Abstract
Primary extraskeletal osteosarcoma of the lung is exceedingly rare and associated with a poor prognosis. This case report presents a patient with circumferential pulmonary ossification secondary to lung extraskeletal osteosarcoma with compressive mediastinal shift who underwent extrapleural pneumonectomy that led to resolution of symptoms. This case offers an approach to the operative management of primary thoracic osteosarcoma and suggests that even patients with advanced disease may be surgical candidates, particularly for symptom relief.
Extraskeletal osteosarcoma (ESOS) is a malignant mesenchymal neoplasm that produces osteoid without direct attachment to bone or periosteum. It is a rare malignant disease and represents approximately 2% to 4% of all osteosarcomas and less than 1% of soft tissue sarcomas.1 The most common locations of presentation are the extremities. Primary thoracic ESOS is a minute subset of ESOS, and those cases arising as a lung primary tumor are extraordinarily rare.
Pulmonary ESOS generally manifests with advanced disease as a result of delayed diagnosis from nonspecific symptoms, including dyspnea, cough, and chest pain. Therapeutic options are limited even among operative candidates because of dense calcifications preventing safe resection. However, symptoms such as mediastinal shift or compressive symptoms of adjacent structures may mandate palliative resections, as in our case in a young, operatively fit patient. Written consent was obtained for the following report.
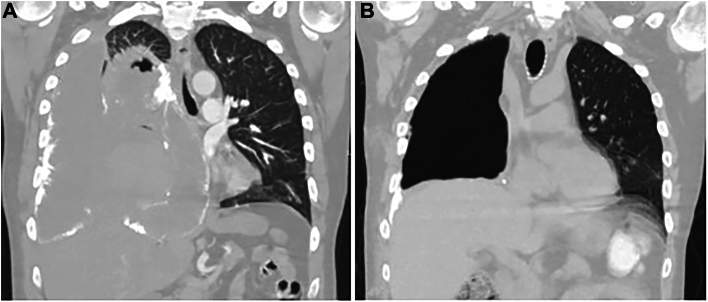
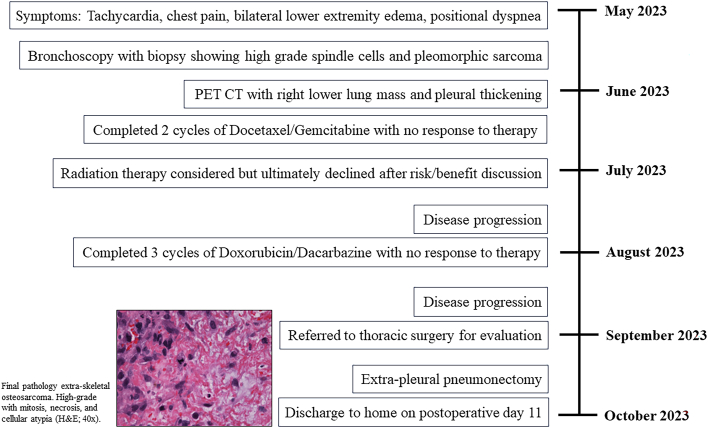
A 48-year-old male, never smoker, patient presented with a 2-month history of cough and was found to have a 4.4-cm biopsy-proven, high-grade, undifferentiated pleiomorphic sarcoma in the right lower lobe invading the bronchus with pleural effusion. He was initially started on a docetaxel-gemcitabine regimen and completed 2 cycles in June 2023. He underwent routine oncologic surveillance with computed tomographic (CT) imaging to assess for disease progression at regular intervals. A CT chest scan in July 2023 showed increased right lower lobe pulmonary masses with concern for bronchopleural fistula, in response to which the chemotherapy regimen was changed to a doxorubicin-dacarbazine combination. His repeat CT chest scan after completion of the first cycle of the doxorubicin-dacarbazine regimen showed overall unchanged pulmonary masses and nodes, and the patient completed 3 cycles of this regimen. Unfortunately, additional imaging in August 2023 showed radiologic progression of disease, and thoracic surgery was consulted for possible surgical resection for symptomatic relief. The mass had obliterated his right lung, with mediastinal shift causing severe cardiac and vascular compression and resulting in persistent tachycardia, positional dyspnea, bilateral lower extremity edema, and chest pain (Figure 1A). After review at our multidisciplinary tumor board and with shared decision making with the patient and family, we proceeded with extrapleural pneumonectomy (EPP), given the lack of alternative therapeutic options and the need for symptom control in an otherwise healthy individual.
Figure 1.
(A) Circumferential calcification of tumor obliterating the right lung and resulting in a mediastinal shift. Thick, calcific plaques on the aorta, inferior vena cava, superior vena cava, and pericardium not shown on current cuts. (B) Postoperatively, the mediastinum reverted to the midline, thereby alleviating the patient’s cardiopulmonary and vascular symptoms.
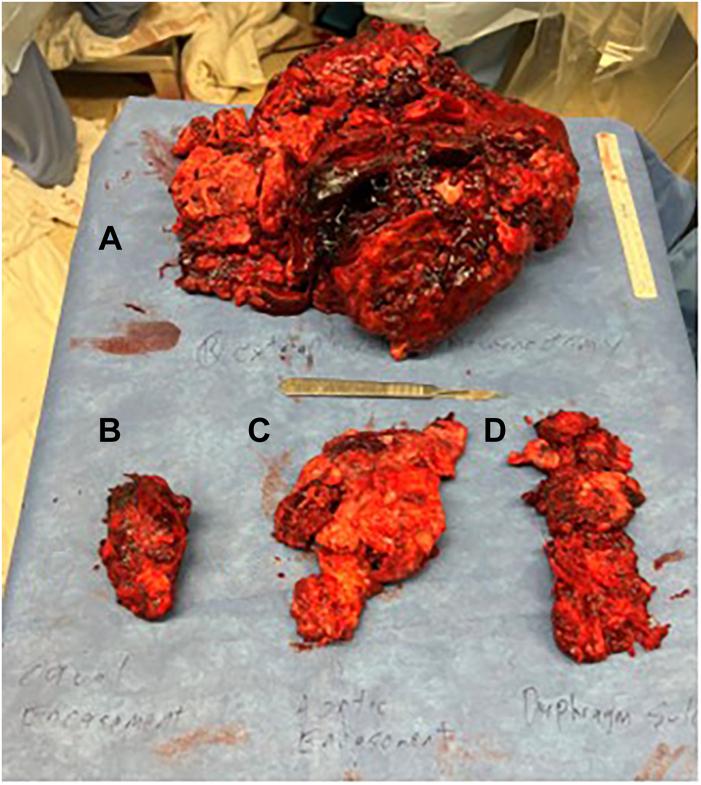
We approached through a right hemiclamshell thoracotomy. On entering the chest, we encountered a sheet of calcification in the interspace that was revealed to be circumferential tumor calcification nearly integrated with the ribs. We dissected the calcified visceral pleura from the ribs throughout the length of the thoracotomy and circumferentially mobilized the lung from the endothoracic fascia, mediastinal structures, and diaphragm to complete the pneumonectomy. The final specimen weighed 2640 g and measured 25.2 × 22.1 × 8.5 cm, of which 22 × 17.4 × 4.6 cm was tumor (Figure 2A). Large shells of calcium were resected separately from the great vessels and pericardium (Figures 2B to 2D). Postoperatively, the patient returned to normal sinus rhythm, had resolution of lower extremity edema, and experienced no further respiratory symptoms. Repeat imaging showed patent vasculature and a midline mediastinum, and the patient was ultimately discharged home (Figure 1B). The final pathology report returned a result of ESOS (Figure 3). The patient was subsequently seen in the thoracic surgery clinic within 5 days of discharge, where he presented with some small breakdown of the incision, with suture repair. He was planned to undergo adjuvant chemoradiation of the chest with surveillance scans after further recovery from surgery at 4 to 6 weeks postoperatively.
Figure 2.
Extrapleural pneumonectomy of circumferentially calcified lung. (A) The final specimen showed nearly complete obliteration of normal lung tissue by tumor and dense calcification circumferentially. The calcifications encasing the (B) vena cava, (C) aorta, and (D) diaphragm were resected separately from the main specimen.
Figure 3.
Timeline of the case report. (CT, computed tomography; H&E, hematoxylin and eosin; PET, positron emission tomography.)
Comment
Osteogenic osteosarcoma is the most common sarcoma subtype; however, extraskeletal sites are rarely documented phenomena, and primary ESOS of the lung is even more uncommon, with approximately 60 reported cases in the literature worldwide.1 Pulmonary ESOS often manifests insidiously in older adults as a large calcified mass, for which the differential diagnosis includes metastatic osteosarcoma, mesothelioma, calcified hamartoma, or calcified primary carcinoma.2 The diagnosis is made on the basis of the following criteria: (1) tumor is composed of a uniform pattern of sarcoma, (2) osteoid or bone must be formed by the sarcoma, and (3) a primary osseous tumor must be excluded.3
Although complete surgical resection improves survival, there is no universally accepted treatment strategy. EPP has been performed almost exclusively for malignant pleural mesothelioma, although we suggest in this report that EPP may be of utility for thoracic osteosarcoma not amenable to other therapies or as a salvage procedure. The following criteria were proposed by Hameury and colleagues4 regarding indications for EPP in patients with sarcoma: (1) recommendation on multidisciplinary tumor board discussion, (2) pleural lesions confined to 1 hemithorax, (3) cases where there are no other alternatives, (4) good performance status, and (5) partial radiologic response to preoperative chemotherapy. We propose that bilateral disease and metastases are also not absolute contraindications to EPP, if the operation is performed for symptom relief and disease control in an otherwise healthy patient.
Multimodality approaches with chemotherapy and radiation can be considered in conjunction with surgery; however, their role in ESOS is controversial. A study by Qi and colleagues5 found no difference in survival among 153 patients who received chemotherapy for ESOS compared with 157 patients who did not receive chemotherapy, regardless of whether they underwent surgical resection. Although nonoperative approaches may be considered in select patients, such as those with distant metastases, there are limited data to support the efficacy of these approaches. Treatment plans should be highly individualized and created in collaboration with a multidisciplinary team.
Given the relative rarity of ESOS, little is known about the specific factors associated with survival. Up to 90% of patients with ESOS present with local recurrences or metastatic disease, and prognosis is overall very poor, with studies citing 5-year survival rates as low as 10%.2 Despite these data, surgery may still be considered in patients as a palliative approach to improve quality of life. Palliative lung resections are generally accepted yet rarely performed operations, with typical indications including hemoptysis, tumor necrosis, vascular occlusion, recurrent pleural effusion, intractable cough, and unrelenting pain. Hameury and colleagues4 analyzed quality of life data among 7 pediatric patients with pulmonary sarcoma who underwent EPP and demonstrated preserved postoperative lung function, with most patients able to return to daily life within 4 weeks, a finding perhaps indicating this as a separable indication for intervening. In our case, the patient experienced resolution of his tachycardia and lower extremity edema and was discharged home with no further respiratory symptoms.
In conclusion, patients with pulmonary ESOS often present in the advanced stages of disease, for which therapeutic options are limited and survival outcomes are poor. Operative intervention should be considered in patients who are otherwise appropriate surgical candidates for the best opportunity for cure or to palliate concurrent symptoms and improve quality of life.
Acknowledgments
Funding Sources
The authors have no funding sources to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Patient Consent
Obtained.
References
- 1.Qian J., Zhang X.Y., Gu P., Shao J.C., Han B.H., Wang H.M. Primary thoracic extra-skeletal osteosarcoma: a case report and literature review. J Thorac Dis. 2017;9:E1088–E1095. doi: 10.21037/jtd.2017.11.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai D., Cai W., Fan G., Yang J., Liu C. Case report: primary extra-skeletal osteosarcoma in the lung and pulmonary artery. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.673494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson P.G., Shields P.G. In: vol. 2. 6th ed. Thomas W., Shields H.C., editors. Lippincott Williams & Wilkins; 2005. Uncommon primary malignant tumors of the lung; pp. 1801–1830. (General Thoracic Surgery). [Google Scholar]
- 4.Hameury F., Marec-Berard P., Eymery M., et al. Pleuropneumonectomy as salvage therapy in children suffering from primary or metastatic sarcomas with pleural localizations. Cancers (Basel) 2021;13:3655. doi: 10.3390/cancers13153655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qi L., Wan L., Ren X., Zhang W., Tu C., Li Z. The Role of chemotherapy in extraskeletal osteosarcoma: a propensity score analysis of the Surveillance Epidemiology and End Results (SEER) database. Med Sci Monit. 2020;26 doi: 10.12659/MSM.925107. [DOI] [PMC free article] [PubMed] [Google Scholar]