZR, Liu F, Wang JK, Shi YJ, Jin YW, Li FY. PI3K-CCL2-CCR2-MDSCs axis: a potential pathway for tumor Clostridia-promoted CD 8(+) T lymphocyte infiltration in bile tract cancers. Neoplasia. 2023;43:100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Flores-Toro JA, Luo D, Gopinath A, Sarkisian MR, Campbell JJ, Charo IF, Singh R, Schall TJ, Datta M, Jain RK, et al. CCR2 inhibition reduces tumor myeloid cells and unmasks a checkpoint inhibitor effect to slow progression of resistant murine gliomas. Proc Natl Acad Sci U S A. 2020;117:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Masuda T, Noda M, Kogawa T, Kitagawa D, Hayashi N, Jomori T, Nakanishi Y, Nakayama KI, Ohno S, Mimori K. Phase I dose-escalation trial to repurpose propagermanium, an oral CCL2 inhibitor, in patients with breast cancer. Cancer Sci. 2020;111:924–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, Toriola AT, Nieman RK, Worley LA, Yano M, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. 2016;17:651–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou X, Fang D, Liu H, Ou X, Zhang C, Zhao Z, Zhao S, Peng J, Cai S, He Y, Xu J. PMN-MDSCs accumulation induced by CXCL1 promotes CD8(+) T cells exhaustion in gastric cancer. Cancer Lett. 2022;532:215598. [DOI] [PubMed] [Google Scholar]
- 173.Wang D, Cheng C, Chen X, Wang J, Liu K, Jing N, Xu P, Xi X, Sun Y, Ji Z, et al. IL-1β is an androgen-responsive target in macrophages for immunotherapy of prostate Cancer. Adv Sci (Weinh). 2023;10:e2206889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Yuan B, Clowers MJ, Velasco WV, Peng S, Peng Q, Shi Y, et al. Targeting IL-1β as an immunopreventive and therapeutic modality for K-ras-mutant lung cancer. JCI Insight. 2022;7:e157788. [DOI] [PMC free article] [PubMed]
- 175.Choksawangkarn W, Graham LM, Burke M, Lee SB, Ostrand-Rosenberg S, Fenselau C, Edwards NJ. Peptide-based systems analysis of inflammation induced myeloid-derived suppressor cells reveals diverse signaling pathways. Proteomics. 2016;16:1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhou Z, Chen X, Li Z, Wang X, Zhang M. Overexpression of S100A9 in tumor stroma contribute to immune evasion of NK/T cell lymphoma and predict poor response rate. Sci Rep. 2021;11:11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Escudier B, Faivre S, Van Cutsem E, Germann N, Pouget JC, Plummer R, Vergote I, Thistlethwaite F, Bjarnason GA, Jones R, et al. A phase II multicentre, Open-Label, proof-of-Concept Study of Tasquinimod in Hepatocellular, ovarian, renal cell, and gastric cancers. Target Oncol. 2017;12:655–61. [DOI] [PubMed] [Google Scholar]
- 178.Feng S, Zhao J, Yang T, Li L. TMPRSS11D/ALR-mediated ER stress regulates the function of myeloid-derived suppressor cells in the cervical cancer microenvironment. Int Immunopharmacol. 2023;124:110869. [DOI] [PubMed] [Google Scholar]
- 179.Tachibana M, Watanabe N, Koda Y, Oya Y, Kaminuma O, Katayama K, Fan Z, Sakurai F, Kawabata K, Hiroi T, Mizuguchi H. Ablation of IL-17A leads to severe colitis in IL-10-deficient mice: implications of myeloid-derived suppressor cells and NO production. Int Immunol. 2020;32:187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Carlson EJ, Savardekar H, Hu X, Lapurga G, Johnson C, Sun SH, Carson WE, Peterson BR. Fluorescent detection of Peroxynitrite produced by myeloid-derived suppressor cells in Cancer and Inhibition by Dasatinib. ACS Pharmacol Transl Sci. 2023;6:738–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li J, Lu Q, Peng M, Liao J, Zhang B, Yang D, Huang P, Yang Y, Zhao Q, Han B, Li J. Water extract from Herpetospermum pedunculosum attenuates oxidative stress and ferroptosis induced by acetaminophen via regulating Nrf2 and NF-κB pathways. J Ethnopharmacol. 2023;305:116069. [DOI] [PubMed] [Google Scholar]
- 182.Li R, Zeng X, Yang M, Feng J, Xu X, Bao L, Ye T, Wang X, Xue B, Huang Y. Antidiabetic DPP-4 inhibitors Reprogram Tumor Microenvironment that facilitates murine breast Cancer Metastasis through Interaction with Cancer cells via a ROS-NF-кB-NLRP3 Axis. Front Oncol. 2021;11:728047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Creelan BC, Gabrilovich DI, Gray JE, Williams CC, Tanvetyanon T, Haura EB, Weber JS, Gibney GT, Markowitz J, Proksch JW, et al. Safety, pharmacokinetics, and pharmacodynamics of oral omaveloxolone (RTA 408), a synthetic triterpenoid, in a first-in-human trial of patients with advanced solid tumors. Onco Targets Ther. 2017;10:4239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res. 2014;2:725–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, Goodman S, Gourin CG, Ha PK, Fakhry C, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, Halama N, Dimitrakopoulou-Strauss A, Haefeli WE, Jäger D, et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic melanoma (TaMe). Oncoimmunology. 2017;6:e1326440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tai LH, Alkayyal AA, Leslie AL, Sahi S, Bennett S, Tanese de Souza C, Baxter K, Angka L, Xu R, Kennedy MA, Auer RC. Phosphodiesterase-5 inhibition reduces postoperative metastatic disease by targeting surgery-induced myeloid derived suppressor cell-dependent inhibition of natural killer cell cytotoxicity. Oncoimmunology. 2018;7:e1431082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Weed DT, Zilio S, Reis IM, Sargi Z, Abouyared M, Gomez-Fernandez CR, Civantos FJ, Rodriguez CP, Serafini P. The reversal of Immune Exclusion mediated by Tadalafil and an anti-tumor vaccine also induces PDL1 Upregulation in Recurrent Head and Neck Squamous Cell Carcinoma: interim analysis of a phase I clinical trial. Front Immunol. 2019;10:1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lin YM, Lu CC, Hsiang YP, Pi SC, Chen CI, Cheng KC, Pan HL, Chien PH, Chen YJ. c-Met inhibition is required for the celecoxib-attenuated stemness property of human colorectal cancer cells. J Cell Physiol. 2019;234:10336–44. [DOI] [PubMed] [Google Scholar]
- 192.Li L, Sluter MN, Yu Y, Jiang J. Prostaglandin E receptors as targets for ischemic stroke: novel evidence and molecular mechanisms of efficacy. Pharmacol Res. 2021;163:105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Karpisheh V, Nikkhoo A, Hojjat-Farsangi M, Namdar A, Azizi G, Ghalamfarsa G, Sabz G, Yousefi M, Yousefi B, Jadidi-Niaragh F. Prostaglandin E2 as a potent therapeutic target for treatment of colon cancer. Prostaglandins Other Lipid Mediat. 2019;144:106338. [DOI] [PubMed] [Google Scholar]
- 194.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC Cancer. 2010;10:464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunol Immunother. 2014;63:847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Peng S, Hu P, Xiao YT, Lu W, Guo D, Hu S, Xie J, Wang M, Yu W, Yang J, et al. Single-cell analysis reveals EP4 as a target for restoring T-Cell infiltration and sensitizing prostate Cancer to Immunotherapy. Clin Cancer Res. 2022;28:552–67. [DOI] [PubMed] [Google Scholar]
- 197.Ishikawa S, Umemura M, Nakakaji R, Nagasako A, Nagao K, Mizuno Y, Sugiura K, Kioi M, Mitsudo K, Ishikawa Y. EP4-induced mitochondrial localization and cell migration mediated by CALML6 in human oral squamous cell carcinoma. Commun Biol. 2024;7:567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Hong DS, Parikh A, Shapiro GI, Varga A, Naing A, Meric-Bernstam F, et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE(2)-receptor E-type 4 (EP4), in patients with advanced cancers. J Immunother Cancer. 2020;8:e000222. [DOI] [PMC free article] [PubMed]
- 199.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Yan G, Zhao H, Zhang Q, Zhou Y, Wu L, Lei J, Wang X, Zhang J, Zhang X, Zheng L, et al. A RIPK3-PGE(2) Circuit mediates myeloid-derived suppressor cell-potentiated colorectal carcinogenesis. Cancer Res. 2018;78:5586–99. [DOI] [PubMed] [Google Scholar]
- 201.Xiao T, Chen Z, Xie Y, Yang C, Wu J, Gao L. Histone deacetylase inhibitors: targeting epigenetic regulation in the treatment of acute leukemia. Ther Adv Hematol. 2024;15:20406207241283277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Cui Y, Cai J, Wang W, Wang S. Regulatory effects of histone deacetylase inhibitors on myeloid-derived suppressor cells. Front Immunol. 2021;12:690207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, Chintala S, Ordentlich P, Kao C, Elzey B, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the Antitumor Effect of PD-1 inhibition in Murine models of Lung and Renal Cell Carcinoma. Clin Cancer Res. 2017;23:5187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Xie Z, Ago Y, Okada N, Tachibana M. Valproic acid attenuates immunosuppressive function of myeloid-derived suppressor cells. J Pharmacol Sci. 2018;137:359–65. [DOI] [PubMed] [Google Scholar]
- 205.Gebhardt C, Simon SCS, Weber R, Gries M, Mun DH, Reinhard R, Holland-Letz T, Umansky V, Utikal J. Potential therapeutic effect of low-dose paclitaxel in melanoma patients resistant to immune checkpoint blockade: a pilot study. Cell Immunol. 2021;360:104274. [DOI] [PubMed] [Google Scholar]
- 206.Mathew AA, Zakkariya ZT, Ashokan A, Manohar M, Keechilat P, Nair SV, Koyakutty M. 5-FU mediated depletion of myeloid suppressor cells enhances T-cell infiltration and anti-tumor response in immunotherapy-resistant lung tumor. Int Immunopharmacol. 2023;120:110129. [DOI] [PubMed] [Google Scholar]
- 207.Zhang Y, Bush X, Yan B, Chen JA. Gemcitabine nanoparticles promote antitumor immunity against melanoma. Biomaterials. 2019;189:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kobayashi Y, Yamada D, Kawai T, Sato Y, Teshima T, Yamada Y, Nakamura M, Suzuki M, Matsumoto A, Nakagawa T, et al. Different immunological effects of the molecular targeted agents sunitinib, everolimus and temsirolimus in patients with renal cell carcinoma. Int J Oncol. 2020;56:999–1013. [DOI] [PubMed] [Google Scholar]
- 209.Duan XL, Guo JP, Li F, Xiu C, Wang H. Sunitinib inhibits PD-L1 expression in osteosarcoma by targeting STAT3 and remodels the immune system in tumor-bearing mice. Future Oncol. 2020;16:1815–24. [DOI] [PubMed] [Google Scholar]
- 210.Liu J, Lin WP, Su W, Wu ZZ, Yang QC, Wang S, Sun TG, Huang CF, Wang XL, Sun ZJ. Sunitinib attenuates reactive MDSCs enhancing anti-tumor immunity in HNSCC. Int Immunopharmacol. 2023;119:110243. [DOI] [PubMed] [Google Scholar]
- 211.Li H, Ding J, Lu M, Liu H, Miao Y, Li L, Wang G, Zheng J, Pei D, Zhang Q. CAIX-specific CAR-T cells and Sunitinib Show Synergistic effects against metastatic renal Cancer models. J Immunother. 2020;43:16–28. [DOI] [PubMed] [Google Scholar]
- 212.Kato T, Wakiyama H, Furusawa A, Choyke PL, Kobayashi H. Near Infrared Photoimmunotherapy; a review of targets for Cancer Therapy. Cancers (Basel). 2021;13:2535. [DOI] [PMC free article] [PubMed]
- 213.Kato T, Fukushima H, Furusawa A, Okada R, Wakiyama H, Furumoto H, Okuyama S, Takao S, Choyke PL, Kobayashi H. Selective depletion of polymorphonuclear myeloid derived suppressor cells in tumor beds with near infrared photoimmunotherapy enhances host immune response. Oncoimmunology. 2022;11:2152248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Khaki Bakhtiarvand V, Ramezani-Ali Akbari K, Amir Jalali S, Hojjat-Farsangi M, Jeddi-Tehrani M, Shokri F, Shabani M. Myeloid-derived suppressor cells (MDSCs) depletion by cabozantinib improves the efficacy of anti-HER2 antibody-based immunotherapy in a 4T1-HER2 murine breast cancer model. Int Immunopharmacol. 2022;113:109470. [DOI] [PubMed] [Google Scholar]
- 215.Feng PH, Lam B, Tseng SH, Kung YJ, Farmer E, Cheng MA, Hung CF. NKG2D-Fc fusion protein promotes antitumor immunity through the depletion of immunosuppressive cells. Cancer Immunol Immunother. 2020;69:2147–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Al-Khami AA, Zheng L, Del Valle L, Hossain F, Wyczechowska D, Zabaleta J, Sanchez MD, Dean MJ, Rodriguez PC, Ochoa AC. Exogenous lipid uptake induces metabolic and functional reprogramming of tumor-associated myeloid-derived suppressor cells. Oncoimmunology. 2017;6:e1344804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Ruan H, Zhang J, Wang Y, Huang Y, Wu J, He C, Ke T, Luo J, Yang M. 27-Hydroxycholesterol/liver X receptor/apolipoprotein E mediates zearalenone-induced intestinal immunosuppression: a key target potentially linking zearalenone and cancer. J Pharm Anal. 2024;14:371–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Yokoyama Y, Estok TM, Wild R. Sirpiglenastat (DRP-104) induces Antitumor Efficacy through Direct, Broad Antagonism of Glutamine Metabolism and stimulation of the Innate and Adaptive Immune systems. Mol Cancer Ther. 2022;21:1561–72. [DOI] [PubMed] [Google Scholar]
- 219.Shi J, Liu C, Luo S, Cao T, Lin B, Zhou M, Zhang X, Wang S, Zheng T, Li X. STING agonist and IDO inhibitor combination therapy inhibits tumor progression in murine models of colorectal cancer. Cell Immunol. 2021;366:104384. [DOI] [PubMed] [Google Scholar]
- 220.Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: IDO inhibitors in cancer therapy. Oncoimmunology. 2020;9:1777625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Muller AJ, Manfredi MG, Zakharia Y, Prendergast GC. Inhibiting IDO pathways to treat cancer: lessons from the ECHO-301 trial and beyond. Semin Immunopathol. 2019;41:41–8. [DOI] [PubMed] [Google Scholar]
- 222.Umansky V, Shevchenko I, Bazhin AV, Utikal J. Extracellular adenosine metabolism in immune cells in melanoma. Cancer Immunol Immunother. 2014;63:1073–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Watanabe T. Realization of osteolysis, angiogenesis, immunosuppression, and drug resistance by extracellular vesicles: roles of RNAs and proteins in their cargoes and of ectonucleotidases of the immunosuppressive adenosinergic noncanonical pathway in the bone marrow niche of multiple myeloma. Cancers (Basel). 2021;13:2969. [DOI] [PMC free article] [PubMed]
- 224.Xia C, Yin S, To KKW, Fu L. CD39/CD73/A2AR pathway and cancer immunotherapy. Mol Cancer. 2023;22:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bao X, Xie L. Targeting purinergic pathway to enhance radiotherapy-induced immunogenic cancer cell death. J Exp Clin Cancer Res. 2022;41:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hamed A, Ghareeb D, Mohamed TM, Hamed M, Nofal MS, Gaber M. Caffeine-folic acid-loaded-chitosan nanoparticles combined with methotrexate as a novel HepG2 immunotherapy targeting adenosine A2A receptor downstream cascade. BMC Complement Med Ther. 2023;23:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lepper A, Bitsch R, Özbay Kurt FG, Arkhypov I, Lasser S, Utikal J, Umansky V. Melanoma patients with immune-related adverse events after immune checkpoint inhibitors are characterized by a distinct immunological phenotype of circulating T cells and M-MDSCs. Oncoimmunology. 2023;12:2247303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kim K, Skora AD, Li Z, Liu Q, Tam AJ, Blosser RL, Diaz LA Jr., Papadopoulos N, Kinzler KW, Vogelstein B, Zhou S. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A. 2014;111:11774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Clavijo PE, Moore EC, Chen J, Davis RJ, Friedman J, Kim Y, Van Waes C, Chen Z, Allen CT. Resistance to CTLA-4 checkpoint inhibition reversed through selective elimination of granulocytic myeloid cells. Oncotarget. 2017;8:55804–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Yu B, Luo F, Sun B, Liu W, Shi Q, Cheng SY, Chen C, Chen G, Li Y, Feng H. KAT6A acetylation of SMAD3 regulates myeloid-derived suppressor cell recruitment, metastasis, and Immunotherapy in Triple-negative breast Cancer. Adv Sci (Weinh). 2021;8:e2100014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zannikou M, Duffy JT, Levine RN, Seblani M, Liu Q, Presser A, et al. IL15 modification enables CAR T cells to act as a dual targeting agent against tumor cells and myeloid-derived suppressor cells in GBM. J Immunother Cancer. 2023;11:e006239. [DOI] [PMC free article] [PubMed]
- 232.Johnson LR, Lee DY, Eacret JS, Ye D, June CH, Minn AJ. The immunostimulatory RNA RN7SL1 enables CAR-T cells to enhance autonomous and endogenous immune function. Cell. 2021;184:4981–e49954914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Meng S, Whitt AG, Stamp BF, Eaton JW, Li C, Yaddanapudi K. Exosome-based cancer vaccine for prevention of lung cancer. Stem Cell Investig. 2023;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Mougel A, Méjean F, Tran T, Adimi Y, Galy-Fauroux I, Kaboré C, Mercier E, Urquia P, Terme M, Tartour E, Tanchot C. Synergistic effect of combining sunitinib with a peptide-based vaccine in cancer treatment after microenvironment remodeling. Oncoimmunology. 2022;11:2110218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Gordy JT, Sandhu AK, Fessler K, Luo K, Kapoor AR, Ayeh SK, Hui Y, Schill C, Chen F, Wang T, et al. IFNα and 5-Aza-2’-deoxycytidine combined with a dendritic-cell targeting DNA vaccine alter tumor immune cell infiltration in the B16F10 melanoma model. Front Immunol. 2022;13:1074644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.