Abstract
Background
Recent reports that the Ross procedure restores normal life expectancy in young adults with aortic valve disease have renewed interest in this complex procedure. Because only a few centers perform a high volume of Ross procedures, there are limited data on the safety of learning and teaching the Ross procedure.
Methods
A total of 234 consecutive adult patients at a single center underwent the Ross procedure performed by an experienced surgeon acting as the primary operator (n = 186; 1994-2021) or mentoring surgeon (n = 48; 2001-2021). Cumulative sum analysis of cardiopulmonary bypass times was performed to evaluate learning curves as primary surgeon and mentored surgeon. Kaplan-Meier analysis was used to estimate long-term survival and freedom from Ross-related reintervention.
Results
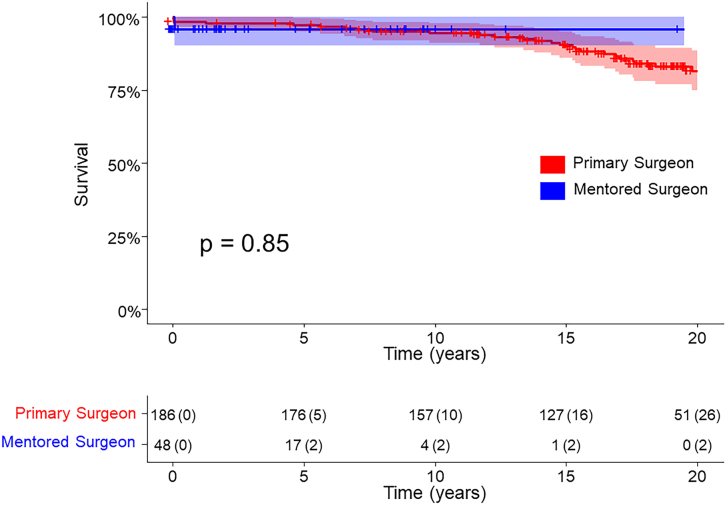
Patients’ mean age was 42 ± 11 years; 169 (72%) were male. Baseline demographic characteristics were similar between cohorts. Operative death occurred in 5 (2%) patients: 3 (2%) as primary surgeon and 2 (4%) as mentor (P = .28). In-hospital reoperation was required in 8 (3%) patients: 6 (3%) as primary surgeon and 2 (4%) as mentor (P = .75). Up to 10 years, there was no difference in survival between primary operator cases and mentored cases (94.4% [91.2%-97.9%]) vs 95.8% [90.3%-100%]; log-rank, P = .85).
Conclusions
Although the Ross procedure is technically complex, it can be taught to experienced aortic root surgeons without compromising short- or long-term outcomes.
In Short.
-
▪
There is growing evidence that the Ross procedure is associated with better long-term survival and freedom from valve-related complications vs bioprosthetic or mechanical aortic valve replacement.
-
▪
This procedure remains uncommonly performed because of its technical complexity, longer operative times, and concern for risk of reintervention with potential for failure of 2 valves.
-
▪
The Ross procedure can be taught without compromising patient survival or valve durability when cardiac surgeons are appropriately mentored.
The Ross procedure restores normal life expectancy for young adults with surgical aortic valve disease without exposing patients to long-term risks of anticoagulation or the risk of structural degeneration of bioprosthetic valves.1 In a propensity score–matched comparison, the Ross procedure is also associated with better long-term survival and freedom from valve-related complications vs bioprosthetic or mechanical aortic valve replacement.2,3 Despite growing supportive evidence, the Ross procedure remains uncommonly performed because of its technical complexity, longer operative times, and concern for risk of reintervention with potential for failure of 2 valves rather than just 1. Few academic centers in the United States perform a high volume of Ross procedures, making it difficult for young surgeons to obtain adequate exposure to the technique.4 This study evaluated the short- and long-term outcomes of patients undergoing the Ross procedure at a center with mentorship by a senior surgeon experienced in the Ross procedure.
Patients and Methods
Patient Population
We retrospectively reviewed consecutive patients who underwent a Ross procedure performed by an experienced Ross surgeon acting as a primary operator (1994-2021) or a mentor to another surgeon (2001-2021). Mentored surgeons were experienced in aortic root and aortic valve reimplantation. Clinical characteristics were obtained retrospectively through review of the patients’ medical records. Long-term vital status was determined through electronic medical records and online obituary searches according to a previously described and validated protocol as well as by phone call.5 This study was approved by the Baylor Scott & White institutional review board (#020-297).
Statistical Analysis
Descriptive statistics including mean, standard deviation, frequency, and percentage were used to summarize the characteristics of study participants. Student t-test or Wilcoxon rank sum test was used to compare the average values of continuous variables between the study groups. Categorical variables were compared by χ2 test. Kaplan-Meier test was used to estimate and to compare the long-term survival and freedom from autograft and homograft reintervention. Cumulative sum analysis was used to evaluate the learning of both groups based on their respective mean cardiopulmonary bypass (CPB) times. All statistical analyses were performed in RStudio.
Results
Patients
There were 234 patients who underwent a Ross procedure from 1994 to 2021 performed by an experienced Ross surgeon acting as the primary operator (n = 186; 1994-2021) or as mentoring surgeon (n = 48; 2001-2021). Operative volumes by year are detailed in Supplemental Figure 1. The mean age of patients was 42 ± 11.2 years; 169 (72%) patients were male. Demographic characteristics and comorbidities of patients were similar between primary and mentored cases. These are detailed in Table 1.
Table 1.
Demographic Characteristics and Comorbidities
| Variable | All Ross Procedures (N = 234) | Primary Cases (n = 186) | Mentored Cases (n = 48) | P Value |
|---|---|---|---|---|
| Age, y | 42 ± 11.2 | 42 ± 11.6 | 41 ± 9.8 | .38 |
| Male | 169 (72) | 137 (74) | 32 (67) | .34 |
| BMI, kg/m2 | 27.9 ± 10.2 | 27.8 ± 11.2 | 28.2 ± 5.5 | .81 |
| Hypertension | 77 (33) | 61 (33) | 16 (33) | .94 |
| Diabetes | 16 (7) | 14 (8) | 2 (4) | .41 |
| COPD | 1 (0) | 1 (1) | 0 (0) | .61 |
| NYHA III/IV | 98 (42) | 85 (46) | 13 (27) | .02 |
| CAD | 12 (5) | 11 (6) | 1 (2) | .28 |
| CKD | 9 (4) | 6 (3) | 3 (6) | .33 |
| Active smoking | 43 (18) | 36 (19) | 7 (15) | .45 |
| LVEF, % | 57 ± 8.3 | 56 ± 8.5 | 60.5 ± 6.6 | <.01 |
| Prior sternotomy | 18 (8) | 16 (9) | 2 (4) | .30 |
| Prior surgery | 21 (9) | 16 (9) | 5 (10) | .70 |
Categorical variables are expressed as number (percentage) and continuous variables as mean ± standard deviation.
BMI, body mass index; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Operative Details and Perioperative Outcomes
There were 26 patients (11%) who underwent a supported root procedure. This became a common practice at this institution after 2017; therefore, all supported Ross procedures were performed as mentored cases.6 The average CPB times (228.8 ± 39.3 minutes vs 192.5 ± 38.2 minutes; P < .01) and cross-clamp times (197.9 ± 33.5 minutes vs 164.5 ± 25.0 minutes; P < .01) were significantly higher in mentored cases because of employment of the supported techniques. The in-hospital mortality was 2% (3/186) in primary operator cases and 4% (2/48) in mentored cases (P = .28; Table 2).
Table 2.
Operative Details
| Variable | All Ross Procedures (N = 234) | Primary Cases (n = 186) | Mentored Cases (n = 48) | P Value |
|---|---|---|---|---|
| Supported root replacement | 26 (11) | 0 (0) | 26 (54) | <.01 |
| Concomitant procedure | 27 (12) | 24 (13) | 3 (6) | .24 |
| Cardiopulmonary bypass time, min | 200 ± 40.7 | 192.5 ± 38.2 | 228.8 ± 39.3 | <.01 |
| Cross-clamp time, min | 171 ± 29.4 | 164.5 ± 25.0 | 197.9 ± 33.5 | <.01 |
| Reoperation | 8 (3) | 6 (3) | 2 (4) | .75 |
| In-hospital mortality | 5 (2) | 3 (2) | 2 (4) | .28 |
Categorical variables are expressed as number (percentage) and continuous variables as mean ± standard deviation.
Long-term Outcomes
Long-term survival was similar between primary operator and mentored cases at up to 10 years (94.4% [95% CI, 91.2%-97.9%] vs 95.8% [95% CI, 90.3%-100%]; log-rank, P = .85; Figure). Freedom from first reintervention on the autograft and homograft is reported in Table 3. There were no differences in rates of reintervention on either valve between cases operated on as primary surgeon and mentored cases up to 10 years.
Figure.
Kaplan-Meier survival of patients undergoing Ross procedure by the surgeon acting as primary operator or a mentor.
Table 3.
Freedom From Autograft and Homograft Reoperation by Kaplan-Meier Analysis
| Variable | 5 years | 10 years | 15 years | 20 years |
|---|---|---|---|---|
| Freedom from autograft reoperation (P = .58) | ||||
| All | 96.7 (94.3-99.1) | 86.3 (81.4-91.5) | 72.9 (65.9-80.5) | 54.7 (45.3-66.1) |
| Primary cases | 96.6 (94-99.3) | 85.4 (80.2-91.0) | 72 (64.9-79.9) | 55.7 (46.5-66.7) |
| Mentored cases | 97.9 (93.8-100) | 97.9 (93.8-100) | … | … |
| Freedom from homograft reoperation (P = .82) | ||||
| All | 96.6 (94.2-99.1) | 96.1 (93.4-98.8) | 92.2 (88.1-96.5) | 82 (75-89.8) |
| Primary cases | 96.1 (93.3-99) | 95.5 (92.5-98.6) | 91.6 (87.3-96.2) | 83.1 (76.4-90.3) |
| Mentored cases | 100 (no events) | 100 (no events) | … | … |
Values are expressed as median (95% CI).
Surgeon Learning Curve
Efficiency based on CPB times was used to assess the surgeon learning curve in isolated Ross procedures. CPB time was chosen to account for pulmonary homograft implantation being done off cross-clamp in some cases, as were minor stitch repairs. It is notable that around 2017, it became common practice at this institution to perform supported Ross procedures for patients with aortic insufficiency. The efficiency learning curves of supported and unsupported Ross procedures are evaluated separately. The mean CPB time for primary operator cases (all unsupported Ross procedures) was 188.1 ± 28.2 minutes. A cumulative sum analysis curve shows that after performing around 33 cases, the surgeon was able to overcome the learning curve as a primary operator with a clear trend toward improved efficiency with decreasing CPB times (Supplemental Figure 2). In mentored cases, the mean CPB time was 211.7 ± 25.3 minutes in unsupported Ross procedures and 245.9 ± 41.2 minutes in supported Ross procedures. With a senior attending mentoring a less experienced surgeon, there was no significant compromise of CPB times observed in either supported or unsupported Ross procedures (Supplemental Figure 3).
Comment
The Ross procedure is a technically complex procedure that can provide patients with excellent long-term survival and low rates of reoperation when it is performed by experienced surgeons.3 The growing evidence of its benefits to young patients with aortic valve disease emphasizes the importance of training surgeons to safely and efficiently perform this procedure. In this study, we found that with appropriate mentorship, experienced aortic root surgeons can perform the Ross procedure with excellent perioperative and long-term survival, with low rates of reoperation, and without compromising efficiency of the procedure.
The learning curve has been assessed with cumulative sum analysis in multiple cardiac procedures.7,8 A single-center study with a Ross program examining the learning curve of surgeons demonstrated that significant improvements in safety and efficiency occurred after 75 to 100 cases with low operative mortality.9 In our study, the learning curve of the primary surgeon reached proficiency at 33 cases and achieved full effect at 70 cases, reinforcing the fact that the Ross procedure is technically difficult to learn. However, our results show that with dedicated mentorship and instruction, cardiac surgeons less experienced in the Ross procedure can quickly achieve efficiency and excellent outcomes. In our experience, an average of 1 aortic root surgery per month is considered a robust experience. As well, the Ross mentor’s identification of surgical mentees who are proficient in aortic root surgery is a component of successful training.
Since the initial procedures in this cohort, there has been modification to the operative technique of the Ross procedure and changes in patient selection discussed in our previous series.10 These changes are reflected in the significantly higher CPB times and rate of ascending aortic replacement as more patients with aortic insufficiency undergo the supported Ross procedure. In addition, a larger mean homograft size in the more recent mentored cases requires more insertion time compared with primary operator cases in our cohort.
Training surgeons is critical if the superior long-term outcomes of the Ross procedure are to be maintained. A study from multi-state-level databases demonstrated that the “living valve” replacement in Ross operations entails clinical benefits when the risks of bioprosthetic and mechanical valves are accounted for.3 Our findings suggest that surgeons can be safely mentored to perform the Ross procedure without compromising patient safety or long-term outcomes.
Limitations
This study is subject to limitations inherent in all retrospective and single-center studies. In addition, the cumulative sum analysis for mentored cases was based on the mean CPB of all cases. Around 2017, performing the Dacron supported Ross technique for aortic insufficiency became standard at our institution as described in our previous series.10 This modification necessitates and therefore increased overall mean bypass times in this analysis. However, before institution of this technique, there remains a clear trend in increased efficiency with more experience. Most mentored cases occurred in the last 10 years, and median follow-up is significantly longer in primary surgeon cases. Last, all Ross procedures performed by the less experienced surgeons have been mentored; therefore, the outcomes of these surgeons independently performing the procedure are not available.
Conclusion
This report shows that the Ross procedure can be taught to experienced aortic root surgeons without compromising patient survival or valve durability when those surgeons are appropriately mentored. It is essential to have institutions with experienced Ross surgeons with excellent outcomes to train newer surgeons. Further studies examining very-long-term outcomes of mentored Ross procedures as well as outcomes of newly independent Ross surgeons are needed.
Acknowledgments
The Supplemental Figures can be viewed in the online version of this article [https://doi.org/10.1016/j.atssr.2022.12.009] on http://www.annalsthoracicsurgery.org.
Funding Sources
This study was funded by a philanthropic gift from Satish and Yasmin Gupta to Baylor Scott & White The Heart Hospital, Plano.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Waived for retrospective review of patient information. Verbal consent as obtained from patients for collecting vitality status from patients by phone call.
Supplementary Data
References
- 1.Ryan W.H., Squiers J.J., Harrington K.B., et al. Long-term outcomes of the Ross procedure in adults. Ann Cardiothorac Surg. 2021;10:499–508. doi: 10.21037/acs-2021-rp-fs-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazine A., David T.E., Rao V., et al. Long-term outcomes of the Ross procedure versus mechanical aortic valve replacement: propensity-matched cohort study. Circulation. 2016;134:576–585. doi: 10.1161/CIRCULATIONAHA.116.022800. [DOI] [PubMed] [Google Scholar]
- 3.El-Hamamsy I., Toyoda N., Itagaki S., et al. Propensity-matched comparison of the Ross procedure and prosthetic aortic valve replacement in adults. J Am Coll Cardiol. 2022;79:805–815. doi: 10.1016/j.jacc.2021.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Bonow R.O., O'Gara P.T. Reconsidering the Ross procedure. JAMA Cardiol. 2021;6:548. doi: 10.1001/jamacardio.2021.0087. [DOI] [PubMed] [Google Scholar]
- 5.Wooley J., Neatherlin H., Mahoney C., et al. Description of a method to obtain complete one-year follow-up in The Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Am J Cardiol. 2018;121:758–761. doi: 10.1016/j.amjcard.2017.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Brinkman W.T., Herbert M.A., Prince S.L., Ryan C., Ryan W.H. Redo autograft operations after the Ross procedure. Ann Thorac Surg. 2012;93:1477–1481. doi: 10.1016/j.athoracsur.2012.01.100. [DOI] [PubMed] [Google Scholar]
- 7.Holzhey D.M., Jacobs S., Walther T., Mochalski M., Mohr F.W., Falk V. Cumulative sum failure analysis for eight surgeons performing minimally invasive direct coronary artery bypass. J Thorac Cardiovasc Surg. 2007;134:663–669. doi: 10.1016/j.jtcvs.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Holzhey D.M., Seeburger J., Misfeld M., Borger M.A., Mohr F.W. Learning minimally invasive mitral valve surgery: a cumulative sum sequential probability analysis of 3895 operations from a single high-volume center. Circulation. 2013;128:483–491. doi: 10.1161/CIRCULATIONAHA.112.001402. [DOI] [PubMed] [Google Scholar]
- 9.Bouhout I., Ghoneim A., Poirier N., et al. Impact of the learning curve on early outcomes following the Ross procedure. Can J Cardiol. 2017;33:493–500. doi: 10.1016/j.cjca.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 10.Shih E, Brinkman WT, Harrington KB, et al. Outcomes of redo operations after Ross procedure. J Thorac Cardiovasc Surg. Published online May 14, 2022. https://doi.org/10.1016/j.jtcvs.2022.04.023 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.