Abstract
Background
Our study examines the relationship between gastroesophageal reflux disease (GERD) and small intestinal bacterial overgrowth (SIBO), focusing on the potential impact of acid-suppressive drugs. We also explore changes in gut microbiota and metabolism in patients with both conditions.
Methods
This study included patients from the Department of Gastroenterology, Beijing Shijitan Hospital, between February 2021 and November 2023. All patients underwent assessments including questionnaires, hydrogen and methane breath tests, and gastroscopy. GERD was diagnosed using the GERD-Q scale and gastroscopy, while SIBO was diagnosed via breath tests. We analyzed the correlation between GERD and SIBO, identified risk factors for SIBO, and examined the gut microbiota using 16S rRNA sequencing to explore the relationship between GERD and SIBO.
Results
The retrospective study included 394 patients.148 with GERD and 287 with positive SIBO results. Among these, 270 had a positive methane (CH4) breath test and 97 had a positive hydrogen (H2) breath test. GERD was more common in patients with positive SIBO (P = 0.007), and the link between CH4 breath tests and GERD was stronger than that with H2 breath tests (P = 0.020). Logistic regression showed GERD is an independent risk factor for SIBO. Short-term, low-dose acid-suppressive drugs did not affect SIBO development. 16S rRNA sequencing of fecal microbiota from 24 patients showed dominant microbiota in SIBO-positive GERD patients included bacteroides uniformis and bacteroides stercoris. Patients with both GERD and SIBO had differential metabolites, mainly associated with ATP-Binding Cassette transporters (ABC transporters).
Conclusion
GERD is strongly linked to SIBO, especially in patients with a positive CH4 breath test. The gut microbiota in GERD and SIBO patients differs from healthy individuals, with bacteroides uniformis as a key marker. Metabolic changes are mainly related to ABC transporter metabolites.
Keywords: gastroesophageal reflux disease, small intestinal bacterial overgrowth, hydrogen-methane breath test, gut microbiota, microbial metabolites
Introduction
Gastroesophageal Reflux Disease (GERD) is one of the most common disorders of the digestive system, characterized by the reflux of gastric contents into the esophagus and, in some cases, into the throat, mouth, or lungs. This condition leads to various discomforting symptoms, including acid reflux and heartburn, which are commonly observed in affected individuals.1 The prevalence of GERD is influenced by a variety of factors, such as geographic region, demographic characteristics, and the methods used in research. Generally, the incidence of GERD is higher in Western countries, while it is lower in Asia and Africa.2 However, in recent years, the incidence of GERD in the Asia-Pacific region has been on the rise.3 According to the American College of Gastroenterology (ACG), approximately 15% to 20% of adults experience gastroesophageal reflux at least once a week. GERD can be classified into two main categories: reflux esophagitis (RE), characterized by mucosal erosion or ulceration of the esophagus, and non-erosive reflux disease (NERD), in which no mucosal injury is observed. The clinical presentation of GERD is diverse, with typical symptoms including acid reflux and heartburn. Non-specific symptoms, such as cough, a burning sensation behind the sternum, the sensation of a foreign body in the throat, and chest pain, are also common. The pathogenesis of GERD involves multiple factors, including dysfunction of the lower esophageal sphincter (LES), abnormal gastric acid secretion, increased sensitivity of the esophageal mucosa to gastric acid, and anatomical abnormalities between the diaphragm and esophagus. In addition, factors such as smoking, obesity, age, alcohol consumption, the use of nonsteroidal anti-inflammatory drugs (NSAIDs), pregnancy, social factors, psychosomatic conditions, and genetic predispositions are recognized as important risk factors for the development of GERD.4 Recent studies have suggested a potential link between GERD and alterations in the gastrointestinal microbiota.5 GERD patients often exhibit impaired gastric acid secretion and abnormal gastric pH, which may facilitate the translocation of bacteria into the small intestine, leading to Small Intestinal Bacterial Overgrowth (SIBO). The dysbiosis of the gut microbiota triggers inflammatory responses, which can further damage the esophageal mucosa. The overgrowth of bacteria associated with SIBO increases metabolic activity in the intestinal lumen, resulting in motility disorders and gas accumulation. These factors, in turn, can delay small bowel transit, impair gastric emptying, and exacerbate or even trigger episodes of esophageal reflux.6
The gut microbiota plays a crucial role in maintaining human health, particularly in areas such as immune regulation, metabolism, digestion, and nutrient absorption.7 Under normal conditions, the small intestine remains relatively sterile, mainly due to the action of digestive fluids like gastric acid and bile,8 as well as the protective barrier properties of the intestinal mucosa, which together inhibit bacterial overgrowth. However, when an imbalance in the gut microbiota occurs, it can lead to the excessive growth of bacteria in the small intestine, a condition known as SIBO. SIBO is characterized by an abnormal increase in the number and/or types of bacteria in the small intestine, typically defined as a bacterial count of ≥ 105 CFU/mL.9 The clinical symptoms of SIBO commonly include abdominal pain, bloating, diarrhea, and irregular bowel movements.9 Currently, the “gold standard” for diagnosing SIBO involves the extraction and culture of small intestinal fluid. However, due to the invasive nature of this method, its clinical application is limited.10 As a result, the hydrogen-methane breath test (H2-CH₄ breath test) is widely recommended as a non-invasive diagnostic tool for SIBO by the ACG. This test involves the oral administration of glucose or lactulose to the patient on an empty stomach, followed by continuous monitoring of H2 and CH₄ concentrations in exhaled breath. An abnormal rise in either hydrogen or methane levels suggests the presence of SIBO.11 When SIBO occurs, the overgrown bacteria ferment undigested food, producing gases such as hydrogen and methane. The accumulation of these gases in the small intestine not only leads to bloating but also increases intestinal pressure. This increased pressure may, in turn, promote the reflux of gastric contents into the esophagus, particularly when the function of the LES is compromised. This exacerbates the symptoms of GERD.11 Therefore, there is a significant interrelationship between SIBO and GERD, and the two conditions are often observed together in clinical practice.
The aim of this study is to address several key gaps in the existing literature. Although previous research12–14 has explored the individual characteristics of GERD and SIBO, as well as the association between SIBO and gut microbiota dysbiosis, most of these studies15 have primarily focused on the increase in bacterial numbers in the small intestine. They have lacked in-depth analysis of the structural composition of the small intestinal microbiota, particularly in the context of comorbidities such as GERD and SIBO. This study represents the first comprehensive analysis of the relationship between GERD and SIBO, investigating the interaction between the two conditions and the potential role of gut microbiota in this relationship. In contrast to previous studies that have examined SIBO or GERD separately, our study combines both conditions to identify common pathological mechanisms and potential mutual influences. By analyzing the differences in microbiota between GERD and SIBO patients, we aim to uncover shared pathological mechanisms, providing new theoretical insights for the diagnosis, prediction, and treatment of GERD and SIBO in clinical practice. Unlike previous research, we also incorporate an analysis of gut microbiota and its metabolites to further explore the relationship between microbiota changes and disease onset. This approach opens up new directions for the clinical application of microbiota-based therapies in GERD and SIBO.
Moreover, there remains significant controversy regarding the relationship between gastric acid-suppressing medications (such as proton pump inhibitors [PPIs], H2-receptor antagonists[H2RAs], and potassium-competitive acid blockers [PCABs]) and SIBO.6,16,17 While some studies suggest that acid-suppressive drugs may promote bacterial overgrowth in the small intestine by reducing gastric acid secretion, altering the intestinal environment, and slowing intestinal motility, the underlying mechanisms are not fully understood. Furthermore, the effects of these medications on SIBO may vary considerably between individuals and under different clinical conditions. Current research primarily focuses on the impact of acid-suppressive medications on the small intestinal microbiota, but the discrepancies in drug types, dosages, and treatment durations have led to inconsistent findings in this field. Therefore, the aim of this study is to systematically investigate the relationship between the use of gastric acid-suppressing medications and the development of SIBO. We specifically focus on factors such as the type of medication, dosage, and duration of use to assess their potential roles in the onset of SIBO. Through a comprehensive analysis, we aim to clarify the mechanisms by which acid-suppressive drugs influence SIBO development, providing clearer guidance for clinical practice and offering a theoretical basis for the formulation of personalized treatment strategies.
Materials and Methods
Study Subjects
This study included patients who visited or were hospitalized at the Department of Gastroenterology, Beijing Shijitan Hospital, between February 2021 and November 2023.
Inclusion Criteria
Retrospective Study Inclusion Criteria
Patients who visited or were hospitalized between February 2021 and September 2023 were screened based on the following criteria:
Age ≥ 18 years;
Underwent hydrogen-methane breath test examination;
Completed gastroscopy examination;
Completed the survey questionnaire and had complete medical history records.
Case-Control Study Inclusion Criteria
Patients who visited or were hospitalized between September 2023 and November 2023 were screened based on the following criteria:
SIBO-negative, Non-GERD group: No subjective symptoms, and no abnormalities found in gastroscopy or hydrogen-methane breath test.
SIBO-positive, Non-GERD group: Positive hydrogen-methane breath test, but did not meet GERD diagnostic criteria.
SIBO-positive, GERD group: Positive hydrogen-methane breath test, diagnosed with GERD.
Exclusion Criteria
Patients were excluded based on the following criteria:
History of gastrointestinal or other abdominal surgeries, renal dysfunction, or other infectious diseases affecting areas outside the gastrointestinal system;
Comorbidities such as inflammatory bowel disease, irritable bowel syndrome, hypothyroidism, intestinal obstruction, Cushing’s syndrome, or other conditions that may affect gastrointestinal function;
Use of antibiotics or medications that affect gastrointestinal motility (eg, domperidone) in the past month;
History of enema treatment or acute enteritis within the past week, or presence of chronic diarrhea, malabsorption, or other gastrointestinal conditions;
Pregnant or lactating women;
History of lactose intolerance;
Failure to comply with the study protocol, lack of informed consent, incomplete personal information that could impact the study’s outcome, or inability to cooperate in completing the study.
Data Collection and Research Methods
Collection of Basic Data
A total of 512 patients completed both gastroscopy and hydrogen-methane breath tests. After excluding patients who did not meet the inclusion criteria, 394 patients were enrolled in the retrospective study. The basic data of the enrolled patients were collected, including age, sex, height, weight, and body mass index (BMI), calculated as BMI = weight (kg) / height (m²). Additional data collected included lifestyle factors (such as smoking and alcohol consumption), medical history (such as hypertension and diabetes), family history, and surgical history. All enrolled patients completed a structured survey questionnaire with the assistance of an attending physician. The questionnaire included the GERD-Q scale. The GERD-Q scale (Table 1) consists of four positive symptoms (heartburn, acid reflux, sleep disturbances, and the use of over-the-counter medications) and two negative symptoms (upper abdominal pain and nausea). Each item was scored on a scale of 0 to 3 based on the frequency of symptoms over the past week, and the total score was the sum of the scores for the six symptoms.
Table 1.
GERD Q Scale
| The frequency of the following symptoms occurring in the past one week | Symptom frequency (score) | |||
|---|---|---|---|---|
| 0 day | 1 day | 2–3 days | 4–7 days | |
| Burning sensation behind the sternum | 0 | 1 | 2 | 3 |
| Gastric contents reflux to the throat or oral cavity | 0 | 1 | 2 | 3 |
| Middle upper abdominal pain | 3 | 2 | 1 | 0 |
| nausea | 3 | 2 | 1 | 0 |
| Affects sleep due to heartburn and/or reflux | 0 | 1 | 2 | 3 |
| In addition to the medication recommended by the doctor, additional medications (such as calcium carbonate, aluminum hydroxide, etc.) are taken to alleviate heartburn and reflux | 0 | 1 | 2 | 3 |
Note: Total score ≥ 8 points for suspected GERD diagnosis.
All 394 subjects were instructed to fast after dinner on the day before blood sample collection to ensure a fasting period of 6 to 12 hours. The blood samples were collected in the early morning under fasting or resting conditions. A total of 1–2 mL of venous whole blood was drawn and placed in an anticoagulant tube for immediate analysis. The tests included alanine aminotransferase (ALT), aspartate aminotransferase (AST), glucose (Glu), total cholesterol (TC), electrolytes (calcium, iron), fasting triglycerides (TG), uric acid (UA), high-density lipoprotein (HDL), low-density lipoprotein (LDL), and other relevant markers. The analysis was conducted using the XS-1000i system (Roche Diagnostics; Shenzhen, China).
Examination Methods and Diagnostic Criteria
Hydrogen-Methane Breath Test
All participants were instructed to fast for 6–12 hours before undergoing the hydrogen-methane breath test. The preparation for the lactulose hydrogen-methane breath test (Quintron Corporation, United States) included the following steps:
Participants were instructed to avoid foods that are slow to digest, high in fiber, or produce excessive hydrogen, such as pasta, beans, and cereals, and to refrain from overeating on the day before the test.
Medication Restrictions: Participants were advised not to take any sedatives or sleep aids the day prior to the test.
Smoking was prohibited for at least one hour before the test.
Participants were required to rinse their mouth thoroughly, brush their teeth, and ensure oral cleanliness before the test.
Test Procedure
Participants began by taking several deep, calm breaths. Following this, they were instructed to inhale deeply through a disposable mouthpiece and exhale slowly. The first breath sample was collected in a bag labeled as “Bag 1” after a baseline measurement. Next, 10 mL of lactulose (10 mL per bottle, Abbott, Netherlands) was orally administered, and the timing began immediately. Subsequent breath samples were collected at 30-minute intervals, with a total of 6 breath samples taken. For accurate results, participants were instructed to ensure a full exhalation into each collection bag. To reduce bacterial interference, participants were advised to rinse their mouth immediately after taking the lactulose.
SIBO Positive Diagnostic Criteria
According to the 2020 American College of Gastroenterology (ACG) consensus,18 the criteria for diagnosing SIBO positivity are as follows:
An increase in H2 concentration of more than 20 ppm from the baseline within 90 minutes after oral lactulose intake.
A CH₄ concentration greater than 10 ppm at any time point after oral lactulose intake.
A positive result is confirmed if either of the above criteria is met.
Gastroscopy Examination
All patients underwent gastroscopy using a video endoscope (GIF-H260/H290; Olympus, Tokyo, Japan) to examine the esophagus, stomach, and duodenum. The endoscopic procedure assessed the appearance of the esophageal and gastric mucosa, the degree of mucosal damage, and other relevant findings. Representative images were captured, and the degree of mucosal injury was evaluated by experienced attending physicians and associate chief physicians. The severity of damage was classified based on the Los Angeles (LA) classification.
GERD Diagnostic Criteria
Based on the 2020 Chinese Consensus on the Diagnosis and Treatment of GERD,19 the following criteria were used to diagnose GERD:
Endoscopy and Biopsy: Evidence of reflux-induced inflammation or Barrett’s esophagus confirmed by endoscopy and histological examination.
24-hour Esophageal pH Monitoring: Detection of abnormal acidic and/or alkaline reflux in the esophagus using 24-hour esophageal pH monitoring.
PPIs Treatment: Significant improvement in symptoms (eg, acid reflux, heartburn) after 1–2 weeks of PPIs therapy, with confirmation of reflux-induced esophageal inflammation on endoscopy after 2–4 weeks.
GERD-Q Questionnaire: A score of ≥8 on the GERD-Q questionnaire is suggestive of GERD.
Any one of these criteria can be used to diagnose GERD.
Endoscopic Classification of GERD
The patients underwent gastrointestinal preparation as required, and the endoscopic examination was performed by our department’s endoscopy center. Based on the endoscopic findings, GERD was classified into two types:20
NERD: No visible mucosal damage in the esophagus on endoscopy, but the patient reports reflux and heartburn symptoms.
RE: Visible mucosal damage in the esophagus caused by reflux. The damage is classified using the LA grading system:
LA-A: One or more mucosal breaks, each less than 5 mm in length.
LA-B: One or more mucosal breaks, with the longest being greater than 5 mm.
LA-C: Mucosal breaks that are confluent but cover less than 75% of the esophageal circumference.
LA-D: Mucosal breaks that are confluent and cover at least 75% of the esophageal circumference.
Barrett’s Esophagus: Columnar epithelium visible on endoscopy, with histological confirmation via biopsy.
Fecal Sample Collection and Detection Method
Based on propensity score matching, a 1:2 ratio of SIBO-negative to SIBO-positive patients was used to select 30 hospitalized patients. 6 Fecal samples were excluded due to quality control issues. Ultimately, 24 patients were included in the study, comprising 5 SIBO-negative patients and 19 SIBO-positive patients. Fresh stool samples (approximately 1–3g) were collected from the middle portion of the stool using a fecal collection kit (Faeces tube 76x20m; Sarstedt Ag & Co. Kg, Germany). The samples were delivered to a −20°C freezer within 2 hours of collection, and then transferred to a −80°C freezer within 24 hours. Informed consent was obtained from all participants prior to enrollment. The study was approved by the Ethics Committee of Beijing Shijitan Hospital, Capital Medical University (Approval No.: sjtky11-1x-2022 (063)).
DNA Extraction and 16S rRNA Gene Amplification
Total DNA was extracted from the stool samples using the TIANamp Stool DNA Kit (TIANGEN, China). The extracted DNA was assessed for quality by 1% agarose gel electrophoresis and quantified using the Nanodrop™ 2000 spectrophotometer (Thermo Scientific, USA). Specific primers targeting the 16S rRNA gene V3-V4 region were used for amplification (Primers: 341F-806R). The PCR amplification conditions were as follows: initial denaturation at 95°C for 3 minutes; denaturation at 95°C for 30 seconds; annealing at 55°C for 30 seconds; extension at 72°C for 45 seconds, repeated for 30 cycles; and final extension at 72°C for 8 minutes. Each sample underwent three independent PCR amplifications. The primers used were as follows: Primer 341F: 5’-CCTACGGGNGGCWGCAG-3’; Primer 806R: 5’-GGACTACHVGGGTWTCTAAT-3’.
After amplification, sequencing libraries were generated using the TIANSeq Fast DNA Library Prep Kit (Illumina, TIANGEN Biotech). The quality of the libraries was assessed using the Qubit™ 2.0 Fluorometer (Thermo Scientific) and the Agilent Bioanalyzer 2100 system. The libraries were then sequenced on the Illumina platform using a 2×250 bp paired-end protocol.
Data Analysis and Microbial Community Reconstruction
For data analysis, the Short Multiple Regions Framework (SMURF) method was employed to reconstruct the microbial community based on 16S rRNA gene sequencing data. SMURF first preprocesses high-throughput sequencing data from multiple amplified regions by filtering out low-quality sequences. Then, k-mers (short nucleotide fragments) are extracted from each amplified region, which overlap between regions and reflect the community composition. SMURF uses the Expectation-Maximization (EM) algorithm to analyze the k-mer set and infer the most likely species composition. Species classification for each sequence is assigned by the majority voting principle. The final output of SMURF includes species classification and relative abundance for each 16S rRNA “group”, providing data support for subsequent community structure and diversity analyses. This method integrates data from multiple amplification regions, enhancing the accuracy of species classification.21–23
Metabolomics Analysis
Approximately 100 mg (±1 mg) of human fecal samples were weighed and then mixed with 500 μL of extraction solvent (methanol: acetonitrile: water, 2:2:1, v/v), containing deuterated internal standards. The mixture was vortexed for 30 seconds, homogenized at 35 hz for 4 minutes, and subjected to ultrasonic treatment in a 4°C water bath for 5 minutes. This step was repeated three times. The samples were incubated at −40°C for 1 hour to precipitate proteins. Subsequently, the samples were centrifuged at 12,000 rpm (relative centrifugal force = 13,800 × g, radius = 8.6 cm) at 4°C for 15 minutes. The supernatant was carefully transferred into new glass vials for analysis. For LC-MS/MS analysis, an UHPLC system (Vanquish, Thermo Fisher Scientific) was employed, coupled with a Waters ACQUITY UPLC BEH Amide column (2.1 mm × 50 mm, 1.7 μm) and connected to an Orbitrap Exploris 120 mass spectrometer (Orbitrap MS, Thermo). The mobile phase consisted of 25 mmol/L ammonium acetate and 25 mmol/L ammonium hydroxide (pH = 9.75) (A) and acetonitrile (B). The autosampler was maintained at 4°C, and the injection volume was 2 μL. The Orbitrap Exploris 120 mass spectrometer acquired MS/MS spectra in Information Dependent Acquisition (IDA) mode, using Xcalibur software (Thermo). In this mode, the software continuously evaluated full-scan MS spectra. The ESI source conditions were as follows: sheath gas flow rate = 50 Arb, auxiliary gas flow rate = 15 Arb, capillary temperature = 320°C, full MS resolution = 60,000, MS/MS resolution = 15,000, collision energy = SNCE 20/30/40, and spray voltage = +3.8 kV (positive) or −3.4 kV (negative). Raw data were converted to mzXML format and analyzed using an internal program developed in R software (version 3.6.2) and XCMS (version 3.5.1) for peak detection, extraction, alignment, and integration. Metabolite identification was carried out using R and BiotreeDB (V3.0).24
Bioinformatics and Statistical Analysis
Basic Information Statistical Analysis
Statistical analysis in this study was conducted using SPSS (version 23.0, IBM Corp., Armonk, NY, USA). The Kolmogorov–Smirnov (K-S) test was used to verify whether the data followed a normal distribution. Continuous variables are presented as mean ± standard deviation, while categorical variables are expressed as percentages. Baseline characteristics and group variables were compared using t-tests for continuous variables and Fisher’s exact test for categorical variables. Chi-square tests were employed to compare the GERD prevalence and digestive system symptoms between SIBO-negative and SIBO-positive groups. Logistic regression and multiple linear regression were used to calculate the multivariable-adjusted odds ratios (OR) and 95% confidence intervals (CI) for SIBO. A two-tailed p-value < 0.05 was considered statistically significant.
Microbiota Analysis
For microbiome analysis, the 16S rRNA gene sequencing of fecal samples was performed to assess the microbial community composition. The composition of individual and group samples was analyzed using Chiplot tools to explore the microbial distribution within individual samples and between groups. All data analyses were carried out using R software (version 3.6.2). Both Alpha diversity and Beta diversity analyses were performed to assess species diversity and abundance. Alpha diversity metrics, including Observed Species, Chao, Shannon, and Simpson indices, were used to evaluate the species richness and evenness within individual samples. These indices reflect the number of different species and their distribution within a given sample. Beta diversity analysis was performed to assess the differences in microbial composition between samples. Prior to clustering, Principal Component Analysis (PCA) was conducted to reduce the dimensionality of the data. PCA was implemented using the stat package and ggbiplot in R. Subsequently, Principal Coordinates Analysis (PCoA) was used to further visualize the differences between samples, and ade and ggplot2 packages in R were used to generate two-dimensional PCoA plots. To further analyze the individual classification and functional annotation differences between groups, Linear discriminant analysis Effect Size (LEfSe) was performed using the Lianchuan BioCloud Platform (2019–2023, Lianchuan Biotechnology Co., Ltd., Hangzhou, China). The LDA score threshold was set to 2. This method identifies biomarkers between different groups and evaluates the effect size and consistency through quantitative analysis and statistical significance tests.25 LEfSe can process data where the number of species or functional annotations is much higher than the sample size and provides biological interpretation of the results. This analysis allows a comprehensive assessment of microbial community differences between groups and aids in understanding their relationship to experimental conditions.
Microbiota Metabolic Analysis
For metabolomics, PERMANOVA (Permutational Multivariate Analysis of Variance) was used to analyze the variance in metabolites across the three groups. PCA and PCoA were conducted to extract the main components from independent variables (X) and dependent variables (Y), calculating their correlations. Orthogonal Partial Least Squares Discriminant Analysis (OPLS-DA) was then applied for further analysis of metabolites. t-tests were used to identify differential metabolites, and the fold change (FC) between groups was calculated. The thresholds for differential metabolites were set to VIP > 0.0, FC > 2.0, and p-value < 0.05. Data visualization, including volcano plots and heatmaps, was performed using Metware Cloud (https://cloud.metware.cn/) and was generated through Python (3.6.6) and R software. To further analyze the biological significance of differential metabolites, the KEGG, HMDB, and LIPID Maps databases were used for metabolite annotation. Metabolites significantly enriched in KEGG metabolic pathways were identified, with a p-value < 0.05 considered as the threshold for significant enrichment.
Correlation Analysis
In the correlation analysis between the gut microbiota and metabolites, Spearman’s rank correlation analysis was used to examine the linear relationship between changes in the microbiome and metabolites. All statistical analyses were performed within the R software (version 3.6.2) environment, and correlations were computed using the same software. This method provided reliable statistical support for further investigating the interactions between the microbiome and metabolites.
Result
Retrospective Study Results
Clinical Demographics and Correlation Analysis of GERD and SIBO
A total of 512 patients completed both gastroscopy and hydrogen methane breath tests, with 118 patients excluded for not meeting the inclusion criteria. Ultimately, 394 patients were included in the study, with their basic demographic data shown in Table 2. Among these patients, 287 (72.8%) were diagnosed with SIBO. Of these, 97 (24.6%) were positive for hydrogen, and 270 (68.5%) were positive for methane on the breath test. The correlation between GERD and SIBO was significant, with a p-value of 0.007, suggesting a notable relationship between the two conditions. Additionally, a significant association was observed between GERD and SIBO-CH₄ with a p-value of 0.020.
Table 2.
Comparison of Basic Information and Serum Biomarkers Between GERD and Non-GERD Patients
| Total | GERD | non-GERD | p value | |
|---|---|---|---|---|
| Age (years) | 55.32 ± 12.397 | 55.590 ± 12.850 | 55.160 ± 12.140 | 0.743 |
| Gender, n (%) | Male | 84 (21.3%) | 118 (29.9%) | 0.097 |
| Female | 64 (16.3%) | 128 (32.5%) | ||
| BMI (kg/m2) | 24.69 ± 4.174 | 24.771 ± 5.185 | 24.641 ± 3.436 | 0.786 |
| SIBO, n (%) | 287 (72.8%) | 191 (48.5%) | 96 (24.4%) | 0.007 |
| SIBO-H2, n (%) | 97 (24.6%) | 62 (15.7%) | 35 (8.9%) | 0.728 |
| SIBO-CH4, n (%) | 270 (68.5%) | 179 (45.4%) | 91 (23.1%) | 0.020 |
| WBC (*1012 /L) | 5.97 ± 1.852 | 6.019 ± 1.53 | 5.941 ± 2.023 | 0.665 |
| ALT (U/L) | 22.665 ± 15.395 | 23.358 ± 17.955 | 22.248 ± 13.649 | 0.518 |
| AST (U/L) | 20.551 ± 8.816 | 20.872 ± 9.01 | 20.358 ± 8.711 | 0.579 |
| GGT (U/L) | 28.553 ± 25.16 | 30.318 ± 28.191 | 27.492 ± 23.143 | 0.305 |
| Alb (g/L) | 42.093 ± 4.364 | 42.443 ± 4.096 | 41.882 ± 4.512 | 0.206 |
| UA (umol/l) | 339.462 ± 86.567 | 351.128 ± 87.993 | 332.443± 85.109 | 0.040 |
| TC (mmol/L) | 4.939 ± 1.343 | 4.927 ± 1.375 | 4.947 ± 1.327 | 0.884 |
| TG (mmol/L) | 1.462 ± 0.709 | 1.427 ± 0.597 | 1.483 ± 0.768 | 0.416 |
| Glu (mmol/L) | 6.291 ± 2.329 | 6.364 ± 2.330 | 6.247 ± 2.332 | 0.628 |
| HDL-C (mmol/L) | 1.171 ± 0.268 | 1.165 ± 0.246 | 1.175 ± 0.281 | 0.711 |
| LDL-C (mmol/L) | 3.008 ± 0.787 | 3.005 ± 0.757 | 3.010 ± 0.805 | 0.957 |
| Smoking, n (%) | 48 (12.2%) | 22 (5.6%) | 26 (6.6%) | 0.135 |
| Drinking alcohol, n(%) | 51 (12.9%) | 26 (6.6%) | 25 (6.3%) | 0.046 |
| Hypertension, n (%) | 95 (24.1%) | 32 (8.1) | 63 (16%) | 0.220 |
| Diabetes, n (%) | 44 (11.2%) | 14 (3.6%) | 30 (7.6%) | 0.254 |
Note: Bold indicates statistically significant values(P < 0.05).
The average age of the 394 patients was 55.32±12.40 years. No significant age differences were observed between those with and without SIBO. The gender distribution was 51.2% male and 48.8% female, with no significant gender differences between SIBO-positive and SIBO-negative groups (P=0.097). The mean BMI was 24.69±4.17, with no statistically significant differences between SIBO-positive and SIBO-negative patients (P=0.786). When analyzing the patients’ medical history, excessive alcohol consumption was found to increase the risk of GERD (P=0.046), which was statistically significant.
Subgroup Analysis of GERD Type and SIBO Association
To further investigate the relationship between GERD and SIBO, a subgroup analysis was performed based on the GERD types and SIBO positivity (H2 or CH4 positive). The results showed that SIBO-CH₄ patients had a more significant association with GERD compared to SIBO-H2 patients (P=0.074, P=0.020).
However, no significant correlation was observed between the severity of GERD and the presence of SIBO-CH₄. This suggests that while methane-positive SIBO is more likely to be associated with GERD, the severity of GERD does not significantly correlate with the methane-positive SIBO status (Tables 2 and 3).
Table 3.
Correlation Analysis Between Different Type of GERD and SIBO
| SIBO type | Non-GERD n (%) |
NERD n (%) |
RE (LA-A) n (%) |
RE (LA-B) n (%) |
RE (LA-C) n (%) |
p value |
|---|---|---|---|---|---|---|
| SIBO+ | 191(48.5%) | 31(7.9%) | 56(14.2%) | 8(2.0%) | 1 (0.3%) | 0.024 |
| SIBO- | 55(14.0%) | 21(5.3%) | 24(6.1%) | 6(1.5%) | 1 (0.3%) | |
| SIBO-CH4+ | 179 (45.4%) | 30 (7.6%) | 53 (13.5%) | 7 (1.8%) | 1 (0.3%) | 0.074 |
| SIBO-CH4- | 67 (17.0%) | 22 (5.6%) | 27 (6.9%) | 7 (1.8%) | 1 (0.3%) | |
| SIBO-H2- | 184 (46.7%) | 38 (9.6%) | 61 (13.5%) | 12 (3.0%) | 2 (0.5%) | 0.913 |
| SIBO-H2+ | 62 (15.7%) | 14 (3.6%) | 19 (4.9%) | 2 (0.6%) | 0 (0.0%) |
Note: Bold indicates statistically significant values(P < 0.05).
Impact of Acid-Suppressing Medications on SIBO
To explore the potential effects of acid-suppressing medications on SIBO, this study conducted a stratified analysis based on the type of medication, dosage, and duration of use. Specifically, the usage of PPIs, H2RAs, and PCABs was analyzed. The results indicated that there was no significant correlation between the use of these medications and the occurrence of SIBO: PPIs usage: P = 0.614; H2RAs usage: P = 0.680; PCABs usage: P = 0.133. Additionally, further stratified analysis showed that neither the dosage (P = 0.847) nor the duration of use (P = 0.807) was significantly associated with the incidence of SIBO. Table 4 presents these findings, which suggest that the type, dosage, and duration of acid-suppressing medications, such as PPIs, H2RAs, and PCABs, do not have a statistically significant impact on the development of SIBO in the study population. However, compared to PPIs and H2RAs, PCABs have a more pronounced effect on SIBO.
Table 4.
Analysis of the Correlation Between Acid-Suppressing Medications and SIBO
| Total | SIBO- | SIBO+ | p value | ||
|---|---|---|---|---|---|
| Patients using acid-suppressing medications, n (%) | 180 (45.8%) | 47 (12.0%) | 133 (33.8%) | 0.648 | |
| Duration of use of acid-suppressing medications (day) | 9.568±13.165 | 9.813±14.632 | 9.448±12.596 | 0.807 | |
| Dosage of acid-suppressing medications (mg/d) | 10.635±14.173 | 10.561±14.655 | 10.662±14.015 | 0.847 | |
| Types of acid-suppressing medications | PPIs | 150 (38.2%) | 43 (10.9%) | 107 (27.2%) | 0.614 |
| P-CABs | 27 (6.9%) | 4 (1.0%) | 23 (5.9%) | 0.133 | |
| H2RAs | 3 (0.8%) | 0 (0%) | 3 (0.8%) | 0.680 | |
Risk Factors for SIBO
To identify the risk factors for SIBO, we developed a new regression model (Table 5), which included known risk factors for SIBO identified in the literature, as well as variables that showed significant results in the univariate logistic regression analysis. These variables included the use of acid-suppressing medications, BMI, smoking, alcohol consumption, and the presence of GERD. The results of the regression analysis indicated that GERD was an independent risk factor for SIBO. Specifically, the OR for the association between GERD and SIBO was 0.508 (95% CI: 0.319–0.809, P=0.004) (Table 5).
Table 5.
Univariate and Multivariate Logistic Regression of SIBO Risk Factors
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Risk factors | OR | 95% CI | p value | OR | 95% CI | p value |
| BMI (kg/m2) | 0.976 | 0.928–1.026 | 0.336 | 0.977 | 0.928–1.029 | 0.383 |
| GERD | 3.473 | 0.338–0.835 | 0.006 | 0.508 | 0.319–0.809 | 0.004 |
| TC (mmol/L) | 0.855 | 0.726–1.007 | 0.061 | 0.849 | 0.719–1.003 | 0.055 |
| Smoking | 0.790 | 0.417–1.495 | 0.469 | 1.016 | 0.442–2.336 | 0.970 |
| Drinking alcohol | 0.893 | 0.459–1.738 | 0.738 | 0.891 | 0.404–1.964 | 0.774 |
| Whether use gastric acid suppressants | 1.110 | 0.710–1.735 | 0.648 | 1.248 | 0.786–1.982 | 0.347 |
Note: Bold indicates statistically significant values(P < 0.05).
Distribution of Gut Microbiota
Basic Characteristics of Gut Microbiota in the Population
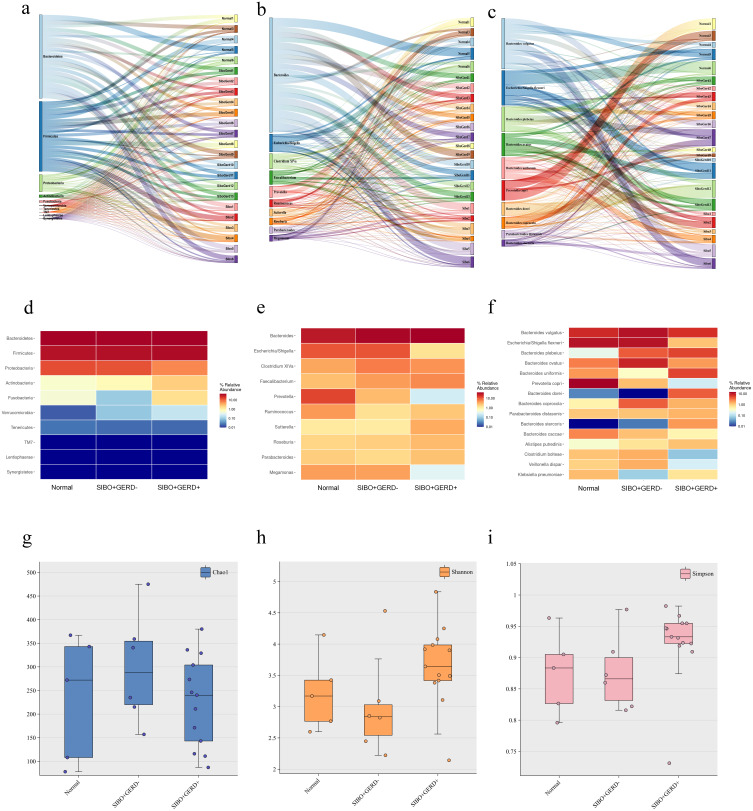
This study selected 30 patients, 10 of whom were SIBO-negative and 20 SIBO-positive, based on propensity score matching (1:2 ratio). However, 6 fecal samples were excluded due to quality control issues, leaving a total of 24 patients included in the final analysis. Ultimately, 24 patients were included in the study, comprising 5 SIBO-negative patients and 19 SIBO-positive patients. These patients were screened from hospitalized cases, and fecal samples were collected for microbiota analysis. The average number of OTUs (Operational Taxonomic Units) in the normal population was 865, while the OTUs in SIBO-negative patients averaged 882. In contrast, patients with SIBO-positive and GERD exhibited a significant increase in OTUs, with an average of 1677. Among the three groups, 326 shared OTUs were identified. At the phylum level (Figure 1a and d), the predominant phyla in all groups were Bacteroidetes, Firmicutes, and Proteobacteria. However, in SIBO patients with GERD, the abundance of Proteobacteria, Actinobacteria, and Fusobacteria was significantly elevated, while the abundance of Verrucomicrobia was notably decreased. In the genus-level analysis (Figure 1b and e), the relative abundance of Escherichia-Shigella, Prevotella, and Miegamonas was significantly reduced in SIBO patients with GERD, while the abundance of the Bacteroides genus increased significantly. At the species level (Figure 1c and f), specific species of Bacteroides, such as bacteroides plebeius and bacteroides uniformis, exhibited a significant increase in SIBO patients with GERD, while the abundance of species such as Escherichia Shigella flexneri and Prevotella copri was significantly reduced.
Figure 1.
Distribution of intestinal microbiome in a single sample at the phylum level (a), top 10 genera by abundance (b), and top 15 species by abundance (c). (d) Distribution of intestinal microbiome at the phylum level for each of the three groups, (e) top 10 genera by abundance, and (f) top 15 species by abundance. (g) Alpha diversity based on the Chao1 index between groups; (h) alpha diversity based on the Shannon index between groups; (i) alpha diversity based on the Simpson index between groups.
Alpha and Beta Diversity of Gut Microbiota
We calculated the alpha diversity of the gut microbiota and found that, compared to the normal population, fecal samples from SIBO-positive and GERD-positive patients did not show significant differences in the Chao1 index, Shannon diversity index, or Simpson index (Figure 1g–i). These results suggest that SIBO and GERD do not significantly alter the within-sample microbial diversity, as measured by these common diversity indices.
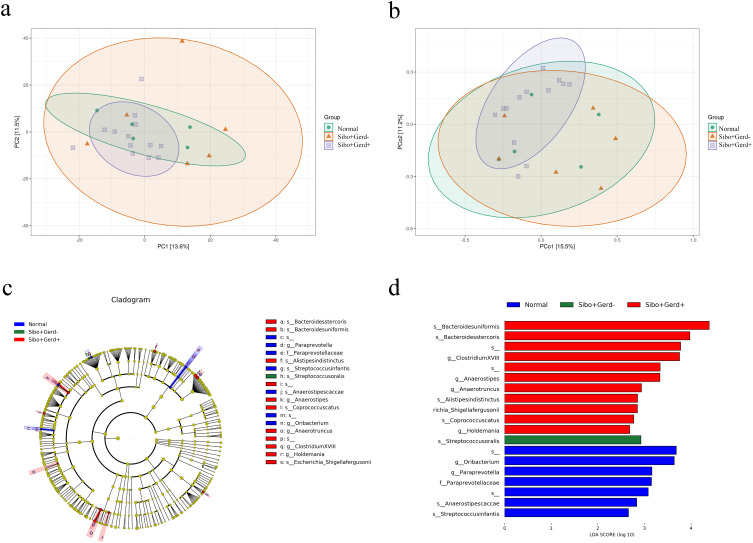
To further evaluate the differences in species complexity among the samples, we conducted beta diversity analysis. Prior to conducting cluster analysis, we applied PCA and PCoA to reduce the dimensionality of the original variables. The results showed significant differences in the fecal microbiota between SIBO patients and those with GERD. Specifically, PCA (P=0.021) and PCoA (P=0.048) analyses indicated that the combination of SIBO and GERD has a significant impact on the overall distribution of gut microbiota (Figure 2a and b).
Figure 2.
(a) Bray-Curtis-based β-diversity analysis; (b) Bray-Curtis-based PCoA (Principal Coordinates Analysis); (c and d) LEfSe analysis showing phylogenetic differences between groups. Only results with LDA > 2 are shown.
LefSe Analysis of Microbial Biomarkers
LefSe analysis identified several key microbial taxa as the main contributors to the distinct fecal microbiota profile in SIBO patients with GERD. These taxa included bacteroides uniformis, bacteroides stercoris, Anaerostipes, clostridium XVIII-, Anaerotruncus, and alistipes indistinctus (Figure 2c and d). The identification of these specific microorganisms suggests that they may play an important role in the dysbiosis of the gut microbiota observed in SIBO combined with GERD patients.
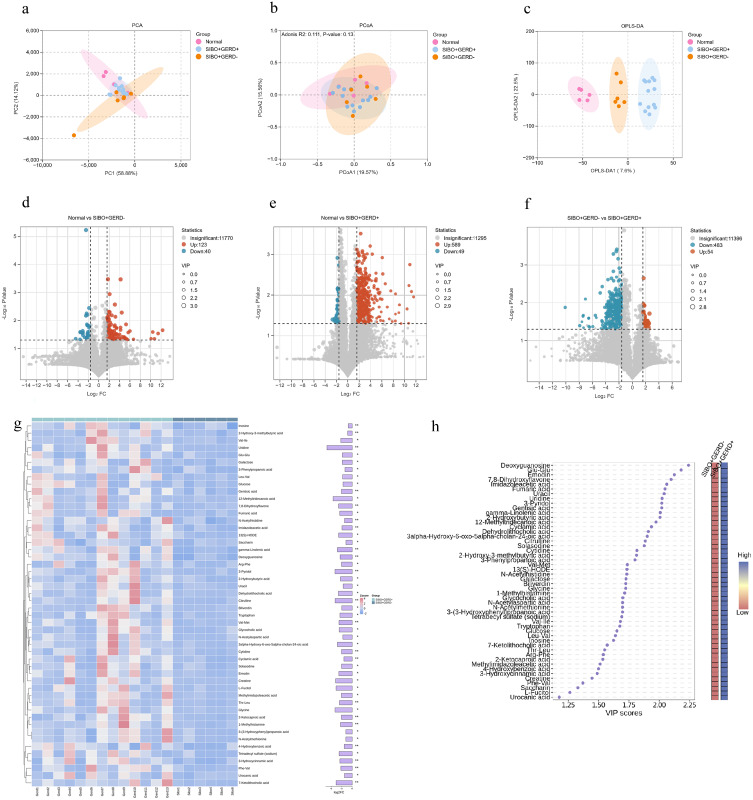
Basic Characteristics of Gut Microbiota Metabolites
PCA and PCoA analyses (Figure 3a and b) showed some differences in the gut microbiota metabolites between SIBO-positive patients, SIBO-positive patients with GERD, and the healthy control group, although these differences did not reach statistical significance. In contrast, OPLS-DA results revealed significant differences in the metabolic profiles of the gut microbiota between the SIBO combined with GERD group, the SIBO positive group, and the normal population (Figure 3c). These findings suggest that the metabolic characteristics of the gut microbiota undergo significant changes in SIBO and its combination with GERD.
Figure 3.
Metabolomic analysis of gut microbiota metabolites in each group (*: p < 0.05; **: p < 0.01). (a) PCA based on intestinal microbiome metabolites; (b) PCoA plot based on intestinal microbiome metabolites; (c) OPLS-DA analysis based on intestinal microbiome metabolites. Volcano plots of differential metabolites among the three groups: (d) healthy control group vs SIBO+GERD-; (e) healthy control group vs SIBO+GERD+; (f) SIBO+GERD- vs SIBO+GERD+. (g) Heatmap of differential metabolites. (h) VIP scores of differential metabolites and corresponding heatmap.
We further examined the differential metabolites among the normal population, SIBO-positive patients, and SIBO with GERD patients using volcano plot analysis (Figure 3d–f). The results revealed that 163 metabolites were differentially abundant between the Normal group and the SIBO-positive group, with 123 metabolites increased and 40 decreased in the SIBO group (Figure 3d). Between the Normal group and the SIBO combined with GERD group, 589 metabolites were differentially abundant, and 49 decreased in the SIBO + GERD group (Figure 3e). In the comparison between SIBO-positive group and SIBO + GERD group, 483 decreased and 54 increased in the SIBO + GERD group (Figure 3f). Further analysis using log-transformation identified the top 50 downregulated metabolites, including Inosine, 2-Hydroxy-3-methylbutyric acid, among others. The top 8 upregulated metabolites included Choline, Chenodeoxycholic acid 3-glucuronide, and others. These metabolites may be involved in the pathogenesis of SIBO and GERD (Figure 3g and h).
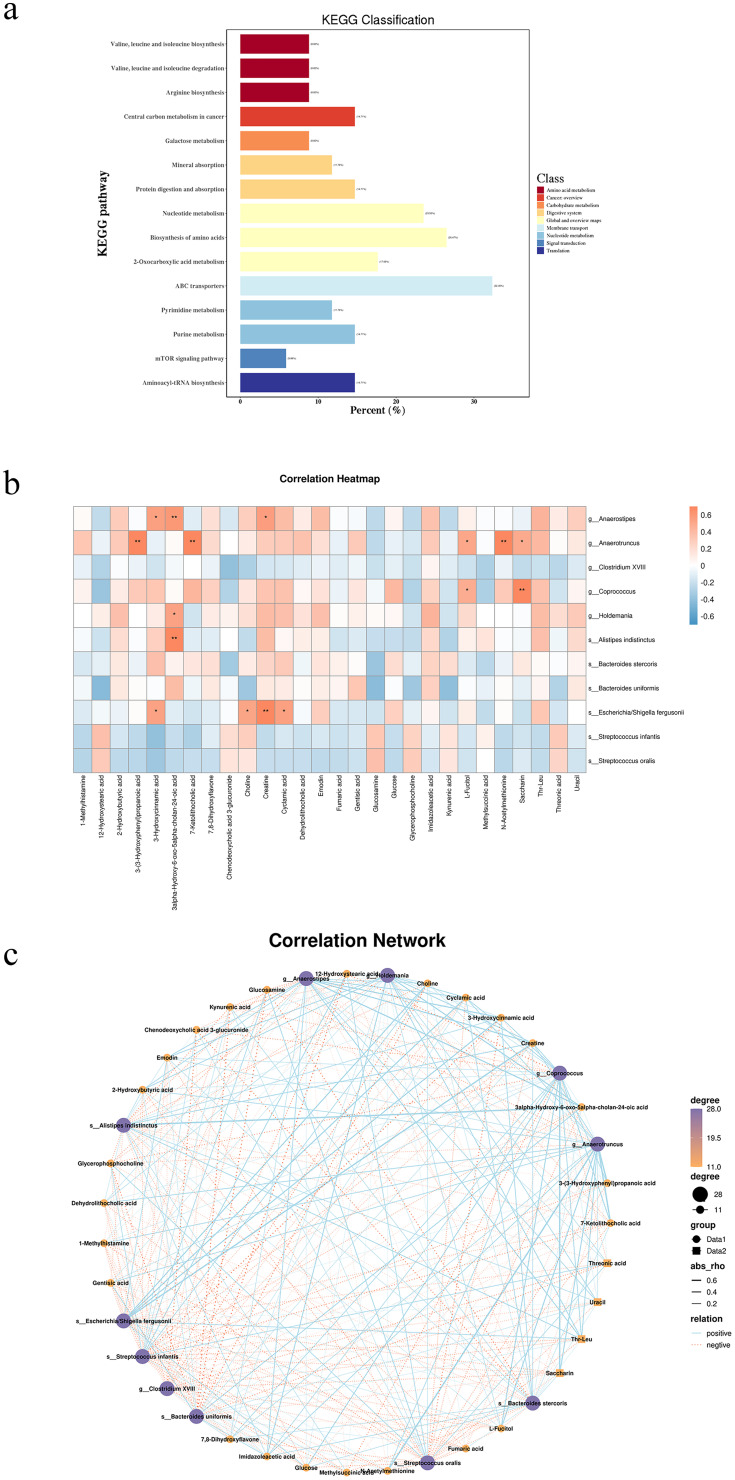
We also conducted a KEGG pathway annotation for the differential metabolites (Figure 4a). The top three enriched pathways were ABC transporters, amino acid biosynthesis, and nucleotide metabolism. These findings suggest that alterations in these metabolic pathways may provide new insights into the pathophysiological mechanisms of these diseases.
Figure 4.
(a) Annotated map of KEGG pathways for differentially abundant metabolites; (b) Heatmap of fecal microbes associated with metabolites. Rows represent differentially abundant microbes, with the right-hand legend providing correlation coefficients, where Orange denotes a positive correlation and blue denotes a negative correlation (*: P < 0.05, **: P < 0.01). (c) Network map of fecal microbes associated with metabolites. Rows represent differentially abundant microorganisms, with the right-hand legend providing correlation coefficients: blue solid line for positive correlation and orange dashed line for negative correlation.
Correlation Analysis Between Gut Microbiota and Metabolites
We further investigated the correlation between the gut microbiota and gut microbiota metabolites in SIBO-positive patients with GERD using Spearman’s rank correlation analysis (Figure 4b and c). The results revealed significant correlations between changes in gut microbiota metabolites and the abundance levels of various bacterial taxa in the gut. Specifically, 3alpha-Hydroxy-6-oxo-5alpha-cholan-24-oic acid was positively correlated with Holdemania and alistipes indistinctus, while Escherichia/shigella fergusonii showed a positive correlation with Choline, Creatine, and Cyclamic acid. These findings suggest that there is a complex interaction between the gut microbiota and its metabolites, which may play a crucial role in the pathogenesis and progression of SIBO and GERD.
Discussion
GERD is a chronic condition where stomach contents reflux into the esophagus, causing symptoms like heartburn, acid reflux, and regurgitation. It is primarily caused by dysfunction of the lower esophageal sphincter, which allows gastric acid to enter the esophagus. Chronic reflux can lead to complications like esophagitis, ulcers, and an increased risk of esophageal cancer. GERD’s causes include overeating, high-fat or spicy foods, obesity, smoking, alcohol use, and excessive NSAID use. Treatment generally involves acid-suppressing medications and lifestyle changes to manage symptoms and reduce acid production.26 An increasing body of evidence suggests that dysbiosis of the gut microbiota may contribute to the onset, progression, and symptomatology of GERD. Among these factors, SIBO is a key manifestation of microbial dysbiosis. SIBO is defined by an abnormal increase in the number or variety of bacteria in the small intestine.27 This condition is often associated with digestive symptoms such as bloating, abdominal pain, diarrhea, and malabsorption, which may further lead to nutritional deficiencies. SIBO is linked to various factors, including anatomical abnormalities of the digestive tract, impaired intestinal motility, and immune dysfunctions.28 However, the role of GERD, particularly in patients using gastric acid-suppressing medications, in the pathogenesis of SIBO is still controversial. Thus, our study aimed to investigate the correlation between SIBO and GERD, the changes in gut microbiota associated with these conditions, and the effects of acid suppressants on both.
In our study, we retrospectively analyzed data from patients who underwent gastroscopy and hydrogen methane breath tests over the past three years. Our findings showed that there is a significant difference in the hydrogen methane breath test positivity rate between GERD patients and non-GERD patients (P=0.007). Furthermore, the incidence of GERD in SIBO-positive patients was significantly higher than in SIBO-negative patients (24.4% vs 13.2%). These results are consistent with previous research suggesting a potential association between GERD and SIBO.29 Notably, subgroup analysis revealed that GERD was more closely associated with SIBO-CH4 than SIBO-H2, suggesting that GERD patients are more likely to develop methane-dominant SIBO. This observation suggests alterations in gastric pH and microbial composition in GERD, particularly in methane-producing subtypes. Our findings align with a study by Kim et al, which demonstrated a link between NERD (non-erosive reflux disease) and SIBO.29 Additionally, a randomized study found that elevated gastric pH is associated with an increased risk of developing SIBO.30 The potential mechanisms are outlined below: under normal conditions, the acidic environment of the stomach (pH 0.9–1.5) plays a crucial role in activating gastric proteases and eliminating most bacteria and pathogens. The secretion of bicarbonate and mucus by the gastric mucosa helps form a protective barrier against acid, maintaining a less acidic pH of 5.5–7 on the mucosal surface.29–32 In GERD patients, however, this protective barrier may be compromised due to increased gastric acid secretion and impaired sphincter function, potentially allowing bacteria to migrate from the stomach to the small intestine, contributing to SIBO.33
In addition, our study found a significant difference in hypercholesterolemia between SIBO-positive and SIBO-negative patients, suggesting that elevated cholesterol may contribute to the development of SIBO. Total cholesterol is involved in important physiological processes such as cell membrane structure, hormone synthesis, and bile acid synthesis. Previous studies34,35 have shown that SIBO can lead to hyperlipidemia by disrupting enterohepatic circulation, which regulates bile acid recycling. Additionally, hydrogen and methane production, as observed in breath tests, has been linked to lipid metabolism.35 High cholesterol may interfere with bile secretion and fat digestion, disrupting the intestinal environment and promoting the overgrowth of harmful bacteria, which may trigger SIBO.34 While previous research suggests associations between smoking, alcohol consumption, obesity, and SIBO,36 our study did not find a significant correlation. This may be due to the single-center design and relatively small sample size, which could limit the ability to control for confounding factors. Thus, while our results differ from some studies, we cannot rule out the potential contribution of these factors to SIBO. Future research with larger sample sizes, multicenter designs, and more rigorous control of confounding variables is needed to better understand the links between smoking, alcohol, obesity, and SIBO.
To clarify the impact of gastric acid-suppressing drugs on the development of SIBO, we conducted a stratified analysis based on drug type, dosage, and duration of use. Specifically, we evaluated the effects of PPIs, H2RAs, and PCABs on SIBO occurrence. Despite our detailed analysis, we found that neither the type of drug nor its dose or duration of use significantly influenced the incidence of SIBO. This suggests that gastric acid suppression, under the conditions studied, may not substantially increase SIBO risk. Several factors explain this result. First, long-term PPIs use is a known risk factor for SIBO, while particularly in patients with refractory GERD,37 most participants in our study used acid suppressants for a short duration (9–10 days), which may not be sufficient to significantly impact the microbiota or SIBO risk. This is consistent with previous studies showing that over 90% of patients do not develop SIBO after 7 days of PPIs therapy.38 Long-term use,39 particularly exceeding one year40 and in elderly patients, has been associated with a higher SIBO incidence. These findings suggest that the duration of acid suppression is more critical than short-term use in determining SIBO risk. Second, the average daily dose of acid suppressants in our cohort was relatively low (9–10 mg), which may have limited any significant effect on gastric acid suppression. Additionally, the potential impact of reduced gastric acid may have been offset by dietary factors, such as the buffering effect of food intake. Lastly, the use of PCABs in our study was limited, as these drugs are relatively new and have not yet been widely adopted at our institution. Consequently, PPIs remain the primary treatment for acid suppression. Future studies with larger sample sizes and longer follow-up durations are needed to more comprehensively assess the role of acid suppressants in the development of SIBO.
We further examined the alterations in gut microbiota associated with GERD and SIBO. Research on the gut microbiota imbalance in patients with both GERD and SIBO is still limited. Previous studies have shown that changes in gastric acid levels can alter microbial communities, primarily consisting of Proteobacteria, Firmicutes, Bacteroidetes, Actinobacteria, and Fusobacteria.41–44 Our study further investigates the impact of gastric and small intestinal changes on the gut microbiota, finding that the predominant microbial communities in GERD and SIBO patients were Bacteroidetes, Firmicutes, and Proteobacteria, consistent with previous research.42–44 Bacteroidetes are involved in the breakdown of complex polysaccharides, providing nutrients for other gut microorganisms. Firmicutes, including lactic acid bacteria like Lactobacillus, ferment carbohydrates to produce lactic acid and help maintain microbiota balance. In contrast, Proteobacteria include pathogenic bacteria such as Escherichia coli and Salmonella, which can disrupt intestinal function.45 Reduced gastric acid levels may promote the growth of these pathogens, increasing the risk of infections.45 Our study also found a significant correlation between Bacteroides and both GERD and SIBO. Bacteroides, anaerobic Gram-negative bacteria, play a key role in digesting polysaccharides, proteins, and fats, thus supporting microbiota balance. Notably, bacteroides uniformis, a probiotic strain, has been shown to improve the gut-adipose tissue axis, reduce body weight and blood lipids, and enhance glucose tolerance in obese mice.46 Bacteroides uniformis also promotes the growth of beneficial bacteria like Bifidobacteria and Lactobacilli, regulates bile acid metabolism, and inhibits pathogenic bacteria such as Escherichia coli and Shigella, helping to stabilize the gut microbiota. We hypothesize that SIBO and GERD patients may have gastrointestinal motility abnormalities, such as delayed gastric emptying or reduced intestinal peristalsis, which prolong bacterial residence time in the gut. This extended exposure may facilitate the growth of Bacteroides and other bacterial communities, exacerbating the imbalance in the gut microbiota.
An interesting unexpected finding, our retrospective study found a strong association between CH₄-producing microbiota and the coexistence of GERD and SIBO. 16S rRNA analysis revealed that Bacteroidetes was one of the primary differentially expressed bacterial groups in GERD patients with SIBO. This suggests that an increase in CH₄-producing bacteria may lead to changes in the Bacteroidetes community. Under normal conditions, CH₄ slows gastrointestinal motility and extends the residence time of food in the intestine, aiding nutrient absorption. Some bacteria produce CH₄ during food breakdown, while others utilize it as an energy source. Our findings suggest that SIBO-CH₄ positivity reflects changes in the Bacteroidetes microbiota, which is linked to the development of GERD. Previous research has shown that Bacteroides bacteria produce more CH₄ than other bacterial populations, supporting our hypothesis that Bacteroides may be the main contributors to increased SIBO-CH₄ levels.47 This further highlights the relationship between CH₄-producing bacteria and gut microbiota imbalance in patients with both GERD and SIBO. We speculate that alterations in the Bacteroidetes microbiota, especially the increase in CH₄-producing bacteria, may influence the progression of GERD by affecting gastrointestinal motility and intestinal dynamics. Additionally, CH₄-producing bacteria may play a key role in maintaining the gut microbiota’s ecological balance, providing new insights into the interactions between GERD and SIBO.
To identify key metabolic changes among the groups, we also performed a multivariate analysis of fecal metabolites. Our findings suggest that the primary metabolic pathway altered in SIBO-positive GERD patients is the ATP-binding cassette (ABC) transporter pathway. ABC transporters are membrane proteins that use ATP hydrolysis to transport various substrates (eg, ions, sugars, lipids, and metabolites) across cell and organelle membranes. Previous studies have identified ABC transporters as therapeutic targets for various diseases,48 and they are considered “gatekeepers” of intestinal health.49 Recent research indicates that ABC transporters can help reduce esophageal reflux by reversing bacterial, inflammatory, and immune-related changes induced by gastroesophageal reflux, suggesting their potential as targets for treating reflux symptoms.50 Moreover, ABC transporters are crucial for transporting essential nutrients like bile acids, lipids, and vitamins, which are vital for maintaining gut microbiota balance. Disruptions in ABC transporter function can cause nutrient imbalances, promoting bacterial overgrowth. Additionally, overexpression of ABC transporters may contribute to intestinal dysbiosis in GERD patients, potentially worsening disease symptoms. In conclusion, our study underscores the importance of ABC transporters in the pathogenesis of GERD and SIBO. Targeting these transporters could offer new therapeutic approaches for managing these conditions.
This study has several limitations. First, as a cross-sectional design, it identifies correlations between GERD, SIBO, and gut microbiota but cannot establish causality. Additionally, the lack of basic experimental validation prevents a mechanistic explanation for these relationships. Future research with longitudinal or experimental models is needed to confirm the causal links between GERD and SIBO. Second, the study’s sample is limited to a single hospital, resulting in a relatively small sample size. This introduces the potential for selection bias and reduces the generalizability of the findings. To enhance the representativeness and external validity of the results, future studies should include larger, multicenter cohorts from diverse regions or institutions. Cross-cultural and cross-regional studies would further validate the applicability of these findings to broader populations.
Conclusion
In conclusion, our study demonstrates a significant association between SIBO and GERD, with GERD identified as an independent risk factor for the development of SIBO. The incidence of GERD is notably higher in SIBO-positive patients, and the risk is further elevated in GERD patients with a positive CH₄ breath test. However, short-term use of acid-suppressing drugs did not appear to significantly affect the occurrence of SIBO. Additionally, the gut microbiota of patients with both GERD and SIBO exhibited substantial alterations, primarily involving bacteroides uniformis and metabolites associated with ABC transporters.
Funding Statement
No funds were received.
Data Sharing Statement
Due to privacy concerns, some of the data in this retrospective study are stored by the authors. Upon request, original data can be obtained via Email from the corresponding author. Data related to the microbial community analysis conducted in this research are available in online repositories. For privacy reasons, all clinical data from this study are kept by the authors, if you require the original data, you can obtain it by sending an Email to the corresponding author. The gut microbiome dataset analyzed during the current study has been submitted to NCBI and is in the process of being reviewed by NCBI, [BioProject ID PRJNA1201557].
Ethics Approval and Informed Consent
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Beijing Shijitan Hospital, Affiliated with Capital Medical University [registration number: sjtky11-1x-2022 (63)]. All patients were informed and agreed. We obtained informed consent from patients or their immediate family members after informing them of the purpose and significance of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflict of interests.
References
- 1.Hunt R, Armstrong D, Katelaris P. et al. Review Team. World Gastroenterology Organisation Global Guidelines: GERD Global Perspective on Gastroesophageal Reflux Disease. J Clin Gastroenterol. 2017;51(6):467–478. doi: 10.1097/MCG.0000000000000854 [DOI] [PubMed] [Google Scholar]
- 2.Eusebi LH, Ratnakumaran R, Yuan Y, Solaymani-Dodaran M, Bazzoli F, Ford AC. Global prevalence of, and risk factors for, gastro-oesophageal reflux symptoms: a meta-analysis. Gut. 2018;67(3):430–440. doi: 10.1136/gutjnl-2016-313589 [DOI] [PubMed] [Google Scholar]
- 3.Fock KM, Talley N, Goh KL, et al. Asia-Pacific consensus on the management of gastro-oesophageal reflux disease: an update focusing on refractory reflux disease and Barrett’s oesophagus. Gut. 2016;65(9):1402–1415. doi: 10.1136/gutjnl-2016-311715 [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Zou DW. Epidemiology and Risk Factors of Gastroesophageal Reflux Disease. Clin Focus. 2017;32:01):1–4. [Google Scholar]
- 5.Ye X, Yu F, Zhou J, Zhao C, Wu J, Ni X. Analysis of the gut microbiota in children with gastroesophageal reflux disease using metagenomics and metabolomics. Front Cell Infect Microbiol. 2023;13:1267192. doi: 10.3389/fcimb.2023.1267192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Revaiah PC, Kochhar R, Rana SV, et al. Risk of small intestinal bacterial overgrowth in patients receiving proton pump inhibitors versus proton pump inhibitors plus prokinetics. JGH Open. 2018;2(2):47–53. doi: 10.1002/jgh3.12045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zmora N, Suez J, Elinav E. You are what you eat: diet, health and the gut microbiota. Nat Rev Gastroenterol Hepatol. 2019;16(1):35–56. doi: 10.1038/s41575-018-0061-2 [DOI] [PubMed] [Google Scholar]
- 8.Manson JM, Rauch M, Gilmore MS. The commensal microbiology of the gastrointestinal tract. Adv Exp Med Biol. 2008;635:15–28. [DOI] [PubMed] [Google Scholar]
- 9.Banaszak M, Górna I, Woźniak D, Przysławski J, Drzymała-Czyż S. Association between gut dysbiosis and the occurrence of SIBO, LIBO, SIFO and IMO. Microorganisms, 11 3 573 doi: 10.3390/microorganisms11030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skrzydło-Radomańska B, Cukrowska B. How to recognize and treat small intestinal bacterial overgrowth? J Clin Med. 2022;11(20):6017. doi: 10.3390/jcm11206017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda Y, Murakami T 2023. Diagnosis by Microbial Culture, Breath Tests and Urinary Excretion Tests, and Treatments of Small Intestinal Bacterial Overgrowth. Antibiotics.Basel: Vol. 122 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng Q, Chen WD, Wang YD. Gut microbiota: an integral moderator in health and disease. Front Microbiol. 2018;9:151. doi: 10.3389/fmicb.2018.00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang K, Wang S, Chen Y, et al. Causal relationship between gut microbiota and risk of gastroesophageal reflux disease: a genetic correlation and bidirectional Mendelian randomization study. Front Immunol. 2024;15:1327503. doi: 10.3389/fimmu.2024.1327503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao FY, Cui LH. Analysis of the correlation between small intestinal bacterial overgrowth and gastroesophageal reflux disease. Med J Chin People’s Liberation Army. 2023;48(09):1076–1080. [Google Scholar]
- 15.Shi YC, Cai ST, Tian YP, et al. Effects of proton pump inhibitors on the gastrointestinal microbiota in gastroesophageal reflux disease. Genomics Proteomics Bioinf. 2019;17(1):52–63. doi: 10.1016/j.gpb.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dial MS. Proton pump inhibitor use and enteric infections. Am J Gastroenterol. 2009;104(Suppl 2):S10–6. doi: 10.1038/ajg.2009.46 [DOI] [PubMed] [Google Scholar]
- 17.Kanu JE, Soldera J. Treatment of Helicobacter pylori with potassium competitive acid blockers: a systematic review and meta-analysis. World J Gastroenterol. 2024;30(9):1213–1223. PMID: 38577188; PMCID: PMC10989498.doi: 10.3748/wjg.v30.i9.1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quigley EMM, Murray JA, Pimentel M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. Gastroenterology. 2020;159(4):1526–1532. doi: 10.1053/j.gastro.2020.06.090 [DOI] [PubMed] [Google Scholar]
- 19.Chinese Society of Gastroenterology, Chinese Medical Association. Chinese expert consensus of gastroesophageal reflux disease in 2020. Chin J Dig. 2020;40(10):649–663. [DOI] [PubMed] [Google Scholar]
- 20.Lundell LR, Dent J, Bennett JR, et al. Gut. Endoscopic assessment of oesophagitis: clinical and functional correlates and further validation of the Los Angeles classification. Gut. 1999; 452:172–180. doi: 10.1136/gut.45.2.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):6494):973–980. doi: 10.1126/science.aay9189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra A, Lai GC, Yao LJ, et al. Microbial exposure during early human development primes fetal immune cells. Cell. 2021;184(13):3394–3409.e20. doi: 10.1016/j.cell.2021.04.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parhi L, Alon-Maimon T, Sol A, et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat Commun. 2020;11(1):3259. doi: 10.1038/s41467-020-16967-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Z, Luo M, Zhang H, Yin Y, Cai Y, Zhu ZJ. Metabolite annotation from knowns to unknowns through knowledge-guided multi-layer metabolic networking. Nat Commun. 2022;13(1):6656. PMID: 36333358; PMCID: PMC9636193.doi: 10.1038/s41467-022-34537-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lyu F, Han F, Changli G, et al. “OmicStudio: a composable bioinformatics cloud platform with real‐time feedback that can generate high‐quality graphs for publication.” iMeta; (2023): n. pag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katzka DA, Kahrilas PJ. Advances in the diagnosis and management of gastroesophageal reflux disease. BMJ. 2020;371:m3786. [DOI] [PubMed] [Google Scholar]
- 27.Bushyhead D, Quigley EM. Small Intestinal Bacterial Overgrowth. Gastroenterol Clin North Am. 2021;50(2):463–474. doi: 10.1016/j.gtc.2021.02.008 [DOI] [PubMed] [Google Scholar]
- 28.Gudan A, Kozłowska-Petriczko K, Wunsch E, Bodnarczuk T, Stachowska E. Small intestinal bacterial overgrowth and non-alcoholic fatty liver disease: what do we know in 2023? Nutrients. 2023;15(6):1323. doi: 10.3390/nu15061323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan Y, Lei Y, Qu Y, et al. Bacteroides uniformis-induced perturbations in colonic microbiota and bile acid levels inhibit TH17 differentiation and ameliorate colitis developments. NPJ Biofilms Microbiomes. 2023;9(1):56. doi: 10.1038/s41522-023-00420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorens J, Froehlich F, Schwizer W, et al. Gut. Bacterial overgrowth during treatment with omeprazole compared with cimetidine: a prospective randomised double blind study. Gut. 1996; 391:54–59. doi: 10.1136/gut.39.1.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–1357. [DOI] [PubMed] [Google Scholar]
- 32.Atuma C, Strugala V, Allen A, Holm L. The adherent gastrointestinal mucus gel layer: thickness and physical state in vivo. Am J Physiol Gastrointest Liver Physiol. 2001;280(5):G922–999. doi: 10.1152/ajpgi.2001.280.5.G922 [DOI] [PubMed] [Google Scholar]
- 33.Bamba S, Imai T, Sasaki M, et al. Altered gut microbiota in patients with small intestinal bacterial overgrowth. J Gastroenterol Hepatol. 2023;38(1):61–69. doi: 10.1111/jgh.16013 [DOI] [PubMed] [Google Scholar]
- 34.Kvit KB, Kharchenko NV, Kharchenko VV, et al. The role of small intestinal bacterial overgrowth in the pathogenesis of hyperlipidemia. Wiad Lek. 2019;72(4):645–649. doi: 10.36740/WLek201904127 [DOI] [PubMed] [Google Scholar]
- 35.Ling Z, Liu X, Cheng Y, Yan X, Wu S. Gut microbiota and aging. Gut Microbiota and Aging Crit Rev Food Sci Nutr. 2022;62(13):3509–3534. doi: 10.1080/10408398.2020.1867054 [DOI] [PubMed] [Google Scholar]
- 36.Sroka N, Rydzewska-Rosołowska A, Kakareko K, Rosołowski M, Głowińska I, Hryszko T. Show me what you have inside-the complex interplay between SIBO and multiple medical conditions-A systematic review. Nutrients. 2022;15(1):90. doi: 10.3390/nu15010090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu J, Qu Q, Yang Y, et al. The Lactulose Breath Test Can Predict Refractory Gastroesophageal Reflux Disease by Measuring Bacterial Overgrowth in the Small Intestine. J Clin Gastroenterol. 2024. PMID: 38896424. doi: 10.1097/MCG.0000000000002031. [DOI] [PubMed] [Google Scholar]
- 38.Durán-Rosas C, Priego-Parra BA, Morel-Cerda E, et al. Incidence of small intestinal bacterial overgrowth and symptoms after 7 days of proton pump inhibitor use: a study on healthy volunteers. Dig Dis Sci. 2024;69(1):209–215. PMID: 37910339.doi: 10.1007/s10620-023-08162-2 [DOI] [PubMed] [Google Scholar]
- 39.Lombardo L, Foti M, Ruggia O, Chiecchio A. Increased incidence of small intestinal bacterial overgrowth during proton pump inhibitor therapy. Clin Gastroenterol Hepatol. 2010;8(6):504–508. doi: 10.1016/j.cgh.2009.12.022 [DOI] [PubMed] [Google Scholar]
- 40.Lim NR, Lim S, Chung WC. A study on the glucose breath test positivity rate and occurrence of small intestine bacterial overgrowth-related symptoms caused by long-term use of Proton Pump Inhibitor (PPI) Versus Potassium-Competitive Acid Blocker (P-CAB) in Elderly Patients. SIBO Between PPI and P-CAB Adv Pharmacol Pharm Sci. 2024;2024:6069151. doi: 10.1155/2024/6069151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stockbrugger RW, Cotton PB, Eugenides N, Bartholomew BA, Hill MJ, Walters CL. Intragastric nitrites, nitrosamines, and bacterial overgrowth during cimetidine treatment. Gut. 1982;23(12):1048–1054. [Gut]. doi: 10.1136/gut.23.12.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, Engstrand L. Comparative analysis of human gut microbiota by barcoded pyrosequencing. PLoS One. 2008;3(7):e2836. doi: 10.1371/journal.pone.0002836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach. Proc Natl Acad Sci U S A, 103 3 732–737 doi: 10.1073/pnas.0506655103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li XX, Wong GL, To KF, et al. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS One. 2009;4(11):e7985. doi: 10.1371/journal.pone.0007985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clooney AG, Bernstein CN, Leslie WD, et al. A comparison of the gut microbiome between long-term users and non-users of proton pump inhibitors. Aliment Pharmacol Ther. 2016;43(9):974–984. doi: 10.1111/apt.13568 [DOI] [PubMed] [Google Scholar]
- 46.Fabersani E, Portune K, Campillo I, et al. Bacteroides uniformis CECT 7771 alleviates inflammation within the gut-adipose tissue axis involving TLR5 signaling in obese mice. Sci Rep. 2021;11(1):11788. doi: 10.1038/s41598-021-90888-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKay LF, Holbrook WP, Eastwood MA. Methane and hydrogen production by human intestinal anaerobic bacteria. Acta Pathol Microbiol Immunol Scand B. 1982;90(3):257–260. doi: 10.1111/j.1699-0463.1982.tb00114.x [DOI] [PubMed] [Google Scholar]
- 48.Fan J, To KKW, Chen ZS, Fu L. ABC transporters affects tumor immune microenvironment to regulate cancer immunotherapy and multidrug resistance. Drug Resist Updat. 2023;66:100905.PMID: 36463807. doi: 10.1016/j.drup.2022.100905 [DOI] [PubMed] [Google Scholar]
- 49.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52(12):1788–1795. PMID: 14633964; PMCID: PMC1773875.doi: 10.1136/gut.52.12.1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Villanueva S, Zhang W, Zecchinati F, Mottino A, Vore M. ABC Transporters in Extrahepatic Tissues: pharmacological Regulation in Heart and Intestine. Curr Med Chem. 2019;26(7):1155–1184. PMID: 29589524.doi: 10.2174/0929867325666180327092639 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to privacy concerns, some of the data in this retrospective study are stored by the authors. Upon request, original data can be obtained via Email from the corresponding author. Data related to the microbial community analysis conducted in this research are available in online repositories. For privacy reasons, all clinical data from this study are kept by the authors, if you require the original data, you can obtain it by sending an Email to the corresponding author. The gut microbiome dataset analyzed during the current study has been submitted to NCBI and is in the process of being reviewed by NCBI, [BioProject ID PRJNA1201557].